Abstract
The American Academy of Sleep Medicine Sleep and Transportation Safety Awareness Task Force responded to the Federal Motor Carrier Safety Administration and Federal Railroad Administration Advance Notice of Proposed Rulemaking and request for public comments regarding the evaluation of safety-sensitive personnel for moderate-to-severe obstructive sleep apnea (OSA). The following document represents this response. The most salient points provided in our comments are that (1) moderate-to-severe OSA is common among commercial motor vehicle operators (CMVOs) and contributes to an increased risk of crashes; (2) objective screening methods are available and preferred for identifying at-risk drivers, with the most commonly used indicator being body mass index; (3) treatment in the form of continuous positive airway pressure (CPAP) is effective and reduces crashes; (4) CPAP is economically viable; (5) guidelines are available to assist medical examiners in determining whether CMVOs with moderate-to-severe OSA should continue to work without restrictions, with conditional certification, or be disqualified from operating commercial motor vehicles.
Citation:
Gurubhagavatula I, Sullivan S, Meoli A, Patil S, Olson R, Berneking M, Watson NF. Management of obstructive sleep apnea in commercial motor vehicle operators: recommendations of the AASM Sleep and Transportation Safety Awareness Task Force. J Clin Sleep Med. 2017;13(5):745–758.
Keywords: motor vehicle operators, obstructive sleep apnea, oral appliance therapy, OSA, transportation safety
1.0 BACKGROUND
The American Academy of Sleep Medicine (AASM) established a Sleep and Transportation Safety Awareness Task Force (STSATF) in 2015 with the goal of engaging transportation stakeholders, including federal and state agencies, to develop educational tools about the dangers of drowsy driving. On March 10, 2016, the Federal Motor Carrier Safety Administration (FMCSA) and Federal Railroad Administration (FRA) issued an Advance Notice of Proposed Rulemaking and request for public comments regarding the evaluation of safety-sensitive personnel for moderate-to-severe obstructive sleep apnea (OSA).1 The STSATF reviewed the Advance Notice of Proposed Rulemaking and submitted comments based on review of available literature from peer-reviewed journals and industry-related studies.2 The paucity of knowledge tailored for sleep medicine professionals became apparent. Therefore, to advance knowledge and provide guidance in this area, the STSATF prepared this article to summarize information submitted to the FMCSA/FRA during the rule-making comment period.
2.0 PREVALENCE OF SLEEP APNEA IN THE GENER AL POPULATION VERSUS CMVOs
2.1 Moderate-to-Severe OSA Is Common Among the General Adult United States Population
In the Wisconsin Cohort Study, Young et al. estimated the prevalence of OSA in middle-aged, working adults in 1994.3 Among women, they found that moderate-to-severe OSA occurred at rates ranging from 3.7% to 4.4%. Among men, rates were two to three times higher, ranging from 6.2% to 11%.3 The strongest risk factor for OSA across both sexes was obesity. An increase in any obesity-related measure of body habitus by one standard deviation above average was associated with a three-fold increase in the risk of having any OSA.
Given the strong association between body composition and OSA, and the rising prevalence of obesity in the United States, more recent investigations have sought to update OSA prevalence estimates. Participants in the Wisconsin Sleep Cohort were invited to complete additional sleep studies at 4-year intervals, and the prevalence of OSA was estimated at follow-up time points as a function of age, sex, and body mass index (BMI). These models were extrapolated to the distribution of BMI scores from a large sample of United States adults participating in the National Health and Nutrition Examination Surveys (NHANES). Estimates of OSA prevalence based on BMI data from the 1988–1994 NHANES were compared against combined data from NHANES 2007–2008 and 2009–2010. These investigations estimated the prevalence of at least moderate OSA to be at 10% and 17% among men aged 30–49 years and 50–70 years, respectively; among women in the same age groups, the respective prevalence rates were 3% and 9%. These more current estimates are substantially higher than they were two decades ago, and reflect proportional increases in prevalence ranging from 14% to 55%, depending on the demographic subgroup of interest.4
Even these newer estimates of OSA prevalence likely underestimate the commonness of the full spectrum of breathing abnormalities during sleep in CMVOs, which also contribute to adverse medical and other consequences. Such consequences include daytime sleepiness, cognitive dysfunction, and cardiovascular disease.5 These other breathing abnormalities include milder OSA, as well as respiratory effort-related arousals, or arousals from sleep due to other phenomena, including snoring or smaller reductions in airflow.
2.2 The Prevalence of OSA Is Higher Among Commercial Truck Drivers Than in the General Population
The prevalence of OSA among commercial truck drivers is estimated to be substantially higher than in the general population. This elevated prevalence is generally attributed to three major risk factors for OSA that are common among commercial drivers; the majority are male, obese, and middle-aged. In studies of commercial truck drivers, OSA prevalence estimates have ranged from 28% to 78%.6–9 In a study commissioned by the FMCSA and conducted in the Philadelphia area, 4,280 commercial drivers were invited to complete a survey, and 1,392 responded. Among respondents, a subgroup of 407 at-risk drivers underwent in-laboratory attended polysomnography (PSG) for OSA. The resulting estimated prevalence of OSA was 28%. In another study of 3,268 commercial truck drivers in Australia, with similar proportion of men and higher proportion of responders, 60% had OSA confirmed by PSG. Further, 24% reported excessive sleepiness, which was related to increased crash risk.7 In a third study conducted with truck drivers employed by a single company in California, 78% were suspected of having OSA based on overnight oximetry.9 This sample had a higher prevalence of obesity than the Australian cohort. In a large study of more than 19,371 drivers at Schneider,6 an online screening tool identified 5,908 individuals (30%) as having high risk for OSA. In subsequent PSG testing, the diagnosis of OSA was made in 2,103 (80%) of those high-risk drivers. A more recent study of 104 individuals who self-identified as holding a commercial drivers' license in the Philadelphia area10 found that almost one-third had severe OSA and 61% had at least moderate OSA based on PSG. Drivers in this more recent sample also had a higher prevalence of obesity relative to samples from prior studies.
2.3 OSA Is Common Among Rail Operators
Although no studies based on objective testing have been done on United States rail workers, data from Brazil indicate that 35% of rail workers have OSA based on PSG.11 In the United States, some data on the prevalence of OSA among rail operators is available as a result of screening programs implemented by Metro-North in New York, after a serious train crash in the Bronx on December 1, 2013. The crash resulted in 4 deaths and 59 nonfatal injuries. An investigation by the National Transportation Safety Board determined that the cause was excessive sleepiness due to untreated, severe OSA.12 A pilot screening program tested all 438 Metro-North engineers and engineers-in-training. Because of the findings of the pilot program, screening was expanded to include conductors and other safety-sensitive personnel. Subsequently, the Human Factors Research Division of the FRA published survey data indicating that 7% of rail employees in safety-sensitive positions report having OSA.13 This self-reported estimate is likely to be low.
As we await the estimates of OSA prevalence for employees working in safety-sensitive transportation positions outside of commercial trucking, the methodology outlined by Peppard et al.4 provides a validated empirical method to extrapolate the prevalence of moderate-to-severe OSA using BMI, age, and sex predictors.
3.0 UNTREATED OSA IS LINKED TO CR ASHES IN BOTH PASSENGER CAR AND COMMERCIAL TRUCK DRIVERS, AND TREATMENT REDUCES THIS RISK
In a meta-analysis commissioned by the FMCSA, data from 10 studies were pooled to estimate overall crash risk among drivers with untreated OSA.14 Overall, the odds that a driver with untreated OSA will have a crash is 243% higher than a driver without OSA. The meta-analysis estimated that 95% of drivers with untreated OSA have a crash risk that ranges from 21% to as high as 489% higher than those without OSA. Furthermore, the same FMCSA-commissioned researchers found that treatment of OSA with continuous positive airway pressure (CPAP)15 (1) lowers crash risk to the same level as that seen in individuals without OSA (risk ratio = 0.278, 95% confidence interval: 0.22 to 0.35; P < .001); (2) improves sleepiness in as few as 2 days, and (3) improves performance on a driving simulator in as few as 2 to 7 days.
More recently, Schneider tracked three groups of commercial truck drivers longitudinally by using a mandatory program that required universal screening under nonpunitive conditions. The Somni-Sage questionnaire was used to screen for the presence or absence of OSA risk, followed by PSG in those at high risk. They compared 1,603 drivers with OSA confirmed by PSG, 403 without OSA based on PSG, and 2,016 matched control drivers who were unlikely to have OSA based on screening. They offered positive airway pressure (PAP) therapy to drivers confirmed to have OSA, and monitored objective adherence rates using builtin monitoring systems. They also tracked preventable, reportable crashes per 100,000 miles driven. Those who did not meet the PAP adherence criterion had a crash rate that was five-fold higher than that of drivers in the matched control group; drivers with OSA who met the PAP adherence criterion had crash rates statistically equivalent to the matched control group.16
A follow-up study at Schneider17 found that, of 255 CMVOs with a diagnosis of OSA and treated with CPAP, 75% had a preventable crash during the study period (pre- or post-CPAP). Among the drivers with crashes, 93% experienced their crash before treatment for OSA, whereas 25% were involved in a crash after CPAP treatment (a 73% reduction in total crashes). In this report, 91% of drivers assessed for CPAP adherence were using CPAP 6 to 7 nights per week. Despite the lack of detail regarding apnea-hypopnea index (AHI) values for those who received a diagnosis of OSA, the report indicated that 44% of drivers with a diagnosis of OSA at the single testing site had severe OSA (AHI range from 34–112 events/h of sleep).
These data suggest a relationship between untreated or under-treated OSA and increased crash risk. Furthermore, the evidence that PAP treatment for OSA lowers crash risk to levels similar to those of drivers who are unlikely to have OSA provides compelling evidence that OSA likely plays a causal role in crashes.
4.0 OSA IS LINKED WITH MORBIDITY AND MORTALITY, WHICH IMPROVE WITH PAP THER APY
OSA is associated with an increase in all-cause mortality.18,19 A meta-analysis of over 25,000 individuals in 12 studies showed relative increased risks of 1.79 for cardiovascular disease, 2.15 for fatal and nonfatal stroke, and 1.92 for death from all causes.20 Among those with OSA, Nieto and colleagues21 found that, compared to individuals who had an AHI < 1.5 events/h, the adjusted odds of hypertension were 20%, 25%, and 37% higher for individuals in mild (5 to 15 events/h), moderate (15 to 30 events/h), and severe (> 30 events/h) AHI categories, respectively.21 OSA is also a cause of “resistant” hypertension,22 defined as failure of hypertensive control despite the use of at least three blood pressure-lowering medications. OSA has been shown to be an independent risk factor for the development of diabetes,23 which is a known risk factor for cardiovascular disease. Cerebrovascular disease is strongly and independently associated with OSA of any severity.24–26 OSA is also associated with reduced quality of life based on a number of measures including physical function, vitality, and health perception.27 There is good evidence OSA is associated with depression,28 with some limited evidence of association with other mental health disorders.
Evidence shows that PAP treatment reverses daytime hypertension in the first few weeks after starting treatment,29–31 reduces mortality after stroke,32 and improves quality of life.33 CPAP also reduces fatal and nonfatal cardiac events,34 and reduces recurrence of atrial fibrillation.35
5.0 THE COST OF SLEEPINESS-RELATED LARGE TRUCK AND R AIL CR ASHES IS HIGH
As previously indicated, treatment of OSA can reduce the risk of crashes. Although commercial truck drivers are safer per vehicle-mile traveled compared to passenger car drivers,36,37 large vehicle crashes are particularly deadly, disabling, and expensive. Commercial truck crashes are estimated to cost between $304,500 to $7 million depending on the size of the truck and severity of the incident.17 These costs are generated by property and vehicular damage, lost wages from absenteeism, and insurance and related medical expenses. Rail safety incidents can cost much more.38,39
6.0 COSTS AND BENEFITS OF SCREENING, EVALUATION, AND MANAGEMENT OF OSA IN SAFETY-SENSITIVE TR ANSPORTATION WORKERS
6.1 Models and Empirical Evidence Available Estimate Potential Costs of Untreated OSA and Cost Savings of OSA Treatment
The AASM recently commissioned Frost & Sullivan, a market research and analytics firm, to perform cost modeling of the economic burden of OSA.40 The total cost of OSA in the United States was divided into four components: medical and mental health comorbidities, workplace accidents, productivity, and motor vehicle accidents. Disease epidemiology, health care utilization and cost, and access to care were factors that contributed to these estimates. Confidence levels were assigned to each component reflecting Frost & Sullivan's assessment of the low- and high-end cost estimates for these components. The total economic effect of commercial and noncommercial accidents in the United States where undiagnosed OSA was a contributing factor was estimated at $26.2 billion in 2015. Cost savings can be realized from comprehensive OSA diagnosis and treatment and include reduction of vehicular damages, lost wages from corresponding absenteeism, property damage, rising insurance premiums, and medical expenses.
When aggressive OSA screening, diagnosis, and treatment are implemented, annual savings to a small trucking company (∼1,000 drivers) is estimated at $19.1 million and, to a large trucking company (∼11,000 drivers), $1.2 billion.
6.2 Costs Incurred by the Safety-Sensitive Transportation Worker: Screening, Testing, and Treatment
Although actual costs to drivers are underreported and likely vary, some cost considerations are presented here.
OSA screening would not be anticipated to incur signifi-cant incremental cost to the pre-employment physical that is conducted by a trained medical examiner at the time of the operator's initial certification. For those requiring additional work-up, total costs to workers come from several sources. These sources include: a sleep medical evaluation (which may be covered in the operator's certification examination; or additional examination may be required), sleep diagnostic testing, potential income loss during the evaluation and initial treatment phase, and if a diagnosis is confirmed, treatment and monitoring. Out-of-pocket costs may be reduced by health insurance coverage, though operators are still responsible for copay or deductible costs, depending on the specifics of the health plan. Substantial variability may exist in these costs, based on region and payer. Some employers have implemented internal programs that cover the cost of evaluation, testing, and treatment, with positive results.6
One approach that has helped reduce the costs of testing is the use of home-based technologies, which are more widely available, and generally more affordable than PSG. Home sleep apnea testing (HSAT) has replaced PSG as the initial diagnostic approach in some regions. Equally, in-laboratory testing to determine best therapeutic PAP pressures has been supplanted in some cases by use of auto-adjusting devices, though in-laboratory PAP testing remains critically important for some patients. HSAT is associated with specific controversies in the CMVO population (see Section 8.2).
After testing and the establishment of a diagnosis of OSA, treatment costs involve medical follow-up and procurement and use of a PAP device and supplies. The cost of PAP typically is covered by health insurance when at least moderate OSA is demonstrated, and even for mild OSA if comorbid conditions are present. Cash prices may range from ∼$400 or higher for a new device with heated humidification and adherence monitoring, plus recurring costs for supplies (mask, tubing, filters) every 3 to 6 months. Typically, these supplies are covered by insurance, with prices starting at ∼$120 for mask/headgear, tubing, and filters. Private sources of assistance are available for procuring CPAP if financial hardship exists, including free or low-cost PAP assistance programs. Multiple commercial entities also offer significantly discounted preowned PAP devices.
Many health insurances also cover the cost of oral appliance (OA) therapy, which may be appropriate for commercial drivers who have milder forms of OSA. After treatment with an OA, a follow-up sleep study may be recommended by the health provider, which may add to the cost of this treatment approach. An important consideration is that PAP therapy requires a power source or battery to operate, whereas OAs generally do not. Access to an adequate power source may be problematic in some settings.
Finally, in evaluating the economic effectiveness of diagnosing and treating OSA, the long-term costs of not treating known OSA should be considered.41 As mentioned, treatment of OSA can improve crash risk and comorbid conditions, including cardiovascular disease.34,35 Cost savings realized through reduced accidents and improved health may counterbalance the cost of OSA detection and treatment, because these medical conditions are costly to manage, as detailed in the following paragraphs.
6.3 Diagnosing and Treating OSA in Commercial Truck Drivers Is Less Expensive Than Leaving OSA Undiagnosed and Untreated
Sleep apnea is an expensive disorder to neglect. In non-CMVO populations, unidentified and/or untreated OSA incurs two-fold higher medical expenses, largely associated with cardiovascular disease.42 To the extent that commercial drivers have been studied, this group appears to be no exception. In a study of commercial drivers undergoing a corporate-driven OSA detection and treatment program, CPAP intervention in 348 drivers with sleep-disordered breathing resulted in a 47.8% reduction in per-member per-month health care spending.43 Treatment of OSA with CPAP resulted in an average savings of $550 per driver per month.43 Hospital admissions were reduced by almost 25% and overall health care dollars spent were cut in half. There was a 73% reduction in preventable driving accidents after instituting a comprehensive OSA screening, diagnosis and treatment program. In another recent study conducted with the Union Pacific Railroad Employees Health Systems, a focused educational campaign on OSA improved health outcomes and led to a measureable reduction in medical expenses—a differential cost savings of $4.9 million for the 2-year study period.44
On a larger scale, undiagnosed OSA cost the United States approximately $149.6 billion in 2015, of which $12.4 billion was related to diagnosis.40 The average cost of treating a patient is $1,190, which is less than the cost of a single emergency department visit for a “moderate” health complaint ($1,200).40 Detailed analyses indicate that treating OSA results in costs that are ∼33% of not treating the disorder, indicating that treatment results in a sizable cost savings.
Costs related to sleepiness-related crashes are summarized in Section 5.0.
7.0 CONSENSUS-BASED OSA SCREENING GUIDELINES LIKELY CAPTURE ONLY A SMALL PORTION OF CASES OF OSA
Guidelines available for screening for OSA in CMVOs have been proposed by several entities, including: (1) a Joint Task Force composed of the National Sleep Foundation, American College of Chest Physicians, and the American College of Occupational and Environmental Medicine;45 and by groups convened by the FMCSA: (2) a Medical Expert Panel,46 a Medical Review Board,47 and jointly by the Medical Review Board and Motor Carrier Safety Advisory Committee48,49 of the FMCSA. They have been summarized recently.50
All of these groups offer ways to keep drivers in service while undergoing evaluation and treatment for OSA. All suggest the use of objective measures, such as BMI, neck size, or the presence of hypertension to identify high-risk individuals who should undergo sleep studies to evaluate for OSA. The emphasis on objective measures is due to the unreliability of self-reported symptoms of OSA, which has been shown in multiple studies.51–53 In one study, even though 78% of those screened had confirmed OSA, none admitted to the common symptoms of snoring and sleepiness.51 Another study of 187 individuals deemed to be at high risk based on physical examination criteria found negative response to Commercial Driver Medical Examination Center (CDME) form questions regarding snoring, sleep disorders, observed apnea and daytime sleepiness.53 In another study, among all individuals with severe OSA, (defined as AHI ≥ 30 events/h) only one reported sleepiness using Ep-worth Sleepiness Scale (ESS) score.52 Finally, Schneider used their own version of an online questionnaire to screen drivers for risk for OSA, and also found that self-reported symptoms were not useful, and cited a specific instance of a driver with an AHI of 164 events/h and a normal ESS.6
To date, we know of no specific recommendations for screening of rail workers in the United States, though studies of foreign rail workers suggests a prevalence of OSA similar to that of commercial drivers.11 The government of Australia requires CMVOs to have a sleep study if they meet either of these conditions: (1) BMI ≥ 40 kg/m2; (2) BMI ≥ 35 kg/m2 AND diabetes OR hypertension needing at least two medications for control. In addition, the Australian government requires a sleep evaluation for any CMVO with evidence of a sleep disorder, such as sleepiness or involvement in a sleepiness-related incident.54
Data show that, by using existing guidelines, health care providers are only capturing the “tip of the iceberg” in terms of the actual numbers of potential OSA cases.51–53,55 By using guidelines provided by the Joint Task Force (which rely on the same BMI threshold of ≥ 35 kg/m2, which was recommended by the Medical Review Board of the Motor Carrier Safety Advisory Committee), only 10% to 13% of commercial truck drivers screen positive,51–53,55 despite the fact that the estimated prevalence of OSA in this group is much higher (see Section 2.2). Furthermore, none of those identified as high-risk then marked “yes” to the question about sleep disorders on the CDME form53; of those identified as high risk who completed the recommended PSG testing, 79% to 100% had confirmed OSA51–53,55; only one individual returned and showed adherence with PAP therapy52; and 34% to 81% did not return to the medical examiner for follow-up evaluation.51–53,55 No trials have explored the reasons for PAP non-adherence in this group, or ways to address it. Programs that mandate treatment,6,16 however, such as the one by Schneider, have shown that adherence improves crash rates.16
Therefore, a recommendation for PSG using existing guidelines yields high rates of positive diagnoses, suggesting that the BMI threshold of ≥ 35 kg/m2 leaves high numbers of at-risk drivers untested. The failure of individuals with confirmed OSA to accept PAP therapy or return for follow-up evaluation suggests that doctor shopping was an issue. The FMCSA has since required that medical examiners undergo training and certification, and be listed in a national registry, to address the issue of doctor shopping.56,57 Investigations are needed to determine whether this registry increases the likelihood of case identification and effective treatment. These collective data support a need for mandated screening, testing, and compliance with therapy.
8.0 SCREENING FOR OSA IN CMVOs AND OTHER SAFETY-SENSITIVE EMPLOYEES
8.1 Screening: General Considerations
It is the opinion of the AASM STSATF that transportation workers with safety-sensitive duties be required to undergo screening for OSA. By safety-sensitive we mean that if the operator were to become suddenly impaired or incapacitated, the operator would pose significant danger to himself or herself, the public, or the environment. CMVOs, therefore, are in this group, because untreated OSA puts them at risk for fall-asleep crashes. However, sleepiness-related crashes (see Section 3.0) do not require that the driver actually fall asleep; driver impairment may take the form of compromised decision making, reduced short-term memory and vigilance, increased impulsivity and risk-taking, and prolonged reaction time.58,59
Currently, both the FRA and FMCSA have existing guidelines regarding who should be screened for OSA. The FRA guidelines include any personnel involved in train movement, dispatching, signal operation, and equipment maintenance who also have key risk factors for OSA, such as obesity. The FMCSA defines drivers as safety-sensitive employees. At this time, the AASM STSATF does not recommend expanding screening beyond these defined groups of safety-sensitive employees.
8.2 Frequency of Screening for OSA Among Transportation Workers With Safety-Sensitive Duties, and Methods of Establishing the Diagnosis of OSA
Routine screening should be conducted as part of the preexisting required fitness-for-duty medical evaluation. If this screening suggests high risk for OSA, we believe a comprehensive sleep evaluation should be performed by a board-certified sleep medicine physician.60 Criteria for referral are summarized in Table 1.
Table 1.
Indications for evaluation by a sleep medicine physician.
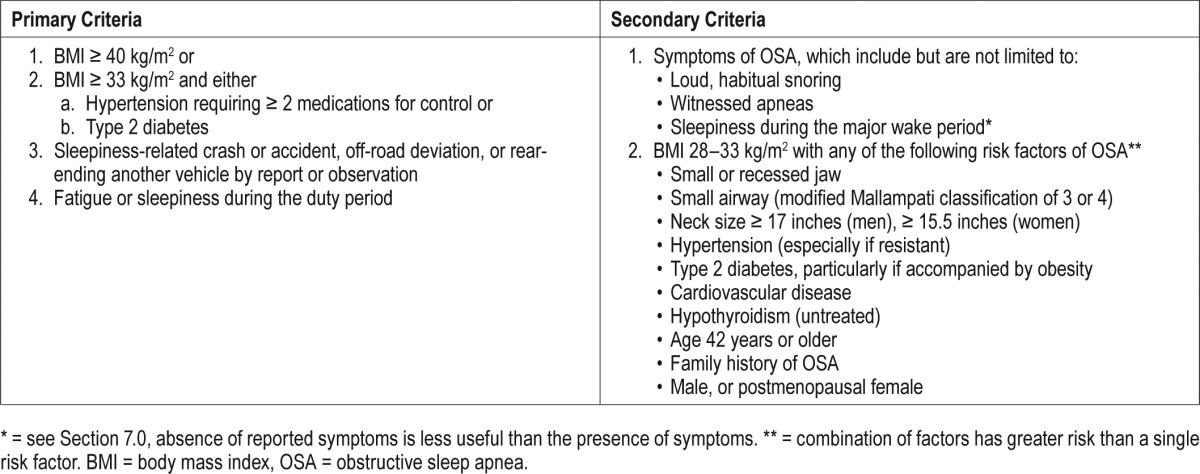
Primary criteria: We recommend that drivers who meet any of the following three criteria be considered high-risk individuals who should be referred to a board-certified sleep medicine specialist for clinical sleep evaluation and diagnostic testing.
Individuals with a BMI ≥ 40 kg/m2
- Individuals who have admitted fatigue or sleepiness during the duty period OR who have been involved in a sleepiness-related crash or accident;
- Factors suggesting a sleepiness-related crash or accident, including a single-vehicle crash, off-road deviation, or rear-ending another vehicle
- Individuals with a BMI ≥ 33 kg/m2 and either
- Hypertension requiring two or more medications for control; or
- Type 2 diabetes
Published data indicate that individuals having either resistant hypertension61,62 or obesity combined with type 2 diabetes63 experience a positive predictive value of having OSA that exceeds 80%.
The aforementioned criteria are recommended as the first or primary determinants of which individuals should undergo referral to a sleep medicine specialist for clinical evaluation and diagnostic testing. These criteria are recommended to capture the most at-risk individuals, the so-called “tip of the iceberg.” Secondary criteria should then be considered in order to capture the next tier of at-risk individuals. Secondary criteria for recommending diagnostic evaluations include the following additional symptoms and risk factors associated with OSA:
Symptoms: potential symptoms of OSA may include but are not limited to: loud, habitual snoring, witnessed apneas, or sleepiness during the major wake period (note that subjective information may be less reliable than objective data, as detailed in Section 7.0, and the absence of reported symptoms is less useful than the presence of symptoms).
- Risk factors: risk factors of OSA may include the following, particularly in those who have lower BMI values (range 28–33 kg/m2). A combination of risk factors denotes greater risk than the presence of a single risk factor alone.
- Factors associated with high-risk:
- Small or recessed jaw
- Small airway (modified Mallampati classification of 3 or 4)
- Neck size ≥ 17 inches (men), ≥ 15.5 inches (women)
- Hypertension (especially if resistant)
- Cardiovascular disease
- Type 2 diabetes (treated or untreated), particularly if accompanied by obesity63
- Hypothyroidism (untreated)
- Other factors:
- Age 42 years or older
- Family history of OSA
- Male, or postmenopausal female
We therefore recommend that operators with (1) BMI ≥ 40 kg/m2, or (2) BMI ≥ 33 kg/m2 with either resistant hypertension or type 2 diabetes undergo a comprehensive sleep evaluation. Others with suggestive symptoms or those who have BMI < 33 kg/m2 may also need evaluation, based on the presence of two or more risk factors listed previously.
The Medical Expert Panel commissioned by the FMCSA recommends that an in-laboratory overnight PSG is the preferred method of diagnosis.46 PSG has the advantage of comprehensive evaluation for other sleep disorders as a possible cause of excessive daytime sleepiness and is considered the diagnostic gold standard. In-laboratory PSG is expensive due to the infrastructure required, and availability may be limited in underserved areas, which may result in a delay in diagnosis of up to 3 months. The AASM echoes this recommendation in that an in-laboratory PSG is the optimal test, particularly when: (1) concern exists for another sleep disorder or (2) pre-test likelihood of OSA is low, because HSAT is less sensitive and may miss milder cases of OSA.
HSAT does offer some distinct advantages, including wider accessibility, greater convenience, lack of necessity to sleep in a laboratory, and lower cost. This method of diagnosis should be considered most valid for “ruling in” likely OSA (identifying true positives) rather than for “ruling out” OSA (identifying true negatives). The typical device is a type 3 monitor, which includes four channels to assess respiratory effort, airflow, oxygen saturation, and heart rate. For those with a high pretest likelihood of OSA, and in the absence of suspicion of another sleep disorder, HSAT would facilitate rapid diagnosis, treatment, and return to duty. In the absence of suspicion for another sleep disorder and in the presence of a high risk of OSA, this method of testing is appropriate. However, limitations of HSAT make it less useful under other conditions.
One important consideration is that HSAT results in a respiratory event index (REI), which is analogous to an AHI but, importantly, based on monitoring time rather than sleep time. As such, the REI from HSAT is often lower than the AHI from PSG, in part due to the use of a larger denominator (monitoring time instead of total sleep time as measured by PSG). HSAT also does not assess hypopneas that result in arousal from sleep without desaturation, nor other types of sleep-disordered breathing events, such as respiratory effort-related arousals. The REI has been used as a surrogate measure of AHI, though the REI may miss milder cases of OSA entirely. This may be particularly true if the individual who is tested does not achieve sufficient sleep. Because HSAT is performed in an unsupervised setting, sleep is presumed but not confirmed. A low REI value, therefore, especially given that individuals were selected for testing based on high risk, should warrant repeat testing, and a subsequent supervised, in-laboratory PSG should be considered.
Technical limitations of HSAT should also be considered in its use. Ten to fifteen percent of HSATs will require a subsequent in-laboratory study due to inconclusive or corrupt data. We recommend HSAT only be performed in conjunction with a comprehensive sleep evaluation by a sleep health professional and not be used for rapid screening. Custody and control should also be considered to ensure that the individual wearing the device is the intended worker who is being evaluated, and that the recorder captures data during the sleep period. Inconclusive results warrant in-laboratory PSG. We do not recommend the type 4 HSAT test, which uses only one to two channels, as there are limited data that these devices are adequate for diagnosis of OSA.
One issue following a diagnostic result is “how long is a sleep study good for?” This is not a question with an absolute answer. The examining physician will need to make a decision based on his or her clinical judgment while monitoring a patient over time. In the Wisconsin Cohort study, a 10% weight gain was associated with a 32% increase in AHI and a six-fold increase in the odds of developing moderate to severe OSA.64 In addition, age is associated with an increase in OSA, with prevalence peaking between 45 to 65 years. Therefore, if a physician notes a significant increase in weight within the age risk window, a new sleep study may be recommended, especially if additional health conditions develop that may be related to OSA, such as diabetes, hypertension, or cardiovascular disease.
8.3 Methods Currently Employed for Providing Training or Other Informational Materials About OSA to Transportation Workers With Safety-Sensitive Duties, and Their Effectiveness in Identifying Workers
There are many print and online resources available to the safety-sensitive worker regarding OSA through the Federal Aviation Administration, FMCSA, AASM, National Sleep Foundation, American Sleep Apnea Association, and others.51,52 Some transportation companies develop their own resources.43,44 For example, as referenced earlier, Schneider's comprehensive OSA program is a well-known model program in the trucking industry with evidence for effectiveness. In general, patient education programs for management of chronic OSA are widely accepted as effective.65
Although educational resources are available and evidence for their effectiveness established, numerous barriers remain in implementing effective screening and treatment of OSA in transportation industries. Reluctance to undergo required evaluation and treatment of OSA persists in the commercial driving community. We believe that such reluctance is due to practical concerns about job security, costs, cultural barriers that limit willingness to pursue medical care, and gaps in education about the seriousness of OSA as a health and safety hazard.
CMVOs with concerns about potential employment repercussions or high expenses for the diagnostic process may underreport or avoid reporting OSA symptoms. The fitness for duty process in and of itself may be perceived as “punitive,” as failing to be considered fit leads to adverse consequences. Although CMVOs can be penalized for making false or misleading statements about disqualifying conditions during an examination by a member of the National Registry of Certified Medical Examiners, false reporting and underreporting have been documented (see Section 7.0).51–53,55 Nonetheless, the lack of symptom disclosure could also lead to denial of insurance coverage for recommended testing.
Efforts to remove aversive pressures of self-reported symptoms would help to identify high-risk individuals in the target population. One study of commercial drivers in 2011 showed that in an anonymous, nonpunitive environment, drivers had a positive Berlin Questionnaire 56% of the time and approximately 21% admitted they fell asleep at stoplights.66 Another study revealed that when privacy and confidentiality were assured, CMVOs offered useful symptom data that complemented objective information, such as BMI, neck circumference, and hypertension10 and increased the diagnostic sensitivity of screening. Symptoms including loud snoring, choking, or gasping during sleep tended to be helpful in identifying OSA in subsets of CMVOs who did not meet the BMI threshold for high risk10 As mentioned in Section 5.0, a mandatory screening and treatment program yielded a 73% reduction in accident rate, when the program was delivered in a nonpunitive environment.37
In summary, we do not recommend reliance on subjective symptom reporting as an effective identification method, and medical examiners should receive specific instruction in objective indicators of OSA risk. Positive symptom data, which are more likely to be available under nonpunitive conditions, may add value to objective information.
Education should be offered as part of screening and evaluation, but should also not be relied on as an effective, independent method for identifying OSA among safety-sensitive transportation workers. Most drivers are either independent owner/operators or belong to smaller companies without the resources to have a comprehensive in-house program. Educational programs, outside of those mandated by corporate or insurance programs, are voluntary. Most of the educational material is neither user-friendly nor written at a level comprehensible by the general public.67
We are unaware of any data that show that educational programs, in and of themselves, succeed in identifying individuals with OSA. Public safety necessitates continuous efforts to improve educational programs while we strive to improve access to care, and work toward more comprehensive federal regulations and policies in this area. We recommend that educational materials, and both screening and treating clinicians, emphasize that OSA is a treatable disorder, and that treatment can reduce crash risk to levels that are similar to those of drivers without OSA.15
8.4 Qualifications or Credentials Necessary for Medical Practitioners Who Perform OSA Screening, Diagnosis, and Treatment of OSA in Commercial Truck or Rail Operators
We recommend that clinicians who screen safety-sensitive employees in the transportation industry are, at a minimum, appropriately licensed and certified in accordance with their credentials. We believe that allopathic and osteopathic physicians, nurse practitioners, and physician assistants would be most appropriate. We do not think that chiropractors have the necessary training in physiology or pharmacology to serve in this role and recommend that they not be utilized. At least four states (Rhode Island, Utah, Washington, and Wyoming) already list the United States Department of Transportation examinations as being outside of the scope of chiropractic medicine.
In accordance with recently established training requirements,68 medical examiners should undergo training regarding the recognition of signs, symptoms, and risk factors for OSA, be proficient in screening, and able to refer those who screen positive for confirmatory diagnostic testing. Drivers identified by medical examiners as at risk for OSA should be referred for diagnosis and treatment to accredited sleep centers. OSA is a medical disorder and therefore should be managed by medical clinicians. Several studies have shown improved treatment compliance, greater patient satisfaction, and greater timeliness in patients treated at accredited sleep centers or by board-certified sleep medicine physicians.69,70
As the delivery of health care continues to evolve, clinical assessment, diagnostic testing, and ongoing follow-up may occur using telemedicine approaches that incorporate HSAT and automatically adjusting PAP therapy. Such approaches may prove to increase access and improve convenience for CMVOs, while reducing costs. We recommend that these tele-medicine evaluations be performed by board-certified sleep medicine physicians.
8.5 Restrictions on Transportation Workers With Safety-Sensitive Duties Who Report Excessive Sleepiness While Performing Safety-Sensitive Duties: General Considerations
Given the health and economic consequences of sleepiness-related crashes, medical examiners or treating physicians should be able to restrict hazardous work duties in high-risk individuals. Consideration should be given to the specific types and timing of work duties (eg, driving steady or rotating night shifts, transporting hazardous materials, long-haul rather than local driving, and extended rather than short driving durations). When the CMVO is deemed to be at high risk for OSA based on screening, but does not yet carry a diagnosis, determining the fitness for duty should be performed on a case-by-case basis. Although we have already reviewed reasons to de-emphasize self-reported symptoms, clinicians should still inquire about symptoms that would suggest an imminent threat to public safety, such as falling asleep while working in their safety-sensitive position or having a sleepiness-related crash.
Absent an imminent threat (such as a known crash that was likely related to sleepiness), however, disqualification is an unrealistic option for most safety-sensitive operators in the absence of a diagnosis. The AASM refers to the United States Department of Transportation's drug testing regulations, which prohibit those in safety-sensitive positions to be pulled from those positions prior to receipt of the final result from the Medical Review Officer,71 suggesting that unconfirmed OSA would be handled similarly.
8.6 Treatment for OSA: Goals and Assessments of Efficacy
The primary goal of any treatment for OSA in CMVOs, whether it is PAP, OA therapy, upper airway surgery, positional therapy, or weight loss, is to demonstrate: (1) resolution of OSA, (2) adherence to therapy, and (3) improved patient symptoms.72 Therefore, the STSATF recommended that acceptable criteria for evaluating efficacy of prescribed OSA treatments address these three elements. Specific measures in each domain include a combination of physician judgement, symptom surveys (which may be useful if positive), objective testing, and adherence data. Employees in safety-sensitive positions face some unique challenges, which include time constraints, financial concerns related to the diagnosis and treatment of sleep disorders, concerns about job loss, and about receiving a treatment that is compatible with their job duties. When considering condition management, the committee recommended that safety-sensitive personnel with OSA have at least annual follow-up with a board-certified sleep medicine specialist.
8.6.1 Improvements in OSA severity
PAP devices have airflow monitoring systems73 that provide an ongoing assessment of not only compliance but the frequency of residual sleep apnea events. This technology, however, is generally unavailable in non-PAP therapies, such as OAs,74 positional therapy, or weight loss. Therefore, when non-PAP therapies are used, a sleep study could be performed while the patient uses the intervention. The STSATF, therefore, recommends that the AHI, respiratory disturbance index (RDI), or REI be ascertained by a sleep study on therapy (eg, OA) or posttherapy (eg, upper airway corrective surgery, weight loss with or without bariatric surgery), and that this measure be used to monitor improvements in OSA severity with the use of an OA or following surgery. Current clinical guidelines advocate that OSA-related therapies target a reduction to an AHI/ RDI/REI < 5 events/h. For those whose posttreatment AHI/ RDI/REI values range from 5 to 20 events/h, clinical judgement is needed to determine fitness for duty.
8.6.2 Adherence to OSA therapy
When PAP is prescribed, progressive improvements in adherence are associated with greater normalization in subjective (eg, ESS) and objective (eg, Multiple Sleep Latency Test) measures of sleepiness as well as sleep-related quality of life (eg, Functional Outcomes in Sleep Questionnaire).63,75 Furthermore, individuals who adhere to treatment for OSA experience reduced incident hypertension76 and improved survival77 compared to individuals who do not.
Objective adherence to treatment is readily monitored by PAP-based therapies. The importance of tracking adherence to therapy has been recognized by previously proposed recommendations announced by the FMCSA in conjunction with an evaluation performed by its Medical Review Board.48 Current guidelines recommend that adherence to PAP therapies be defined as use of PAP for ≥ 4 hours per night for ≥ 70% of nights. This definition has been adopted by the Centers for Medicare and Medicaid Services and some third-party insurers for reimbursement for PAP devices and PAP-related supplies. CMVOs should be urged, however, that greater use of these devices, for the full duration of sleep, is expected to provide greater benefits in reducing sleepiness and improving daytime functioning.
8.6.3 Improved patient symptoms
Sleepiness is the cardinal OSA-related symptom that is the most concerning in transportation personnel in safety- sensitive positions. Improvement in sleepiness is a primary goal of OSA treatment in safety-sensitive personnel in the transportation industry. However, subjective assessments of sleepiness, with tools such as the ESS78 have limited utility in the employment setting where underreporting of symptoms has been documented.51–53,55 Therefore, symptom data should be considered in the context of a comprehensive assessment performed by a clinician.
Objective measures of sleepiness, such as the Multiple Sleep Latency Test, or of alertness, such as the Maintenance of Wakefulness Test, have been proposed to assess for ongoing sleepiness or alertness. If these tests yield an increased tendency to fall asleep or an inability to stay awake during the major wake period, they may indicate that a CMVO may not be fit to drive. However, these tests have large ranges for normative data and the potential to yield false-negative results, as well as the lack of assessment in an ongoing, “real-time” basis, thus limiting their usefulness in assessing the risk for a sleepiness-related accident.
Given these limitations, the STSATF would advocate that no specific measure be used alone to assess the criteria of improved patient symptoms. Rather, a comprehensive evaluation at an accredited sleep center should be used, which may include a combination of subjective and objective testing as deemed appropriate by the evaluating clinician. Objective data include assessments for improvements in OSA severity, adherence to treatment, and improvements in clinical outcomes such as blood pressure. Assessments performed by a sleep medicine specialist or primary care clinician can help inform the medical examiner's determination of fitness for duty.
9.0 MANDATE AND THE HIGH-RISK DRIVER
The STSATF recommends that transportation workers with safety-sensitive duties should be required to undergo screening for OSA and, if OSA is confirmed, receive treatment.
10.0 RECOMMENDATIONS REGARDING OSA FOR TR ANSPORTATION WORKERS WITH SAFETY-SENSITIVE DUTIES: GENER AL CONSIDER ATIONS
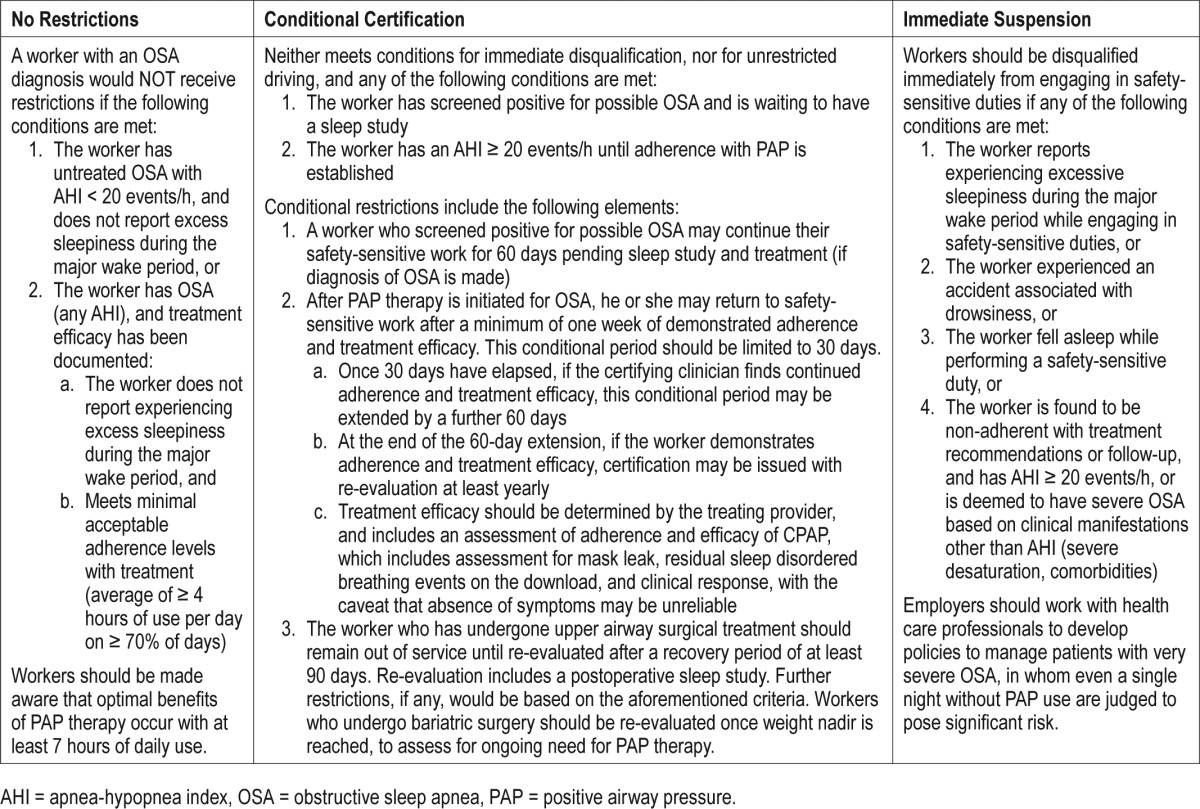
Some transportation workers with safety-sensitive duties in whom OSA is diagnosed may continue to work without restrictions, but for others, either immediate disqualification or conditional certification may be appropriate. The criteria for each are outlined in the next paragraphs and summarized in Table 2. Because some CVMOs work steady night or rotating shifts, excessive sleepiness refers to that which occurs during the “major wake period.”
Table 2.
Criteria for determining whether certification to operate a motor vehicle should be unrestricted, conditional, or denied.
10.1 Task Force Recommendations: PAP Versus Non-PAP Therapies
The STSATF recommended PAP treatment for most patients in whom OSA was diagnosed. PAP is established, effective, and the most studied mode of treatment. Treatment with PAP reduces crash risk,15,16,79 improves health, and decreases mortality, as discussed previously.
Prompt PAP therapy is advised for those with AHI ≥ 20 events/h. Data linking crash risk for those with AHI ≥ 20 events/h are more consistent, whereas data linking crashes to AHI values in the mild to moderate range is less consistent.44 Workers with milder OSA (AHI 5 to 20 events/h) may still benefit from PAP treatment, particularly if a comprehensive evaluation suggests more severe disease (such as level of de-saturation, history of a sleepiness-related crash, level of daytime sleepiness, sleepiness while engaging in safety-sensitive duties, or the presence of comorbidities), and CMVOs should be encouraged to explore this option. Moreover, workers who have an AHI between 5 and 20 events/h should be required to seek treatment if they have experienced a sleepiness-related crash, or if they report sleepiness while engaging in safety-sensitive duties.
We emphasize that AHI thresholds referenced in this document are based on data generated from type 1 in-laboratory PSG. As previously mentioned, when home OSA testing is performed, the resulting REI is only an approximation of AHI, and may be an underestimate. Although currently there are no good data establishing specific cutoffs based on REI, most third-party payers recognize that an REI ≥ 10 events/h represents treatable OSA. Lower thresholds, however, may be appropriate in the presence of comorbidities, symptoms related to OSA, or severe desaturation.
Recent data suggest that close follow-up of recently diagnosed patients improves long-term adherence in commercial and noncommercial drivers.80 Therefore, we recommend the stepped conditional certification approach.81 Disturbingly, the Schneider study cited earlier showed that approximately 60% of drivers with OSA who did not adhere to treatment simply quit, in order to avoid the potential effect on their driving career. Such drivers may choose to continue to drive for another carrier without revealing data regarding a prior diagnosis of OSA or prior treatment, because the CDME form82 relies on self-reporting. The possibility that drivers who are aware that they have untreated OSA may still choose to continue driving underscores the need for follow-up after diagnosis.
Those with AHI 5–20 events/h, in the absence of serious sequelae (severe desaturation, history of sleepiness-related crash, severe daytime sleepiness with ESS ≥ 16,45 or the presence of comorbidities) may consider non-PAP treatment modalities, including upper airway surgery or an OA. We recognize that OAs are an effective treatment for OSA for some patients.74 Although OAs may be better tolerated by some and are portable, current data are insufficient to make recommendations regarding the effect of OAs on occupational and driving safety, and so these devices are not recommended for those with AHI ≥ 20 events/h, or those with lower values of AHI, yet who are deemed to have more severe clinical manifestations of disease.
10.2 Recommendations for Workers Treated With PAP
All CMVOs who are treated with PAP should be counseled against driving while sleepy, and instructed about available countermeasures. These are summarized elsewhere.83 They should also be instructed to avoid alcohol or sedating medications. For those who have positional apnea, position therapy should also be instructed. Instructions about good sleep hygiene and adequate sleep duration should also be provided (ie, 7 to 9 hours per day for adults).84 Those who are overweight or obese should be encouraged to engage in a program of gradual weight loss.
10.2.1 No restrictions
A worker with an OSA diagnosis would not receive restrictions if the following conditions are met:
The worker has untreated OSA in the mild-to-moderate range, with an AHI of less than 20 events/h, and the worker does not report experiencing excessive sleepiness during the major wake period, or
- The efficacy of treatment of the worker's OSA has been documented. This includes an assessment of both compliance to treatment and evidence of effectiveness.
- Minimally acceptable adherence with treatment would be defined as ≥ 4 hours per day of use on ≥ 70% of days.
- Effectiveness is demonstrated by review of data from the PAP device that assesses for residual sleep apnea events and mask leak, and by a review of symptoms and physical examination (eg, blood pressure measurement).
Workers should be advised that optimal benefits of PAP therapy occur with 7 or more hours of daily use.
10.2.2 Immediate suspension from safety-sensitive activities
Workers should be advised to refrain immediately from engaging in safety-sensitive duties if any of the following conditions are met:
The worker reports experiencing excessive sleepiness during the major wake period while engaging in safety-sensitive duties, or
The worker experienced an accident associated with drowsiness, or
The worker fell asleep while performing a safety-sensitive duty, or
The worker has been found to be noncompliant with treatment recommendations or follow-up, and has AHI ≥ 20 events/h, or is otherwise deemed to have severe OSA based on clinical manifestations other than AHI (eg, degree of desaturation, comorbidity), until treatment efficacy is established. Employers should work with health care professionals to develop policies to manage patients with very severe OSA, in whom even a single night without PAP use are judged to pose significant risk.
10.2.3 Conditional certification
Workers may be granted conditional certification if they do not meet conditions for immediate suspension from duties, and any of the following conditions are met:
The worker has screened positive for possible OSA and is waiting to have a sleep study.
The worker has an AHI ≥ 20 events/h, until adherence with PAP is demonstrated.
Conditional certification should include the following elements:
A worker who has screened positive for possible OSA may continue in their safety-sensitive work for 60 days, while awaiting sleep study and, if the worker is diagnosed with OSA, for establishing efficacious treatment.
- After OSA is diagnosed and the patient has started PAP therapy, he or she may return to safety-sensitive work after a minimum of one week of demonstrated adherence and treatment efficacy. This conditional period should be limited to 30 days.
- The worker should return to the certifying clinician after 30 days have elapsed on PAP therapy, and, if continued compliance and treatment efficacy are demonstrated, a further extension to this conditional period of up to 60 days may be granted.
- At the end of the 60-day extension, if the worker shows compliance and treatment efficacy, certification may be issued with re-evaluation at least yearly.
- Efficacy of therapy should be determined by the treating provider, and includes an assessment of not only adherence, but other measures including an assessment for mask leak, and residual apneas and hypopneas obtained through tracking software from the PAP device. In addition, symptoms of OSA, such as daytime sleepiness, snoring, choking or gasping, or apneas witnessed by others during sleep, should be reviewed. Though they are not helpful if absent, the presence of residual symptoms warrants additional evaluation by a board-certified sleep medicine physician. Blood pressure measurement may also provide helpful evidence of treatment efficacy or lack thereof.
- The physician may recommend additional visits other than those at the 30- and 60-day time points, to support adherence and achieve adequate efficacy if needed.
10.3 Recommendations for Workers Treated With Surgery
Experience with surgical interventions indicates that improvements in symptoms and AHI after sleep surgeries are not universal, and follow-up sleep study would likely be indicated to assess individual benefits.
The worker who has undergone surgical treatment should remain out of service for at least a 90-day recovery period and then be re-evaluated. We recommend that this re-evaluation includes a postoperative sleep study. Further restrictions, if any, would be based on the aforementioned criteria.
The worker who undergoes bariatric surgery should be tested when the weight nadir is reached, to assess for residual OSA and determine whether there is ongoing need for PAP therapy. This occurs at approximately 6 months, and may vary between individuals.
10.4 Recommendations for Workers Treated With Oral Appliance Therapy
A sleep study showing the efficacy of OA therapy is advised for patients in whom the risk of untreated apnea is deemed to be high. Research regarding the validity of adherence monitoring technologies for OA therapy is under way.85
11.0 SUMMARY AND CONCLUSIONS
In response to the FMCSA and FRA's Advance Notice of Proposed Rulemaking regarding the evaluation of safety-sensitive personnel for moderate-to-severe OSA, the AASM STSATF has taken the position that: (1) moderate-to-severe OSA is common among CMVOs and contributes to an increased risk of crashes; (2) objective screening methods are available and preferred for identifying at-risk drivers, with the most commonly used indicator being BMI; (3) treatment in the form of CPAP is effective and reduces crashes; (4) CPAP is economically viable; (5) guidelines are available to assist medical examiners in determining whether CMVOs in whom moderate-to-severe OSA is diagnosed should continue to work without restrictions, with conditional certification, or be disqualified from operating commercial motor vehicles. These guidelines allow for some operators to continue driving while undergoing evaluation and treatment of OSA.
DISCLOSURE STATEMENT
This was not an industry supported study. Drs. Berneking, Gurubhagavatula, Meoli, Olson, Patil and Sullivan have indicated no financial conflicts of interest. Dr. Watson is a consultant for SleepScore Labs.
ACKNOWLEDGMENTS
The authors thank Mr. Ted Thurn and Mr. Branden Stearns for their assistance in preparing the manuscript.
ABBREVIATIONS
- AASM
American Academy of Sleep Medicine
- AHI
apnea-hypopnea index
- BMI
body mass index
- CDME
Commercial Driver Medical Examination Center
- CMVO
commercial motor vehicle operator
- CPAP
continuous positive airway pressure
- ESS
Epworth Sleepiness Scale
- FMCSA
Federal Motor Carrier Safety Administration
- FRA
Federal Railroad Administration
- HSAT
home sleep apnea testing
- NHANES
National Health and Nutrition Examination Surveys
- OA
oral appliance
- OSA
obstructive sleep apnea
- PSG
polysomnography
- PAP
positive airway pressure
- RDI
respiratory disturbance index
- REI
respiratory event index
- STSATF
Sleep and Transportation Safety Awareness Task Force
REFERENCES
- 1.Federal Motor Carrier Safety Administration, Federal Railroad Administration. Evaluation of Safety Sensitive Personnel for Moderate-to-Severe Obstructive Sleep Apnea. Federal Register website. [Accessed March 9, 2017]. https://www.federalregister.gov/documents/2016/03/10/2016-05396/evaluation-of-safety-sensitive-personnel-for-moderate-to-severe-obstructive-sleep-apnea. Published March 10, 2016.
- 2.American Academy of Sleep Medicine. American Academy of Sleep Medicine - Comments. regulations.gov website. [Accessed March 9, 2017]. https://www.regulations.gov/document?D=FRA-2015-0111-0065. Published June 10, 2016.
- 3.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328(17):1230–1235. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 4.Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol. 2013;177(9):1006–1014. doi: 10.1093/aje/kws342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Quan SF, Gillin JC, Littner M, Shepard J. Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. The report of an American Academy of Sleep Medicine Task Force. Sleep. 1999;22(5):667–689. [PubMed] [Google Scholar]
- 6.Berger M, Varvarigou V, Rielly A, Czeisler CA, Malhotra A, Kales SN. Employer-mandated sleep apnea screening and diagnosis in commercial drivers. J Occup Environ Med. 2012;54(8):1017–1025. doi: 10.1097/JOM.0b013e3182572e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Howard ME, Desai AV, Grunstein RR, et al. Sleepiness, sleep-disordered breathing, and accident risk factors in commercial vehicle drivers. Am J Respir Crit Care Med. 2004;170(9):1014–1021. doi: 10.1164/rccm.200312-1782OC. [DOI] [PubMed] [Google Scholar]
- 8.Pack A, Dinges D, Maislin G. A Study of Prevalence of Sleep Apnea among Commercial Truck Drivers. Washington, DC: Federal Motor Carrier Safety Administration; 2002. [Accessed March 9, 2017]. Publication no. DOT-RT-02-030. https://ntl.bts.gov/lib/55000/55400/55447/DOT-RT-02-030.pdf. Published May 2002. [Google Scholar]
- 9.Stoohs RA, Bingham LA, Itoi A, Guilleminault C, Dement WC. Sleep and sleep-disordered breathing in commercial long-haul truck drivers. Chest. 1995;107(5):1275–1282. doi: 10.1378/chest.107.5.1275. [DOI] [PubMed] [Google Scholar]
- 10.Platt AB, Wick LC, Hurley S, et al. Hits and misses: screening commercial drivers for obstructive sleep apnea using guidelines recommended by a joint task force. J Occup Environ Med. 2013;55(9):1035–1040. doi: 10.1097/JOM.0b013e318298fb0e. [DOI] [PubMed] [Google Scholar]
- 11.Koyama RG, Esteves AM, Oliveira e Silva L, et al. Prevalence of and risk factors for obstructive sleep apnea syndrome in Brazilian railroad workers. Sleep Med. 2012;13(8):1028–1032. doi: 10.1016/j.sleep.2012.06.017. [DOI] [PubMed] [Google Scholar]
- 12.McKay MP. Fatal consequences: obstructive sleep apnea in a train engineer. Ann Fam Med. 2015;13(6):583–586. doi: 10.1370/afm.1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Raslear TG. Prevalence and treatment of OSA in safety-critical railroad employees. J Sleep Disord Ther. 2014;3:179. [Google Scholar]
- 14.Tregear S, Reston J, Schoelles K, Phillips B. Obstructive sleep apnea and risk of motor vehicle crash: systematic review and meta-analysis. J Clin Sleep Med. 2009;5(10):573–581. [PMC free article] [PubMed] [Google Scholar]
- 15.Tregear S, Reston J, Schoelles K, Phillips B. Continuous positive airway pressure reduces risk of motor vehicle crash among drivers with obstructive sleep apnea: systematic review and meta-analysis. Sleep. 2010;33(10):1373–1380. doi: 10.1093/sleep/33.10.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burks SV, Anderson JE, Bombyk M, et al. Nonadherence with employer-mandated sleep apnea treatment and increased risk of serious truck crashes. Sleep. 2016;39(5):967–975. doi: 10.5665/sleep.5734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.2015 Pocket Guide to Large Truck and Bus Statistics. Federal Motor Carrier Safety Administration website. [Accessed March 9, 2017]. https://www.fmcsa.dot.gov/safety/data-and-statistics/commercial-motor-vehicle-facts. Published April 2015.
- 18.Marshall NS, Wong KK, Cullen SR, Knuiman MW, Grunstein RR. Sleep apnea and 20-year follow-up for all-cause mortality, stroke, and cancer incidence and mortality in the Busselton Health Study cohort. J Clin Sleep Med. 2014;10(4):355–362. doi: 10.5664/jcsm.3600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Young T, Finn L, Peppard PE, et al. Sleep disordered breathing and mortality: eighteen-year follow-up of the Wisconsin sleep cohort. Sleep. 2008;31(8):1071–1078. [PMC free article] [PubMed] [Google Scholar]
- 20.Wang X, Ouyang Y, Wang Z, Zhao G, Liu L, Bi Y. Obstructive sleep apnea and risk of cardiovascular disease and all-cause mortality: a meta-analysis of prospective cohort studies. Int J Cardiol. 2013;169(3):207–214. doi: 10.1016/j.ijcard.2013.08.088. [DOI] [PubMed] [Google Scholar]
- 21.Nieto FJ, Young TB, Lind BK, et al. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study. Sleep Heart Health Study. JAMA. 2000;283(14):1829–1836. doi: 10.1001/jama.283.14.1829. [DOI] [PubMed] [Google Scholar]
- 22.Calhoun DA, Jones D, Textor S, et al. Resistant hypertension: diagnosis, evaluation, and treatment. A scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research. Hypertension. 2008;51(6):1403–1419. doi: 10.1161/HYPERTENSIONAHA.108.189141. [DOI] [PubMed] [Google Scholar]
- 23.Botros N, Concato J, Mohsenin V, Selim B, Doctor K, Yaggi HK. Obstructive sleep apnea as a risk factor for type 2 diabetes. Am J Med. 2009;122(12):1122–1127. doi: 10.1016/j.amjmed.2009.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arzt M, Young T, Finn L, Skatrud JB, Bradley TD. Association of sleep-disordered breathing and the occurrence of stroke. Am J Respir Crit Care Med. 2005;172(11):1447–1451. doi: 10.1164/rccm.200505-702OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Redline S, Yenokyan G, Gottlieb DJ, et al. Obstructive sleep apnea-hypopnea and incident stroke: the sleep heart health study. Am J Respir Crit Care Med. 2010;182(2):269–277. doi: 10.1164/rccm.200911-1746OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yaggi HK, Concato J, Kernan WN, Lichtman JH, Brass LM, Mohsenin V. Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med. 2005;353(19):2034–2041. doi: 10.1056/NEJMoa043104. [DOI] [PubMed] [Google Scholar]
- 27.Yang EH, Hla KM, McHorney CA, Havighurst T, Badr MS, Weber S. Sleep apnea and quality of life. Sleep. 2000;23(4):535–541. [PubMed] [Google Scholar]
- 28.Akashiba T, Kawahara S, Akahoshi T, et al. Relationship between quality of life and mood or depression in patients with severe obstructive sleep apnea syndrome. Chest. 2002;122(3):861–865. doi: 10.1378/chest.122.3.861. [DOI] [PubMed] [Google Scholar]
- 29.Durán-Cantolla J, Aizpuru F, Montserrat JM, et al. Continuous positive airway pressure as treatment for systemic hypertension in people with obstructive sleep apnoea: randomised controlled trial. BMJ. 2010;341:c5991. doi: 10.1136/bmj.c5991. [DOI] [PubMed] [Google Scholar]
- 30.Martinez-Garcia MA, Capote F, Campos-Rodriguez F, et al. Effect of CPAP on blood pressure in patients with obstructive sleep apnea and resistant hypertension: the HIPARCO randomized clinical trial. JAMA. 2013;310(22):2407–2415. doi: 10.1001/jama.2013.281250. [DOI] [PubMed] [Google Scholar]
- 31.Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med. 2002;165(9):1217–1239. doi: 10.1164/rccm.2109080. [DOI] [PubMed] [Google Scholar]
- 32.Martinez-Garcia MA, Soler-Cataluna JJ, Ejarque-Martinez L, et al. Continuous positive airway pressure treatment reduces mortality in patients with ischemic stroke and obstructive sleep apnea: a 5-year follow-up study. Am J Respir Crit Care Med. 2009;180(1):36–41. doi: 10.1164/rccm.200808-1341OC. [DOI] [PubMed] [Google Scholar]
- 33.Bolitschek J, Schmeiser-Rieder A, Schobersberger R, Rosenberger A, Kunze M, Aigner K. Impact of nasal continuous positive airway pressure treatment on quality of life in patients with obstructive sleep apnoea. Eur Respir J. 1998;11(4):890–894. doi: 10.1183/09031936.98.11040890. [DOI] [PubMed] [Google Scholar]
- 34.Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365(9464):1046–1053. doi: 10.1016/S0140-6736(05)71141-7. [DOI] [PubMed] [Google Scholar]
- 35.Kanagala R, Murali NS, Friedman PA, et al. Obstructive sleep apnea and the recurrence of atrial fibrillation. Circulation. 2003;107(20):2589–2594. doi: 10.1161/01.CIR.0000068337.25994.21. [DOI] [PubMed] [Google Scholar]
- 36.Large Truck and Bus Crash Facts 2014. Federal Motor Carrier Safety Administration website. [Accessed March 9, 2017]. https://www.fmcsa.dot.gov/safety/data-and-statistics/large-truck-and-bus-crash-facts-2014. Published March 2016. Updated April 15, 2016.
- 37.Fatality Analysis Reporting System (FARS) Encyclopedia. National Highway Traffic Safety Administration website. [Accessed March 9, 2017]. https://www-fars.nhtsa.dot.gov/Main/index.aspx.
- 38.Hursh SR, Fanzone JF, Raslear TG. Analysis of the Relationship between Operator Effectiveness Measures and Economic Impacts of Rail Accidents. Federal Railroad Administration website. [Accessed March 9, 2017]. https://www.fra.dot.gov/eLib/details/L01301. Published May 2011.
- 39.National Cooperative Highway Research Program Report 755: Comprehensive Costs of Highway-Rail Grade Crossing Crashes. Transportation Research Board website. [Accessed March 9, 2017]. http://www.trb.org/Main/Blurbs/169061.aspx. Published 2013. Updated March 23, 2016.
- 40.Frost & Sullivan; American Academy of Sleep Medicine. Hidden health crisis costing America billions: underdiagnosing and undertreating obstructive sleep apnea draining health care system. American Academy of Sleep Medicine website. [Accessed March 9, 2017]. http://www.aasmnet.org/sleepapnea-economic-impact.aspx. Published August 8, 2016.
- 41.Gurubhagavatula I, Nkwuo JE, Maislin G, Pack AI. Estimated cost of crashes in commercial drivers supports screening and treatment of obstructive sleep apnea. Accid Anal Prev. 2008;40(1):104–115. doi: 10.1016/j.aap.2007.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tarasiuk A, Reuveni H. The economic impact of obstructive sleep apnea. Curr Opin Pulm Med. 2013;19(6):639–644. doi: 10.1097/MCP.0b013e3283659e1e. [DOI] [PubMed] [Google Scholar]
- 43.Berger MB, Sullivan W, Owen R, Wu C. A Corporate Driven Sleep Apnea Detection and Treatment Program: Results and Challenges. Protecting Professional Drivers website. [Accessed March 9, 2017]. http://ppdsleep.com/sleep-dispoders-and-sleep-apnea-publications/. Published 2005.
- 44.Potts KJ, Butterfield DT, Sims P, Henderson M, Shames CB. Cost savings associated with an education campaign on the diagnosis and management of sleep-disordered breathing: a retrospective, claims-based US study. Popul Health Manag. 2013;16(1):7–13. doi: 10.1089/pop.2011.0102. [DOI] [PubMed] [Google Scholar]
- 45.Hartenbaum N, Collop N, Rosen IM, et al. Sleep apnea and commercial motor vehicle operators: statement from the joint Task Force of the American College of Chest Physicians, American College of Occupational and Environmental Medicine, and the National Sleep Foundation. J Occup Environ Med. 2006;48(9Suppl):S4–S37. doi: 10.1097/01.jom.0000236404.96857.a2. [DOI] [PubMed] [Google Scholar]
- 46.Ancoli-Israel S, Czeisler CA, George CF, Guilleminault C, Pack AI. Expert Panel Recommendations: Obstructive Sleep Apnea and Commercial Motor Vehicle Driver Safety. Federal Motor Carrier Safety Administration website. [Accessed March 9, 2017]. https://cms.fmcsa.dot.gov/regulations/medical/expert-panel-recommendations-obstructive-sleep-apnea-and-commercial-motor. Published 2008.
- 47.January 28, 2008 MRB Meeting Summary. Federal Motor Carrier Safety Administration website. [Accessed March 9, 2017]. https://www.fmcsa.dot.gov/january-28-2008-mrb-meeting-summary. Published April 7, 2008. Updated November 24, 2009.
- 48.Proposed Recommendations on Obstructive Sleep Apnea. Federal Motor Carrier Safety Administration website. [Accessed March 9, 2017]. https://www.fmcsa.dot.gov/regulations/notices/2012-9555. Published April 20, 2012.
- 49.Final Report: OSA (MCSAC/MRB February 2012 meeting) (Task 11-05) Federal Motor Carrier Safety Administration website. [Accessed March 9, 2017]. https://www.fmcsa.dot.gov/advisory-committees/mcsac/final-report-osa-mcsacmrb-february-2012-meeting-task-11-05-0. Published February 21, 2012.
- 50.Colvin LJ, Collop NA. Commercial motor vehicle driver obstructive sleep apnea screening and treatment in the united states: an update and recommendation overview. J Clin Sleep Med. 2016;12(1):113–125. doi: 10.5664/jcsm.5408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dagan Y, Doljansky JT, Green A, Weiner A. Body mass index (BMI) as a first-line screening criterion for detection of excessive daytime sleepiness among professional drivers. Traffic Inj Prev. 2006;7(1):44–48. doi: 10.1080/15389580500412994. [DOI] [PubMed] [Google Scholar]
- 52.Parks P, Durand G, Tsismenakis AJ, Vela-Bueno A, Kales S. Screening for obstructive sleep apnea during commercial driver medical examinations. J Occup Environ Med. 2009;51(3):275–282. doi: 10.1097/jom.0b013e31819eaaa4. [DOI] [PubMed] [Google Scholar]
- 53.Talmage JB, Hudson TB, Hegmann KT, Thiese MS. Consensus criteria for screening commercial drivers for obstructive sleep apnea: evidence of efficacy. J Occup Environ Med. 2008;50(3):324–329. doi: 10.1097/JOM.0b013e3181617ab8. [DOI] [PubMed] [Google Scholar]
- 54.National Standard for Health Assessment of Rail Safety Workers October 2012. National Transport Commission Australia website. [Accessed March 9, 2017]. https://www.ntc.gov.au/rail/safety/national-standard-for-health-assessment-of-rail-safety-workers/. Published October 2012. Updated March 16, 2013.
- 55.Xie W, Chakrabarty S, Levine R, Johnson R, Talmage JB. Factors associated with obstructive sleep apnea among commercial motor vehicle drivers. J Occup Environ Med. 2011;53(2):169–173. doi: 10.1097/JOM.0b013e3182068ceb. [DOI] [PubMed] [Google Scholar]
- 56.Gurubhagavatula I. Does the rubber meet the road? Addressing sleep apnea in commercial truck drivers. Sleep. 2012;35(11):1443–1444. doi: 10.5665/sleep.2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang C, Berger M, Malhotra A, Kales SN. Portable diagnostic devices for identifying obstructive sleep apnea among commercial motor vehicle drivers: considerations and unanswered questions. Sleep. 2012;35(11):1481–1489. doi: 10.5665/sleep.2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Aloia MS, Arnedt JT. Mechanisms of sleepiness in obstructive sleep apnea. In: Pack AI, editor. Sleep Apnea: Pathogenesis, Diagnosis and Treatment. 2nd ed. New York, NY: Informa Healthcare; 2012. [Google Scholar]
- 59.Durmer JS, Dinges DF. Neurocognitive consequences of sleep deprivation. Semin Neurol. 2005;25(1):117–129. doi: 10.1055/s-2005-867080. [DOI] [PubMed] [Google Scholar]
- 60.Epstein LJ, Kristo D, Strollo PJ, Jr, et al. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med. 2009;5(3):263–276. [PMC free article] [PubMed] [Google Scholar]
- 61.Logan AG, Perlikowski SM, Mente A, et al. High prevalence of unrecognized sleep apnoea in drug-resistant hypertension. J Hypertens. 2001;19(12):2271–2277. doi: 10.1097/00004872-200112000-00022. [DOI] [PubMed] [Google Scholar]
- 62.Pedrosa RP, Drager LF, Gonzaga CC, et al. Obstructive sleep apnea: the most common secondary cause of hypertension associated with resistant hypertension. Hypertension. 2011;58(5):811–817. doi: 10.1161/HYPERTENSIONAHA.111.179788. [DOI] [PubMed] [Google Scholar]
- 63.Foster GD, Sanders MH, Millman R, et al. Obstructive sleep apnea among obese patients with type 2 diabetes. Diabetes Care. 2009;32(6):1017–1019. doi: 10.2337/dc08-1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Peppard PE, Young T, Palta M, Dempsey J, Skatrud J. Longitudinal study of moderate weight change and sleep-disordered breathing. JAMA. 2000;284(23):3015–3021. doi: 10.1001/jama.284.23.3015. [DOI] [PubMed] [Google Scholar]
- 65.Warsi A, Wang PS, LaValley MP, Avorn J, Solomon DH. Self-management education programs in chronic disease: a systematic review and methodological critique of the literature. Arch Intern Med. 2004;164(15):1641–1649. doi: 10.1001/archinte.164.15.1641. [DOI] [PubMed] [Google Scholar]
- 66.Smith B, Phillips BA. Truckers drive their own assessment for obstructive sleep apnea: a collaborative approach to online self-assessment for obstructive sleep apnea. J Clin Sleep Med. 2011;7(3):241–245. doi: 10.5664/JCSM.1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Graber MA, Roller CM, Kaeble B. Readability levels of patient education material on the World Wide Web. J Fam Pract. 1999;48(1):58–61. [PubMed] [Google Scholar]
- 68.National Registry of Certified Medical Examiners website. [Accessed March 9, 2017]. https://nationalregistry.fmcsa.dot.gov/NRPublicUI/home.seam.
- 69.Parthasarathy S, Haynes PL, Budhiraja R, Habib MP, Quan SF. A national survey of the effect of sleep medicine specialists and American Academy of Sleep Medicine accreditation on management of obstructive sleep apnea. J Clin Sleep Med. 2006;2(2):133–142. [PubMed] [Google Scholar]
- 70.Parthasarathy S, Subramanian S, Quan SF. A multicenter prospective comparative effectiveness study of the effect of physician certification and center accreditation on patient-centered outcomes in obstructive sleep apnea. J Clin Sleep Med. 2014;10(3):243–249. doi: 10.5664/jcsm.3518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.DOT Rule 49 CFR Part 40 Section 40.21. United States Department of Transportation website. [Accessed March 9, 2017]. https://www.transportation.gov/odapc/part40/40_21.
- 72.Weaver TE, Chasens ER. Continuous positive airway pressure treatment for sleep apnea in older adults. Sleep Med Rev. 2007;11(2):99–111. doi: 10.1016/j.smrv.2006.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Berry RB, Kushida CA, Kryger MH, Soto-Calderon H, Staley B, Kuna ST. Respiratory event detection by a positive airway pressure device. Sleep. 2012;35(3):361–367. doi: 10.5665/sleep.1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ramar K, Dort LC, Katz SG, et al. Clinical practice guideline for the treatment of obstructive sleep apnea and snoring with oral appliance therapy: an update for 2015. J Clin Sleep Med. 2015;11(7):773–827. doi: 10.5664/jcsm.4858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Antic NA, Catcheside P, Buchan C, et al. The effect of CPAP in normalizing daytime sleepiness, quality of life, and neurocognitive function in patients with moderate to severe OSA. Sleep. 2011;34(1):111–119. doi: 10.1093/sleep/34.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Barbe F, Duran-Cantolla J, Sanchez-de-la-Torre M, et al. Effect of continuous positive airway pressure on the incidence of hypertension and cardiovascular events in nonsleepy patients with obstructive sleep apnea: a randomized controlled trial. JAMA. 2012;307(20):2161–2168. doi: 10.1001/jama.2012.4366. [DOI] [PubMed] [Google Scholar]
- 77.Campos-Rodriguez F, Pena-Grinan N, Reyes-Nunez N, et al. Mortality in obstructive sleep apnea-hypopnea patients treated with positive airway pressure. Chest. 2005;128(2):624–633. doi: 10.1378/chest.128.2.624. [DOI] [PubMed] [Google Scholar]
- 78.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14(6):540–545. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 79.Sassani A, Findley LJ, Kryger M, Goldlust E, George C, Davidson TM. Reducing motor-vehicle collisions, costs, and fatalities by treating obstructive sleep apnea syndrome. Sleep. 2004;27(3):453–458. doi: 10.1093/sleep/27.3.453. [DOI] [PubMed] [Google Scholar]
- 80.Bouloukaki I, Giannadaki K, Mermigkis C, et al. Intensive versus standard follow-up to improve continuous positive airway pressure compliance. Eur Respir J. 2014;44(5):1262–1274. doi: 10.1183/09031936.00021314. [DOI] [PubMed] [Google Scholar]
- 81.Colvin LJ, Dace GA, Colvin RM, Ojile J, Collop N. Commercial motor vehicle driver positive airway pressure therapy adherence in a sleep center. J Clin Sleep Med. 2016;12(4):477–485. doi: 10.5664/jcsm.5670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Medical Examination Report For Commercial Driver Fitness Determination 649-F (6045) Federal Motor Carrier Safety Administration website. [Accessed March 9, 2017]. https://www.fmcsa.dot.gov/sites/fmcsa.dot.gov/files/docs/Medical_Examination_Report_for_Commercial_Driver_Fitness_Determination_649-F(6045).pdf.
- 83.MacLean AW, Davies DR, Thiele K. The hazards and prevention of driving while sleepy. Sleep Med Rev. 2003;7(6):507–521. doi: 10.1016/s1087-0792(03)90004-9. [DOI] [PubMed] [Google Scholar]
- 84.Hirshkowitz M, Whiton K, Albert SM, et al. National Sleep Foundation's updated sleep duration recommendations: final report. Sleep Health. 2015;1(4):233–243. doi: 10.1016/j.sleh.2015.10.004. [DOI] [PubMed] [Google Scholar]
- 85.Bonato R, Bradley DC. Introducing a novel micro-recorder for the detection of oral appliance compliance: DentiTrac. Sleep Diagnosis and Therapy. 2013;8(3):12–15. [Google Scholar]


