Abstract
Background:
Th9 cells are a newly discovered CD4+ T helper cell subtype, characterized by high interleukin (IL)-9 secretion. Growing evidences suggest that Th9 cells are involved in the pathogenic mechanism of multiple sclerosis (MS). Mast cells are multifunctional innate immune cells, which are perhaps best known for their role as dominant effector cells in allergies and asthma. Several lines of evidence point to an important role for mast cells in MS and its animal models. Simultaneously, there is dynamic “cross-talk” between Th9 and mast cells. The aim of the present study was to examine the IL-9-mast cell axis in experimental autoimmune encephalomyelitis (EAE) and determine its interaction after neutralizing anti-IL-9 antibody treatment.
Methods:
Female C57BL/6 mice were randomly divided into three groups (n = 5 in each group): mice with myelin oligodendrocyte glycoprotein (MOG)-induced EAE (EAE group), EAE mice treated with anti-IL-9 antibody (anti-IL-9 Abs group), and EAE mice treated with IgG isotype control (IgG group). EAE clinical score was evaluated. Mast cells from central nervous system (CNS) were detected by flow cytometry. The production of chemokine recruiting mast cells in the CNS was explored by reverse transcription-polymerase chain reaction (RT-PCR). In mice with MOG-induced EAE, the expression of IL-9 receptor (IL-9R) complexes in CNS and spleen mast cells was also explored by RT-PCR, and then was repeating validated by immunocytochemistry. In vitro, spleen cells from EAE mice were cultured with anti-IL-9 antibody, and quantity of mast cells was counted by flow cytometry after co-culture.
Results:
Compared with IgG group, IL-9 blockade delayed clinical disease onset and ameliorated EAE severity (t = −2.217, P = 0.031), accompany with mast cells infiltration decreases (day 5: t = −8.005, P < 0.001; day 15: t = −11.857, P < 0.001; day 20: t = −5.243, P = 0.001) in anti-IL-9 Abs group. The messenger RNA expressions of C-C motif chemokine ligand 5 (t = −5.932, P = 0.003) and vascular cell adhesion molecule-1 (t = −4.029, P = 0.004) were significantly decreased after IL-9 neutralization in anti-IL-9 Abs group, compared with IgG group. In MOG-induced EAE, the IL-9R complexes were expressed in CNS and spleen mast cells. In vitro, splenocytes cultured with anti-IL-9 antibody showed significantly lower levels of mast cells in a dose-dependent manner, compared with splenocytes cultured with anti-mouse IgG (5 μg/ml: t = −0.894, P = 0.397; 10 μg/ml: t = −3.348, P = 0.019; 20 μg/ml: t = −7.639, P < 0.001).
Conclusions:
This study revealed that IL-9 neutralization reduced mast cell infiltration in CNS and ameliorated EAE, which might be relate to the interaction between IL-9 and mast cells.
Keywords: Experimental Autoimmune Encephalomyelitis, Interleukin-9, Mast Cell, Multiple Sclerosis, Th9
Introduction
Multiple sclerosis (MS) is an inflammatory demyelinating disease of the central nervous system (CNS) with a complex etiology.[1] The pathogenesis of MS is not completely understood, and it is believed that a combination of genetic, environmental, and infectious factors trigger autoimmune activity and cause MS onset.[2] Experimental autoimmune encephalomyelitis (EAE) is a mouse model for MS, wherein disease is mediated by CD4+ T helper cells, macrophages, and other immune cells.[3]
Th9 cells are a newly discovered CD4+ T helper cell subtype, characterized by high interleukin (IL)-9 secretion.[4,5] Growing evidences suggest that Th9 cells are involved in the pathogenic mechanism of MS. Moreover, it has been reported that myelin oligodendrocyte glycoprotein (MOG)-specific Th9 cells can induce EAE on adoptive transfer,[6] whereas anti-IL-9 neutralizing antibody treatment attenuates EAE.[7]
Mast cells are multifunctional innate immune cells, which are perhaps best known for their role as dominant effector cells in allergies and asthma.[8] However, mast cells have been widely overlooked in other autoimmune diseases.[9] Nowadays, several lines of evidence point to an important role for mast cells in MS and its animal models.[10,11] Mast cells are present in inflammatory demyelinating plaques in the brain and spinal cord of MS patients, while a unique mast cell protease (tryptase) and histamine are elevated in cerebrospinal fluid.[12] Furthermore, EAE severity can be reduced by preventing mast cell activation through administration of depletory vasoactive amines (specifically, reserpine) in mast cell granules.[13] These clinical- and laboratory-based studies support a prominent role for mast cells in MS pathogenesis.
Simultaneously, there is dynamic “cross-talk” between mast cells and lymphocytes (regulatory/effector T-cells and B-cells) for integrated control of immune damage and development of the immune response.[14] Besides, the interaction between Th9 and mast cells has been confirmed in other autoimmune diseases. Kearley et al.[15] found that IL-9 governs allergen inducing mast cell number in the lung, and has pronounced effects on chronic allergic inflammation, thereby suggesting an important role for the IL-9-mast cell axis in autoimmune disease pathology. However, the involvement of IL-9-mast cell interaction in MS pathogenesis remains unknown. Here, the aim of the present study was to examine the IL-9-mast cell axis in EAE, and determine its interaction after neutralizing anti-IL-9 antibody treatment.
Methods
Reagents
Rat MOG 35–55 peptide (MEVGWYRSPFSRVVHLYRNGK; >95% purity) was synthesized by Xi’an Biotechnology Co., (Xi’an, China). Heat-killed Mycobacterium tuberculosis H37Ra was obtained from Difco (USA). Complete Freund's adjuvant (CFA) was obtained from Sigma Aldrich (USA), and pertussis toxin (PTX) from Alexis (Germany). The following antibodies were used in this study: FITC-anti-mouse CD45 (eBioscience, USA), PE-Cyanine5-anti-mouse CD117 (eBioscience), anti-mouse IL-9 (BE0181; BioXCell, USA), anti-mouse IgG2a isotype control (BE0085; BioXCell), anti-mouse IL-9 receptor (IL-9R) (SC699; Santa Cruz, USA), anti-mouse IL-2Rγ (SC668; Santa Cruz), anti-mouse IgG isotype control (GTX35009; Santa Cruz), and donkey pAb to Rb IgG Alexa Flour 555 (Ab150074; Abcam, USA).
Animals
Female C57BL/6 mice, aged between 6 and 10 weeks and weighing 16–18 g, were provided by the Medical Laboratory Animal Center of Guangdong Province (Foshan City, China). Mice were bred in the Laboratory Animal Center of Sun Yat-sen University (Guangzhou, China). The animal experimental protocol was approved by the Animal Experiment Committee of Sun Yat-sen University.
Experimental autoimmune encephalomyelitis and anti-interleukin-9 monoclonal antibody treatment
Female C57BL/6 mice were randomly divided into three groups (n = 5 in each group): Mice with MOG-induced EAE (EAE group), EAE mice treated with anti-IL-9 antibody (anti-IL-9 Abs group), and EAE mice treated with IgG isotype control (IgG group). MOG-induced EAE was induced as described previously.[16] Briefly, on days 0 and 7, mice were injected subcutaneously with a 0.2 ml emulsion containing 200 μg MOG 35–55 peptide in phosphate buffer saline combined with an equal volume of CFA containing 300 μg heat-killed M. tuberculosis H37Ra, respectively in the bilateral inguinal and axillary regions. On the day of immunization and 2 days after immunization, mice were injected with PTX intraperitoneally (300 ng/mouse). Starting from the day before immunization, anti-IL-9 antibody or IgG isotype control was injected intraperitoneally every other day for the whole course of 30 days.[17] Clinical EAE was graded daily on a scale of 1–5 using previously established standard criteria:[18] 0, normal; 1, flaccid tail; 2, moderate hind or front leg weakness; 3, severe hind or front leg weakness; 4, complete paralysis of limb(s); and 5, moribund.
Sample preparation
Purified cells containing mast cells were harvested from the CNS and spleen, as described previously.[17] Briefly, tissue was gently mashed and resuspended in 2 ml 70% buffered Percoll (Amersham Pharmacia Biotech, USA). After centrifugation, the suspension at the bottom of the tube was mainly composed of mast cells and red cells. This suspension was harvested, washed, and incubated in 3 ml red blood cell lysis buffer (Qiagen, Crawley, UK).
Flow cytometry
CD45+ CD117+ mast cells from CNS and spleen were harvested from purified cells by flow cytometry. Purified cells were stained with specific monoclonal antibodies (mAbs). All mAbs for flow cytometry were purchased from eBioscience. The protocol was the same as described previously.[17]
Reverse transcription-polymerase chain reaction
Total RNA was extracted from CNS and spleen mast cells. Total RNA (0.2 μg) was denatured for 5 min at 65°C and reverse transcription performed. Real-time reverse transcription-polymerase chain reaction (RT-PCR) was performed using gene specific primers [Table 1], as described previously.[17] An internal control (β-actin) was used to calculate relative expression of targeted genes and standardize complementary DNA loaded in each sample.
Table 1.
Primers used for PCR amplification
| Genes | Primers |
|---|---|
| β-Actin | |
| Forward primers | 5’ GGCTGTATTCCCCTCCATCG3’ |
| Reverse primers | 5’ CCAGTTGGTAACAATGCCATGT3 |
| IL-2Rγ | |
| Forward primers | 5’ TTCTCCCTGCCTAGTGTGGA3’ |
| Reverse primers | 5’ GGACAGGCTGGCTCCATTTA3’ |
| IL-9R | |
| Forward primers | 5’ TCCTGGTTCCTGATCTACAGC3’ |
| Reverse primers | 5’ TGTGTTTGATTTCAGTCACCTGG3’ |
| CCL-2 | |
| Forward primers | 5’ CAGCAGGTGTCCCAAAGAAG3’ |
| Reverse primers | 5’ AAGTGCTTGAGGTGGTTGTG3’ |
| CCL-5 | |
| Forward primers | 5’ ATATGGCTCGGACACCACTC3’ |
| Reverse primers | 5’ CACACACTTGGCGGTTCCTT3’ |
| Vcam-1 | |
| Forward primers | 5’ CTTGTGGAAATGTGCCCGAA3’ |
| Reverse primers | 5’ TAGAGTGCAAGGAGTTCGGG3’ |
| Pecam-1 | |
| Forward primers | 5’ GTCATTGGAGTGGTCATCGC3’ |
| Reverse primers | 5’ GCTTCCACACTAGGCTCAGA3’ |
| SCF | |
| Forward primers | 5’ TGGAAGAAAACGCACCGAAG3’ |
| Reverse primers | 5’ TGGAATCTTTCTCGGGACCT3’ |
CCL2: C-C motif chemokine ligand 2; CCL5: C-C motif chemokine ligand 5; IL-2Rγ: Interleukin-2 receptor γ; IL-9R: Interleukin-9 receptor; PCR: Polymerase chain reaction; Pecam1: Platelet and endothelial cell adhesion molecule 1; SCF: Supercoiling factor; Vcam-1: Vascular cell adhesion molecule 1.
Immunocytochemistry
Spleen mast cells purified by flow cytometry sorting were plated on 24-well chamber slides and fixed with paraformaldehyde for 30 min. Cells were then incubated with DAPI (Invitrogen, USA), anti-IL-9R (Santa Cruz Biotechnology), and anti-IL-2Rγ (Santa Cruz Biotechnology) antibodies. Next, cells were incubated with Alexa Fluor555-conjugated secondary antibodies (Abcam) for 30 min. Cells were examined using a deconvolution fluorescence microscope system (Zeiss, Germany).[17]
Splenocyte culture
Splenocyte cultures were prepared as described previously.[19] Splenocytes were harvested from EAE mice (2–3 scores) 5 days after MOG immunization. They were then divided into equal cell numbers (0.5 × 106 cells) in wells, and incubated with different concentrations (5 μg/ml, 10 μg/ml, and 20 μg/ml) of anti-IL-9 antibody or anti-mouse IgG for 7 h.
Statistical analysis
Data were expressed as mean ± standard error. Results among three groups were analyzed by one-way analysis of variance. Two groups were compared using Student's t-test. All statistical analysis was performed using SPSS version 13.0 for Windows (SPSS Inc., Chicago, IL, USA). A P < 0.05 was considered statistically significant.
Results
Anti-interleukin-9 antibody treatment suppressed experimental autoimmune encephalomyelitis
To determine the efficacy of anti-IL-9 antibody treatment in EAE, we examined EAE clinical score. It was shown that IL-9 blockade delayed clinical disease onset and ameliorated EAE severity in anti-IL-9 Abs group, compared withIgG group (t = −2.217, P = 0.031; Figure 1). These results confirmed that IL-9 neutralization was an effective therapeutic strategy for EAE.
Figure 1.
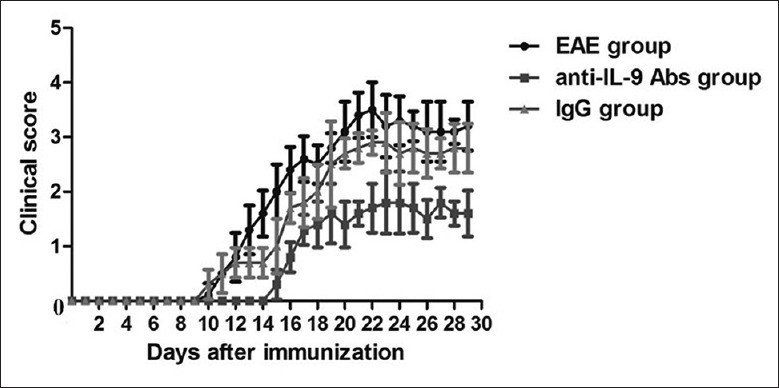
Anti-IL-9 antibody treatment suppressed EAE. IL-9 blockade delayed clinical disease onset and ameliorated EAE severity. Data are shown as mean ± SE. EAE: Experimental autoimmune encephalomyelitis; SE: Standard error; IL: Interleukin.
Anti-interleukin-9 antibody treatment decreased mast cell infiltration of the central nervous system
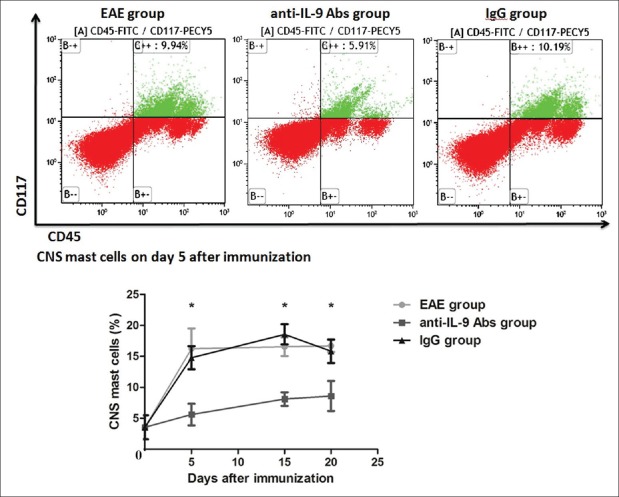
To determine the mast cell population that infiltrated the CNS, purified cells (mainly composed of mast cells) were harvested from the CNS (i.e., cerebrum, cerebellum, brain stem, and spinal cord) on days 0, and 5, 15, and 20 after EAE immunization. CD45+ CD117+ mast cells were detected by flow cytometry. CNS mast cells maintained a high level throughout the disease course in EAE group [Figure 2]. However, mast cells were significantly reduced from day 5 and remained low in anti-IL-9 Abs group, compared with IgG group (day 5: t = −8.005, P < 0.001; day 15: t = −11.857, P < 0.001; day 20: t = −5.243, P = 0.001).
Figure 2.
Anti-IL-9 antibody treatment decreased mast cell infiltration of the CNS. Purified cells (mainly composed of mast cells) were harvested from the CNS on days 0, and 5, 15, and 20 after EAE immunization. CD45+ CD117+ mast cells were detected by flow cytometry. CNS mast cells maintained a high level throughout the disease course in EAE group. However, mast cells were significantly reduced from day 5, and remained low in anti-IL-9 Abs group, compared with IgG group. Data are shown as mean ± SE. *P < 0.01, anti-IL-9 Abs group versus IgG group. CNS: Central nervous system; EAE: Experimental autoimmune encephalomyelitis; SE: Standard error; IL: Interleukin.
Interleukin-9 blockade reduced production of chemokine recruiting mast cells in the central nervous system
IL-9 induces various chemotactic factors in mice and humans.[20,21] Among them, platelet and endothelial cell adhesion molecule 1 (Pecam1), supercoiling factor (SCF), vascular cell adhesion molecule 1 (Vcam-1), C-C motif chemokine ligand 2 (CCL2), and C-C motif chemokine ligand 5 (CCL5, RANTES) are the main chemokine recruiting mast cells.[22] Thus, in anti-IL-9 Abs and IgG groups, the production of these chemokines in EAE after IL-9 blockade were examined by RT-PCR; and messenger RNA (mRNA) expressions of the chemokines in CNS tissue of EAE were also detected 5 days after immunization. It was found that after IL-9 neutralization, mRNA expressions of CCL5 and Vcam-1 were significantly decreased in anti-IL-9 Abs group, compared with IgG group (CCL5: t = −5.932, P = 0.003; Vcam-1: t = −4.029, P = 0.004; Figure 3).
Figure 3.
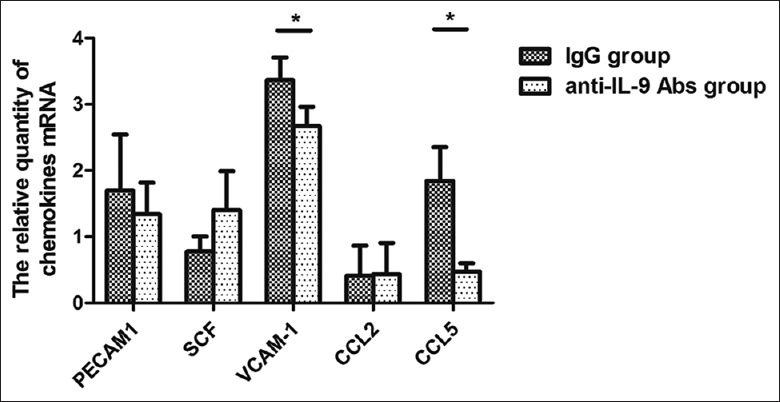
IL-9 blockade reduced production of chemokine recruiting mast cells in the CNS. Five days after immunization, mRNA expressions of Pecam1, SCF, Vcam-1, CCL2, and CCL5 in CNS tissue of EAE mice were detected by RT-PCR. After IL-9 neutralization, mRNA expressions of CCL5 and Vcam-1 were significantly decreased in anti-IL-9 Abs group, compared with IgG group. *P < 0.01. Pecam1: Platelet and endothelial cell adhesion molecule 1; SCF: Supercoiling factor; Vcam-1: Vascular cell adhesion molecule 1; CCL2: C-C motif chemokine ligand 2; CCL5: C-C motif chemokine ligand 5; CNS: Central nervous system; EAE: Experimental autoimmune encephalomyelitis; IL: Interleukin; RT-PCR: Reverse transcription-polymerase chain reaction; mRNA: Messenger RNA.
Interleukin-9 receptor complex was expressed in central nervous system and spleen mast cells
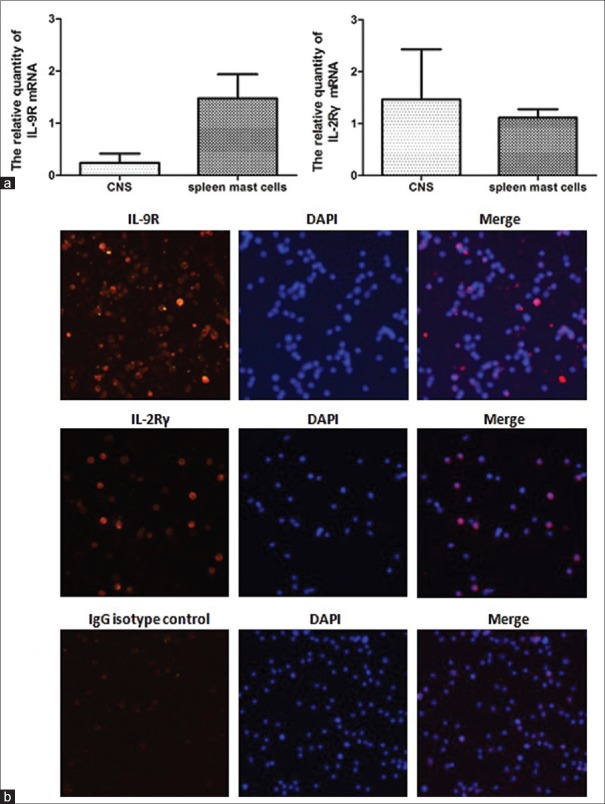
To determine the IL-9 target that interacts with CNS and mast cells, we examined the expression of IL-9R complexes (which are mainly composed of IL-9R and IL-2Rγ[23,24]) in CNS tissue and spleen mast cells from EAE mice 5 days after MOG immunization. RT-PCR showed mRNA expressions of both IL-9R and IL-2Rγ in CNS and spleen mast cells [Figure 4].
Figure 4.
The IL-9R complexes (mainly composed of IL-9R and IL-2Rγ) were expressed in CNS and spleen mast cells. (a) RT-PCR showed mRNA expressions of both IL-9R and IL-2Rγ in CNS and spleen mast cells. (b) Immunocytochemistry further confirmed protein expressions of IL-9R and IL-2Rγ in spleen mast cells of EAE (original magnification ×200). IL-9R: Interleukine-9 receptor; IL-2Rγ: Interleukin-2 receptor γ; CNS: Central nervous system; EAE: Experimental autoimmune encephalomyelitis; RT-PCR: Reverse transcription-polymerase chain reaction; mRNA: Messenger RNA.
Immunocytochemistry further confirmed the protein expressions of IL-9R and IL-2Rγ in spleen mast cells of EAE mice [Figure 4]. These results suggested that the IL-9R complex was expressed in CNS and spleen mast cells, and indicated that CNS and mast cells were the targets of IL-9 in EAE.
In vitro effect of anti-interleukin-9 antibody on spleen mast cells
To determine whether anti-IL-9 antibody has a direct effect on mast cells, we cultured splenic cells with anti-IL-9 antibody or anti-mouse IgG in vitro. Splenocytes were harvested from EAE mice 5 days after MOG immunization. After co-culture for 7 h, mast cell numbers were counted by flow cytometry. Compared with splenocytes cultured with anti-mouse IgG, the splenocytes cultured with anti-IL-9 antibody showed significantly lower levels of mast cells in a dose-dependent manner; and this trend was particularly evident with anti-IL-9 antibody concentrations up to 20 μg/ml (5 μg/ml: t = −0.894, P = 0.397; 10 μg/ml: t = −3.348, P = 0.019; 20 μg/ml: t = −7.639, P < 0.001; Figure 5).
Figure 5.
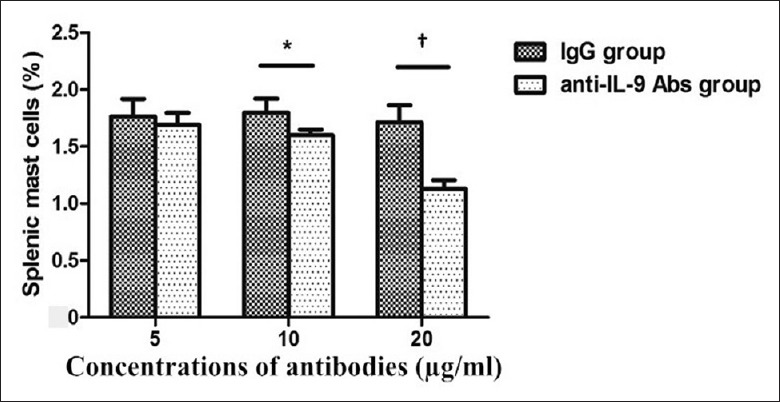
In vitro, the effect of anti-IL-9 antibody on splenic mast cells. Splenocytes were harvested from experimental autoimmune encephalomyelitis mice 5 days after MOG immunization. After co-culture with anti-IL-9 antibody or anti-mouse IgG for 7 h, mast cell number was counted by flow cytometry. Splenic mast cells cultured with anti-IL-9 antibody showed significantly lower levels in a dose-dependent manner. This trend was particularly evident with anti-IL-9 antibody concentrations up to 20 μg/ml. *P < 0.05; †P < 0.01. IL: Interleukin; MOG: Myelin oligodendrocyte glycoprotein.
Discussion
In addition to Th1, Th2, and Th17 cells, another effector T cell subset has recently been described, namely Th9 cells.[4,5] Previous studies have shown that Th9 cells are capable of inducing EAE.[6] Mast cells originate in bone marrow from lineage-specific multipotent hematopoietic progenitors.[25,26] They display functional diversity depending on the tissue in which they differentiate. Their distribution enables them to be among the first cells of the immune response to interact with environmental antigens and allergens, invading pathogens, or toxic compounds.[27,28] In recent years, research for mast cells has dramatically changed our perception of their function and role. A growing body of evidence showed that mast cells are implicated in a broad spectrum of autoimmune responses, including inflammatory demyelinating diseases.[10,11,29,30,31]
Remarkably, in EAE, both Th9 cells and mast cells play an effector function against blood-brain barrier disruption.[17,32] Moreover, Th9 and mast cell interaction has been confirmed in other autoimmune diseases. For example, in an adoptive transfer model of allergic inflammation, Th9 cell recipients showed significantly higher mast cell accumulation and expression of mast cell proteases. Further, mast cell accumulation was dependent on IL-9, suggesting that Th9 cells and IL-9 played an important role in mast cell accumulation and activation.[33] Another study found that anti-IL-9 antibody treated mice were protected from airway remodeling with a concomitant reduction in mature mast cell number and activation, also demonstrating an important role of the IL-9-mast cell axis in chronic asthma pathology.[15] However, the presence of the IL-9-mast cell interaction in MS pathogenesis remains unknown.
In this study, IL-9 blockade delayed onset of clinical disease, ameliorated EAE severity, and was accompanied by decreasing mast cell infiltration of the CNS. Our results indicated that IL-9 was an important factor for EAE pathogenesis and could affect CNS mast cell quantity. This demonstrated that both IL-9 and mast cells contributed to the autoimmune response of EAE. Consistent with our study, genetically ablating IL-9R or blocking IL-9 with a neutralizing antibody attenuated EAE due to fewer Th17 cells within the CNS.[7,34] Appropriately, another study suggested that IL-9 signaling deficiency exacerbated EAE.[35] Thus, IL-9 might have multi-effects in EAE pathogenesis. At present, the exact role of mast cells in CNS autoimmune disease is highly debated, in particular with regards to basic data for research, which has often shown contradictory outcomes when using different animal models.[10,11] The controversial aspects for mast cells might be due to their multifunctional characteristics, with mast cell function appearing plastic and reliant on the specific tissue microenvironment. For example, de Vries et al.[36] reported that mast cell degranulation resulted in transient loss of regulatory T cell (Treg) suppressor activity, and disrupted peripheral tolerance in an allograft tolerance model. Lu et al.[37] found that IL-9 activated mast cells to mediate regional immune suppression in a tolerant allograft model, while neutralization of IL-9 greatly accelerated allograft rejection in tolerant mice. Indeed, mast cell contact with a wide array of cytokine signaling pathways allowed them to respond to different kinds of stimuli within specific microenvironments. Because of this plastic response in mast cells, the role they playing in autoimmune disease was more complex than expected. The mechanisms by which mast cells contribute to these controversial phenomenon needs further investigation. Nonetheless, it is evident that mast cells are involved in the pathogenesis of inflammatory demyelinating diseases.[10,11]
To exclude factors that might affect the mast cell microenvironment in vivo, and to examine the direct effect of anti-IL-9 antibody on mast cells, we co-cultured anti-IL-9 antibody and splenocytes in vitro. Consequently, we found that neutralizing IL-9 with anti-IL-9 antibody decreased mast cells in cultured splenocytes. This further supported the hypothesis that IL-9 had a direct effect on mast cells.
The pathways by which IL-9 blockade decreases mast cell infiltration in EAE have not been examined. Previous studies have shown that IL-9 enhanced expression of various chemokines for tissue cell accumulation.[38,39] For example, Zhou et al.[17] observed that IL-9 induced astrocytic production of C-C motif chemokine ligand 20 (CCL-20) and subsequent migration of Th17 cells into the CNS, resulting in an EAE immune response. Therefore, we speculated that IL-9 effects on mast cells may also involve chemokine regulation. Vcam-1, Pecam1, SCF, CCL-2, and CCL-5 (RANTES) are the main chemokines recruiting mast cells.[22] Thus, we examined the expression of these chemokines, confirming their expression in CNS tissue from EAE mice, and showing significantly decreased CCL-5 and Vcam-1 production after IL-9 blockade. These findings suggested that IL-9 neutralization reduced mast cell infiltration by decreasing CCL-5 and Vcam-1 expression, and provided additional evidence for an interaction between IL-9 and mast cells.
Corroboratively, there is a structural basis for the interaction between IL-9 and mast cells. Previously, Fontaine et al.[40] reported that IL-9R was detected on the surface of neurons. Our study confirmed this, and further showed IL-9R complex expression in CNS and splenic mast cells. Overall, these results suggested that CNS and splenic mast cells were the targets of IL-9, and suggested an important role for the IL-9-mast cell axis in EAE pathology.
We have not explored the downstream biological effect of mast cells under IL-9 regulation in the present study. In addition, Th9 cells produce large amounts of IL-9 and are the main source of IL-9. Jäger et al.[6] has shown that MOG-reactive Th9 cells infiltrated the CNS on adoptive transfer, suggesting that Th9 cells might produce IL-9 to start the cascade in inflammatory demyelinating disease. Nevertheless, the presence of a Th9-mast cell interaction in EAE pathogenesis remains unknown. Further investigation on the precise interaction between Th9 and mast cells may provide new insights into the pathogenesis of inflammatory demyelinating diseases.
In conclusion, this study showed that IL-9 blockade ameliorated EAE, and was accompanied by decreased mast cell infiltration and reduced CCL-5 and Vcam-1 expression in the CNS. This suggested that IL-9 neutralization reduced CNS mast cell infiltration and ameliorated EAE, which might lead to decreased CCL-5 and Vcam-1 expressions. Simultaneously, the IL-9R complexes were expressed in CNS and spleen mast cells, demonstrating that mast cells were the target of IL-9, which provided additional evidence for an interaction between IL-9 and mast cells in EAE. However, the downstream biological effects of mast cells under IL-9 regulation need further investigation.
Financial support and sponsorship
This study was supported by a grant from the Natural Science Foundation of China (No. 81301028).
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Xin Chen
References
- 1.Compston A, Coles A. Multiple sclerosis. Lancet. 2008;372:1502–17. doi: 10.1016/S0140-6736(08)61620-7. doi: 10.1016/S0140-6736(08)61620-7. [DOI] [PubMed] [Google Scholar]
- 2.Chen H, Liu SM, Zhang XX, Liu YO, Li SZ, Liu Z, et al. Clinical features of patients with multiple sclerosis and neuromyelitis optica spectrum disorders. Chin Med J. 2016;129:2079–84. doi: 10.4103/0366-6999.189046. doi: 10.4103/0366-6999.189046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Procaccini C, De Rosa V, Pucino V, Formisano L, Matarese G. Animal models of Multiple sclerosis. Eur J Pharmacol. 2015;759:182–91. doi: 10.1016/j.ejphar.2015.03.042. doi: 10.1016/j.ejphar. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dardalhon V, Awasthi A, Kwon H, Galileos G, Gao W, Sobel RA, et al. IL-4 inhibits TGF-beta-induced Foxp3+ T cells and, together with TGF-beta, generates IL-9+ IL-10+ Foxp3(−) effector T cells. Nat Immunol. 2008;9:1347–55. doi: 10.1038/ni.1677. doi: 10.1038/ni.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Veldhoen M, Uyttenhove C, van Snick J, Helmby H, Westendorf A, Buer J, et al. Transforming growth factor-beta ‘reprograms’ the differentiation of T helper 2 cells and promotes an interleukin 9-producing subset. Nat Immunol. 2008;9:1341–6. doi: 10.1038/ni.1659. doi: 10.1038/ni.1659. [DOI] [PubMed] [Google Scholar]
- 6.Jäger A, Dardalhon V, Sobel RA, Bettelli E, Kuchroo VK. Th1, Th17, and Th9 effector cells induce experimental autoimmune encephalomyelitis with different pathological phenotypes. J Immunol. 2009;183:7169–77. doi: 10.4049/jimmunol.0901906. doi: 10.4049/jimmunol.0901906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li H, Nourbakhsh B, Ciric B, Zhang GX, Rostami A. Neutralization of IL-9 ameliorates experimental autoimmune encephalomyelitis by decreasing the effector T cell population. J Immunol. 2010;185:4095–100. doi: 10.4049/jimmunol.1000986. doi: 10.4049/jimmunol.1000986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Galli SJ, Nakae S, Tsai M. Mast cells in the development of adaptive immune responses. Nat Immunol. 2005;6:135–42. doi: 10.1038/ni1158. doi: 10.1038/ni1158. [DOI] [PubMed] [Google Scholar]
- 9.Xu Y, Chen G. Mast cell and autoimmune diseases. Mediators Inflamm 2015. 2015 doi: 10.1155/2015/246126. 246126. doi: 10.1155/2015/246126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Costanza M, Colombo MP, Pedotti R. Mast cells in the pathogenesis of multiple sclerosis and experimental autoimmune encephalomyelitis. Int J Mol Sci. 2012;13:15107–25. doi: 10.3390/ijms131115107. doi: 10.3390/ijms131115107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Conti P, Kempuraj D. Important role of mast cells in multiple sclerosis. Mult Scler Relat Disord. 2016;5:77–80. doi: 10.1016/j.msard.2015.11.005. doi: 10.1016/j.msard.2015.11.005. [DOI] [PubMed] [Google Scholar]
- 12.Karagkouni A, Alevizos M, Theoharides TC. Effect of stress on brain inflammation and multiple sclerosis. Autoimmun Rev. 2013;12:947–53. doi: 10.1016/j.autrev.2013.02.006. doi: 10.1016/j.autrev.2013. [DOI] [PubMed] [Google Scholar]
- 13.Dietsch GN, Hinrichs DJ. The role of mast cells in the elicitation of experimental allergic encephalomyelitis. J Immunol. 1989;142:1476–81. [PubMed] [Google Scholar]
- 14.Mekori YA, Hershko AY, Frossi B, Mion F, Pucillo CE. Integrating innate and adaptive immune cells: Mast cells as crossroads between regulatory and effector B and T cells. Eur J Pharmacol. 2016;778:84–9. doi: 10.1016/j.ejphar.2015.03.087. doi: 10.1016/j.ejphar.2015.03.087. [DOI] [PubMed] [Google Scholar]
- 15.Kearley J, Erjefalt JS, Andersson C, Benjamin E, Jones CP, Robichaud A, et al. IL-9 governs allergen-induced mast cell numbers in the lung and chronic remodeling of the airways. Am J Respir Crit Care Med. 2011;183:865–75. doi: 10.1164/rccm.200909-1462OC. doi: 10.1164/rccm. 200909-1462OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang J, Takeuchi H, Sonobe Y, Jin S, Mizuno T, Miyakawa S, et al. Inhibition of midkine alleviates experimental autoimmune encephalomyelitis through the expansion of regulatory T cell population. Proc Natl Acad Sci U S A. 2008;105:3915–20. doi: 10.1073/pnas.0709592105. doi: 10.1073/pnas.0709592105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou Y, Sonobe Y, Akahori T, Jin S, Kawanokuchi J, Noda M, et al. IL-9 promotes Th17 cell migration into the central nervous system via CC chemokine ligand-20 produced by astrocytes. J Immunol. 2011;186:4415–21. doi: 10.4049/jimmunol.1003307. doi: 10.4049/jimmunol.1003307. [DOI] [PubMed] [Google Scholar]
- 18.Kono DH, Urban JL, Horvath SJ, Ando DG, Saavedra RA, Hood L. Two minor determinants of myelin basic protein induce experimental allergic encephalomyelitis in SJL/J mice. J Exp Med. 1988;168:213–27. doi: 10.1084/jem.168.1.213. doi: 10.1084/jem.168.1.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Denney L, Kok WL, Cole SL, Sanderson S, McMichael AJ, Ho LP. Activation of invariant NKT cells in early phase of experimental autoimmune encephalomyelitis results in differentiation of Ly6Chi inflammatory monocyte to M2 macrophages and improved outcome. J Immunol. 2012;189:551–7. doi: 10.4049/jimmunol.1103608. doi: 10.4049/jimmunol.1103608. [DOI] [PubMed] [Google Scholar]
- 20.Dong Q, Louahed J, Vink A, Sullivan CD, Messler CJ, Zhou Y, et al. IL-9 induces chemokine expression in lung epithelial cells and baseline airway eosinophilia in transgenic mice. Eur J Immunol. 1999;29:2130–9. doi: 10.1002/(SICI)1521-4141(199907)29:07<2130::AID-IMMU2130>3.0.CO;2-S. doi: 10.1002/(SICI)1521-4141(199907)29:07<2130::AID-IMMU2130>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 21.Soussi-Gounni A, Kontolemos M, Hamid Q. Role of IL-9 in the pathophysiology of allergic diseases. J Allergy Clin Immunol. 2001;107:575–82. doi: 10.1067/mai.2001.114238. doi: 10.1067/mai.2001.114238. [DOI] [PubMed] [Google Scholar]
- 22.Taub D, Dastych J, Inamura N, Upton J, Kelvin D, Metcalfe D, et al. Bone marrow-derived murine mast cells migrate, but do not degranulate, in response to chemokines. J Immunol. 1995;154:2393–402. [PubMed] [Google Scholar]
- 23.Kimura Y, Takeshita T, Kondo M, Ishii N, Nakamura M, Van Snick J, et al. Sharing of the IL-2 receptor gamma chain with the functional IL-9 receptor complex. Int Immunol. 1995;7:115–20. doi: 10.1093/intimm/7.1.115. doi: 10.1093/intimm/7.1.115. [DOI] [PubMed] [Google Scholar]
- 24.Demoulin JB, Uyttenhove C, Van Roost E, DeLestré B, Donckers D, Van Snick J, et al. A single tyrosine of the interleukin-9 (IL-9) receptor is required for STAT activation, antiapoptotic activity, and growth regulation by IL-9. Mol Cell Biol. 1996;16:4710–6. doi: 10.1128/mcb.16.9.4710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Galli SJ, Grimbaldeston M, Tsai M. Immunomodulatory mast cells: Negative, as well as positive, regulators of immunity. Nat Rev Immunol. 2008;8:478–86. doi: 10.1038/nri2327. doi: 10.1038/nri2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gurish MF, Austen KF. Developmental origin and functional specialization of mast cell subsets. Immunity. 2012;37:25–33. doi: 10.1016/j.immuni.2012.07.003. doi: 10.1016/j.immuni.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 27.Janssens AS, Heide R, den Hollander JC, Mulder PG, Tank B, Oranje AP. Mast cell distribution in normal adult skin. J Clin Pathol. 2005;58:285–9. doi: 10.1136/jcp.2004.017210. doi: 10.1016/j.immuni.2012.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abraham SN, St. John AL. Mast cell-orchestrated immunity to pathogens. Nat Rev Immunol. 2010;10:440–52. doi: 10.1038/nri2782. doi: 10.1038/nri2782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Galli SJ, Tsai M. IgE and mast cells in allergic disease. Nat Med. 2012;18:693–704. doi: 10.1038/nm.2755. doi: 10.1038/nm.2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Walker ME, Hatfield JK, Brown MA. New insights into the role of mast cells in autoimmunity: Evidence for a common mechanism of action? Biochim Biophys Acta. 2012;1822:57–65. doi: 10.1016/j.bbadis.2011.02.009. doi: 10.1016/j.bbadis.2011.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rodewald HR, Feyerabend TB. Widespread immunological functions of mast cells: Fact or fiction? Immunity. 2012;37:13–24. doi: 10.1016/j.immuni.2012.07.007. doi: 10.1016/j.immuni.2012.07.007. [DOI] [PubMed] [Google Scholar]
- 32.Sayed BA, Christy AL, Walker ME, Brown MA. Meningeal mast cells affect early T cell central nervous system infiltration and blood-brain barrier integrity through TNF: A role for neutrophil recruitment? J Immunol. 2010;184:6891–900. doi: 10.4049/jimmunol.1000126. doi: 10.4049/jimmunol.1000126. [DOI] [PubMed] [Google Scholar]
- 33.Sehra S, Yao W, Nguyen ET, Glosson-Byers NL, Akhtar N, Zhou B, et al. TH9 cells are required for tissue mast cell accumulation during allergic inflammation. J Allergy Clin Immunol. 2015;136:433–40.e1. doi: 10.1016/j.jaci.2015.01.021. doi: 10.1016/j.jaci.2015.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nowak EC, Weaver CT, Turner H, Begum-Haque S, Becher B, Schreiner B, et al. IL-9 as a mediator of Th17-driven inflammatory disease. J Exp Med. 2009;206:1653–60. doi: 10.1084/jem.20090246. doi: 10.1084/jem.20090246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Elyaman W, Bradshaw EM, Uyttenhove C, Dardalhon V, Awasthi A, Imitola J, et al. IL-9 induces differentiation of TH17 cells and enhances function of FoxP3+ natural regulatory T cells. Proc Natl Acad Sci U S A. 2009;106:12885–90. doi: 10.1073/pnas.0812530106. doi: 10.1073/pnas.0812530106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.de Vries VC, Wasiuk A, Bennett KA, Benson MJ, Elgueta R, Waldschmidt TJ, et al. Mast cell degranulation breaks peripheral tolerance. Am J Transplant. 2009;9:2270–80. doi: 10.1111/j.1600-6143.2009.02755.x. doi: 10.1111/j.1600-6143.2009.02755.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lu LF, Lind EF, Gondek DC, Bennett KA, Gleeson MW, Pino-Lagos K, et al. Mast cells are essential intermediaries in regulatory T-cell tolerance. Nature. 2006;442:997–1002. doi: 10.1038/nature05010. doi: 10.1038/nature05010. [DOI] [PubMed] [Google Scholar]
- 38.Ding X, Cao F, Cui L, Ciric B, Zhang GX, Rostami A. IL-9 signaling affects central nervous system resident cells during inflammatory stimuli. Exp Mol Pathol. 2015;99:570–4. doi: 10.1016/j.yexmp.2015.07.010. doi: 10.1016/j.yexmp.2015.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhuang JC, Wu L, Qian MZ, Cai PP, Liu QB, Zhao GX, et al. Variants of interleukin-7/interleukin-7 receptor alpha are associated with both neuromyelitis optica and multiple sclerosis among Chinese Han population in Southeastern China. Chin Med J. 2015;128:3062–8. doi: 10.4103/0366-6999.169093. doi: 10.4103/0366-6999.169093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fontaine RH, Cases O, Lelièvre V, Mesplès B, Renauld JC, Loron G, et al. IL-9/IL-9 receptor signaling selectively protects cortical neurons against developmental apoptosis. Cell Death Differ. 2008;15:1542–52. doi: 10.1038/cdd.2008.79. doi: 10.1038/cdd.2008.79. [DOI] [PubMed] [Google Scholar]