Abstract
A role for red and processed meat in the development of colorectal cancer has been proposed based largely on evidence from observational studies in humans, especially in those populations consuming a westernized diet. Determination of causation specifically by red or processed meat is contingent upon identification of plausible mechanisms that lead to colorectal cancer. We conducted a systematic review of the available evidence to determine the availability of plausible mechanistic data linking red and processed meat consumption to colorectal cancer risk. Forty studies using animal models or cell cultures met specified inclusion criteria, most of which were designed to examine the role of heme iron or heterocyclic amines in relation to colon carcinogenesis. Most studies used levels of meat or meat components well in excess of those found in human diets. Although many of the experiments used semi-purified diets designed to mimic the nutrient loads in current westernized diets, most did not include potential biologically active protective compounds present in whole foods. Because of these limitations in the existing literature, there is currently insufficient evidence to confirm a mechanistic link between the intake of red meat as part of a healthy dietary pattern and colorectal cancer risk.
Impact statement
Current recommendations to reduce colon cancer include the reduction or elimination of red or processed meats. These recommendations are based on data from epidemiological studies conducted among cultures where meat consumption is elevated and consumption of fruits, vegetables, and whole grains are reduced. This review evaluated experimental data exploring the putative mechanisms whereby red or processed meats may contribute to colon cancer. Most studies used levels of meat or meat-derived compounds that were in excess of those in human diets, even in cultures where meat intake is elevated. Experiments where protective dietary compounds were used to mitigate the extreme levels of meat and meat-derived compounds showed protection against colon cancer, with some essentially negating the impact of meat in the diet. It is essential that better-designed studies be conducted that use relevant concentrations of meat or meat-derived compounds in complex diets representative of the foods consumed by humans.
Keywords: Red meat, processed meat, cancer, heme iron, heterocyclic amines, nitrates, N-nitroso compounds, Western dietary pattern
Introduction
The morbidity and mortality associated with cancer are a major health concern around the world. Human observational studies explore factors that might be involved in promoting or reducing cancer. Lifestyle factors including diet, physical activity, and smoking are associated with cancer risk1; however, epidemiologic data are not sufficient to demonstrate a cause and effect or elucidate mechanisms contributing to carcinogenesis.
Recently, a Working Group of the International Agency for Research on Cancer (IARC) concluded that sufficient epidemiologic data exist to classify processed meat as carcinogenic.2 However, they stated that uncontrollable factors contributing to “chance, bias, and confounding” reduce the evidence for carcinogenicity of unprocessed red meat, and that there was limited evidence for the carcinogenicity of red meat.2 In fact, two recent publications investigating the association between red meat and risk of various cancers found relative risk increases mostly below 40% and often less than 20% with many non-significant findings.3,4 Uncertainty around the interpretation of epidemiologic evidence in this area is increased by broad food categorizations and incomplete descriptions of specific food products.5 Inconsistent definitions of meat and variable data collection methodologies make direct data comparisons problematic.6 Problems also arise from inaccuracies in self-reporting of food intake, lack of biomarkers for meat intake and of reliable nutrient composition databases used to interpret dietary intake data.7 In contrast, the 2016 IARC Working Group concluded that the mechanistic evidence provided strong support for the carcinogenicity of red meat but the level of support was only moderate for processed meat, even though they viewed the evidence in experimental animals to be inadequate.2 Weak positive associations from epidemiologic studies, and mechanistic evidence of varying strength and consistency, further erode confidence in a causal relationship between red meat and cancer as determined by accepted causation criteria.8 As the proposed strongest evidence for a link between meat intake and cancer incidence is found for colorectal cancer (CRC), this systematic review of recent mechanistic literature will focus on this cancer site.
Materials and methods
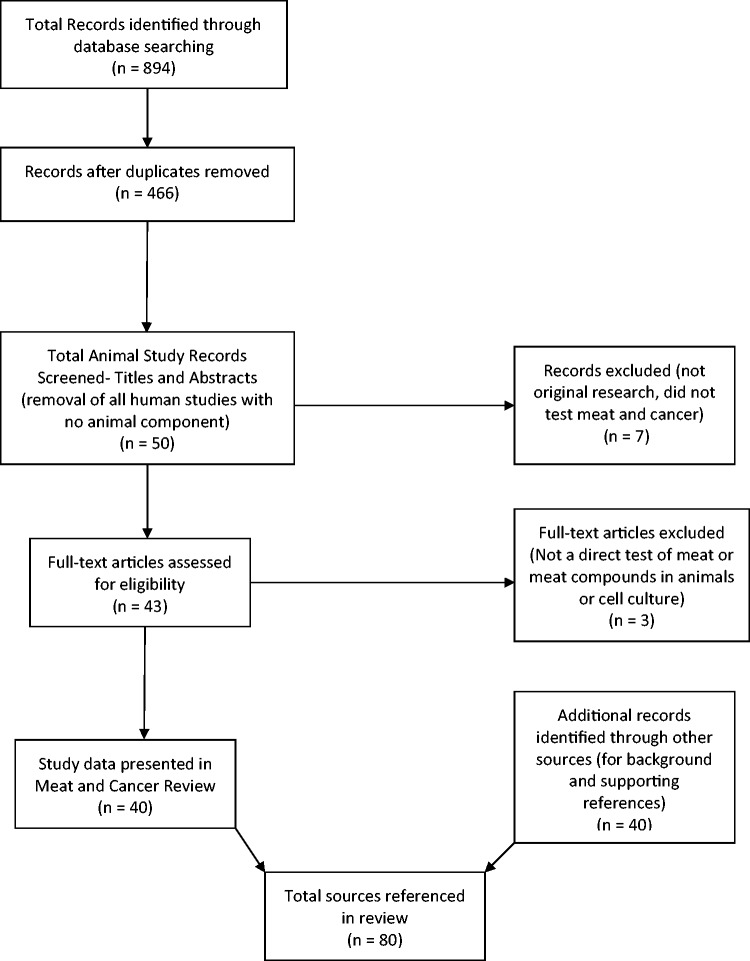
The following databases were searched: Agricola, AGRIS, Biosis, CAB Abstracts, Food Science and Technology Abstracts (FSTA), Medline, Pubmed, and Web of Science. Search terms included: meat, red meat, processed meat, or beef in combination with colon neoplasm, CRC, rectal cancer, colon tumor, and colon carcinogenesis or colon tumorigenesis. The search was limited to papers published from January 2004 through June 2016. Although using this time frame means this is not an exhaustive review of all possible publications, it provides a critical review of the recent literature. This produced 894 results, and once search results were combined and duplicates removed the remaining 466 publications was screened at the level of title and abstract to determine eligibility (Figure 1). Publications that appeared to meet inclusion criteria or for which eligibility could not be determined from the title or abstract, were obtained for full-text review. The inclusion criteria applied were: (1) animal cancer models or cell culture studies, (2) used meat or meat components as treatments, and (3) were original research papers. Exclusion criteria included: (1) review papers, editorials, book chapters, meeting abstracts, proceeding papers, or news items, (2) public health studies, (3) pharmacologic actions, (4) cattle diseases, (5) human clinical or epidemiology studies, or (6) did not use well-defined “meat” or meat components as the interventions. Bibliographies of relevant publications discovered in the searches were reviewed to determine if additional publications were available that had not been otherwise identified.
Figure 1.
Flow diagram of the selection process used to identify mechanistic studies that addressed the impact of meat or meat-derived compounds on colon cancer
Results and discussion
A total of 40 studies met our criteria to identify experimental studies examining the relationship between consumption of red or processed meat (or components) and altered colon physiology or carcinogenicity.
Heme iron
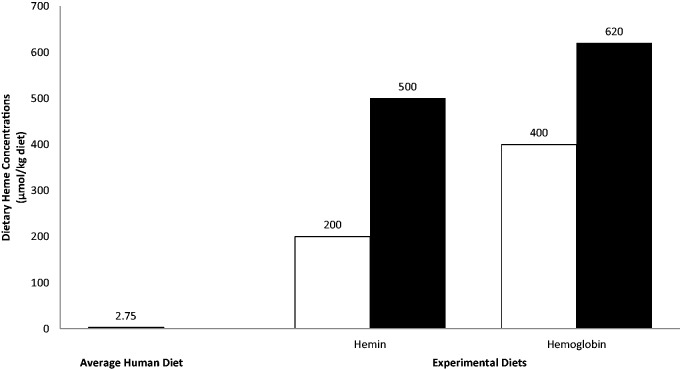
One attribute of red meat that has been studied extensively to determine its potential contribution to colon cancer development is iron. Most studies evaluate iron impacts using hemoglobin or other heme compounds as a surrogate for the heme-containing proteins in meat, such as myoglobin and cytochromes.9 The concentration of heme used in these studies is an important factor to consider in order to make conclusions regarding diet’s influence on colon cancer. Assuming an estimated meat intake of 220 g/d in the US,7 the average diet would include approximately 3.08 mg of heme, which is equivalent to 0.005 mmol of heme and 0.00275 mmol/kg of a mixed diet. Eighteen studies examining heme iron-related mechanisms met our inclusion criteria (see Table 1).
Table 1.
Studies examining heme and nitroso-compounds as a mechanism for promotion of colorectal carcinogenesis
| Study | Animals/duration | Treatment group diets | Test article | Carcinogen used | Findings | Conclusions |
|---|---|---|---|---|---|---|
| Chenni et al.10 | Fisher 344 Rats Male 4 weeks old 4 diets; n = 10/diet Fed 100 days | Control diet was AIN-76 + 2.7 g/kg dibasic calcium phosphate and 5% safflower oil; Experimental diets included Nitrite, Hb, Hb + Nitrite | Freeze dried hemoglobin (0.63% in one study and 1% in the other) | None | Hemoglobin did not alter food intake or BW; hemoglobin increased TBARS; high nitrite treatment slightly decreased TBARS; no difference in TBARS of rats fed Hb + 0.4 g/L nitrite; fecal water of heme rats was cytotoxic and adding nitrite did not alter cytotoxicity; Hb increased fecal ATNC and adding nitrite + Hb caused a 3-fold increase in ATNC levels; increase of ATNC was due to iron nitrosyl; nitrite treatment caused an increase in ATNC but not due to iron nitrosyl; urinary DHN-MA excretion was 2- and 6-times the control for 0.63% and 1% hemoglobin rats, respectively; no difference in DHN-MA excretion between HB and Hb + nitrite groups | Nitrite at levels in human saliva does not affect lipid peroxidation; sodium nitrite and hemoglobin increased ATNC levels |
| Davis et al.11 | A/J mice Female 6 weeks old Study 1: n = 11 in ANC group and n = 6 in the control group 17–19 weeks Study 2: n = 23 in the ANC group and n = 12 in the control group 38-39 weeks | Westernized, high fat semi-purified diet (with reduced calcium and vitamin D) | ANC doses ranged from 85 nmol/g diet – 3600 nmol/g | Azoxymethane (10 mg/kg or 5 mg/kg) | ANC derived from hot dog extracts increased ACF formation in the shorter study; formation of mucin-depleted foci (MDF) was not impacted by consuming ANC; no tumors developed in any mice. | ANC contributed to formation of early lesions of colon cancer but no tumors were found. |
| de Vogel et al.12 | Pathogen free Wistar Rats Male 8 weeks old 2 diets; n = 16/diet Fed 2 weeks | Purified, humanized diets (40% fat and 20 mmol calcium/kg), Control vs. Control + hemin | 0.5 mmol hemin/kg added to diet Food was given immediately before dark to prevent heme degradation | None | 10-fold increase in fecal water cytolytic activity, hemin impaired reabsorption capacity of colon epithelium, increased surface cells with a necrotic appearance, increased colonocyte proliferation with an expanded proliferation | Hemin alters epithelial cell homeostasis; hemin levels used exceed levels of meat hemoglobin in a typical human diet. |
| Gueraud et al.13 | Fisher 344 rats Female 4 weeks old 5 diets; n = 6/diet Fed 17 days | AIN-76 diet and low-calcium (0.8 g/kg) and 5% of oil (corn oil during acclimatization then hydrogenated coconut oil, safflower oil, and Menhaden fish oil for experimental diets). | Experimental diets contained either hemin (0.94 g/kg) or ferric citrate (0.36 g/kg) and had a similar content of iron (80 mg/kg). | None | MDA and DHN-MA were dependent on the dietary factors tested; 8-iso-PGF2α was affected; differences in biomarkers attributed to lipid peroxides in food or during digestion. Fecal water from rats fed hemin or fish oil diets were highly cytotoxic. | Oxidative biomarkers were dependent on the source of the lipid in the diet. Hemin promoted greater lipid oxidation when fish oil was included in the diet. |
| Ijssennagger et al.14 | C57BL/J Mice Male 8 weeks old 2 diets; n = 9/diet Fed 2 weeks | Westernized control diet (40% fat from palm oil and 30 µmol/g calcium); Control + 0.5 µmol/g heme | Heme (0.5 µmol/g) | None | Heme increased proliferation and decreased apoptosis; directly affected surface cells, but not crypt cells; caused differential expression of multiple genes. | Heme impacts proliferation through surface cell signaling to crypt cells. |
| Ijssennagger et al.15 | WT and PPARα KO mice 7–9 weeks old 4 diets; n = 6/diet Fed 14 days | WT-heme, WT-no heme, KO-heme, KO-no heme | Heme (0.5 µmol/g) | None | No effect of genetype on lipid peroxidation, cytotoxicity, or proliferation; lipid metabolism genes (including PPARα target genes) are induced by heme even in PPARα KO mice | PPARα is not involved in heme-induced proliferation; cytotoxic stress is more likely the cause of elevated proliferation in heme fed mice |
| Ijssennagger et al.16 | C57BL/J Mice Male 8 weeks old 2 diets; n = 8/diet Fed 2 weeks | Westernized control diet (40% fat from palm oil and 30 µmol/g calcium); Control + 0.5 µmol/g heme | Heme (0.5 µmol/g) | None | Heme increased cytolytic activity of fecal water, heme increased ROS, cell proliferation, and stress response genes (Hmox1, Catalase, and glutathione); heme down regulated Wif1, IL-5, Ihh, induced Ki67, cyclins, and apoptosis inhibitors; heme promoted an increase in gram negative bacteria and altered the ratio of gram negative to gram positive bacteria; heme increased Slpi and Alpi expression | These high heme levels are not representative of human diets. Suggests that heme does impact microbiota but does not appear to alter crosstalk between bacteria and host. |
| Ijssennagger et al.17 | C57BL/J Mice Male 8 weeks old 3 diets; n = 8/diet Fed 14 days | Westernized control diet (40% fat from palm oil and 30 µmol/g calcium); Control + 0.5 µmol/g heme; Control + 0.2 µmol/g heme | Heme (0.2 µmol/g or 0.5 µmol/g) | None | Heme induced proliferation, which was greater with the 0.5 µmol/g diet, but % proliferating cells/crypt were similar between the diets; Heme increased TBARS (TBARS was increased at day 2 and remained increased through day 14); increased cytotoxicity was not detected until day 7; heme increased proliferation at day 7; multiple effects on gene expression started being detected on day 4 | Oxidative stress did not impact signaling, it occurred in parallel with elevated cytotoxicity. |
| Ijssennagger et al.18 | C57BL6/J mice 8 weeks old 4 diet; n = 9/diet Fed 14 days | Control – 40% energy from fat (mainly palm-oil), low calcium (30 µmol/g) Heme – control + 0.5 µmol/g heme Control + Antibiotics –ampicillin (1 g/L), neomycin (1 g/L), and metronidazole (0.5 g/L) Heme + Antibiotics – 40 0.5 µmol/g heme + ampicillin (1 g/L), neomycin (1 g/L), and metronidazole (0.5 g/L) | 0.5 µmol/g heme | None | Heme-induced proliferation was dependent on the gut microbiota; antibiotics reduced proliferation but did not affect heme-induced luminal cytotoxicity. Antibiotics block heme-induced differential expression of oncogenes, tumor suppressors, and cell turnover genes, implying that antibiotic treatment prevented the heme dependent cytotoxicity. | The gut microbiota is involved in heme induced epithelial proliferation. |
| Martin et al.19 | Study 1: Fisher 344 rats Male 5 weeks old 2 diets; n = 16/diet Fed 14 days Study 2: Fisher 344 rats Female 5 weeks old 3 diets; n = 10/diet Fed 14 days | AIN76 diet containing dibasic calcium phosphate (3.4 g/kg) Half of each diet group received an antibiotic cocktail (4 mg/mL kanamycin, 0.35 mg/mL gentamicin, 8500 U/mL colistin, 2.15 mg/mL metronidazole, and 0.45 mg/mL vancomycin) by gavage daily for 17 days. Study 1: Control diet contains 0.036% ferric citrate; Hemoglobin Study 2: Control – 0.036% ferric citrate; Hemoglobin; Hemin | Hemoglobin and hemin groups received the same amount of heme (1.5 mmol/g diet. | None | Antibiotics reduced crypt height and proliferation; hemoglobin increased fecal TBARs, which were suppressed by the antibiotics; hemin yielded similar results in second study. | Microbiota play a role in the heme-induced proliferation and lipid peroxide formation. |
| Mirvish et al.20 | Swiss-Webster Mice Male 6–7 weeks old 18 treatment groups Fed for 7 days | Control (commercial diet); hot dog diet; TD-94045 purified diet with additives (hemin, Na ascorbate, ellagic acid, a-tocopherol, omeprazole); NaNO2 in the drinking water | Hemin (125 mg/kg diet or 1 g/kg diet); hot dog diet (180 g/kg diet) 18% by weight of hot dogs (fresh weight) mixed into the TD-01407 diet | None | Nitrite increased fecal N-nitroso-compounds (ANC); hemin + nitrite increased ANC even more than nitrite alone; omeprazole + ascorbate decreased ANC levels; nitrosothiols made up 13-24% of ANC in the feces of mice | Fecal (colonic) ANC are probably derived from the combination of dietary ANC and those generated digestion, and generation of ANC is likely catalyzed by acid and hemin. |
| Pierre et al.21 | Fisher 344 Rats Female 4 weeks old 4 diets; n = 5/diet Fed 100 days (long term); Fed 15 days (short term) | AIN-76 diet with varying concentrations of heme by the addition of 60% meat (chicken, beef or blood sausage) to the diet; balanced for nutrients (except blood sausage diet which could not be balanced for iron) | Beef, chicken, or blood sausage | Long term study: Azoxymethane (20 mg/kg) | Long term: blood sausage increased DHN-MA urinary output 73-fold, beef increased DHN-MA urinary output by 4.6-fold, chicken diet did not increase DHN-MA; beef and blood sausage increased MDF compared to the control diet, especially with blood sausage; Short term: DHN-MA excretion increased with blood sausage. | Blood sausage increased urinary DHN-MA relative to beef and control diets; MDF development ws associated with DHN-MA levels |
| Pierre et al.22 (see Table 3) | ||||||
| Pierre et al.23 | Fisher 344 Rats Female 4 weeks old Study 1: 5 diets; n = 5/diet; Study 2: 2 diets; n = 10/diet Study 1: Fed 14 days; Study 2: Fed 100 days | Study 1: control low salt, hemin high salt, hemoglobin high salt, control high salt, ham high salt Study 2: control and ham | AIN 76 low calcium diets with ham (55% of total diet, cured, cooked, freeze dried, = 0.25 µmol heme and 0.36 µmol heme (short vs long studies), heme and hemoglobin diets = 0.25 µmol heme | Study 2: 1,2 dimethylhydrazine (190 mg/kg) | Ham increased number of MDF and ACF; long term ham diet increased lipid peroxidation and cytotoxicity of fecal water; ham, heme and hemoglobin all increased lipid peroxidation in the short term study but the effect was smaller for hemoglobin; fecal water was not cytotoxic for hemoglobin fed rats; ham and hemin increased urinary DHN-MA excretion, which did not occur with hemoglobin | Ham and hemin produce similar short term effects that were not observed in the hemoglobin group, therefore these two may be more cytotoxic than hemoglobin found in fresh red meat. |
| Santarelli et al.24 | Fisher 344 Rats Female 4 weeks old Study 1: 90 rats in 17 groups (n = 10 control, or = 5 experimental diets); Study 2: 50 rats in 5 groups (n = 10/group) | Control- 10 g fat/100 g diet = same protein, fat, iron contents as meat diets. Study 1: 16 models of cured pork; high vs low heme (dark or light meat), cooked meat vs raw meat, nitrite added vs none added, air exposed packaging vs anaerobic packaging (crossed the 4 factors in a 2 × 2 × 2 × 2 design) Study 2: dark, cooked, oxidized meat; dark, cooked, nitrite, oxidized; dark, cooked, nitrite, anaerobic; dark, raw, anaerobic; control (15 g lipids/100 g diet and 40 g casein/100 g diet) | Dark meat pork (15-17 mg heme/100 g); Light meat (0.36-2 mg heme/100 g); Cooked meat (heated to 70℃); Raw meat (heated to 50℃); Nitrite (cured with NaCl = 0.6 g sodium nitrite/100 g salt); Anaerobic meat (packaged under vacuum immediately after processing) | Study 2: 1,2 dimethylhydrazine (180 mg/kg) | Study 1: fecal water from processed meat fed rats contained 2-5 times more lipid peroxidation products than controls; dark meat, cooking, and aerobic storage increased TBARS; nitrite addition decreased TBARS; DHN-MA urinary excretion was higher with processed meats; the factors increased TBARS, cooking temp and nitrite increased cytotoxicity; cooking temp affected urinary DHN-MA; Color of meat/heme level and cooking temperature modified pH, added nitrite modified nitrosyl heme concentration, all factors except oxidation influenced pro-oxidant activity, added nitrite modified hexanal concentration. Study 2: Processed meat diets increased number of ACF compared to control diets; advanced ACF were noted in rats fed oxidized diets; dark cooked meat with nitrite, oxidized fed rats had more MDFs compared to the control and compared to the dark cooked meat nitrite, anaerobic and the dark cooked meat, no nitrite, oxidized fed rats; dark cooked meat with nitrite, oxidized fed rats had more heme and more ATNC in the fecal water but less lipid oxidation products and lower cytotoxicity | Processed meats with heme, nitrite, cooked to 70℃ and oxidized increase the number of preneoplastic lesions. |
| Sødring et al.25 | A/JMin/+mice Male and female 3 weeks old 4 diets; n = 19-21/diet Fed 56 days | AIN-93M, with reduced calcium (15 µmol/g), no added vitamin D, and soy protein instead of casein. Hemin (model of red meat – 0.5 µmol/g iron and 2.8 µmol/g sodium) Hemin + Nitrite (model of processed meat – 2.8 µmol/g sodium nitrite, 0.5 µmol/g iron) Nitrite – 2.8 µmol/g sodium nitrite, 0.5 µmol/g iron Control – 0.5 µmol/g iron and 2.8 µmol/g sodium | Hemin and the hemin + nitrite diets contained 0.5 µmol/g hemin, a ferric form of heme iron with a chloride ligand. | None | Hemin decreased the number of colonic lesions in the A/JMin/+ mouse, but increased tumors in the small intestine; nitrite did not have an effect in the colon but suppressed tumor growth in the small intestine. | Hemin reduced colon tumors, whereas nitrite had no effects in the colon but decreased small intestinal tumors. |
| Van Hecke et al.26 | In vitro digestion model | Uncured and nitrite-cured pork; Raw, cooked (65℃, 15 min), overcooked (90℃, 30 min) | Raw, cooked or overcooked pork | None | Nitrite served as an antioxidant during digestion except when meat was overcooked; intense cooking elevated NOC-specific DNA adducts in the colonic digesta and this was affected by the source of fecal innoculum used | Overcooking leads to elevated levels of DNA adducts in colonic digesta. |
| Zhou et al.27 | A/J mice and CF-1 mice Female 4–5 weeks old 4 diets; n = 8-20/diet Fed 21–25 weeks | Study 1: AIN93G diet and 0.5 or 1.0 g NaNO2/L drinking water, or with 1 g NaNO2/L water plus 250 mg hemin/kg diet Study 2: Control diet or 1.0, 1.25, or 1.5 g NaNO2/L drinking water Study 3: hot dogs manufactured with the addition of NaNO2 (18% by weight) | Study 3: hot dogs | Study 3: Azoxymethane | Study 1: no differences in ACF among mice fed 0, 0.5, or 1.0 g NaNO2/L water; Study 2: Non-linear ACF response to NaNO2 levels; Study 3: hot dogs (18% of diet), inhibited ACF formation | Study does not support a role of NaNO2 in an increased risk for of developing early colon cancer lesions. |
ABC: colonic abberant crypts; ACF: aberrant crypt foci; AIN: American Institute of Nutrition; AOM: azoxymethane; ANOVA: Analysis of variance; Apc min: adenomatous polyposis coli gene minus; ATNC: apparent total nitrso compound; DHN-MA: 1,4-Dihydroxynonane Mercapturic Acid; Hb:hemoglobin; HO-1:;Heme oxygenase 1; HSP-25: heat shock protein 25; IU: international unit; KO:knock out; mRNA: messenger RNA; MDA: Malondialdehyde; NaNO2:sodium nitrite; PGF2alpha: prostaglandin F2; TBARS: thiobarbituric acid-reactive substances; WT:wild type.
Three studies evaluated the impact of heme iron on colon physiology. In each study, rodents were provided a Westernized diet with or without 0.5 mmol of heme/kg diet for 14 days. de Vogel et al.12 found a 10-fold increase in the concentration of sodium and 4-fold increase in potassium in the feces, suggesting hemin reduced epithelial absorptive capacity. Fecal water-soluble components derived from hemin fed rats led to higher lysis of erythrocytes, suggesting hemin promoted a cytotoxic environment. The resulting epithelial surface injury induced by hemin or its metabolites led to an increase in cell proliferation and a reduction in apoptosis to maintain epithelial barrier function. Ijssennagger et al.14–18 conducted several experiments to explore the impact of heme iron on colon physiology. In the first of their studies reviewed here, they determined that heme elevated proliferation and reduced apoptosis in colonocytes, while also selectively elevating expression of genes involved in mediating oxidative stress and heme metabolism in the luminal surface cells but not in the cells located within the crypts.14 Expression of some genes involved in promoting cell proliferation was elevated and expression of some genes involved in the inhibition of proliferation was decreased in cells lining the crypts in mice consuming heme diets. The data suggest heme altered surface epithelial cells through oxidative stress mechanisms, and the signals produced by these cells may have been transmitted to those in crypts, contributing to increased proliferation and reduced apoptosis. In another experiment, Ijssennagger et al.15 explored heme’s effect on hyperproliferation and PPARα-regulated gene expression in wild type and PPARα knock-out mice. PPARα is a non-selective nuclear hormone receptor that binds fatty acids (including oxidized fatty acids), and impacts expression of genes involved in responding to oxidative stress created by a variety of inputs, including lipid peroxides. Differential PPARα target gene expression was detected in luminal surface cells, but not in cells lining the crypts. They observed increased cytotoxicity of fecal water in erythrocytes, and demonstrated an increase in fecal TBARS, which reflected an elevation in lipid peroxidation products. PPARα knock-out and wild-type mice showed similar responses to heme, suggesting that the impact of heme consumption is not mediated through PPARα. However, it is possible that the effects of this signaling molecule may be mediated through lipid peroxidation products, because the expression patterns of lipid-metabolism related genes are similar in the knock-out and wild-type mice consuming heme. This experiment also demonstrated that hyperproliferation was not the result of an altered antioxidant response in the knock-out mice, suggesting oxidative stress, per se, is not responsible for colon epithelial hyperplasia, but that it may be due to cytotoxic stress.
Ijssennagger et al.17 also evaluated responses of the colon to different doses of heme (0.2 or 0.5 mmol heme/kg diet for 14 days) and lengths of exposure (0.2 mmol heme/kg diet for 0, 2, 4, 7 or 14 days) when fed to mice. Both heme levels induced similar changes in gene expression and increased proliferation of colonocytes, but the proliferation increase was greater for the 0.5 mmol/kg level. The 0.2 mmol/kg heme time course study resulted in increased fecal TBAR levels, which occurred by day 2. Fecal water cytotoxicity only became different from control values on days 7 and 14, which is when colonocyte proliferation was elevated above controls. These results suggest that oxidative stress is an acute response to heme, whereas cytotoxicity and hyperproliferation are delayed effects. Altered expression of genes involved in lipid metabolism occurred by day 2, whereas those showing changes at day 4 were involved in proliferation and other cancer/neoplasia pathways. Changes in heme-sensing gene expression did not occur until after day 4. These data suggest an acute and a longer term response to heme, and that responses to heme result from cytotoxic stress, as opposed to oxidative stress.
A study by Gueraud et al.13 evaluated the impact of hemin (0.94 g/kg) or ferric citrate (0.36 g/kg) in diets (low calcium levels, 0.8 g/kg, compared to the recommended amount of 5 g/kg) containing 5% oil from corn, hydrogenated coconut oil, safflower oil, or fish oil on lipid oxidation in rats. A parallel study evaluated cytotoxicity of fecal water from these animals on mouse colon cells. Malondialdehyde (MDA, a lipid oxidation product) and a urinary metabolite of 4-hydroxynonenal (4-HNE) were elevated when hemin was included in the fish oil diet, whereas the combination of safflower oil with hemin only elevated urinary 4-HNE metabolites. Hemin and hydrogenated coconut oil did not affect lipid oxidation products. These data suggest that hemin is a stronger oxidative catalyst than ferric citrate, and that hemin in combination with polyunsaturated fatty acid-rich lipids, such as fish oil, produces a greater increase in lipid oxidation compared to hemin in combination with other lipid sources high in monounsaturated or saturated fatty acids.
Pierre et al.21 investigated urinary excretion of 1, 4-dihydroxynonane mercapturic acid (DHN-MA), a metabolite of the lipid peroxidation product 4-HNE, in Fisher 344 rats. Chicken, beef or blood sausage was included in the diet (low calcium, 2.7 g/kg dibasic calcium phosphate) of Fischer 344 rats treated with azoxymethane (AOM, a colon-specific carcinogen). DHN-MA excretion increased in rats fed blood sausage diets compared to all other diets, and excretion corresponded to the number of preneoplastic lesions in AOM-treated rats. Urinary 8-iso-PGF2A was moderately increased in rats fed a high heme diet. In general, urinary excretion of DHN-MA is an indicator of a normal detoxification pathway, and without other comparators it is not possible to determine whether this level of excretion is associated with CRC risk. In fact, if this compound was present in the urine as the result of iron induced oxidation, the relationship would be with the whole body status of iron, not the colon luminal content. It is well established that the type/source of iron in the diet, as well as other nutrients, significantly impacts iron bioavailability and status. Therefore, iron status measured in both human and animal studies are needed in order to understand the relationships between urinary excretion of any compound and dietary heme iron. This study did not (nor did any of the animal studies in this review) report the iron status of the animals. Of most concern in the present study is the use of blood sausage as a source of heme iron, and its use to represent meat in the experimental diets. As described in a subsequent section of this review, the amount of heme iron from hemoglobin, as well as total iron, is dramatically higher in the blood sausage diet than in other foods. The composition of blood sausage is also drastically different with regard to a number of other nutrients, making it a poor experimental model for red meat in general. In this study, the reported iron content of animal diets containing blood sausage is more than 6-fold greater than the control diet.
Pierre et al.22 utilized a Fisher 344 rat model along with a colon carcinogen in an initiation-promotion protocol. Rats were fed a modified AIN-76 diet that included 60% red meat (a level which far exceeds typical human intake) as a heme source with supplements including calcium, olive oil, or antioxidants. Aberrant crypt foci (ACF) and mucin-depleted foci (MDF), putative tumor biomarkers detectable at early stages of colon cancer,28 as well as urinary DHN-MA were determined. Fecal water TBARS was quantitated and cytotoxicity determined in mouse tumor cells. Cytotoxicity, fecal water TBARS and urinary DHN-MA were increased by consumption of diets containing 60% red meat. The high beef diet increased ACF and MDF compared to the control diet, an effect inhibited by the addition of dietary calcium. Calcium also normalized fecal TBARS and fecal water cytotoxicity, but it did not reduce urinary DHN-MA levels. Unexpectedly, rats fed the high-calcium control diet had more ACF and MDF compared to those fed the low-calcium control diet. Supplementation with antioxidants or olive oil failed to normalize ACF and MDF in the high meat diet group. The disparate effects of calcium, in addition to the lack of effect from antioxidant/olive supplementation, bring into question the role of oxidative stress caused by the extreme level of meat used in the diets in this study. The authors used a carcinogen in all animals, and therefore no comparisons to healthy control animals were possible.
Ijssennagger et al.16 explored the impact of heme on colon microbiota and host epithelial cell physiology. Mice were provided a Westernized diet with 0.5 mmol of heme/kg for 14 days. Microbial characterizations were performed using a microarray approach, instead of using sequencing procedures that are the current norm. The authors discovered no changes in the density of microbiota, yet there was a change in the ratio of Gram-negative to Gram-positive bacteria from 0.7 in the control mice to a ratio of 2.2 in the heme fed mice. Patterns of gene expression from the microbiota suggest the mice consuming heme had an increased capacity to reduce nitrates in mice consuming heme, which may lead to elevated levels of nitroso compounds (NOC) being produced in the colons of these mice through a process that is dependent upon the microbial metabolism. They followed up this study using a similar experimental design but with the inclusion of broad-spectrum antibiotics in order to confirm that the response to heme is dependent on colonic microbiota.18 They found that antibiotic treatment eliminated the heme-induced hyperproliferation of colonocytes as well as the differential expression of oncogenes and tumor suppressor genes. They concluded from these observations that the colon microbiota was required for heme-induced hyperproliferation and hyperplasia.
The importance of bacteria to hemoglobin-promoted colon cancer was also explored by Martin et al.19 in a series of studies. In one study, rats were given a control (0.036% ferric citrate) or hemoglobin diet (1.5 µmol of heme/g); all diets contained low levels of calcium and approximately 0.136 µmol of iron/g. Half of the rats received an antibiotic cocktail to minimize microbial populations. In a second study, the experimental diet contained hemin (1.5 µmol of heme/g diet). Hemoglobin and hemin both increased fecal TBARS, which were suppressed by antibiotics. The reduction in oxidation products caused by antibiotics indicates colon microbiota would be responsible for lipid peroxides induced by free heme iron. Heme iron provided by hemoglobin did not increase colonocyte proliferation. The authors suggest free heme iron may promote cell proliferation, and that myoglobin in meat would likely produce a response similar to that obtained with hemoglobin in this study.
N-NOCs and their interactions with heme
Pierre et al.23 used rats to evaluate the impact of ham (100 day study), and attributes of ham (salt, nitrite, hemoglobin, 2 week study) on the formation of ACF. Heme intake for rats consuming the ham, hemoglobin, and hemin diets in the short-term study was similar. The ham (cured, cooked and freeze dried) resulted in more lesions than occurred in the control rats. Ham, as well as hemin produced elevated lipid peroxidation products in the colon and greater fecal water cytotoxicity. Although hemoglobin also increased lipid peroxides, it was much lower than with ham and hemin, and fecal water from animals consuming hemoglobin was not cytotoxic. Ham and hemin diets also increased the urinary levels of a lipid peroxide metabolite relative to the control, but hemoglobin did not affect this urinary metabolite.
Santarelli et al.24 used the same experimental endpoints (2 week or 100 day) to evaluate the impact of pork (light or dark cuts) that were cooked or raw, with or without added nitrite, and stored aerobically or oxidized by air exposure prior to feeding. Both cooking and added nitrite increased fecal water cytotoxicity. Dark cuts, cooking, nitrite, and oxidation increased lipid peroxides in fecal water; however, only cooking elevated urinary lipid peroxide metabolites. Oxidized, nitrite-containing cooked meat prepared from dark cuts produced more ACF. This combination of factors also produced by far the greatest level of N-NOC and heme in the feces, but did not significantly alter fecal water cytotoxicity. The combination of experimental factors used in this experiment replicates the situation of a cooked ham kept in the refrigerator prior to being consumed. Therefore, the type of packaging and storage conditions may be an important contributor to the proposed stimulation of colon cancer by processed meats.
Van Hecke et al.26 used an in vitro digestion model to simulate digestion in the mouth, stomach, duodenum and colon. They prepared uncured and nitrite-cured pork, which was used to measure the level of oxidized lipids and proteins prior to and after digestion of raw, cooked or overcooked samples. Uncured, cooked, and overcooked samples contained elevated levels of MDA and 4-HNE, compared to the uncured raw pork prior to digestion. Lipid oxidation products were reduced in nitrite containing samples, and this was not affected by cooking. Following duodenal digestion, uncured overcooked samples contained elevated MDA, indicating that cooking increased lipid aldehydes. The overcooked nitrite-cured product had elevated lipid aldehydes relative to the raw or cooked nitrite-cured product. After colon digestion, all aldehydes (except MDA and heptanal) were lower than was present in the duodenal digesta. More intense heating led to greater production of protein oxidation products before and after digestion. Nitrite decreased these products before digestion, but protein oxidation products were elevated in nitrite cured, overcooked duodenal digesta, but not in the colon digesta.
The A/J Min/+ mouse is a routinely used model to evaluate the influence of diet on colon carcinogenesis. These mice are heterozygous for the loss of APC, a tumor suppressor that typically develops an inactivating mutation in human colon cancers. Most tumors of APC mutant models develop in the small intestine, and thus it does not completely represent the dynamics associated with the mixture of diet and microbiota present in the colon. Sødring et al.25 used this model to evaluate the role of dietary hemin (0.5 µmol/g diet) alone or in combination with nitrite (2.8 µmol/g diet of sodium nitrite) on tumor development. This experiment used a basal diet containing low levels of calcium, vitamin D and fat, which was fed for eight weeks. Diets containing only hemoglobin were a model of unprocessed red meat and diets containing both hemin and nitrite served as a model of processed meat. Hemin reduced the number of ACF and tended to reduce the number and size of tumors in the colon, but nitrite did not affect colon tumorigenesis. Tumor number and load in the small intestine did not differ among the diets, but tumor size in the small intestine was reduced by dietary nitrite. The authors suggest nitrite may have mitigated tumorigenesis in the small intestine and that hemin does not promote colon tumor development in the context of a lower fat diet.
Mirvish et al.20 used mice to determine the levels of N-NOCs in the feces after being treated with nitrite, nitrate or hemin alone or in combination with antioxidant molecules, or in the form of hotdogs. The level of N-NOCs reached a maximum concentration of about 65 nmol/g feces when sodium nitrite (NaNO2) was provided alone (2 g/L water), and was elevated above the control values with the 1 g/L level (∼20 nmol/g feces). When 2 g/L of NaNO2 was combined with hemin (250 mg/kg diet), the fecal levels were in excess of 150 nmol/g, suggesting that combinations of compounds present in processed meats potentiate formation of these potential carcinogens. Zhou et al.27 also tested the colon responses to exposure to various levels of NaNO2 delivered in either drinking water or nitrite present in hot dogs. In the first experiment, they found no changes in ACF formation in AJ mice with 0.5 or 1.0 g/L of NaNO2, with or without hemin. In a second experiment where NaNO2 was included at 1.0, 1.25 or 1.5 g/L, there was a tendency for a dose-dependent development of ACF in CF-1 mice. However, there was no difference in ACF formation between the 1.5 g/L treatment and the control group. In their final experiment, diets for CF-1 mice were formulated to contain 18% hot dogs, which resulted in a tendency for ACF formation to be reduced by the hot dog diet relative to the control diet. A similar experiment by Davis et al.11 determined the impact of N-NOCs isolated from hot dogs on induction of ACF in mice after 8 or 17–34 weeks. They found increased ACF in one experiment, but no appreciable change in numbers of these lesions in a second experiment.
Chenni et al.10 used rats to determine the impact of consuming hemoglobin (6.3 or 10.0 g/kg of diet) in a relatively low fat diet (5%) on fecal water cytotoxicity and whether the level of fecal NOC compounds was altered by the presence of nitrite during a 100-day study. The study used nitrite levels that are more than 10–100 times higher than the levels found in human saliva/stomach but much lower levels of fat than are found in typical human diets.10 Nitrite alone had no effect on cytotoxicity, but did elevate the fecal level of non-iron containing NOCs. Adding hemoglobin to the diet elevated cytotoxicity and resulted in a minor increase in fecal NOCs. When hemoglobin was combined with nitrite, there was a small increase in cytotoxicity and an increase in fecal NOCs (although not to the same extent as with the nitrite alone). The NOCs produced with the combined treatment were primarily iron containing, in contrast to those produced with nitrite alone. The increases in NOCs observed were not very large, and based on comparison to other studies to determine the levels needed to promote cancer, these reported changes would not be sufficient to promote disease development.29 Importantly, iron-NOC complexes are less likely to promote cancer, suggesting that endogenous nitrite normally found in human saliva derived from nitrates found in the diet (including diets rich in vegetables) may help protect against an increase in cancer risk associated with meats containing hemoglobin.10 These authors also demonstrated that very low calcium levels (as most of the referenced studies have used) allow NOC formation to occur, but when elevated calcium levels are used, NOC levels are greatly reduced. Nitrite did not impact the level of lipid peroxides detected in the urine, which were elevated by hemoglobin. Data from this work demonstrate the importance of considering the effects of other dietary components when attempting to determine the effect of meat consumption on gut health.
Cooking effects on pro-mutagenic compounds
As mentioned previously, processing (preservation or cooking) can incorporate or develop mutagens and carcinogens in meat which have been shown to enhance carcinogenesis.30 However, not all studies evaluating the impact of cooking practices have found a large change in risk associated with those processes.31 The classes of compounds formed during high-temperature or open-flame cooking include heterocyclic amines (HCAs) and polycyclic aromatic hydrocarbons (PAHs). The HCA in meat includes 2-amino-3-dimethylimidazo [4,5-f]quinoxaline (IQ), 2-amino-3,8-dimethylimidazo [4,5-f]quinoxaline (MeIQx), 2-amino-3,4,8-trimethylimidazo [4,5-f]quinoxaline (DiMeIQx), and 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP). The most prominent PAH in meat is benzo(a)pyrene (BaP). Activation of these pro-carcinogens is initiated by cytochrome P450 enzymes followed by further conversions in several metabolic pathways. The level and activity of these enzymes are influenced by a multitude of compounds in our diets, including the beneficial dietary bioactives. Estimations of the normal levels of PhIP consumption vary with some reporting a range of 0.1–13.8 µg/day32 and others reporting an estimate of 72 ng/day.33 These levels are much lower than those used in studies to understand the mechanisms, whereby PhIP serves as a carcinogen. In the colon, studies have focused on DNA adduct formation and eventual mutations, and on the regulation of colon cell homeostasis. Eight studies met our inclusion criteria for mechanistic investigation of HCAs related to red and processed meat and CRC (Table 2). No experimental studies using BaP were identified.
Table 2.
Studies examining dietary HCAs as a mechanism for promotion of colorectal carcinogenesis
| Study | animal/cells/treatment | Treatment group diets | Meat/meat-related compound | Carcinogen used | Findings | Conclusions |
|---|---|---|---|---|---|---|
| Bastide et al.34 | F344 Rats; C57BL/6J Apc −/+ mice; Apc +/+ mice 4 weeks old 4 diets; n = 20/diet (rats) 2 diets; n = 10-15/diet (mice) Fed 100 days (rats); 49 days (APC mice); 14 days (C57BL/6J mice) | AIN76 control diet; Rats – 1% hemoglobin, HCA (PhIP, 50 mg/kg; MeIQx, 25 mg/kg), or both with sodium nitrate and nitrite (0.17 g/L of NaNO2 and 0.23 g/L of NaNO3) added to drinking water vs. nitrate free drinking water; Mice - control diet or a 2.5% hemoglobin diet | PhIP; MeIQx; hemoglobin | Azoxymethane | Diets with hemoglobin rather than HCA or nitrates/nitrites significantly increased MDF per colon in rats. Nitrates/nitrites increased ATNC but heme-based diets resulted in a decrease in ATNC. Hemoglobin diets (2.5%) increased the small intestinal tumor load in mice. No effects of hemoglobin in the colon. Heme diets increased TBARs. No neoplasia was induced in normal Apc+/+ mice by hemoglobin. Hemoglobin only induced more AB in Apc+/+ mice in epithelium, but it was not clear if AB index was correlated with TBARs. | Hemoglobin induced lipid peroxides and led to more early lesions of colon cancer in rats, but had no affect on colon tumors in APC mice; exposures to both heme and HCAs far exceed that expected in a typical human diet |
| Cheung et al.35 | Humanized (hCYP1A) and C57BL/6J wild type mice Male and Female 5–8 weeks old Gavage single dose PhIP; with or without DSS in drinking water to induce inflammation | AIN-93M control diet PhIP (100 or 200 mg/kg BW) (oral gavage); 1 or 1.5% DSS in drinking water | PhIP | None | All hPYP1A mice treated with 200 mg/kg PhIP + 1.5% DSS had colon tumors at 12–21 weeks; 87% of tumors at 6–10 weeks were adenocarcinomas; colon tumors were not observed in mice treated with PhIP only; 100 mg/kg PhIP + 1% DSS had low tumor occurrence but ACF were observed beginning at 6 weeks of treatment; overexpression of B-catenin, c-Myc, cyclin D1, iNOS, and COX2 in hCYP1A tumor samples; strong nuclear localization of B-catenin | Single dose PhIP treatment of 200 mg/kg followed by 1 week of 1.5% DSS treatment in hCYP1A mice was most effective in induction of colonic adenomas and adenocarcinomas with optimal time point of tumor formation at 6–10 weeks |
| Kuhnel et al.36 | Fisher 344 Rats Male 6 weeks old 3 diets; n = 40/diet Fed 10 months | Standard Chow (control); control + 0.1 ppm PhIP; control + 100 ppm PhIP | PhIP | None | No signs of active or chronic inflammation in the colon; no difference in T-lymphocytes; no difference in hyperplastic and dysplastic crypts in control vs PhIP | PhIP does not induce inflammation at physiologically relevant concentrations; this concentration is not sufficient to initiate colon carcinogenesis; HCAs alone are probably not the factor that contributes most to the epidemiological association between red meat and colon cancer |
| Nicken et al.37 | Fisher 344 Rats Male 8–10 weeks old | PhIP (10 umol/L) included in Ussing chamber media | Serosa-mucosa transport was significantly higher compared to mucosa-serosa transport resulting in a net secretion of PhIP; no significant difference in transport genes | PhIP is actively (opposed to passively) secreted into the lumen of the colon; human consumption of HCAs are low and their bioavailability is also low | ||
| Nowak et al.38 | Probiotic bacteria Feces from children, adults, elderly | PhIP (0.25%) or IQ (0.1%) | Cytotoxicity was greatest for fecal water from elderly; addition of PhIP or IQ did not alter cytotoxicity; probiotics reduced cytotoxicity; fecal samples contained no detectable PhIP or IQ | Aging changes the cytotoxic nature of feces and probiotics are able to reduce cytotoxicity. These HCAs were not detectable in feces and had no effect on cytotoxicity. | ||
| Wang et al.39 | Fisher 344 Rats Male 4–5 weeks old Gavaged 14 weeks (2 weeks PhIP, 4 HF, 2 PhIP, 4 HF, 2 PhIP) | Vehicle control; oral gavage of PhIP | PhIP (50 mg/kg BW) daily, which is about 400 ppm PhIP in the diet | None | Proliferation increased in the colon of PhIP treated rats compared to the controls; apoptotic cells were distributed throughout the crypt in PhIP rats, whereas controls had apoptotic cells primarily in the luminal region; PhIP increased the nuclear distribution of B-catenin relative to the controls; PhIP increased Ctnnb1 (30%) and c-myc (65%) mRNA and protein expression; there was no change in mRNA or protein levels for cyclin D1 or c-jun | This level of PhIP exposure alters colonocyte proliferation and apoptosis, which may occur in part because of changes in genes/proteins involved in regulating these processes. |
| Wang et al.40 | Fisher 344 Rats Male 3-4 weeks old Fed 52 weeks | 3 cycles of PhIP/High fat diet cycles (2 weeks of PhIP treatment on a low-fat diet followed by 4 weeks on the HF diet) after 3 cycles rats were swtiched to standard diet for remainder of study | PhIP | None | Nox/Duox mRNA highly expressed in tumors compared to adjacent normal tissue (Nox1, Nox4, Doux2); Nox1 and Nox4 proteins elevated 2-3 fold in tumors vs adjacent normal tissue; increased protein expression of NFκB-p50 and NFκB-p65; increased IL1B, IL6, TNFα, TNFR1 mRNA | PhIP induced colon tumors show increased Nox/Duox expression and NFκB activation |
AB:anaphase bridge; ACF: aberrant crypt foci; AIN: American Institute of Nutrition; AOM: azoxymethane; Apc min: adenomatous polyposis coli gene minus; AXRF: arabinoxylan-rich fraction; ATNC: apparent total nitrso compound; BNF: β-naphthoflavone; BrdU: Bromodeoxyuridine; COX2: cyclooxygenase 2; CYP1A1: Cytochrome P450, family 1, subfamily A, polypeptide 1; DSS: dextran sodium sulfate; DHN-MA: 1,4-Dihydroxynonane Mercapturic Acid; Duox: dual oxidase 2; HCA:heterocyclic amines; HPLC:high pressure liquid chromatography; IFG-1: insulin-like growth factor 1; IL-6:interlukin 6; ILB1:interlukin B1; IP: intraperitoneal; IQ: 2-Amino-3methylimidazo[4,5-f]quinolone; mRNA: messenger RNA; MDA: Malondialdehyde; MeIQx: 2-Amino-3,8-dimethylimidazo[4,5-f]quinoxaline; SCFAs: short chain fatty acids; Mptx: Mucosal pentraxin; MMP-2: matrix metalloproteinase; NaNO2:sodium nitrite; NaNO3: sodium nitrate; NFkB: nuclear factor kappa-light-chain-enhancer of activated B cells; Nox: NADPH oxidase; PhIP: 2-Amino-1-methyl-6-phenylimiazo[4,5-b]pyridine; SSB: single strand breaks; TBARS: thiobarbituric acid-reactive substances; TIMP-2: tissue inhibitor of matrix metalloproteinase; TNFα: tumor necrosis factor; TNFR1: tumor necrosis factor receptor 1; TXB2: Thromboxane B2.
Wang et al.39 determined the response to intermittent exposures to PhIP (50 mg/kg body weight by oral gavage, ∼400 ppm in the diet) or a high fat diet (30% by weight) on colon epithelial cells shortly after PhIP exposure to evaluate their role in early carcinogenesis. The authors treated rats with PhIP for two weeks, followed by four weeks of a high fat diet, which was repeated twice. Rats were terminated 24 h after the third PhIP treatment period. It is not clear why the authors used this protocol, as it seems that either intermittent exposure to PhIP in a fairly uniform high fat diet or having PhIP exposure during the high fat periods would better reflect typical human dietary patterns. PhIP elevated proliferation throughout the colon, but it induced apoptosis within the crypt while reducing it on the luminal surface. Apoptosis normally occurs in the top part of the crypt and on the luminal surface, so this carcinogen reversed the normal pattern. This response has been demonstrated with other carcinogens that stimulate apoptotic removal of DNA damaged cells, which occurs throughout the crypt, including at the base of the crypt where the adult colon stem cells reside.41–43 Wang et al.40 followed up this experiment using the same design in which they discovered that expression of select genes (e.g., NADPH oxidase, Nox) is elevated. They also noted that downregulation of Nox expression reduced cell cycle activity and allowed apoptosis to occur. These results demonstrate that observations from the meat/hemoglobin studies indicating proliferation is promoted and apoptosis is inhibited may similarly be induced by other components formed in cooked meats. However, it should be noted that the level of PhIP used in these studies is extremely high relative to normal levels of exposure (below 0.1 ppm in the diet).
Kuhnel et al.36 determined whether exposure to 0.1 or 100 ppm PhIP for 10 months induced tumor formation and colon inflammation in rats. Colon lesions were found in 30% of rats at 6 months (not significant) and 60% of rats at 10 months with the consumption of 100 ppm PhIP, yet at 0.1 ppm, PhIP did not significantly increase colon lesions at either 6 or 10 months. Colon inflammation was not found in rats consuming either diet. These data suggest that human diet relevant exposures to PhIP do not appear to create an excessive risk for transformations capable of causing tumors within the 10-month time frame used in this study.
Bastide et al.34 evaluated the impact of PhIP (50 µg/kg diet) and MeIQx (25 µg/kg diet), heme iron (1% of diet), and NaNO2/NaNO3 (0.17/0.23 g/L water) on various outcomes that might contribute to colon carcinogenesis. Heme iron increased early colon lesions induced by AOM injection; however, the mixture of HCA and NOCs induced by nitrite and nitrate in the water did not. In contrast, heme did not affect colon tumor formation, even though small intestinal tumors were increased in the Min/+ mice. Heme iron consumption increased lipid peroxide metabolites in the urine, which led the authors to conclude that heme iron promoted colon carcinogenesis through lipid peroxide formation.
The goal of Cheung et al.35 was to study the conversion of PhIP into the final carcinogen, and colon tumorigenesis using a humanized mouse expressing the human P450 gene involved in PhIP metabolism instead of the mouse gene. The goal was to better approximate the metabolic outcomes occurring in humans and determine how these products influence inflammation-associated colon carcinogenesis. Mice were treated with PhIP (100 or 200 mg of PhIP/kg body weight) by oral gavage, and 7 days later exposed to dextran sodium sulfate (DSS; 1 or 1.5% to induce inflammation) daily for 1 week prior to termination at times between 6 and 24 weeks. Another study terminated the mice between 16 and 40 weeks after PhIP treatment (200 mg/kg body weight). Mice expressing the human P450 gene treated with 200 mg/kg PhIP, exposed to 1.5% DSS, and terminated between 12 and 21 weeks had colon tumors, whereas the wild-type mice did not. The 1.0% DSS combination with 100 mg/kg PhIP caused only 9% of the mice to have tumors at 24 weeks, whereas 200 mg/kg PhIP alone did not cause any tumors to form between weeks 16 and 40. These data indicate that higher doses of PhIP in combination with an inflammatory challenge are highly effective at producing colon tumors when the metabolic conversion of these compounds occurs using human enzymes. However, lower doses of PhIP, or PhIP treatment without inflammation are not as effective, but still induce the early, non-neoplastic lesions of colon cancer that are considered biomarkers of eventual tumors. These results document a potential model of human metabolic conversion of carcinogens, and indicate that PhIP may serve as a better carcinogen in humans than is currently estimated by data generated in normal rodents. A similar caveat regarding the relevance of elevated doses used in this study must be kept in mind, as they do not reflect normal human exposures.
Nicken et al.37 conducted a study to understand the absorption and secretion of PhIP in the intestine. The goal of their work was to determine why excessively high levels of PhIP are required to induce tumorigenesis. They used rat intestinal segments in an Ussing chamber, which allows the study of absorptive and secretory characteristics of molecules dissolved in the solutions on each side of the tissue. They discovered that very little PhIP is absorbed by the small intestine; however, it is actively secreted into the luminal side of the distal colon from the serosal side. These observations suggest that the relatively low levels of PhIP consumed by humans, in combination with the low rate of absorption, may not be a major contributor to colon cancer risk.
Mitigating the potential increase in fecal water cytotoxicity caused by meat consumption may be possible if probiotics are used to induce a more beneficial distribution of microbiota in the colon. To test this theory, Nowak et al.38 used feces collected from children, adults and seniors, which was incubated with PhIP or IQ, with or without probiotics. They discovered that cytotoxicity was greatest in samples from the elderly and least for samples from children. Cytotoxicity was not changed by the addition of PhIP or IQ, relative to the samples incubated without these compounds, and overall probiotics were able to reduce cytotoxicity. The protection conferred by the probiotics was dependent upon the individual fecal sample used, suggesting that the starting microbiota has a strong impact upon the negative or positive responses associated with PhIP/IQ exposure and probiotic interventions. Interestingly, the fecal samples used in these experiments did not contain PhIP or IQ, or the levels were below the level of detection.
Mechanistic evidence – Protection conferred by other dietary components
Much of the experimental work to determine the effect of red or processed meat on colon cancer development has been conducted using semipurified diets that primarily include either compounds isolated from meats or purified compounds representing those components. However, these paradigms do not allow an understanding of how the colon responds to meat in combination with other foods that contain many different biologically active compounds. There are a large number of nutrients and compounds in the diet that suppress cancer.44 Such biologically active compounds are known to influence cell proliferation and cell death,45,46 as well as carcinogen activation and detoxification.47 The remainder of this review will discuss results from studies where attempts were made to counteract the impact of meat by including other dietary components that may suppress colon carcinogenesis (Table 3).
Table 3.
Protection conferred by other dietary components
| Study | Animal/Treatment | Diets | Meat | Carcinogen used | Findings | Conclusions |
|---|---|---|---|---|---|---|
| Allam et al.48 | Fischer 344 rats Female 4 weeks old Study 1 (short term): 8 diets; n = 5/diet Study 2 (short term): 5 diets; n = 5/diet Study 3 (long term): 2 diets; n = 20/diet Fed 14 days (short term) Fed 3 months (long term) | Modified AIN76 diet Study 1: 60% meat (wet wt), freeze dried, and low calcium (20 mmol/kg) supplemented with calcium phosphate at 33, 55, 90, 150, 250 mmol/kg, or calcium carbonate or calcium gluconate at 250 mmol/kg; Study 2 calcium carbonate diets with hemoglobin(0.63 g/100 g diet); Study 3 calcium carbonate (33 and 100 mmol/kg) versus control | freeze dried beef meat (∼0.6 mmol heme/g meat) | Study 3 - 1,2-dimethylhydrazine (precursor of AOM) | In short term study, 150 and 250 mmol/kg calcium phosphate reduced fecal water cytotoxicity and TBARS. Calcium carbonate was more effective than calcium phosphate. In long term study, a diet containing 100 mmol/kg calcium carbonate did not promote ACF in the colon 101 d after a dimethylhydrazine injection | Calcium carbonate effectively mitigated impact of heme intake |
| Belobrajdic et al.49 | Pigs Male 9 weeks old 2 diets; N = 5/diet Fed 4 weeks | Red meat control; red meat with arabinoxylan-rich fraction (AXRF) from wheat (370 g/kg diet) | Beef steak trimmed of fat (300 g/kg diet) Cooked until lightly browned, minced, frozen | None | AXRF supplemented pigs had increased SCFA in cecum compared to controls; AXRF lowered cecal pH and raised colon pH; AXRF lowered colonocyte DNA damage; cecal digesta p-cresol concentrations were lower with AXRF; AXRF group had lower phenol concentrations in the mid and distal colon compared to the control; AXRF reduced abundance of Prevotella clusters, Bacteroidetes phylum, and Clostridial clusters; control group elevated species of Proteobacteria phylum, Fusobacteria, Clostridial clusters I, II, XI, Bacteroidetes fragilis, and B. distasonis | There may be a beneficial effect due to consuming AXRF with meat in the diet. |
| de Vogel et al.50 | Wistar Rats Male 8 weeks old Study 1: 4 diets; n = 8/diet Study 2: 4 diets; n = 8/diet Fed 14 days | Study 1: control, heme, spinach, heme + spinach Study 2: control, heme, heme + spinach, heme + chlorophyll | Heme (0.5 mmol/kg diet) | Heme fed rats had a 50% increase in rate of DNA replication compared to controls; spinach inhibited heme induced colonocyte proliferation; heme fed rats showed evidence of necrosis in surface cells; heme excretion increased with the addition of spinach; chlorophyll mimicked the effect of spinach in preventing heme induced epithelial proliferation; spinach and chlorophyll both inhibited the increased cytotoxicity of fecal water seen with heme treatment; chlorophyll blocked the formation of the “heme factor” almost completely (this effect was similar with spinach) | Spinach and chlorophyll inhibited heme induced stimulation of colonocyte proliferation; spinach, and the chlorophyll it contains may protect against changes induced by high levels of heme | |
| de Vogel et al.50 | SPF Wistar Rats Male 8 weeks old 5 diets; n = 8/diet Fed 2 weeks | Control, Heme, Heme + Na-chlorophyllin, Heme + Na, Cu-chlorophyllin, Heme + chlorophyll | Heme (0.5 mmol/kg diet) | Heme supplementation increased proliferation compared to controls; supplementation with natural chlorophyll but not chlorophyllins inhibited the heme effects on proliferation; Na-chlorophyllin decreased heme-induced cytotoxicity of fecal water and natural chlorophyll completely blocked heme induced cytotoxicity; heme excretion was low in the control, heme, and heme + chlorophyllin groups but chlorophyll groups had a >50% heme detection in the feces; TBARS of heme and chlorophyllin groups were increased 1.5-2 fold but chlorophyll addition reduced the formation of lipid radicals | Natural chlorophyll is able to induce a protective effect in a heme diet but chlorophyllins are not as effective | |
| O’Callaghan et al.51 | Sprague-Dawley rats Male Adult 12 diets; n = 8/diet Fed 4 weeks | 15, 25, 35% red meat, 13, 22, 30% white meat, with or without HAMS | Beef steak trimmed of fat Cooked at 150℃ until lightly browned, dried at 45℃ for 48 h | Colonocyte telomere length decreased as the % of red meat increased and colonocyte telomere length was shortest with the highest red meat % and no HAMS treatment; addition of HAMS prevented telomere length shortening in the red meat group; white meat also showed a dose dependent decrease in telomere length but it was not significant; HAMS resulted in longer telomeres; telomere lengths were not different between the 15% red and 15% white meat groups; red and white meat increased DSB and SSB dose dependently and damage was greater with red meat; HAMS induced more SCFA production and lowered the concentrations of phenols and cresols; increased MDA concentrations was correlated with shorter telomeres; MDA and acetate concentrations impacted telomere length more than other variables studied | Increased red meat intake shortens colonocyte telomeres and RS is able to attenuate this reduction; levels of oxidative stress are related to the shortening of colonic telomeres; increased SCFA from RS is associated with a reduction of MDA levels and a decrease in telomere shortening and DNA damage | |
| Paturi et al.52 | Sprague-Dawley rats Male 4 weeks old 6 diets; n = 10/diet Fed 8 weeks | Cellulose, potato fiber, or potato-resistant starch for 2 wk without beef (Phase I diets), followed by feeding the same diets for 6 wk with 25% cooked beef (Phase II diets) | Cooked beef, 76.7% protein and 21.6% fat (DM basis) | None | Potato fiber resulted in lower Bacteroides-Prevotella-Porphyromonas. Colonic Bifidobacterium spp. and/or Lactobacillus spp. were higher with potato fiber and potato-resistant starch than with cellulose. Beneficial changes were observed in SCFA concentrations in response to potato fiber. Phenol and p-cresol concentrations were lower in the cecum and colon with potato fiber. An increase in goblet cells per crypt and longer crypts were found in the colon of rats fed potato fiber and potato-resistant starch diets. Fermentable carbohydrates had no effect on colonic DNA damage. | Potato fiber or potato-resistant starch has distinctive effects in the large bowel when fed in combination with red meat. Consuming nondigestible carbohydrates along with red meat is likely one of several dietary factors that contribute to maintenance of normal colonic health. |
| Pierre et al.22 | Fisher 344 Rats Female 4 weeks old 8 diets; n = 10/diet Fed 100 days | Control (low Ca), Beef (Low Ca), control + Ca, Beef + Ca, control + olive oil, beef + olive oil, control + antioxidant, beef + antioxidant | Beef meat contained 0.6 umol/g heme; 60% of diet (wet wt, freeze-dried) | 1,2-dimethylhydrazine | Calcium suppressed beef-meat induced ACF and MDF promotion but it did not reduce mean number of crypts per lesion; antioxidants and olive oil reduced MDF number but not to the extent of calcium and these factors did not affect ACF number; the high calcium control diet (no meat) had more MDF and ACF than control low calcium diets and more crypts per lesion; beef meat plus calcium showed little heme in the fecal water; calcium almost normalized lipid peroxidation, which was not seen with the addition of olive oil or antioxidants; neither calcium or olive oil reduced DHN-MA excretion in beef fed rats, but antioxidants did slightly decrease DHN-MA excretion | Perturbations associated with hemin consumption may be prevented by improved dietary calcium levels |
| Pierre et al.53 | Fisher 344 Rats Female 5 weeks old Study 1: 7 diets; n = 5/diet Study 2: 3 diets; n = 16, 10, 10/diet Study 1: Fed 14 days Study 2: Fed 100 days | Study 1: experimental cured meat + protective agents (rutin, carnosol, alpha-tocopherol, calcium carbonate, inulin, trisodium pyrophosphate) or none (control) Study 2: control meat diet, control + calcium carbonate, control + alpha-tocopherol | Cured meat (dark cooked pork with nitrite and oxidized) added to AIN76 diet (40% protein, 15% fat, 0.27% calcium) Cured with 2 g salt/100 g, heated to 70℃, exposed to air for 5 days | Study 1: None Study 2: 1,2 dimethylhydrazine | Study 1: All tested additives decreased fecal water TBARS and urinary DHN-MA concentrations; addition of CaCO3, rutin, or alpha-tocopherol increased survival of Apc+/+ cells compared to Apc min/+ cells; only inulin did not decrease fecal water cytotoxicty; fecal water of control + inulin was cytotoxic to the Apc +/+ but not the Apc min/+ cells Study 2: the addition of CaCO3 and alpha-tocopherol decreased MDF but not ACF per colon compared to control meat group; CaCO3 decreased all tested biomarkers but alpha-tocopherol only decreased heme concentration in fecal water and DHN-MA in the urine; fecal ATNC was decreased by both additives | The increase of early colon cancer lesions in rats by cured meat can be suppressed by dietary calcium or alpha-tocopherol; potential for cancer promotion due to cured meat could be negated by increased consumption of food rich in calcium |
| Toden et al.54 | Sprague-Dawley Rats Male Adult 6 diets; n = 8/diet Fed 4 weeks | Either 15% casein, 25% casein, or 25% meat; +/−25% HAMS | Beef steak trimmed of fat or chicken breast trimmed of fat Cooked at 150℃ until lightly browned, dried at 45℃ for 48 hours | Cecal pH was lower with meat than casein in the absence of HAMS, but no pH difference when HAMS was included; HAMS increased fecal output relative to controls and significantly higher for the 25% meat diet group; pH of the feces was lowered by HAMS but not affected by protein type; 25% meat treatment had higher DNA damage compared to 15% casein; DNA damage was greater in non-HAMS groups; no difference in DNA damage among diets in the presence of HAMS; all SCFA were increased with the inclusion of HAMS | Substitution of red meat for casein elevates the extent of colon DNA damage; increased dietary protein (casein or beef) increases colonic DNA damage, especially in the absence of RS | |
| Toden et al.55 | Sprague-Dawley Rats Male Adult 12 diets; n = 8/diet Fed 4 weeks | Either 15, 25, or 35% cooked beef or 13, 22, or 30% cooked chicken, with or without 20% HAMS | Beef steak trimmed of fat or chicken breast trimmed of fat Cooked at 150℃ until lightly browned, dried at 45℃ for 48 hours | Dose dependent increase in colonic DNA SSB and DSB in control starch animals fed either red or white meat, and was higher for red than white meat; HAMS prevented the increase; red meat led to increased apoptosis compared to white control diets; cecal butyrate was higher in red meat HAMS (correlation between cecal butyrate and apoptosis was found) | Red meat leads to great increases in colonic DNA damage compared to white meat but HAMS protects against this damage | |
| Toden et al.56 | Sprague-Dawley Rats Male Adult 12 diets; n = 8/diet Fed 4 weeks | Either 15, 25, or 35% cooked beef or 13, 22, or 30% cooked chicken, with or without 20% HAMS | Beef steak trimmed of fat or chicken breast trimmed of fat Cooked at 150℃ until lightly browned, dried at 45℃ for 48 hours | Beef resulted in higher plasma levels of MDA relative to chicken, but levels were reduced by HAMS; leptin levels were higher with beef than chicken, and were reduced by HAMS; chicken, but not beef, increased IGF-1 and HAMS had no effect; chicken increased insulin, relative to beef diets containing HAMS; MMP-2 and TIMP-2 levels were higher with HAMS, but TIMP-2 was highest for chicken without HAMS; SSB and DSB DNA damage correlated with plasma MDA concentrations and DNA DSB correlated with colonic MDA concentrations; beef was positively associated with DSB for plasma and colonic MDA; hepatic portal plasma butyrate concentration was correlated with plasma MDA concentration | Changes in various factors that may affect cancer development were differentially affected by meat source, with some affected by beef and others affected by chicken. Including HAMS suppressed several impacts of either meat. | |
| Van der Meer- van Kraaij et al.57 | Wistar rats Male 9 weeks old 4 diets; n = 16/diet Fed 2 weeks | AIN-93 Control (20 mmol Ca/kg); heme (20 mmol Ca/kg and 0.5 mmol heme/kg); calcium (100 mmol Ca/kg); and heme + calcium (100 mmol Ca/kg and 0.5 mmol heme/kg) | None | None | Heme increased the cytotoxicity of fecal water and elevated colon epithelial proliferation. Calcium reduced cytotoxicity and inhibited heme-induced effects. Mptx was the strongest differentially expressed gene (down-regulated by dietary heme and up-regulated by calcium). | Calcium normalized many variables shown to respond to inclusion of heme in the diet. |
| Van Hecke et al.58 | In vitro digestions, used in Caco-2, HT-29 and HCT-116 cultures 80 digests; n = 3 replicates per digest | Low fat or high fat meat diets combined with 0, 5, 10, 15, or 20 mg α-tocopherol; quercetin; silibinin; ascorbic acid; gallic acid, ferulic acid, chlorogenic acid, or caffeic acid | Beef (low fat); Beef with added pork fat (high fat) | None | Lipid peroxidation products in the digesta was higher with high fat meat, and induced greater cytotoxicity in the cell lines; lipophilic compounds were all antioxidants, but hydrophilic compounds were either antioxidants or pro-oxidants depending on the doses and fat content | Numerous dietary factors influence lipid peroxidation product formation during cooking and digestion; some mitigate cytotoxicity and genotoxicity associated with digests from high or low-fat beef/pork. |
| Winter et al.59 | C57BL/J Mice Male 8 weeks old 6 diets; n = 12/diet Fed 4 weeks | Low casein; high casein; low casein + RS; Low meat; High meat; High meat + RS | Lean, minced steak Cooked at medium temp with mixing to prevent burning; oven dried, ground into a powder | No affect of protein level on fecal pH; red meat increased p-cresol, propionate and total SCFA concentrations compared to casein; RS lowered pH, ammonia, and phenol concentration and increased SCFAs; no effect of amount or type of protein on cell proliferation or apoptosis; RS increased proliferation and reduced apoptosis; DNA adducts were higher in mice consuming red meat compared to casein but protein amount had no effect; RS reduced DNA adduct formation; there was a positive relationship between DNA adduct formation and the levels of p-cresol and fecal pH; propionate, butyrate and total SCFA correlated inversely with distal colon apoptosis; and apoptosis positively correlated with fecal pH | High protein diets increase DNA adducts in the colon and the type of protein has a greater effect than the amount of protein; Supplementation with fermentable CHO reduced formation of DNA adducts | |
| Winter et al.60 | C57BL/J Mice Male 8 weeks old 4 diets; n = 16/diet Fed 4 weeks (short term); 18 months (long term) | Modified AIN-76 diets (15% casein) Control, Control + 10% HAMS, Heme, Heme + 10% HAMS | Heme (0.2 µmol/g)- approximates heme content of a high red meat diet Diets placed in sealed containers and stored at 4℃ | None | Long term heme mice weighed less; heme increased proliferation in short term but not long term study; in long term study there was no change in apoptosis, or DNA adducts with heme, but HAMS increased proliferation; heme lowered apoptosis in older mice, cell proliferation increased with age; heme led to increased DNA adducts | Heme increased DNA adducts and cell proliferation, and reduced apoptosis; HAMS promoted good bacteria and reduced formation of toxic products from protein over short periods but changes are not sustained over time; heme was not sufficient to induce colon cancer |
ACF:: aberrant crypt foci; AIN: American Institute of Nutrition; AOM: azoxymethane; Apc min: adenomatous polyposis coli gene minus; AXRF: arabinoxylan-rich fraction; ATNC: apparent total nitrso compound; BrdU: Bromodeoxyuridine; CLA-FFA: conjugated linoleic acid free fatty acid; CLA-TG: conjugated linoleic acid triglyceride; CMT: cell media type; CRC: colorectal cancer; DHN-MA: 1,4-Dihydroxynonane Mercapturic Acid; DSB: double strand breaks; HAMS: High amylose maize starch; IFG-1: insulin-like growth factor 1; IL-6:interlukein 6; LAMS: low amylose maize starch; MDA: Malondialdehyde; SCFAs: short chain fatty acids; Mptx: Mucosal pentraxin; MMP-2: matrix metalloproteinase; SSB: single strand breaks; TBARS: thiobarbituric acid-reactive substances; TIMP-2: tissue inhibitor of matrix metalloproteinase; TNFα: tumor necrosis factor; TXB2: Thromboxane B2.
Winter et al.59 examined whether inclusion of resistant starch (reaches the colon like dietary fiber) in red meat-containing diets would impact microbial fermentation products or epithelial cell proliferation and apoptosis in the colon of mice after either three or four weeks. When compared to casein, consumption of cooked and dried red meat (20.43 or 40.9% to achieve 15 or 30% protein, respectively) increased fecal short chain fatty acid concentrations and the protein metabolite p-cresol (a genotoxic compound). Adding resistant starch to the diets lowered fecal pH, ammonia and phenol concentrations but increased the level of all short chain fatty acids measured. Changing the level or source of protein (meat vs. casein) had no effect on colon epithelial proliferation, the number of cells within crypts or the rate of apoptosis. However, resistant starch added to any diet increased epithelial proliferation and crypt height, but reduced apoptosis. The DNA adduct O6-methyl guanine was increased by meat, but resistant starch reduced adducts to levels similar to that observed in the casein fed mice. A positive correlation between the level of p-cresol and formation of the DNA adduct was observed. Their work leads to the conclusion that inclusion of a readily fermentable fiber along with even relatively high levels of red meat would mitigate some of the potentially damaging effects of protein metabolites derived from red meats in the colon. Winter et al.60 also evaluated the ability of resistant starch to counteract the effects of hemin (0.2 µmol/g diet) in mice consuming a Westernized diet containing low levels of calcium, vitamin D and methyl donors, and high in fat. They discovered there was no difference in colon epithelial cell proliferation, apoptosis, crypt height, or DNA adduct levels measured at 18 months, whereas after a shorter period (three weeks) hemin increased proliferation. Including resistant starch in the diets during the 18-month experiment increased proliferation and crypt height, but did not affect apoptosis or DNA adduct formation. They found no significant change in the incidence of colon tumors due to either the hemin or resistant starch treatments.
Chlorophyll-containing foods were the subject of studies conducted by de Vogel et al.50,61 using Westernized diet compositions. These two-week studies evaluated the effect of spinach, natural chlorophyll, or molecular analogs of chlorophyll (chlorophyllins) on heme-induced perturbations of the colon. Chlorophyllins are food grade analogs of chlorophyl in which the magnesium molecule in chlorophyll is replaced by a sodium or copper molecule. Heme content increased colon epithelial cell proliferation and fecal water cytotoxicity; however, both spinach and natural chlorophyll reduced these outcomes to normal or below normal values. The addition of spinach greatly increased heme excretion in feces well beyond that observed with the heme alone, suggesting spinach was altering heme metabolism, and possibly preventing heme’s conversion to some of the potential carcinogenic compounds discussed previously. The sodium or copper chlorophyllins were not as protective as natural forms of the chlorophyll molecule or spinach. The levels of chlorophyll used in these experiments would equate to the consumption of 450 g/day of spinach in humans, which is not reasonable. Yet, when the level of chlorophyll consumed in a omnivorous diet rich in plant foods is considered, this value may not be excessive.
Dietary fiber or resistant starch are able to mitigate some of the changes in colon epithelial proliferation and DNA adduct formation, such as single and double strand breaks that are associated with meat consumption.49,54,55 Red meat (beef) was found to induce more DNA strand breaks than white meat (chicken), but resistant starch was protective for both types of meat when it was included at 15, 25, or 35% of the diet.54,55 Toden et al.55 also reported changes in microbial metabolites resulting from the consumption of resistant starch, and the extent of the effect was dependent upon the amount of meat consumed by the rats. Red meat with resistant starch produced more cecal butyrate than when resistant starch was combined with white meat. This is important because butyrate has been shown to induce apoptosis, which during tumorigenesis is a positive outcome as apoptosis in those conditions can be targeted to DNA damaged cells.41,62,63 Cecal and fecal p-cresol concentrations (a toxic microbial protein metabolite) were elevated with red meat, which did not occur with white meat, but resistant starch reduced the level of p-cresol. A follow-up study indicated that changes in the colon metabolic profile, DNA damage level, and physiology were related to alterations in circulating mediators of inflammation.56 Paturi et al.52 found that microbial populations and their metabolites were impacted by the addition of potato fiber or potato resistant starch to a diet provided to rats that contained 25% cooked beef. The potato treatments also led to an increase in the number of cells lining the colon, suggesting there was a stimulation of proliferation or a reduction in apoptosis. The study by Belobrajdic et al.49 used pigs as a model because of the similarities between pig and human intestinal physiology. They found that addition of an arabinoxylan-rich fraction of wheat to a 30% red meat diet reduced DNA strand breaks in the proximal, middle, and distal colon, with the greatest reduction occurring in the distal colon. Arabinoxylan reduced p-cresol in all segments of the colon, but had no effect on the levels of phenol or ammonia. This group also sequenced the colon microbiota and demonstrated beneficial shifts in the populations when the arabinoxylan was included in the diet. O’Callaghan et al.51 was concerned with the impact of meat (beef or chicken) consumption (15, 25, or 35% of diet) on telomeres located at the end of DNA strands. Telomeres protect the genome against genomic instability and cellular senescence. Telomere shortening has been reported to be a characteristic of DNA found in colon tumors. They discovered that telomere length shortened in proportion to the amount of meat in the diet of rats, with red meat causing greater shortening than white meat. However, inclusion of resistant starch in the diet mitigated all but a small fraction of the reduction in telomere length that occurred with red meat.
Elevated calcium levels have long been known to be associated with reduced incidence of colon cancer, even though the mechanisms involved in conferring protection are not completely understood at this time.64 Several animal studies have been conducted to determine if supplementing calcium would negate the promotion of colon cancer induced by consuming fresh or cured meat. The calcium requirement to support normal maintenance and growth of laboratory animals is 5 g/kg (124.8 mmol/kg) diet. Colon epithelial cell proliferation and gene expression, as well as fecal water cytotoxicity and lipid peroxide content, and the formation of preneoplastic lesions have been the focus of these studies. The study by van der Meer-van Kraaij et al.57 used rats to determine if providing 100 mmol calcium phosphate/kg diet (compared to 20 mmol calcium phosphate/kg diet) for two weeks protected against various biomarkers of altered colon physiology induced by heme (0.5 mmol/kg diet). The elevated calcium level mitigated the heme-induced cytotoxicity and almost restored colon epithelial cell proliferation to normal levels. Heme induced the differential expression of multiple genes involved in a variety of pathways and calcium reduced the impact of heme on gene expression, but did not restore expression to normal levels for most of those genes. Pierre et al.22 incorporated red meat (60%) and 31 g/kg calcium phosphate into diets in order to determine their independent and combined effects on several colon biomarkers, including the formation of preneoplastic lesions. Including calcium phosphate with the beef reversed the increase in lesion formation induced by the beef alone. Including calcium phosphate in the diet reversed the increase in fecal lipid peroxides and cytotoxicity of fecal water from meat-fed rats. However, this study discovered that a control diet including elevated levels of calcium phosphate stimulated formation of the preneoplastic lesions. Allam et al.48 followed up the prior study22 by comparing the effects of various concentrations of calcium phosphate with those from calcium carbonate in diets containing either meat (60% beef) or heme (0.6 mmol/g). They found that calcium carbonate (100 µmol/g) was protective against preneoplastic lesion formation both with and without 60% beef in the diet. Finally, Pierre et al.53 evaluated the impact of including calcium and α-tocopherol on the effects of cured pork (47% in the diet for 100 days) on colon tumor formation in carcinogen-injected rats. Calcium reduced both lipid peroxides and N-NOCs, but the α-tocopherol reduced only the N-NOCs. Both treatments did not affect the number of overall preneoplastic lesions in the rats, but did reduce the number of a special class of lesions that contain few mucin-producing cells. Therefore, calcium appears to help reduce the impact of high levels of meat or heme intake, but care should be taken to use calcium carbonate and not calcium phosphate to achieve this goal.
Van Hecke et al.58 used an in vitro digestion system to determine if lipophilic and hydrophilic reducing compounds alter lipid peroxidation and cytotoxicity of a low fat (1%) or high fat (15%) beef diet. Pork fat was added to the low fat product to make the high-fat beef product. Lipid peroxidation products were elevated in the cooked high fat product, relative to the low fat product, if it did not contain any of the reducing compounds. Lipid peroxides were reduced with the addition of most hydrophilic compounds added or by quercetin, but not by α-tocopherol or silibinin in the low-fat product digest. In the high-fat product digests, all lipophilic compounds (except silibinin) were able to reduce the lipid peroxides, but the level of 4-HNE was elevated by ascorbic acid and gallic acid. Phenolic molecules reduced MDA levels, with the smallest reduction occurring with chlorogenic acid. The data suggest that some biologically active compounds present in the diet are capable of reducing lipid peroxidation products, even when the diet contains high levels of fat.
Impact of study designs and experimental approaches
Most studies were short-term in nature using two to four week designs, with only a few animal studies lasting up to 100 days (Table 1). The majority of studies used isolated sources of heme rather than meat per se. Purified or semi-purified diets were used in all designs and many manipulated the fat, calcium, and anti-oxidant contents. While many were designed to represent a “Westernized diet,” few mimicked a human diet with regard to nutrient levels or diversity of potential chemopreventive compounds normally found in human diets. Selection of animal models is a critical element of study designs to evaluate the impact of diet on colon carcinogenesis. The importance of this issue should not be understated, but discussion beyond that already provided is not the goal of this review (see Johnson and Fleet65 for a thorough review of the subject).
Sources of heme used in experimental designs
The use of hemin and other heme-containing chemicals as test agents may not accurately reflect heme iron intake from red and processed meat. Red meat contains heme iron from myoglobin, cytochromes and hemoglobin. The content and proportion of these heme-containing proteins vary widely according to species of origin, age of the animal, and anatomical location of the source.66 In many red meats, such as beef, myoglobin contributes up to 50% of the heme-iron, making myoglobin particularly important for studies intended to represent typical meat intake.67 Isolated forms of heme do not accurately represent the effect that cooking, and other common forms of denaturation used in food preparation, have on the heme-containing meat protein.68,69 The combination of heme-containing proteins as they occur in red meat was evaluated only in studies that provided meat.21,22,59 Myoglobin is a ubiquitous and significant form of heme iron in red meat and the use of surrogates, such as hemin or isolated hemoglobin, are not equivalent to meat.
Following digestion, any source of heme iron is converted to protoporphyrin IX, and as such, should be treated equally within the intestinal lumen. Hemin, a protoporphyrin IX derived from oxidized heme, which contains ferric iron (Fe3+) with a chloride ligand is used clinically in the management of porphyria, which is an inherited disease in which there is an excess production of porphyrins such as hemoglobin.70 Cooking contributes to oxidation of ferrous iron in meat, and oxidized iron (ferric) is less bioavailable causing more to reach the colon. Pierre et al.23 provides a direct comparison of diets supplemented with hemin vs. hemoglobin. Fecal heme concentrations were higher in the hemoglobin-fed rats than in hemin-fed rats. The authors concluded the differences in fecal heme concentrations were due to the increase in fecal water promoted by hemin,71 which effectively diluted the heme. Hemoglobin did not cause an increase in fecal water content. Analysis of the ACF and MDF data showed that hemin induced large MDF instead of ACF, while hemoglobin promoted ACF but not MDF.23 The cytolytic activity of fecal water increased 50-fold in rats fed a hemin diet, but not in hemoglobin-fed rats.
Pierre et al.23 noted that freeze-drying leads to elevated levels of lipid peroxides in processed meat (e.g., ham); they suggest freeze-drying should be avoided. With respect to model selection, they recommend that hemin be used to represent the impact of processed meats and that hemoglobin be used to represent the impact of fresh red meats in future studies aimed to determine the effect of meat consumption on carcinogenesis.
Levels of heme iron or meat in the diets
Establishing appropriate levels of meat or its components in experimental studies is critical if extrapolation to the outcomes resulting from human consumption patterns is to be feasible. Studies using hemin or heme all included the product in the diet at either 0.2 or 0.5 mmol/kg diet.12,14–17 Meat heme concentrations are, on average, 1.4 mg/100 g meat,72 which equates to 0.0227 mmol/kg meat. On the assumption that the total estimated meat (all sources) intake is 220 g/d in the US,7 the average diet would include 3.08 mg of heme, which is equivalent to 0.005 mmol of heme or 0.00275 mmol/kg of a mixed diet (∼1.82 kg) eaten each day by humans. Therefore, the concentrations of hemin used in the diets for these studies were approximately 9–22 times the amount found in meat, and a much larger excess when considering meat consumed in a mixed meal (Figure 2). The study using freeze-dried hemoglobin incorporated it into the diet at either 0.63% or 1.0%, which equates to 0.10 or 0.16 mmol/kg diet.10 Because hemoglobin contains four heme molecules, this would be equivalent to approximately 0.40 or 0.62 mmol/kg of heme in the diet, which is between 145 and 225 times levels found in human diets.
Figure 2.
Comparison of the heme concentration in average human diets and the range (low = white column, high = black column) of concentrations used in experimental diets containing hemin or hemoglobin
According to Pierre et al.,21 the reason that effects seen in humans were less than in rats in their study could be explained by differences in heme doses, and in protective agents found in a human omnivore diet. Effective doses of heme are much different in rats than in humans when considering body size, weight, and metabolic rates.73 In addition, diets given to human control groups rarely have an absolute exclusion of nutrients or food groups that can be achieved in experimental animals. Finally, rat diets are composed of purified components and void of chemopreventive compounds derived from fruits, vegetables, and grains. Those compounds inhibit both peroxidation and carcinogenesis.21
The use of blood sausage (blood pudding) as a source of heme iron is also problematic. Blood sausage is not a muscle meat, and according to the recipe reported by Pierre et al.74 contains no muscle meat products. Blood, by definition, contains a nutrient profile that is different from muscles and, as used in these studies, provides over 25 times the hemoglobin and over 6 times the iron content of the high-meat diets.21,74
Diet design
Treatments were often added to semi-purified diets, which for most of the studies were formulated to approximate a “Westernized dietary pattern” that includes an elevated fat content (40%) and low calcium levels.12,14–17 Two studies used diets with 5%10 or 20%59 fat. It is well established that dietary calcium, and particularly residual calcium in the colon suppresses the efficiency of AOM-induced tumorigenesis.64 Therefore, most diets used in these studies were based on a low-calcium modification of an AIN diet in an effort to exacerbate tumorigenesis. Such reductions are severe and would result in deficient calcium intakes (∼128–352 mg/d).
The experiment utilizing cooked meat incorporated either 20.43 or 40.90 g/100 g of a semi-purified diet.59 The meat (lean rump steak) had been minced and cooked at a medium temperature until browned, followed by drying at an unknown temperature overnight and grinding into a powder, which was then incorporated into the diet. The mixed diets were stored in airtight containers at 4℃ prior to use and fresh food was provided daily. If we use the current US average distribution of all meats consumed relative to the overall diet (noted previously), meat consumed primarily in the fully hydrated form makes up approximately 12.1% of the mass consumed each day. Therefore, the experimental diets contained more than 1.69- to 3.38-fold the amount of meat consumed by humans each day.
Use of a carcinogen to initiate experimental animals
Hemoglobin is not a carcinogen. Many studies in this review include data from AOM-treated rats, a model of sporadic colon cancer. AOM is a complete carcinogen meaning all animals will eventually develop tumors. During this process, colon epithelial cells undergo pathogenesis from minor lesions (ACF), to adenoma and malignant adenocarcinoma.72,75 The ability to test the effect of outside factors on tumor yield, make the model appropriate for chemoprevention studies. In order to compare normal colon metabolism and responses to meat intake to that in a cancer-initiated state, it is necessary to include appropriate controls (animals injected with saline), which was not done in all studies.
Limitations of fecal water assays
Several of the included studies measured the amount of heme iron and TBARS in fecal water, followed by determining the cytotoxic potential of fecal water in an in vitro cell assay. Implications are that as heme iron intake increases, the amount of heme iron in fecal water increases, which will in turn cause oxidative damage to colon mucosa. However, none of the studies measured lipid peroxidation in the colonic mucosa from the animals, which is necessary to verify that the oxidative state was altered in colonocytes.
Limitations of apparent total nitrso compound determination
The “apparent total nitroso compounds” (ATNC) assay has been used by many investigators as a proxy measurement of carcinogenic N-nitrosamines (NOC).76–80 This assay was used in some studies reported in this review (see Tables 1 and 2) seeking to find a mechanistic basis for the reported epidemiologic associations between meat intake and colon cancer. However, this is a broad category of compounds comprising a complex mixture of nitrite-derived products, including N- nitrosamines, S-nitrosothiols, N-nitrosamides, nitrosoguanidines, and iron-nitrosyl species.81–83 These compounds, all of which are measured in ATNC assays, have a broad range of metabolic activities. Bryan et al.81 suggested that a number of improvements in the assay are needed to detect individual compounds and discriminate between carcinogenic and non-carcinogenic N-nitrosamines. Therefore, measurement of total ATNC may not be a useful indicator of carcinogenic potential in studies of meat and CRC.
In summary, the mechanisms whereby heme iron or HCAs from red and processed meat may enhance biomarkers and other factors related to CRC remain open as experimental and theoretical gaps and weaknesses exist that will require further research. None of the various mechanisms tested by studies included in this review, including oxidative stress, inflammation, cytotoxicity and perturbations to the normal process of apoptosis, are supported by evidence sufficient to confirm a mechanistic link between red meat intake and CRC risk.
Recommendations for future research
The major limitation to most of the preclinical research reviewed is the greatly elevated levels of meat or meat components included in the diets, or delivered through oral gavage. Although many of the experiments have used semipurified diets designed to mimic the nutrient loads in current Westernized diets, it is not possible to capture the benefits associated with consuming the potential biologically active protective compounds present in whole foods using these paradigms. The inherent diversity in composition associated with any whole foods and their normally higher water content makes conducting experiments with them a challenge. Importantly, most experiments do not attempt to compare the response to include different levels of meat in an otherwise healthy dietary pattern (rich in fruits, vegetables, and whole grains) to what happens when meat is added to an unhealthy dietary pattern. The studies summarized indicate that when diets contain elevated levels of chlorophyll, readily fermentable fibers, or calcium carbonate, there is little impact of including reasonable levels of meat on colon health. It is also possible that other biologically active compounds present in fruits, vegetables and whole grains would provide protection. Together, these patterns of responses indicate the probable involvement of colon microbiota in influencing whether red meat is involved in colon tumorigenesis, a subject that deserves further attention. These data directly point to the fact that it is the response to the overall dietary characteristics that drives associations between red meat consumption and CRC risk and that future studies must use experimental designs that capture the complexity of dietary patterns in our attempts to define the potential relationship between meat consumption and colon cancer, and the mechanisms involved.
Acknowledgements
The authors wish to thank Dr. Mary Van Elswyk and Charli Weatherford for help with editing and formatting. National Cattlemen’s Beef Association, a contractor to the Beef Checkoff, provided financial support in the form of an unrestricted educational grant to perform this critical review of the literature.
Authors’ contributions
Both authors participated in reviewing the literature and writing of the manuscript; SKL performed the literature search; SKL and NDT conducted the selection/elimination determination; SKL and NDT reviewed the selected manuscripts; SKL and NDT wrote the manuscript.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
There was no funding to support this minireview.
References
- 1.Minozzi S, Armaroli P, Expina C, Villain P, Wiseman M, Schuz J, Segnan N. European code against cancer 4th edition: Process of reviewing the scientific evidence and revising the recommendations. Cancer Epidemiol 2015; 39(Suppl 1): S11–9. [DOI] [PubMed] [Google Scholar]
- 2.Bouvard V, Loomis D, Guyton KZ, Grosse Y, El Ghissassi F, Benbrahim-Tallaa L, Guha NHM, Straif K. Carcinogenicity of consumption of red and processed meat. Lancet Oncol 2015; 16: 1599–600. [DOI] [PubMed] [Google Scholar]
- 3.Lippi G, Mattiuzzi C, Cervellin G. Meat consumption and cancer risk: A critical review of published meta-analyses. Crit Rev Oncol Hematol 2016; 97: 1–14. [DOI] [PubMed] [Google Scholar]
- 4.Richi EB, Baumer B, Conrad B, Darioli R, Schmid A, Keller U. Health risks associated with meat consumption: A review of epidemiological studies. Int J Vitam Nutr Res 2015; 85: 70–8. [DOI] [PubMed] [Google Scholar]
- 5.De Meyer D, Mertens B, De Smet S, Ulens M. Mechanisms linking colorectal cancer to the consumption of (processed) red meat: A review. CritRev Food Sci Nutr 2016; 56: 2747–66. [DOI] [PubMed] [Google Scholar]
- 6.Fehrenbach KS, Righter AC, Santo RE. A critical examination of the available data sources for estimating meat and protein consumption in the USA. Pub Health Nutr 2015; 19: 1358–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oostindjer M, Alexander J, Amdam GV, Andersen G, Bryan NS, Chen D, Corpet DE, De Smet S, Dragsted LO, Haug A, Karlsson AH, Kleter G, de Kok TM, Kulseng B, Milkowski AL, Martin RJ, Pajari A-M, Paulsen JE, Pickova J, Rudi K, Sødring M, Weed DL, Egelandsdal B. The role of red and processed meat in colorectal cancer development: A perspective. Meat Sci 2014; 97: 583–96. [DOI] [PubMed] [Google Scholar]
- 8.Klurfed DM. Research gaps in evaluating the relationship of meat and health. Meat Sci 2015; 109: 86–95. [DOI] [PubMed] [Google Scholar]
- 9.IOM. Dietary reference intakes for vitamin a, vitamin k, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc, Washington, DC: National Academy Press, 2001. [PubMed] [Google Scholar]
- 10.Chenni FZ, Tache S, Naud N, Gueraud F, Hobbs DA, Kunhle GGC, Pierre FH, Corpet DE. Heme-induced biomarkers associated with red meat promotion of colon cancer are not modulated by the intake of nitrite. Nutr Cancer 2013; 65: 227–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davis ME, Lisowyj MP, Zhou L, Wisecarver JL, Gulizia JM, Shostrom VK, Naud N, Corpet DE, Mirvish SS. Induction of colonic aberrant crypts in mice by feeding apparent N-nitroso compounds derived from hot dogs. Nutr Cancer 2012; 64: 342–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Vogel J, Van-Eck WB, Sesink ALA, Jonker-Termont D, Kleibeuker J, van der Meer R. Dietary heme injures surface epithelium resulting in hyperproliferation, inhibition of apoptosis and crypt hyperplasia in rat colon. Carcinogenesis 2008; 29: 398–403. [DOI] [PubMed] [Google Scholar]
- 13.Gueraud F, Tache S, Steghens JP, Milkovic L, Borovic-Sunjic S, Zarkovic N, Gaultier E, Naud N, Helies-Toussaint C, Pierre F, Priymenko N. Dietary polyunsaturated fatty acids and heme iron induce oxidative stress biomarkers and a cancer promoting environment in the colon of rats. Free Rad Biol Med 2015; 83: 192–200. [DOI] [PubMed] [Google Scholar]
- 14.Ijssennagger N, Rijnierse A, de Wit N, Jonker-Termont D, Dekker J, Muller M, van der Meer R. Dietary haem stimulates epithelial cell turnover by downregulating feedback inhibitors of proliferation in murine colon. Gut 2012a; 61: 1041–9. [DOI] [PubMed] [Google Scholar]
- 15.Ijssennagger N, de Wit N, Muller M, van der Meer R. Dietary heme-mediated PPAR alpha activation does not affect the heme-induced epithelial hyperproliferation and hyperplasia in mouse colon. PLoS One 2012b; 7: e43260–e43260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ijssennagger N, Derrien M, van Doorn GM, Rijnierse A, van den Bogert B, Muller M, Dekker J, Kleerebezem M, van der Meer R. Dietary heme alters microbiota and mucosa of mouse colon without functional changes in host-microbe cross-talk. PLoS One 2012c; 7: e49868–e49868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ijssennagger N, Rijnierse A, de Wit NJ, Boekschoten MV, Dekker J, Schonewille A, Muller M, van der Meer R. Dietary heme induces acute oxidative stress, but delayed cytotoxicity and compensatory hyperproliferation in mouse colon. Carcinogenesis 2013; 34: 1628–35. [DOI] [PubMed] [Google Scholar]
- 18.Ijssennagger N, Belzer C, Hooiveld GJ, Dekker J, van Mil SWC, Muller M, Kleerebezem M, van der Meer R. Gut microbiota facilitates dietary heme-induced epithelial hyperproliferation by opening the mucus barrier in colon. Proc Natl Acad SciU S A 2015; 112: 10038–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martin OC, Lin C, Naud N, Tache S, Raymond-Letron I, Corpet DE, Pierre FH. Antibiotic suppression of intestinal microbiota reduces heme-induced lipoperoxidation associated with colon carcinogenesis in rats. Nutr Cancer 2015; 67: 119–25. [DOI] [PubMed] [Google Scholar]
- 20.Mirvish SS, Davis ME, Lisowyj MP, Gaikwadt NW. Effect of feeding nitrite, ascorbate, hemin, and omeprazole on excretion of fecal total apparent N-nitroso compounds in mice. Chem Res Toxicol 2008; 21: 2344–51. [DOI] [PubMed] [Google Scholar]
- 21.Pierre F, Peiro G, Tache S, Cross AJ, Bingham SA, Gasc N, Gottardi G, Corpet DE, Gueraud F. New marker of colon cancer risk associated with heme intake: 1,4-dihydroxynonane mercapturic acid. Cancer Epidemiol Biomark Prevent 2006; 15: 2274–9. [DOI] [PubMed] [Google Scholar]
- 22.Pierre F, Santarelli R, Tache S, Gueraud F, Corpet DE. Beef meat promotion of dimethylhydrazine-induced colorectal carcinogenesis biomarkers is suppressed by dietary calcium. Br J Nutr 2008; 99: 1000–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pierre FHF, Santarelli RL, Allam O, Tache S, Naud N, Gueraud F, Corpet DE. Freeze-dried ham promotes azoxymethane-induced mucin-depleted foci and aberrant crypt foci in rat colon. Nutr Cancer 2010; 62: 567–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Santarelli RL, Vendeuvre J-L, Naud N, Taché S, Guéraud F, Viau M, Genot C, Corpet DE, Pierre FHF. Meat processing and colon carcinogenesis: Cooked, nitrite-treated, and oxidized high-heme cured meat promotes mucin-depleted foci in rats. Cancer Prevent Res 2010; 3: 852–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sødring M, Oostindjer M, Egelandsdal B, Paulsen JE. Effects of hemin and nitrite on intestinal tumorigenes in the A/J Min/+ mouse model. PLoS One 2015; 10: e0122880–e0122880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Van Hecke T, Vossen E, Hemeryck LY, Vanden Bussche J, Vanhaecke L, De Smet S. Increased oxidative and nitrosative reactions during digestion could contribute to the association between well-done red meat consumption and colorectal cancer. Food Chem 2015; 187: 29–36. [DOI] [PubMed] [Google Scholar]
- 27.Zhou L, Zahid M, Anwar MM, Pennington KL, Cohen SM, Wisecarver JL, Shostrom V, Mirvish SS. Suggestive evidence for the induction of colonic aberrant crypts in mice fed sodium nitrite. Nutr Cancer 2016; 68: 105–12. [DOI] [PubMed] [Google Scholar]
- 28.Pretlow TP, Barrow BJ, Ashton WS, O'Riordan MA, Pretlow TG, Jurcisek JA, Stellato TA. Aberrant crypts: Putative preneoplastic foci in human colonic mucosa. Cancer Res 1991; 51: 1564–7. [PubMed] [Google Scholar]
- 29.Parnaud G, Pignatelli B, Peiffer G, Tache S, Corpet DE. Endogenous N-nitroso compounds, and their precursors, present in bacon, do not initiate or promote aberrant crypt foci in the colon of rats. Nutr Cancer 2000; 38: 74–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Corpet DE. Red meat and colon cancer: Should we become vegetarians, or can we make meat safer? Meat Sci 2011; 89: 310–6. [DOI] [PubMed] [Google Scholar]
- 31.Di Maso M, Talamini R, Bosetti C, Montella M, Zucchetto A, Libra M, Negri E, Levi F, La Vecchia C, Franceschi S, Serraino D, Polesel J. Red meat and cancer risk in a network of case–control studies focusing on cooking practices. Ann Oncol 2013; 24: 3107–12. [DOI] [PubMed] [Google Scholar]
- 32.Wakabayashi K, Ushiyama H, Takahashi M, Nukaya H, Kim SB, Hirose M, Ochiai M, Sugimura T, Nagao M. Exposure to heterocyclic amines. Environ Health Perspect 1993; 99: 129–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Augustsson K, Skog K, Jagerstad M, Steineck G. Assessment of human exposure to heterocyclic amines. Carcinogenesis 1997; 18: 1931–5. [DOI] [PubMed] [Google Scholar]
- 34.Bastide NM, Chenni F, Audebert M, Santarelli RL, Tache S, Naud N, Baradat M, Jouanin I, Surya R, Hobbs DA, Kuhnle GG, Raymond-Letron I, Gueraud F, Corpet DE, Pierre FH. A central role for heme iron in colon carcinogenesis associated with red meat intake. Cancer Res 2015; 75: 870–9. [DOI] [PubMed] [Google Scholar]
- 35.Cheung C, Loy S, Li GX, Liu AB, Yang CS. Rapid induction of colon carcinogenesis in CYP1A-humanized mice by 2-amino-1-methyl-6-phenylimidazo 4,5-b pyridine and dextran sodium sulfate. Carcinogenesis 2011; 32: 233–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kuhnel D, Taugner F, Scholtka B, Steinberg P. Inflammation does not precede or accompany the induction of preneoplastic lesions in the colon of 2-amino-1-methyl-6-phenylimidazo 4,5-b pyridine-fed rats. Arch Toxicol 2009; 83: 763–8. [DOI] [PubMed] [Google Scholar]
- 37.Nicken P, Schroder B, von Keutz A, Breves G, Steinberg P. The colon carcinogen 2-amino-1-methyl-6-phenylimidazo 4,5-b pyridine (PhIP) is actively secreted in the distal colon of the rat: An integrated view on the role of PhIP transport and metabolism in PhIP-induced colon carcinogenesis. Arch Toxicol 2013; 87: 895–904. [DOI] [PubMed] [Google Scholar]
- 38.Nowak A, Czyzowska A, Stanczyk M. Protective activity of probiotic bacteria against 2-amino-3-methyl-3H-imidazo 4,5-f quinoline (IQ) and 2-amino-1-methyl-6-phenyl-1H-imidazo 4,5-b pyridine (PhIP) – An in vitro study. Food Addit Contam Part A 2015; 32: 1927–38. [DOI] [PubMed] [Google Scholar]
- 39.Wang R, Dashwood WM, Lohr CV, Fischer KA, Nakagama H, Williams DE, Dashwood RH. Beta-catenin is strongly elevated in rat colonic epithelium following short-term intermittent treatment with 2-amino-1-methyl-6-phenylimidazo 4,5-b pyridine (PhIP) and a high-fat diet. Cancer Sci 2008; 99: 1754–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang R, Dashwood WM, Nian H, Lohr CV, Fischer KA, Tsuchiya N, Nakagama H, Ashktorab H, Dashwood RH. NADPH oxidase overexpression in human colon cancers and rat colon tumors induced by 2-amino-1-methyl-6-phenylimidazo 4,5-b pyridine (PhIP). Int J Cancer 2011; 128: 2581–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hong MY, Bancroft LK, Turner ND, Davidson LA, Murphy ME, Carroll RJ, Chapkin RS, Lupton JR. Fish oil decreases oxidative DNA damage by enhancing apoptosis in rat colon. Nutr Cancer 2005; 52: 166–75. [DOI] [PubMed] [Google Scholar]
- 42.Hong MY, Turner ND, Murphy ME, Carroll RJ, Chapkin RS, Lupton JR. In vivo regulation of colon cell proliferation, differentiation, apoptosis and P27Kip1 by dietary fish oil and butyrate in rats. Cancer Prevent Res 2015; 8: 1076–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim E, Davidson LA, Zoh RS, Patil BS, Jayaprakasha GK, Callaway ES, Allred CD, Turner ND, Chapkin RS. Homeostatic responses of colonic LGR5 stem cells following acute in vivo exposure to a genotoxic carcinogen. Carcinogenesis 2016; 37: 206–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yebra-Pimentel I, Fernandez-Gonzalez R, Martinez-Carballo E, Simal-Gandara J. A critical review about the health risk assessment of PAHs and their metabolites in foods. Crit Rev Food Sci Nutr 2015; 55: 1383–405. [DOI] [PubMed] [Google Scholar]
- 45.Vanamala J, Leonardi T, Patil BS, Taddeo SS, Murphy ME, Pike LM, Chapkin RS, Lupton JR, Turner ND. Suppression of colon carcinogenesis by bioactive compounds in grapefruit. Carcinogenesis 2006; 27: 1257–65. [DOI] [PubMed] [Google Scholar]
- 46.Warren CA, Paulhill KJ, Davidson LA, Lupton JR, Taddeo SS, Hong MY, Carroll RJ, Chapkin RS, Turner ND. Quercetin may suppress rat aberrant crypt foci formation by suppressing inflammatory mediators that influence proliferation and apoptosis. J Nutr 2009; 139: 101–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zanini S, Marzotto M, Giovinazzo F, Bassi C, Bellavite P. Effects of dietary components on cancer of the digestive system. Crit Rev Food Sci Nutr 2015; 55: 1870–85. [DOI] [PubMed] [Google Scholar]
- 48.Allam O, Bahuaud D, Tache S, Naud N, Corpet DE, Pierre FHF. Calcium carbonate suppresses haem toxicity markers without calcium phosphate side effects on colon carcinogenesis. Br J Nutr 2011; 105: 384–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Belobrajdic DP, Bird AR, Conlon MA, Williams BA, Kang S, McSweeney CS, Zhang D, Bryden WL, Gidley MJ, Topping DL. An arabinoxylan-rich fraction from wheat enhances caecal fermentation and protects colonocyte DNA against diet-induced damage in pigs. Br J Nutr 2012; 107: 1274–82. [DOI] [PubMed] [Google Scholar]
- 50.de Vogel J, Jonker-Termont DS, van Lieshout EM, Katan MB, van der Meer R. Green vegetables, red meat and colon cancer: Chlorophyll prevents the cytotoxic and hyperproliferative effects of haem in rat colon. Carcinogenesis 2005; 26: 387–93. [DOI] [PubMed] [Google Scholar]
- 51.O’Callaghan NJ, Fenech M, Conlon MA, Topping DL, Toden S, Bird AR. Colonocyte telomere shortening is greater with dietary red meat than white meat and is attenuated by resistant starch [electronic resource]. Clin Nutr 2012; 31: 60–4. [DOI] [PubMed] [Google Scholar]
- 52.Paturi G, Nyanhanda T, Butts CA, Herath TD, Monro JA, Ansell J. Effects of potato fiber and potato-resistant starch on biomarkers of colonic health in rats fed diets containing red meat. J Food Sci 2012; 77: H216–H23. [DOI] [PubMed] [Google Scholar]
- 53.Pierre FHF, Martin OCB, Santarelli RL, Tache S, Naud N, Gueraud F, Audebert M, Dupuy J, Meunier N, Attaix D, Vendeuvre JL, Mirvish SS, Kuhnle GCG, Cano N, Corpet DE. Calcium and alpha -tocopherol suppress cured-meat promotion of chemically induced colon carcinogenesis in rats and reduce associated biomarkers in human volunteers. Am J Clin Nutr 2013; 98: 1255–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Toden S, Bird AR, Topping DL, Conlon MA. Resistant starch prevents colonic DNA damage induced by high dietary cooked red meat or casein in rats. Cancer Biol Ther 2006; 5: 267–72. [DOI] [PubMed] [Google Scholar]
- 55.Toden S, Bird AR, Topping DL, Conlon MA. High red meat diets induce greater numbers of colonic DNA double-strand breaks than white meat in rats: Attenuation by high-amylose maize starch. Carcinogenesis 2007; 28: 2355–62. [DOI] [PubMed] [Google Scholar]
- 56.Toden S, Topping DL, Conlon MA, Belobrajdic DP, Bird AR. Effects of Dietary Beef and Chicken With and without high amylose maize starch on blood malondialdehyde, interleukins, IGF-I, insulin, leptin, MMP-2, and TIMP-2 concentrations in rats. Nutr Cancer 2010; 62: 454–65. [DOI] [PubMed] [Google Scholar]
- 57.van der Meer-van Kraaij C, Kramer E, Jonker-Termont D, Katan MB, van der Meer R, Keijer J. Differential gene expression in rat colon by dietary heme and calcium. Carcinogenesis 2005; 26: 73–9. [DOI] [PubMed] [Google Scholar]
- 58.Van Hecke T, Wouters A, Rombouts C, Izzati T, Berardo A, Vossen E, Claeys E, Van Camp J, Raes K, Vanhaecke L, Peeters M, De Vos WH, De Smet S. Reducing compounds equivocally influence oxidation during digestion of a high-fat beef product, which promotes cytotoxicity in colorectal carcinoma cell lines. J Agricult Food Chem 2016; 64: 1600–9. [DOI] [PubMed] [Google Scholar]
- 59.Winter J, Nyskohus L, Young GP, Hu Y, Conlon MA, Bird AR, Topping DL, Le Leu RK. Inhibition by resistant starch of red meat-induced promutagenic adducts in mouse colon. Cancer Prevent Res 2011; 4: 1920–8. [DOI] [PubMed] [Google Scholar]
- 60.Winter J, Conlon MA, Le Leu RK, Gratz SW, Young GP, Hu Y. Accumulation of promutagenic DNA adducts in the mouse distal colon after consumption of heme does not induce colonic neoplasms in the western diet model of spontaneous colorectal cancer [electronic resource]. Mol Nutr Food Res 2014; 58: 550–8. [DOI] [PubMed] [Google Scholar]
- 61.de Vogel J, van der Meer R, Katan MB, Jonker-Termont DSML. Natural chlorophyll but not chlorophyllin prevents heme-induced cytotoxic and hyperproliferative effects in rat colon. J Nutr 2005; 135: 1995–2000. [DOI] [PubMed] [Google Scholar]
- 62.Cho Y, Turner ND, Davidson LA, Chapkin RS, Carroll RJ, Lupton JR. A chemoprotective fish oil/pectin diet enhances apoptosis via Bcl-2 promoter methylation in rat azoxymethane-induced carcinomas. Exp Biol Med 2012; 237: 1387–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cho Y, Turner ND, Davidson LA, Chapkin RS, Carroll RJ, Lupton JR. Colon cancer cell apoptosis is induced by combined exposure to the n-3 fatty acid DHA and butyrate through promoter methylation. Exp Biol Med 2014; 239: 302–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Huncharek M, Muscat J, Kupelnick B. Colorectal cancer risk and dietary intake of calcium, vitamin D, and diary products: A meta-analysis of 26,335 cases from 60 observational studies. Nutr Cancer 2009; 61: 47–69. [DOI] [PubMed] [Google Scholar]
- 65.Johnson RL, Fleet JC. Animal models of colorectal cancer. Cancer Metastasis Rev 2013; 32: 39–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lawrie RA, Ledward DA. Lawrie's meat science, 7th ed Cambridge, England: Woodhead Publishing, 2006. [Google Scholar]
- 67.McKenna DR, Mies PD, Baird BE, Pfeiffer KD, Ellebracht JW, Savell JW. Biochemical and physical factors affecting discoloration characteristics of 19 bovine muscles. Meat Sci 2005; 70: 665–82. [DOI] [PubMed] [Google Scholar]
- 68.Suman SP, Faustman C, Lee SA, Tang J, Sepe HA, Vasudevan P, Annamalai T, Manojkumar M, Marek P, Venkitanarayanan KS. Effect of erythorbate, storage andhigh-oxygen packaging on premature browning in ground beef. Meat Sci 2005; 69: 363–9. [DOI] [PubMed] [Google Scholar]
- 69.Joseph P, Suman SP, Li S, Beach CM, Steinke L, Fontaine M. Characterization of bison (Bison bison) myoglobin. Meat Sci 2010; 84: 71–8. [DOI] [PubMed] [Google Scholar]
- 70.Anderson KE, Bloomer JR, Bonkovsky HL, Kuschner JP, Pierach CA, Pimstone NR, Desnick RJ. Recommendations for the diagnosis and treatment of the acute porphyrias. Ann Int Med 2005; 142: 439–50. [DOI] [PubMed] [Google Scholar]
- 71.Sesink AL, Termont DS, Kleibeuker JH, Van der Meer R. Red meat and colon cancer. Cancer Res 1999; 59: 5704–9. [PubMed] [Google Scholar]
- 72.Valenzuela C, de Romana DL, Olivares M, Moreales MS, Pizarro F. Total iron and heme iron content and their distribution in beef meat and viscera. Biol Trace Elem Res 2009; 132: 103–11. [DOI] [PubMed] [Google Scholar]
- 73.Reagan-Shaw S, Nihal M, Ahmad N. Dose translation from animal to human studies revisisted. FASEB J 2008; 22: 659–61. [DOI] [PubMed] [Google Scholar]
- 74.Pierre F, Freeman A, Tache S, van der Meer R, Corpet DE. Beef meat and blood sausage promote the formation of azoxymethane-induced mucin-depleted foci and aberrant crypt foci in rat colon. J Nutr 2004; 134: 2711–6. [DOI] [PubMed] [Google Scholar]
- 75.Chen J, Huang XF. The signal pathways in azoxymethane-induced colon cancer and preventive implications. Cancer Biol Ther 2009; 8: 1313–7. [DOI] [PubMed] [Google Scholar]
- 76.Kuhnle GG, Story GW, Reda T, Mani AR, Moore KP, Lunn JC, Bingham SA. Diet-induced endogenous formation of nitroso compounds in the GI tract. Free Rad Biol Med 2007; 43: 1040–7. [DOI] [PubMed] [Google Scholar]
- 77.Cross AJ, Pollock JR, Bingham SA. Haem, not protein or inorganic iron is responsible for endogenous intestinal n-nitrosation arising from red meat. Cancer Res 2003; 63: 2358–60. [PubMed] [Google Scholar]
- 78.Bingham SA, Pignatelli B, Pollock JR, Ellul A, Malaveille C, Gross G, Runswick S, Cummings JH, O'Neill IK. Does increased endogenous formation of N-nitroso compounds in the human colon explain the association between red meat and colon cancer? Carcinogenesis 1996; 17: 515–23. [DOI] [PubMed] [Google Scholar]
- 79.Bingham SA, Hughes R, Cross AJ. Effect of white versus red meat on endogenous N-nitrosation in the human colon and further evidence of a dose response. J Nutrition 2002; 132: 3522S–5S. [DOI] [PubMed] [Google Scholar]
- 80.Hughes R, Pollock JR, Bingham S. Effect of vegetables, tea, and soy on endogenous N-nitrosation, fecal ammonia, and fecal water genotoxicity during a high red meat diet in humans. Nutr Cancer 2002; 42: 70–7. [DOI] [PubMed] [Google Scholar]
- 81.Bryan NS, Alexander DD, Coughlin JR, Milkowski AL, Boffetta P. Ingested nitrate and nitrite and stomach cancer risk: An updated review. Food Chem Toxicol 2012; 50: 3646–65. [DOI] [PubMed] [Google Scholar]
- 82.Feelisch M, Rassaf T, Mnaimneh S, Singh N, Bryan NS, Jourd'Heuil D, Kelm M. Concomitant S-, N-, and heme-nitros(yl)ation in biological tissues and fluids: implications for the fate of NO in vivo. FASEB J 2002; 16: 1775–85. [DOI] [PubMed] [Google Scholar]
- 83.Hogg N. Red meet and colon cancer: Heme proteins and nitrite in the gut. Free Rad Biol Med 2007; 43: 1037–9. [DOI] [PubMed] [Google Scholar]