Abstract
Objective
This review summarizes a portion of the discussions of an NIH Workshop (Bethesda, MD, 2015) entitled, “Self-Regulation of Appetite, It's Complicated,” which focused on the biological aspects of appetite regulation.
Methods
Here we summarize the key biological inputs of appetite regulation and their implications for body weight regulation.
Results
These discussions offer an update of the long-held, rigid perspective of an “adipocentric” biological control, taking a broader view that also includes important inputs from the digestive tract, from lean mass, and from the chemical sensory systems underlying taste and smell. We are only beginning to understand how these biological systems are integrated and how this integrated input influences appetite and food eating behaviors. The relevance of these biological inputs was discussed primarily in the context of obesity and the problem of weight regain, touching on topics related to the biological predisposition for obesity and the impact that obesity treatments (dieting, exercise, bariatric surgery, etc.) might have on appetite and weight loss maintenance. Finally, we consider a common theme that pervaded the workshop discussions, which was individual variability.
Conclusions
It is this individual variability in the predisposition for obesity and in the biological response to weight loss that makes the biological component of appetite regulation so complicated. When this individual biological variability is placed in the context of the diverse environmental and behavioral pressures that also influence food eating behaviors, it is easy to appreciate the daunting complexities that arise with the self-regulation of appetite.
Introduction
Our biology provides an important collection of neuroendocrine inputs that affect hunger, satiety and the ongoing flux of ingested nutrients (1, 2). This biological regulation of appetite is part of a larger homeorhetic system that governs energy reserves and functional mass. Its regulation is very complex, engaging a number of tissues, organs, hormones, and neural circuits throughout the body in a feedback loop between the brain and peripheral tissues. In its simplest description, the brain sends out signals that affect food eating behaviors, nutrient absorption, energy storage, and eventually expended energy. In turn, nutrient and energy sensing systems in the gut, liver, adipose and other peripheral tissues inform the brain about the immediate energy needs, the levels of stored energy, and the metabolic requirements, which are required to meet the demands of the organism.
In this review, we examine aspects of this feedback system which are involved in promoting and regulating appetite and eating behaviors. In particular, we have summarized the perspectives of four members of a 2015 NIH Working Group entitled, “Self-Regulation of Appetite, It's Complicated,” who discussed biological mechanisms inherent in appetite control. This review is not meant to provide a comprehensive overview of the entire body of literature on this topic. Rather, this work represents the synthesis and integration of these perspectives as presented at the 2015 Workshop held in Bethesda, MD.
Key Biological Inputs of Appetite Regulation
Over the course of the last 50 years scientific thinking about the mechanisms of appetite control has changed dramatically. In the 1950s and 1960s the hypothalamic ‘dual centre’ hypothesis was believed to provide a comprehensive account of the initiation and inhibition of food intake e.g. Anand & Brobeck (3). Following technological advances in the identification of neurotransmitter pathways in the brain, the two-centre hypothesis was replaced by a model based on aminergic systems (4). More recently, much of the field has embraced a theory of appetite control based on an interaction between adipose tissue and peripheral episodic signals from gut-derived signals (5), as this perspective has provided a working framework for understanding certain aspects of appetite regulation in the context of obesity, weight loss, and weight regain.
Here, we break down the critical components of this “adipocentric” perspective and build upon them to generate a broader, more complex view of the biological inputs of appetite regulation. This working group centered its discussion on four aspects of biology that inform appetite regulation: 1) long term energy reserves; 2) nutrient sensing and availability; 3) functional mass metabolic requirements; and 4) the establishment of taste and food preference.
Long Term Energy Reserves of Adipose Tissue
The best characterized signals reflecting the long term energy reserves within the body are thought to be leptin and insulin (6). Leptin is secreted directly from adipose tissues in relation to the level of adiposity, while circulating insulin levels increase with peripheral insulin resistance that commonly develops with increasing adiposity. Together, leptin and insulin bind their respective receptors in the arcuate nucleus of the hypothalamus and brain stem regions to adjust key neural circuits of energy balance regulation to reduce food intake and increase energy expenditure. These hormones also convey their message to additional areas that include the prefontal cortex, the hippocampus, the ventral tegmental area, the nucleus accumbens, the amygdala, and other regions that are involved in learning, memory, decision making, and the rewarding aspects of eating behaviors (1, 7). Taken together, the overlapping signals reflecting energy reserves are integrated at a number of levels in a manner that exerts a concerted inhibitory impact on appetite and food eating behaviors (Figure 1).
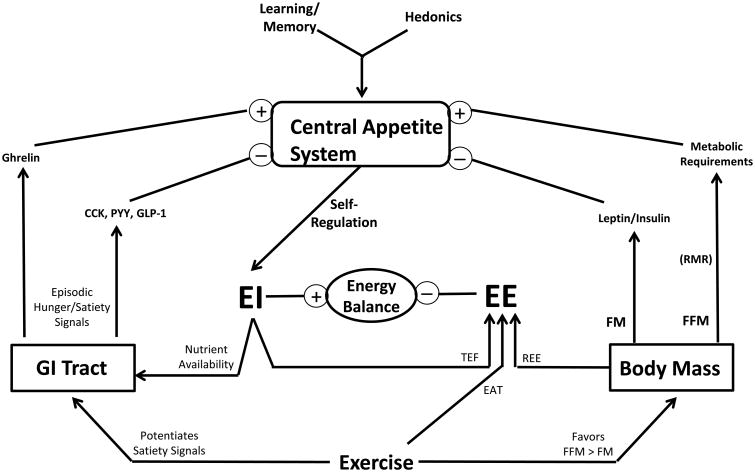
Figure 1. Biological Inputs of Appetite Regulation.
The key biological affectors of appetite are placed in the context of the energy balance relationship (energy intake, EI; expended energy, EE; thermic effect of food, TEF; exercise activity thermogenesis, EAT; resting energy expenditure, REE; resting metabolic rate, RMR)). Separate effects of fat-free mass (FFM) and fat mass (FM) denote stimulatory and inhibitory inputs, respectively. The gut provides feedback through neural and endocrine paths that involve the episodic hunger and satiety signals coincident to nutrient availability and the prandial state. These biological inputs operate in a neural architecture established early in life that dictates food preferences. Exercise may influence appetite through its impact on these biological inputs, but its overall impact is variable and complicated by compensatory food eating behaviors. The built-in redundancies, complexities, and individual variability, with each aspect of food preference and these feedback systems, which are rooted in the underlying genetics, establish a daunting biological complexity to the nature of appetite control.
While it is clear that the actions of leptin and insulin are important in appetite regulation, there are caveats that must be made about their conveyance of information about adiposity. First, this tonic inhibitory influence on appetite has a particularly strong influence under conditions when the energy reserves of adipose tissue are depleted and leptin and insulin levels in circulation are low. Under chronic conditions of energy surplus, the central and peripheral resistance to the actions of these hormones lessens their influence on appetite regulation. Second, the levels of both hormones are influenced by the prandial state of the organism. As such, low levels that would be observed during a chronically energy depleted state, like caloric restriction, would resolve very quickly with sustained overfeeding. Third, the signals that these hormones convey to the neural circuits affecting appetite are constantly integrated with the more immediate messages about nutrient availability, overlaid with the sustained influence of the energy demands of the functional mass, and placed in the context of other biological architecture of appetite regulation dictating food preference and reward.
Nutrient Availability: Gut-Derived Signals
There is extensive evidence that the gut provides important episodic inputs for the regulation of appetite that coincide with the prandial state and nutrient availability (Figure 1). There is a panoply of hormones secreted from peripheral tissues that have putative effects in appetite regulation (8). With the exception of ghrelin, the endocrine factors released from the gut promote satiety and/or satiation. Gut hormones secreted from the gastrointestinal (GI) enteroendocrine cells act as autocrine, paracrine and endocrine regulators of energy and glucose homeostasis via the circulation and indirectly via afferent nerves (9), targeting similar neural circuits that are affected by leptin and insulin.
The anorectic hormone peptide YY3-36 (PYY) and glucagon-like peptide-1 (GLP-1), an incretin hormone, are secreted in response to nutrient ingestion from enteroendocrine L-cells present throughout the GI tract. PYY has potent anorectic effects, with exogenous administration shown to reduce food intake in normal weight and obese humans (10). Robust evidence from experimental imaging and translational studies have identified that PYY mediates its anorectic effects predominantly by acting upon central appetite-regulating circuits and regions involved in food reward (11). GLP-1 also has appetite-suppressing effects and modulates neural activity within homeostatic and reward brain centers, in a manner additive to PYY (12). Circulating levels of the orexigenic hormone ghrelin, produced by P/D1 cells in oxyntic glands in the gastric fundus, increase in the fasted state and decrease post-meal. Ghrelin increases hunger and energy intake and leads to activation of neuronal pathways within homeostatic and reward centers involved in appetite regulation to control energy intake (13).
Bile acids, in addition to their role in lipid metabolism are now recognized to play a key role in regulating energy balance and metabolism primarily via the nuclear farnesoid X receptor (FXR) and the G-protein coupled receptor TGR5 (14). Bile acids acting via TGR5 stimulate L-cell secretion of GLP-1 and PYY. In addition, bile acids directly and indirectly through the FXR-induced antimicrobial peptides, regulate the gut microbiota composition which in turn has been extensively linked to the pathogenesis of obesity and type 2 diabetes. Thus, there is a complex interplay between the gut microbiome, bile acids and gut hormones. However, the relative importance of each of these factors in regulating appetite and the directionality of the relationship between these remains to be determined.
Functional Mass Metabolic Requirements
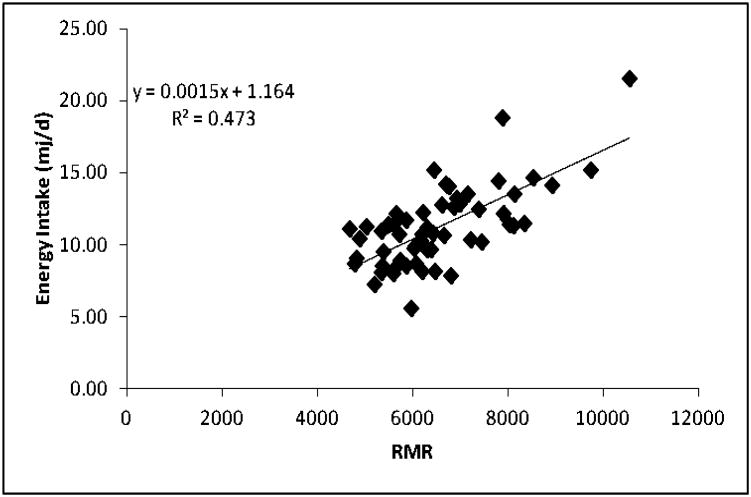
This association between fat free mass (FFM) and eating behavior has implications for an energy balance approach to appetite control, and for the relationship between energy expenditure (EE) and energy intake (EI) as described by Edholm and others (15, 16). It is well established that FFM is the primary determinant of resting metabolic rate (RMR), and that RMR is the largest component (60-70%) of daily EE (17) From a homeostatic standpoint, an ongoing and recurring drive to eat arising from the physiological demand for energy (e.g. RMR) appears logical, as this energy demand would remain relatively stable between days and would ensure the maintenance and execution of key biological and behavioral processes. Consequently, it might be predicted that RMR could be associated with the quantitative aspect of eating behavior and with daily EI. When this was examined (18), it was demonstrated that RMR was a significant determinant of the size of a self-determined meal, and of daily EI (when measured objectively and quantified – Figure 2). In addition, RMR was associated with the intensity of hunger objectively rated on hand held electronic data capture instruments (19).
Figure 2. Relationship between RMR and Self-Determined Intake.
Scatter plot and standardized β-coefficient to illustrate the relationship between resting metabolic rate RMR and daily energy intake in 59 individuals. While the underlying mechanisms remain elusive, this is one of many studies showing a strong association between fat free mass, the metabolic requirements of the body, and the drive to eat.
Consequently, these findings – that are broadly consistent with the early predictions of Edholm – have demonstrated an association between the major components of daily EE and daily EI. In other words, they demonstrate that appetite control could be a function of energy balance. Importantly, the major findings have been replicated in completely independent large data sets that included participants from different ethnic groups showing a huge range of EI (20), and from participants of variable body mass indices (BMIs) allowed to freely select their own diet under meticulously controlled semi-free living conditions (21). These confirmatory reports suggest that the associations are robust and are not restricted to a particular group of people measured in a specific geographical location.
This putative role for FFM does not preclude a role that fat mass (FM) plays in appetite control. Rather, there is likely a con-joint influence of FFM and FM on appetite control (22), which is shown in Figure 1). The implications stemming from this effect of body composition is that FM has an inhibitory influence on EI, but the strength of this tonic inhibition is moderated by insulin and leptin sensitivity (23). As people overconsume (due to cultural obesogenic influences), FM increases and the consequential increase in leptin and insulin resistance weakens the inhibitory influence of FM on appetite. This amounts to a ‘dis-inhibition’, so that accumulating FM fails to suppress EI and permits more eating (over-consumption). Indeed there is good evidence that low insulin sensitivity reduces post-prandial satiety and weakens meal to meal appetite control (24). In addition, clear positive associations of FFM and EI, and negative associations of FM and EI, have been demonstrated – but overlooked – in a comprehensive analysis carried out by Lissner et al. (25) more than 25 years ago.
The complexity imposed by the FFM-related component of appetite control is difficult to appreciate, because we have yet to establish a clear mechanism by which the energy requirements of functional mass are conveyed to the neural centers that influence food intake. Our lack of understanding of this mechanistic link clearly deserves more research, as it has long complicated the broader picture of the biological control of appetite.
The Establishment of Food and Taste Preference
The biological inputs of appetite regulation stemming from FFM, FM, and nutrient sensing in the GI tract, operate in a neural architecture with established preferences for taste and food (Figure 1). Taste and olfactory signals influence food selection and, consequently, EI (26). Relevant to appetite regulation, there is emerging evidence for interplay between signals of energy homeostasis and taste and smell. Insulin, leptin, GLP-1, PYY and ghrelin have been found in saliva and their cognate receptors identified on taste buds and olfactory neurons. In preclinical studies, increasing saliva PYY concentrations has been shown to alter food preference, reduce caloric intake and reduce body weight (27).
Scientific investigations during the past century reveal that humans are born with well-developed taste and olfactory systems (28, 29). Children prefer higher concentrations of sugars and salt, especially during periods of growth (30)—the adult pattern of preference emerges only in mid-adolescence(31)—and tasting something sweet can blunt expressions of pain for infants(32) and children(33), but not for most adults (33). Although bitter taste differs widely among individuals, at psychophysical and genetic levels, children are more sensitive to the taste of some bitters(34), which sweet and salty taste can partially mask or block (35, 36).
Early in life, children learn the rules of cuisine—how to eat, what to eat, when to eat, and what foods are supposed to taste like (37, 38). And this learning most often occurs in the context of the changing dynamic between mother and child(39) and the food environment in which they live (40, 41). Before their first taste of foods, they learn, like other animals, what their mother's diet “tastes like” since dietary flavors are transmitted to amniotic fluid and then mother's milk (42). These multiple routes of flavor learning suggest that there is redundancy of dietary information, providing complementary routes for the young to learn about the types of safe foods available in the environment before they themselves begin to eat (or forage on) foods (43, 44). Mothers eating diets rich in healthy foods transmit these flavors to amniotic fluid and mother's milk—when you feed a mother, you feed a child. Such early flavor lead to greater acceptance of those foods at weaning (45, 46). In contrast, infants fed formula learn to prefer its unique but monotonous profile and may have more difficulty initially accepting flavors not found in formula, such as those of fruits and vegetables (44). Thus the detrimental consequences of not being exposed to a variety of flavors of healthful foods when young cannot be understated.
Opportunities to get children to accept a variety of healthy foods do not end with pregnancy and breastfeeding, but continues throughout complementary feedings. Research begun in the 1980s (37, 38) revealed that regardless whether breastfed or formula fed, infants continue to learn to like the flavor of foods through several mechanisms. First, infants learn by repeated exposure to the same food. Infants exposed to the same fruit or vegetable, for different lengths of time (37, 38), ate significantly more of that fruit or vegetable compared with their initial acceptance of it. Merely looking at the food was not sufficient; children had to taste the food to learn to like it (47). Rapid increases in intake in infants after repeated exposure contrast with slower changes seen in toddlers (48), further highlighting the importance of starting early (49). Second, infants learn by repeated exposure to dietary variety. Eight days of tasting a variety of pureed fruits resulted in greater intake of a novel fruit (pears), similar to that observed in infants with repeated exposure to pears (50). Similar effects were observed after tasting a variety of pureed vegetables (50, 51). Such functional plasticity, one of the main characteristics of the brain, highlights the ability to change ingestive behavior based on experience (52).Experiencing the flavors of healthy foods, when part of the family's diet and food environment, helps children develop preferences for these foods. During childhood, they learn what to eat, how to eat and when foods are eaten on what occasions- they learn the rules of cuisine for their families. The food habits established during infancy track into childhood and adolescence for both nutrient-dense and nutrient-poor foods (53, 54, 55, 56, 57). Such dietary patterns, which begin to be identified during childhood (58), are significant determinants of the quality of the adult diet (59, 60). In addition, providing foods low in salt and sugars (both nutritive and non-nutritive) may help protect the developing child from excess intake later in life (61, 62). However, because consumption of vegetables and fruits is below recommended levels among many families (63, 64), some children are deprived of early sensory experiences with healthy foods, parental modeling, and food environments needed to learn to like these foods (38). In other words, to influence the establishment of taste preferences and acceptance of a variety of healthy foods, focus needs to be on feeding infants and children in the context of their family food environment.
Implications for Obesity and Body Weight Regulation
Biological Preference for Obesogenic Foods
Chemical senses of taste and smell determine the flavor of our foods and serve as our body's primary gatekeepers, determining whether to accept or reject a foreign substance, and if accepted, to inform the gastrointestinal system about the quality and quantity of the impending rush of nutrients or toxins (65). Understanding the biopsychology of these, the chemical senses that determine flavor, provide a foundational first step to prevent the development of many chronic diseases that derive in large part from poor food choices, particularly excess caloric intake of sweet, fatty and salty foods. Contrary to the potential for early preventive effects of exposure to the diverse flavors of healthy foods, unhealthy eating habits are rampant among the youngest members of our society (66). From the age of two years, Americans are more likely to eat a manufactured sweet than a fruit or vegetable on a given day (67). Dietary patterns established early in life (66), increasing an individual's risk for chronic non-communicable diseases, are the leading cause of death and disability worldwide (68). Thus, the importance of promoting healthy sustainable food habits from an early age cannot be underestimated.
Why is it so difficult to develop healthy food habits and to change unhealthy ones? Modern patterns of food choice that are antithetical to health must be considered. Two factors predispose toward obesogenic diets: (a) inborn, evolutionarily driven taste preference genes that make us vulnerable to the modern food environment rich in sugar, salt, and fat, and (b) the detrimental consequences of not being exposed to a variety of healthful foods in early childhood. However, there is inherent plasticity in the chemical senses. Although there are inborn responses (e.g., newborns like sweet taste, reject bitter), the senses interact with early experiences to determine what are appropriate and liked foods, which in turn ensures children are not restricted to a narrow range of foodstuffs (42, 69). That is, the biological drive to avoid bitter and prefer salty and sweet foods may have served children well in a feast-or-famine setting, but today their biology makes them especially vulnerable to environments abundant in highly processed and palatable foods, rich in added sugars, non-nutritive sweeteners, and salt. Devoid of experience with the taste of alternatives as fruits and vegetables, some may never learn to like flavors associated with healthy foods. (37, 38).
Genetic Predisposition for Obesity and Biologic Variation
There is emerging evidence for interplay between common genetic variants and circulating gut hormone levels, with altered gut hormone levels contributing to the obesity-risk effects of genetic variants. For example, children and adults with the obesity-risk-variant of rs9939609, linked to the fat-mass and obesity-associated (FTO) gene, exhibit increased appetite, increased EI and a preference for energy dense foods. Normal weight subjects homozygous for the obesity-risk rs9939609 variant (AA subjects) exhibit an attenuated postprandial suppression of ghrelin. increased hunger, and altered brain responses to food cues within regions that drive eating behavior, compared to subjects with the low-risk variant (TT subjects) (70). In other studies, the melanocortin-4 receptor (MC4R), known to play a role both in monogenic and common obesity, has been shown to be present on enteroendocrine P/D1 cells that produce ghrelin and L-cells that secrete PYY and GLP-1. MC4R agonists have been shown to alter circulating levels of PYY and GLP-1, thus delineating an interaction between genetics and gut hormones (71).
There is also increasing evidence for an interaction between dietary factors, genetic variation and obesity susceptibility. In randomized control trials of different diets a person's genotype has been shown to modify the effect of the diet intervention on weight loss, weight maintenance and changes in related metabolic traits such as lipids, insulin resistance and hypertension. For example, in the Preventing Overweight Using Novel Dietary Strategies (POUNDS lost) study increased protein diets were found to be more efficacious in subjects with the obesity-risk variants of FTO (72). Interestingly, protein has been shown to reduce ghrelin levels more than fat and carbohydrate (73) thus potentially increased protein diets may act via reducing the high ghrelin levels in FTO at-risk subjects. However, the impact of high-protein diets on gut hormone levels based on genotype has not been evaluated.
From an evolutionary perspective, key genes, like FTO and MC4R are likely representative of a vast number of genes that are critical for establishing the neural networks and endocrine systems inherent to the homeorhetic control of body weight. The long-held, but frequently debated, “thrifty genotype” hypothesis asserts that evolutionary pressures have over time selected for one or more multi-factorial genotypes that favored survival selective advantage through numerous feast/famine cycles in the past, but favor the development of obesity and metabolic diseases in the current environment with a readily available food supply (74). Compounding this genetic selection may be a more acute epigenetic insults from the environment that passed from one generation to the next through perinatal programming (75). While there remains about the origins of the predisposition for obesity, a polygenic and multi-factorial epigenetic variability between individials lays the foundation for the individual variability in the predisposition to become obese, our ability to self-regulate our food intake, and how our bodies respond to our attempt to lose weight through dieting.
Biological Adaptations to Dieting (76)
One of the most relevant circumstances in which the biological inputs emerge is in response to calorie-restricted weight loss (77). Almost every aspect of the homeorhetic system controlling body weight adapts to this challenge, culminating in an elevated appetite, suppressed energy expenditure, and a biological pressure to regain the lost weight (78). Proactively restricting ingested energy requires that endogenous stores be mobilized to meet the energetic demands of the individual. In response to this metabolic challenge, peripheral tissues send a signal, primarily through a decline in circulating levels of leptin and insulin, that energy stores are depleted. In addition, neuroendocrine signals from the gut send a signal of low nutrient availability, as ghrelin levels increase and the satiety signals of PYY, CCK, and GLP-1 decline. Along with changes in these surrogate signals, the nutrients in circulation also decline as they are absorbed and cleared with greater efficiency. This decline can be sensed in nutrient sensing nodes in both the peripheral tissues and in the brain (ventromedial hypothalamus) strengthen the signal of low nutrient availability that is conveyed by signals from the GI tract. These nutrient and neuroendocrine signals converge to convey an overwhelming signal that nutrients are in short supply and that energy reserves are depleted.
The consequence is that feelings of hunger emerge and the sensitivity to satiety signals from the periphery declines. At the same time, the brain sends signals out to peripheral tissues, primarily through the sympathetic nervous system, to enhance metabolic efficiency, reduce the metabolic demands, and prepare peripheral tissues to replete energy reserves when food becomes more available. The weight reduced state is thus characterized by elevated appetite and suppressed energy expenditure. To maintain weight loss, an individual must proactively ignore the strong feelings of hunger and limit their intake to the level that their metabolic requirements are suppressed. Studies in animal models of weight regain have shown that these biological pressures on appetite and metabolism can be very strong and persistent (79, 80). They do not resolve with time after weight loss; rather, they may even strengthen with time during weight loss maintenance. More studies of long-term metabolic recalibration after weight loss in humans are needed.
Biological Responses to Bariatric Surgery
Bariatric surgery is currently the only effective treatment for severe obesity, which is defined by a BMI equal to or greater than 40kg/m2, or greater than 35 kg/m2 in the presence of obesity-related complications (81). In contrast to weight loss though calorie-restricted dieting, bariatric surgery poses an effective treatment for severe obesity with significant weight loss, most often sustained in the long-term.
Bariatric surgery involves surgical manipulation of the GI tract, which alters nutrient flow and impacts upon GI biology. These changes engender beneficial effects upon energy and glucose homeostasis (9). The multi-factorial mechanisms promoting weight loss and improved metabolism following Roux-en-Y gastric bypass (RYGB) and sleeve gastrectomy (SG), the two most commonly performed procedures are incompletely understood. However, it is now clear that the beneficial effects of bariatric surgery are not achieved through restriction and malabsorption alone. Decreased EI as a result of altered eating behavior is the main driver for weight loss in humans (82). Reduced appetite, changes in subjective taste and food preference and altered neural response to food cues are thought to drive altered eating behavior. The biological mediators underlying these changes remain incompletely understood but gut-derived signals as a consequence of altered nutrient and/or biliary flow and/or microbiome changes are key candidates. In contrast to the effect of caloric restriction through dieting, RYGB and SG lead to a marked post-meal elevation of PYY and GLP-1 with a concomitant decrease in ghrelin (83). These gut hormone changes are thought to contribute to the marked reduction in appetite, reduced interest in food, and attenuated sweet taste palatability, which occur post-surgery. In addition, the variation in weight loss response following surgery is thought to be related to differences in gut hormone response with good responders exhibiting higher circulating PYY and GLP-1 levels coupled with lower ghrelin compared to poor responders (84). For the vast majority of patients, bariatric surgery offers sustained marked weight reduction coupled with unparalleled health benefits. Gaining an understanding of the mechanisms underlying these sustained weight loss and metabolic benefits holds the key to developing novel non-surgical treatments for obesity and type 2 diabetes (T2D).
Biological Responses to Exercise
Many studies have assessed EI during the manipulation of exercise. Most of these studies have been acute in nature i.e. often single dose, single day experiments (for a review, see (85) or (86)). The clear outcome is that exercise has little effect on EI within a single day (87). However, as the exercise is continued over several days the system begins to respond and a small compensatory rise in EI has been observed in both men and women (88, 89). Comparisons between participants undergoing high, medium and low volume sessions of exercise indicated a graded and proportional (but partial) compensatory increase in EI which accounted for approximately 30% of the EE (88). However there was a large range of individual variability. This variation became clearer when daily exercise sessions were continued for 16 days with participants showing between 0% and 60% compensation in EI for the exercise EE (89). As anticipated this variable response was reflected in small changes in body weight.
For medium term studies, in which mandatory exercise sessions were performed daily for 12 weeks in overweight and obese individuals (90), an average weight loss of approximately 3.3 kg was recorded but with weight change varying between –14.7 kg and +1.7 kg. This outcome is quite remarkable because the weight gain of some participants was achieved despite the performance of supervised and measured exercise sessions (5 days per week for 12 weeks). Therefore, even though all participants completed the exercise sessions (with total exercise-induced EE calculated at 28 – 29,000 kcal), there was a large variation in the change in body weight and composition. The variability in body weight changes following 12 weeks of supervised aerobic exercise has subsequently been replicated in a larger number of overweight and obese individuals (see Figure 3) and in several other trials of the effects of exercise on weight loss in obese people. More significant than the change and variability in body weight is the effect of exercise on body composition. The weight lost is almost entirely adipose tissue, where as the weight gain is reflected in lean mass (FFM) which is apparent in both men and women (91). Clearly, with exercise as an intervention, there are conflicting inputs to the regulation of appetite that involving both the biological feedback systems and the psychology of compensatory eating behaviors, which contributes to the complicated nature of self-regulation.
Figure 3. Individual Variability in the Response to Supervised Exercise.
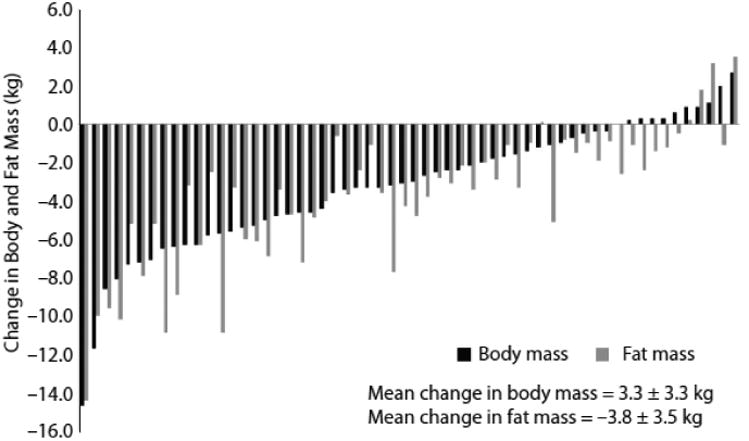
Data taken from the studies of King et al (90, 97) showing the wide variation in body weights and body fat in a group of overweight and obese individuals who completed 12 weeks of supervised and measured physical activity (5 sessions per week) designed to expend 2 MJ per session. These studies revealed that the effect of exercise on appetite regulation involves at least 2 processes: an increase in the overall (orexigenic) drive to eat and a concomitant increase in the satiating efficiency of a fixed meal. The individual variability in the overall response is likely rooted, at least in part, in how exercise differentially affects these two processes between individuals.
Common Theme in the Biological Regulation of Appetite – Individual Variability
There are a number of reasons why the biological control of appetite is, on its own, so complicated. First, the epigenetic and genetic variability between individuals impart nuances in how the energy homeorhetic system is established and how it responds to metabolic challenges. This may involve unique differences between the sexes that may have implications for how this feedback system functions. The control system is then subject to change through the lifespan (including the aging process), overall health and disease processes, and the ever changing milieu of environmental and behavioral stressors that are imposed. Compounding this individual variability is the vast number of different types of nutrients that must be sensed along the gastrointestinal tract and the wide variety of signals that are generated as these nutrients are consumed, absorbed, cleared, stored, and metabolized (92, 93). The numerous signals are integrated with a large amount of built-in redundancy to areas of the brain involved in both homeostatic and hedonic food eating behaviors (1, 7). When the complexities of the biological aspects of appetite control are considered in context of the behavioral and environmental diversity in which they operate, it is not surprising to find that individuals will vary quite dramatically in their ability to self-regulate the food they eat. The complicated nature of self-regulation poses a considerable problem for devising public health (and intervention) strategies for dealing with eating behavior change in relation to the obesity epidemic (94). This complexity rhetorically answers: “Why is it so hard”?
The description of susceptible and resistant phenotypes draws attention to the wide diversity in the pattern of the human eating response in the face of an obesogenic culture. Perhaps this should not be surprising given the great variability in the nutritional patterns adopted by the human species in extreme ranges of climate and habitat. Thus, inherent diversity in the variability in biologic mechanisms and environments, and responses effecting appetite regulation has implications for methodology used in studying these phenomena.
One methodological issue concerns the use of the statistical mean—or other measures of central tendency—to describe responses to interventions or treatments. In many studies of body weight, like the exercise intervention presented here (Figure 3), the average weight or body composition change would be regarded as the most important parameter of intervention effect. However, as Dilnot has (95) has pointed out, science is often weakened by subscribing to the ‘tyrany of the average’. Very often the mean outcome fails to adequately reveal the true effect of the intervention or treatment (weight loss response to enforced exercise is a good example). A truer reflection of intervention effects is described by the diversity of responses that encourages a deeper examination of the internal processes responsible. This implies that one unique explanation cannot account for all outcomes.
This issue draws attention, once again, to the nomothetic and idiographic approaches to scientific explanation (96). What should be the balance between seeking a common unifying principle and a regard for individual differences (quantitative and qualitative)? In light of this question, it may be an appropriate time for a paradigm shift to focus attention on individual variability rather than on the mean value of any set of responses. In scientific research the mean response is the statistical parameter associated with the elucidation of scientific principles. However, given cause-effect relationships and other features normally seen as the objectives of scientific inquiry, the great diversity of the human eating response suggests that we are dealing with a phenomenon for which the average may be inappropriate. This means that traditional use of research methodology may be missing much that is truly important in explaining human appetitive behavior. The identification of phenotypes—their behavioral expression, underlying physiology, genetics and interactions—constitutes a partial step toward a recognition of the variability inherent in human energy balance behaviors.
Conclusion
In summary, the biological control of appetite involves a host of nutrient sensors, endocrine factors, and neural signals, which together convey a message reflecting both long term energy reserves, metabolic requirements of the body, and the type and availability of nutrients to meet the immediate needs of the body's tissues. Their influence on appetite is apparent early in life and is inherently integrated with the control of EE. Control of both aspects of energy balance are riddled with complex redundancy. Moreover, this homeorhetic system both affects and is affected by other systems known to influence appetite through hedonic inputs, as well as learning and memory, which, among other things, establishes our preferences and aversions to certain types of flavors and foods. Underlying the complexities of this integration are the complications of individual variability that are rooted in genetic heterogeneity and the diverse environmental pressures, behavioral choices, and personal experiences that occur throughout the lifespan. In most individuals under normal conditions, biological influences on appetite exert a subtle, underlying tone of control, responding to eating behaviors that are modified by environmental pressures. However, their influence can emerge to become a driving force affecting the type and amount of food we eat under certain circumstances, as is the case with modern food patterns and di1eting. Thus when developing novel strategies to impact appetite regulation, individual variation and the complexity of inputs must be considered.
Acknowledgments
The authors are supported by grants from the National Institutes of Health (P30 DK48520, P50 HD073063, R01 CA164166 to PSM; R01 HD072307 to JAM), from Rosetrees Trust (to RLB), and from the BBSRC (BBS/B/05079 and BB/G005524/1 to JEL).
References
- 1.Faulconbridge LF, Hayes MR. Regulation of energy balance and body weight by the brain: a distributed system prone to disruption. Psychiatr Clin North Am. 2011;34:733–745. doi: 10.1016/j.psc.2011.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Riediger T. The receptive function of hypothalamic and brainstem centres to hormonal and nutrient signals affecting energy balance. The Proceedings of the Nutrition Society. 2012;71:463–477. doi: 10.1017/S0029665112000778. [DOI] [PubMed] [Google Scholar]
- 3.Anand BK, Brobeck JR. Hypothalamic control of food intake in rats and cats. Yale J Biol Med. 1951;24:123–140. [PMC free article] [PubMed] [Google Scholar]
- 4.Vucetic Z, Reyes TM. Central dopaminergic circuitry controlling food intake and reward: implications for the regulation of obesity. Wiley Interdiscip Rev Syst Biol Med. 2010;2:577–593. doi: 10.1002/wsbm.77. [DOI] [PubMed] [Google Scholar]
- 5.Badman MK, Flier JS. The gut and energy balance: visceral allies in the obesity wars. Science. 2005;307:1909. doi: 10.1126/science.1109951. [DOI] [PubMed] [Google Scholar]
- 6.Varela L, Horvath TL. Leptin and insulin pathways in POMC and AgRP neurons that modulate energy balance and glucose homeostasis. EMBO Rep. 2012;13:1079–1086. doi: 10.1038/embor.2012.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zheng H, Lenard NR, Shin AC, Berthoud HR. Appetite control and energy balance regulation in the modern world: reward-driven brain overrides repletion signals. International Journal of Obesity. 2009;33(Suppl 2):S8–13. doi: 10.1038/ijo.2009.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cummings DE, Overduin J. Gastrointestinal regulation of food intake. J Clin Invest. 2007;117:13–23. doi: 10.1172/JCI30227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scott WR, Batterham RL. Roux-en-Y gastric bypass and laparoscopic sleeve gastrectomy: understanding weight loss and improvements in type 2 diabetes after bariatric surgery. Am J Physiol Regul Integr Comp Physiol. 2011;301:R15–27. doi: 10.1152/ajpregu.00038.2011. [DOI] [PubMed] [Google Scholar]
- 10.Manning S, Batterham RL. The role of gut hormone peptide YY in energy and glucose homeostasis: twelve years on. Annual review of physiology. 2014;76:585–608. doi: 10.1146/annurev-physiol-021113-170404. [DOI] [PubMed] [Google Scholar]
- 11.Batterham RL, ffytche DH, Rosenthal JM, Zelaya FO, Barker GJ, Withers DJ, et al. PYY modulation of cortical and hypothalamic brain areas predicts feeding behaviour in humans. Nature. 2007;450:106–109. doi: 10.1038/nature06212. [DOI] [PubMed] [Google Scholar]
- 12.Manning S, Pucci A, Batterham RL. GLP-1: a mediator of the beneficial metabolic effects of bariatric surgery? Physiology. 2015;30:50–62. doi: 10.1152/physiol.00027.2014. [DOI] [PubMed] [Google Scholar]
- 13.Muller TD, Nogueiras R, Andermann ML, Andrews ZB, Anker SD, Argente J, et al. Ghrelin. Molecular metabolism. 2015;4:437–460. doi: 10.1016/j.molmet.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Penney NC, Kinross J, Newton RC, Purkayastha S. The role of bile acids in reducing the metabolic complications of obesity after bariatric surgery: a systematic review. Int J Obes (Lond) 2015;39:1565–1574. doi: 10.1038/ijo.2015.115. [DOI] [PubMed] [Google Scholar]
- 15.Edholm OG, Fletcher JG, Widdowson EM, McCance RA. The Energy Expenditure and Food Intake of Individual Men. British Journal of Nutrition. 1955;9:286–300. doi: 10.1079/bjn19550040. [DOI] [PubMed] [Google Scholar]
- 16.Edholm O. Energy balance in man. Studies carried out by the Division of Human Physiology, National Institute for Medical Research. Journal of Human Nutrition (UK) 1977;31:413–431. [PubMed] [Google Scholar]
- 17.Ravussin E, Lillioja S, Anderson TE, Christin L, Bogardus C. Determinants of 24-hour energy expenditure in man. Methods and results using a respiratory chamber. The Journal of clinical investigation. 1986;78:1568–1578. doi: 10.1172/JCI112749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Caudwell P, Finlayson G, Gibbons C, Hopkins M, King N, Naslund E, et al. Resting metabolic rate is associated with hunger, self-determined meal size, and daily energy intake and may represent a marker for appetite. American Journal of Clinical Nutrition. 2013;97:7–14. doi: 10.3945/ajcn.111.029975. [DOI] [PubMed] [Google Scholar]
- 19.Gibbons C, Caudwell P, Finlayson G, King N, Blundell J. Validation of a new hand-held electronic data capture method for continuous monitoring of subjective appetite sensations. International Journal of Behavioral Nutrition and Physical Activity. 2011;8:57–64. doi: 10.1186/1479-5868-8-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weise C, Hohenadel M, Krakoff J, Votruba S. Body composition and energy expenditure predict ad-libitum food and macronutrient intake in humans. International Journal of Obesity. 2013 doi: 10.1038/ijo.2013.85.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hopkins M, Finlayson G, Duarte C, Whybrow S, Ritz P, Horgan GW, et al. Modelling the associations between fat-free mass, resting metabolic rate and energy intake in the context of total energy balance. International Journal of Obesity. 2016;40:312–318. doi: 10.1038/ijo.2015.155. [DOI] [PubMed] [Google Scholar]
- 22.Blundell JE, Caudwell P, Gibbons C, Hopkins M, Naslund E, King N, et al. Role of resting metabolic rate and energy expenditure in hunger and appetite control: a new formulation. Disease Models & Mechanisms. 2012;5:608–613. doi: 10.1242/dmm.009837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Blundell J, Caudwell P, Gibbons C, Hopkins M, Naslund E, King N, et al. Body composition and appetite: fat-free mass (but not fat-mass or BMI) is positively associated with self-determined meal size and daily energy intake in humans. British Journal of Nutrition. 2012;107:445–459. doi: 10.1017/S0007114511003138. [DOI] [PubMed] [Google Scholar]
- 24.Flint A, Gregersen NT, Gluud LL, Moller BK, Raben A, Tetens I, et al. Associations between postprandial insulin and blood glucose responses, appetite sensations and energy intake in normal weight and overweight individuals: a meta-analysis of test meal studies. British Journal of Nutrition. 2007;98:17–25. doi: 10.1017/S000711450768297X. [DOI] [PubMed] [Google Scholar]
- 25.Lissner L, Habicht JP, Strupp BJ, Levitsky D, Haas JD, Roe D. Body composition and energy intake: do overweight women overeat and underreport? The American Journal of Clinical Nutrition. 1989;49:320–325. doi: 10.1093/ajcn/49.2.320. [DOI] [PubMed] [Google Scholar]
- 26.Cummings DE. Taste and the regulation of food intake: it's not just about flavor. Am J Clin Nutr. 2015;102:717–718. doi: 10.3945/ajcn.115.120667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Acosta A, Hurtado MD, Gorbatyuk O, La Sala M, Duncan D, Aslanidi G, et al. Salivary PYY: a putative bypass to satiety. PLoS One. 2011;6:e26137. doi: 10.1371/journal.pone.0026137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Forestell CA, Mennella JA. The ontogeny of taste perception and preference throughout childhood. In: Doty RL, editor. Handbook of Olfaction and Gustation. 3rd. Wiley-Liss; New York: 2015. [Google Scholar]
- 29.Schaal B, Hummel T, Soussignan R. Olfaction in the fetal and premature infant: functional status and clinical implications. Clin Perinatol. 2004;31:261–285. vi–vii. doi: 10.1016/j.clp.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 30.Mennella JA, Finkbeiner S, Lipchock SV, Hwang LD, Reed DR. Preferences for salty and sweet tastes are elevated and related to each other during childhood. PLoS One. 2014;9:e92201. doi: 10.1371/journal.pone.0092201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mennella JA, Lukasewycz LD, Griffith JW, Beauchamp GK. Evaluation of the Monell forced-choice, paired-comparison tracking procedure for determining sweet taste preferences across the lifespan. Chem Senses. 2011;36:345–355. doi: 10.1093/chemse/bjq134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Harrison D, Stevens B, Bueno M, Yamada J, Adams-Webber T, Beyene J, et al. Efficacy of sweet solutions for analgesia in infants between 1 and 12 months of age: a systematic review. Arch Dis Child. 2010;95:406–413. doi: 10.1136/adc.2009.174227. [DOI] [PubMed] [Google Scholar]
- 33.Pepino MY, Mennella JA. Sucrose-induced analgesia is related to sweet preferences in children but not adults. Pain. 2005;119:210–218. doi: 10.1016/j.pain.2005.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mennella JA, Pepino MY, Duke FF, Reed DR. Age modifies the genotype-phenotype relationship for the bitter receptor TAS2R38. BMC Genet. 2010;11:60. doi: 10.1186/1471-2156-11-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mennella JA, Reed DR, Mathew PS, Roberts KM, Mansfield CJ. “A spoonful of sugar helps the medicine go down”: bitter masking by sucrose among children and adults. Chemical Senses. 2015;40:17–25. doi: 10.1093/chemse/bju053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mennella JA, Reed DR, Roberts KM, Mathew PS, Mansfield CJ. Age-related differences in bitter taste and efficacy of bitter blockers. PLoS One. 2014;9:e103107. doi: 10.1371/journal.pone.0103107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Birch LL, Doub AE. Learning to eat: birth to age 2 y. Am J Clin Nutr. 2014;99:723S–728S. doi: 10.3945/ajcn.113.069047. [DOI] [PubMed] [Google Scholar]
- 38.Mennella JA, Reiter AR, Daniels LM. Vegetable and fruit acceptance during infancy: Impact of ontogeny, genetics, and early experiences. Adv Nutr. 2016;7:211S–219S. doi: 10.3945/an.115.008649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Neville MC, Anderson SM, McManaman JL, Badger TM, Bunik M, Contractor N, et al. Lactation and neonatal nutrition: defining and refining the critical questions. J Mammary Gland Biol Neoplasia. 2012;17:167–188. doi: 10.1007/s10911-012-9261-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Drewnowski A, Rehm CD. Socioeconomic gradient in consumption of whole fruit and 100% fruit juice among US children and adults. Nutr J. 2015;14:3. doi: 10.1186/1475-2891-14-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Aggarwal A, Cook AJ, Jiao J, Seguin RA, Vernez Moudon A, Hurvitz PM, et al. Access to supermarkets and fruit and vegetable consumption. Am J Public Health. 2014;104:917–923. doi: 10.2105/AJPH.2013.301763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mennella JA. The chemical senses and the development of flavor preferences in humans. In: Hale TW, Hartmann PE, editors. Textbook on Human Lactation. Hale Publishing; Amarillo, TX: 2007. pp. 403–414. [Google Scholar]
- 43.Provenza FD, Launchbauch KL. Foraging on the edge of chaos. In: Launchbaugh KL, Sanders KD, Mosley JC, editors. Grazing behavior of livestock and wildlife. J. C. Mosley; Idaho Forest, Wildlife and Range Experiment Station Bulletin No.70 University of Idaho, Moscow, Idaho: 1999. pp. 1–12. [Google Scholar]
- 44.Mennella JA, Trabulsi JC, Inamdar LB. The sensory world of formula-fed infants: differences among artificial milk feedings in flavor learning and satiation. In: Preedy VR, Watson RR, Zibadi S, editors. Handbook of dietary and nutritional aspects of bottle feeding Wageningen. Academic Publishers; Wageningen, The Netherlands: 2014. pp. 95–116. [Google Scholar]
- 45.Forestell CA, Mennella JA. Early determinants of fruit and vegetable acceptance. Pediatrics. 2007;120:1247–1254. doi: 10.1542/peds.2007-0858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mennella JA, Jagnow CP, Beauchamp GK. Prenatal and postnatal flavor learning by human infants. Pediatrics. 2001;107:E88. doi: 10.1542/peds.107.6.e88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Birch LL, McPhee L, Shoba BC, Pirok E, Steinberg L. What kind of exposure reduces children's food neophobia? Looking vs. tasting. Appetite. 1987;9:171–178. doi: 10.1016/s0195-6663(87)80011-9. [DOI] [PubMed] [Google Scholar]
- 48.Birch LL, Marlin DW. I don't like it; I never tried it: effects of exposure on two-year-old children's food preferences. Appetite. 1982;3 doi: 10.1016/s0195-6663(82)80053-6. [DOI] [PubMed] [Google Scholar]
- 49.Birch LL, Anzman-Frasca S, Paul IM. Starting early: obesity prevention during infancy. Nestle Nutr Inst Workshop Ser. 2012;73:81–94. doi: 10.1159/000341300. [DOI] [PubMed] [Google Scholar]
- 50.Mennella JA, Nicklaus S, Jagolino AL, Yourshaw LM. Variety is the spice of life: strategies for promoting fruit and vegetable acceptance during infancy. Physiol Behav. 2008;94:29–38. doi: 10.1016/j.physbeh.2007.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gerrish CJ, Mennella JA. Flavor variety enhances food acceptance in formula-fed infants. Am J Clin Nutr. 2001;73:1080–1085. doi: 10.1093/ajcn/73.6.1080. [DOI] [PubMed] [Google Scholar]
- 52.Kolb B, Gibb R. Brain plasticity and behaviour in the developing brain. J Can Acad Child Adolesc Psychiatry. 2011;20:265–276. [PMC free article] [PubMed] [Google Scholar]
- 53.Skinner JD, Carruth BR, Wendy B, Ziegler PJ. Children's food preferences: a longitudinal analysis. J Am Diet Assoc. 2002;102:1638–1647. doi: 10.1016/s0002-8223(02)90349-4. [DOI] [PubMed] [Google Scholar]
- 54.Nicklaus SBV, Chabanet C, Issanchou S. A prospective study of food preferences in childhood. Food Quality and Preference. 2004;15:805–819. [Google Scholar]
- 55.Singer MR, Moore LL, Garrahie EJ, Ellison RC. The tracking of nutrient intake in young children: the Framingham Children's Study. Am J Public Health. 1995;85:1673–1677. doi: 10.2105/ajph.85.12.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lioret S, McNaughton SA, SSpence AC, Crawford D, Campbell KJ. Tracking of dietary intakes in early childhood: the Melbourne InFANT Program. European journal of clinical nutrition. 2013;67:275–281. doi: 10.1038/ejcn.2012.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Northstone K, Emmett PM. Are dietary patterns stable throughout early and mid-childhood? A birth cohort study. Br J Nutr. 2008;100:1069–1076. doi: 10.1017/S0007114508968264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kaikkonen JE, Mikkila V, Magnussen CG, Juonala M, Viikari JS, Raitakari OT. Does childhood nutrition influence adult cardiovascular disease risk?--insights from the Young Finns Study. Annals of medicine. 2013;45:120–128. doi: 10.3109/07853890.2012.671537. [DOI] [PubMed] [Google Scholar]
- 59.Mikkila V, Rasanen L, Raitakari OT, Pietinen P, Viikari J. Longitudinal changes in diet from childhood into adulthood with respect to risk of cardiovascular diseases: The Cardiovascular Risk in Young Finns Study. European journal of clinical nutrition. 2004;58:1038–1045. doi: 10.1038/sj.ejcn.1601929. [DOI] [PubMed] [Google Scholar]
- 60.Mikkila V, Rasanen L, Raitakari OT, Marniemi J, Pietinen P, Ronnemaa T, Viikari J. Major dietary patterns and cardiovascular risk factors from childhood to adulthood. The Cardiovascular Risk in Young Finns Study. Br J Nutr. 2007;98:218–225. doi: 10.1017/S0007114507691831. [DOI] [PubMed] [Google Scholar]
- 61.Strategies to reduce sodium intake in the United States. National Academies Press; Washington, DC: 2010. Institute of Medicine, Committee on Strategies to Reduce Sodium Intake Food and Nutrition Board. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Swithers SE. Artificial sweeteners are not the answer to childhood obesity. Appetite. 2015 doi: 10.1016/j.appet.2015.03.027. [DOI] [PubMed] [Google Scholar]
- 63.Fowler JK, Evers SE, Campbell MK. Inadequate dietary intakes among pregnant women. Can J Diet Pract Res. 2012;73:72–77. doi: 10.3148/73.2.2012.72. [DOI] [PubMed] [Google Scholar]
- 64.Krebs-Smith SM, Guenther PM, Subar AF, Kirkpatrick SI, Dodd KW. Americans do not meet federal dietary recommendations. J Nutr. 2010;140:1832–1838. doi: 10.3945/jn.110.124826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sclafani A. Sweet taste signaling in the gut. Proc Natl Acad Sci U S A. 2007;104:14887–14888. doi: 10.1073/pnas.0707410104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Saavedra JM, Deming D, Dattilo A, Reidy K. Lessons from the feeding infants and toddlers study in North America: what children eat, and implications for obesity prevention. Ann Nutr Metab. 2013;62 Suppl 3:27–36. doi: 10.1159/000351538. [DOI] [PubMed] [Google Scholar]
- 67.Siega-Riz AM, Deming DM, Reidy KC, Fox MK, Condon E, Briefel RR. Food consumption patterns of infants and toddlers: where are we now? J Am Diet Assoc. 2010;110:S38–51. doi: 10.1016/j.jada.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 68.Lim SS, Vos T, Flaxman AD, Danaei G, Shibuya K, Adair-Rohani H, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2224–2260. doi: 10.1016/S0140-6736(12)61766-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mennella JA, Beauchamp GK. The role of early life experiences in flavor perception and delight. In: Dube L, Bechara A, Dagher A, Drewnowski A, Lebel J, James P, et al., editors. Obesity prevention The role of society and brain on individual behavior. Elsevier; London: 2010. pp. 203–218. [Google Scholar]
- 70.Karra E, O'Daly OG, Choudhury AI, Yousseif A, Millership S, Neary MT, et al. A link between FTO, ghrelin, and impaired brain food-cue responsivity. J Clin Invest. 2013;123:3539–3551. doi: 10.1172/JCI44403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Panaro BL, Tough IR, Engelstoft MS, Matthews RT, Digby GJ, Moller CL, et al. The melanocortin-4 receptor is expressed in enteroendocrine L cells and regulates the release of peptide YY and glucagon-like peptide 1 in vivo. Cell metabolism. 2014;20:1018–1029. doi: 10.1016/j.cmet.2014.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhang X, Qi Q, Zhang C, Smith SR, Hu FB, Sacks FM, et al. FTO genotype and 2-year change in body composition and fat distribution in response to weight-loss diets: the POUNDS LOST Trial. Diabetes. 2012;61:3005–3011. doi: 10.2337/db11-1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Foster-Schubert KE, Overduin J, Prudom CE, Liu J, Callahan HS, Gaylinn BD, et al. Acyl and total ghrelin are suppressed strongly by ingested proteins, weakly by lipids, and biphasically by carbohydrates. J Clin Endocrinol Metab. 2008;93:1971–1979. doi: 10.1210/jc.2007-2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Neel JV. Diabetes mellitus: a “thrifty” genotype rendered detrimental by “progress”? Am J Hum Genet. 1962;14:353–362. [PMC free article] [PubMed] [Google Scholar]
- 75.Hales CN, Barker DJ. The thrifty phenotype hypothesis. Br Med Bull. 2001;60:5–20. doi: 10.1093/bmb/60.1.5. [DOI] [PubMed] [Google Scholar]
- 76.Wells JC. Commentary: The thrifty phenotype and the hierarchical preservation of tissues under stress. Int J Epidemiol. 2013;42:1223–1227. doi: 10.1093/ije/dyt130. [DOI] [PubMed] [Google Scholar]
- 77.MacLean PS, Wing RR, Davidson T, Epstein L, Goodpaster B, Hall KD, et al. NIH working group report: Innovative research to improve maintenance of weight loss. Obesity (Silver Spring) 2015;23:7–15. doi: 10.1002/oby.20967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Maclean PS, Bergouignan A, Cornier MA, Jackman MR. Biology's response to dieting: the impetus for weight regain. Am J Physiol Regul Integr Comp Physiol. 2011;301:R581–600. doi: 10.1152/ajpregu.00755.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.MacLean PS, Higgins JA, Johnson GC, Fleming-Elder BK, Donahoo WT, Melanson EL, et al. Enhanced metabolic efficiency contributes to weight regain after weight loss in obesity-prone rats. Am J Physiol Regul Integr Comp Physiol. 2004;287:R1306–1315. doi: 10.1152/ajpregu.00463.2004. [DOI] [PubMed] [Google Scholar]
- 80.MacLean PS, Higgins JA, Jackman MR, Johnson GC, Fleming-Elder BK, Wyatt HR, et al. Peripheral metabolic responses to prolonged weight reduction that promote rapid, efficient regain in obesity-prone rats. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1577–1588. doi: 10.1152/ajpregu.00810.2005. [DOI] [PubMed] [Google Scholar]
- 81.Gloy VL, Briel M, Bhatt DL, Kashyap SR, Schauer PR, Mingrone G, et al. Bariatric surgery versus non-surgical treatment for obesity: a systematic review and meta-analysis of randomised controlled trials. BMJ. 2013;347:f5934. doi: 10.1136/bmj.f5934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Odstrcil EA, Martinez JG, Santa Ana CA, Xue B, Schneider RE, Steffer KJ, et al. The contribution of malabsorption to the reduction in net energy absorption after long-limb Roux-en-Y gastric bypass. Am J Clin Nutr. 2010;92:704–713. doi: 10.3945/ajcn.2010.29870. [DOI] [PubMed] [Google Scholar]
- 83.Yousseif A, Emmanuel J, Karra E, Millet Q, Elkalaawy M, Jenkinson AD, et al. Differential effects of laparoscopic sleeve gastrectomy and laparoscopic gastric bypass on appetite, circulating acyl-ghrelin, peptide YY3-36 and active GLP-1 levels in non-diabetic humans. Obesity surgery. 2014;24:241–252. doi: 10.1007/s11695-013-1066-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Dirksen C, Jorgensen NB, Bojsen-Moller KN, Kielgast U, Jacobsen SH, Clausen TR, et al. Gut hormones, early dumping and resting energy expenditure in patients with good and poor weight loss response after Roux-en-Y gastric bypass. Int J Obes (Lond) 2013;37:1452–1459. doi: 10.1038/ijo.2013.15. [DOI] [PubMed] [Google Scholar]
- 85.Hopkins M, King NA, Blundell JE. Acute and long-term effects of exercise on appetite control: is there any benefit for weight control? Current Opinion in Clinical Nutrition & Metabolic Care. 2010;13:635. doi: 10.1097/MCO.0b013e32833e343b. [DOI] [PubMed] [Google Scholar]
- 86.Donnelly JE, Herrmann SD, Lambourne K, Szabo AN, Honas JJ, Washburn RA. Does increased exercise or physical activity alter ad-libitum daily energy intake or macronutrient composition in healthy adults? A systematic review. PloS one. 2014;9:e83498. doi: 10.1371/journal.pone.0083498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Blundell J, King N. Effects of exercise on appetite control: loose coupling between energy expenditure and energy intake. International journal of obesity Supplement. 1998;22:S22–S29. [PubMed] [Google Scholar]
- 88.Stubbs R, Sepp A, Hughes D, Johnstone A, King N, Horgan G, et al. The effect of graded levels of exercise on energy intake and balance in free-living women. International Journal of Obesity. 2002;26:866–869. doi: 10.1038/sj.ijo.0801874. [DOI] [PubMed] [Google Scholar]
- 89.Whybrow S, Hughes DA, Ritz P, Johnstone AM, Horgan GW, King N, et al. The effect of an incremental increase in exercise on appetite, eating behaviour and energy balance in lean men and women feeding ad libitum. British Journal of Nutrition. 2008;100:1109–1115. doi: 10.1017/S0007114508968240. [DOI] [PubMed] [Google Scholar]
- 90.King NA, Hopkins M, Caudwell P, Stubbs R, Blundell JE. Individual variability following 12 weeks of supervised exercise: identification and characterization of compensation for exercise-induced weight loss. International Journal of Obesity. 2008;32:177–184. doi: 10.1038/sj.ijo.0803712. [DOI] [PubMed] [Google Scholar]
- 91.Caudwell P, Gibbons C, Hopkins M, King N, Finlayson G, Blundell J. No Sex Difference in Body Fat in Response to Supervised and Measured Exercise. Medicine & Science in Sports & Exercise. 2013;45:351–358. doi: 10.1249/MSS.0b013e31826ced79. [DOI] [PubMed] [Google Scholar]
- 92.Geraedts MC, Troost FJ, Saris WH. Gastrointestinal targets to modulate satiety and food intake. Obesity reviews: an official journal of the International Association for the Study of Obesity. 2011;12:470–477. doi: 10.1111/j.1467-789X.2010.00788.x. [DOI] [PubMed] [Google Scholar]
- 93.Sclafani A, Ackroff K. Role of gut nutrient sensing in stimulating appetite and conditioning food preferences. Am J Physiol Regul Integr Comp Physiol. 2012;302:R1119–1133. doi: 10.1152/ajpregu.00038.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ridley M. Nature via nurture: Genes, experience, and what makes us human. HarperCollins Publishers; 2003. [Google Scholar]
- 95.Dilnot A. The tiger that isn't: seeing through a world of numbers. Profile Books. 2008 [Google Scholar]
- 96.Allport GW. Personality: A psychological interpretation. 1937 [Google Scholar]
- 97.King NA, Caudwell PP, Hopkins M, Stubbs JR, Naslund E, Blundell JE. Dual-process action of exercise on appetite control: increase in orexigenic drive but improvement in meal-induced satiety. The American Journal of Clinical Nutrition. 2009;90:921–927. doi: 10.3945/ajcn.2009.27706. [DOI] [PubMed] [Google Scholar]