The results of this work could alter the present paradigm of basing clinical pediatric heart failure (HF) treatment on outcomes of adult HF clinical trials. The use of serum-treated primary cardiomyocytes may define age-specific mechanisms in pediatric HF with the potential to identify unique age-appropriate and disease-specific therapy.
Keywords: pediatric dilated cardiomyopathy, cellular hypertrophy, fetal gene program, exosomes
Abstract
Stimulation of the renin-angiotensin-aldosterone system (RAAS) and β-adrenergic receptors plays an important role in adult heart failure (HF). Despite the demonstrated benefits of RAAS inhibition and β-adrenergic receptor blockade in adult HF patients, no substantial improvement in survival rate has been observed in children with HF. This suggests that the underlying disease mechanism is uniquely regulated in pediatric HF. Here, we show that treatment of human-induced pluripotent stem cell-derived cardiomyocytes (iPSC-CMs) and neonatal rat ventricular myocytes (NRVMs) with serum from pediatric dilated cardiomyopathy (DCM) patients induces pathological changes in gene expression, which occur independently of the RAAS and adrenergic systems, suggesting that serum circulating factors play an important role in cardiac remodeling. Furthermore, exosomes purified from DCM serum induced pathological changes in gene expression in NRVMs and iPSC-CMs. Our results suggest that DCM serum exosomes mediate pathological responses in cardiomyocytes and may propagate the pediatric HF disease process, representing a potential novel therapeutic target specific to this population.
NEW & NOTEWORTHY The results of this work could alter the present paradigm of basing clinical pediatric heart failure (HF) treatment on outcomes of adult HF clinical trials. The use of serum-treated primary cardiomyocytes may define age-specific mechanisms in pediatric HF with the potential to identify unique age-appropriate and disease-specific therapy.
Listen to this article's corresponding podcast at https://ajpheart.podbean.com/e/exosomes-in-pediatric-dilated-cardiomyopathy/.
heart failure (HF) is a major public health issue that is increasing in prevalence and is associated with high mortality and frequent hospitalization, and imposes a major societal economic burden. Despite the demonstrated benefits of present medical therapy in adult HF patients, no substantial improvement in survival rate has been observed in children with HF (9), suggesting that underlying cellular mechanisms are uniquely regulated in this patient population. Although the majority of HF in adults is caused by ischemic heart disease, pediatric HF is a heterogeneous disease process with dilated cardiomyopathy (DCM) as the most common indication for heart transplantation in children over the age of 1 (29). In children with DCM, left ventricular systolic pump function of the heart is impaired, leading to progressive cardiac enlargement and hypertrophy, a process called cardiac remodeling. Cardiomyocyte hypertrophy has been viewed as a compensatory mechanism to normalize stress and enhance cardiac performance. However, the late-phase “remodeling” process leads to ventricular dilation and progressive decline in cardiac output (7).
On the basis of adult and animal model studies, it is widely accepted that the renin-angiotensin-aldosterone system (RAAS) and β-adrenergic receptor systems are major components of pathological remodeling (3, 5, 13). Pathological remodeling is characterized by changes in gene expression that include upregulation of atrial natriuretic factor (ANF) and B-type natriuretic peptide (BNP) (28). We have previously shown that pathological changes in gene expression are recapitulated in the pediatric failing heart (16). Here we show that treatment of neonatal rat ventricular myocytes (NRVMs) treated with serum from children with DCM induce expression of ANF and BNP, independently of the RAAS or β-adrenergic receptor systems.
Recently, studies have shown that exosomes are involved in mediating paracrine effects in cells. Exosomes are small, 30 to 100-nm membrane vesicles, released by live cells (donor cells) into biofluids (plasma/serum, urine, cerebrospinal fluid, and saliva), which contain cargoes that include coding and noncoding RNAs, proteins, and signaling molecules (10, 21). These cargoes can be transported to recipient cells, inducing an intracellular response. We show that treatment of primary cardiomyocytes with pediatric DCM serum-derived exosomes results in upregulation of ANF and BNP, which can be prevented by pretreatment with the exosome uptake inhibitor cytochalasin D (cytD) (2). Our results suggest that serum circulating factors play an important role in modulating cardiac remodeling and may represent potential novel therapeutic targets specific to this population.
MATERIALS AND METHODS
Human samples.
Pediatric dilated cardiomyopathy (DCM) subjects were boys and girls of all ages under 18 who donated their serum at the time of cardiac transplantation. Samples of pediatric nonfailing (NF) serum were obtained from donor children with normal heart function. A detailed description of all pediatric subjects is listed in Table 1.
Table 1.
Pediatric subject characteristics and analyses
| Patient Characteristics |
Medications |
Device | ||||||
|---|---|---|---|---|---|---|---|---|
| ID | Sex | Age at Blood Collection, yr | EF/FS | Digoxin | ACE Inhibitor/AT1R Blocker | β-Blocker | Inotropes | VAD |
| Nonfailing (NF) | ||||||||
| 1 | M | 9.7 | NA | N | N | N | N | N |
| 2a | F | 11.8 | NA | N | N | N | N | N |
| 3 | M | 4.3 | NA | N | N | N | N | N |
| Dilated cardiomyopathy (DCM) | ||||||||
| 1 | M | 9.0 | EF 20% | N | N | N | N | YB |
| 2 | M | 11.6 | NA | N | Y | N | Y | N |
| 3 | M | 5.1 | FS 4% | N | N | Y | N | YB |
| 4 | M | 5.2 | EF 21% | N | Y | Y | Y | N |
| 5 | M | 9.8 | EF 7% | N | Y | N | Y | N |
| 6 | M | 2.9 | NA | N | Y | N | Y | N |
| 7a | M | 17.2 | NA | N | Y | N | N | YH |
| 8a | F | 0.6 | NA | N | N | N | N | YB |
| 9 | F | 5.3 | EF 20% | N | N | Y | N | YB |
| 10 | F | 0.4 | EF 20% | N | Y | Y | Y | N |
| 11 | F | 0.7 | EF 24% | Y | Y | N | Y | N |
Inotropes include the following: phosphodiesterase 3 (PDE3), dopamine (Dop), dobutamine (Dob), and epinephrine (Epi). Ventricular assist device (VAD) includes Berlin (B) and Heartware (H). M, male; F, female; Y, yes; N, no; EF, ejection fraction; FS, fractional shortening; NA, not available.
Exosome precipitation and quantification.
Exosomes were isolated from the serum of 11 pediatric DCM patients and 3 NF control children by using either miRCURY Isolation Kit (Exiqon) or differential centrifugation. According to manufacturer’s protocol, serum was centrifuged at 10,000 g for 5 min to remove the cell debris, and the indicated amount of Precipitation Buffer was added into the supernatant. The buffer/serum mixture was incubated at 4°C for 2 h, followed by centrifugation at 1,500 g for 30 min. The exosome pellet was resuspended in 100 μl PBS for further analysis.
Exosomes were also isolated from serum by differential centrifugation: serum was centrifuged at 4°C at 1,200 g for 15 min, at 10,000 g for 20 min, and at 20,000 g for 20 min to remove cell debris and protein contamination, followed by filtration with a 0.22-μm filter (Millipore) to remove microvesicles. The supernatant was then centrifuged at 100,000 g for 70 min at 4°C. The exosome pellet was resuspended in 100 μl PBS for further analysis.
Exosome quantitation was measured by using the EXOCET exosome quantitation kit (System Biosciences, Mountain View, CA) according to manufacturer’s instructions.
Nanoparticle track analysis.
Nanoparticle track analysis (NTA) was performed on NanoSight NS500 instrument (Marvern, MA). Exosomes was diluted 100-fold in 1 ml PBS. Samples were then infused into the NanoSight NS500 instrument at rate of 25 (arbitrary units) by using a syringe pump. Data was collected for 30 s, and five movies were recorded for each sample and then analyzed with NanoSight NTA 3.1 software.
Cell culture and serum/exosome treatment.
NRVMs were isolated from ventricle of 1- to 3-day-old Sprague Dawley rats (Charles River) by enzymatic digestions as described previously (28). NRVMs were treated with indicated concentration of serum/exosomes and incubated at 37°C for 72 h. The amount of the exosome fraction used to treat the cells was based on equal starting volumes of NF and DCM serum and not on number of exosomes. NRVMs were preincubated with 10 µM losartan (angiotensin receptor blocker), 100 nM prazosin (α1-adrenergic receptor blocker), or 1 µM propranolol (β-adrenergic receptor blocker) for 30 min before serum treatment. Efficiency of receptor blockers was tested by treatment of cells with receptor-specific agonists [10−6 M isoproterenol (iso) for β-adrenergic receptor; 10−5 M phenylephrine (PE) for α-adrenergic receptor, and 10−6 M angiotension II (ANG II) receptor] for 72 h in the presence of each receptor blocker. NRVMs were pretreated with 0.25 µM cytochalasin D (CytD) for 30 min before exosome treatment. Protocol for animal work is in accordance with PHS Animal Welfare Assurance, ID A3269-01, and approved by the Institutional Animal Care and Use Committee.
iPSC-CMs were a generous gift from Dr. Joseph Wu (Stanford Cardiovascular Institute Biobank). Cells were cultured at 37°C with 5% CO2 as previously described (31). Cells were treated with serum at day 2 postbeating initiation.
RNA isolation and RT-PCR analysis.
NRVMs were homogenized in TRIzol (ThermoFisher Scientific), and RNA was extracted as per manufacturer’s conditions. cDNA was synthesized by using iScript cDNA synthesis kit (Bio-Rad, Hercules, CA) according to manufacturer’s instructions. Gene expression was measured by RT-PCR as described before (16, 27), and expression levels of all transcripts were normalized to 18S rRNA.
Cell size measurement.
Cell size was measured as described (25). In short, NRVMs were treated with serum from pediatric DCM patients and NF controls and incubated at 37°C for 72 h. NRVMs were fixed with 3.2% paraformaldehyde in PBS. Cells were permeabilized with NP-40 (0.1%), blocked with 1% BSA fraction V (Fisher), and stained with antibodies against α-actinin (Sigma). Coverslips were incubated with fluorescein-conjugated anti-mouse secondary antibodies (Jackson ImmunoResearch Laboratory) and mounted on slides using SlowFade Gold antifade reagent containing DAPI (Invitrogen). Two fluorescent channels were collected for quantification of cell size: fluorescein (α-actinin) and DAPI (nuclei). Objects were initially defined using the nuclear channel, and cytoplasm was then segmented based on the fluorescein channel. Mean fluorescein intensity was calculated for each cell. NRVMs were selected using threshold values for mean fluorescein fluorescence to filter out the residual fibroblasts. Finally, cell area was calculated for each valid cell based on the fluorescein channel.
Confocal microscopy.
Exosomes were labeled with BODIPY TR ceramide (FITC-type probe) according to manufacturer’s recommendations (Invitrogen, Carlsbad, CA), followed by removal of unbound dye from labeled exosomes with Exosome Spin Columns MW3000 (Invitrogen). Controls were BODIPY-labeled PBS.
Fluorescent-labeled exosome uptake was performed on live NRVMs, which were grown on 35-mm glass bottom plates (MatTek Corporation, Ashland, MA) and incubated in a temperature-controlled CO2 chamber. The images were taken at the indicated times by using Olympus FLUOVIEW FV1000 confocal laser scanning microscope with the excitation filter at 583 nm and emission filter at 614 nm. Surface-bound exosomes were removed by extensive washing of the cells.
Western blot analysis.
Western blot analysis was used to analyze exosome surface marker αCD63 as directed by manufacturer’s instruction with minor modifications. Briefly, exosome pellet was treated with 200 µl RIPA buffer and vortexed for 15 s. Twenty micrograms of exosomal protein was loaded. The membrane was incubated with primary rabbit anti-CD63 (System Bio) at 4°C overnight, followed by incubation with the secondary antibody, HRP-labeled goat anti-rabbit IgG at room temperature for 1 h. Detection was carried by out by enhanced chemiluminescence.
Statistical analysis.
Quantitative results are shown as the means ± SE. Statistical analysis was performed with Statview software (SAS Institute, Cary, NC) between NF and DCM groups. P values < 0.05 were considered statistically significant. For each assay, at least three batches of NRVMs were used.
Human studies approval.
All human studies were approved by the Institutional Review Boards of the University of Colorado Anschutz Medical Campus (12-1197).
RESULTS
DCM serum treatment induces pathological changes in gene expression in NRVMs.
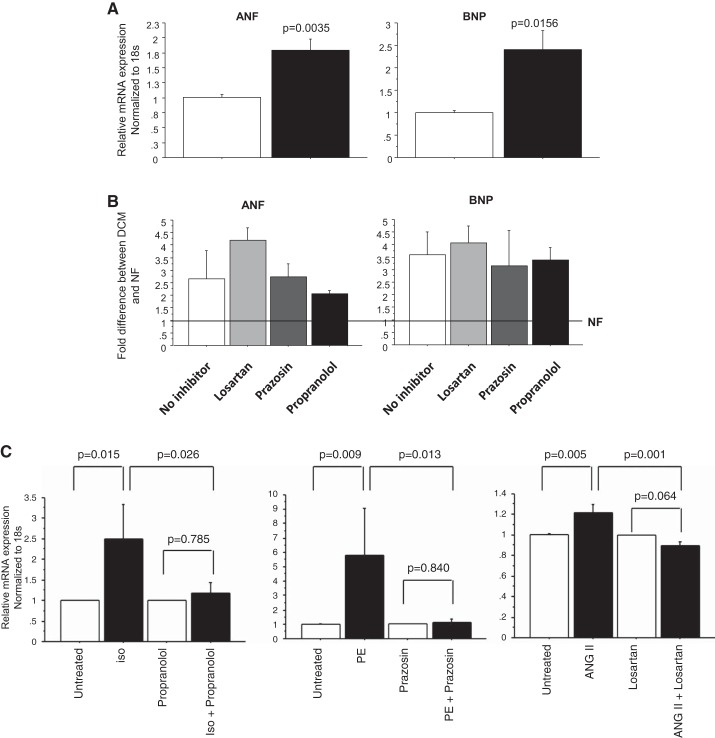
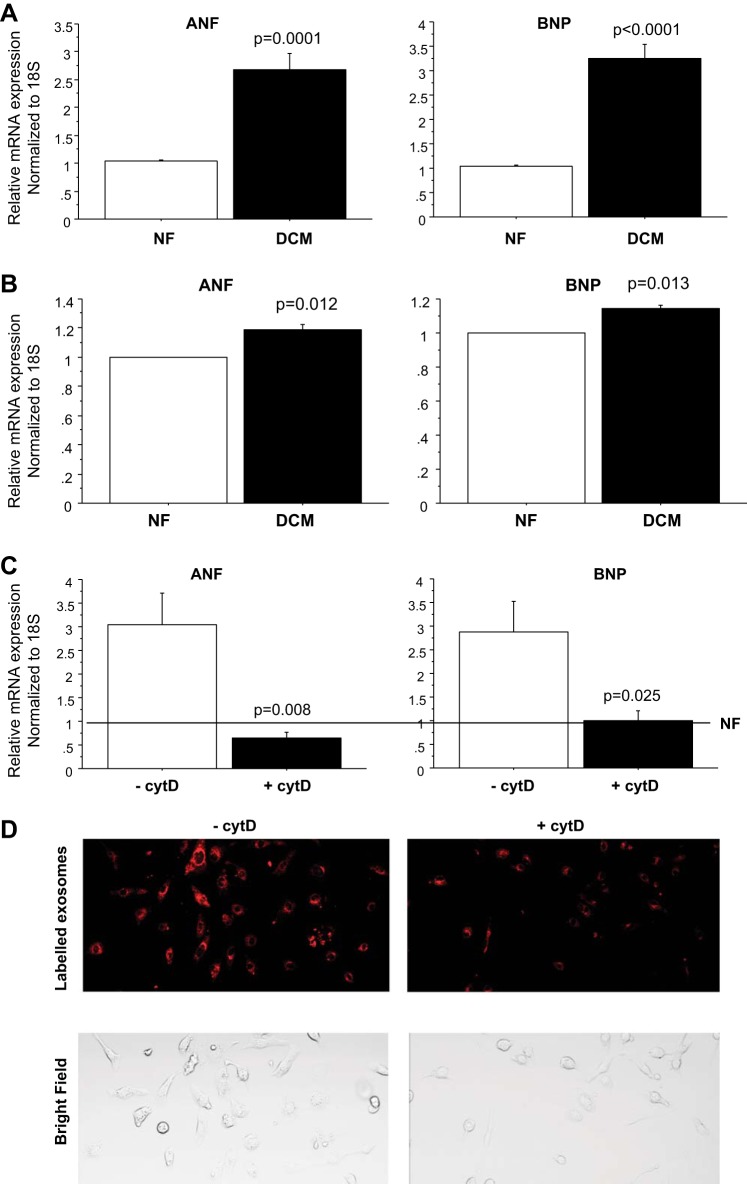
To determine the effect of pediatric DCM serum in inducing pathological remodeling, NRVMs were treated with serum from DCM patients and NF controls. A detailed description of all pediatric subjects is listed in Table 1. Serum treatment resulted in the upregulation of ANF and BNP (Fig. 1A) [DCM: ANF: 1.80 ± 0.19, P = 0.0035; BNP: 2.40 ± 0.42, P = 0.0156 (normalized to NF)].
Fig. 1.
Pediatric dilated cardiomyopathy (DCM) serum causes pathological remodeling in neonatal rat ventricular myocytes (NRVMs). A: pathological gene expression analysis in NRVMs. Serum from three nonfailing (NF) and seven DCM patients was used to treat NRVMs for 72 h, followed by RNA extraction and RT-PCR. Expression levels of all transcripts were normalized to 18S rRNA, and the values in each plot are means of seven independent experiments. B: fold difference (DCM vs. NF) of gene expression in NRVMs treated with serum in the presence of cell surface receptor blockers. NRVMs were pretreated with cell surface receptor blockers for 30 min before serum treatment. The values in each plot are means of four independent experiments. No significant differences in gene expression were detected between treatments with DCM serum in the presence/absence of the various inhibitors. Line at 1 represents mRNA expression level in NF controls in the presence/absence of the inhibitors. C: surface receptor-specific blockers effectively prevent the activation of atrial natriuretic factor (ANF) caused by receptor-specific agonists. NRVMs were preincubated with 10 µM losartan, 100 nM prazosin, or 1 µM propranolol for 30 min before agonist treatment [10−6M isoproterenol (iso: β-adrenergic receptor agonist); 10−5 M phenylephrine (phenylephrine: α-adrenergic receptor agonist); 10−6M angiotensin II (ANG II: angiotensin receptor agonist)]. Cells were treated for 72 h in the presence of each receptor blocker. The values in each plot are means of at least three independent experiments. BNP, B-type natriuretic peptide.
Numerous clinical studies have shown that myocardial remodeling is affected by stimulation of RAAS and myocardial β-adrenergic receptors (12). To determine if RAAS or adrenergic receptors were involved in the serum-mediated response, NRVMs were pretreated with cell surface receptor inhibitors, including an angiotensin II receptor blocker (losartan), α1-adrenoceptor blocker (prazosin), and β-adrenoceptor blocker (propranolol) for 30 min before serum treatment. The results showed that changes in gene expression were not affected by inhibitor treatment (Fig. 1B). Efficiency of receptor blockade was tested by treatment of cells with receptor-specific agonists. Propranolol, prazosin, and losartan efficiently prevented upregulation of ANF and BNP by isoproterenol, phenylephrine, or angiotensin II respectively (Fig. 1C).
Treatment with DCM serum promotes cardiomyocyte hypertrophy.
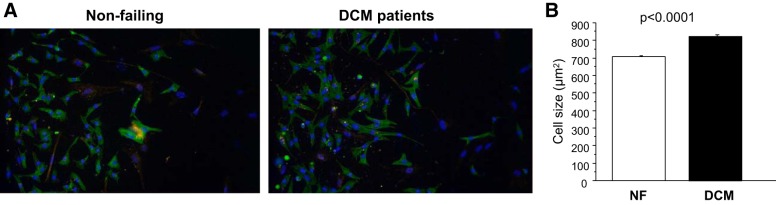
In addition to the induction of genes normally expressed during development, cellular hypertrophy is another hallmark of pathological remodeling. To determine if treatment with serum from DCM patients results in increased cell size in vitro, cell area of NRVMs was measured by using α-actinin staining after 72 h of serum treatment (Fig. 2). As shown in Fig. 2B, there was an increase in cell area of DCM serum-treated NRVMs compared with that of NF serum-treated cells (NF: 705 ± 6.5 μm2; DCM: 825 ± 8.7 μm2, P < 0.001).
Fig. 2.
DCM serum treatment increases NRVM area. A: NRVMs were treated with serum from DCM patients and NF controls for 72 h at 37°C. B: cell area was measured for individual cells based on α-actinin staining. The total area was then divided by the number of myocytes in each well, yielding an average cell area for each well, expressed in square micrometers. Twenty-five wells were measured for NF and F, individually.
Characterization of exosomes isolated from serum of NF controls and DCM patients.
To investigate if the serum effect was mediated by exosomes, exosomes were isolated from serum samples by using either a commercial kit or differential centrifugation (see materials and methods). The exosome surface marker CD63 was detected in the exosome pellet isolated from both methods (Fig. 3).
Fig. 3.
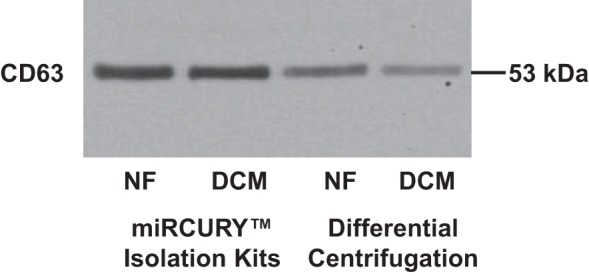
Serum exosome contains CD63. Exosomes were precipitated from serum of NF controls and pediatric DCM patients by using either water capture–based commercial kit or differential centrifugation. Presence of CD63 was determined by Western blot analysis.
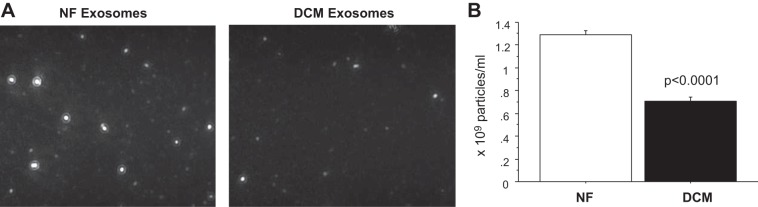
The number of exosomes was measured by NanoSight, which uses NTA to characterize the size and number of nanoparticles in solution (Fig. 4A and Supplemental Material video).1 As shown in Fig. 4B, the number of exosomes isolated from NF serum was 1.29 × 109 ± 3.51 × 107 exosomes/ml, and exosomes isolated from pediatric DCM serum was 7.07 × 108 ± 3.43 × 107 exosomes/ml. EXOCET exosome quantitation kit was also used to estimate exosome numbers isolated from serum, and the results also show that the number of exosomes isolated from serum of DCM patients is lower than that of NF control. The number of exosomes isolated from NF serum was 1.7 × 1,010 exosomes/ml, whereas ~5.3 × 109 exosomes/ml were isolated from pediatric DCM serum. This suggests that exosome content, not simply an increase in exosome number, contributes to the pathological response and myocyte hypertrophy demonstrated by exosome treatment of NRVMs.
Fig. 4.
DCM serum contains fewer exosomes than serum from NF controls. A: screen shot from 1:100 diluted exosome samples isolated from serum of NF controls and DCM patients by using miRCURY Isolation Kit (Exiqon). B: numbers of exosomes isolated from serum of NF controls and DCM patients based on nanoparticle track analysis (NTA). The values in each plot are means of five separate NTAs.
Exosome uptake by NRVMs was time and concentration dependent.
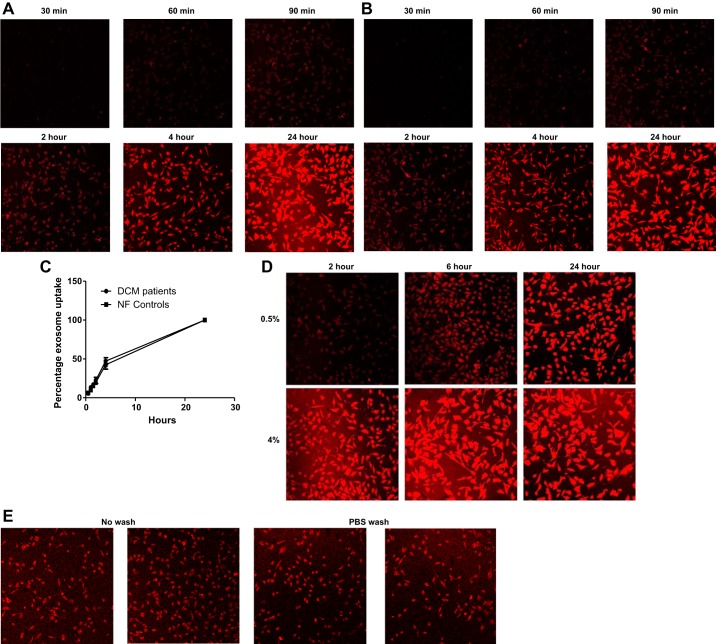
The time- and concentration-dependent manner of exosome uptake into NRVMs was investigated. Initially, exosome uptake over time was analyzed by using 2% NF and DCM exosome fractions. As shown in Fig. 5, A and B, the accumulation of labeled exosomes from serum of NF control and pediatric DCM patients was detected as early as 1 h after exosome treatment, and uptake was observed over time up to 24 h. Exosome uptake (2%) was linear in the initial 4 h of incubation, and fluorescence intensity was close to saturation at 24 h. The accumulation of exosomes from pediatric NF controls showed a similar uptake pattern compared with exosomes from pediatric DCM patients (Fig. 5C). In addition, exosome uptake was concentration dependent, and maximum uptake was observed after a 6-h incubation with 4% exosomes, whereas 24 h was needed for complete uptake with 0.5% exosome treatment (Fig. 5D).
Fig. 5.
Exosome uptake is time and dose dependent. A: uptake (2%) of fluorescent-labeled exosomes from NF controls in NRVMs was monitored at indicated time points. The values in plot are means of two independent experiments. B: uptake (2%) of fluorescent-labeled exosomes from pediatric DCM serum in NRVMs was monitored at indicated time points. C: the uptake efficiency in 24 h was comparable in NRVMs treated with exosomes from NF or pediatric DCM serum. D: different concentrations of fluorescent-labeled exosomes from NF controls were used to treat NRVMs for 2, 6, and 24 h. E: exosomes are taken up into NRVMs. To determine if exosomes are taken up by NRVMs and are not loosely attached to cell surface, cells were washed with PBS, and the fluorescence intensity was compared with the unwashed cells after 2-h exosome treatment at 37̊C.
To ensure the observed signal reflected intracellular localization of exosomes, and not exosomes bound to the cell membrane, cells were washed three times after 2 h of incubation with exosomes, and fluorescence intensity was comparable to the unwashed cells. No decrease in signal intensity suggests intracellular localization of exosomes (Fig. 5E).
Exosome and serum treatment induces similar changes in pathological gene expression.
To determine if exosomes from DCM patients induced pathological changes in gene expression, mRNA levels of ANF and BNP were investigated in primary cardiomyocytes treated with exosome fractions. As shown in Fig. 6A, treatment of NRVMs with 2% exosome fractions from DCM patients resulted in increased gene expression (DCM: ANF: 2.69 ± 0.21 P = 0.0001; BNP: 3.26 ± 0.29, P < 0.0001) similar to serum treatment in NRVMs (Fig. 1A). In addition, DCM exosome treatment of human iPSC-CMs resulted in upregulation of ANF and BNP compared with NF serum treatment [DCM: ANF: 1.15 ± 0.04, P = 0.012; BNP: 1.21 ± 0.06, P = 0.013 (normalized to NF)] (Fig. 6B).
Fig. 6.
Exosomes induce pathological changes in gene expression in NRVMs and human human-induced pluripotent stem cell-derived cardiomyocytes (iPSC-CMs). A: pathological gene expression analysis in NRVMs. Exosomes from 3 NF and 11 DCM patients were used to treat NRVMs for 72 h, followed by RNA extraction and RT-PCR. Expression levels of all transcripts were normalized to 18S rRNA, and the values in each plot were means of eight independent experiments. B: pathological gene expression analysis in human iPSC-CMs. iPSC-CMs were treated with exosomes from two NF and three individual DCM patients. The values in each plot were means of four independent assays. C: fold difference (DCM vs. NF) of gene expression in NRVMs in the presence of exosome uptake inhibitor cytD. NRVMs were pretreated with 0.25 µM cytD for 30 min before exosome treatment. The values in each plot were means of three independent experiments. Line at 1 represents the result of treatment with NF control exosomes in the presence/absence of cytD. D: intensity of labeled exomes is decreased in cytD-treated cells. NRVMs were pretreated with 0.25 µM cytD for 30 min before exosome treatment. Uptake of fluorescent-labeled exosomes isolated from NF controls in NRVMs was monitored after 5-h incubation.
We also measured if varying amounts of exosome have different effects on changes in gene expression. Exosomes of 0.1 and 2% were used to treat NRVMs, and the results show no statistical significance in the gene expression in cells treated with different concentrations of exosome (data not shown).
To confirm that pathological gene expression is induced by exosomes, NRVMS were pretreated with cytochalasin D (cytD), an inhibitor of exosome uptake (2), for 30 min before exosome treatment. The results showed that cytD treatment prevents pathological changes in gene expression (Fig. 6C). CytD effect on exosome uptake is shown in Fig. 6D.
DISCUSSION
DCM is the most common cause of HF in children. Although treatment for adults with HF is well established, no improvement in mortality has been observed in children in the last three decades (9). Importantly, our published studies show a distinct molecular profile in children with HF that could contribute to age-related differences in response to therapy, including differences in response to phosphodiesterase treatment, β–adrenergic receptor system adaptation, the fibrotic gene profile, and miRNA expression (4, 15, 16, 18, 26, 32). Together, these findings suggest that the underlying cellular mechanisms are uniquely regulated in children compared with adults with HF. However, these age-related differences are not currently considered in the treatment of pediatric HF, potentially contributing to poor outcomes and hindering the development of new drug therapies that could prevent or reverse the progression of the disease.
Here, we report that circulating factors are important in modulating pediatric cardiac remodeling. Our results show that treatment of primary cardiomyocytes with serum from DCM patients induces changes in gene expression with similar directionality as in diseased hearts (Fig. 1A) (16). Importantly, serum-mediated changes are independent of the RAAS or adrenergic system (Fig. 1B). Furthermore, DCM serum treatment generates pathological hypertrophy in cells (Fig. 2). Our results suggest that factors other than circulating catecholamines or ANG II can promote cellular pathological remodeling. Paracrine effects on cardiac remodeling can also be mediated by growth factors (33), suggesting that growth factors may be present in the pediatric DCM circulation and be involved in the disease process.
In addition, our results demonstrate that exosomes are involved in the serum-mediated effect in vitro. We show that exosomes precipitated from serum of pediatric DCM patients promote changes in gene expression similar to serum-mediated pathological remodeling (Fig. 6, A and B). Exosomes are 30 to 100-nm membrane vesicles, secreted by a variety of cell types, which have been reported to play an important role in cell-to-cell communications (1, 19), altering gene expression in recipient cells. They are enriched in several molecules, including miRNA, mRNA, membrane trafficking molecules, and signal transduction proteins from the donor cells (10, 21).
A pure exosomal preparation is not trivial, and different exosome isolation methods exist, i.e., isolation kit that is based on capturing of water molecules that form the hydrate envelope of exosomes, and differential centrifugation. As shown in Fig. 3, exosomes were isolated from serum by two different methods: differential centrifugation or miRCURY Isolation Kits (Exiqon) (20, 23). Western blotting was used to detect the presence of exosomes by detecting the exosomes surface marker CD63, and although more exosomes were recovered by using the water capture–based method, exosomes isolated by using both methodologies resulted in similar changes in gene expression (data not shown).
To determine the number of exosomes isolated from pediatric DCM serum and NF controls, we used NTA to characterize exosomes in solution. Our results show that the number of exosomes was lower in the pediatric DCM patients than in NF controls. These results are highly consistent with EXOCET exosome quantitation, which measures the activity of acetyl-CoA acetylcholinesterase that is known to be enriched in exosomes (24).
A recent study showed that exosome uptake in bladder cancer cells is dose and time dependent (6). In our study, we also demonstrated that exosome uptake by NRVMs occurs in a dose- and time-dependent manner (Fig. 5). Moreover, NF and DCM exosome uptake was similar in NRVMs (Fig. 5C), and uptake of 4% exosome fraction by NRVMs was more efficient than for 0.5% exosome fraction within 24 h (Fig. 5D). However, there were no significant differences in gene expression in NRVMs treated with 0.1 or 2% exosome fractions for 72 h (data not shown). This suggests that although higher exosome amounts seem to result in faster uptake, even low exosome amounts are sufficient to induce changes in gene expression.
To confirm that pathological changes in gene expression were mediated by exosomes, NRVMs were treated with cytD, an exosome uptake inhibitor (2), before serum treatment. Our results show that pathological gene expression can be inhibited by cytD (Fig. 6D). A previous study showed that cytD treatment of recipient cells did not affect the interaction of exosomes with the cell surface but prevented uptake (8). These experiments were performed using GFP-containing exosomes, and in response to cytD treatment, GFP was still bound to the cilia but was not internalized. Therefore, cytD treatment resulted in exosomes attached to the cell surface but not internalized. Therefore, labeled exosomes are still bound to the cell surface. As shown in Supplemental Fig. S3, upon cytD treatment the labeled exosomes were still detected although intensity was decreased. However, cytD also acts as an actin polymerization inhibitor, and the reduced exosome-mediated pathological effect can be caused indirectly via perturbation of other cellular processes, such as cellular polarization, migration, or cell cycle (17).
Recent studies have shown that exosomes are involved in a variety of diseases (21). Cancer studies have shown that exosomes are important in promoting the metastatic process (14), underscoring their role in cancer pathology. The effect of exosomes in cardiovascular diseases is still controversial. Exosomes isolated either from conditioned media of embryonic stem cells in culture (11) or from plasma of wild-type rats and healthy volunteers (30) can have a cardioprotective effect in the setting of ischemic injury and myocardial infarction. In contrast, Bang et al. (2) reported that exosomes can be detrimental by promoting the export of fibroblast-derived miR-21 from cardiac fibroblasts to cardiomyocytes, affecting the expression of target genes within cardiomyocytes and leading to cellular hypertrophy. Furthermore, a recent study showed that exosomes containing the ANG II type I receptor (AT1R) are released from cardiomyocytes of mice undergoing cardiac pressure overload and modulate blood pressure responses in AT1R knockout mice (20), highlighting the role of exosomes in promoting cellular communication. These results suggest that different exosomal contents are likely to modulate molecular responses in recipient cells. In our studies, we showed that exosomes isolated from pediatric HF serum can promote pathological cardiac remodeling, underscoring the importance of serum circulating factors in affecting myocellular regulation.
Limitations.
There are limitations to this study. First, the present study is based on in vitro experiments, and the effect of exosomes in vivo has not been tested. In addition, exosome content has not been characterized, and it is unclear if the remodeling effect is mediated by RNAs or proteins. Finally, because of the limited number of patients, the conclusions of this study cannot be extended to all pediatric DCM patients and different HF etiologies.
Conclusions.
Our results strongly suggest that exosomes derived from serum of pediatric DCM patients are involved in the remodeling process observed in primary cardiomyocytes. Identification of serum circulating factors will increase our understanding of molecular mechanisms in pediatric DCM and has the potential to identify novel therapies specific to this population.
GRANTS
This study was supported by the Millisor Chair in Pediatric Heart Disease, the Boedecker Foundation, a gift from the Nair Family, American Heart Association Grant-In-Aid 13GRNT16950045, and National Heart, Lung, and Blood Institute (NHBLI) Grants HL-126928 and HL-107715. NHBLI Grant T32-HL-7171-37 (PI: Stenmark) covered X. Jiang's salary. This study was also supported by National Institutes of Health/NCATS Colorado CTSA Grant UL1-TR-001082 and an American Physiological Society/NHLBI STRIDE 2014 Summer Research Fellowship (to J. Sucharov). Contents are the authors’ sole responsibility and do not necessarily represent official National Institutes of Health views.
DISCLOSURES
B. Stauffer had research support from Forest Laboratories, Incorporated. C. Sucharov is a scientific founder and shareholder at miRagen, Incorporated. C. Sucharov, S. Miyamoto, and B. Stauffer are scientific founders and shareholders at CoramiR, Incorporated. No potential conflicts of interest have been identified.
AUTHOR CONTRIBUTIONS
X.J., B.L.S., S.D.M., and C.C.S. conception and design of research; X.J. and J.S. performed experiments; X.J. and C.C.S. analyzed data; X.J., B.L.S., S.D.M., and C.C.S. interpreted results of experiments; X.J. and C.C.S. prepared figures; X.J. drafted manuscript; X.J., J.S., B.L.S., S.D.M., and C.C.S. approved final version of manuscript; B.L.S., S.D.M., and C.C.S. edited and revised manuscript.
Supplementary Material
ACKNOWLEDGMENTS
The authors acknowledge Dr. Joseph Wu for providing human iPS-CM and Linda Cagle, Kathleen Spanjer, and Dr. Brian Freed for providing the serum samples in the study.
Footnotes
Supplemental Material for this article is available at the American Journal of Physiology-Heart and Circulatory Physiology website.
REFERENCES
- 1.Ailawadi S, Wang X, Gu H, Fan GC. Pathologic function and therapeutic potential of exosomes in cardiovascular disease. Biochim Biophys Acta 1852: 1–11, 2015. doi: 10.1016/j.bbadis.2014.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bang C, Batkai S, Dangwal S, Gupta SK, Foinquinos A, Holzmann A, Just A, Remke J, Zimmer K, Zeug A, Ponimaskin E, Schmiedl A, Yin X, Mayr M, Halder R, Fischer A, Engelhardt S, Wei Y, Schober A, Fiedler J, Thum T. Cardiac fibroblast-derived microRNA passenger strand-enriched exosomes mediate cardiomyocyte hypertrophy. J Clin Invest 124: 2136–2146, 2014. doi: 10.1172/JCI70577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bristow MR, Minobe W, Rasmussen R, Larrabee P, Skerl L, Klein JW, Anderson FL, Murray J, Mestroni L, Karwande SV, Fowler M, Ginburg M. Beta-adrenergic neuroeffector abnormalities in the failing human heart are produced by local rather than systemic mechanisms. J Clin Invest 89: 803–815, 1992. doi: 10.1172/JCI115659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chatfield KC, Sparagna GC, Sucharov CC, Miyamoto SD, Grudis JE, Sobus RD, Hijmans J, Stauffer BL. Dysregulation of cardiolipin biosynthesis in pediatric heart failure. J Mol Cell Cardiol 74: 251–259, 2014. doi: 10.1016/j.yjmcc.2014.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dzau VJ. Clinical implications for therapy: possible cardioprotective effects of ACE inhibition. Br J Clin Pharmacol 28, Suppl 2: 183S–187S, 1989. doi: 10.1111/j.1365-2125.1989.tb03594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Franzen CA, Simms PE, Van Huis AF, Foreman KE, Kuo PC, Gupta GN. Characterization of uptake and internalization of exosomes by bladder cancer cells. BioMed Res Int 2014: 619829, 2014. doi: 10.1155/2014/619829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Frey N, Katus HA, Olson EN, Hill JA. Hypertrophy of the heart: a new therapeutic target? Circulation 109: 1580–1589, 2004. doi: 10.1161/01.CIR.0000120390.68287.BB. [DOI] [PubMed] [Google Scholar]
- 8.Heusermann W, Hean J, Trojer D, Steib E, von Bueren S, Graff-Meyer A, Genoud C, Martin K, Pizzato N, Voshol J, Morrissey DV, Andaloussi SE, Wood MJ, Meisner-Kober NC. Exosomes surf on filopodia to enter cells at endocytic hot spots, traffic within endosomes, and are targeted to the ER. J Cell Biol 213: 173–184, 2016. doi: 10.1083/jcb.201506084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kantor PF, Abraham JR, Dipchand AI, Benson LN, Redington AN. The impact of changing medical therapy on transplantation-free survival in pediatric dilated cardiomyopathy. J Am Coll Cardiol 55: 1377–1384, 2010. doi: 10.1016/j.jacc.2009.11.059. [DOI] [PubMed] [Google Scholar]
- 10.Khalyfa A, Gozal D. Exosomal miRNAs as potential biomarkers of cardiovascular risk in children. J Transl Med 12: 162, 2014. doi: 10.1186/1479-5876-12-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khan M, Nickoloff E, Abramova T, Johnson J, Verma SK, Krishnamurthy P, Mackie AR, Vaughan E, Garikipati VN, Benedict C, Ramirez V, Lambers E, Ito A, Gao E, Misener S, Luongo T, Elrod J, Qin G, Houser SR, Koch WJ, Kishore R. Embryonic stem cell-derived exosomes promote endogenous repair mechanisms and enhance cardiac function following myocardial infarction. Circ Res 117: 52–64, 2015. doi: 10.1161/CIRCRESAHA.117.305990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krum H, Abraham WT. Heart failure. Lancet 373: 941–955, 2009. doi: 10.1016/S0140-6736(09)60236-1. [DOI] [PubMed] [Google Scholar]
- 13.Lionetti V, Bianchi G, Recchia FA, Ventura C. Control of autocrine and paracrine myocardial signals: an emerging therapeutic strategy in heart failure. Heart Fail Rev 15: 531–542, 2010. doi: 10.1007/s10741-010-9165-7. [DOI] [PubMed] [Google Scholar]
- 14.Liu Y, Cao X. Organotropic metastasis: role of tumor exosomes. Cell Res 26: 149–150, 2016. doi: 10.1038/cr.2015.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Medina E, Sucharov CC, Nelson P, Miyamoto SD, Stauffer BL. Molecular changes in children with heart failure undergoing left ventricular assist device therapy. J Pediatr. In press. doi: 10.1016/j.jpeds.2016.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miyamoto SD, Stauffer BL, Nakano S, Sobus R, Nunley K, Nelson P, Sucharov CC. Beta-adrenergic adaptation in paediatric idiopathic dilated cardiomyopathy. Eur Heart J 35: 33–41, 2014. doi: 10.1093/eurheartj/ehs229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mulcahy LA, Pink RC, Carter DR. Routes and mechanisms of extracellular vesicle uptake. J Extracell Vesicles 3: 3, 2014. doi: 10.3402/jev.v3.24641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nakano SJ, Miyamoto SD, Movsesian M, Nelson P, Stauffer BL, Sucharov CC. Age-related differences in phosphodiesterase activity and effects of chronic phosphodiesterase inhibition in idiopathic dilated cardiomyopathy. Circ Heart Fail 8: 57–63, 2015. doi: 10.1161/CIRCHEARTFAILURE.114.001218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ono K, Kuwabara Y, Han J. MicroRNAs and cardiovascular diseases. FEBS J 278: 1619–1633, 2011. doi: 10.1111/j.1742-4658.2011.08090.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pironti G, Strachan RT, Abraham D, Mon-Wei Yu S, Chen M, Chen W, Hanada K, Mao L, Watson LJ, Rockman HA. Circulating exosomes induced by cardiac pressure overload contain functional angiotensin II type 1 receptors. Circulation 131: 2120–2130, 2015. doi: 10.1161/CIRCULATIONAHA.115.015687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol 200: 373–383, 2013. doi: 10.1083/jcb.201211138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rekker K, Saare M, Roost AM, Kubo AL, Zarovni N, Chiesi A, Salumets A, Peters M. Comparison of serum exosome isolation methods for microRNA profiling. Clin Biochem 47: 135–138, 2014. doi: 10.1016/j.clinbiochem.2013.10.020. [DOI] [PubMed] [Google Scholar]
- 24.Savina A, Vidal M, Colombo MI. The exosome pathway in K562 cells is regulated by Rab11. J Cell Sci 115: 2505–2515, 2002. [DOI] [PubMed] [Google Scholar]
- 25.Spiltoir JI, Stratton MS, Cavasin MA, Demos-Davies K, Reid BG, Qi J, Bradner JE, McKinsey TA. BET acetyl-lysine binding proteins control pathological cardiac hypertrophy. J Mol Cell Cardiol 63: 175–179, 2013. doi: 10.1016/j.yjmcc.2013.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stauffer BL, Russell G, Nunley K, Miyamoto SD, Sucharov CC. miRNA expression in pediatric failing human heart. J Mol Cell Cardiol 57: 43–46, 2013. doi: 10.1016/j.yjmcc.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sucharov CC, Dockstader K, McKinsey TA. YY1 protects cardiac myocytes from pathologic hypertrophy by interacting with HDAC5. Mol Biol Cell 19: 4141–4153, 2008. doi: 10.1091/mbc.E07-12-1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sucharov CC, Mariner PD, Nunley KR, Long C, Leinwand L, Bristow MR. A β1-adrenergic receptor CaM kinase II-dependent pathway mediates cardiac myocyte fetal gene induction. Am J Physiol Heart Circ Physiol 291: H1299–H1308, 2006. doi: 10.1152/ajpheart.00017.2006. [DOI] [PubMed] [Google Scholar]
- 29.Thrush PT, Hoffman TM. Pediatric heart transplantation-indications and outcomes in the current era. J Thorac Dis 6: 1080–1096, 2014 10.3978/j.issn.2072-1439.2014.06.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vicencio JM, Yellon DM, Sivaraman V, Das D, Boi-Doku C, Arjun S, Zheng Y, Riquelme JA, Kearney J, Sharma V, Multhoff G, Hall AR, Davidson SM. Plasma exosomes protect the myocardium from ischemia-reperfusion injury. J Am Coll Cardiol 65: 1525–1536, 2015. doi: 10.1016/j.jacc.2015.02.026. [DOI] [PubMed] [Google Scholar]
- 31.Wilson KD, Sun N, Huang M, Zhang WY, Lee AS, Li Z, Wang SX, Wu JC. Effects of ionizing radiation on self-renewal and pluripotency of human embryonic stem cells. Cancer Res 70: 5539–5548, 2010. doi: 10.1158/0008-5472.CAN-09-4238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Woulfe KC, Siomos AK, Nguyen H, SooHoo M, Galambos C, Stauffer BL, Sucharov C, Miyamoto S, SooHoo M, Galambo C, Stauffer BL, Sucharov C, Miyamoto S. Fibrosis and fibrotic gene expression in pediatric and adult patients with idiopathic dilated cardiomyopathy. J Card Fail. In press. doi: 10.1016/j.cardfail.2016.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yoshioka J, Prince RN, Huang H, Perkins SB, Cruz FU, MacGillivray C, Lauffenburger DA, Lee RT. Cardiomyocyte hypertrophy and degradation of connexin43 through spatially restricted autocrine/paracrine heparin-binding EGF. Proc Natl Acad Sci USA 102: 10622–10627, 2005. doi: 10.1073/pnas.0501198102. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.