Abstract
The innate immune system has been implicated in both AKI and CKD. Damaged mitochondria release danger molecules, such as reactive oxygen species, DNA, and cardiolipin, which can cause NLRP3 inflammasome activation and upregulation of IL-18 and IL-1β. It is not known if mitochondrial damage persists long after ischemia to sustain chronic inflammasome activation. We conducted a 9-month study in Sprague–Dawley rats after 45 minutes of bilateral renal ischemia. We detected glomerular and peritubular capillary rarefaction, macrophage infiltration, and fibrosis at 1 month. Transmission electron microscopy revealed mitochondrial degeneration, mitophagy, and deformed foot processes in podocytes. These changes progressed over the study period, with a persistent increase in renal cortical expression of IL-18, IL-1β, and TGF-β, despite a gradual decline in TNF-α expression and macrophage infiltration. Treatment with a mitoprotective agent (SS-31; elamipretide) for 6 weeks, starting 1 month after ischemia, preserved mitochondrial integrity, ameliorated expression levels of all inflammatory markers, restored glomerular capillaries and podocyte structure, and arrested glomerulosclerosis and interstitial fibrosis. Further, helium ion microscopy vividly demonstrated the restoration of podocyte structure by SS-31. The protection by SS-31 was sustained for ≥6 months after treatment ended, with normalization of IL-18 and IL-1β expression. These results support a role for mitochondrial damage in inflammasome activation and CKD and suggest mitochondrial protection as a novel therapeutic approach that can arrest the progression of CKD. Notably, SS-31 is effective when given long after AKI and provides persistent protection after termination of drug treatment.
Keywords: Podocytes, innate immunity, inflammasome, microvascular rarefaction, SS- 31, Elamipretide
Patients who survive AKI have a greater risk for CKD.1 Currently, treatment of CKD is limited to aggressive control of hypertension with inhibitors of the renin-angiotensin-aldosterone system and reducing nitrogen load to the kidney. Although there are a number of experimental interventions in clinical trials, they have to be administered before or shortly after the acute injury,2 and some are offset by serious adverse effects.3–7 The Acute Dialysis Quality Initiative XIII Work Group recently concluded there are no therapeutic interventions specifically targeting progression from AKI to CKD.8 Given the increasing global burden of CKD,9 novel strategies that can halt the progression of CKD and restore normal renal structure and function are needed.8
Inflammation plays a critical role in the initiation and progression of renal fibrosis.8 Injured epithelial cells secrete proinflammatory cytokines to recruit inflammatory cells to sites of injury to drive tissue repair. Profibrotic cytokines, such as TGF-β, stimulate matrix-producing cells to produce extracellular matrix. Antifibrotic treatments have mostly targeted TGF-β, but TGF-β is a pleiotropic cytokine with vital anti-inflammatory functions, and blocking TGF-β activity may incite immune activation.10 It is thus imperative that novel targets be identified that can arrest the chronic inflammation and progressive fibrosis.
The innate immune system has recently been implicated in both acute and chronic kidney injury.11,12 Necrotic cells release danger-associated molecular patterns (DAMPs) that can trigger an inflammatory form of cell death mediated by Nod-like receptors (NLRs).13 These NLRs form multimeric protein complexes termed inflammasomes, of which NLRP3 is the best understood for its involvement in chronic and acute kidney disease.14,15 The NLRP3 inflammasome triggers activation of caspase-1, which then processes the proinflammatory cytokines pro–IL-1β and pro–IL-18 into their bioactive mature forms. The inflammasome may be considered the final common pathway that sustains the inflammation–fibrosis cycle in CKD, and IL-1β and IL-18 represent potential therapeutic targets for CKD.16
Inflammasome activation in response to a variety of stimuli appears to converge upon mitochondrial reactive oxygen species (ROS).17–19 Mitochondrial DNA and N-formyl peptides represent two other sources of mitochondrial DAMPs.20 Compounds that cause mitochondrial damage enhance NRLP3 activation, whereas compounds that reduce mitochondrial ROS inhibit inflammasome activation.21–23 Further, NLRP3 appears to bind directly to cardiolipin, a phospholipid that is only expressed on the inner mitochondrial membrane, suggesting that mitochondrial damage and the translocation of cardiolipin to the outer mitochondrial membrane (OMM) may provide the docking signal for inflammasome assembly and activation.24 Ischemia causes mitochondrial injury,25 but it is not known if mitochondrial damage persists long after ischemia to account for sustained inflammasome activation.
SS-31 (also known as elamipretide) is a synthetic tetrapeptide (D-Arg-2′6′-dimethylTyr-Lys-Phe-NH2) that selectively targets cardiolipin on the inner mitochondrial membrane to protect cristae curvature, stabilizes mitochondrial structure, facilitates electron transport, and minimizes ROS production.26–28 SS-31 is very effective in minimizing ischemic AKI and preventing the development of interstitial fibrosis and glomerulosclerosis.25,29 It is not known if later administration of SS-31 can stop the progression of CKD. We conducted a 9-month study in rats subjected to bilateral renal ischemia. Significant mitochondrial damage was found in endothelial cells, podocytes, and proximal tubular cells 9 months after ischemia. Treatment with SS-31, starting 1 month after ischemia and maintained for 6 weeks, protected mitochondrial integrity and halted the progression of glomerulosclerosis and tubulointerstitial fibrosis. The protection by SS-31 was sustained for at least 6 months after treatment ended. Upregulation of IL-1β and IL-18 at 9 months after ischemia was normalized by SS-31 treatment, suggesting that SS-31 reduced CKD by protecting mitochondria and preventing inflammasome activation.
Results
SS-31 Reverses Tubulointerstitial Fibrosis and Glomerulosclerosis after Acute Ischemia
Adult rats (n=53) were subjected to bilateral renal ischemia for 45 minutes and then allowed to recover for 4 weeks. Mortality was approximately 30% with 45 minutes ischemia, but normal renal function was fully restored after 4 weeks.29 Surviving animals (n=37) were randomly assigned to treatment with saline or SS-31 (2 mg/kg per day) for 1.5 months via a subcutaneously implanted osmotic pump (see Table 1).
Table 1.
Experimental study groups
| Treatment Groups | Treatment Begins 1 mo after Ischemia | Study Terminates after 1.5 mo Drug Treatment | Study Terminates 6 mo after Treatment Termination |
|---|---|---|---|
| Saline | 19 | 13 | 6 |
| SS-31, 2 mg/kg per d | 18 | 12 | 6 |
All data presented as n. A total of 50 rats were included in this study. Rats that survived the acute ischemic challenge (n=37) were randomized to receive either saline or SS-31 for 1.5 months.
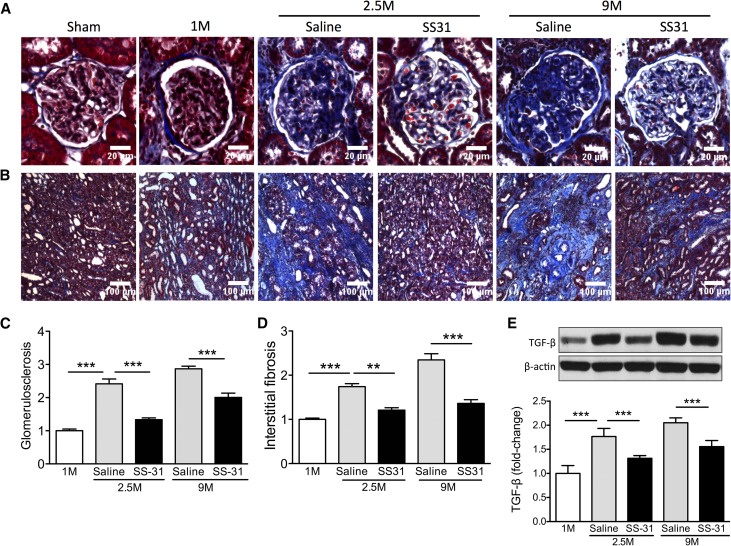
We previously reported that this duration of ischemia results in tubular necrosis and significant increase in serum creatinine, BUN, and fractional excretion of Na+ and K+.25 Although full recovery of renal function is achieved <1 week after ischemia, interstitial fibrosis is seen in the outer medulla 1 month later.29 In this study, we found progressive increase in interstitial fibrosis and glomerulosclerosis through 9 months after ischemia (Figure 1, A–D) that were associated with continuing increase in TGF-β expression in renal cortical tissue (Figure 1E). Six weeks of treatment with SS-31, starting 1 month after ischemia, significantly blunted the upregulation of TGF-β (Figure 1E, Supplemental Figure 1) and halted the progression of interstitial fibrosis and glomerulosclerosis (Figure 1, A–D). This antifibrotic effect of SS-31 was sustained for >6 months after treatment (Figure 1, A–D).
Figure 1.
SS-31 halts progression of interstitial fibrosis and glomerulosclerosis after acute renal ischemia. Rats were treated with SS-31 (2 mg/kg per day) or saline for 1.5 months starting 1 month after acute ischemia. (A) Representative images of trichrome stain of the renal cortex showing progressive increase in glomerulosclerosis at 1 month (1M), 2.5 months (2.5M), and 9 months (9M) after acute ischemia. Treatment with SS-31 starting at 1M prevented progression of fibrosis at 2.5M, and protection was seen even 6 months after termination of treatment. (B) Representative images of trichrome stain of the inner stripe of the outer medulla (ISOM) showing prevention of fibrosis by SS-31 treatment. (C) Quantification of trichrome stain in glomeruli at various times after ischemia. Data shown as mean±SEM; n=4/group; ***P<0.001. (D) Quantification of trichrome stain in ISOM at various times after ischemia. Data shown as mean±SEM; n=4/group; **P<0.01; ***P<0.001. (E) Representative Western blot and densitometric analysis of TGF-β expression in renal cortical tissue. Data shown as mean±SEM; n=4/group; ***P<0.001. (See Supplemental Figure 1 for all Western blots).
SS-31 Treatment Limits Inflammatory Response after Ischemia
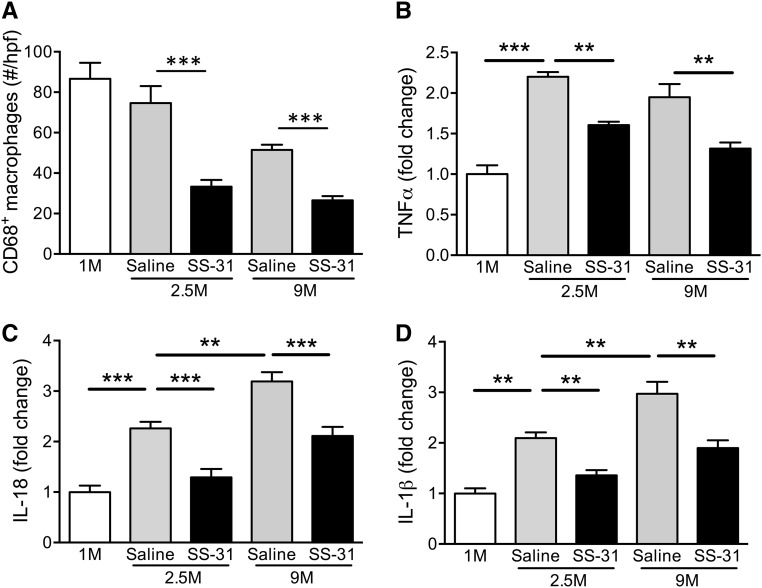
We previously reported >15-fold increase in TNF-α and interstitial infiltration of lymphocytes and macrophages 1 month after acute ischemic injury.29 Here we report further increase in TNF-α expression in renal cortical tissue and glomerular infiltration of CD68+ macrophages 2.5 months after acute ischemia (Figure 2, A and B, Supplemental Figure 1). This inflammatory response remained high but began to decline by 9 months. SS-31 significantly reduced TNF-α expression and macrophage infiltration at 2.5 months, and this effect was sustained for >6 months after termination of treatment.
Figure 2.
SS-31 reduces inflammation after acute renal ischemia. Rats were treated with SS-31 (2 mg/kg per day) or saline for 1.5 months starting 1 month after acute ischemia. (A) The number of CD68+ macrophages per glomerulus at various times after ischemia. The number shown reflects the average determined from ten glomeruli per sample and averaged. Data shown as mean±SEM; n=4/group; ***P<0.001. (B) Representative Western blot and densitometric analysis of TNF-α expression in renal cortical tissue at various times after acute ischemia. Data shown as mean±SEM; n=4/group; **P<0.01; ***P<0.001. (C) Representative Western blot and densitometric analysis of IL-18 expression in renal cortical tissue at various times after acute ischemia. Data shown as mean±SEM; n=4/group; **P<0.01; ***P<0.001. (D) Representative Western blot and densitometric analysis of IL-1β expression in renal cortical tissue at various times after acute ischemia in saline and SS-31-treated rats, versus age-matched controls. Data shown as mean±SEM; n=4/group; **P<0.01. (See Supplemental Figure 1 for all Western blots).
The eventual decline in TNF-α and macrophage infiltration led us to question if inflammasome activation plays a role in sustaining the continual upregulation of TGF-β. The expression of IL-18 and IL-1β continued to increase at 9 months after ischemia, but they were abolished by 6 weeks of SS-31 treatment (Figure 2, C and D, Supplemental Figure 1).
SS-31 Reverses Endothelial Injury after Ischemia
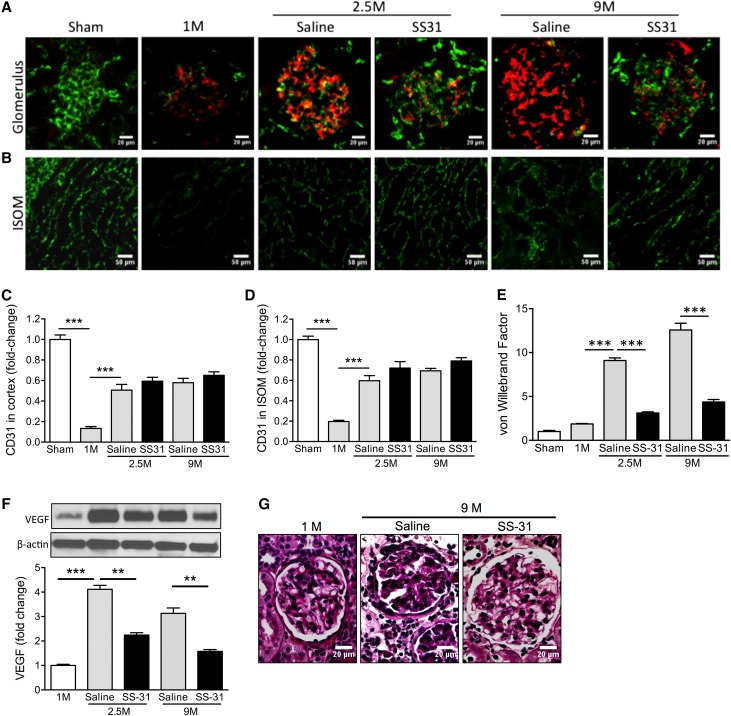
Renal ischemia causes peritubular microvascular rarefaction that contributes to further tissue damage.29–31 There was significant loss of CD31 immunostaining in the renal cortex 1 month after ischemia, primarily in the glomeruli (Figure 3, A and C). There was also significant loss of CD31 in the inner stripe of the outer medulla (Figure 3, B and D). Despite partial recovery of CD31 expression in the renal cortex by 2.5 months, there was persistent upregulation of vascular endothelial growth factor even 9 months after ischemia (Figure 3E, Supplemental Figure 1), suggesting prolonged tissue hypoxia. The dramatic upregulation of vWF expression in post-ischemic glomeruli (Figure 3, A and F) indicates persistent endothelial injury after acute ischemia. vWF is a glycoprotein that mediates platelet adhesion to the subendothelium at sites of vascular injury, and it is normally not expressed in human glomeruli.32 We found no vWF expression in normal rat glomeruli, but vWF was clearly present in glomeruli at 1 month and progressively increased over 9 months after ischemia (Figure 3, A and F). Treatment with SS-31 did not improve CD31 expression (Figure 3, C and D), but significantly reduced vWF and vascular endothelial growth factor expression at 2.5 and 9 months, and the effect persisted for 6 months after treatment (Figure 3, E and F). Mesangial deposition of vWF is associated with mesangial matrix expansion and fibrin deposition,33 and periodic acid–Schiff staining showed that SS-31 prevented mesangial expansion at 9 months (Figure 3G).
Figure 3.
SS-31 reduces endothelial injury after acute ischemia. Rats were treated with SS-31 (2 mg/kg per day) or saline for 1.5 months starting 1 month after acute ischemia. (A) Representative microscopic images of glomeruli showing endothelial cell marker CD31 (green) and vWF (red) in saline- and SS-31-treated rats at 1 month (1M), 2.5 months (2.5M), and 9 months (9M) after ischemia. (B) Representative microscopic images of inner stripe of outer medulla (ISOM) showing CD31 (green) in saline and SS-31-treated rats at 1M, 2.5M, and 9M after ischemia. (C) Densitometric analysis of CD31 staining in renal cortex showing significant decrease in endothelial cell marker 1M after ischemia and partial recovery by 2.5M. SS-31 treatment had no effect on CD31 recovery. Data shown as mean±SEM; n=4/group; ***P<0.001. (D) Densitometric analysis of CD31 staining in ISOM showing significant decrease in endothelial cell marker 1M after ischemia and partial recovery by 2.5M. SS-31 treatment had no effect on CD31 recovery. Data shown as mean±SEM; n=4/group; ***P<0.001. (E) Representative Western blot and densitometric analysis of vascular endothelial growth factor (VEGF) expression in renal cortical tissue at various times after acute ischemia. Data shown as mean±SEM; n=4/group; **P<0.01; ***P<0.001. (See Supplemental Figure 1 for all Western blots). (F) Densitometric analysis of vWF stain in renal cortex showing marked increase in endothelial injury marker that was sustained over 9M after ischemia. SS-31 treatment abolished the increase. Data shown as mean±SEM; n=4/group; ***P<0.001. (G) Representative images of periodic acid–Schiff stain of glomeruli at 1M after ischemia, and at 9M in saline versus SS-31-treated rats.
SS-31 Protects Glomerular Capillary Loops and Fenestrations after Ischemia by Protecting Endothelial Mitochondria
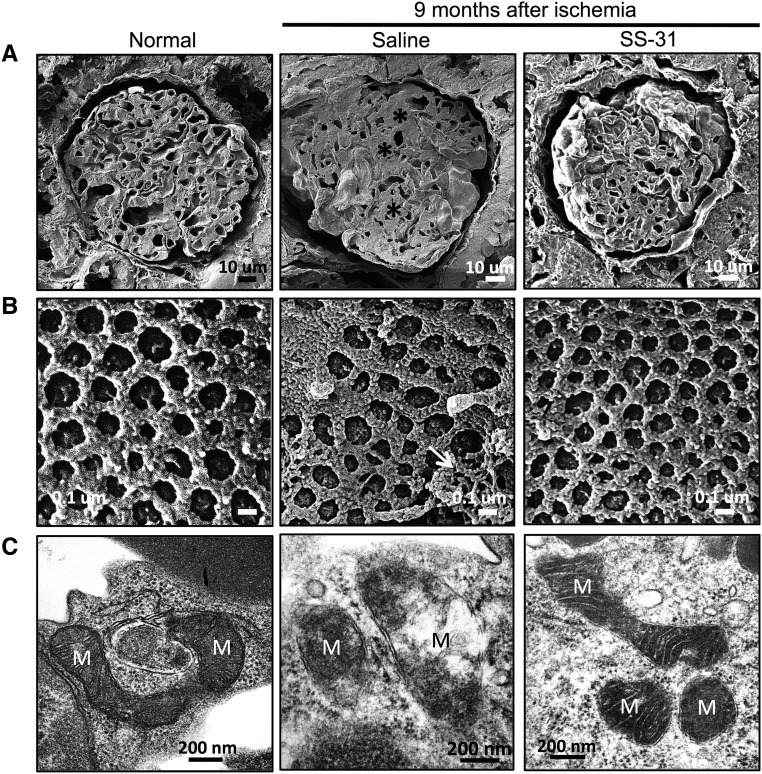
We used helium ion microscopy (HIM) to further investigate damage to glomerular capillaries and endothelial fenestrations after acute ischemia. HIM provides subnanometer resolution images of biologic tissues with greater detail than scanning electron microscopy.34,35 Figure 4A shows a representative cross-section of an age-matched (11 months) normal rat glomerulus with numerous capillary loops. In contrast, mesangial expansion and compressed capillary loops were observed 9 months after ischemia. SS-31 restored majority of capillary loops and reduced mesangial volume. Closer examination of the capillary endothelial surface shows uniform fenestrations in a normal glomerulus (Figure 4B). In contrast, there was loss of fenestrations and extensive adhesive material on the endothelial surface 9 months after ischemia. SS-31 restored normal fenestrations and reduced adhesive material, consistent with reduction of vWF expression.
Figure 4.
SS-31 reduces glomerular capillary compression and damage to endothelial fenestrations after acute ischemia. Rats were treated with SS-31 (2 mg/kg per day) or saline for 1.5 months starting 1 month after acute ischemia. Glomerular capillary structure was examined by HIM. (A) Representative cross-sectional images of glomeruli at 9M after acute ischemia from saline and SS-31-treated rats, compared with age-matched normal rats. Ischemia caused capillary compression because of mesangial expansion (*) in saline-treated rats. Capillary loops are much more apparent in SS-31-treated rats and similar to image from normal rat. (B) Representative HIM images showing endothelial fenestrations from saline versus SS-31 rats compared with age-matched normal rats. Fenestrations are uniform in normal rats, whereas saline-treated rats show distorted fenestrations and adhesive material (white arrow). SS-31 treatment restored normal fenestration pattern. (C) Representative electron micrograph showing glomerular endothelial cell mitochondria from saline versus SS-31 rats at 2.5M after ischemia, compared with normal rat. Saline-treated ischemic cells show degenerative changes in mitochondria with disruption of cristae architecture, reduced matrix density, and some loss of OMM. SS-31 treatment restored normal endothelial mitochondria. (More examples are provided in Supplemental Figure 2). M, mitochondria.
Ischemia causes extensive mitochondria damage in renal endothelial cells.29 Normal glomerular endothelial mitochondria are elongated, with dense cristae membranes and a dark matrix (Figure 4C, Supplemental Figure 2). Significant degenerative changes were still found in endothelial cells 2.5 months after ischemia, with loss of cristae membranes and matrix density, and evidence of rupture of the OMM. These degenerative changes were abolished by 6 weeks of treatment with SS-31.
SS-31 Prevents Podocyte Stress after Ischemia
Podocytes are secretory cells that contain a well developed Golgi system, many mitochondria, prominent lysosomes, and abundant endoplasmic reticulum (ER) responsible for the folding and trafficking of proteins (Figure 5A).36 Transmission electron microscopy showed pronounced dilation of ER lumen, an indication of ER stress, in many podocytes 1 month after ischemia (Figure 5B) compared with sham rats (Figure 5A). By 2.5 months, the podocytes were highly vacuolated and there was a general loss of cytoplasmic density (Figure 5C). Podocyte stress was still apparent 9 months after ischemia (Figure 5D). However, 6 weeks of SS-31 restored normal ER structure (Figure 5E) and this persisted for at least 6 months after treatment (Figure 5F).
Figure 5.
SS-31 prevents podocyte stress after acute ischemia. Rats were treated with SS-31 (2 mg/kg per day) or saline for 1.5 months starting 1 month after acute ischemia. Podocyte structure was examined by transmission electron microscopy. (A) Representative image of a normal podocyte showing dense cytoplasm containing large number of endoplasmic reticulum (ER; arrows) and mitochondria. (B) Representative image obtained 1 month after ischemia showing pronounced dilation of ER (arrows). (C) Representative image obtained 2.5 months after ischemia showing loss of cytoplasmic density and large number of vacuoles. (D) Representative image obtained 9 months after ischemia with numerous dilated ER (arrows). (E) Representative image obtained 2.5 months after ischemia following short-term SS-31 treatment showing normal cytoplasmic structures. (F) Representative image obtained from an SS-31-treated rat 9 months after ischemia showing persistent podocyte protection with normal ER (arrows).
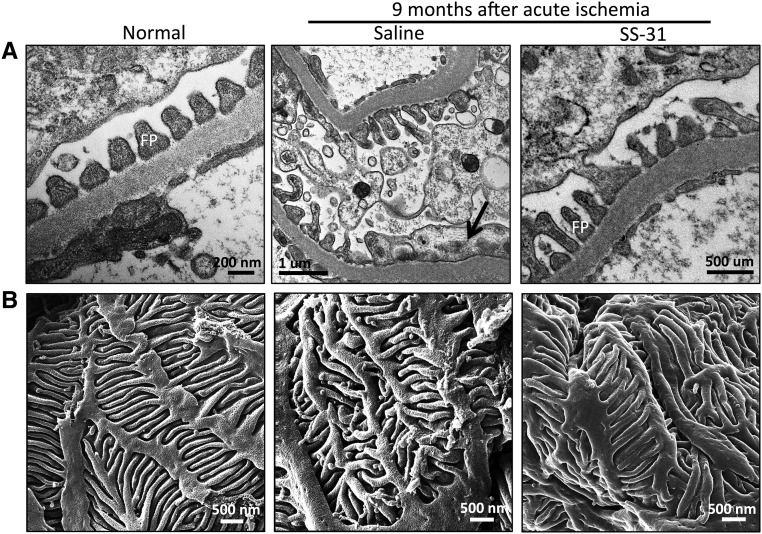
SS-31 Protects Podocyte Foot Processes after Ischemia
Podocytes have a complex cellular architecture with interdigitating foot processes maintained by a precise organization of actin filaments in the cellular cytoplasm. Podocyte swelling and effacement of foot processes have been reported after 45 minutes of ischemia in rats, but little is known about long-term changes after acute ischemia.37 Transmission electron microscopy revealed swollen podocytes and foot process effacement 9 months after acute ischemia compared with age-matched controls (Figure 6A). Condensation of actin microfilaments can be seen in the flattened foot process. Short-term SS-31 treatment restored normal foot processes. HIM provided a much clearer image of the damage to podocytes (Figure 6B). In normal podocytes, the foot processes are long and tightly interdigitated to provide an effective filtration barrier. Ischemia resulted in shortened and deformed foot processes that did not interdigitate well. Podocyte foot processes were normalized with SS-31 treatment.
Figure 6.
SS-31 preserves podocyte foot processes after acute ischemia. Rats were treated with SS-31 (2 mg/kg per day) or saline for 1.5 months starting 1 month after acute ischemia. Podocyte structure was examined 9 months after ischemia. (A) Representative images of podocyte foot processes obtained by transmission electron microscope. Podocyte swelling and foot process effacement are common 9 months after acute ischemia compared with age-matched control. Condensation of actin microfilaments can be seen in the flattened foot processes (black arrow). Short-term SS-31 treatment restores normal foot processes. (B) Representative images of podocyte foot processes obtained by helium ion microscope. In normal podocytes, the foot processes are long and tightly interdigitated. Ischemia resulted in shortened and deformed foot processes. Short-term SS-31 treatment restores normal structural organization of foot processes.
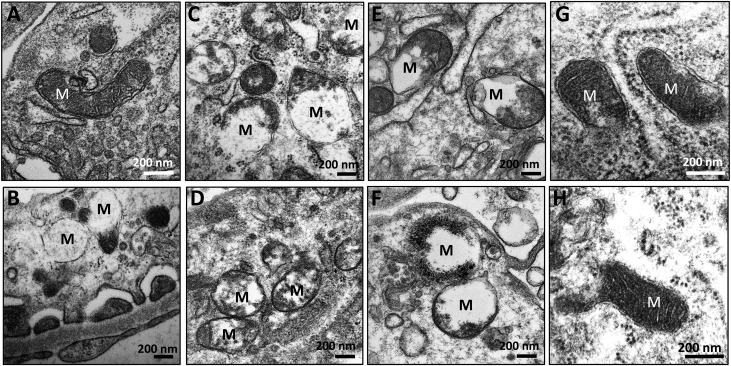
SS-31 Reverses Ischemia-Induced Mitochondria Damage in Podocytes
Mitochondria play a major role in providing ATP for maintaining the actin cytoskeleton in podocytes.38,39 The collapse of the podocyte actin cytoskeleton at 9 months suggests sustained mitochondrial damage after an acute event. Normal podocyte mitochondria are elongated with cristae membranes and a dark matrix (Figure 7A). One month after ischemia, mitochondria showed degenerative changes with loss of cristae membranes and matrix swelling (Figure 7B). These degenerative changes were extensive and persisted at 2.5 months, with some mitochondria showing destruction of the OMM (Figure 7C). At 9 months, most mitochondria were small with only remnants of cristae membranes and a clear matrix, whereas others underwent extensive proteolysis with just some residual matrix density (Figure 7, D–F). Six weeks of SS-31 treatment restored mitochondria structure (Figure 7G), and mitochondria remained normal even 9 months after ischemia (Figure 7H).
Figure 7.
SS-31 reverses ischemia-induced mitochondria damage in podocytes. Rats were treated with SS-31 (2 mg/kg per day) or saline for 1.5 months starting 1 month after acute ischemia. Podocyte mitochondria were examined 9 months after ischemia using transmission electron microscopy. (A) Representative image from normal podocyte showing elongated mitochondria with cristae membranes and a dark matrix. (B) Representative image obtained 1 month after ischemia showing mitochondria with degenerative changes including loss of cristae membranes and matrix swelling. (C) Representative image obtained 2.5 months after ischemia showing persistent mitochondrial damage. (D–F) Representative images obtained 9 months after ischemia. Many mitochondria are small with remnants of cristae membranes and a clear matrix, whereas others have undergone extensive proteolysis. (G) Representative image obtained from an SS-31-treated rat 2.5 months after ischemia showing normal mitochondria cristae and matrix density. (H) Representative image from an SS-31-treated rat 9 months after ischemia showing normal podocyte mitochondria. M, mitochondria.
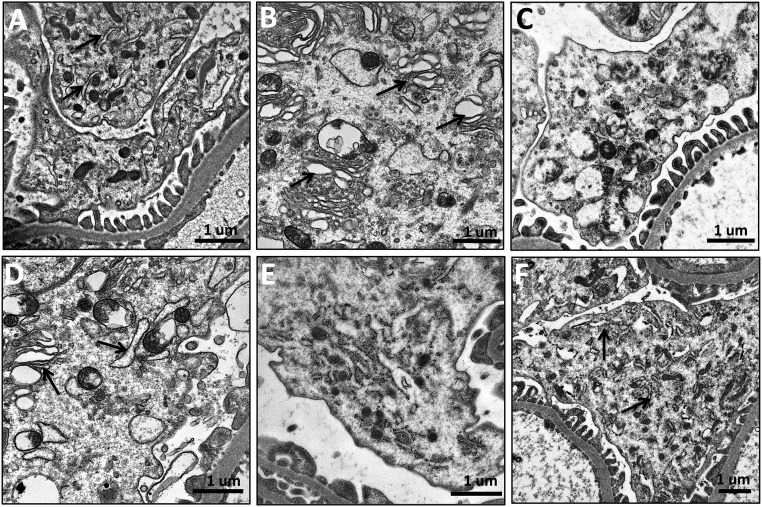
Persistent Upregulation of Autophagy in Podocytes after Ischemia
Accumulating evidence indicates that ER stress may trigger autophagy.40 Postmitotic podocytes exhibit autophagy under basal conditions,41 but the effect of ischemia on podocyte autophagy is unknown. Autophagic vacuoles containing organelles and cytoplasmic contents can be seen in podocytes 1 month after ischemia (Figure 8A). Figure 8B shows an intact mitochondrion inside an autophagosome. Mitophagy is still upregulated 9 months after acute ischemia, with interaction between lysosomes and mitochondria (Figure 8, C and D). Autophagolysosomes containing digested material and myelin bodies are shown in Figure 8E. Finally, Figure 8F shows an example of dilated ER wrapping around mitochondria to cause mitochondrial fission.42
Figure 8.
Persistent upregulation of autophagy in podocytes after acute ischemia. Rats were subjected to bilateral renal ischemia for 45 minutes and podocytes examined by transmission electron microscopy. (A) Autophagic vacuoles (arrows) containing organelles and cytoplasmic contents in podocytes 1 month after ischemia. (B) An intact mitochondrion can be seen inside an autophagosome. (C and D) Interaction between lysosomes (arrows) and mitochondria can be seen 9 months after ischemia. (E) Autophagolysosomes containing digested material and myelin bodies 9 months after ischemia. (F) Dilated endoplasmic reticulum wrapping around mitochondria causing mitochondrial fission. M, mitochondria.
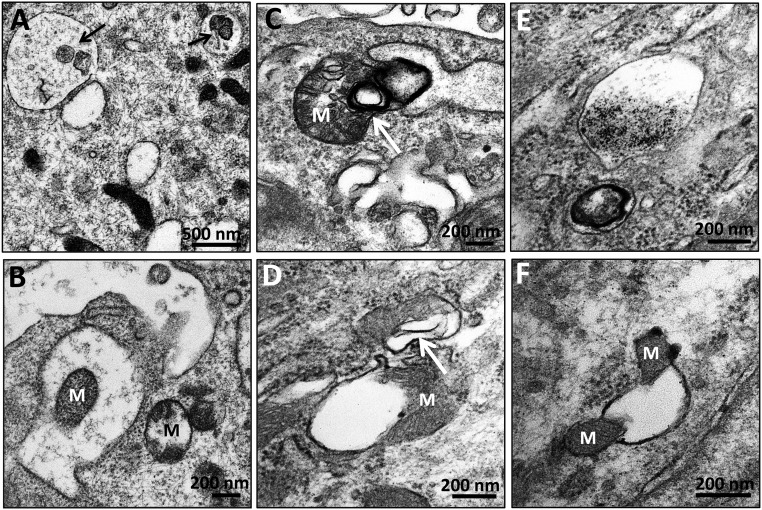
SS-31 Protects Proximal Tubule Mitochondria
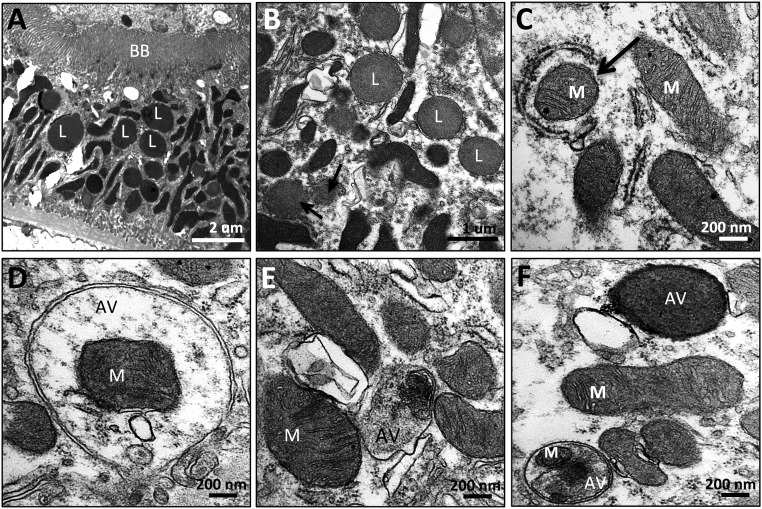
Proximal tubules are highly susceptible to ischemic injury, with rapid loss of brush border and cell detachment.25,26 However, tubular cells can regenerate, and brush borders are intact 9 months after acute ischemia (Figure 9A). Unlike the elongated mitochondria found in normal proximal tubules, post-ischemic proximal tubule mitochondria are small and highly disorganized, and there is an abundance of lysosomes (Figure 9B). There is also evidence of mitophagy, with early and complete autophagosomes around mitochondria (Figure 9, C and D). Some autophagolysosomes contain cytoplasmic content or are completely homogenized (Figure 9, E and F). Autophagy was not observed in proximal tubules treated with SS-31.
Figure 9.
Mitophagy in proximal tubules after acute ischemia. Rats were treated with SS-31 (2 mg/kg per day) or saline for 1.5 months starting 1 month after acute ischemia. Proximal tubules were examined by transmission electron microscopy. (A and B) Representative images obtained 9 months after acute ischemia showing normal brush border (BB). Mitochondria are small and highly disorganized, and there is an abundance of lysosomes (L). (C and D) Representative images showing early and complete autophagic vesicles (AV) surrounding mitochondria (M). (E and F) Representative images showing autophagic vacuoles containing mitochondria and other cytoplasmic content.
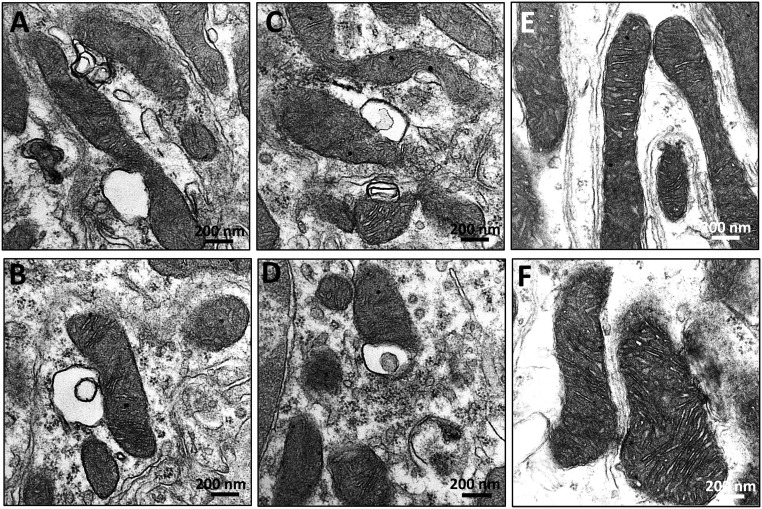
In addition to mitophagy, some proximal tubule mitochondria appear relatively normal but have membrane vesicles in close apposition to the OMM (Figure 10, A–D). These vesicles may be dilated ER that are in close apposition to the OMM, commonly termed mitochondria-associated membranes. These structures were not seen in rats that received SS-31 treatment (Figure 10, E and F).
Figure 10.
Mitochondria-associated membranes in proximal tubules after ischemia. Rats were treated with SS-31 (2 mg/kg per day) or saline for 1.5 months starting 1 month after acute ischemia. Proximal tubules were examined by transmission electron microscopy 9 months after ischemia. (A–D) Representative images showing membrane vesicles in close apposition to the OMM. (E and F) Representative images from SS-31-treated samples did not show these mitochondria-associated membranes.
Discussion
We previously reported peritubular and glomerular capillary loss, inflammation, tubulointerstitial fibrosis, and glomerulosclerosis 1 month after acute ischemia.29 We confirm those earlier findings in this study, and further show persistent endothelial injury and microvascular rarefaction up to 9 months after acute ischemia, accompanied by podocyte damage, inflammation, and progressive glomerular and interstitial fibrosis. Although renal function was not assessed in this study, significant proteinuria has been reported in rats 9 months after the same duration of ischemia.43
Podocytes are terminally differentiated epithelial cells that form part of the filtration barrier. Even though there was no evidence of podocyte loss at 9 months, the cell bodies were swollen and foot processes were deformed and retracted, as revealed vividly by HIM. The complex structure of foot processes depends on ordered actin filament bundles, and the assembly and maintenance of the actin cytoskeleton require ATP.44 The breakdown of the actin cytoskeleton suggests bioenergetics failure in podocytes long after the acute ischemic insult. Ischemia causes mitochondrial swelling and loss of cristae membranes in all renal cells,25,26,29 but it has not been appreciated that mitochondrial damage can persist for as long as 9 months. Damage to podocyte mitochondria is extensive and most show loss of cristae membranes and homogenization, consistent with proteolytic degradation by mitochondrial proteases.45
The accumulation of damaged mitochondria in podocytes suggests that mitochondrial quality control remains impaired long after acute ischemia. Mitochondria undergo regular cycles of degradation and biogenesis in order to maintain an efficient supply of ATP to the cell. Mitophagy serves as a rapid removal mechanism for whole dysfunctional mitochondria or a large number of damaged mitochondria. Autophagosomes containing mitochondria and autophagolysosomes were apparent in both podocytes and proximal tubules 9 months after ischemia. However, a large number of podocyte mitochondria appear to undergo proteolytic degradation suggesting that mitophagy might be impaired, and damaged proteins are eliminated by mitochondrial proteases.46
Our results suggest that persistent mitochondrial damage underlies the progression of CKD, and this was confirmed with the mitoprotective agent SS-31. Treatment with SS-31 before ischemia protected endothelial and epithelial mitochondria, preserved peritubular and glomerular capillaries, and prevented inflammation and fibrosis.29 Here we show that SS-31, started 1 month after ischemia, can repair mitochondria in endothelial cells, podocytes, and tubular cells. This led to restoration of peritubular and glomerular capillaries, preservation of podocyte architecture, suppression of inflammation, and fibrosis. SS-31 abolished the increase in TNF-α and TGF-β over the 6-week treatment interval. Remarkably, renal protection persisted for at least 6 months after termination of SS-31 treatment. No other experimental treatment has been shown to accomplish this when given so late after AKI. Most experimental treatments evaluated in animal studies are given prior to or shortly after acute injury, although the clinical reality is that most patients present with established disease.
SS-31 protects mitochondrial structure by interacting with cardiolipin on the inner mitochondrial membrane and protecting cristae structure.26,47,48 Cardiolipin promotes membrane folding and promotes respiratory complexes to form supercomplexes to facilitate electron transfer, promote ATP synthesis, and reduce electron leak. Dysfunctional mitochondria generate ROS, and cardiolipin is particularly susceptible to lipid peroxidation. In the presence of ROS, cytochrome c acts as a peroxidase and causes cardiolipin peroxidation and cristae degradation. SS-31 acts as a multifunctional antioxidant by minimizing electron leak, scavenging excess electrons, and inhibiting cytochrome c peroxidase activity.28,47,49 SS-31 has been reported to restore cardiolipin content and mitochondrial cristae structure, and improve mitochondrial energetics, in experimental models of chronic kidney and heart failure.50–52 The recovery of podocyte bioenergetics allowed repair of the actin cytoskeleton and podocyte structure.
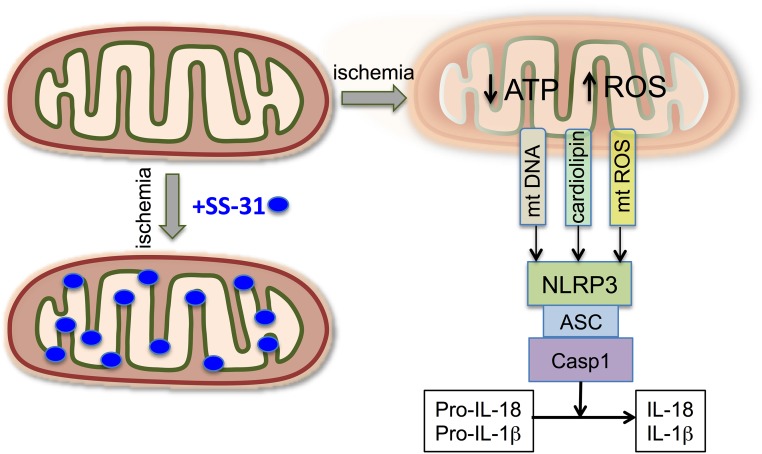
Inflammation plays a major role in sustaining fibrosis after acute injury. However, TNF-α and macrophage infiltration were already on the decline at 9 months. Inflammasome activation may be responsible for sustaining inflammation long after acute ischemia. Mitochondria play an integral role in the assembly and activation of the inflammasome.14,19 Release of mitochondrial DNA, ROS, and cardiolipin from damaged mitochondria can activate the NLRP3 inflammasome (Figure 11).13,23,24 There was sustained increase in IL-18 and IL-1β expression 9 months after acute ischemia, supporting a role for inflammasome activation in the progression to CKD. Cardiolipin peroxidation causes the translocation of cardiolipin to the OMM where it has been proposed to serve as a docking site for inflammasome assembly, whereas mitochondrial ROS activates the inflammasome to produce caspase-1.17,24 Mitochondria-associated membranes are also involved in the formation of the inflammasome,53 and they were prominent in proximal tubules 9 months after ischemia. By inhibiting mitochondrial ROS production, preventing cardiolipin peroxidation, and protecting mitochondrial cristae structure, SS-31 prevented the upregulation of IL-1β and IL-18 after acute renal ischemia (Figure 11). Inflammasome activation also contributes to renal inflammation and fibrosis in the mouse unilateral ureteral obstruction (UUO) model,14,54 and SS-31 can prevent interstitial fibrosis after UUO.55 SS-31 has also been reported to attenuate inflammasome activation in alveolar cells in response to mechanical ventilation,56 and in the brain of aging mice in response to isoflurane.57
Figure 11.
SS-31 protects mitochondrial integrity and prevents inflammasome activation and upregulation of IL-18 and IL-1β. Ischemia causes mitochondria damage resulting in decreased ATP production, increased ROS production, and cardiolipin peroxidation. Damage to mitochondrial membranes leads to the release of mitochondrial DNA (mt DNA), mitochondrial reactive oxygen species (mt ROS), and cardiolipin that are known to activate the NLRP3 inflammasome. The NLRP3 inflammasome, together with the adaptor protein (ASC), triggers activation of caspase-1 (Casp1), which then processes the proinflammatory cytokines pro–IL-1β and pro–IL-18 into their bioactive mature forms (IL-1β and IL-18). SS-31 (blue dots) targets cardiolipin, inhibits cardiolipin peroxidation, and protects mitochondrial cristae membrane.26 By preserving mitochondrial integrity, SS-31 not only preserves ATP production but also prevents NLRP3 inflammasome activation and normalizes IL-18 and IL-1β expression. ASC, Apoptosis-associated speck-like protein containing a caspase-recruitment domain.
Several inhibitors of IL-1β and IL-18 are in clinical development, as well as inhibitors of the NLRP3 inflammasome and caspase-1.58,59 Targeting upstream of the inflammasome might prove more effective than single anticytokine therapies and carry less risk. SS-31 has been shown to be highly effective in preventing acute and chronic kidney injury, including ischemia-reperfusion injury, UUO, contrast dye, endotoxemia, and streptozotocin-induced diabetic kidney injury.25,29,55,60–62 SS-31 has also been reported to reverse progressive CKD after chronic ischemia caused by renovascular stenosis, and reduce glomerulopathy and tubular injury caused by high-fat diet.63,64 SS-31 (also known as MTP-131, elamipretide, and Bendavia) may represent a novel treatment for CKD in addition to BP control.
Concise Methods
Animals
The study was performed using male Sprague–Dawley rats (Charles River Laboratories, Wilmington, MA) weighing 250–300 g. Animals were housed in 12:12-hour light/dark cycle and allowed free access to water and standard rat chow. Care of the rats before and during the experimental procedures was conducted in accordance with the policies of the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals. All protocols had received prior approval from the Weill Cornell Medical College Institutional Animal Care and Use Committee.
Materials and Materials
SS-31 was supplied by Stealth Peptides Inc., Newton Centre, MA. Unless otherwise specified, reagents and assay kits were purchased from Sigma-Aldrich (St. Louis, MO).
Ischemia-Reperfusion Experimental Protocol
Rats were anesthetized with ketamine/xylazine and bilateral renal ischemia was induced by application of nontraumatic microvascular clamps around both left and right renal pedicles for 45 minutes, as previously described in detail.25 After ischemia, the animals were allowed to recover for 1 month. Surviving rats were randomly assigned to 6 weeks of treatment with SS-31 (2.0 mg/kg per day) or saline by subcutaneously implanted osmotic pump (Alza, Cupertino, CA). Rats were euthanized either immediately after drug treatment or 6 months after termination of drug treatment. Kidneys were harvested for biochemical and histologic analysis. Kidney tissues were frozen at −80°C for Western blotting or embedded in OCT-cryostat sectioning medium containing 30% sucrose for immunohistochemistry. Other samples were fixed in 4% paraformaldehyde for paraffin sections and ultrastructural examination by transmission electron microscopy or HIM.
Renal Histopathology
Paraffin sections (3 μm) were stained with periodic acid–Schiff and examined by light microscopy (Nikon Eclipse TE2000-U). Masson trichrome staining was used to assess interstitial fibrosis and glomerulosclerosis.
Assessment of Renal Microvasculature
Microvascular density and endothelial injury were assessed by immunohistochemical staining of CD31 and vWF, respectively. Frozen OCT blocks were cut into 8 μm sections, fixed in acetone for 4–5 minutes at room temperature, and incubated with anti-mouse CD31 (BD Biosciences, San Jose, CA) and anti-rabbit vWF (Sigma-Aldrich) overnight at 4°C. Sections were then incubated with anti-mouse IgG-conjugated Alexa Fluor-488 (Invitrogen, Carlsbad, CA) or goat anti-rabbit IgG-conjugated with biotin (Vector Laboratories, Burlingame, CA), and then treated with streptavidin-conjugated Alexa Fluor-594 (Invitrogen) for 30 minutes at room temperature, covered with VECTASHIELD Mounting Medium with DAPI (Vector Laboratories), and imaged by fluorescence microscopy (Nikon Eclipse TE2000-U). For each animal, ten representative glomeruli were selected in the renal cortex, and the green fluorescence intensity of the CD31 immunostain was quantified (ImageJ; National Institutes of Health) and averaged. Similarly, ten representative areas of the inner stripe of the outer medullar were quantified and averaged.
Assessment of Renal Inflammation
Macrophages were assessed by immunohistochemical staining of 4 μm paraffin sections for CD68. The kidney sections were incubated with anti-mouse CD68 (Abcam, Inc., Cambridge, MA) overnight at 4°C and incubated with HRP-conjugated anti-mouse IgG (Dako, Carpinteria, CA) for 30 minutes at room temperature, and color developed by DAB substrate kit (Vector Laboratories). The number of CD68+ cells were counted from five different fields for each sample and averaged.
The cytokines TNF-α, TGF-β1, IL-18 and IL-1β were quantified by Western blotting. Kidney homogenates were prepared according to standard protocol (Santa Cruz Biotechnology, Santa Cruz, CA), suspended in loading buffer, and subjected to 4%–15% SDS-PAGE. The resolved proteins were transferred to an Immuno-Blot polyvinylidene difluoride membrane (Bio-Rad, Hercules, CA). After electroblotting, the membrane was incubated overnight with primary antibodies against TNF-α (αβ1793; Abcam, Inc.), TGB-β (sc-146; Santa Cruz Biotechnology), IL-18 (MAB521; R&D Systems, Minneapolis, MN), and IL-1β (MB5011; R&D Systems). Membranes were further incubated for 1 hour with HRP-conjugated secondary antibodies (Dako). Protein bands were detected with an enhanced chemiluminescence detection system (Bio-Rad) and autoradiography. Bands were evaluated for integrated density values on Image Lab Software (Bio-Rad).
Transmission Electron Microscopy
Pieces of renal tissue were fixed in 4% paraformaldehyde, postfixed in 1% osmium tetroxide, dehydrated in graded alcohols, and embedded in Epon. Ultrathin sections (200–400 Å) were cut on nickel grids, stained with uranyl acetate and lead citrate, and examined using a digital electron microscope (JEM-1400; JEOL Ltd.).
HIM
Kidney tissues were sectioned at 500 μm on a Leica vibrating microtome. The slices were cut into smaller, wedge-shaped pieces so that they would remain flat during the drying process. Tissues were dehydrated through a graded PBS-MeOH series at 4°C, according to previously published methods.35 Tissues were subjected to critical point drying with CO2. Dried samples were mounted on scanning electron microscope stubs using carbon sticky tabs and stored in a desiccator until imaging. HIM was carried out on the Carl Zeiss Orion Plus helium ion microscope (Carl Zeiss GmbH, Jena, Germany) operating at 30 KeV acceleration voltage, with a beam current of approximately 0.5 pA. A 10 nm layer of Au was evaporated on the samples to minimize local charging effects. For all images taken, an electron flood gun was used for charge neutralization at an accelerating voltage of 900 eV. The vacuum reading in the analysis chamber was 2×10−7 torr.
Statistical Analyses
Results are expressed as means±SEM. Statistical analysis was carried out using Prism software (GraphPad Software Inc., San Diego, CA). Multiple group comparisons were performed using ANOVA followed by a Tukey post hoc test. A P value <0.05 was considered statistically significant.
Disclosures
The SS peptide described in this article has been licensed for commercial research and development to Stealth Peptides Inc. (now Stealth BioTherapeutics, Newton, MA), a clinical stage biopharmaceutical company, in which H.H.S. and the Cornell Research Foundation have financial interests. H.H.S. is the inventor of SS-31 and the Scientific Founder of Stealth BioTherapeutics.
Supplementary Material
Acknowledgments
We thank Juan Pablo Jimenez of the Electron Microscopy & Histology Core Facility at Weill Cornell Medical College for his technical assistance.
This work was supported, in part, by the Research Program in Mitochondrial Therapeutics at Weill Cornell Medicine. S.L. and Y.S. acknowledge support from the Social Profit Network. L.C.F., T.G., and V.M. acknowledge support from National Science Foundation grant Division of Materials Research 1126468. V.M. was also supported by a Graduate Assistance in Areas of National Need fellowship.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “Preventing the Progression of AKI to CKD: The Role of Mitochondria,” on pages 1327–1329.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2016070761/-/DCSupplemental.
References
- 1.Coca SG, Singanamala S, Parikh CR: Chronic kidney disease after acute kidney injury: A systematic review and meta-analysis. Kidney Int 81: 442–448, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kaushal GP, Shah SV: Challenges and advances in the treatment of AKI. J Am Soc Nephrol 25: 877–883, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gobe GC, Bennett NC, West M, Colditz P, Brown L, Vesey DA, Johnson DW: Increased progression to kidney fibrosis after erythropoietin is used as a treatment for acute kidney injury. Am J Physiol Renal Physiol 306: F681–F692, 2014 [DOI] [PubMed] [Google Scholar]
- 4.Dagher PC, Mai EM, Hato T, Lee SY, Anderson MD, Karozos SC, Mang HE, Knipe NL, Plotkin Z, Sutton TA: The p53 inhibitor pifithrin-α can stimulate fibrosis in a rat model of ischemic acute kidney injury. Am J Physiol Renal Physiol 302: F284–F291, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haase VH: Hypoxia-inducible factor signaling in the development of kidney fibrosis. Fibrogenesis Tissue Repair 5[Suppl 1]: S16, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kelsen S, He X, Chade AR: Early superoxide scavenging accelerates renal microvascular rarefaction and damage in the stenotic kidney. Am J Physiol Renal Physiol 303: F576–F583, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kohan DE, Barton M: Endothelin and endothelin antagonists in chronic kidney disease. Kidney Int 86: 896–904, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Basile DP, Bonventre JV, Mehta R, Nangaku M, Unwin R, Rosner MH, Kellum JA, Ronco C; ADQI XIII Work Group : Progression after AKI: Understanding maladaptive repair processes to predict and identify therapeutic treatments. J Am Soc Nephrol 27: 687–697, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hill NR, Fatoba ST, Oke JL, Hirst JA, O’Callaghan CA, Lasserson DS, Hobbs FD: Global prevalence of chronic kidney disease - A systematic review and meta-analysis. PLoS One 11: e0158765, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Varga J, Pasche B: Transforming growth factor beta as a therapeutic target in systemic sclerosis. Nat Rev Rheumatol 5: 200–206, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gonçalves GM, Castoldi A, Braga TT, Câmara NO: New roles for innate immune response in acute and chronic kidney injuries. Scand J Immunol 73: 428–435, 2011 [DOI] [PubMed] [Google Scholar]
- 12.Anders HJ, Schaefer L: Beyond tissue injury-damage-associated molecular patterns, toll-like receptors, and inflammasomes also drive regeneration and fibrosis. J Am Soc Nephrol 25: 1387–1400, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iyer SS, Pulskens WP, Sadler JJ, Butter LM, Teske GJ, Ulland TK, Eisenbarth SC, Florquin S, Flavell RA, Leemans JC, Sutterwala FS: Necrotic cells trigger a sterile inflammatory response through the Nlrp3 inflammasome. Proc Natl Acad Sci USA 106: 20388–20393, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vilaysane A, Chun J, Seamone ME, Wang W, Chin R, Hirota S, Li Y, Clark SA, Tschopp J, Trpkov K, Hemmelgarn BR, Beck PL, Muruve DA: The NLRP3 inflammasome promotes renal inflammation and contributes to CKD. J Am Soc Nephrol 21: 1732–1744, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kurts C, Panzer U, Anders HJ, Rees AJ: The immune system and kidney disease: Basic concepts and clinical implications. Nat Rev Immunol 13: 738–753, 2013 [DOI] [PubMed] [Google Scholar]
- 16.Turner CM, Arulkumaran N, Singer M, Unwin RJ, Tam FW: Is the inflammasome a potential therapeutic target in renal disease? BMC Nephrol 15: 21, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tschopp J, Schroder K: NLRP3 inflammasome activation: The convergence of multiple signalling pathways on ROS production? Nat Rev Immunol 10: 210–215, 2010 [DOI] [PubMed] [Google Scholar]
- 18.van Bruggen R, Köker MY, Jansen M, van Houdt M, Roos D, Kuijpers TW, van den Berg TK: Human NLRP3 inflammasome activation is Nox1-4 independent. Blood 115: 5398–5400, 2010 [DOI] [PubMed] [Google Scholar]
- 19.Zhou R, Yazdi AS, Menu P, Tschopp J: A role for mitochondria in NLRP3 inflammasome activation. Nature 469: 221–225, 2011 [DOI] [PubMed] [Google Scholar]
- 20.West AP, Shadel GS, Ghosh S: Mitochondria in innate immune responses. Nat Rev Immunol 11: 389–402, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jabaut J, Ather JL, Taracanova A, Poynter ME, Ckless K: Mitochondria-targeted drugs enhance Nlrp3 inflammasome-dependent IL-1β secretion in association with alterations in cellular redox and energy status. Free Radic Biol Med 60: 233–245, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heid ME, Keyel PA, Kamga C, Shiva S, Watkins SC, Salter RD: Mitochondrial reactive oxygen species induces NLRP3-dependent lysosomal damage and inflammasome activation. J Immunol 191: 5230–5238, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nakahira K, Haspel JA, Rathinam VA, Lee SJ, Dolinay T, Lam HC, Englert JA, Rabinovitch M, Cernadas M, Kim HP, Fitzgerald KA, Ryter SW, Choi AM: Autophagy proteins regulate innate immune responses by inhibiting the release of mitochondrial DNA mediated by the NALP3 inflammasome. Nat Immunol 12: 222–230, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Iyer SS, He Q, Janczy JR, Elliott EI, Zhong Z, Olivier AK, Sadler JJ, Knepper-Adrian V, Han R, Qiao L, Eisenbarth SC, Nauseef WM, Cassel SL, Sutterwala FS: Mitochondrial cardiolipin is required for Nlrp3 inflammasome activation. Immunity 39: 311–323, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Szeto HH, Liu S, Soong Y, Wu D, Darrah SF, Cheng FY, Zhao Z, Ganger M, Tow CY, Seshan SV: Mitochondria-targeted peptide accelerates ATP recovery and reduces ischemic kidney injury. J Am Soc Nephrol 22: 1041–1052, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Birk AV, Liu S, Soong Y, Mills W, Singh P, Warren JD, Seshan SV, Pardee JD, Szeto HH: The mitochondrial-targeted compound SS-31 re-energizes ischemic mitochondria by interacting with cardiolipin. J Am Soc Nephrol 24: 1250–1261, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Szeto HH, Liu S, Soong Y, Birk AV: Improving mitochondrial bioenergetics under ischemic conditions increases warm ischemia tolerance in the kidney. Am J Physiol Renal Physiol 308: F11–F21, 2014 [DOI] [PubMed] [Google Scholar]
- 28.Zhao K, Zhao GM, Wu D, Soong Y, Birk AV, Schiller PW, Szeto HH: Cell-permeable peptide antioxidants targeted to inner mitochondrial membrane inhibit mitochondrial swelling, oxidative cell death, and reperfusion injury. J Biol Chem 279: 34682–34690, 2004 [DOI] [PubMed] [Google Scholar]
- 29.Liu S, Soong Y, Seshan SV, Szeto HH: Novel cardiolipin therapeutic protects endothelial mitochondria during renal ischemia and mitigates microvascular rarefaction, inflammation, and fibrosis. Am J Physiol Renal Physiol 306: F970–F980, 2014 [DOI] [PubMed] [Google Scholar]
- 30.Sutton TA, Fisher CJ, Molitoris BA: Microvascular endothelial injury and dysfunction during ischemic acute renal failure. Kidney Int 62: 1539–1549, 2002 [DOI] [PubMed] [Google Scholar]
- 31.Basile DP, Donohoe D, Roethe K, Osborn JL: Renal ischemic injury results in permanent damage to peritubular capillaries and influences long-term function. Am J Physiol Renal Physiol 281: F887–F899, 2001 [DOI] [PubMed] [Google Scholar]
- 32.Pusztaszeri MP, Seelentag W, Bosman FT: Immunohistochemical expression of endothelial markers CD31, CD34, von Willebrand factor, and Fli-1 in normal human tissues. J Histochem Cytochem 54: 385–395, 2006 [DOI] [PubMed] [Google Scholar]
- 33.Nakayama T, Sato W, Yoshimura A, Zhang L, Kosugi T, Campbell-Thompson M, Kojima H, Croker BP, Nakagawa T: Endothelial von Willebrand factor release due to eNOS deficiency predisposes to thrombotic microangiopathy in mouse aging kidney. Am J Pathol 176: 2198–2208, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ward BW, Notte JA, Economou NP: Helium ion microscope: A new tool for nanoscale microscopy and metrology. J Vac Sci Technol B 24: 2871–2874, 2006 [Google Scholar]
- 35.Rice WL, Van Hoek AN, Păunescu TG, Huynh C, Goetze B, Singh B, Scipioni L, Stern LA, Brown D: High resolution helium ion scanning microscopy of the rat kidney. PLoS One 8: e57051, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pavenstädt H, Kriz W, Kretzler M: Cell biology of the glomerular podocyte. Physiol Rev 83: 253–307, 2003 [DOI] [PubMed] [Google Scholar]
- 37.Wagner MC, Rhodes G, Wang E, Pruthi V, Arif E, Saleem MA, Wean SE, Garg P, Verma R, Holzman LB, Gattone V, Molitoris BA, Nihalani D: Ischemic injury to kidney induces glomerular podocyte effacement and dissociation of slit diaphragm proteins Neph1 and ZO-1. J Biol Chem 283: 35579–35589, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Abe Y, Sakairi T, Kajiyama H, Shrivastav S, Beeson C, Kopp JB: Bioenergetic characterization of mouse podocytes. Am J Physiol Cell Physiol 299: C464–C476, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Imasawa T, Rossignol R: Podocyte energy metabolism and glomerular diseases. Int J Biochem Cell Biol 45: 2109–2118, 2013 [DOI] [PubMed] [Google Scholar]
- 40.Cybulsky AV: The intersecting roles of endoplasmic reticulum stress, ubiquitin-proteasome system, and autophagy in the pathogenesis of proteinuric kidney disease. Kidney Int 84: 25–33, 2013 [DOI] [PubMed] [Google Scholar]
- 41.Hartleben B, Gödel M, Meyer-Schwesinger C, Liu S, Ulrich T, Köbler S, Wiech T, Grahammer F, Arnold SJ, Lindenmeyer MT, Cohen CD, Pavenstädt H, Kerjaschki D, Mizushima N, Shaw AS, Walz G, Huber TB: Autophagy influences glomerular disease susceptibility and maintains podocyte homeostasis in aging mice. J Clin Invest 120: 1084–1096, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Marchi S, Patergnani S, Pinton P: The endoplasmic reticulum-mitochondria connection: one touch, multiple functions. Biochim Biophys Acta 1837: 461–469, 2014 [DOI] [PubMed] [Google Scholar]
- 43.Barrera-Chimal J, Pérez-Villalva R, Ortega JA, Sánchez A, Rodríguez-Romo R, Durand M, Jaisser F, Bobadilla NA: Mild ischemic injury leads to long-term alterations in the kidney: Amelioration by spironolactone administration. Int J Biol Sci 11: 892–900, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Janmey PA, Hvidt S, Oster GF, Lamb J, Stossel TP, Hartwig JH: Effect of ATP on actin filament stiffness. Nature 347: 95–99, 1990 [DOI] [PubMed] [Google Scholar]
- 45.Koppen M, Langer T: Protein degradation within mitochondria: Versatile activities of AAA proteases and other peptidases. Crit Rev Biochem Mol Biol 42: 221–242, 2007 [DOI] [PubMed] [Google Scholar]
- 46.Langer T, Käser M, Klanner C, Leonhard K: AAA proteases of mitochondria: Quality control of membrane proteins and regulatory functions during mitochondrial biogenesis. Biochem Soc Trans 29: 431–436, 2001 [DOI] [PubMed] [Google Scholar]
- 47.Birk AV, Chao WM, Bracken C, Warren JD, Szeto HH: Targeting mitochondrial cardiolipin and the cytochrome c/cardiolipin complex to promote electron transport and optimize mitochondrial ATP synthesis. Br J Pharmacol 171: 2017–2028, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Szeto HH, Birk AV: Serendipity and the discovery of novel compounds that restore mitochondrial plasticity. Clin Pharmacol Ther 96: 672–683, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Birk AV, Chao WM, Liu S, Soong Y, Szeto HH: Disruption of cytochrome c heme coordination is responsible for mitochondrial injury during ischemia. Biochim Biophys Acta 1847: 1075–1084, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shi J, Dai W, Hale SL, Brown DA, Wang M, Han X, Kloner RA: Bendavia restores mitochondrial energy metabolism gene expression and suppresses cardiac fibrosis in the border zone of the infarcted heart. Life Sci 141: 170–178, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sabbah HN, Gupta RC, Kohli S, Wang M, Hachem S, Zhang K: Chronic therapy with elamipretide (MTP-131), a novel mitochondria-targeting peptide, improves left ventricular and mitochondrial function in dogs with advanced heart failure. Circ Heart Fail 9: e002206, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Eirin A, Ebrahimi B, Kwon SH, Fiala JA, Williams BJ, Woollard JR, He Q, Gupta RC, Sabbah HN, Prakash YS, Textor SC, Lerman A, Lerman LO: Restoration of mitochondrial cardiolipin attenuates cardiac damage in swine renovascular hypertension. J Am Heart Assoc 5: e003118, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.van Vliet AR, Verfaillie T, Agostinis P: New functions of mitochondria associated membranes in cellular signaling. Biochim Biophys Acta 1843: 2253–2262, 2014 [DOI] [PubMed] [Google Scholar]
- 54.Bani-Hani AH, Leslie JA, Asanuma H, Dinarello CA, Campbell MT, Meldrum DR, Zhang H, Hile K, Meldrum KK: IL-18 neutralization ameliorates obstruction-induced epithelial-mesenchymal transition and renal fibrosis. Kidney Int 76: 500–511, 2009 [DOI] [PubMed] [Google Scholar]
- 55.Mizuguchi Y, Chen J, Seshan SV, Poppas DP, Szeto HH, Felsen D: A novel cell-permeable antioxidant peptide decreases renal tubular apoptosis and damage in unilateral ureteral obstruction. Am J Physiol Renal Physiol 295: F1545–F1553, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wu J, Yan Z, Schwartz DE, Yu J, Malik AB, Hu G: Activation of NLRP3 inflammasome in alveolar macrophages contributes to mechanical stretch-induced lung inflammation and injury. J Immunol 190: 3590–3599, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wu J, Li H, Sun X, Zhang H, Hao S, Ji M, Yang J, Li K: A mitochondrion-targeted antioxidant ameliorates isoflurane-induced cognitive deficits in aging mice. PLoS One 10: e0138256, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Baldwin AG, Brough D, Freeman S: Inhibiting the inflammasome: A chemical perspective. J Med Chem 59: 1691–1710, 2016 [DOI] [PubMed] [Google Scholar]
- 59.Ozaki E, Campbell M, Doyle SL: Targeting the NLRP3 inflammasome in chronic inflammatory diseases: Current perspectives. J Inflamm Res 8: 15–27, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Duan SB, Yang SK, Zhou QY, Pan P, Zhang H, Liu F, Xu XQ: Mitochondria-targeted peptides prevent on contrast-induced acute kidney injury in the rats with hypercholesterolemia. Ren Fail 35: 1124–1129, 2013 [DOI] [PubMed] [Google Scholar]
- 61.Li G, Wu J, Li R, Yuan D, Fan Y, Yang J, Ji M, Zhu S: Protective effects of antioxidant peptide SS-31 against multiple organ dysfunctions during endotoxemia. Inflammation 39: 54–64, 2016 [DOI] [PubMed] [Google Scholar]
- 62.Hou Y, Li S, Wu M, Wei J, Ren Y, Du C, Wu H, Han C, Duan H, Shi Y: Mitochondria-targeted peptide SS-31 attenuates renal injury via an antioxidant effect in diabetic nephropathy. Am J Physiol Renal Physiol 310: F547–F559, 2016 [DOI] [PubMed] [Google Scholar]
- 63.Szeto HH, Liu S, Soong Y, Alam N, Prusky GT, Seshan SV: Protection of mitochondria prevents high-fat diet-induced glomerulopathy and proximal tubular injury. Kidney Int 90: 997–1011, 2016 [DOI] [PubMed] [Google Scholar]
- 64.Eirin A, Ebrahimi B, Zhang X, Zhu XY, Woollard JR, He Q, Textor SC, Lerman A, Lerman LO: Mitochondrial protection restores renal function in swine atherosclerotic renovascular disease. Cardiovasc Res 103: 461–472, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.