Abstract
Sex‐determining region Y‐box 2 (SOX2) is an essential factor involved in the self‐renewal and pluripotency of embryonic stem cells and has functions in cell survival and progression in many types of cancers. Here, we found that several endometrial cancer cell lines expressed SOX2, which was required for cell growth. Additionally, SOX2 overexpression regulated the expression of cyclin‐dependent kinase inhibitor 1A (CDKN1A), and SOX2 specifically bound to p21 promoter DNA in endometrial cancer cell lines expressing SOX2. Expressions of SOX2 in endometrial cancer patients were significantly correlated with histological grade and poor prognosis. Moreover, low p21 together with high SOX2 expressions in advanced endometrial cancer patients were associated with the most unfavorable outcomes of patients. These results indicated that simultaneous measurement of SOX2 and p21 expression in endometrial cancer patients may be a useful biomarker for patient prognosis.
Keywords: Biomarker, cell cycle, endometrial cancer, p21, sex‐determining region Y‐box 2
Endometrial cancer is one of the most frequent gynecological malignancies worldwide.1 Most of the endometrial cancer patients are diagnosed as International Federation of Gynecology and Obstetrics (FIGO) stage I (Table S1).1, 2 These patients with early stage disease can often be cured by complete surgical removal of the tumor with or without adjuvant chemotherapy. However, about 20% of endometrial cancer cases are diagnosed as the advanced stage, in which tumors have spread to the abdominal or pelvic cavities and/or lymph nodes. The survival rates of patients with advanced disease are poor; in particular, the 5‐year overall survival rate for endometrial cancers with FIGO stage III and IV cancers is 20%–26%.2, 3, 4 An effective chemotherapy for endometrial cancer has not yet been established and the efficacy of radiotherapy is far from satisfactory;1, 5, 6 indeed, radiotherapy does not extend overall survival of patients with this disease.7, 8, 9
Several reports have established that multiple transcriptional factors, including sex‐determining region Y‐box 2 (SOX2), octamer‐binding transcription factor (OCT‐3/4), and Nanog, comprise a set of essential factors promoting the self‐renewal and pluripotency of embryonic stem (ES) cells;10, 11 these factors form a key regulatory network controlling the identity and replication of ES cells with other coregulators.12 In addition, Kruppel‐like factor 4 (KLF4), Myc, and LIN28, have been reported to be critical factors for the establishment of induced pluripotent stem cells from somatic cells.13, 14, 15
These transcriptional factors regulating the pluripotency of normal stem cells are essential for maintenance of several types of cancer cells.16 In addition, SOX2 has been reported to be essential for cell survival and cancer progression and initiation in many types of cancers, including glioblastoma,17 breast cancer,18 pancreatic cancer,19, 20 gastric cancer,21 melanoma,22 lung cancer,23 osteosarcoma,24 and Ewing sarcoma.25 Recently, we also demonstrated that SOX2 expression was required for the growth and survival of ovarian cancer cells.26 In most types of cancers, SOX2 acts as an oncogenic transcriptional factor, and thus it may become a novel therapeutic target in these cancers.22, 23, 27, 28, 29 However, the roles of SOX2 in endometrial cancer are still unclear.30, 31
In this study, we examine the role and function of SOX2 in uterine endometrial cancer. We found that SOX2 expression was correlated with poor outcomes in patients with endometrial cancer, and that SOX2 was a critical factor for the proliferation of endometrial cancer cells.
Materials and Methods
Clinical specimens
All 258 uterine endometrial endometrioid adenocarcinoma tumors were resected from patients who had undergone initial surgery at Niigata University Medical and Dental Hospital between 1 January 2006 and 31 December 2013. All patients provided informed consent for participation in the study, and all procedures were performed under a protocol approved by the Ethics Committee of Niigata University. Histopathological diagnoses were based on World Health Organization criteria, tumor grading was based on Gynecologic Oncology Group criteria, and disease stage was assigned according to the FIGO staging system.
Immunohistochemical (IHC) analyses
Primary or mouse xenograft tumors were fixed in neutral formalin, embedded in paraffin, and used for staining with hematoxylin and eosin. For immunostaining, the sections were stained following standard IHC methods, as previously described,26 with slight modifications. Briefly, after deparaffinization, antigen retrieval was carried out with Target Retrieval Solution (10 mM citrate buffer, pH 6.0; Dako) in a microwave for 30 min at 96°C. Subsequently, the sections were incubated with primary anti‐SOX2, anti‐p21 (1:200 dilution; Cell Signaling Technology, Danvers, MA, USA), anti‐ p53 or anti‐Ki‐67 antibodies (1:200 dilution; Dako) and biotinylated secondary antibodies (Vector Laboratories, Burlingame, CA, USA), followed by incubation with ABC reagent (Dako) and 3,3’‐diaminobenzidine (DAB; Sigma, St. Louis, MO, USA). Slides were counterstained with hematoxylin.
IHC scoring
The evaluation of immunostaining was performed independently by two observers (K. Yamawaki and T.I.). Images were taken using a BX51 microscope (Olympus, Tokyo, Japan). Five random bright‐field, high‐magnification images were evaluated for each sample. Staining of SOX2 and p21 was scored according to nuclear staining of cancer cells. The staining intensity was visually evaluated on a scale of 0 (no staining), 1 (weak staining), 2 (moderate staining), and 3 (strong staining) (Figs 1a and 5a). Samples with moderate or strong nuclear staining (staining intensity scored as 2 or 3) in at least 10% of cancer cells were regarded as “IHC‐positive”, and samples with absent or weak staining (staining intensity scored as 0 or 1) and less than 10% staining of cells were regarded as “IHC‐negative”. Nuclear staining for p53 and Ki‐67 was performed and the stained cancer cells were counted; when more than half of the cancer cells were stained, the sample was regarded as “IHC‐positive”.32
Figure 1.
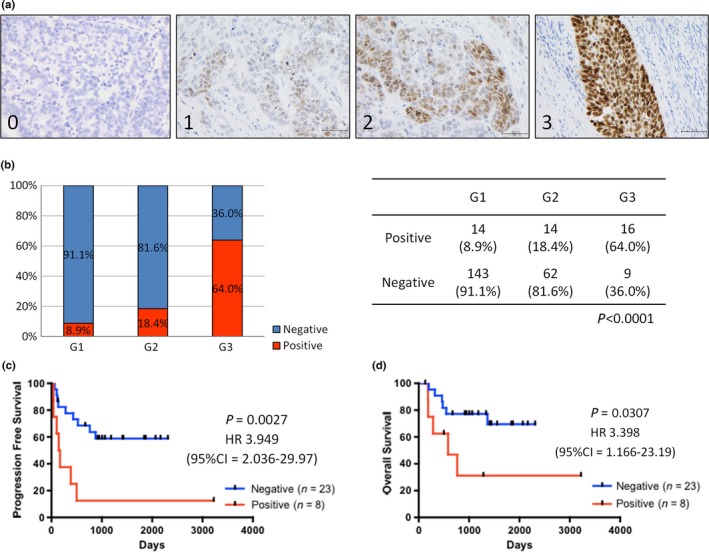
SOX2 expression is correlated with histological grade and poor prognosis in endometrial cancer. (a) Representative immunostaining results for SOX2 (score 0–3). Scale bars: 100 μm. (b) Distribution of SOX2 expression in different histological grades of endometrial cancer (n = 258). (c,d). Kaplan–Meier analyses of progression‐free survival (c) and overall survival (d) in patients with advanced‐stage endometrial cancer. The patients were stratified into SOX2‐positive (red lines, n = 8) and SOX2‐negative (blue lines, n = 23) groups.
Cell culture
HEC1, HEC1A, HEC59, HEC151, HEC50B, Ishikawa, and PA‐1 cells were obtained from the Japanese Collection of Research Bioresources Cell Bank (Japan). JHUEM3 and JHUEM7 cells were obtained from RIKEN BioResource Center (Japan). AN3CA cells were obtained from American Type Culture Collection (Manassas, VA, USA). EN cells were a kind gift from Dr. Isaka (Tokyo Medical and Dental University, Japan).33 EN cells were cultured in Dulbecco's modified Eagle medium (DMEM)/F‐12 (Invitrogen, Carlsbad, CA, USA), and other cancer cells were cultured in DMEM (Invitrogen), supplemented with 10% fetal bovine serum (Invitrogen), penicillin, and streptomycin (Gibco). All cells were maintained at 37°C in an atmosphere containing 5% CO2.
Western blot analyses
Cells were lysed in RIPA buffer (50 mM Tris at pH 8.0, 150 mM NaCl, 1% Nonidet P‐40, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate [SDS], and 1 mM EDTA) supplemented with protease inhibitors (Roche). The lysates were separated by SDS‐polyacrylamide gel electrophoresis on 5%–20% gradient gels and transferred to polyvinylidene difluoride (PVDF) membranes (Millipore). Western blot analyses were performed as previously described.34 Nuclear extracts and extracts of other subcellular fractions were prepared using an NE‐PER Nuclear and Cytoplasmic Extraction Reagent Kit (Thermo Scientific, Rockford, IL, USA) according to the manufacturer's instructions.
Immunocytochemical analyses
Adherent cells were fixed in 4% paraformaldehyde in phosphate‐buffered saline (PBS) for 15 min, washed with 1× PBS, permeabilized in PBS/0.1% Triton X‐100 for 5 min at 4°C, and blocked with PBS containing 3% bovine serum albumin (BSA). The fixed cells were then used for immunostaining with anti‐SOX2 and anti‐phalloidin antibodies (Cell Signaling Technology) and stained with 4’,6‐diamidino‐2‐phenylindole (DAPI).
Real‐time polymerase chain reaction (PCR) analyses
RNA was isolated from cells with an miRNeasy Mini kit (Qiagen, Valencia, CA, USA), and cDNA was synthesized from total RNA using a PrimeScript First Strand cDNA Synthesis Kit (Takara, Shiga, Japan). Gene expression levels were measured using SYBR Premix Ex Taq II (Takara) with primers targeting the SOX2 promoter (HA173580; TAKARA) and Taqman gene expression assays (Applied Biosystems, Foster City, CA, USA) with the primer/probe set (Hs01053049_s1) according to the manufacturer's instructions. Levels of glyceraldehyde 3‐phosphate dehydrogenase (GAPDH) were measured to calculate delta Ct values.
siRNA transfection
HEC59, EN, and JHUEM7 cell (2 × 105 cells/well) were transfected with 4 pmol siRNA targeting SOX2 mRNA in the presence of 20 μL Hiperfect (Qiagen) for 2 days. SOX2 siRNAs (si#6: 5′‐CTGCCGAGAATCCATGTATAT‐3′, si#7: 5′‐CCAUGGGUUCGGUGGUCAATT‐3′) and control siRNA were purchased from Qiagen.
Cell viability assay
Cell growth was quantified by measuring the amounts of cellular ATP using CellTiter‐Glo Luminescent Cell Viability Assays (Promega, Madison, WI, USA) according to the manufacturer's instructions. The intensity of luminescence was measured using a FLUOROSCAN instrument (Thermo Scientific).
Xenograft establishment
Cells were dissociated into single cells with trypsin/ethylenediaminetetraacetic acid (EDTA; Gibco), suspended in 100 μL medium containing 50% Matrigel (BD Biosciences, Bedford, MA, USA), and used for subcutaneous injection into the flanks of NOG (NOD/Shi‐scid IL‐2rgnull) mice (Central Institute for Experimental Animals, Kawasaki, Japan) with a 27‐gauge needle. Mice were monitored every 2–3 days until 5 weeks postinjection. All animal experiments and protocols were approved by the Animal Care and Use Committees of Niigata University and performed in accordance with institutional policies.
Cell cycle analysis and cell sorting
Fixed cells in methanol were stained with 25 μg/mL propidium iodide and 50 μg/mL RNase, as previously described.35 All flow cytometry and cell sorting analyses were carried out using a FACS Aria II (BD Biosciences). Growing cells were incubated with 5 μg/mL Hoechst 33342 (Sigma) for 1 h at 37°C in the dark. After trypsinization, cells were sorted based on the amount of DNA.36, 37
Chromatin immunoprecipitation (ChIP) assay
ChIP was conducted with a SimpleChIP Enzymatic Chromatin IP Kit (#9003; Cell Signaling Technology) according to the manufacturer's recommendations. Immunoprecipitation was carried out using anti‐SOX2 antibodies (#5024; Cell Signaling Technology), normal rabbit IgG (#2729; Cell Signaling Technology) as a negative control, and anti‐histone H3 antibodies (#4620; Cell Signaling Technology) as a positive control. Quantification of DNA by real‐time PCR was performed as described above with primers targeting the CDKN1A promoter (#6449; Cell Signaling Technology) and RPL30 promoter (#7014; Cell Signaling Technology).
Statistical analysis
Clinicopathological parameters were analyzed using Fisher's exact test. Univariate survival analysis was performed using the Kaplan‐Meier method, and the significance of difference between groups was analyzed using the log‐rank test. Multivariate survival analysis was carried out using Cox proportional hazards regression model. For survival analysis, patients who also had other types of cancer, for example, ovarian cancer, or were treated with chemotherapy before surgery were excluded, and a total of 241 patients, including 201 patients with stage I cancer and 31 patients with advanced stage cancer, were subjected to survival analysis (Table S2). Differences with P‐values of less than 0.05 were considered statistically significant. All statistical analyses were performed using GraphPad Prism software (GraphPad Software Inc., La Jolla, CA, USA) or JMP software (SAS Institute Inc., Cary, NC, USA).
Results
SOX2 expression is correlated with histological grade and poor prognosis in endometrial cancer
To investigate the clinical importance of SOX2 in endometrial cancer, we evaluated SOX2 expression levels in 258 human uterine endometrial endometrioid adenocarcinoma tissues by IHC (Fig. 1a). Patient characteristics are summarized in Table S1. The expression of SOX2 in endometrial cancer tissues were detected more in high‐grade patients than low‐grade ones, and there were significantly correlated with histological grade (Fig. 1b; P < 0.0001, Fisher's exact test); 64.0% (16/25 cases) of high‐grade (grade 3) endometrial cancer cases exhibited positive staining for SOX2, whereas only 8.9% (14/158 cases) of low‐grade (grade 1) cases were positive for SOX2 staining. These results were consistent with the findings of previous reports of many types of cancers, including cancers of head and neck, and ovary.18, 38, 39 The correlation between SOX2 expression and histological grade was also consistent in both early and advanced stage disease (Table S2).
There were no significant differences in the rates of SOX2 positivity between early and advanced stage groups (Fig. S1a). Kaplan–Meier analyses of 31 advanced stage endometrial cancer patients (FIGO stages III and IV) showed that SOX2 expression was associated with poor prognosis (Fig. 1c,d; P = 0.0027 for progression‐free survival [PFS] and P = 0.0307 for overall survival [OS]). Moreover, multivariate analyses of clinical factors indicated that SOX2 positive staining served as an independent prognostic factor (Table S3). In 201 cases of FIGO stage I endometrial cancer, PFS of SOX2‐positive patients was significantly shorter than that of SOX2‐negative patients (Fig. S1b; P = 0.0049), and univariate analyses, not multivariate analyses, of clinical factors indicated that SOX2 positive staining served as a prognostic factor for PFS (Table S4). However, these two groups do not show any significant difference in OS, potentially because of the good prognosis of patients with early stage endometrial cancer patients,2 or the limited number of cases (Fig. S1c).
It was reported that p53 status is one of the markers useful for this discrimination between low‐grade and high‐grade endometrioid carcinoma.40 We evaluated the p53 protein expression of the same clinical tissue samples shown in Figure 1(b) (Fig. S1d). Of the total 258 cases, only 13 cases (5.0%) expressed high levels of p53 expression, which is a hallmark of p53 mutation41 (Fig. S1e). On the other hand, out of 25 cases of high‐grade (Grade 3) endometrioid carcinoma, four cases (16.0%) expressed high levels of p53 expression (Fig. S1f), which is in accord with the relatively high incidence of p53 mutation in high‐grade endometrioid carcinoma. Although levels of both p53 and SOX2 increased in high‐grade cases, we did not observe statistically significant relationship between p53 status and SOX2 levels (Fig. S1e,f).
SOX2 is overexpressed in a subset of endometrial cancer cells
Next, we investigated the expression of SOX2 in 10 endometrial cancer cell lines using western blotting and real‐time PCR. A teratocarcinoma cell line PA‐1 was used as a positive control for SOX2 expression analysis. We detected SOX2 expression in three endometrial cancer cell lines, HEC59, EN, and JHUEM7 (Fig. 2a). In particular, EN cells expressed high levels of SOX2 comparable to that in PA‐1 cells. SOX2 protein and mRNA levels in these cancer cell lines were correlated with each other (Fig. S2a). Based on these results, we used HEC59, EN, and JHUEM7 cells as typical SOX2‐positive endometrial cancer cell lines for further experiments.
Figure 2.
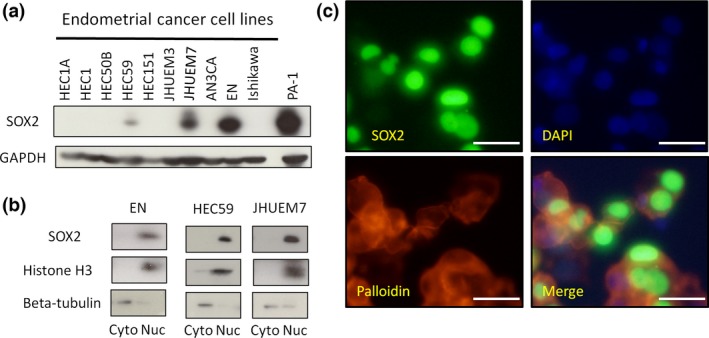
SOX2 expression in endometrial cancer cells. (a) The expression levels of SOX2 in human endometrial cancer cells, as determined by western blot analyses. PA‐1: teratocarcinoma cells used as a positive control for SOX2 expression. (b) Western blot analysis of nuclear and cytoplasmic extracts from endometrial cancer cells. Anti‐histone H3 and anti‐beta‐tubulin antibodies were used as markers of the nucleus and cytoplasm, respectively. (c) Immunostaining of EN cells for SOX2, DAPI, and phalloidin. Primary antibodies are listed in Table S5. Scale bars: 50 μm.
To investigate the subcellular localization of SOX2 protein in endometrial cancer cells, we performed western blot analysis after subcellular fractionation and immunostaining with anti‐SOX2 antibodies. These analyses showed that SOX2 was predominantly localized in the nucleus of HEC59, EN, and JHUEM7 cells; weak SOX2 expression was observed in the cytoplasm (Fig. 2b,c, Fig. S2b). Intriguingly, the expression of SOX2 in respective cells derived from the same cell lines varied (Fig. 2c, Fig. S2b,c). To further characterize this observation, we purified a single cell from EN cells, and the purified cell was cultured for 7 days, following which SOX2 expression in cells derived from a single cell was measured. Nuclear expression of SOX2 in respective EN cells derived from a single cell varied (Fig. S2d). In addition, we divided endometrial cancer cells according to the cell cycle phase (G1 phase and G2/M phase) using Hoechst 33342 staining (Fig. S2e). SOX2 protein and mRNA expression was increased in EN cells during the G1 phase (Fig. 2f,g). These results suggested that SOX2 expression fluctuated during endometrial cancer propagation, which may contribute to heterogeneous SOX2 expression in clinical endometrial cancer tissues (Fig. 1a).
SOX2 expression is required for endometrial cancer cell growth
In order to examine whether SOX2 contributed to endometrial cancer cell proliferation, we evaluated cell proliferation after knockdown of SOX2 using siRNA. Knockdown of SOX2 expression in SOX2‐positive cells reduced the proliferation of SOX2‐positive cells, including HEC59, EN, and JHUEM7 cells (Fig. 3a,b, Fig. S3a–c).
Figure 3.
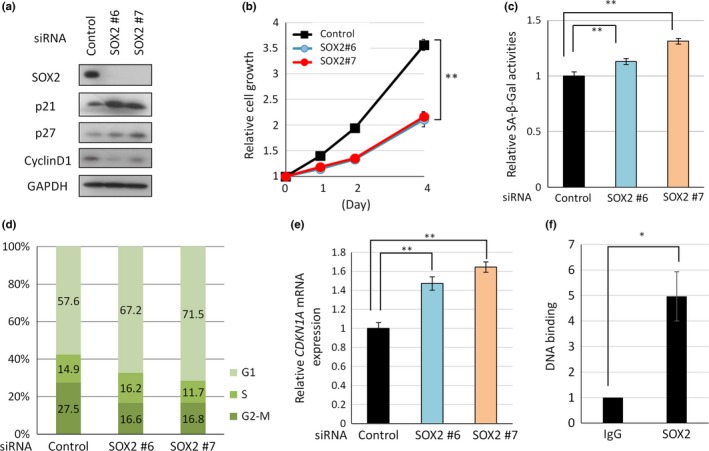
SOX2 expression is required for endometrial cancer cell growth via CDKN1A. (a) Western blot analyses. (b) Time course of cancer cell proliferation. (c) Senescence‐associated β‐galactosidase activity analysis in HEC59 cells transfected with SOX2 siRNA or control siRNA. (d) Cell cycle analysis of EN cells was performed using a FACS Aria II instrument after staining of the cells with propidium iodide. (e) CDKN1A mRNA expression levels in HEC59 cells transfected with siRNA targeting SOX2, as analyzed by quantitative real‐time PCR. (f) Chromatin immunoprecipitation assays were used to detect the SOX2‐CDKN1A interaction in HEC59 cells.
SOX2‐knockdown in endometrial cancer cells increased cell size and altered cell morphology (spreading over the dish), which are reminiscent of senescent cells (data not shown). In fact, SOX2‐knockdown cells expressed a senescence marker protein, i.e., β‐galactosidase (Fig. 3c, Fig. S3d). Moreover, because cell cycle arrest is a hallmark of cell senescence, cell cycle analysis was performed.42 This analysis showed that cells accumulated in the G1 phase after knockdown of SOX2 expression (Fig. 3d). On the other hand, SOX2‐knockdown in endometrial cancer cells did not increase the sub‐G1 fraction, representing apoptotic cells (data not shown). Furthermore, knockdown of SOX2 expression in endometrial cancer cells increased the expression of senescence‐associated cell cycle inhibitor p21 but not p27 (Fig. 3a,e, Fig. S3a,e). Taken together, these results indicated that SOX2 was required for cell cycle progression and for the inhibition of p21 expression in endometrial cancer cells.
To examine whether SOX2 inhibits p21 expression at the transcription level, we examined whether SOX2 binds to the promoter DNA of CDKN1A gene encoding p21 protein. ChIP analysis detected specific binding of SOX2 to the CDKN1A promoter DNA in both EN and HEC59 cells (Fig. 3f and Fig. S3f). These results indicated that SOX2 represses transcription of p21/CDKN1A gene through binding to CDKN1A promoter DNA in EN and HEC59 cells.
Because p21 is a potent inhibitor of cell cycle progression, we evaluated the relationship between SOX2 expression and Ki‐67 expression in order to examine whether SOX2 expression stimulates cell cycle progression via p21 inhibition. Indeed, in all 258 clinical endometrial cancer samples by IHC (Fig. 4a), expression of Ki‐67 was higher in SOX2‐positive cases than in SOX2‐negative ones (Fig. 4b; P < 0.0001, Fisher's exact test).
Figure 4.
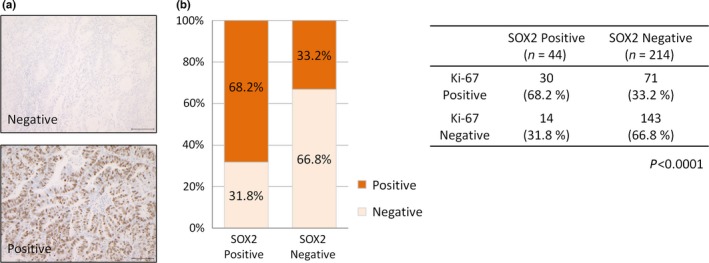
Ki‐67 expression is correlated with SOX2 expression in endometrial cancer. (a) Representative immunostaining images for Ki‐67‐negative (top) and Ki‐67‐positive (bottom) cases. Scale bars: 100 μm. (b) Distribution of Ki‐67 expression in SOX2‐positive (n = 44) and SOX2‐negative endometrial cancer cases (n = 214).
SOX2 expression mediates tumor formation in vivo
Next, we explored whether SOX2 expression in endometrial cancer cells contributed to tumorigenicity in vivo. EN cells were transfected either with SOX2 siRNA or the control siRNA, and the cells were then inoculated into the flanks of immunocompromised NOG mice. The control EN cells began to generate palpable tumors of up to 5 mm in diameter from 1 week after inoculation; tumors were observed in 8 of 10 inoculated sites at 5 weeks (Fig. 5a). In contrast, SOX2‐knockdown EN cells generated smaller tumors that were detectable at 4–5 weeks after injection in only 2 of 12 inoculated sites (Fig. 5b,c). The generated tumors originated from control and SOX2‐knockdown EN cells were histologically indistinguishable (Fig. 5a,d). These results indicated that the expression of SOX2 in endometrial cancer cells augments the tumorigenicity in vivo.
Figure 5.
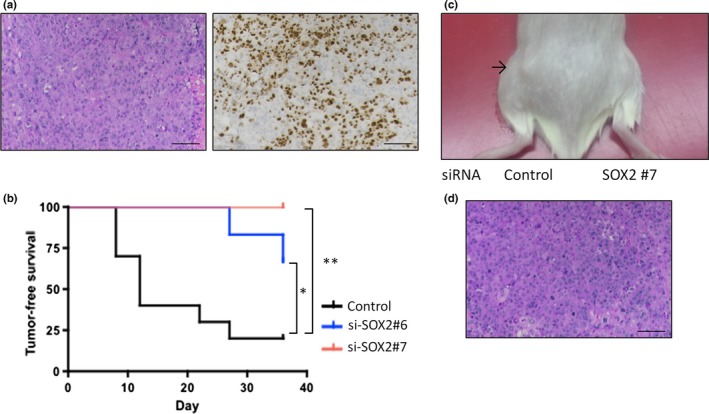
SOX2 expression mediates tumor formation in vivo. (a) HE and SOX2 immunostaining of xenograft tumors of EN cells. Scale bars: 100 μm. (b) Xenograft tumor‐free survival curve in immune‐deficient mice after injection of EN cells transfected with SOX2 siRNA (#6 and #7) or control siRNA. (c) Representative images of tumor‐bearing mice 5 weeks after injection of EN cells. The black arrow near the left flank indicates a xenograft tumor of EN cells transfected with control siRNA. There was no tumor in the right flank after injection of cells transfected with SOX2 siRNA (#7). (d) HE staining of xenograft tumors of SOX2‐knockdown EN cells (#6).
Evaluation of combined SOX2 and p21 expression in advanced endometrial cancer
The data above indicated that SOX2 in endometrial cancer cells inhibits expression of cell cycle inhibitor p21. Hence, we examined the SOX2 expression was inversely associated with that of p21. IHC analysis of p21 expression (Fig. 6a) of the 31 advanced endometrial cancer cases (Fig. 1d) stratified them into the four groups based on the levels of p21/SOX2 expression (Fig. S4). While the inverse relationship between p21 and SOX2 expression was not clear, further classification based on p53 status suggest that SOX2 expression may be inversely related to that of p21 in cases with high levels of p53 (Fig. S4).
Figure 6.
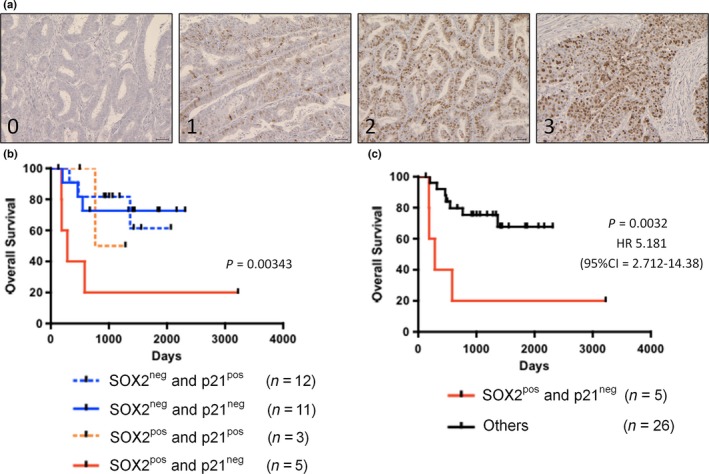
Combined expression of SOX2 and p21 is correlated with poor prognosis in advanced endometrial cancer. (a) Representative immunostaining for p21 (score 0–3). Scale bars: 100 μm. (b,c) Kaplan–Meier analyses of overall survival in patients with advanced‐stage endometrial cancer. (b) Patients were stratified into four groups: (i) SOX2‐positive and p21‐negative (red lines, n = 5); SOX2‐positive and p21‐positive (red dotted lines, n = 3); SOX2‐negative and p21‐negative (blue lines, n = 11), and SOX2‐negative and p21‐positive (red dotted lines, n = 12) groups. (c) The patients were also stratified into two groups: (i) SOX2‐positive and p21‐negative (red lines, n = 5) and others (black lines, n = 26) groups.
Finally, we determined whether the expressions of SOX2 and p21 in patients with endometrial cancer act as a useful marker for cancer prognosis. Kaplan–Meier analyses showed that the SOX2‐positive/p21‐negative group showed the most unfavorable prognosis with regard to overall survival (OS; Fig. 6b). In addition, the SOX2‐positive/p21‐negative patients showed shorter OS than the other patients (Fig. 6c). These results suggest that the simultaneous evaluation of SOX2 and p21 in advanced endometrial cancer patients provides more accurate information for patient prognosis than either alone, regardless of p53 status.
Discussion
In this study we investigated the role of SOX2, an essential multipotent transcription factor, in endometrial cancer. Our findings indicated that expression of SOX2 in endometrial cancer patients were correlated with unfavorable histological cancer grade and poor prognosis. We also found that SOX2 augmented cell proliferation of endometrial cancer cells, and p21 was a target of SOX2 in endometrial cancer. Consistent with these observations, SOX2‐positive/p21‐negative cancers predicted the most unfavorable prognosis in advanced endometrial cancer.
Since the outcomes of advanced endometrial cancer patients are poor, novel prognosis markers are needed to improve therapeutic strategies for advanced cancer. Poorly differentiated tumors have been shown to express high levels of several stemness‐related factors that are typically enriched in ES cells.43, 44 SOX2 is overexpressed in poorly differentiated tumors more often than in well‐differentiated tumors in many types of cancers, indicating that SOX2 may act as an effective biomarker predicting the prognosis of cancer patients.18, 19, 30, 39, 45, 46, 47 Our results were consistent with these findings, and indicated for the first time that SOX2 expression was significantly correlated with histological grade endometrial cancer tissues in both early and advanced stage disease. Thus, SOX2 is a promising candidate biomarker for endometrial cancer.
SOX2 tightly regulates cancer cell proliferation, apoptotic signaling, invasion, and migration in many cancer types.17, 18, 19, 20, 21, 22, 23, 24, 25 Previous studies have shown that SOX2 is required for positive cell cycle progression and the expression of some cell cycle regulatory genes in various cancer cells. SOX2 augments the proliferation of pancreatic cancer via repressing the expression of cell cycle inhibitors CDKN1A, CDKN1B, and augmenting the expression of cell cycle stimulator CCND3.19 Knockdown of SOX2 expression in prostate cancer reduces cell proliferation and leads to upregulation of cell cycle inhibitor CDKN1B and downregulation of cell cycle stimulator CCNE1.29 Our results suggested that SOX2 mediated cancer cell proliferation and that CDKN1A was a direct target of SOX2 in endometrial cancer cells, indicating that SOX2 may be an attractive therapeutic target in endometrial cancer and other types of solid tumors.
Previous studies showed that cancer patients lacking p21 expression tend to have a poorer prognosis than patients with p21 expression in several types of cancer including endometrial cancer.48, 49 In particular, we revealed that endometrial cancer with high expression level of SOX2 and low expression level of p21 represents an aggressive malignant subgroup with significantly shortened survival. Some other factors including p53,50 smad,51 and myc52 might affect the p21 expression in some cases. Although future prospective studies are needed to validate our results, our findings suggested that simultaneous evaluation of SOX2 and p21 in endometrial cancer may be a useful biomarker for predicting the prognosis of patients. In summary, our findings supported that SOX2 and p21 may be an attractive biomarker for endometrial cancer.
Disclosure Statement
The authors have no conflict of interest.
Supporting information
Fig. S1. Comparison of SOX2 and p53 expression in endometrial cancer.
Fig. S2. SOX2 expression in human endometrial cancer cell lines.
Fig. S3. SOX2 is required to modulate the proliferation of endometrial cancer cells.
Fig. S4. Stratification of SOX2/p21 immunostaining based on p53 expression.
Table S1. Clinicopathological factors of all 258 patients with uterine corpus endometrioid adenocarcinoma presented in Figure 1b.
Table S2. Clinicopathological factors of 241 patients with uterine corpus endometrioid adenocarcinoma presented in Figure 1c, 1d, and Supplementary Figure S1.
Table S3. Univariate (a) and multivariate analyses (b) of prognostic factors for overall survival (OS) and progression‐free survival (PFS) in 31 cases of advanced‐stage cancer presented in Figure 1c and 1d.
Table S4. Univariate (a) and multivariate analyses (b) of prognostic factors for overall survival (OS) and progression‐free survival (PFS) in 201 cases of stage endometrial cancer presented in Supplementary Figure S1b and S1c.
Table S5. List of antibodies used in this study.
Acknowledgements
The authors wish to thank Naoyuki Yamaguchi and Anna Ishida for technical assistance, Keiichi Isaka for EN cells, and Teiichi Motoyama for pathological assessment. NOG mice were provided by the Central Institute for Experimental Animals (Kawasaki, Japan). This research was supported by Grant‐in‐Aid for Challenging Exploratory Research (T. E.), for Scientific Research (C) (M. Y., and M. S.), and for Young Scientists (B) (T. I.) from the Japan Society for the Promotion of Science.
Cancer Sci 108 (2017) 632–640
Funding InformationJapan Society for the Promotion of Science, (Grant / Award Number: ‘15K10707‘,’15K15598‘,’15K20132‘,’16K11133‘)
References
- 1. Morice P, Leary A, Creutzberg C, Abu‐Rustum N, Darai E. Endometrial cancer. Lancet 2016; 387: 1094–108. [DOI] [PubMed] [Google Scholar]
- 2. Aoki D. Annual report of Gynecologic Oncology Committee, Japan Society of Obstetrics and Gynecology, 2013. J Obstet Gynaecol Res 2014; 40: 338–48. [DOI] [PubMed] [Google Scholar]
- 3. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin 2015; 65: 5–29. [DOI] [PubMed] [Google Scholar]
- 4. Rauh‐Hain JA, Del Carmen MG. Treatment for advanced and recurrent endometrial carcinoma: combined modalities. Oncologist 2010; 15: 852–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Colombo N, Preti E, Landoni F et al Endometrial cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow‐up. Ann Oncol 2013; 24(Suppl 6): vi33–8. [DOI] [PubMed] [Google Scholar]
- 6. Huang HJ, Tang YH, Chou HH et al Treatment failure in endometrial carcinoma. Int J Gynecol Cancer 2014; 24: 885–93. [DOI] [PubMed] [Google Scholar]
- 7. Creutzberg CL, van Putten WLJ, Koper PCM et al Surgery and postoperative radiotherapy versus surgery alone for patients with stage‐1 endometrial carcinoma: multicentre randomised trial. Lancet 2000; 355: 1404–11. [DOI] [PubMed] [Google Scholar]
- 8. Creutzberg CL, Nout RA, Lybeert ML et al Fifteen‐year radiotherapy outcomes of the randomized PORTEC‐1 trial for endometrial carcinoma. Int J Radiat Oncol Biol Phys 2011; 81: e631–8. [DOI] [PubMed] [Google Scholar]
- 9. Kong A, Johnson N, Kitchener HC, Lawrie TA. Adjuvant radiotherapy for stage I endometrial cancer: an updated Cochrane systematic review and meta‐analysis. J Natl Cancer Inst 2012; 104: 1625–34. [DOI] [PubMed] [Google Scholar]
- 10. Boyer LA, Lee TI, Cole MF et al Core transcriptional regulatory circuitry in human embryonic stem cells. Cell 2005; 122: 947–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Boiani M, Scholer HR. Regulatory networks in embryo‐derived pluripotent stem cells. Nat Rev Mol Cell Biol 2005; 6: 872–84. [DOI] [PubMed] [Google Scholar]
- 12. Chambers I, Tomlinson SR. The transcriptional foundation of pluripotency. Development 2009; 136: 2311–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006; 126: 663–76. [DOI] [PubMed] [Google Scholar]
- 14. Takahashi K, Tanabe K, Ohnuki M et al Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 2007; 131: 861–72. [DOI] [PubMed] [Google Scholar]
- 15. Yu J, Vodyanik MA, Smuga‐Otto K et al Induced pluripotent stem cell lines derived from human somatic cells. Science 2007; 318: 1917–20. [DOI] [PubMed] [Google Scholar]
- 16. Ishiguro T, Sato A, Ohata H, Sakai H, Nakagama H, Okamoto K. Differential expression of nanog1 and nanogp8 in colon cancer cells. Biochem Biophys Res Commun 2012; 418: 199–204. [DOI] [PubMed] [Google Scholar]
- 17. Oppel F, Muller N, Schackert G et al SOX2‐RNAi attenuates S‐phase entry and induces RhoA‐dependent switch to protease‐independent amoeboid migration in human glioma cells. Mol Cancer 2011; 10: 137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chen Y, Shi L, Zhang L et al The Molecular mechanism governing the oncogenic potential of SOX2 in breast cancer. J Biol Chem 2008; 283: 17969–78. [DOI] [PubMed] [Google Scholar]
- 19. Herreros‐Villanueva M, Zhang JS, Koenig A et al SOX2 promotes dedifferentiation and imparts stem cell‐like features to pancreatic cancer cells. Oncogenesis 2013; 2: e61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jia X, Li X, Xu Y et al SOX2 promotes tumorigenesis and increases the anti‐apoptotic property of human prostate cancer cell. J Mol Cell Biol 2011; 3: 230–8. [DOI] [PubMed] [Google Scholar]
- 21. Hutz K, Mejias‐Luque R, Farsakova K et al The stem cell factor SOX2 regulates the tumorigenic potential in human gastric cancer cells. Carcinogenesis 2014; 35: 942–50. [DOI] [PubMed] [Google Scholar]
- 22. Santini R, Pietrobono S, Pandolfi S et al SOX2 regulates self‐renewal and tumorigenicity of human melanoma‐initiating cells. Oncogene 2014; 33: 4697–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Xiang R, Liao D, Cheng T et al Downregulation of transcription factor SOX2 in cancer stem cells suppresses growth and metastasis of lung cancer. Br J Cancer 2011; 104: 1410–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Basu‐Roy U, Seo E, Ramanathapuram L et al Sox2 maintains self renewal of tumor‐initiating cells in osteosarcomas. Oncogene 2012; 31: 2270–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ren C, Ren T, Yang K et al Inhibition of SOX2 induces cell apoptosis and G1/S arrest in Ewing's sarcoma through the PI3K/Akt pathway. J Exp Clin Cancer Res 2016; 35: 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ishiguro T, Sato A, Ohata H et al Establishment and characterization of an in vitro model of ovarian cancer stem‐like Cells with an enhanced proliferative capacity. Cancer Res 2016; 76: 150–60. [DOI] [PubMed] [Google Scholar]
- 27. Boumahdi S, Driessens G, Lapouge G et al SOX2 controls tumour initiation and cancer stem‐cell functions in squamous‐cell carcinoma. Nature 2014; 511: 246–50. [DOI] [PubMed] [Google Scholar]
- 28. Vanner Robert J, Remke M, Gallo M et al Quiescent Sox2+ cells drive hierarchical growth and relapse in sonic hedgehog subgroup medulloblastoma. Cancer Cell 2014; 26: 33–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lin F, Lin P, Zhao D et al Sox2 targets cyclinE, p27 and survivin to regulate androgen‐independent human prostate cancer cell proliferation and apoptosis. Cell Prolif 2012; 45: 207–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pitynski K, Banas T, Pietrus M, Milian‐Ciesielska K, Ludwin A, Okon K. SOX‐2, but not Oct4, is highly expressed in early‐stage endometrial adenocarcinoma and is related to tumour grading. Int J Clin Exp Pathol 2015; 8: 8189–98. [PMC free article] [PubMed] [Google Scholar]
- 31. Wong OG, Huo Z, Siu MK et al Hypermethylation of SOX2 promoter in endometrial carcinogenesis. Obstet Gynecol Int 2010. doi: 10.1155/2010/682504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Alkushi A, Lim P, Coldman A, Huntsman D, Miller D, Gilks CB. Interpretation of p53 immunoreactivity in endometrial carcinoma: establishing a clinically relevant cut‐off level. Int J Gynecol Pathol 2004; 23: 129–37. [DOI] [PubMed] [Google Scholar]
- 33. Isaka K, Nishi H, Sagawa Y et al Establishment of a new human cell line (EN) with TP53 mutation derived from endometrial carcinoma. Cancer Genet Cytogenet 2003; 141: 20–5. [DOI] [PubMed] [Google Scholar]
- 34. Okamoto K, Ishiguro T, Midorikawa Y et al miR‐493 induction during carcinogenesis blocks metastatic settlement of colon cancer cells in liver. EMBO J 2012; 31: 1752–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ohata H, Ishiguro T, Aihara Y et al Induction of the stem‐like cell regulator CD44 by Rho kinase inhibition contributes to the maintenance of colon cancer‐initiating cells. Cancer Res 2012; 72: 5101–10. [DOI] [PubMed] [Google Scholar]
- 36. Juan G, Hernando E, Cordon‐Cardo C. Separation of live cells in different phases of the cell cycle for gene expression analysis. Cytometry 2002; 49: 170–5. [DOI] [PubMed] [Google Scholar]
- 37. Quintana AM, Zhou YE, Pena JJ, O'Rourke JP, Ness SA. Dramatic repositioning of c‐Myb to different promoters during the cell cycle observed by combining cell sorting with chromatin immunoprecipitation. PLoS ONE 2011; 6: e17362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gonzalez‐Marquez R, Llorente JL, Rodrigo JP et al SOX2 expression in hypopharyngeal, laryngeal, and sinonasal squamous cell carcinoma. Hum Pathol 2014; 45: 851–7. [DOI] [PubMed] [Google Scholar]
- 39. Wang X, Ji X, Chen J et al SOX2 enhances the migration and invasion of ovarian cancer cells via Src kinase. PLoS ONE 2014; 9: e99594. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 40. Murali R, Soslow RA, Weigelt B. Classification of endometrial carcinoma: more than two types. Lancet Oncol 2014; 15: e268–78. [DOI] [PubMed] [Google Scholar]
- 41. Cole AJ, Dwight T, Gill AJ et al Assessing mutant p53 in primary high‐grade serous ovarian cancer using immunohistochemistry and massively parallel sequencing. Sci Rep 2016; 6: 26191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Collado M, Blasco MA, Serrano M. Cellular senescence in cancer and aging. Cell 2007; 130: 223–33. [DOI] [PubMed] [Google Scholar]
- 43. Hassan KA, Chen G, Kalemkerian GP, Wicha MS, Beer DG. An embryonic stem cell‐like signature identifies poorly differentiated lung adenocarcinoma but not squamous cell carcinoma. Clin Cancer Res 2009; 15: 6386–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ben‐Porath I, Thomson MW, Carey VJ et al An embryonic stem cell‐like gene expression signature in poorly differentiated aggressive human tumors. Nat Genet 2008; 40: 499–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Li X, Xu Y, Chen Y et al SOX2 promotes tumor metastasis by stimulating epithelial‐to‐mesenchymal transition via regulation of WNT/beta‐catenin signal network. Cancer Lett 2013; 336: 379–89. [DOI] [PubMed] [Google Scholar]
- 46. Piva M, Domenici G, Iriondo O et al Sox2 promotes tamoxifen resistance in breast cancer cells. EMBO Mol Med 2014; 6: 66–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Chou YT, Lee CC, Hsiao SH et al The emerging role of SOX2 in cell proliferation and survival and its crosstalk with oncogenic signaling in lung cancer. Stem Cells 2013; 31: 2607–19. [DOI] [PubMed] [Google Scholar]
- 48. Ito K, Sasano H, Matsunaga G et al Correlations between p21 expression and clinicopathological findings, p53 gene and protein alterations, and survival in patients with endometrial carcinoma. J Pathol 1997; 183: 318–24. [DOI] [PubMed] [Google Scholar]
- 49. Shiozaki A, Nakashima S, Ichikawa D et al Prognostic significance of p21 expression in patients with esophageal squamous cell carcinoma. Anticancer Res 2013; 33: 4329–35. [PubMed] [Google Scholar]
- 50. Saegusa M, Hashimura M, Suzuki E, Yoshida T, Kuwata T. Transcriptional up‐regulation of Sox9 by NF‐kappaB in endometrial carcinoma cells, modulating cell proliferation through alteration in the p14(ARF)/p53/p21(WAF1) pathway. Am J Pathol 2012; 181: 684–92. [DOI] [PubMed] [Google Scholar]
- 51. Bauer J, Sporn JC, Cabral J, Gomez J, Jung B. Effects of activin and TGFbeta on p21 in colon cancer. PLoS ONE 2012; 7: e39381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kress TR, Sabo A, Amati B. MYC: connecting selective transcriptional control to global RNA production. Nat Rev Cancer 2015; 15: 593–607. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Comparison of SOX2 and p53 expression in endometrial cancer.
Fig. S2. SOX2 expression in human endometrial cancer cell lines.
Fig. S3. SOX2 is required to modulate the proliferation of endometrial cancer cells.
Fig. S4. Stratification of SOX2/p21 immunostaining based on p53 expression.
Table S1. Clinicopathological factors of all 258 patients with uterine corpus endometrioid adenocarcinoma presented in Figure 1b.
Table S2. Clinicopathological factors of 241 patients with uterine corpus endometrioid adenocarcinoma presented in Figure 1c, 1d, and Supplementary Figure S1.
Table S3. Univariate (a) and multivariate analyses (b) of prognostic factors for overall survival (OS) and progression‐free survival (PFS) in 31 cases of advanced‐stage cancer presented in Figure 1c and 1d.
Table S4. Univariate (a) and multivariate analyses (b) of prognostic factors for overall survival (OS) and progression‐free survival (PFS) in 201 cases of stage endometrial cancer presented in Supplementary Figure S1b and S1c.
Table S5. List of antibodies used in this study.


