Abstract
Background
Pancreatic ductal adenocarcinoma utilizes the CCL2/CCR2 chemokine axis to facilitate recruitment of tumor associated macrophages to sculpt an immunosuppressive tumor microenvironment. This pathway has prognostic implications in pancreas cancer, and blockade of CCR2 restores anti-tumor immunity in pre-clinical models. This provided the rationale for a clinical study in pancreatic adenocarcinoma to determine the safety and recommended phase 2 oral dosage of the CCR2 inhibitor PF-04136309 in combination with chemotherapy (FOLFIRINOX).
Methods
In this single-center, open label, phase Ib clinical trial patients age ≥ 18 years with treatment naïve borderline resectable or locally advanced, biopsy-proven pancreatic ductal adenocarcinoma, Eastern Cooperative Oncology Group performance status <2, measurable disease by Response Evaluation Criteria in Solid Tumors Version 1.1, and normal end organ function were eligible for enrollment. FOLFIRINOX (oxaliplatin, 85 mg/m2; irinotecan, 180 mg/m2; leucovorin, 400 mg/m2, and bolus fluorouracil 400 mg/m2 followed by 2,400 mg/m2 46 hour continuous infusion) was administered every 2 weeks for a total of six treatment cycles. To determine the recommended phase 2 dose, PF-04136309 was orally administered at a starting dose of 500 mg twice daily in a standard 3+3 dose de-escalation design with an expansion phase planned at the recommended phase 2 dose. Both FOLFIRINOX and PF-04136309 were simultaneously initiated with a total treatment duration of 3 months. The primary endpoints were to determine the recommended phase 2 dose and toxicity of PF-04136309 in combination with FOLFIRINOX. All patients in the dose de-escalation and expansion phase received the recommended phase 2 dose of PF-04136309 were combined for assessment of treatment toxicity by an intention to treat analysis. For tissue specimen comparison in corollary studies, a group of patients receiving FOLFIRINOX alone were enrolled and evaluated for treatment related toxicity. This study has been completed and is registered at ClinicalTrials.gov; number NCT01413022.
Results
From April 19th, 2012 through November 12th, 2014 a total of 47 patients were enrolled. The dose de-escalation group (n=6) received PF-04136309 at 500 mg administered orally twice daily. No dose-limiting toxicities were observed and this was established as the recommended phase 2 dose. The expansion phase cohort (n=33) and patients in the dose de-escalation arm receiving PF-04136309 at the recommended phase 2 dose (n=6) were combined for assessment of treatment related toxicity. No therapy related deaths occurring during the study interval. Early termination as the result of treatment related toxicity occurred in 2 of the 39 patients (5%) in the FOLFIRINOX plus PF-04136309 arm. Grade ≥3 adverse events reported in ≥10% of the patients receiving PF-04136309 included neutropenia in 27 patients (69%), febrile neutropenia in 7 patients (18%), lymphopenia in 4 patients (10%), diarrhea in 6 patients (15%), and hypokalemia in 7 patients (18%). Among patients receiving FOLFIRINOX alone (n=8), a total of 6 patients were evaluated for treatment toxicity, with 2 patients receiving the intended therapy but not monitored for adverse events due to insurance coverage issues and excluded. Therapy was terminated due treatment related toxicity in 1 of the 6 patients (17%) receiving FOLFIRINOX alone. Grade ≥3 adverse events reported in ≥10% of patients receiving FOLFIRINOX alone were neutropenia in 6 cases (100%), febrile neutropenia in 1 case (17%), anemia in 2 cases (33%), lymphopenia in 1 cases (17%), diarrhea in 2 cases (33%), hypoalbuminemia in 1 case (17%), and hypokalemia in 3 cases (50%). An objective tumor response was seen in 16 of 33 patients (49%) receiving FOLFIRINOX plus PF-04136309 that had repeat imaging available, with local tumor control achieved in 32 of 33 patients (97%). In the FOLFIRINOX alone arm there were no objective responses among 5 patients with repeat imaging, with 4 out of 5 patients (80%) demonstrating stable disease.
Interpretation
CCR2 targeted therapy with PF-04136309 in combination with FOLFIRINOX is safe and tolerable. Corollary studies suggest that CCR2 blockade reduces TAM and alters the TME, providing rationale for future clinical studies of this promising treatment modality.
Introduction
Pancreatic ductal adenocarcinoma (PDAC) is currently the 4th leading cause of cancer related death.1 The majority of patients present with advanced disease, either metastatic or locally unresectable, and for the minority of patients that proceed to resection disease recurrence rates are greater than 75%.2 Despite recent advances utilizing conventional chemotherapy, durable responses remain elusive.3,4
PDAC is characterized by a desmoplastic stroma that is rich in leukocytes.5 This immune component contains a paucity of tumor infiltrating lymphocytes (TIL) and is predominately bone marrow derived myeloid cells including tumor associated macrophages (TAM) that are critical for tumor immune evasion, treatment resistance, and disease progression.6,7 Chemokine pathways recruit bone marrow derived cells to sites of inflammation in normal physiology, but are co-opted by PDAC and other cancers to mobilize myeloid cells to the tumor microenvironment (TME).8,9
The chemokine CCL2 is responsible for the recruitment of CCR2+ inflammatory monocytes (IM) from the bone marrow to the peripheral blood where they ultimately migrate to pancreatic tumors and become immunosuppressive TAM. We have previously shown that the CCL2/CCR2 chemokine axis has prognostic significance in human PDAC, with the ratio of peripheral blood to bone marrow IM being prognostic of post-resection survival.7 Targeting this pathway, via either CCR2 inhibition or CCL2 neutralizing antibodies, has demonstrated efficacy in several pre-clinical tumor models including PDAC.6,7,10,11
Based on this strong rationale, we conducted a phase Ib clinical trial in patients with borderline resectable (BR) and locally advanced pancreatic cancer (LAPC), examining the safety and efficacy of an orally dosed, small molecule CCR2 inhibitor (PF-04136309) in combination with standard chemotherapy (FOLFIRINOX).
Methods
Study design and participants
This phase Ib open-label, non-randomized, single institution clinical trial enrolled patients age ≥18 years with treatment naïve, biopsy-proven, borderline resectable (BR) and locally advanced pancreatic adenocarcinoma (LAPC) with measurable disease by Response Evaluation in Solid Tumors, Version 1.1. BR and LAPC designation was determined using the Americas Hepato-Pancreato-Biliary Association (AHPBA) 2009 consensus guidelines. No upper age limit was established for enrollment in the study. Patients were required to have an Eastern Collaborative Oncology Group (ECOG) performance score of 1 or less and an estimated life expectancy >6 months at time of enrollment. Inclusion criteria required evidence of normal bone marrow function (absolute neutrophil count≥1,500/mcl, platelets≥100,000/mcl, hemoglobin≥9·0 g/dl) and end-organ function (creatinine clearance >60 ml/min, a serum bilirubin less than 1·5x upper limit of normal, and a normal International Normalized Ratio (INR) for patients not on anticoagulant therapy). Baseline laboratory tests were obtained for eligibility screening prior to enrollment and reassessed at the start of each 14 day treatment interval. Exclusion criteria included any prior or current treatment, evidence of metastasis, duodenal/ampullary adenocarcinoma, neuroendocrine tumor, or a prognosis of survival <6 months. Additional exclusion criteria included pregnancy and a history of malignancy in prior 3 years, excluding basal or squamous cell carcinoma of the skin treated with local excision only or carcinoma in situ of the cervix. Patients taking chronic oral steroids were also excluded from the study, however steroid use for the prophylactic treatment of chemotherapy related nausea and inhaled steroids were permitted. Placement of biliary stents prior to enrollment was allowed if liver function returned to permissible levels for inclusion. Informed consent was obtained for all enrolled patients under an institutional review board (IRB) approved protocol at Washington University School of Medicine (St. Louis, MO).
Procedures
The FOLFIRINOX regimen (oxaliplatin [85 mg/m2], irinotecan [180 mg/m2], leucovorin [400 mg/m2], and bolus fluorouracil [400 mg/m2] followed by 46 hour infusion [2,400 mg/m2]) was administered every 2 weeks for a total of 6 cycles. PF-04136309 was administered orally and taken twice daily at the recommended phase 2 dose for 6 two week cycles concurrent with the FOLFIRINOX regimen schedule. The recommended phase 2 dose for PF-04136309 in combination with FOLFIRINOX was determined in a standard 3+3 dose de-escalation design. Dose limiting included grade 4 neutropenia >7 days, febrile neutropenia, thrombocytopenia requiring ≥2 transfusions during two cycles of chemotherapy. Non-hematologic DLT included any grade 3 or 4 clinically significant adverse event during the first two chemotherapy cycles with the following exceptions: grade 3 or 4 elevation of liver enzymes or bilirubin, grade 3 or 4 nausea, vomiting, or diarrhea that resolved with supportive care within 7 days, grade 3 mucositis or fatigue lasting less than 7 days, and grade 3 or 4 laboratory abnormalities that were not clinically significant. Any treatment related toxicity resulting in a delay of therapy >21 days cumulatively during the first cycle or >14 days cumulatively during the second cycle was also considered a DLT. The recommended phase 2 dose was used in an expansion cohort, with patients from the dose de-escalation group receiving the recommended phase 2 dose and the expansion phase pooled for analysis.
Treatment related adverse events were assessed from time of treatment initiation every 14 days until 30 days after last therapeutic cycle was completed or death. Toxicity grade was determined by the revised National Cancer Institute (NCI) Common Terminology Criteria for Adverse Events (CTCAE) version 4·0 and centrally reviewed on a weekly basis through the Developmental Therapeutics Committee at Washington University School of Medicine (St. Louis, MO). Patients allocated to either FOLFIRINOX alone or FOLFIRINOX plus PF-04136309 were treated for the complete 12-week intended duration of therapy unless one of the following criteria occurred; death, confirmed disease progression, treatment related adverse event that may result in severe or permanent harm, serious non-compliance, change in the patient’s general or medical condition that renders the patient unable to continue on the study, lost to follow-up, or withdrawal of informed consent.
Pre-specified dose reductions and treatment delays in either the FOLFIRINOX regimen and/or PF-04136309 were permitted based on the toxicity and grade. All treatment was delayed until toxicities had resolved to ≤; grade 1. No more than two dose reductions of each agent were permitted. Dose reductions in the FOLFIRINOX regimen for neutropenia were permitted during the first two treatment cycles because G-CSF administration was not permitted prior to the second treatment cycle as to not interfere with interpretation of corollary studies. Following the second treatment cycle G-CSF administration was permitted. PF-04136309 dose reductions were permitted for grade 3 or greater vomiting and diarrhea.
All radiologic evaluations were performed by a centralized panel of independent, blinded board certified radiologists at the Mallinckrodt Institute of Radiology at Washington University School of Medicine (St. Louis, MO). Baseline pancreatic protocol CT scans were obtained ≤;28 days prior to initiating treatment. Patients receiving >2 cycles of treatment were evaluable for treatment response with repeat imaging obtained within 2–4 weeks of completing the last treatment cycle. Patients receiving less than 2 cycles of intended therapy or without repeat imaging were excluded from the analysis of treatment response.
Bone marrow, peripheral blood, and endoscopic ultrasound (EUS) guided fine needle aspiration (FNA) tumor biopsies were collected at baseline and after completion of treatment cycle 2 (+/−5 days). Following the FOLFIRINOX only arm and dose de-escalation group receiving PF-04136309 at the recommended phase 2 dose, bone marrow and repeat FNA tumor biopsies were collected on an optional basis per patient preference. Tumor biopsies were collected sequentially by an interventional gastroenterologist with experience in the procedure. All FNA samples were also confirmed to be PDAC on cytologic examination by a trained pathologist. FNA biopsies were immediately frozen, except for the final two passes, which were used for flow cytometry analysis. Total RNA was extracted from the frozen matched FNA tumor samples via TRIzol (Life Technologies) then digested with DNase and cleaned with the RNeasy Mini Kit (Qiagen). RNA was reverse transcribed into cDNA and quantitative real time PCR (qRT-PCR) performed using predesigned TaqMan primers (Life Technologies) on a 7500 Fast Thermal Cycler (Applied Biosystems). Target gene expression was normalized to GAPDH, HPRT1, and β-actin using the comparative CT (ΔΔCT) method. Mononuclear cells were isolated from bone marrow and blood by Ficoll density gradient centrifugation within one hour of collection. These cells were assessed for viability and quantified prior to being stored at −80 C. Prior to analysis samples were thawed and viability and cell counts repeated. For flow cytometry analysis of fresh FNA tumor biopsies, samples were immediately collected and mechanically dissociated and strained through a 70 micron nylon filter (Fisher Scientific) to create single cell suspensions. To prevent nonspecific antibody binding, all flow cytometry samples were incubated with human Fc receptor blocking solution (Human TruStain FcX, BioLegend) followed by addition of fluorophore-conjugated anti-human antibodies. The following antibodies were obtained from BioLegend: CD45 (HI30), CD11b (M1/70), CD115 (9-4D2-1E4), CD14 (HCD14), CD15 (W6D3), HLA-DR (L243), CCR2 (G10F5), CD3 (HIT3a), CD4 (OKT4), CD8 (SK1). For intracellular staining, cells were permeabilized and stained with FoxP3 (259D) per manufacturer’s instructions (BD Bioscience). Flow cytometry was performed on an LSRII (BD Biosciences) and analyzed using FloJo Version X software (Tree Star, Inc).
Outcomes
The primary endpoint of the study was to define the safety, tolerability, and recommended phase 2 dosing of PF-04136309 in combination with FOLFIRINOX. Treatment related adverse events were assessed using the revised National Cancer Institute (NCI) Common Terminology Criteria for Adverse Events (CTCAE) version 4·0. Secondary objectives included determining the partial response rate (PR) and tumor control rate (TCR= stable disease [SD] + partial response [PR] + complete response [CR]) of FOLFIRINOX plus PF-04136309 using RECIST, version 1·1. Exploratory objectives included quantifying the prevalence and functional impact of inflammatory monocytes (IM) and tumor associated macrophages (TAM) in the bone marrow, peripheral blood, and tumor following treatment with FOLFIRINOX alone or in combination with PF-04136309.
Statistical Analysis
Statistical analysis was performed using SAS version 9·1 (SAS Institute, Inc.) and GraphPad Prism version 4 (GraphPad Software, Inc.) with a significance level of α=0·05. We compared characteristics by assignment to FOLFIRINOX and FOLFIRINOX plus PF-04136309. Data was determined to follow a Gaussian distribution by the D’Agostino and Pearson omnibus normality test. For comparing continuous variables, the Student’s t-test or Mann-Whitney test was used for normally and non-Gaussian distributed data respectively. Categorical variables were compared using Fischer Exact Chi Square test. Exact 95% confidence intervals (2-sided) were calculated and provided where indicated.
Analysis of the primary outcome were analyzed by an intention to treat analysis. Patients that were unable to be assessed for treatment related toxicity following allocation were excluded from the analysis as this data was unavailable. Demographic and clinical characteristics of the patient population and adverse event by grade are summarized using descriptive statistics. For patients receiving PF-04136309, the dose de-escalation group receiving the recommended phase 2 dose and expansion cohort were pooled for purposes of analysis.
Treatment response in patients receiving FOLFIRINOX plus PF-04136309 was analyzed by a prespecified 2-sided hypothesis test for proportions. 32 patients receiving PF-04136309 were required to be assessed for treatment response by RECIST to obtain a power of 80% (β=0·2). The null hypothesis of a 25% RECIST partial response (PR) rate with FOLFIRINOX alone was determined prior to the start of the study and compared to the observed PR rate in those receiving FOLFIRINOX plus PF-04136309. The study was designed to detect an improvement of ≥20% above this pre-specified hypothesis or an absolute PR ≥45% in the FOLFIRINOX plus PF-04136309 arm. Response rates were analyzed by a modified per protocol analysis with patients not completing >2 cycles of intended therapy or without repeat imaging excluded from the analysis.
This trial is registered with ClinicalTrials.gov, number NCT01413022.
Role of funding source
The funding source provided compound for clinical use in the study and approved the trial design. The trial was initiated by the corresponding author and study design, data acquisition, analysis, and interpretation were performed independently by the authors listed. The manuscript was written by the authors independently. The funding source had no role in the writing of the submitted manuscript, but it was made available to the funder prior to submission with no editorial rights. Authors of the study had full access to the data, performed the writing of the report, and agreed upon its final content. The work reported in this study was also funded in part by grant support from the National Institute of Health (NIH), awarded to DCL as principal investigator (NIH 5R01CA168863). TMN & DES were supported by the NIH T32 CA 009621 grant. None of the authors are employed by the NIH. The corresponding author had full access to all of the data and the final responsibility to submit for publication.
Results
Patient characteristics
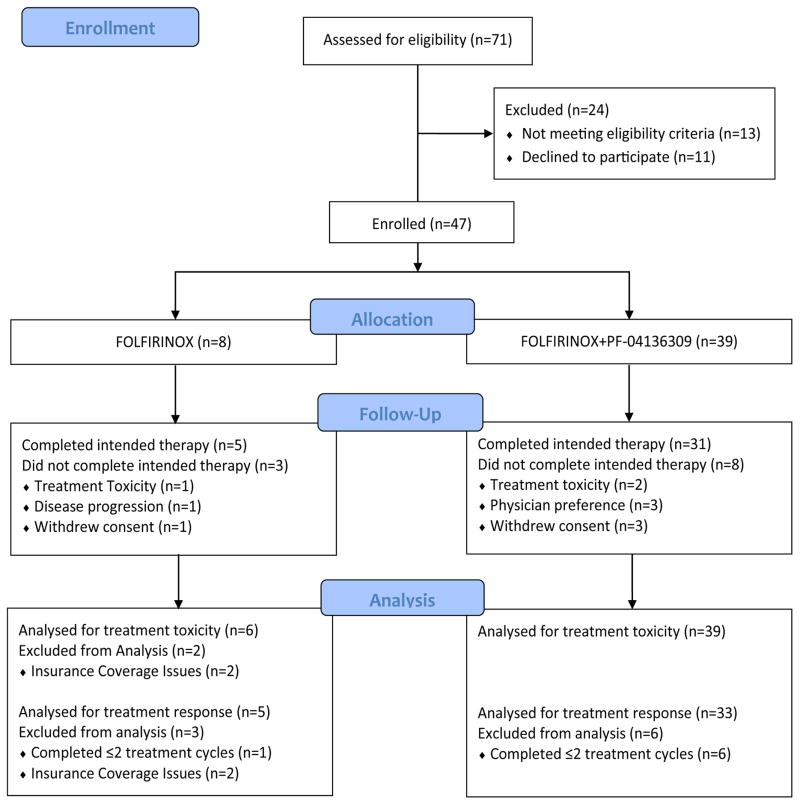
Between April 19th, 2012 and November 12th, 2014 a total of 71 patients were screened and 47 patients were enrolled (Fig 1). Characteristics of both treatment groups are reviewed in Table 1. The median age was 63 years (range: 41–75) in the FOLFIRINOX only group (n=8) and 60 years (range: 45–73) in the group receiving FOLFIRINOX in combination with PF-04136309 (n=39). The ECOG performance status was ≤;1 for all enrolled patients (n=47). The median number of treatment cycles completed was 6 in both groups. Most patients presented with LAPC (n=6 [75%] vs n=31 [79%]), with the majority of lesions located in the pancreatic head or neck (n=6 [75%] vs n=30 [77%]). Placement of a biliary stent prior to treatment was required in 2 of the 8 patients (25%) receiving FOLFIRINOX and in 24 of the 39 patients (61·5%) in the FOLFIRINOX plus PF-04136309 group.
Figure 1.
Trial Schema
Table 1.
| FOLFIRINOX (n=8) | FOLFIRINOX+PF-04136309 (n=39) | |
|---|---|---|
| Age (years) | 63 (41–75) | 60 (45–73) |
|
| ||
| Sex -no. (%) | ||
| Male | 2 (25%) | 21 (54%) |
| Female | 6 (75%) | 18 (46%) |
|
| ||
| Race -no. (%) | ||
| Caucasian | 7 (87%) | 31 (79%) |
| Black | 1 (13%) | 7 (18%) |
| Other | 0 | 1 (3%) |
|
| ||
| Tumor Classification -no. (%) | ||
| Borderline Resectable | 2 (25%) | 8 (21%) |
| Locally Advanced | 6 (75%) | 31 (79%) |
|
| ||
| Tumor Location -no. (%) | ||
| Head | 6 (75%) | 30 (77%) |
| Tail | 2 (25%) | 9 (23%) |
|
| ||
| CA19-9 (Units/ml) | 362 (20–5,000) | 175 (<10–9,166) |
|
| ||
| ECOG Performance Status | ||
| 0 | 1 (13%) | 15 (39%) |
| 1 | 7 (87%) | 24 (61%) |
|
| ||
| Treatment Cycles | 6 (2–6) | 6 (1–6) |
|
| ||
| Received G-CSF -no. (%) | ||
| Yes | 3 (38%) | 22 (56%) |
| No | 5 (62%) | 17 (44%) |
|
| ||
| Biliary Stent –no. (%) | ||
| Yes | 2 (25%) | 24 (61%) |
| No | 6 (75%) | 15 (38%) |
Treatment tolerability
Patients who received at least one cycle of FOLFIRINOX were evaluable for toxicity. A total of 47 patients were enrolled in the study and 45 patients assessed for treatment related toxicity, with 2 patients receiving FOLFIRINOX alone withdrawing consent after allocation due to insurance coverage issues and excluded from the analysis.
The median duration patients in the FOLFIRINOX alone group (n=6) were evaluated for toxicity was 72·0 days (IQR: 49·5 to 89·0) with a median of 6 treatment cycles (range: 2–6) completed. In the FOLFIRINOX plus PF-04136309 arm (n=39) patients were followed for treatment related adverse events for a median of 77 days (IQR: 70 to 90) and completed a median of 6 treatment cycles (range: 1–6). No difference in median dose intensity of the FOLFIRINOX regimen was seen between cohorts (Supplementary Appendix pg. 1). Mandated dose reductions of irinotecan due to grade 3 or 4 diarrhea resulted in irinotecan having a lower dose intensity compared to oxaliplatin. In the FOLFIRINOX plus PF-04136309 arm, 31 of 39 patients (79·5%) completed all six FOLFIRINOX treatment cycles and 6 of the 39 patients (15·4%) did not require dose reductions. The intended treatment was completed in 3 of the 6 patients (50%) in the group receiving FOLFIRINOX alone evaluated for treatment related adverse events, with 1 of the 6 patients (16.7%) not requiring therapeutic dose reduction.
Dose reductions of PF-04136309 were permissible per the study protocol and occurred in 6 of 39 cases (15·4%). These were the result of PF-04136309 being held for grade 3 or higher diarrhea (Table 2). If this improved to grade 1 or less, then PF-04136309 was decreased to 375 mg twice daily. Per patients’ self-reported medication logs, the median PF-04136309 dose actually taken was 444·2 mg (range: 169·6–500) twice daily (Supplementary Appendix; pg. 1).
Table 2.
| FOLFIRINOX (n=6) | FOLFIRINOX plus PF-04136309 (n=39) | |||||||
|---|---|---|---|---|---|---|---|---|
| Grade 1 or 2 | Grade 3 | Grade 4 | Grade 5 | Grade 1 or 2 | Grade 3 | Grade 4 | Grade 5 | |
| Hematologic | ||||||||
| Neutropenia | 0 | 2 (33%) | 4 (67%) | 0 | 2 (5%) | 15 (38%) | 12 (31%) | 0 |
| Febrile neutropenia | 0 | 0 | 1 (17%) | 0 | 0 | 5 (13%) | 2 (5%) | 0 |
| Anemia | 4 (67%) | 2 (33%) | 0 | 0 | 34 (87%) | 3 (8%) | 0 | 0 |
| Lymphopenia | 2 (33%) | 1 (17%) | 0 | 0 | 20 (51%) | 4 (10%) | 0 | 0 |
| Thrombocytopenia | 4 (67%) | 0 | 0 | 0 | 19 (49%) | 1 (3%) | 1 (3%) | 0 |
| Non-Hematologic | ||||||||
| Nausea | 3 (50%) | 0 | 0 | 0 | 23 (59%) | 1 (3%) | 0 | 0 |
| Vomiting | 2 (33%) | 0 | 0 | 0 | 13 (33%) | 1 (3%) | 0 | 0 |
| Diarrhea | 4 (67%) | 2 (33%) | 0 | 0 | 19 (49%) | 6 (15%) | 0 | 0 |
| Mucositis | 3 (50%) | 0 | 0 | 0 | 16 (41%) | 1 (3%) | 0 | 0 |
| Alopecia | 4 (67%) | 0 | 0 | 0 | 28 (72%) | 0 | 0 | 0 |
| Peripheral neuropathy | 1 (17%) | 0 | 0 | 0 | 25 (64%) | 0 | 0 | 0 |
| Hypoalbuminemia | 4 (67%) | 1 (17%) | 0 | 0 | 25 (64%) | 2 (5%) | 0 | 0 |
| Hypokalemia | 1 (17%) | 3 (50%) | 0 | 0 | 17 (44%) | 5 (13%) | 2 (5%) | 0 |
| ELEVATED ASPARTATE Aminotransferase | 2 (33%) | 0 | 0 | 0 | 12 (31%) | 2 (5%) | 0 | 0 |
A total of 45 of the 47 patients (95·8%) enrolled in the study were assessed for treatment related adverse events, with 2 patients in the FOLFIRINOX only cohort unable to be followed for treatment related toxicity due to insurance issues and not included in the analysis. No treatment related deaths occurred. Treatment related adverse events are reviewed in Table 2. Adverse events with severity grade 3 or greater in patients receiving PF-04136309 included neutropenia in 27 of 39 patients (69·2%), with 22 patients (56·4%) receiving G-CSF support. Febrile neutropenia was reported in 7 out of 39 patients (18·0%). Among patients receiving FOLFIRINOX alone evaluated for treatment toxicity (n=6), grade 3 or greater neutropenia occurred in all 6 patients (100%), with 3 (50%) receiving G-CSF support and febrile neutropenia in 1 of 6 patients (17%). The most frequent grade 3 or greater non-hematologic toxicities in the FOLFIRINOX plus PF-04136309 cohort included diarrhea in 6 of the 39 patients (15·4%) and hypokalemia in 7 of 39 patients (18·0%). These were also the most common non-hematologic adverse events in the FOLFIRINOX alone (n=6), with 2 of the 6 (33%) patients having diarrhea and 3 of the 6 patients (50%) having hypokalemia of severity grade 3 or greater.
Termination due to treatment related toxicity was reported in 1 of 6 patients (16·7%) and 2 of 39 patients (5·1%) in the FOLFIRINOX and FOLFIRINOX plus PF-04136309 groups respectively. The remainder of cases in the FOLFIRINOX plus PF-04136309 group not included in the evaluation of tumor response were the result of consent withdrawal (n=4) and all occurred prior to completing the second treatment cycle.
Repeat imaging was obtained in patients receiving ≥2 cycles of intended treatment but discontinued therapy for any reason and were included in the analysis for treatment response. This occurred in 2 of the 6 FOLFIRINOX patients (25%) and were the result of disease progression in 1 patient and diverticulitis in 1 patient. In the FOLFIRINOX plus PF-04136309 group, 2 of the 39 patients (5·1%) had early termination of therapy as the result of cholangitis in 1 patient and the other due to infectious colitis.
Treatment Response
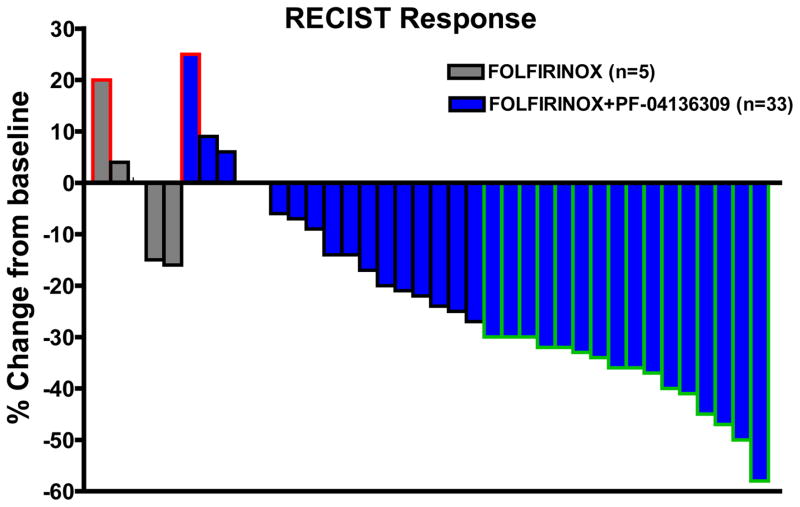
Patients were considered evaluable for treatment response by RECIST if they had received at least 2 cycles of treatment and had repeat imaging for comparison with baseline. The change in tumor size from baseline by RECIST for all evaluable patients receiving FOLFIRINOX plus PF-04136309 (n=33) demonstrates 16 of 33 patients (48·5% [95% CI: 30·80 to 66·54]) achieved PR (Fig 2). This is significantly higher than our pre-specified, expected PR rate of 25% with FOLFIRINOX alone (p=0·006). Tumor control (TCR=CR+PR+SD) was achieved in 32 of 33 patients (97·0% [95% CI: 84·24 to 99·92]) receiving PF-04136309 in combination with FOLFIRINOX, with only 1 out 33 patients having disease progression and no reports of distant metastasis during the study. No objective treatment responses were seen among evaluable cases receiving FOLFIRINOX alone (n=5) and one patient had distant metastasis while on therapy (TCR=80%). Post treatment tumor markers were available in 25 patients receiving PF-04136309 with elevated (>35 U/ml) serum CA19-9 levels at baseline. Analysis of this data showed an overall significant reduction in tumor markers following FOLFIRINOX plus PF-04136309 and 5 patients returned to within normal levels following treatment (Supplemental Appendix; pg. 8).
Figure 2. FOLFIRINOX+PF-04136309 Treatment Response.
Waterfall plot depicting percent change from baseline for all evaluable patients (n=38). FOLFIRINOX alone (n=5) denoted in grey and FOLFINOX plus PF-04136309 (n=33) in blue. Disease progression (red border), stable disease (black border), and partial response (green border) as defined by RECIST. Patient with disease progression receiving FOLFIRINOX alone had distant metastasis.
Among patients evaluated for treatment response, 13 of 33 (39·4%) were downsized sufficiently for resection. Ultimately, 10 of the 33 patients (30·3%) were able to undergo successful operative excision of the primary tumor. An R0 resection was obtained in 7 of the 10 resected patients (70·0%) and 5 of the 10 cases (50·0%) had negative lymph nodes on pathologic examination.
Additional neoadjuvant chemoradiation, initiated at the discretion of a multi-disciplinary tumor board and was performed in 6 of the 10 resected cases (60%) and occurred after repeat imaging was obtained for determining the objective response rate reported in this study.
In the FOLFIRINOX plus PF-04136309 arm evaluated for treatment response 7 of the 33 (21.2%) patients were classified as borderline resectable (BR) at time of enrollment with 5 of the 7 patients (71·4%) having a partial response and 1 patient (14.3%) having local disease progression (Supplemental Appendix; pg. 9). Resection was performed in 5 of the 7 BR cases (71·4%) and occurred without additional chemoradiation in 2 of these cases. The remaining 26 out of 33 (78·8%) patients had locally advanced pancreatic cancer (LAPC) and an objective response obtained in 11 out of 26 of these cases (42.3%), with no instances of disease progression reported. Surgical resection occurred in 5 of the 26 LAPC patients (19·2%) and was performed in 2 cases without chemoradiation.
Corollary Studies
Analysis of bone marrow and peripheral blood
We hypothesized that the clinical activity seen with CCR2 inhibition was the result of a reduction in inflammatory monocyte (IM) recruitment from the bone marrow. To test this assertion we examined the prevalence of IM in the blood and bone marrow of patients treated with FOLFIRINOX alone or in combination with PF-04136309. Representative flow cytometry illustrates the impact of CCR2 blockade on these respective compartments (Supplemental Appendix; pg. 2).
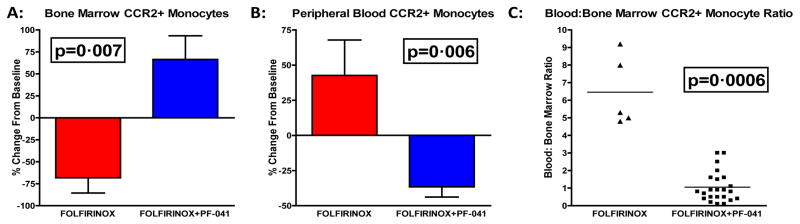
Since PDAC overexpresses CCL2, we predicted that IM would decrease in the bone marrow as they are recruited into the circulation by the primary tumor.7 However, to exclude the possibility that cytotoxic chemotherapy (FOLFIRINOX) would ablate CCR2+ monocytes, we performed flow cytometry analysis with matched baseline and post-treatment bone marrow aspirates from patients receiving FOLFIRINOX alone (n=5) and FOLFIRINOX plus PF-04136309 (n=23). This demonstrated a mean decrease of −68·2% (95% CI:−116·4 to −20·0) from baseline in bone marrow CCR2+ monocytes following FOLFIRINOX alone (p=0·007; Fig 3A). In contrast the addition of PF-04136309 resulted in a mean increase of 66·43% (95% CI: 10·56 to 122·3) in bone marrow CCR2+ monocytes relative to baseline.
Figure 3. CCR2 blockade prevents PDAC mediated mobilization of bone marrow derived inflammatory monocytes into the peripheral circulation.
(A) Percent change in bone marrow CCR2+ monocytes from matched baseline of FOLFIRINOX only (n=5) and FOLFIRINOX+PF-04136309 (n=23). (B) Percent change in peripheral blood CCR2+ monocytes from matched baseline of FOLFIRINOX (n=6) and FOLFIRINOX+PF-04136309 (n=34). (C) Ratio of blood to bone marrow CCR2+ monocytes as a percentage of CD45% cells following 2 cycles of treatment with FOLFIRINOX (n=5) or FOLFIRINOX+PF-04136309 (n=23). Error bars reflect ±SEM. P-values determined by Mann-Whitney test.
Next, we assessed the impact of PF-04136309 on CCR2+ monocytes in the peripheral blood from patients receiving FOLFIRINOX alone (n=6) and FOLFIRINOX plus PF-04136309 (n=34). The FOLFIRINOX plus PF-04136309 cohort revealed a mean decrease of −36·56% (95% CI: −51·4 to −21·72) in circulating CCR2+ monocytes from baseline (p=0·006; Fig 3B). FOLFIRINOX treatment alone had a mean increase of 42·67% (95% CI: −22·06 to 107·4) from baseline CCR2+ monocytes in the periphery.
Assessment of the absolute number of CCR2+ monocytes in the bone marrow and blood confirmed these findings, with a significant increase in the absolute number of IM in the bone marrow and reduction in the absolute number of circulating IM following FOLFIRINOX plus PF-04136309 compared to FOLFIRINOX alone (Supplemental Appendix; pg. 4). Furthermore, no difference was seen in the absolute number of baseline IM in the bone marrow or peripheral blood between those receiving FOLFIRINOX alone compared to patients receiving PF-04136309, excluding this as possible explanation for our findings (Supplemental Appendix; pg. 3).
As our previous work showed that the ratio of blood to bone marrow CCR2+ monocytes is prognostic of survival, we assessed if PF-04136309 was effective in reducing this parameter.7 The mean ratio of blood to bone marrow CCR2+ monocytes was 1·06 (95% CI: 0·68 to 1·43) following treatment with FOLFIRINOX plus PF-04136309 (n=23). This was significantly decreased compared to the FOLFIRINOX only cohort that had a mean ratio of 6·46 (95% CI: 3·97 to 8·95) following therapy (p=0.0006; Fig 3C).
To examine if changes in CCR2+ IM correlated with treatment response, we assessed the bone marrow and peripheral blood compartments for differences between patients receiving PF-04136309 that achieved a RECIST objective response compared to non-responders (Supplementary Appendix; pg. 5). Bone marrow IM were increased by a mean of 69·8% (95% CI: −2·71 to 142·3%) from baseline in patients achieving a RECIST PR following FOLFIRINOX plus PF-04136309 (n=10). Although this was higher than the 30·31% (95% CI: −16·11 to 76·73) increase from baseline in patients without an objective tumor response receiving PF-04136309 (n=13), this did not achieve statistical significance. However, in the peripheral blood, CCR2+ monocytes were significantly decreased in patient that achieved PR following PF-04136309 plus FOLFIRINOX (n=15) by a mean of −51·33% (95% CI: −62·15 to −40·5) from baseline compared to a mean reduction of −4·21% (95% CI: −23·72 to 15·30) from baseline in patients without an objective response that also received FOLFIRINOX plus PF-04136309 (n=19). Overall, patients with PR receiving PF-04136309 (n=10) had a trend towards a lower peripheral blood to bone marrow IM ratio with a mean ratio of 0·76 (95% CI: 0·45 to 1·07) compared to a mean ratio of 1·29 (95% CI: 0·65 to 1·92) in patients with stable disease receiving PF-04136309 (n=13).
Analysis of the tumor microenvironment
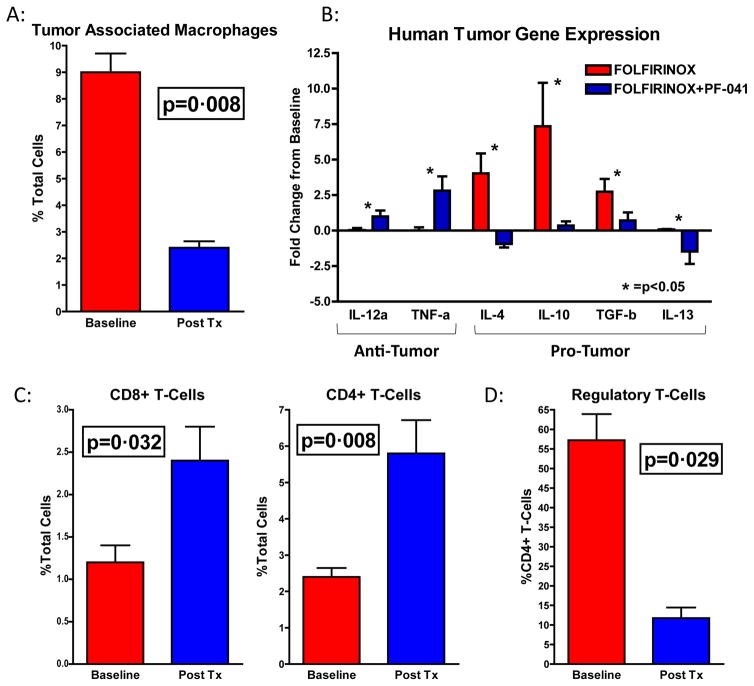
To assess if the impact of CCR2 blockade in the periphery was associated with a reduction of tumor associated macrophages (TAM) in the tumor microenvironment (TME), we performed flow cytometry analysis on baseline and post-treatment tumor biopsies (Supplemental Appendix; pg. 6). Analysis of matched samples (n=6) showed a mean reduction in TAM from 9·0% (95% CI: 7·04 to 10.96) to 2·4% (95% CI: 1·72 to 3·08) of total cells following treatment with PF-04136309 (p=0·008; Fig 4A).
Figure 4. TAM are decreased following treatment with FOLFIRINOX+PF-04136309 promoting an anti-tumor immune response.
(A) Graph represents TAM population as a percentage of total cells (n=6). (B) qRT-PCR from tumors following FOLFIRINOX (n=6) and FOLFIRINOX+PF-04136309 (n=14). Percentage reflects change compared to matched baseline sample. (C) Graphs represent CD8+ (left) and CD4+ (right) tumor infiltrating T-cells following FOLFIRINOX+PF-04136309 (n=6). (D) Graph depicts FoxP3+ regulatory T-cells as percentage of CD4+ lymphocytes. * =p<0.05. Error bars depict ±SEM. P-values determined by Mann-Whitney test.
Quantitative real time PCR analysis on matched FNA tumor biopsies following FOLFIRINOX alone (n=5) or FOLFIRINOX plus PF-04136309 (n=14) was performed and demonstrated the reduction in CCR2+ TAM was associated with a significant increase, relative to matched baseline samples, in immunostimulatory IL-12 and TNFα levels in patients receiving FOLFIRINOX plus PF-04136309 compared to FOLFIRINOX controls (* =p<0·05; Fig 4B). In contrast, the immunosuppressive factors IL-4, IL-10, IL-13, and TGF-β were all upregulated following FOLFIRINOX and this effect was decreased following CCR2 blockade with PF-04136309.
To explore if these changes in the TME had an effect on tumor infiltrating lymphocytes (TIL) we examined both the effector CD8+ and CD4+ T-cell populations (Supplemental Appendix; pg. 7). Treatment with FOLFIRINOX plus PF-04136309 increased CD8+ TIL from a mean of 1·2% (95% CI: 0·65 to 1·76) to 2·4% (95% CI: 1·29 to 3·51) of total cells (p=0·032; Fig 4C). Helper CD4+ T-cells were also more abundant following PF-04136309 with an increase from 2·4% (95% CI: 1·72 to 3·08) at baseline to 5·8% (95% CI: 3·26 to 8·35) of total cells (p=0·008; Fig 4C). Analysis of the FoxP3 regulatory T-cell (Treg) population at baseline revealed a mean of 57.3% (95% CI: 36.1 to78.4) of CD4+ lymphocytes at baseline. However, following treatment with PF-04136309 Tregs were reduced to 11.8% (95% CI: 3.1 to 20.4) of CD4+ TILs (p=0·029; Fig 4D).
Discussion
In this phase Ib clinical trial, the recommended phase 2 orally administered dose of PF-04136309 was determined to be 500 mg twice daily and was safely tolerated in combination with the FOLFIRINOX regimen. Analysis of prespecified secondary objectives found the addition of PF-04136309 resulted in a higher than expected objective response rate in borderline resectable (BR) and locally advanced pancreatic cancer (LAPC) patients. PF-04136309, an orally available small molecule CCR2 inhibitor, prevented inflammatory monocyte (IM) egress from the bone marrow and reduced tumor associated macrophage (TAM) infiltrate at the primary tumor. The subsequent reversal of immune suppression within the tumor microenvironment (TME) and influx of tumor infiltrating lymphocytes (TIL) suggests a restoration of anti-tumor immunity. Furthermore, PF-04136309 resulted in an overall reduction in circulating CCR2+ IM from baseline and this effect was more pronounced in patients that achieved an objective response by RECIST. While interpretation of this is limited by the small population sampled in this study, this biomarker evidence suggests that the clinical activity seen with PF-04136309 correlated with target engagement. The results of this current study supports a potential therapeutic impact utilizing a targeted therapy to disrupt the CCL2/CCR2 chemokine axis in PDAC.
No consensus management for neoadjuvant treatment of LAPC currently exists and the treatment paradigm for BR disease is currently evolving to frequently include chemotherapy or radiation prior to resection.12 While FOLFIRINOX has demonstrated an improvement in overall survival compared to Gemcitabine in patients with metastatic disease, no randomized clinical trial has evaluated this regimen alone in our study population.4 However, a large retrospective study of the FOLFIRINOX regimen in patients with BR and LAPC by Marthey, et al. demonstrated a 28% RECIST response rate and a TCR of 84%.13 These findings are consistent with other retrospective studies as well as reports from our own institution which showed a PR of 27·8% and TCR of 83·3% following FOLFIRINOX in this patient population.14–17 While our results using PF-04136309 compare favorably and suggest a therapeutic benefit compared to standard chemotherapy alone, this current study is insufficient and larger, randomized clinical trials are currently being planned. Furthermore, the emergence of nab-paclitaxel plus gemcitabine for the treatment of metastatic disease was published after we had initiated our trial, but both FOLFIRINOX and gemcitabine in combination with CCR2 blockade has demonstrated promise in pre-clinical models (Personal Communication; authors) and should be explored in clinical studies.3
TAM are abundant in the TME and correlate with survival in multiple malignancies including prostate, breast, colorectal, and pancreas.18 PDAC not only recruits myelomonocytic cells to the tumor, but also induces an immunosuppressive, alternatively activated macrophage phenotype within the TME that supports tumor growth and progression.19 This polarization of recruited CCR2+ monocytes is demonstrated in human PDAC, where CD14+ TAMs isolated from pancreatic tumors, but not the peripheral blood, were capable of suppressing T-cell proliferation.7 The mechanisms by which these bone marrow derived cells suppress an endogenous anti-tumor immune response has been well described via the production of arginase and reactive oxygen species.20,21 Our study suggests that targeting IM mobilization from the bone marrow alleviates these immunosuppressive mechanisms and fosters an anti-tumor immune response. However, it remains unclear if the increase in TIL seen in this study is due to recruitment or expansion of an already present T-cell population. Further work delineating the mechanism by which TAM affect the adaptive immune response to tumors may lead to additional applications of targeting TAM in the clinical setting.
Recent clinical trials using macrophage colony stimulating factor 1 receptor (CSF1R) blockade strategies have reported a reduction in TAM and improved clinical response in tenosynovial giant-cell tumors.22,23 However, the efficacy of CSF1R inhibition is dependent on the agent’s ability to infiltrate the TME. In PDAC, high interstitial pressures due to desmoplasia impedes perfusion and decreases drug delivery to malignant cells, potentially limiting the therapeutic impact of CSF1R blockade.24 In contrast, CCR2 inhibition prevents IM mobilization from the bone marrow and TAM trafficking into the tumor so the mechanism of action is not dependent on intra-tumoral concentrations of inhibitor. Prior clinical trials targeting the CCL2/CCR2 axis in metastatic prostate cancer with a monoclonal αCCL2 antibody did not show an improvement in clinical response.25 However, the therapy failed to effectively engage the desired target and pharmacokinetic data revealed a rapid dissociation of the antibody, resulting in an undesired increase in serum CCL2 concentrations.26 In this current study, we utilized a CCR2 small molecule antagonist and it is feasible that by targeting the receptor we are able to overcome the limitations observed in these prior trials. Another potential obstacle of targeting the CCL2/CCR2 axis is illustrated by studies demonstrating that cessation of αCCL2 therapy resulted in increased pulmonary metastasis and decreased survival in a murine model of breast cancer.27 Future clinical trial designs incorporating prolonged CCR2 inhibitor monotherapy are currently being planned and will address this potential mechanism of treatment resistance.
Conventional chemotherapy is associated with myelosuppressive properties and may affect CCR2+ monocyte development and recruitment. However, evidence suggests that the global inflammatory reaction induced by cytotoxic agents may induce bone marrow derived cell recruitment.28 In a murine breast cancer model, conventional chemotherapy resulted in tumor production of both CSF1 and IL-34, increasing immunosuppressive TAM via a CSF1R dependent mechanism.29 In a similar model, intravital microscopy revealed an increase in TAM following chemotherapy, which was driven by elevated stromal CCL2 production.30 In PDAC patients there was no difference in the circulating CCR2+ monocytes between patients receiving chemotherapy and those that did not.7 This data supports our findings that chemotherapy alone is not sufficient to decrease tumor recruitment of IM from the bone marrow (Fig 3).
PF-04136309 in combination with FOLFIRINOX did not result in additional toxicity at the recommended phase 2 dose (Table 2). CCR2 blockade demonstrated a reduction in the TAM infiltrate and evidence of an endogenous anti-tumor immune response (Fig 4). The results from this study strongly suggest that CCR2 targeted therapy impacts the biology of PDAC and future clinical trials should explore this promising therapeutic strategy.
Supplementary Material
Research in context.
Evidence before this study
At the start of our study in 2012 there was no consensus treatment for borderline resectable (BR) and locally advanced pancreatic cancer (LAPC). To date, the topic has not been definitively resolved. We searched PubMed between 2009–2015 and ClinicalTrials.gov (without date restriction) with the following search terms: “FOLFIRINOX”, “pancreatic adenocarcinoma”, “CCL2”, “CCR2”, “PF-04136309”, “inflammatory monocyte”, and “tumor associated macrophage”. No language restriction was used. Surgery is currently the only curative treatment option for pancreatic ductal adenocarcinoma (PDAC) and is obtained in a paucity of patients that present with disease. Several retrospective reports suggest FOLFIRINOX is an appropriate neoadjuvant treatment in patients with BR and LAPC that have good functional status, but no randomized clinical trial has reported data on the topic. Immunosuppressive tumor associated macrophages (TAM) are critical in sculpting the tumor microenvironment (TME) in PDAC patients. TAM are derived from circulating inflammatory monocytes (IM) and the CCL2/CCR2 chemokine axis is critical in the recruitment of IM from the bone marrow to the TME. Strategies preventing TAM trafficking to the TME via CCR2 inhibition have shown promise in preclinical PDAC models. To make progress in this recalcitrant disease, novel treatment strategies are greatly needed to improve patient outcomes.
Added value of this study
Using an orally dosed, small molecule CCR2 inhibitor (PF-04136309), we demonstrate that targeting the CCL2/CCR2 chemokine axis in PDAC prevents IM egress from the bone marrow and decreases TAM in the TME. The addition of CCR2 blockade to FOLFIRINOX was safe and the evidence suggests an improvement in tumor response and locoregional control rates. Our corollary studies also suggest the mechanism of action of CCR2 inhibition is mediated through the reversal of immune suppression within the TME.
Implications of all the available evidence
No neoadjuvant therapy currently provides a durable clinical impact in PDAC patients. CCR2 inhibition is a promising therapeutic strategy and should be explored in further clinical trials.
Acknowledgments
Funding: Work supported in part by a research grant from the Washington University/Pfizer Biomedical Collaborative.
The investigators would like to recognize the contributions of the patients who participated in the study for their time and commitment in advancing our understanding of pancreatic cancer. This work was funded in part by a research grant through the Washington University/Pfizer Biomedical Collaborative (PW0457). TMN, AWG, DES, RZP, DGD, RCF, WGH, SPG, & DCL acknowledge support from the Siteman Cancer Center Frontier Fund Team Science Award. TMN & DES were supported by the NIH T32 CA 009621 grant. TMN, DES, BAB, RZP, SPG, & DCL acknowledge support from NIH 5R01CA168863.
Footnotes
Contributors
TMN and AWG contributed equally to this work. TMN, DES, BAB, RPZ, AWG, DGD, SPG, and DCL contributed to the study design, data collection, analysis and interpretation. AWG, ACL, RS, BRT, KHL, RCF, SMS, WGH, and DCL were clinical investigators and conducted the clinical study. BMC, ATT, RKN, LAW, MY, and KJF contributed to the collection and analysis of the data. The initial draft of the manuscript was written by TMN with review and revisions made available to all authors. All authors had full access to the data in the study, agreed upon the final content, and made the decision to submit for publication. DCL, the corresponding author, had full access to all of the data and the final responsibility to submit for publication.
Declaration of interests
DCL received research grant support through the Washington University School of Medicine/Pfizer Biomedical Collaborative. AWG is a consultant for Pfizer, Merrimack, and Newlink. The other authors declared no conflicts of interest.
References
- 1.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64(1):9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Vincent A, Herman J, Schulick R, Hruban RH, Goggins M. Pancreatic cancer. Lancet. 2011;378(9791):607–20. doi: 10.1016/S0140-6736(10)62307-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369(18):1691–703. doi: 10.1056/NEJMoa1304369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364(19):1817–25. doi: 10.1056/NEJMoa1011923. [DOI] [PubMed] [Google Scholar]
- 5.Clark CE, Hingorani SR, Mick R, Combs C, Tuveson DA, Vonderheide RH. Dynamics of the immune reaction to pancreatic cancer from inception to invasion. Cancer Res. 2007;67(19):9518–27. doi: 10.1158/0008-5472.CAN-07-0175. [DOI] [PubMed] [Google Scholar]
- 6.Mitchem JB, Brennan DJ, Knolhoff BL, et al. Targeting tumor-infiltrating macrophages decreases tumor-initiating cells, relieves immunosuppression, and improves chemotherapeutic responses. Cancer Res. 2013;73(3):1128–41. doi: 10.1158/0008-5472.CAN-12-2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sanford DE, Belt BA, Panni RZ, et al. Inflammatory monocyte mobilization decreases patient survival in pancreatic cancer: a role for targeting the CCL2/CCR2 axis. Clin Cancer Res. 2013;19(13):3404–15. doi: 10.1158/1078-0432.CCR-13-0525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shi C, Pamer EG. Monocyte recruitment during infection and inflammation. Nat Rev Immunol. 2011;11(11):762–74. doi: 10.1038/nri3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol. 2012;12(4):253–68. doi: 10.1038/nri3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Qian BZ, Li J, Zhang H, et al. CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature. 2011;475(7355):222–5. doi: 10.1038/nature10138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhao L, Lim SY, Gordon-Weeks AN, et al. Recruitment of a myeloid cell subset (CD11b/Gr1 mid) via CCL2/CCR2 promotes the development of colorectal cancer liver metastasis. Hepatology. 2013;57(2):829–39. doi: 10.1002/hep.26094. [DOI] [PubMed] [Google Scholar]
- 12.Katz MH, Shi Q, Ahmad SA, et al. Preoperative modified FOLFIRINOX (mFOLFIRINOX) followed by chemoradiation (CRT) for borderline resectable (BLR) pancreatic cancer (PDAC): initial results from alliance trial A021101. J Clin Oncol. 2015;33(suppl) abstr 4008. [Google Scholar]
- 13.Marthey L, Sa-Cunha A, Blanc JF, et al. FOLFIRINOX for locally advanced pancreatic adenocarcinoma: results of an AGEO multicenter prospective observational cohort. Ann Surg Oncol. 2015;22(1):295–301. doi: 10.1245/s10434-014-3898-9. [DOI] [PubMed] [Google Scholar]
- 14.Faris JE, Blaszkowsky LS, McDermott S, et al. FOLFIRINOX in locally advanced pancreatic cancer: the Massachusetts General Hospital Cancer Center experience. Oncologist. 2013;18(5):543–8. doi: 10.1634/theoncologist.2012-0435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boone BA, Steve J, Krasinskas AM, et al. Outcomes with FOLFIRINOX for borderline resectable and locally unresectable pancreatic cancer. J Surg Oncol. 2013;108(4):236–41. doi: 10.1002/jso.23392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hosein PJ, Macintyre J, Kawamura C, et al. A retrospective study of neoadjuvant FOLFIRINOX in unresectable or borderline-resectable locally advanced pancreatic adenocarcinoma. BMC Cancer. 2012;12:199. doi: 10.1186/1471-2407-12-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peddi PF, Lubner S, McWilliams R, et al. Multi-institutional experience with FOLFIRINOX in pancreatic adenocarcinoma. JOP. 2012;13(5):497–501. doi: 10.6092/1590-8577/913. [DOI] [PubMed] [Google Scholar]
- 18.Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell. 2010;141(1):39–51. doi: 10.1016/j.cell.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mantovani A, Sozzani S, Locati M, Allavena P, Sica A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002;23(11):549–55. doi: 10.1016/s1471-4906(02)02302-5. [DOI] [PubMed] [Google Scholar]
- 20.Nagaraj S, Schrum AG, Cho HI, Celis E, Gabrilovich DI. Mechanism of T cell tolerance induced by myeloid-derived suppressor cells. J Immunol. 2010;184(6):3106–16. doi: 10.4049/jimmunol.0902661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bronte V, Zanovello P. Regulation of immune responses by L-arginine metabolism. Nat Rev Immunol. 2005;5(8):641–54. doi: 10.1038/nri1668. [DOI] [PubMed] [Google Scholar]
- 22.Cassier PA, Italiano A, Gomez-Roca CA, et al. CSF1R inhibition with emactuzumab in locally advanced diffuse-type tenosynovial giant cell tumours of the soft tissue: a dose-escalation and dose-expansion phase 1 study. Lancet Oncol. 2015;16(8):949–56. doi: 10.1016/S1470-2045(15)00132-1. [DOI] [PubMed] [Google Scholar]
- 23.Tap WD, Wainberg ZA, Anthony SP, et al. Structure-guided blockade of CSF1R kinase in tenosynovial giant-cell tumor. N Engl J Med. 2015;373(5):428–37. doi: 10.1056/NEJMoa1411366. [DOI] [PubMed] [Google Scholar]
- 24.Olive KP, Jacobetz MA, Davidson CJ, et al. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science. 2009;324(5933):1457–61. doi: 10.1126/science.1171362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pienta KJ, Machiels JP, Schrijvers D, et al. Phase 2 study of carlumab (CNTO 888), a human monoclonal antibody against CC-chemokine ligand 2 (CCL2), in metastatic castration-resistant prostate cancer. Invest New Drugs. 2013;31(3):760–8. doi: 10.1007/s10637-012-9869-8. [DOI] [PubMed] [Google Scholar]
- 26.Fetterly GJ, Aras U, Meholick PD, et al. Utilizing pharmacokinetics/pharmacodynamics modeling to simultaneously examine free CCL2, total CCL2 and carlumab (CNTO 888) concentration time data. J Clin Pharmacol. 2013;53(10):1020–7. doi: 10.1002/jcph.140. [DOI] [PubMed] [Google Scholar]
- 27.Bonapace L, Coissieux MM, Wyckoff J, et al. Cessation of CCL2 inhibition accelerates breast cancer metastasis by promoting angiogenesis. Nature. 2014;515(7525):130–3. doi: 10.1038/nature13862. [DOI] [PubMed] [Google Scholar]
- 28.Zitvogel L, Apetoh L, Ghiringhelli F, Kroemer G. Immunological aspects of cancer chemotherapy. Nat Rev Immunol. 2008;8(1):59–73. doi: 10.1038/nri2216. [DOI] [PubMed] [Google Scholar]
- 29.DeNardo DG, Brennan DJ, Rexhepaj E, et al. Leukocyte complexity predicts breast cancer survival and functionally regulates response to chemotherapy. Cancer Discov. 2011;1(1):54–67. doi: 10.1158/2159-8274.CD-10-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nakasone ES, Askautrud HA, Kees T, et al. Imaging tumor-stroma interactions during chemotherapy reveals contributions of the microenvironment to resistance. Cancer Cell. 2012;21(4):488–503. doi: 10.1016/j.ccr.2012.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.