Abstract
Incubation of synthetic 2-methylneryl diphosphate (2-MeNPP, 10) with 2-methylisoborneol synthase (MIBS) gave a mixture of products that differed significantly from that derived from the natural substrate (E)-2-methylgeranyl disphosphate (3, 2-MeGPP. The proportion of (−)-2-methylisoborneol (1) decreased from 89% to 17% while that of 2-methylenebornane (4) increased from 10% to 26%, with the relative yields of the isomeric homo-monoterpenes 2-methyl-2-bornene (5) and 1-methylcamphene (6) remaining essentially unchanged (<1% each), as determined by chiral gas chromatographic-mass spectrometric (GC-MS) analysis. The majority of the product mixture resulting from the MIBS-catalyzed cyclization of 2-MeNPP (10) consisted of the anomalous monocyclic homo-monoterpenes (±)-2-methylllimonene (15, 39%) and 2-methyl-α-terpineol (13, 10%), as well as the acylic derivatives 2-methylnerol (11, 7%) and 2-methyllinalool (14, <1%). The steady state kinetic parameters of the MIBS-catalyzed reaction, determined using [1-3H]-2-MeNPP, were kcat 0.0046 ± 0.0003 s−1, Km 18 ± 6 μM, and kcat/Km 2.55 × 102 M−1s−1. By comparison, the natural substrate 2-MeGPP had a kcat 0.105 ± 0.007 s−1, Km 95 ± 49 μM, and kcat/Km 1.11 × 103 M−1s−1. Taken together with earlier X-ray crystallographic studies of MIBS, as well as previous investigations of the mechanistically related plant monoterpene cyclase, bornyl diphosphate synthase, these results provide important insights into the binding and cyclization of both native substrates and intermediates and their analogues.
Keywords: terpene synthase, homo-monoterpene, 2-methylisoborneol, 2-methylneryl diphosphate
Introduction
Since the discovery of 2-methylisoborneol (2-MIB, 1) by Gerber in 1969, there has been considerable interest in the detection, bioremediation, and biosynthesis of this volatile, odiferous homo-monoterpene (Figure 1).1–4 Possessing a musty odor and muddy off-taste, as well as an extremely low threshold of detection by humans (<10 ng/L), 2-MIB is produced by a wide range of Gram-positive actinomycetes, myxobacteria, and cyanobacteria, being second in occurrence only to the common odoriferous sesquiterpene geosmin.5
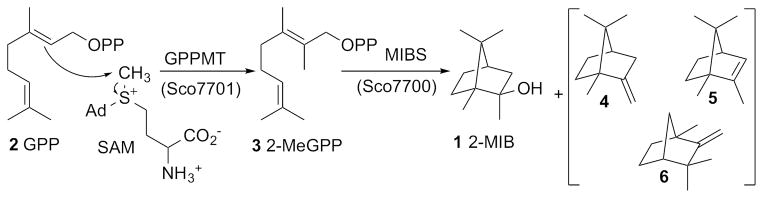
Figure 1.
Biosynthesis of 2-methylisoborneol (1).
The elucidation of the biosynthesis of 2-methylisoborneol at the molecular genetic and biochemical level was independently reported in 2008 by two research groups (Figure 1).6–8 The 2-MIB biosynthetic gene cluster in Streptomyces coelicolor was shown to harbor a terpene synthase gene (sco7700) translationally coupled to the gene for an S-adenosyl-L-methionine (SAM)-dependent C-methyl transferase, (sco7701), as well as a third gene (sco7699) encoding a protein of still unknown function, annotated only as a nucleotide-binding protein.7 This three-gene biosynthetic operon is highly conserved across the genome sequences of more than three dozen bacterial species.3,5,6 The SAM-dependent geranyl diphosphate C-methyltransferase (GPPMT) (Sco7701) was shown to catalyze the first step in the biosynthetic pathway in S. coelicolor, the electrophilic methylation of the olefinic C-2 position of geranyl diphosphate (GPP, 2) to form (E)-2-methylgeranyl diphosphate (2-MeGPP, 3).7 Sco7700, 2-methylisoborneol synthase (MIBS), catalyzes the Mg2+-dependent multistep cyclization of the acyclic 2-MeGPP precursor to the bicyclic alcohol 2-MIB (1) which is accompanied by small amounts of the bicyclic homo-monoterpenes 2-methylenebornane (4), 2-methylbornene (5), and 1-methylcamphene (6), The only other enzyme known to utilize 2-MeGPP as substrate is the closely related 2-methylenebornane synthase (Pfl_1841) of Pseudomodas fluorescens PfO-1.9 These findings were fully consistent with the results of independent whole-cell precursor incorporation experiments involving the feeding of [methyl-13C]methionine and samples of deuterated mevalonate to the myxobacterium Nannocycstis exedens and MS analysis of the resulting 2-methylisoborneol in the head space volatiles.4
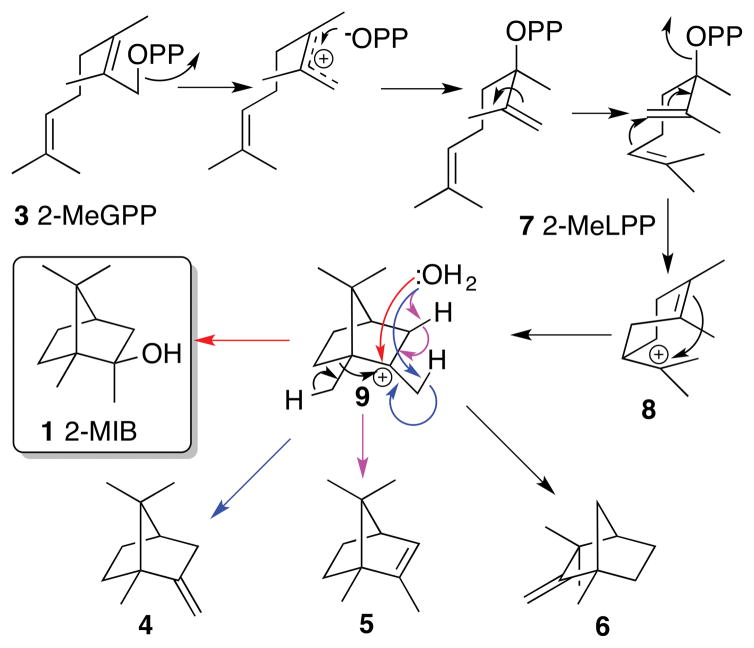
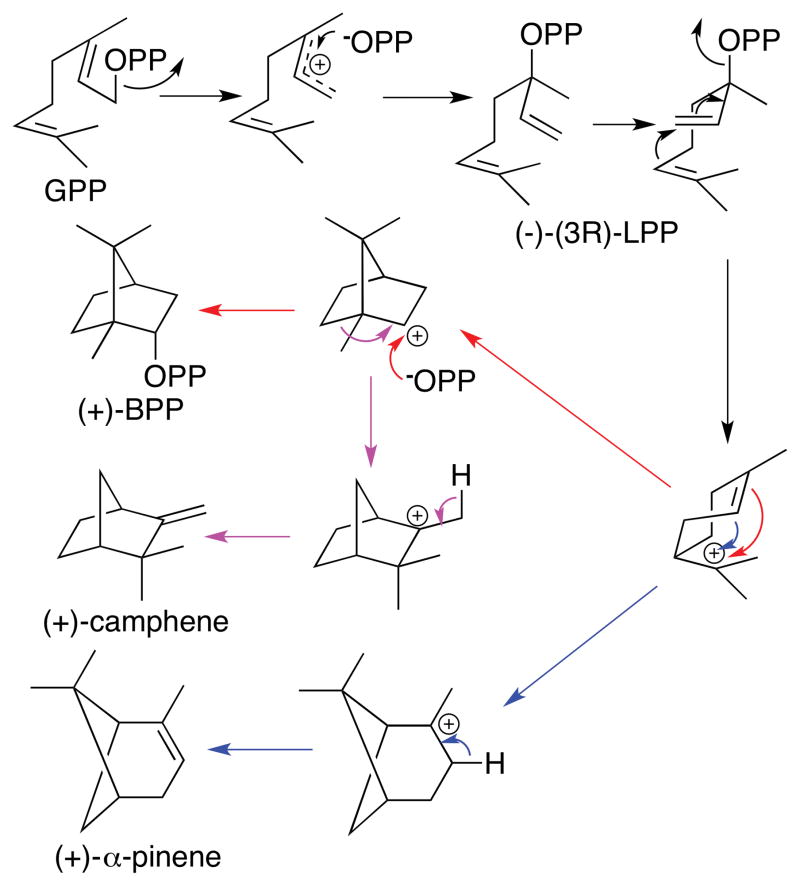
The proposed 2-methylisoborneol synthase mechanism is based on close analogy to the well-documented cyclization mechanisms established for a wide range of monoterpene synthases (Figure 2).10 MIBS initiates the electrophilic cyclization by ionizing the Mg2+-complexed substrate 2-MeGPP (3) to generate the corresponding allylic cation–pyrophosphate ion pair which then collapses to the transoid conformer of the allylic isomer (3R)-2-methyllinalyl diphosphate (2-MeLPP, 7). Rotation about the C-2,3-bond generates the cisoid conformer of 7, folded in the anti-endo-boat conformation, which then undergoes ionization and cyclization to generate the (4R)-2-methyl-α-terpinyl cation (8). Electrophilic attack on the cyclohexenyl double bond of 8 generates the 2-methyl-2-bornyl cation (9), which is then quenched on the exo face by bound water to generate 2-methylisoborneol (1). Competing deprotonation of intermediate 9 or its proximal rearrangement product readily accounts for formation of the co-products 4, 5, and 6. The MIBS cyclization mechanism, including the deduced stereochemistry and conformations of the various intermediates, closely resembles that firmly established for (+)-bornyl diphosphate synthase/α-pinene synthase (BPPS) from Salvia officinalis which converts geranyl diphosphate (GPP, 2) to (+)-bornyl diphosphate ((+)-BPP) (75 %) as well as a mixture of the monoterpene hydrocarbons (+)-α-pinene, (+)-camphene, (±)-limonene and terpinolene (25% total olefins) (Figure 3).11–13 There are of course two key differences between the reactions catalyzed by MIBS and by BPPS: 1) MIBS cyclizes 2-MeGPP instead of GPP. Although MIBS can cyclize GPP to a mixture of monocyclic and bicyclic monoterpenes, the observed kcat is four orders of magnitude lower than that for the natural methylated substrate 2-MeGPP.7 2) The final step in the MIBS-catalyzed cyclization cascade is the quenching of the 2-methyl-2-bornyl cation exclusively on the exo face by a bound water (Figure 2), while in the BPPS-catalyzed reaction, the paired pyrophosphate ion is recombined with the bornyl cation exclusively by endo attack (Figure 3).
Figure 2.
Mechanism of the MIBS-catalyzed cyclization of 2-methylgeranyldiphosphate (3) to 2-methylisoborneol (1).
Figure 3.
Mechanism of bornyl diphosphate/α-pinene synthase.
High resolution crystal structures have recently been determined for MIBS in both an unliganded state and in complex with a number of substrate or intermediate analogues.14,15 The 1.80 – 1.95 Å structures of MIBS bound to geranyl-S-thiolodiphosphate and 2-fluorogeranyl diphosphate, respectively, show each analogue coordinated with two 2 Mg2+ ions and bound in extended conformations in which the 6,7-double bond of each analogue is twisted away from the parallel relationship expected for the postulated anti-endo-boat conformation of the actual cyclizing cisoid (3R)-2-MeLPP intermediate (Figure 2).14 Although this deviation may simply be the result of crystallographic trapping of a thermodynamically stable, rather than a kinetically or mechanistically relevant conformer, more interestingly it reflects the fact that the required 180° rotation of the 2-propenyl substituent about the C-2,3 bond of the anti-endo-boat conformer of the (3R)-2-methyl linalyl diphosphate intermediate is sterically forbidden by a clash with the 6,7-double bond that is augmented by the presence of the C2-methyl substituent. It is thus probable that adoption of the endo-boat conformation takes place subsequent to conversion of the transoid to the cisoid conformer of 2-MeLPP. Such extended 6,7-double bond conformations of bound substrate analogues have previously been observed in the structures of a number of canonical monoterpene synthases.16,17 Also thought-provoking were the unexpected results from co-crystallization of MIBS with racemic 2-fluorolinalyl diphosphate (2-FLPP), which had been predicted to be an unreactive analogue of the natural (3R)-2-methyllinalyl diphosphate intermediate due to the strongly electron-withdrawing 2-fluoro substituent. The 2.00 Å structure of the resulting complex revealed that the MIBS-bound 2-FLPP had undergone a reverse allylic rearrangement to generate bound 2-fluoroneryl diphosphate in complex with 2 Mg2+ ions.15 Also unexpectedly, it was found that in solution MIBS converted the supposedly unreactive 2-FLPP to camphor, no doubt formed by cyclization of 2-FLPP and spontaneous elimination of HF from enzymatically-generated 2-fluoro-2-isoborneol. The observation of enzyme-generated 2-fluoroneryl diphosphate trapped within the active site of MIBS raises the question whether the cis geometric isomer of 2-FGPP is a true intermediate of the MIBS-catalyzed ionization and cyclization of 2-FLPP, the nominally unreactive analogue of the natural intermediate 2-MeLPP (7), or merely an anomalous shunt metabolite of the reverse allylic diphosphate rearrangement. We have therefore now examined the incubation of MIBS with 2-methylneryl diphosphate (2-MeNPP, 10), the cis isomer of the natural substrate, 2-methylgeranyl diphosphate (2-MeGPP, 3), and report the results below.
Results
Preparation of 2-methylneryl diphosphate (10) and (±)-2-methyl-α-terpineol (13)
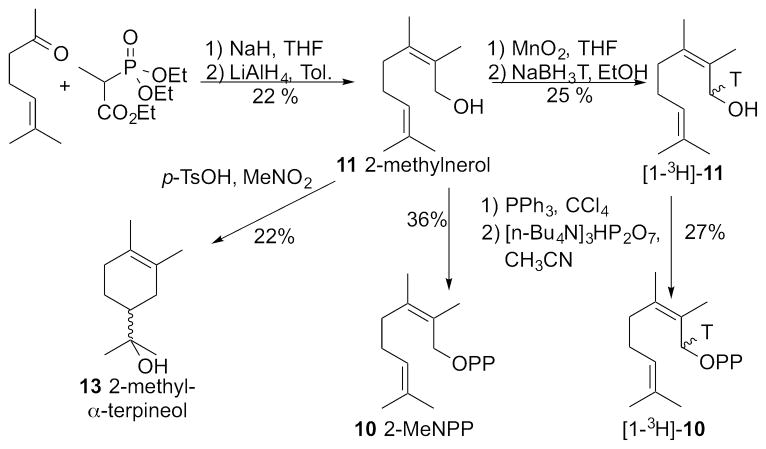
To prepare 2-methylneryl diphosphate (11, 2-MeNPP), we used a modification of the previously reported synthesis of the trans isomer 2-methylgeranyl diphosphate (3, 2-MeGPP) (Figure 4).7 Wadsworth-Horner-Emmons reaction of 6-methyl-5-hepten-2-one with triethyl 2-phosphonopropionate followed by LiAlH4 reduction of the resulting mixture of Z and E conjugated esters gave a 1:1 mixture of (Z)-2-methylnerol (11) and (E)-2-methylgeraniol (12) which were cleanly resolved by flash column chromatography (see SI for details). The purified 2-methylnerol (11) was reacted with triphenylphosphine and CCl4 in an Appel reaction and the resulting 2-methylneryl chloride was then directly reacted without further purification with tris(tetrabutylammonium) hydrogen pyrophosphate to give 2-methylneryl diphosphate (10, 2-MeNPP). The corresponding [1-3H]-2-methylneryl diphosphate ([1-3H]-10, [1-3H]-2-MeNPP) was similarly prepared by oxidation of the mixture of 2-methylnerol and 2-methylgeraniol to the derived mixture of aldehydes, followed by reduction with NaBH3T and chromatographic separation of the resulting mixture of [1-3H]-2-methylnerol and [1-3H]-2-methylgeraniol using AgNO3-impregnated silica gel.
Figure 4.
Synthesis of 2-methylneryl diphosphate (10, 2-MeNPP) and 2-methyl-α-terpineol (13).
For the planned enzyme incubations, we also needed an authentic reference sample of the homo-monoterpene alcohol (±)-2-methyl-α-terpineol (13),18 which was readily prepared by biomimetically-modeled, p-TsOH-catalyzed solvolysis of 2-methylnerol (11) in nitromethane (Figure 4).
In vitro incubation of 2-methylneryl diphosphate with 2-methylisoborneol synthase
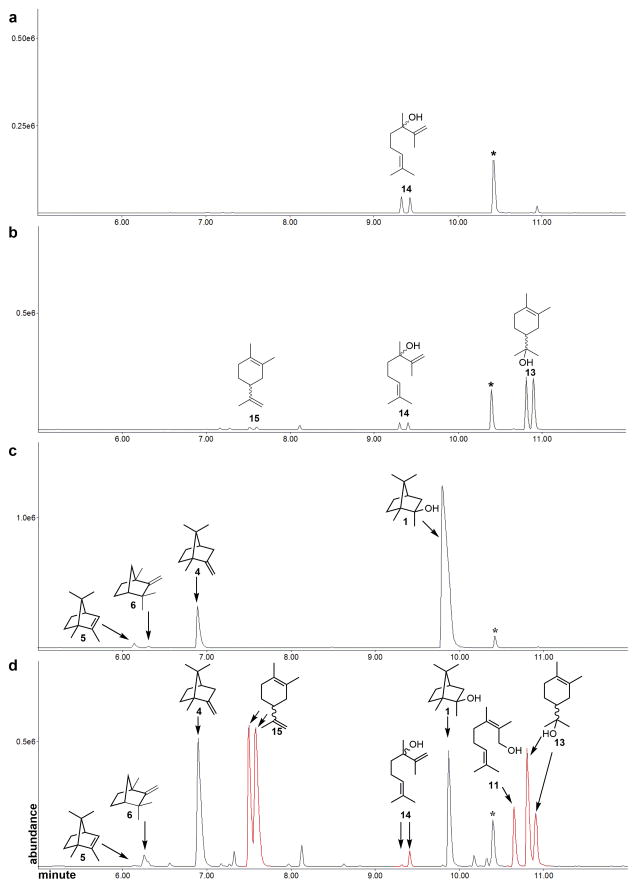
Before examining the cyclization of 2-methylneryl diphosphate by methylisoborneol synthase, we carried out individual control incubations in the absence of enzyme with 2-MeGPP and 2-MeNPP and analyzed the pentane-soluble extracts by chiral GC-MS in order to identify and quantify the formation of Mg2+-catalyzed solvolysis products (Figures 5a and 5b). The control incubation with 2-MeGPP produced only racemic (±)-2-methyllinalool (14). The exclusive formation of this tertiary allylic alcohol contrasts with the well-known Mg2+-catalyzed solvolysis of GPP itself, which typically gives a 3:1 mixture of linalool and geraniol.18 The preferred formation of 2-methyllinalool (14) by solvolysis of 2-MeGPP presumably reflects the higher free energy of 2-methylgeraniol due to the increased steric hindrance about the tetra-substituted double bond compared to geraniol. By contrast, the control Mg2+-catalyzed solvolysis of 2-MeNPP gave a mixture of three racemic products, consisting of acyclic (±)-2-methyllinalool (14) as well as the monocyclic (±)-2-methyllimonene (15), and (±)-2-methyl-α-terpineol (13) (Figure 5b). The Mg2+-catalyzed formation of both (±)-13 and (±)-15 from 2-MeNPP, but not from 2-MeGPP, is consistent with the fact that the (E)-allylic diphosphate 2-MeGPP (3) is geometrically incapable of direct cyclization. As a positive control, incubation of 2-MeGPP (3) with 2-methylisoborneol synthase and chiral GC-MS analysis confirmed the formation of (−)-2-methyisoborneol (2-MIB, 1) as the major product (89 %) accompanied by minor amounts of enantiomerically pure 2-methylenebornane (4) (10 %), 1-methylcamphene (6) (<1 %) and 2-methyl-2-bornene (5) (<1 %), as previously observed (Figure 5c and Table 1).6,7
Figure 5.
Chiral GC-MS analysis (TIC) of incubations with methylisoborneol synthase (MIBS). a) Control incubation of 2-MeGPP (3) in Mg2+-containing buffer without MIBS. b) Control incubation of 2-MeNPP (10) in Mg2+-containing buffer without MIBS. c) Incubation of MIBS with 2-MeGPP (3). d) Incubation of MIBS with 2-MeNPP (10). Previously unobserved product peaks are shown in red. Peaks denoted with * are geraniol internal standard.
Table 1.
Distribution of products from the incubation of 2-MeGPP (3) and 2-MeNPP (10) with 2-methylisoborneol synthase.
| Product (%) | ||||||||
|---|---|---|---|---|---|---|---|---|
| substrate | MIB (1) | 4 | 5 | 6 | 13 | (±)-15 | 11 | 14 |
| 2-MeGPP (3) | 89 | 10 | <1 | <1 | NDa | ND | ND | ND |
| 2-MeNPP (10) | 17 | 26 | <1 | <1 | 10b (10/0)c | 39b (17/22)c | 7 | <1b (0/<1)c |
ND, not detected;
percent corrected for background Mg2+-catalyzed solvolysis;
percent first eluted peak/percent second eluted peak.
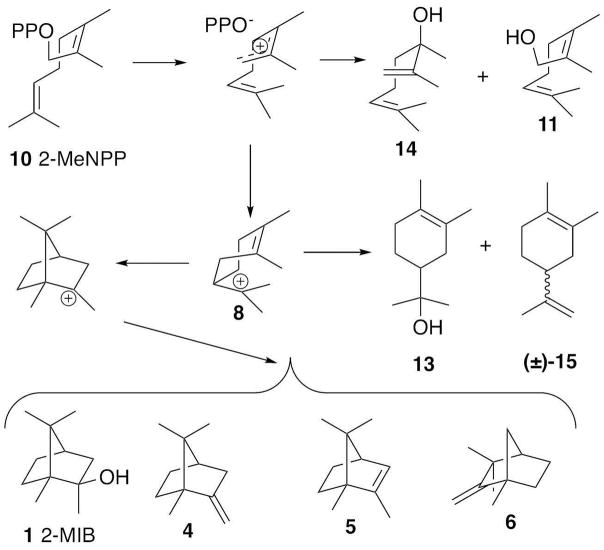
Incubation of 2-MeNPP (10) with MIBS and analysis by chiral GC-MS revealed the production of the same group of homochiral bicyclic homo-monoterpenes, (−)-2-methylisoborneol (1, 17%), 2-methylenebornane (13, 26%), 1-methylcamphene (6) (<1 %) and 2-methyl-2-bornene (5) (<1 %), but in both substantially different proportions and lower overall yield compared to 2-MeGPP (Figures 5d and 6). Significantly, four additional, previously unobserved products constituting >56% of the overall mixture were formed: monocyclic 2-methyllimonene (15, 39 %) and 2-methyl-α-terpineol (13, 10 %) along with the acyclic alcohols 2-methylnerol (11, 7%) and 2-methyllinalool (14, <1%) (Table 1, Figures 5d and 6). Interestingly, correction for background Mg2+-catalyzed solvolysis of the 2-MeNPP substrate revealed that both 2-methyl-α-terpineol (13) and 2-methyllinalool (14) were formed as single enantiomers, while 2-methyllimonene (15) was generated as a racemic mixture. The absolute configurations of the enzymatically-generated products 13 and 14 were not determined.
Figure 6.
Mechanism of the MIBS-catalyzed transformation of 2-MeNPP (10).
Steady-state kinetics
The steady-state kinetic parameters for the MIBS-catalyzed conversion of [1-3H]-2-MeNPP (10) to total pentane soluble homo-monoterpene products were determined and directly compared with those re-determined for the natural substrate [1-3H]-2-MeGPP (3) under identical conditions (Table 2). While the turnover number, kcat, for 2-MeNPP (0.0045 s−1) was ~20-fold lower than that for 2-MeGPP, the observed kcat/Km, of 255 M−1s−1 for 2-MeNPP was only 4-fold lower than that for 2-MeGPP (1105 M−1s−1), reflecting a partially compensating ~5-fold decrease in the observed Km for 2-MeNPP compared to 2-MeGPP.8
Table 2.
Steady-state kinetic parameters for total product formation from incubation with methylisoborneol synthase.
| Substrate | kcat (s−1) | Km (μM) | kcat/Km (M−1s−1) |
|---|---|---|---|
| 2-MeGPP (3) | 0.105 ± 0.007 | 95 ± 49 | 1110 |
| 2-MeNPP (10) | 0.0046 ± 0.0003 | 18 ± 6 | 255 |
Discussion
Although (Z)-2-methylneryl diphosphate (10, 2-MeNPP) shows only a modest 4-fold decrease in the specificity constant kcat/Km compared to the natural substrate, the trans geometric isomer (E)-2-methylgeranyl diphosphate (3, 2-MeGPP), the substantially altered product distributions resulting from incubation of 2-MeNPP with 2-methylisoborneol synthase establish that 2-MeNPP is a poor surrogate for 2-MeGPP. Thus, while 2-MeNPP could, in principle, be ionized to the same cisoid allylic cation – pyrophosphate ion pair as the presumptive intermediate (3R)-2-methyllinalyl diphosphate (7, 2-MeLPP), in practice incubation of 2-MeNPP with 2-methylisoborneol synthase generated a substantially altered array of bicyclic and monocylic homo-monoterpene products. Thus, although cyclization of 2-MeNPP generated only a single enantiomer of each of the four natural bicyclic natural products, 2-methylisoborneol (1), 2-methylenebornane (4), 2-methylbornene (5), and 1-methylcamphene (6), the relative proportion of 1 was reduced more than 5-fold from 89% to 17% of the total product mixture, only partially offset by an increase in the fraction of 2-methylenebornane (4) which increased from 10% to 26% (Table 1). The majority of the product mixture resulting from the MIBS-catalyzed cyclization of 2-MeNPP (10) consisted of the anomalous monocyclic homo-monoterpenes (±)-2-methyllimonene (15, 39%) and a single enantiomer of 2-methyl-α-terpineol (13, 10%), as well as the acylic derivatives 2-methylnerol (11, 7%) and a single enantiomer of 2-methyllinalool (14, <1%). Interestingly, while 13, 14, and 15 are not detected among the products of the in vitro incubation of 2-methylisoborneol synthase with 2-MeGPP, all three of these homo-monoterpenes, of unspecified chiral purity, have been reported as minor components of the volatile headspace extracts of a small number of 2-methylisoborneol-producing actinomycetes.19,20
Substantial changes in product distribution have been reported when the cis isomer neryl diphosphate is incubated with monoterpene synthases in place of the natural trans allylic substrate, geranyl diphosphate. For example, in spite of only minor differences in overall kcat, incubation (+)-BPP/(+)-pinene synthase with NPP instead of GPP resulted in a decrease in the proportion of the bicyclic monoterpenes α-pinene and camphene from 79% to 23%, while the fraction of the monocyclic products limonene and α-terpinolene exhibited a substantial increase from 15% to 71% of total monoterpenes.21 The enhanced formation of abortive cyclization products is almost certainly the consequence of aberrant positioning of 2-MeNPP and NPP in the active sites of the respective homo-monoterpene or monoterpene synthases, compared to the binding of the native substrates 2-MeGPP or GPP.
The fact that cyclization of 2-MeNPP by 2-methylisoborneol synthase generates the naturally occurring enantiomers of 2-methylisoborneol (1), 2-methylenebornane (4), 2-methylbornene (5), and 1-methylcamphene (6) indicates that at least a portion of this anomalous substrate is bound to MIBS in a position and conformation that is compatible with, but certainly not identical to, the precise folding of the natural substrate 2-MeGPP (3) and the derived intermediate (3R)-2-MeLPP (7) in the MIBS active site. Although the 2-methylllimonene (15) and 2-methyl-α-terpineol (13) formed from 2-MeNPP are both formally derivable from a common 2-methyl-α-terpinyl cation, the fact that 15 is generated as a racemic mixture while 13 is produced as a single enantiomer indicates clearly that they cannot both be derived from a single monocyclic carbocation intermediate. The surrogate 2-MeNPP substrate therefore must be bound in at least two distinct conformations during MIBS catalyzed cyclization. The fact that only a single enantiomer of each of bicyclic, monocyclic, and acyclic alcohols 1, 13, and 14 is produced by incubation of 2-MeNPP with MIBS also suggests that the same bound water molecule is responsible for quenching the corresponding carbocation intermediates.
Experimental Section
Materials
Reagents and solvents were purchased from Sigma-Aldrich or Fisher Scientific, were of the highest quality available, and were used without further purification. NaBH3T solution (6 μmol, 80 Ci/mmol specific activity, 500 mCi total in 1 mL of 0.01 N NaOH) was purchased from American Radiolabeled Chemicals. Isopropylthio-D-galactopyranoside (IPTG) was purchased from Invitrogen. Ni-NTA affinity resin was from Qiagen. Amicon Ultra Centrifugal Filter Units (Amicon Ultra-15 10,000 MWCO) were purchased from Millipore. Purified SCO7700 protein was overexpressed and purified as previously described.7
Methods
Gas chromatography–mass spectrometry (GC–MS) analyses were performed using an Agilent 5977A Series GC/MSD instrument (70 eV, electron impact), a 1-μL injection volume, and a 3 min solvent delay. Achiral GC–MS conditions used an HP-5ms capillary column (0.25 mm ID × 30 m length × 0.25 μm film, Agilent Technologies) and a temperature program with a 2 min hold at 60 °C, a 20 °C min−1 increment to 280 °C, followed by a 2 min hold at 280 °C. Chiral GC–MS separations were preformed using a CP-ChiralSil-Dex column (0.32 mm ID × 25 m length × 0.25 μm film, Agilent) and a temperature program with a 1 min hold at 50 °C, a 10 °C min−1 increment to 200 °C, followed by a 1 min hold at 200 °C. Compounds detected by GC–MS were directly compared to their authentic standards using the MassFinder 4.2.1 program (http://www.massfinder.com). Retention indices were measured using C8–C20 and C10–C40 alkane standards.
Liquid chromatography-mass spectrometry (LC-MS) analyses were performed using a Finnigan LXQ LC-MS instrument in negative ESI mode, with a Waters Symmetry C18 column (35 μM, 2.1 × 50 mm), a 10 μL injection volume and 1 min signal delay. The method used was a 1 min isocratic gradient of 95:5 water:acetonitrile, a gradient to 5:95 water:acetonitrile over 5 min, followed by isocratic elution with 5:95 water:acetonitrile for 9 min. NMR spectra were obtained using a Bruker Avance AV400 NMR spectrometer operating at a 400 MHz 1H frequency or a Bruker Avance AV300 NMR spectrometer operating at a 300 MHz 1H frequency. Liquid scintillation counting was performed using a Beckman-Coulter LS6500 scintillation counter and Opti-Flour scintillation cocktail from Perkin-Elmer.
All proteins were handled at 4 °C unless otherwise stated. Protein concentrations were determined according to the method of Bradford with bovine serum albumin as the standard.22 Protein purity was estimated using SDS PAGE gel electrophoresis and visualized using Coomassie Blue stain according to the method of Laemmli.23
2-methylneryl diphosphate (10)
The cis isomer 2-methylnerol (11) was obtained by preparation of a 1:1 mixture of 2-methylnerol (11) to 2-methylgeraniol (12) and separation of the isomeric alcohols using column chromatography as previously described.7 The purified 2-methylnerol was then converted to 2-methylneryl diphosphate (10), using the method developed by Poulter.24 Triphenylphosphine (1.27 g, 4.75 mmol) was added to a stirring solution of 2-methylnerol (11) (200 mg, 1.19 mmol) in anhydrous CCl4 (16 mL) and the mixture was refluxed for 24 h. The solvent was evaporated in vacuo and the resulting slurry was triturated with pentane (30 mL) and filtered by suction filtration. Concentration of the pentane filtrate at room temperature provided crude 2-methylneryl chloride, which was used in the subsequent reaction without further purification. Tris(tetrabutylammonium) hydrogen pyrophosphate (1.58 g, 2.38 mmol) was added to the 2-methylneryl chloride in dry CH3CN (10 mL) under nitrogen and the reaction was stirred at room temperature for 2 days. The solvent was removed under a gentle stream of N2 to give a viscous, light brown liquid, followed by the addition of 30 mL of 0.05 M KHCO3 and extraction with ether (5 × 5 mL) to remove organic impurities. The resulting aqueous phase was applied to a DEAE-Sephadex column that had been equilibrated with 0.05 M KHCO3. After loading, the column was washed with 100 mL of 0.05 M KHCO3 and eluted with a linear gradient of 0.05 M KHCO3 (300 mL) to 1.0 M KHCO3 (300 mL) at a flow rate of 2.0 mL/min. Fractions (35 mL) were collected and lyophilized and those containing 2-methylneryl diphosphate (10) were dissolved in a minimum amount of dH2O and applied to a CHP-20P column equilibrated with dH2O. The column was run with a stepwise gradient of 100 mL of dH2O, 100 mL of 5% aqueous CH3CN, 100 mL of 10% aqueous CH3CN, 100 mL of 20 % aqueous CH3CN in H2O and 100 mL of 60 % aqueous CH3CN in H2O. Fractions were lyophilized to yield 105 mg of 2-methylneryl diphosphate (10) as a pale yellow solid (27 % yield). Rf = 0.35 (6:3:1 propanol to NH4OH to water). 1H NMR (400 MHz, D2O) δ 5.08 (t, J = 4.5 Hz, 1H, H-6), 4.31 (d, J = 4.0 Hz, 2H, H-1), 2.05 (m, 2H, H-4), 1.98 (m, 2H, H-5), 1.61 (s, 3H, H-11), 1.58 (s, 3H, H-9), 1.55 (s, 3H, H-10), 1.49 (s, 3H, H-8). 31P {1H} NMR (161 MHz, D2O) δ −6.6 (d, J = 22.5 Hz, Pβ), −10.4 (d, J = 22.5 Hz, Pα). 13C {1H} NMR (75 MHz, D2O): δ 16.1 (CH3), 17.0 (CH3), 18.2 (CH3), 24.9 (CH3), 26.6 (CH2), 33.3 (CH2), 66.3 (CH2), 124.1 (CH), 133.8 (C), 135.6 (C), 160.3 (C). LC-MS (neg ion ESIMS) m/z 326.99.
[1-3H]-2-Methylneryl diphosphate ([1-3H]-10) and [1-3H]-2-methylgeranyl diphosphate ([1-3H]-3)
A 1.0 mL solution of NaBH3T (6 μmol, 80 Ci/mmol, 500 mCi in 1 mL of 0.01 N NaOH) was added to a round bottom flask. The reagent vial was rinsed with 4 × 1 mL of 0.01 N NaOH which was added to the solution and then placed under argon, cooled in an ice bath. An ice-cold 1:1 mixture of 2-methylneral to 2-methylgeranial (425 mg, 2.55 mmol), freshly prepared as previously described,7 in dry EtOH (7.5 mL) was added slowly over 10 min. The reaction was stirred at 0 °C for 3 h, and the remaining aldehydes were reduced by the slow addition of unlabeled NaBH4 powder (75 mg, 2.0 mmol). The resulting solution was warmed to room temperature and stirred overnight. After a total of 22 h, the reaction was quenched by careful transfer of the mixture into ice-cold half-saturated aqueous NH4Cl (10 mL). The aqueous layer was extracted with Et2O (5 × 10 mL) and the combined extracts were dried, filtered, and concentrated to give 310 mg of yellowish liquid (72% crude chemical yield, 375 mCi, 75 % radiochemical yield). The residue was purified by flash chromatography on 10 % (w/w) AgNO3-impregnated silica gel [EtOAc/hexanes (1:9); 4 × 16-cm column] giving 100 mg of [1-3H]-2-methylnerol ([1-3H]-11, 25 % chemical yield, 128 mCi, 26 % radiochemical yield) and 210 mg of [1-3H]-2-methylgeraniol ([1-3H]-12, 48 % chemical yield, 247 mCi, 49 % radiochemical yield). The purified [1-3H]-2-methylnerol ([1-3H]-11) and [1-3H] 2-methylgeraniol ([1-3H]-12) were individually converted to the corresponding diphosphate esters using the same method employed for the synthesis of unlabeled 10 and 3 to yield 98 mg of [1-3H]-2-methylneryl diphosphate ([1-3H]-10), 37 % chemical yield, 6.2 mCi total radioactivity, 2.2 % radiochemical yield, 28 mCi/mmol specific activity) and 200.1 mg of [1-3H]2-methylgeranyl diphosphate ([1-3H]-3, 36 % chemical yield, 105 mCi total radioactivity, 36 % radiochemical yield, 232.8 mCi/mmol specific activity). The NMR spectra of ([1-3H]-10 and of [1-3H]-3 matched those of unlabeled 10 and 3.7
2-Methyl-α-terpineol (13)
p-TsOH (40 mg, 0.20 mmol) was added to a solution of 2-methylnerol (200 mg, 1.19 mmol) in 10 mL of MeNO2. The reaction mixture was stirred at rt for 1 h and monitored by TLC (5:1 hexanes/ethyl acetate). The crude product was concentrated in vacuo and purified by column chromatography (5:1 hexanes/ethyl acetate) yielding 44 mg of 2-methyl-α-terpineol (13, 22% yield). The NMR data matched those previously reported for 13 prepared by an alternative method.19 1H NMR (400 MHz, CDCl3) δ 1.98, (m, 2H, H-6), 1.85 (m, 2H, H-3), 1.72 (m, 1H, H-5), 1.65 (s, 6H, H-10, 11), 1.48 (m, 2H, H-4), 1.22 (s, 6H, H-8, 9). 13C {1H} NMR (100 MHz, CDCl3) δ 19.4 (C-9, CH3), 19.5 (C-8, CH3), 24.3 (C-3, CH2), 26.3 (C-6, CH2), 27.3 (C-4, CH2), 32.7 (C-11, CH3), 33.2 (C-10, CH3), 45.8 (C-5, CH), 72.9 (C-7, C), 125.0 (C-2, C), 125.8 (C-1, C). GC-MS (EI) m/z 168.1.
Incubations with 2-methylisoborneol synthase (SCO7700)
Purified 2-methylisoborneol synthase (SCO7700) (10 μM) was added to a glass test tube containing 3.0 mL of assay buffer (50 mM PIPES, 100 mM NaCl, 15 mM MgCl2, 5 mM β-mercaptoethanol and 20 % glycerol, pH 6.7) and 60 μM of either 2-methylneryl diphosphate (10) or 2-methylgeranyl diphosphate (3). The enzymatic reaction mixture was overlaid with 3.0 mL of pentane and incubated at 30 °C for 2 h. The enzymatic products were extracted with 3 × 3.0 mL of pentane and the organic extracts dried over Na2SO4, filtered, concentrated in vacuo to 100 μL and analyzed by GC-MS. Control reactions, measuring the background Mg2+-catalyzed hydrolysis of each substrate, were conducted similarly as above minus the addition of SCO7700 to the incubation mixture. An internal geraniol standard (25 pmoles) was added to the organic extracts of the enzymatic and control incubations after extraction.
Steady-state kinetics
Kinetic analyses were performed in 1 mL of assay buffer (50 mM PIPES, 15 mM MgCl2, 100 mM NaCl, 5 mM β-mercaptoethanol, 20 % glycerol, pH 6.7) with either 2-methylneryl diphosphate (10, 10 – 500 μM) and [1-3H]-2-methylneryl diphosphate ([1-3H]-10, 8.4 Ci/mol) or 2-methylgeranyl diphosphate (3, 10 – 500 mM) and [1-3H]2-methylgeranyl diphosphate (([1-3H]-3, 16.5 Ci/mol). The reactions were initiated by the addition of 0.20 μM of SCO7700 protein, overlaid with 1 mL of pentane and incubated at 30 °C for 17 min. The reactions were quenched by the addition of 75 μL of 500 mM EDTA (pH 8.0) and vortexing for 30 s. The pentane layer was loaded onto a silica column (2 cm) in a Pasteur pipette and forced through with a stream of nitrogen into a scintillation vial containing 7 mL of Opti-Fluor. The enzymatic reaction mixture was extracted 3 more times with 1 mL of ether, and all organic extracts were passed through the silica column and collected. The combined extracts were assayed by liquid scintillation counting using a Beckman-Coulter LS6500 scintillation counter. Kinetic constants were calculated using the program Kaleidagraph 4.0 and were fit to the Michaelis-Menten equation. Reported standard deviations in the steady-state kinetic parameters represent the calculated statistical errors in the nonlinear, least squares regression analysis.
Supplementary Material
Acknowledgments
This work was supported by NIH grant GM30301 (D.E.C.). We thank Tun-Li Shen for assistance with mass spectrometry and Russell Hopson for assistance with NMR spectroscopy.
Footnotes
This article is dedicated to Professor Satoshi Ōmura, 2015 Nobel Laureate in Physiology or Medicine, in honor of his profound contributions to basic science and the improvement of human health as well as his longstanding support of the arts, and in deep appreciation for his continuing support and friendship.
References
- 1.Gerber NN. A volatile metabolite of actinomycetes, 2-methylisoborneol. J Antibiot. 1969;22:508–509. doi: 10.7164/antibiotics.22.508. [DOI] [PubMed] [Google Scholar]
- 2.Jüttner F, Watson SB. Biochemical and ecological control of geosmin and 2-methylisoborneol in source waters. Appl Environ Microbiol. 2007;73:4395–4406. doi: 10.1128/AEM.02250-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Giglio S, Chou WK, Ikeda H, Cane DE, Monis PT. Biosynthesis of 2-methylisoborneol in cyanobacteria. Environ Sci Technol. 2011;45:992–998. doi: 10.1021/es102992p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dickschat JS, et al. Biosynthesis of the off-flavor 2-methylisoborneol by the myxobacterium Nannocystis exedens. Angew Chem Int Ed Engl. 2007;46:8287–8290. doi: 10.1002/anie.200702496. [DOI] [PubMed] [Google Scholar]
- 5.Yamada Y, et al. Terpene synthases are widely distributed in bacteria. Proc Natl Acad Sci U S A. 2015;112:857–862. doi: 10.1073/pnas.1422108112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Komatsu M, Tsuda M, Omura S, Oikawa H, Ikeda H. Identification and functional analysis of genes controlling biosynthesis of 2-methylisoborneol. Proc Natl Acad Sci U S A. 2008;105:7422–7427. doi: 10.1073/pnas.0802312105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang CM, Cane DE. Biochemistry and molecular genetics of the biosynthesis of the earthy odorant methylisoborneol in Streptomyces coelicolor. J Am Chem Soc. 2008;130:8908–8909. doi: 10.1021/ja803639g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang CM, Cane DE. Biochemistry and molecular genetics of the biosynthesis of the earthy odorant methylisoborneol in Streptomyces coelicolor (corrn to J Am Chem Soc 130, 8908 (2008)) J Am Chem Soc. 2010;132:9509. doi: 10.1021/ja803639g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chou WK, Ikeda H, Cane DE. Cloning and characterization of Pfl_1841, a 2-methylenebornane synthase in Pseudomonas fluorescens PfO-1. Tetrahedron. 2011;67:6627–6632. doi: 10.1016/j.tet.2011.05.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wise ML, Croteau R. Comprehensive Natural Products Chemistry. In: Cane David E., editor. Isoprenoids Including Carotenoids and Steroids. Vol. 2. Elsevier; 1999. pp. 97–153. [Google Scholar]
- 11.Croteau R, Satterwhite DM, Cane DE, Chang CC. Biosynthesis of monoterpenes: Enantioselectivity in the enzymatic cyclization of (+)- and (−)-linalyl pyrophosphate to (+)- and (−)-bornyl pyrophosphate. J Biol Chem. 1986;261:13438–13445. [PubMed] [Google Scholar]
- 12.Croteau R, Satterwhite DM, Cane DE, Chang CC. Enantioselectivity in the enzymatic cyclization of (+)- and (−)-linalyl pyrophosphate to (+)- and (−)-pinene and (+)- and (−)-camphene. J Biol Chem. 1988;263:10063–10071. [PubMed] [Google Scholar]
- 13.Wise ML, Savage TJ, Katahira E, Croteau R. Monoterpene synthases from common sage (Salvia officinalis) cDNA isolation, characterization, and functional expression of (+)-sabinene synthase, 1,8-cineole synthase, and (+)-bornyl diphosphate synthase. J Biol Chem. 1998;273:14891–14899. doi: 10.1074/jbc.273.24.14891. [DOI] [PubMed] [Google Scholar]
- 14.Koksal M, Chou WK, Cane DE, Christianson DW. Structure of 2-methylisoborneol synthase from Streptomyces coelicolor and implications for the cyclization of a noncanonical C-methylated monoterpenoid substrate. Biochemistry. 2012;51:3011–3020. doi: 10.1021/bi201827a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koksal M, Chou WK, Cane DE, Christianson DW. Unexpected reactivity of 2-fluorolinalyl diphosphate in the active site of crystalline 2-methylisoborneol synthase. Biochemistry. 2013;52:5247–5255. doi: 10.1021/bi400797c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Whittington DA, et al. Bornyl diphosphate synthase: structure and strategy for carbocation manipulation by a terpenoid cyclase. Proc Natl Acad Sci U S A. 2002;99:15375–15380. doi: 10.1073/pnas.232591099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hyatt DC, et al. Structure of limonene synthase, a simple model for terpenoid cyclase catalysis. Proc Natl Acad Sci U S A. 2007;104:5360–5365. doi: 10.1073/pnas.0700915104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.George-Nascimento C, Pont-Lezicka R, Cori O. Nonenzymic formation of nerolidol from farnesyl pyrophosphate in the presence of bivalent cations. Biochem Biophys Res Commun. 1971;45:119–124. doi: 10.1016/0006-291x(71)90058-1. [DOI] [PubMed] [Google Scholar]
- 19.Brock NL, Ravella SR, Schulz S, Dickschat JS. A detailed view of 2-methylisoborneol biosynthesis. Angew Chem Int Ed Engl. 2013;52:2100–2104. doi: 10.1002/anie.201209173. [DOI] [PubMed] [Google Scholar]
- 20.Citron CA, Barra L, Wink J, Dickschat JS. Volatiles from nineteen recently genome sequenced actinomycetes. Org Biomol Chem. 2015;13:2673–2683. doi: 10.1039/c4ob02609h. [DOI] [PubMed] [Google Scholar]
- 21.Croteau R, Satterwhite DM. Biosynthesis of monoterpenes. Stereochemical implications of acyclic and monocyclic olefin formation by (+)- and (−)-pinene cyclases from sage. J Biol Chem. 1989;264:15309–15315. [PubMed] [Google Scholar]
- 22.Bradford M. A rapid and sensitive method for the quantitation of microgram quantitites of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 23.Laemmli UK. Cleavage of structural proteins during the assembly of the head of Bacteriophage T4. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 24.Davisson VJ, et al. Phosphorylation of isoprenoid alcohols. J Org Chem. 1986;51:4768–4779. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.