Abstract
The killer phenomenon is defined as the ability of some yeast to secrete toxins that are lethal to other sensitive yeasts and filamentous fungi. Since the discovery of strains of Saccharomyces cerevisiae capable of secreting killer toxins, much information has been gained regarding killer toxins and this fact has substantially contributed knowledge on fundamental aspects of cell biology and yeast genetics. The killer phenomenon has been studied in Pichia membranifaciens for several years, during which two toxins have been described. PMKT and PMKT2 are proteins of low molecular mass that bind to primary receptors located in the cell wall structure of sensitive yeast cells, linear (1→6)-β-d-glucans and mannoproteins for PMKT and PMKT2, respectively. Cwp2p also acts as a secondary receptor for PMKT. Killing of sensitive cells by PMKT is characterized by ionic movements across plasma membrane and an acidification of the intracellular pH triggering an activation of the High Osmolarity Glycerol (HOG) pathway. On the contrary, our investigations showed a mechanism of killing in which cells are arrested at an early S-phase by high concentrations of PMKT2. However, we concluded that induced mortality at low PMKT2 doses and also PMKT is indeed of an apoptotic nature. Killer yeasts and their toxins have found potential applications in several fields: in food and beverage production, as biocontrol agents, in yeast bio-typing, and as novel antimycotic agents. Accordingly, several applications have been found for P. membranifaciens killer toxins, ranging from pre- and post-harvest biocontrol of plant pathogens to applications during wine fermentation and ageing (inhibition of Botrytis cinerea, Brettanomyces bruxellensis, etc.).
Keywords: Pichia membranifaciens, PMKT, PMKT2, killer toxin, biochemical characteristics, mechanism of action, biocontrol, applications
1. Introduction
Killer yeast strains have the characteristic of secreting toxins of proteinaceous nature that are lethal to sensitive yeast cells and filamentous fungi. The killer phenomenon in yeasts was first discovered in Saccharomyces cerevisiae [1] but soon shown to be present in many other genera of yeast [2,3,4]. Until now, the killer phenomenon has been discovered in almost one hundred species of more than twenty genera [5]. Over 11 different killer toxins have been described, and they are produced by representatives of such genera as Hanseniaspora, Pichia, Saccharomyces, Torulaspora, Ustilago, Williopsis, etc. (Table 1) [6,7,8,9,10].
Table 1.
Characteristics of killer toxins from genus other than Pichia.
| Killer Yeast | Strain (Killer Toxin) | Molecular Mass (kDa) | Glycoprotein Nature | Genetic Basis | Primary Receptors | Mode of Action | References |
|---|---|---|---|---|---|---|---|
| Candida nodaensis | PYCC 3198 (CnKT) | - | No | Chromosome | - | - | [45] |
| Debaryomyces robertsiae | CBS6693 (-) | >100.0 | dsDNA (pWR1A pWR1B) | Chitin | - | [46] | |
| Hanseniaspora uvarum | 470 (-) | 18.0 | No | dsRNA | (1→6)-β-d-glucans | - | [47] |
| Kluyveromyces lactis | IFO1267 (Zymocin) | α (97.0); β (31.0); γ (28.0) |
Yes (α), No (β, γ) | dsDNA (pGKL1) | Chitin <β2> | rRNA fragmentation | [48] |
| Kluyveromyces phaffii | DBVPG 6076 (KpKt) | 33.0 | Yes | Chromosome | (1→6)-β-d-glucans; (1→3)-β-d-glucans |
Glucanase activity | [49] |
| Saccharomyces cerevisiae | KL88 (K1) |
α (9,5); β (9.0) |
No | M1-dsRNA | (1→6)-β-d-glucans | Ionic channels formation in plasma membrane | [50] |
| - (K2) |
α (21.0); β (5.0) |
Yes | M2-dsRNA | (1→6)-β-d-glucans | Increase in plasma membrane permeability | [51,52] | |
| CBS8112 (K28) |
α (10,5); β (11.0) |
Yes | M28-dsRNA | Mannoproteins | Toxin entry by endocytosis and cell cycle arresting in S phase | [53] | |
| - (Klus) |
α (?), β (?) | Yes | Mlus-dsRNA | - | - | [54] | |
| 111 (KHR) |
20.0 | - | Chromosome | - | - | [55] | |
| - (KHS) |
75.0 | - | - | - | Increase of membrane permeability to ions | [3] | |
| Schwanniomyces occidentalis | ATCC 44252 (-) |
α (7.4); β (4.9) |
No | Chromosome | Mannoproteins | Plasma membrane damage | [36] |
| Ustilago maydis | P1 (KP1) |
(19.0) α, β | - | dsRNA (P1) | - | Increase of membrane permeability to ions | [56] |
| P2 (KP2) |
11.1 | - | - | - | Increase of membrane permeability to ions | [3] | |
| P4 (KP4) |
13.6 | - | dsRNA (P4) | - | Inhibition of Ca+ channels | [57] | |
| P6 (KP6) |
α (8.6); β (9.1) |
- | dsRNA (P6) | - | K+ depletion | [58] | |
| Williopsis saturnus var. mrakii | IFO 0895 (HM-1) |
10.7 | No | Chromosome | (1→6)-β-d-glucans; (1→3)-β-d-glucans |
Inhibition of b-1,3-glucan synthase | [59,60] |
| Williopsis saturnus var. saturnus | IFO 0117 (HYI) |
9.5 | No | Chromosome | - | (1→3)-b-D-glucans sintase inhibition | [61,62] |
| Zygosaccharomyces bailii | 412 (KT412) |
10.0 | No | dsRNA | Mannoproteins | Plasma membrane damage | [63] |
| Torulaspora delbrueckii | Kbarr (Kbarr-1) |
α (?), β (?), γ (?) |
Yes | dsRNA | - | - | [64] |
| NPCC 1033 (TdKT) | >30.0 | - | - | (1→3)-β-d-glucans | Cell wall disruption and apoptotic death processes | [9] |
Killer yeasts are widespread in nature where they can be found in percentages that several fold exceed those found in laboratory strains, indicating the existence of a competitive advantage. Microbes have evolved to compete over the neighborhoods of their same niche. One of the mechanisms to gain advantage that have been described is shown by some killer yeast through the presence of dsRNA viruses. These mycoviruses encode killer toxins that provide benefits to the producing cells by killing sensitive yeast cells. Killer toxin production has been related in S. cerevisiae with the presence of two dsRNA viruses: L-A, the helper virus, and the M killer virus that encodes a killer toxin that determines its phenotype (K1, K2, K28 or Klus). Although killer toxins could imply an important competitive advantage, it has also been demonstrated that there is a fitness cost for carrying mycoviruses [11].
In addition to the cytoplasmic dsRNA viruses mentioned above, a variety of yeast species (Kluyveromyces lactis, P. acaciae, P. inositovora, D. robertsiae, Trichosporon pullulans, etc.) produce linear dsDNA plasmids, probably not encapsulated, that encoded killer toxins. Furthermore, chromosomally encoded killer toxins (Williopsis saturnus, Pichia anomala, Pichia kluyveri, Pichia membranifaciens, etc.) are also found widespread in nature. Most of these toxins exceed the focus of the presented review but their characteristics and potential applications can be reviewed in the tables showed (Table 1, Table 2 and Table 3).
Table 2.
Killer toxins from genus Pichia.
| Killer Yeast | Strain/s (Killer Toxin) | Molecular Mass (kDa) | Glycoprotein Nature | Genetic Basis | Primary Receptors | Mode of Action | Reference |
|---|---|---|---|---|---|---|---|
| P. acaciae | NRRLY-18665 (PaT) | α (110), β (39), γ (38) | Yes | dsDNA (pPac1-2) | Chitin | Cell cycle arrest in G1, chitinase activity | [30] |
| P. anomala | NCYC434 (Panomycocin) |
49.0 | Yes | ? | (1→3)-β-d-glucans | (1→3)-β-d-glucan hydrolysis | [65] |
| ATCC 96603/K36/UP25F (PaKT) | 85.0 | - | Chromosome | β-Glucan | - | [66] | |
| DBVPG 3003 (Pikt) | 8.0 | - | Chromosome | (1→6)-β-d-glucans | - | [67] | |
| YF07b (-) | 47.0 | - | Chromosome | - | (1→3)-β-d-glucanase activity | [68] | |
| VKM-Y (WAKTa/b) | - | - | - | - | - | [69] | |
| P. farinosa | KK1 (SMKT) |
α (6.6), β (7.9) | Yes | Chromosome | - | Membrane permeabilization | [70] |
| P. inositovora | NRRL Y-18709 (-) | 3 subunits > 100.0 | - | dsDNA (pPin1-3) | Chitin | rRNA fragmentation | [71] |
| P. kluyveri | 1002 (-) | 19.0 | Yes | Chromosome | - | Membrane permeabilization | [37] |
| DBVPG 5826 (Pkkp) | 54.0 | - | - | Cell wall receptor | - | [8] | |
| P. kudriavzevii | RY55 (-) | 39.8 | - | - | - | - | [72] |
| P. membranifaciens | CYC 1106 (PMKT) | 18.0 | No | Chromosome | (1→6)-β-d-glucans | Membrane permeabilization/apoptosis | [19,41,73,74,75,76] |
| CYC 1086 (PMKT2) | 30.0 | - | - | Mannoproteins | Cell cycle arrest/apoptosis | [20,38] | |
| Pichia ohmeri | 158 (-) | <3.0 | - | Chromosome | - | Loss of cellular integrity | [33] |
Table 3.
Potential applications of killer toxins.
| Applications | Organism/s | Toxin | General Description | References |
|---|---|---|---|---|
| Biological models | Saccharomyces cerevisiae | K28 | Model for the study of proteins, lipids, and mechanisms required on endocytosis and retrograde trafficking in A/B toxins (as Ricin, Shiga, and Cholera toxins) | [166] |
| Biotyping | Killer strains panel | - | Fingerprinting and clustering yeast strains (genera Debaryomyces, Kluyveromyces, Saccharomyces, and Zygosaccharomyces) by use of killer toxin sensitive patterns | [167] |
| Saccharomyces cerevisiae | - | Typing S. cerevisiae strain by combined use of RAPD and killer toxin sensitivity patterns | [168] | |
| Cryptococcus laurentii (CBS 139) | - | Biotyping varieties of Cryptococcus neomorfans (var. neoformans and var. gattii) by differential killer toxin sensitivity patterns | [169] | |
| Killer strains panel | - | Combined use of mtDNA-RFLP patterns and killer toxin biotype to study wine S. cerevisiae strain diversity in different viticulture regions | [170] | |
| Killer strains panel | - | Fingerprinting of Saccharomyces wine yeast by differential killer sensitivity | [171] | |
| Pichia mrakii | K9 | Use Pichia genera killer toxins to differentiate members belonging to the Nocardia asteroides complex (N. asteroides, N. farcinica, and N. nova) | [172] | |
| Pichia lynferdii | K76 | |||
| Killer strains panel | - | Use of toxins produced by a selected panel of killer yeast to discriminate strains belonging to genus Candida by their killer sensitive patterns | [173] | |
| Antimycotics for the treatment of human infections | Pichia anomala | PaKT | Using killer toxin-like anti-idiotypic antibodies of P. anomala killer toxin (PaKT-antilds) to treat treating human Pneumocystis carinii pneumonia. | [174] |
| Zygosaccharomyces bailii | Zygocin | Antifungal effect of Zygocin, a killer toxin produced by Z. bailii, on a broad-spectrum of sensitive fungal cells (Candida, Pichia, Hanseniaspora, Fusarium genera) | [104] | |
| Kodamaea ohmeri (HB55 and HB88) | - | Inhibition of C. neoformans (vars. neoformans, grubii and gattii), both clinical and environmental isolates, by two Brazilian yeasts selected for being able to inhibit human pathogenic fungi | [175] | |
| Filobasidium capsuligenum | Fc-1 | Use of a novel toxin with activity against the opportunistic fungal pathogen C. neoformans, as a therapeutic agent for the treatment of cryptococcosis | [176] | |
| Pichia anomala | K10 MAbs | Apply of antiidiotypic monoclonal antibodies (KT MAbs) from P. anomala against Aspergillus fumigatus. | [177] | |
| Williopsis mrakii var. mrakii (IFO 0895) | HM-1 | Activity of the killer toxin HM-1 from W. markii var. mrakii (formerly known as Hansenula mrakii) against Candida genus yeasts | [147] | |
| W. saturnus var. mrakii (MUCL 41968) | WmKT | Application of killer toxin (WmKT) secreted by W. saturnus var. mrakii, which is active against a wide range of pathogens. | [178] | |
| Antimycotics for treatment of animal infections | Kluyveromyces siamensis (HN12-1) | - | Use of a killer toxin of K. siamensis against Metschnikowia bicuspidata WCY, agent for the milky disease in crab | [179] |
| Pichia anomala (YF07b) | - | Apply of a killer toxin produced by the marine yeast P. anomala, against pathogenic yeast cells in crab, artemia, and shrimp | [68] | |
| Prevention of contaminants in fermentation industries | Saccharomyces cerevisiae | - | Effect of killer strains of S. cerevisiae on the growth of sensitive strains during wine fermentation, to improve the selected wine strain implantation. Sparkling wines quality improvement | [180,181] |
| Kluyveromyces phaffii (DBVPG 6076) | - | Use of K. phaffii killer toxin to control apiculate spoilage wine yeast (Hanseniaspora uvarum) | [182] | |
| Torulaspora delbrueckii (NPCC 1033) | TdKT | Application of a novel killer toxin (TdKT) from T. delbrueckii as a biocontrol tool in winemaking against different wine spoilage yeasts | [9] | |
| Ustilago maydis (CYC 1410) | - | Use of U. maydis toxins for the biocontrol of Brettanomyces bruxellensis for the reduction of aroma defects caused by this spoilage yeast | [10] | |
| Candida nodaensis (PYCC 3198) | CnKT | Application of CnKT in the preservation of salt-fermented foods, because of its high stability to salinity | [45] | |
| D. hansenii, K. marxianus, P. anomala, P. guilliermondii, S. cerevisiae | - | Control of olive table fermentation by the selection of killer yeast and their toxins which are able to suppress indigenous spoilage yeast growth | [86] | |
| Williopsis saturnus var. saturnus | - | Use of W. saturnus var. saturnus to inhibit spoilage yeast, as S. cerevisiae and K. marxianus, as a biopreservative agent on cheese making (in laboratory conditions) | [183] | |
| Wickerhamomyces anomalus (D2) | PiKT | Application of KwKT killer toxin produced by K. wickerhamii and PiKT produced by W. anomalus killer against B. bruxellensis during wine fermentation. | [184] | |
| Kluyveromyces wickerhamii (D15) | KwKT | |||
| Candida pyralidae (IWBT Y1140) | CpKT1 | Control of B. bruxellensis by C. pyralidae killer toxin in wine fermentation. | [185] | |
| Pichia membranifaciens | PMKT | Use of PMKT to control Zygosaccharomyces spp. contamination in winemaking. | [186] | |
| Wine quality improvement | S. cerevisiae (AWRI 796) | K2 | Interaction of S. cerevisiae killer strain (AWRI 796) and sensitive strain (Tyr 303) to accelerate the autolysis in the sparkling wine production to improve the final wine quality | [187] |
| Wickerhamomyces anomalus (CBS 1982, CBS5759) | - | Application of W. anomalus and S. cerevisiae in a mixed culture to positively influence chemical composition (higher amounts of polyphenol compounds and lower amounts of malic acid) and sensory features of produced apple wines | [188] | |
| Biological control | Kluyveromyces lactis (PCK27) | - | Use of killer strain K. lactis PCK27, to inhibit P. anomala, aerobic spoilage yeast in silage making | [144] |
| Williopsis mrakii | Mycocin HMK | Utilization of killer toxin HMK of W. mrakii, expressed in Aspergillus niger, to control both silage spoilage and yoghurt spoilage caused by yeasts | [90] |
The reasons why killer yeasts are immune to their own toxins remain to be elucidated for most killer systems. However, in particular cases it has been solved. For example, K28 immunity occurs via the ubiquitination of re-internalized mature toxin and unprocessed precursor moieties complexes that are degraded by the proteasome [12]. Furthermore, Pichia acaciae, Kluyveromyces lactis and Debaryomyces robertsiae cytoplasmic virus like elements encode toxic anticodon nucleases along with specific proteins that confer toxin immunity [13,14]. The potential use of killer yeasts and their toxins has been intended for various fields of application such as the alcohol fermentation industries (brewery, winery, and distillery), fermented vegetables, biological control of post-harvest diseases, yeast bio-typing, as antimycotics in the medical field and they have been used as model systems to understand eukaryotic polypeptide processing and expression of eukaryotic viruses [10,11,12,13,14,15,16,17,18,19,20,21].
Currently, around 1500 different species of yeasts are known [22] and one of the largest yeast genera is the genus Pichia within the context of number of species. The number of species included in Pichia genus has changed considerably since Hansen (1904) first described the genus Pichia [22,23,24,25]. Pichia species are widely distributed and are present in natural habitats and also as spoilage yeasts in several foods and beverages [26,27] and even, human pathogens [28,29].
Killer phenomenon has been discovered and studied in several species of Pichia that show killer activity against yeasts or fungi of clinical concern (Table 2). Toxins of the genus Pichia are either associated with cytoplasmic genetic elements, such as dsDNA virus-like elements or chromosomally encoded [30,31,32]. Gene products of both extranuclear and chromosomally encoded toxins, are diverse in their molecular mass. There has been described monomeric toxins of Pichia species from less than 3 kDa (P. ohmeri killer toxin) to trimeric toxins of more than 187 KDa (P. acaciae PaT) [29,33,34], although, similar to what occurs globally for killer toxins, the molecular mass is not generally high (20–50 KDa) in Pichia toxins.
The stability of most killer toxins has been shown to be restricted to acidic pH values and low temperatures [35,36,37,38]. In this regard, toxins of the Pichia genus are not different from the rest of killer toxins; to date, no Pichia toxins with stability to high temperature or wide pH ranges have been described.
Known killer toxins display diverse modes of actions, such as membrane-damaging agents, glucanase activity, inhibitors of β-1,3-glucansynthase, cell cycle arrest, inhibition of calcium uptake, or tRNase activity; and toxins of the Pichia genus exhibit most of them [39,40,41,42].
In initial works, it was found that the majority of yeasts isolated from spontaneously fermenting olive brines possessed the killer character and that strains of Pichia membranifaciens, the dominant species, were particularly active in the presence of salt, eventually influencing the development of some spontaneous fermentations [43,44]. The reasons for such an increased killer activity was elucidated in the study of the mechanism of action of the first P. membranifaciens toxin analyzed, called PMKT (produced by the strain CYC 1106). Additional studies indicated that P. membranifaciens CYC 1086, presented a different killer behavior, secreting PMKT2. This review is about the killer system of P. membranifaciens; and in particular, it focuses in the findings described during the last 20 years about PMKT and PMKT2, until now, the only toxins described for this species. Significant progress in the determination of the nature of the killer toxins, their toxic mechanism and approaches for the potential use of such toxins has only been done very recently. In this review, are concentrated the studies carried out for the characterization on PMKT and PMKT2, their primary and secondary receptors in sensitive strains, toxin mechanisms of action, and cellular responses of sensitive cells to killer toxins. Finally, the potential applications and future perspectives of killer toxins, with special focus in P. membranifaciens killer toxins, are described and discussed.
2. Isolation of Pichia membranifaciens Killer Strains
The frequency and distribution of yeasts in fermentations of several olive brines is well referenced [77,78], but investigations have habitually been related with negative effects [79,80,81]. Scarce relevance has been given to the potential positive roles that most significant yeast species can develop in olive brines, including interactions among different yeast species and with other microorganisms, particularly the predominant bacterial populations responsible for the lactic acid fermentation of olives. The majority of yeasts isolated from olive brines bore the killer character, and the strains of P. membranifaciens, the dominant species, were found to be particularly active [43,82]. In this anthropic environment, halotolerant yeast populations develop [43] at pH values around 4.5 and temperatures that have been observed to be compatible with the killer character shown by most yeast isolates of such environments. The presence of NaCl, in concentrations similar to that found in olive brines, increase the killer character and augment the killer spectra of yeasts isolated from this environment, assessing the relevance of the killer phenomenon and indicating that the killer character could be a competitive advantage in this environment [44]. Furthermore, the presence of killer yeast in olive brines is found to be very high (39%), indicating that, in these particular fermentative conditions, the killer character could be particularly relevant for competition [83].
The isolated P. membranifaciens strains were screened against a collection of several sensitive strains, for the presence of different P. membranifaciens killer phenotypes, determining the presence of, at least, two clearly different ones [20] and confirming the fact that different toxins can be produced by strains of the same yeast species. Two strains of P. membranifaciens, CYC 1106 and CYC 1086, were selected because of their different killer spectrum of resistance/sensitivity and their higher activity against the panel of potentially sensitive yeasts used. In conclusion, the strains of P. membranifaciens of our laboratory produced two different toxins that were named PMKT (secreted by P. membranifaciens CYC 1106) and PMKT2 (secreted by P. membranifaciens CYC 1086). These toxins are the main objective of the investigations recovered in the present work.
3. Production of PMKT and PMKT2
Having established that the secreted toxins increased in culture liquid as growth progressed and then stabilized when the yeast population reached the stationary phase (as considered for primary metabolites), the toxins of P. membranifaciens were purified from samples taken at the early stationary phase, where the toxins concentration was observed to be higher [84]. The production of killer toxins in complex media (which contained peptone, malt extract and yeast extract) and minimal media (containing growth factors, mineral salts and trace elements) has been compared. Although production of PMKT in complex media is higher than in minimal media, the specific activity is higher (seven-fold higher) in the latter case, according to similar observations that were reported previously [85,86] and probably due to the greater stability of the toxin in a media with high contain in peptides and proteins. However, for the purpose of PMKT2 production and purification, P. membranifaciens CYC 1086 is developed in complex medium because PMKT2 is purified easier [20,39].
In addition, with the aim of increasing toxin production, stabilizing agents such as polyols, detergents and protease inhibitors were added. Results indicated that the non-ionic detergents, in particular Brij-58, increased the killer activity present in the extracellular media after growth. Accordingly, the synthetic medium with Brij-58, was used for purification of PMKT [19]. Probably, non-ionic detergents stabilize the killer toxin produced or inhibit the adsorption by (1→6)-β-d-glucans. (1→6)-β-d-glucans in yeast cell walls are known to function as primary receptors of some killer toxins in sensitive cells [47,49,50,51,52,59,75,87]. Killer cells have these (1→6)-β-d-glucans, and it is easy to understand that some amount of the secreted toxins can be attached by (1→6)-β-d-glucans and are not released to the culture medium. The same can be applied to KRE1 mutants, which show a 50% reduction in cell wall (1→6)-β-d-glucans that are superkillers due to an increased secretion of the K1 toxin of S. cerevisiae [88].
Production of toxins by killer yeasts with an increased degree of activity also depends on the culture conditions of pH and temperature. Briefly, the maximum killer toxin production for both toxins was achieved at acidic pH values of around 4.5 at 20 °C, accordingly to the activity and stability properties described later in this review for both toxins.
4. Homogeneity Purification and Biochemical Characterization of PMKT and PMKT2
The homogeneity purification of the killer toxins of P. membranifaciens, as well as other proteins and enzymes, depends, not only on the characteristics of the killer toxin, but also on the complexity of the protein extract from where they have to be purified. Furthermore, both the well-reported lability of secreted toxins from killer yeasts and their aggregation tendency are usual problems found during killer toxin purification, especially in the case of PMKT [19,20]. Accordingly, in spite of the similar biochemical characteristics of PMKT and PMKT2, the purification of both toxins is achieved using different approaches. PMKT is purified from P. membranifaciens CYC 1106 in four steps and being necessary the use of an affinity column of (1→6)-β-d-glucan-epoxy-Sepharose 6B [19] whereas PMKT2 is easily purified to homogeneity from P. membranifaciens CYC 1086 [20]. Biochemical characteristics of PMKT and PMKT2 have been determined.
Both toxins are observed to be monomeric non-glycosylated proteins of 18 kDa (PMKT) and 30 kDa (PMKT2) with isoelectric points of around 3.9 for PMKT and 3.7 for PMKT2. In addition, both toxins are found to be similar in their behavior at different pH and temperature values. Protein denaturation involves changes in protein structure (generally an unfolding) with the loss of activity. The stability of most killer toxins has been shown to be strongly dependent on acidic pH values (pH < 5.5–6.0) and low temperatures (<20–25 °C), and mechanical shaking is also destructive [35,36,37,38]. PMKT is stable only within a pH interval of 3.0 and 4.8 and it is rapidly inactivated at temperatures above 20 °C, in accordance with the loss of PMKT production during yeast growth in pH and temperatures above these values [19,20]. PMKT2 had physical chemical properties similar to PMKT: it was active at acidic pH values (2.5–4.8) and temperatures below 20 °C, although, it was less labile during short periods of time at higher temperatures and neutral pH values than PMKT. However, differences in these properties between killer toxins indicate that they are biochemically distinct [38,89]. In this regard, HM-1 killer toxin, produced by W. saturnus var. mrakii IFO0895 (previously named as Hansenula mrakii) is special in its wide range of stability to pH and temperature, as it remains active after exposure to 100 °C for 10 min and pH values comprised between 2 and 11 [59,90]. Probably, the observed internal disulfide bridges give to this toxin its higher stability [91,92]. Tilletiopsis albenscens killer toxin seems to be stable in a broad pH range of 3.5–8.0 [93].
5. Killing Mechanism of Action
5.1. Primary Receptors in Sensitive Yeast Cells
It is widely accepted that cell surface polysaccharides act as receptors for bacteria, viruses, and toxins [52,94]. Yeast killer toxins have different mechanisms of action but most toxins are able to kill sensitive yeasts in a two-step process that is receptor-mediated. There are two kinds of killer toxin receptors: the primary, usually located on the cell wall, and the secondary receptor, that exist on the plasma membrane of the sensitive cells. The interaction with the first receptor within the cell wall of a sensitive target cell involves a fast and energy-independent binding [20,49,75,76,95,96,97,98,99]. Cell walls are mainly composed by carbohydrates, some of them free and some linked to proteins. Overall, the main components of the yeast cell wall are (1→3)-β-d-glucans, (1→6)-β-d-glucans, mannoproteins and chitin, which is concentrated in the bud-scar region [100,101].
A diversity of cell wall receptors for killer toxins exist, evidencing that all the main cell wall components of yeasts could function as the primary receptor for a killer toxin. For example, (1→6)-β-d-glucans act as cell wall receptors for K1 and K2 killer toxins of S. cerevisiae [44,52], for Hanseniaspora uvarum killer toxin [102] and for Pichia anomala DBVPG3003. (1→3)-β-d-glucans are primary receptors for Pichia anomala NCYC434 [65], mannoproteins are receptors for KT28 of S. cerevisiae and also Zygosaccharomyces bailii killer toxin [63,96,103,104], and, finally, chitin is the cell receptor for Kluyveromyces lactis killer toxin [97]. In agreement with that, reported mutations in a gene/genes involved in the synthesis or architecture of the cell wall are observed to be resistant to killer toxins. Kre-Killer Resistant mutants have been obtained for K1 [105] and, in the same way, mnn-Mannoprotein mutants for K28 [106]. Mutational analyses revealed that both subunits of K1, α and β, are involved in glucan binding [107,108]. It has been indicated that the primary receptors in the cell wall facilitate the primary contact of the killer toxins with the sensitive yeasts or even mediates close contacts between the toxin and secondary receptors on the plasma membrane [96].
Using both in vivo and in vitro approaches, in previous works [75], it was confirmed the role of (1→6)-β-d-glucans as primary targets for PMKT and the utility of such interaction as an effective means for PMKT purification [19]. Finally, it was shown that the adsorption of PMKT to purified (1→6)-β-d-glucans occur within the first 2 min of contact, indicating, as previously hypothesized, that this process is an energy-independent binding and toxin-specific, since polysaccharides with the following linkages: (1→3)-β-, (1→4)-β-, (1→4)-α-, or (1→6)-α- were unable to bind PMKT.
Yeast mannans may be covalently linked to cell wall proteins, so that the term “mannoproteins” adequately reflects their status in the yeast cell wall. Mannoproteins have been described as antigenic determinants, also functioning as receptors or even as support for other receptors of different nature [109,110]. Conversely to what was found for PMKT, in a complementary study for the determination of the binding capacity of different yeast cell-wall fractions, cell wall glucan fractions do not bind PMKT2, whereas mannoproteins and mannan purified from sensitive yeasts were able to, showing the existence of a cell wall receptor of different nature for PMKT2 [20].
5.2. Secondary Receptors for P. membranifaciens Killer Toxins
It has been described that, for most toxins, a second step that implies toxin transfer to the plasma membrane and subsequent bind to the secondary receptor mediates killing. The number of studies conducted for the determination of the presence and description of secondary receptors are scarce. However, the evidences accumulated during years of investigation about the effects of killer toxins on sensitive yeast cells corroborated that, in addition to primary receptors, specific and essential secondary receptors should be involved in the killing mechanism. In the case of K1 toxin, the most studied killer toxin, the resistance of whole cells of kre mutants to K1 and the sensitivity of the spheroplasts of kre mutants and wild-type cells, indicate the existence of these secondary receptors [98,111]. K1 toxin, after its binding to cell wall (1→6)-β-d-glucans [85], interacts with Kre1p, an O-glycosylated GPI-anchored cell wall protein. Mutational analyses reveal that solely α subunit interacts with Kre1p. ΔKRE1 mutants show complete resistance to K1, indicating that Kre1p acts as a receptor in the cytoplasmic membrane [21,98,99,103,112].
In addition, a few receptors for other toxins have been described. The H/KDEL receptor Erd2p mediates K28 toxin binding and uptake in yeast spheroplasts, a viral A/B toxin bearing an HDEL motif at the β-subunit. In vivo toxicity to K28 depends on the existence of Erd2p [113]. In the case of Kluyveromyces lactis zymocin, Ipt1p seems to be involved as a membrane receptor. KTI6, which is allelic to IPT1 (coding for mannosyl-diinositolphospho-ceramide [M(IP)2C] synthase), join in a step that follows recognition of the primary receptor (chitin) of the toxin but precedes the role of the multi-subunit Elongator, involved in histone acetylation and transcription, the most probable toxin target [114].
Speaking specifically about Pichia toxins, only the membrane receptor for the PMKT toxin has been described. Some unidentified receptors have been hypothesized for other Pichia toxins, in an attempt to explain the binding capability and sensitivity of toxin-treated spheroplasts [115,116]. PMKT resembles K1 mechanism of action, using a similar strategy to reach the cytoplasmic membrane, involving a two-step interaction with the primary receptors (1→6)-β-d-glucans) and cell wall-localized secondary receptors which are also plasma membrane-associated. PMKT utilizes as the secondary membrane receptor a GPI-anchored protein of the cell wall [76]. This protein, called Cwp2p, can be purified using PMKT-affinity chromatography, indicating the existence of an in vitro interaction between both proteins. It was found, in a collection of 288 viable deletion mutants for proteins related to the cell periphery, as appeared in the CYGD (Comprehensive Yeast Genome Database), that 15 ORFs increased protoplast resistance to the toxin when deleted. This agrees with the hypothesis that Cwp2p is a secondary receptor for PMKT. Furthermore, complete yeast cells and cwp2Δ protoplasts are resistant and also unable to bind PMKT, demonstrating once again that Cwp2p is necessary for PMKT toxicity. In addition, it should be indicated that, in its definitive state, Cwp2p is bind to (1→6)-β-d-glucans and this is the most probable way by which PMKT interact closely with Cwp2p, the plasma membrane receptor located in the cell wall. It has been also demonstrated, using a collection of deletion mutants related to GPI (Glycosyl Phosphatidyl Inositol) anchor biosynthesis, that Cwp2p is present in both cellular locations the plasma membrane and cell wall, as usually occur for most GPI-proteins of the yeast cell wall [76,98]. The fact that, when exposed to PMKT, GPI anchoring-defective mutants are much more resistant, and so are protoplasts from wild-type strains previously incubated with phosphatidylinositol-specific phospholipase C (to remove GPI-anchored proteins), indicate that the precursor of Cwp2p, which is GPI-anchored, is involved in PMKT mechanism of action. Furthermore, carboxyfluorescein-entrapped liposomes that include purified Cwp2p are observed to release carboxyfluorescein in the presence of PMKT while liposomes without Cwp2p do not. This is consistent with the idea that the presence of another effector is not necessary in the proposed model. Cwp2p, could facilitate PMKT insertion into the plasma membrane bilayer, as has been proposed for other toxin receptors [117,118] and in accordance to the observed effects in PMKT-intoxicated cells [41]. The N-terminal amino acidic sequence of Cwp2p presents a PIR repeat and, due to the presence of multiple serine and threonine residues, the rest of Cwp2p is O-glycosylated and thus, probably bears several phosphodiester groups. At acidic pH values (those pH values where there is killer activity), PMKT bears positive charges and so on could be strongly bound to the negative charges present on Cwp2p [76]. Overall, data suggested that Cwp2p plays a central role in the activity of PMKT, acting as a bridge between the primary receptors and the plasma membrane and promoting cellular toxicity. Finally, after reaching the cytoplasmic membrane, PMKT exerts its toxin action by cation channel formation and disruption of transmembrane gradients [41]. Until now, it has not been possible to determine the existence of a secondary receptor for PMKT2.
5.3. Cellular Responses to Killer Toxins
5.3.1. PMKT
Many targets to the killer toxin action in sensitive cells have been identified and, in consequence, some molecular mechanisms of killing action have been also determined. The first study for determining some aspects of the killing action of PMKT was intended to assess the importance of the addition of salt (which is common in the production of traditional fermented foods) in the expression of the killer phenotype [44]. In preliminary studies, we observed that sodium chloride in concentrations of up to 6% (wt/vol) eventually enhanced the toxicity and enlarged the killer spectra of a few of the yeasts tested. This observation raised the question of whether this higher activity was due to increased killer toxin production or increased sensitivity of the strains affected. In addition, it has been also shown that the killing action of some other killer yeasts isolated from high salinity environments are related on the presence of NaCl. For example, SMK toxin produced by Pichia farinosa KK1 shows its maximum killer activity in the presence of 2.0 M NaCl [70]. Furthermore, CnKT, produced by Candida nodaensis, presents a strong salt-dependent phenotype [45] and the same can also be observed for Mrakia frigida 2E00797 and Williopsis saturnus WC91-2 killer toxins [119,120]. However, so far, little has been studied concerning the reasons why, in these particular cases, the killing activity is dependent on NaCl.
PMKT finds its greatest similarity in K1, which share certain biochemical characteristics such as similar isoelectric point and molecular mass. In a similar way to the mode of action in which K1 acts from outside the cell after reaching the cytoplasmic membrane, PMKT exerts its lethal effect by ion channel formation and disruption of cytoplasmic membrane gradients [19,121,122,123]. PMKT binds to the cell wall (1→6)-β-d-glucans in the first 2–3 min after toxin addition, and then a lag phase of approximately one hour is necessary to observe reductions in cell viability, probably reflecting the time necessary for the development of the mechanism of action, gradual intoxication and death. The lag phase observed after cell wall binding and previously to cell death, is supposed to involve several sequential interactions and effects: interaction with secondary receptors, ion channel formation in the plasma membrane, alteration of transmembrane gradients and deenergization of cells and eventually, formation of large unspecific plasma membrane pores that permit the passage of other intracellular molecules [124]. During the first 30 min of incubation with PMKT, the pHi of toxin-treated sensitive cells and control cells is observed to be similar, thereafter, pHi is progressively acidified, reaching values of about 4.6–4.7 after 0.5–1 h incubation with PMKT. Then a leakage of K+ out of the cells starts (1–1.5 h after exposure to PMKT) and simultaneously, a flow of Na+ began. In the same way as K+, the intake of Na+ is directly related with killing [41]. The disruption of ionic equilibrium across the cytoplasmic membrane may could generate an augmented mortality of the cells in the presence of salt. The increased permeability of the cytoplasmic membrane to protons could consequently generate the influx of ions through the plasma membrane. Any compound accumulated in or outside the cell against its concentration gradient will equilibrate and a flow could occur. These findings are also in agreement with those studies that observed in yeast spheroplasts and artificial liposomes treated with K1 that the ion channels caused by K1 do not distinguish between Na+ and K+ [122,125].
In addition, PMKT causes in liposomes a conductance when added at low pH, showing a characteristic channel activity. Furthermore, all the common physiological ions are observed to flow through the pores caused by PMKT in the selectivity sequence: K+ > Na+ > Li+ > Ca2+ > Cl−. Finally, changes in plasma membrane integrity (determined as permeability to propidium iodide) are observed when cellular death was advanced in time, being probably a late consequence of PMKT treatment [41].
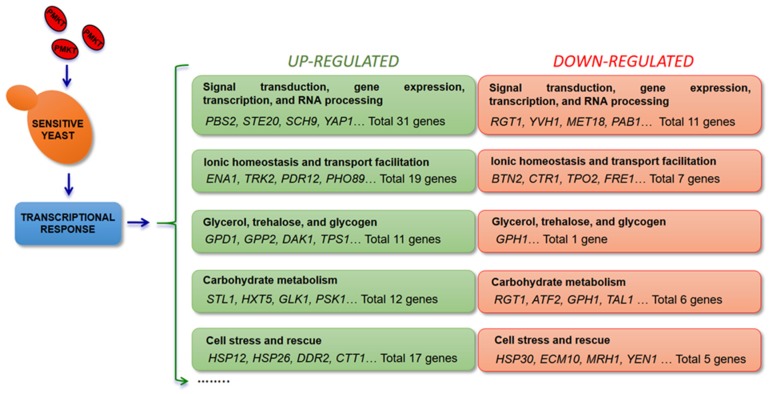
Yeasts response to harsh physical chemical factors (oxidative stress, heat, osmotic shock, nutrient availability, extreme pH, toxins, heavy metals, etc.) by activating a wide variety of defenses that include general or specific regulatory pathways, activating multiple MAPK cascades, which transform stimuli into appropriate physiological responses [126,127,128]. In order to confirm the physiological events described for PMKT toxicity [41], the transcriptional response of the sensitive cells was studied with a view to obtain information of the mechanisms of killing action. In addition, the results were confirmed by studying the response of deleted mutants in all those genes that were observed to be significantly up- or down-regulated when sensitive cells were treated with PMKT [73]. When the global gene expression response of the yeast S. cerevisiae to PMKT was explored, 146 genes showed an altered expression in response to PMKT.
PMKT-treated cells with a PMKT dosage of 1205 AU/mL (considered to be a high dose as stated below) and untreated cells were selected for mRNA isolation and determination of the transcriptional response. The most important functional group with 31 up-regulated genes is the group related to signal transduction, gene expression, transcription and RNA processing, followed by the group of genes related to ionic homeostasis, transport facilitation, carbohydrate metabolism, and cell stress and rescue. Both, the phosphatases and dehydrogenases implicated in the glycerol production pathway are also up-regulated in response to PMKT. In addition, many similarities are found when the group of up-regulated genes in response to the toxin [73] and those induced by osmotic stress [129] are compared. In fact, most PMKT-induced genes encode proteins involved in counteracting the physiological changes induced by an osmotic shock, thus indicating the existence of an osmotic-related stimulus inside the cells when treated with the toxin. Genes that were related to salt tolerance in response to PMKT were CTT1, HSP12, GPD1, GPP2, TRK2, PDR12, ENA1, SCH9, HAL9, YAP1, XBP1, and STL1 (Figure 1). Furthermore, ENA1, TRK2, PHO89, ZRT1 and PDR12 are a group of genes that surely are found to be up-regulated because of the leakage of ions and small metabolites induced by the toxin due to the generation of non-specific channels in a cellular response to compensate the leakage over a short period of time [41,73].
Figure 1.
Analysis of the transcriptional response of S. cerevisiae to PMKT. Genes, sorted into functional categories, induced after a PMKT exposure by more than 3-fold and repressed by more than two-fold.
In S. cerevisiae, it is known that the response to rapid changes in the osmolarity relies in the activation of the HOG (High Osmolarity Glycerol) pathway, which is manifested by the phosphorylation of Hog1p. Hog1p encodes for the MAPK that is activated by phosphorylation and then translocated to the nucleus to counteract with several environmental stimuli [130]. HOG1Δ mutants are hypersensitive to such environmental stresses, which cause Hog1p activation [130,131]. Several transcription factors have been found to be among the substrates of Hog1p [132], so the phosphorylation and the consequent activation of Hog1p also regulates gene expression profiles [133]. Hog1p phosphorylation has been studied in order to determine whether the observed transcriptional response in the presence of PMKT was connected with the HOG pathway or not [73]. Hog1p phosphorylation is observed rapidly in PMKT-intoxicated cells. Phosphorylation is present in the first ten minutes, and during the rest of the exposure to PMKT, indicating constant activation of the signaling pathway. Accordingly, HOG1Δ mutants are found to be hypersensitive to PMKT.
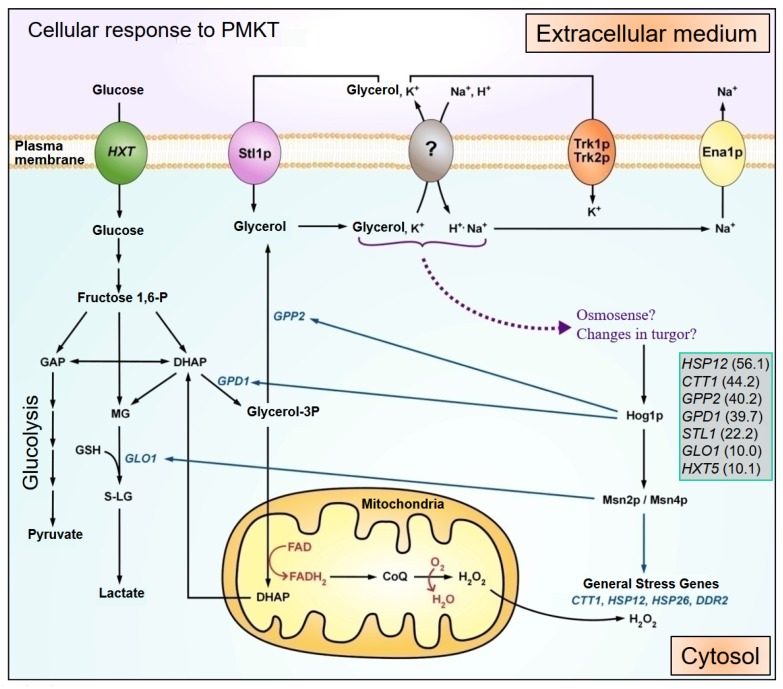
Glycerol is the main compatible osmolyte that accumulate rapidly as a consequence of activation of Hog1p and the transcriptional response is correlated with known responses to osmotic shocks. The mRNA coding for enzymes, GPD1 and GPP2, involved in glycerol production, were augmented in the presence of PMKT, agreeing with the glycerol increment observed in response to PMKT, clearly revealing compatible osmolyte accumulation to counteract the toxin (Figure 2).
Figure 2.
Cellular response to PMKT exposure deduced from the analysis of the transcriptional response of S. cerevisiae to the toxin. Once PMKT has reached the cell wall through an interaction with (1→6)-β-d-glucans, PMKT interacts with the cytoplasmic membrane through the interaction with cwp2p (not shown in the figure). This interaction, through a mechanism of unknown nature (?), leads to disruption of electrochemical gradients. Cellular receptors are not included in the scheme for simplification. PMKT cause the formation of unregulated channels through which cations and small metabolites (e.g., K+, H+, Na+, glycerol) flow. These changes in pHi, turgor or ionic concentrations generates a transcriptional response through the activation of Hog1p. Genes (CTT1, HSP12, HSP26, and DDR2), related with a general stress response, and genes (GPD1 and GPP2), involved in glycerol synthesis, are transcribed to compensate the toxic effects of PMKT. Cations and glycerol, among others, can leak out from sensitive cells generating cell death. Highlighted in a box are the induction levels of the most up-regulated genes. Abbreviations: DHAP (dihydroxyacetone phosphate), G3P (glycerol-3-phosphate), FBP (fructose-1,6-bisphosphate), MG (methylglyoxal), CoQ (coenzyme Q), S-LG (S-d-lactoylglutathione), GSH (glutathione).
In conclusion, the most important number of up-regulated genes are core environmental stress response genes, in relation with the HOG pathway, showing that the global transcriptional response to PMKT is probably due to an alteration of the transmembrane gradients and so on cellular homeostasis [41]. Deletion mutants of up-regulated genes in response to PMKT are also observed to show hypersensitive phenotypes to PMKT, direct evidence that they are surely involved in the mechanism of action of PMKT [73], an effect also observed in HOG signaling-defective cells of S. cerevisiae when exposed to K1 [134], suggesting a response coincident to other hyperosmotic stresses being effective to counteract K1 toxicity. However, GPD1Δ mutants, and others defective in intracellular glycerol production, are hypersensitive to PMKT, but this is not the case for K1 and no downstream effectors of Hog1p relevant for K1 resistance could be identified [134]. It has been described that, for most toxins, a second energy-dependent step operates, involving toxin transference to the cytoplasmic membrane and subsequent interaction with the secondary membrane receptor.
5.3.2. PMKT2
The fact that PMKT2 presented a different spectrum of killer activity, as indicated above, soon suggested that the molecular mechanism of action of PMKT2 might be different. In previous works [20], the binding of PMKT2 to mannoproteins, its primary receptors, was seen to occur in the first two or three minutes after PMKT2 incorporation and then, cellular viability reduced (80% after 5 h of exposure to PMKT2). Furthermore, none of the cellular events observed, when sensitive cells are intoxicated with PMKT, occurred with PMKT2. Leakage of K+, Na+ influx and reduction of pHi were not observed during the initial exposure to PMKT2, indicating that the nature of the interaction is not related with the formation of unregulated cation-permeable channels. The observed increase of membrane permeability to H+, K+, Na+ after 4–5 h in contact with PMKT2 is considered an indirect effect, since they initiate concomitantly or after cell death, reflecting that they are not primary effects.
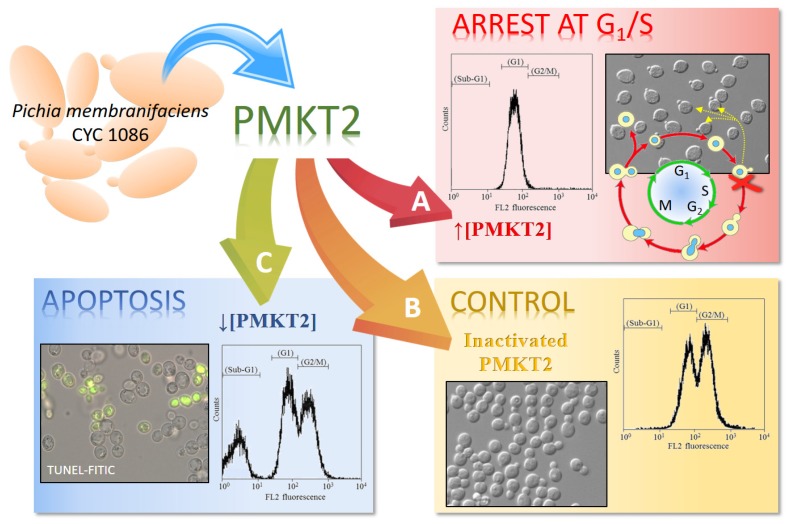
The reason so many dead cells remain non-permeabilized during a long time of exposure to PMKT2 was elucidated when the cell cycle was studied. Flow cytometry studies of sensitive yeast cells exposed to PMKT2 show cells with arrested at an early S-phase with a nascent bud and pre-replicated 1n DNA content (Figure 3). The execution point for lethality was determined with more precision by checking the sensitivity/resistance of cells arrested at different cell cycle stages. Alpha-factor-arrested cells at the G1-phase or G2-phase-arrested cells by treatment with methyl benzimidazol-2-yl-carbamate, are resistant to PMKT2, indicating that sensitive yeasts are not affected in these stages. Furthermore, in both cases, sensitive cells rescued from the arrest are inhibited by the toxin, suggesting, all together, that G2-phase-released cells start to divide, but die in the presence of the toxin after initiating the S-phase, adopting a terminal phenotype. To explain the late ion movements across plasma membrane, we hypothesize that, during the first moments of toxin action, the cell cycle arrest does not cause cytoplasmic membrane permeabilization and then, the arrested cells became converted unviable and plasma membrane permeabilization happens [42].
Figure 3.
Mechanisms of the killing activity of PMKT2 depending on toxin dosage. (A) High concentrations of PMKT2 caused cell cycle arrest at G1/S. Flow cytometry analyses of DNA content of S. cerevisiae revealed that cells treated with high doses of PMKT2 were arrested at an early S phase with a nascent bud. (B) Asynchronously growing cells exposed to inactivated toxin remained fully viable. (C) Cells treated with low concentrations of PMKT2 lead to the typical markers of apoptosis. Figure adapted and reproduced with permission (Santos et al. 2013) [42].
In short, the killing of action of PMKT, as well as several other yeast killer toxins, is related to peptide membrane permeation properties [16,41,125], whereas the mechanism of PMKT2 is related to cell cycle arrest [42].
5.4. The Dual Mechanism of Action of PMKT and PMKT2
It has been described how the killing of S. cerevisiae sensitive cells by P. membranifaciens killer toxins may be expressed as a combination of two mechanisms depending on toxin dosage. High doses (between 1250 and 2000 AU/mL) of PMKT and PMKT2 are observed to induce the aforementioned effects on sensitive yeast cells. By contrast, low doses (200 AU/mL) of the toxins lead to a cell death process in S. cerevisiae accompanied by cytological and biochemical indicators of apoptotic cell death (PCD, Programmed Cell Death). The observed cell death process is characterized by the presence of an initial apoptotic state that rapidly evolves to a necrotic state. The rate at which this process occurs is proportional to the amount of killer toxin used in the study. Low PMKT concentrations (up to 200 AU/mL) lead to a higher number of apoptotic cells, whereas higher concentrations (from 300 to 3000 AU/mL) cause both apoptosis and necrosis. Necrotic cells are usually characterized by a permeabilized plasma membrane (usually detected by propidium iodide stain), whereas the plasma membrane that have initiated a PCD phenotype is usually intact. As a result of these investigations, it can be determined that cells appear to start the process of death with a PCD response, die as a consequence of apoptosis, and then, as a late consequence, become necrotic [74]. In these experimental conditions, cell death is characterized by cytological, genetic and biochemical indicators of apoptosis, namely, an increment of reactive oxygen species (ROS), DNA strand breaks, externalization of phosphatidylserine, metacaspase activation and cytochrome c release, confirming the hypothesis of PCD induction.
It has been also demonstrated that the secondary receptors in the sensitive cells have the same function in both experimental conditions. CWP2Δ mutants are resistant to pro-apoptotic, as well as for pro-ionophoric amounts of PMKT, also in agreement with the observations obtained for Kre1p, the plasma membrane secondary receptor involved in the K1 mechanism of action [76,135], indicating that Cwp2p is always involved in the killing action [76,135].
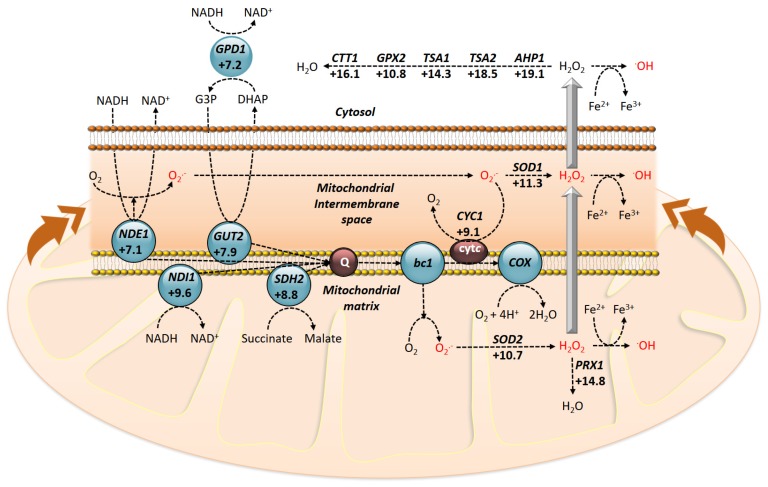
In the comparison studies between the two experimental conditions, it has been determined whether the transcriptional response of the cells is coincident. Therefore, using DNA microarrays, global gene expression has been also evaluated in the presence of pro-apoptotic concentrations of PMKT. The results obtained from DNA microarrays indicate that genes related with an oxidative stress response are induced, showing that the transcriptional response is not coincident with that obtained for pro-ionophoric doses of PMKT. The most highly induced genes are, in order, DCS2, FLR1, GSH1, APH1, VPS13, TSA2, GLR1, ECM4, AAD14 and GTO3, most of which are observed to be induced by different oxidative compounds. Several genes encoding for ROS detoxifying enzymes, such as CTT1, PRX1, TSA1, and SOD1, are also observed to be up-regulated (activations comprised between +16.1 and +11.3), indicating an oxidative stress that can be observed by analyzing the ROS production with dihydroethidium in the presence of PMKT (Figure 4).
Figure 4.
Transcriptional response of S. cerevisiae after exposure to pro-apoptotic concentrations of PMKT. Respiratory chain of S. cerevisiae with the indication of sites where ROS are formed and the main mitochondrial enzymes involved. Numbers indicate the levels of induction of some genes coding for such representative enzymes. Figure adapted and reproduced with permission (Santos et al. 2011) [74].
Taking into account the previous research on PMKT, we raised the possibility that PMKT2 also presented the aforementioned duality in its mode of action. This hypothesis was confirmed following the same experimental approach used for PMKT. Briefly, we described how the mechanism of killing of S. cerevisiae cells may be expressed as a sum of two mechanisms that depend on the used PMKT2 doses. High doses induce cell cycle arrest as already mentioned and low doses, as well as described for PMKT, induced a PCD process accompanied by cellular and genetic indicators of PCD in S. cerevisiae [42].
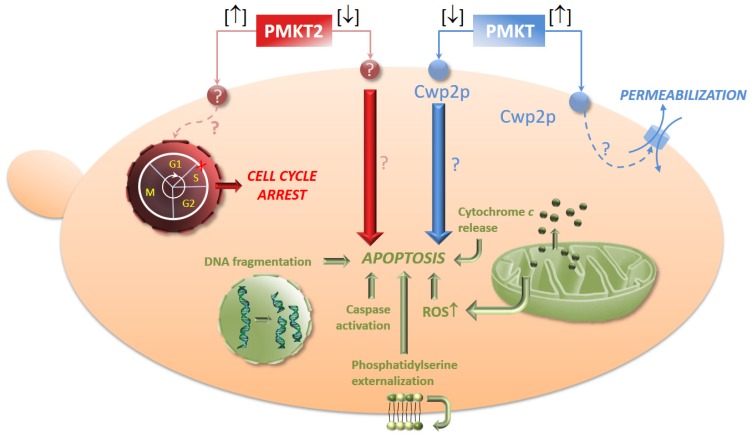
It remains to be determined why low concentrations of both toxins, PMKT and PMKT2, alter the oxidative homeostasis of sensitive cells and cause apoptosis (Figure 5). The mechanism by which PCD is induced in response to P. membranifaciens toxins, as other killer toxins, is as yet unknown, but it proves to be a challenging research field for the future.
Figure 5.
Schematic representation of the most important events known to be induced in S. cerevisiae after exposure to high [↑] and low [↓] concentrations of PMKT and PMKT2. Figure adapted and reproduced with permission (Santos et al. 2011; Santos et al. 2013) [42,74].
6. Applications, Conclusions and Future Perspectives of P. membranifaciens Killer Toxins
Although killer toxins were originally described and studied for their ecological function in the early 1960s [1], and they promptly received much attention from geneticists for the understanding of their genetic basis at the end of this decade [86,136], their biocidal nature has an inherent applicability in clinical, food and biocontrol fields that was not systematically explored until the 1990s (for medicine: [137,138,139]; for food: [140,141,142,143]; for biocontrol: [144,145,146]). As mentioned at the beginning, P. membranifaciens killer yeasts exhibit a broad action spectrum including yeasts of biotechnological concern (Brettanomyces/Dekkera, Candida boidinii, Zygosaccharomyces) and phytopatogenic fungi (Fusarium proliferatum, Botrytis cinerea), as well as other killer yeasts [21,104].
To a large extent, the potential applications that have been proposed for yeast killer toxins have been conditioned by the aforementioned recognized lability of such toxins. The proposed applications are largely restricted to those that provide physical chemical conditions compatible with the stability of killer toxins, that is, those that can be performed at relatively low pH and low temperature values (Table 3). Although the majority of killer toxins (W. mrakii var. mrakii HM-1, W. saturnus toxin, P. anomala PaKT and Z. bailii zygocin) have shown activity against pathogenic yeasts, such as Candida albicans, Cryptococcus neoformans, etc., they have been suggested to be potentially useful as potential antifungal agents for infections caused by fungi, their direct application is of limited real importance because of their instability at physiological temperatures and pH values and their antigenicity [39,104,146,147,148,149,150,151]. Once again, only the killer toxins that display a remarkable stability (e.g., the Williopsis toxins) could be therapeutically used, but restricted to topical applications such as on superficial skin infections [151]. In this context, the role and use of P. membranifaciens and its killer toxins to control human or animal infections and other clinical diseases is still uncertain.
Since the use of P. membranifaciens killer toxins seems to be not really useful at clinical stages, their use for biocontrol purposes has a great interest in agro-food industry. The applications of the biocide activity of P. membranifaciens were initially unveiled by Masih et al. (2001), observing that the mycelium of the B. cinerea treated with P. membranifaciens failed to develop the characteristic grey mould symptoms when it was re-inoculated onto grapevine [152]. This destructive process was suggested to be mediated by the production of exo- and endo-β-1,3-glucanases, as was also demonstrated by Masih et al. (2002) [153]. However, as described by Santos and colleagues in both grapevine and apple matrixes, this antagonistic phenomenon could also be mediated by killer toxin (PMKT) activity [19,154]. The co-existence of different mechanisms, including hydrolytic enzymes and killer toxin production, is one of the reasons that justifies the interest and versatility of P. membranifaciens as a biocontrol agent against B. cinerea, since it was also proved effective in postharvest pears [155].
The existence of similar and additional antagonistic mechanisms in other yeasts and their use to control B. cinerea spoilage have also been reported. For example, the production of exo-glucanase toxins in W. anomalus was reported by Parafati et al. (2016) [156], but the production of certain volatile organic compounds (i.e., 2-phenylethanol) has also been suggested as determinant for the biocontrol activity against B. cinerea found in this species and in others such as Candida intermedia, Sporidiobolus pararoseus, Starmerella bacilaris and S. cerevisiae [157,158,159,160,161,162].
In a similar context, P. membranifaciens has also been used as a biological tool for the control of other crop-fruit-associated fungus pathogens such as Penicillium expansum in postharvest peaches (by rapid colonization and competition for space and nutrients in fruit wounds) [163] and in postharvest pears (by nutrient and space competition and the production of chitinase, glucanase and other killer activities) [155]; Colletotrichum acutatum causing anthracnose rot in postharvest loquat fruit (by producing chitinase and β-1,3-glucanase) [164]; Penicillium roqueforti in storage wheat grain (with no conclusions about the mechanism of action) [160]; and Penicillium italicum, Penicillium digitatum and Colletotrichum gloeosporioides causing blue and green mold and anthracnose in citrus fruit (with no conclusions about the mechanism of action) [165].
The activity of P. membranifaciens towards the wine spoilage yeast B. bruxellensis was firstly observed by Yap et al. (2000) [189], and this fact was later confirmed by Santos et al. (2009), who verified the inhibition of P. membranifaciens CYC 1086 and its killer toxin PMKT2 against B. bruxellensis in simulated winemaking conditions [20]. Apart from the use of P. membranifaciens CYC 1086 and PMKT2, killer toxins from Ustilago maydis [10], Torulaspora delbrueckii (TdKT) [9], Kluyveromyces wickerhamii (Kwkt), Wickerhamomyces anomalus (Pikt) [183] and Candida pyralidae (CpKT1) [184] and the production of pulcherriminic acid by Metschnikowia pulcherrima (without a real killer activity but depleting biologically available iron from the medium) [190] have been reported as useful to control Brettanomyces spoilage. Continuing in the enology context, results from Alonso et al. (2015) and Labbani et al. (2015) conclude that a synergistic effect was operating using PMKT [185] and Pkkp (from P. kluyveri) [8] killer toxins, respectively, in combination with potassium metabisulfite, against wine spoilage yeasts such as Zygosaccharomyces or Dekkera/Brettanomyces species. Recently, Velazquez and coworkers described the utility of mixed-inoculation with killer and sensitive yeast strains to speed cell death during second fermentation of sparkling-wines to release of certain compounds from autolyzed yeast cells [181].
In summary, the killer activity of P. membranifaciens shows a broad spectrum of potential applications, sustained by a considerable diversity of mechanisms of action. However, a marked lability of these killer toxins restricts their application to specific environments and matrixes providing adequate chemical physical conditions. These requirements are basically found in food, post-harvest, and fermentative environments, even though the industrial application of killer toxins is far from a reality, since their large-scale production is still expensive. For this reason, the current tendency is only based in applying killer yeasts instead of their toxins. Furthermore, killer resistant strains have been described since the initial studies on killer toxins and, in many surveys, it is possible to found, over a panel of potential sensitive yeast isolates, that some strains are resistant to that potential biocontrol agents [20]. In accordance, these toxins could be less valuable for biocontrol in real applications.
For those purposes, regarding toxin stability in both production and application stages, adaptive and directed evolution approaches are of great interest in the development and isolation of phenotypically improved variants. It is possible to evolve populations in laboratory conditions, where it is important that the selection conditions match the industrial parameters as closely as possible [191]. Since temperature and pH are the main limiting factors, especially for clinical applications, adaptive processes should be carried out with P. membranifaciens killer strains to obtain toxins with increased stability at human physiological conditions. Additionally, killer toxins identified as leads for therapeutic uses should also be modulated to reduce their antigenicity [192]. For that purpose, the molecular structure and mechanism of action of P. membranifaciens killer toxins should be studied in depth, in order to identify key residues causing toxicity and antigenicity, and determine their stability. Finally, the cellular response of the target pathogenic/spoilage microorganism to killer toxins needs to be studied species by species to understand the basis of their susceptibility.
Additional studies are currently under development to increase the background of knowledge accumulated during the last two decades on the features of P. membranifaciens killer toxins, in the hope of understanding how to work with these killer toxins that have potential biotechnological applications. Elucidation of the molecular mechanisms of their action will be helpful to develop the strategies to fight more and more harmful spoilage yeasts.
Acknowledgments
The studies referenced in this review were supported by Grants from the European Community (AIR CT93-0830), Universidad Complutense-Community of Madrid (PR1/03-11632; CCG07-UCM/AGR-2612, GR45/06; GR69/06; GR74/07; GR58/08, GR35/10-A), and CDTI-MINECO-Centre for the Development of Industrial Technology (IDI-20130192; IDI-20140448; IDI-20160102).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Bevan E., Makower M. The physiological basis of the killer character in yeast. Proc. Int. Congr. Genet. 1963;1:202–203. [Google Scholar]
- 2.Marquina D., Santos A., Peinado J. Biology of killer yeasts. Int. Microbiol. 2002;5:65–71. doi: 10.1007/s10123-002-0066-z. [DOI] [PubMed] [Google Scholar]
- 3.El-Benna A., El-Sahn M., Shehata M. Yeasts producing killer toxins: An overview. Alex J. Food Sci. Technol. 2011;8:41–53. [Google Scholar]
- 4.Liu Y., Rousseaux S., Tourdot-Marechal R., Sadoudi M., Gougeon R., Schmitt-Kopplin P., Alexandre H. Wine microbiome, a dynamic world of microbial interactions. Crit. Rev. Food Sci. Nutr. 2017;57:856–873. doi: 10.1080/10408398.2014.983591. [DOI] [PubMed] [Google Scholar]
- 5.Golubev W. Antagonistic interactions among yeasts. In: Péter G., Rosa C., editors. Biodiversity and Ecophysiology of Yeasts. Springer; Berlin, Germany: 2006. pp. 197–219. [Google Scholar]
- 6.Schmitt M.J., Breinig F. The viral killer system in yeast: From molecular biology to application. FEMS Microbiol. Lett. 2002;26:257–276. doi: 10.1111/j.1574-6976.2002.tb00614.x. [DOI] [PubMed] [Google Scholar]
- 7.Meinhardt F., Klassen R. Yeast killer toxins: Fundamentals and applications. In: Anke T., Weber D., editors. Physiology and Genetics. Volume 15. Springer; Berlin, Germany: 2009. pp. 107–130. [Google Scholar]
- 8.Labbani F.-Z.K., Turchetti B., Bennamoun L., Dakhmouche S., Roberti R., Corazzi L., Meraihi Z., Buzzini P. A novel killer protein from Pichia kluyveri isolated from an algerian soil: Purification and characterization of its in vitro activity against food and beverage spoilage yeasts. Antonie Leeuwenhoek. 2015;107:961–970. doi: 10.1007/s10482-015-0388-4. [DOI] [PubMed] [Google Scholar]
- 9.Villalba M.L., Sáez J.S., del Monaco S., Lopes C.A., Sangorrín M.P. TdKT, a new killer toxin produced by Torulaspora delbrueckii effective against wine spoilage yeasts. Int. J. Food Microbiol. 2016;217:94–100. doi: 10.1016/j.ijfoodmicro.2015.10.006. [DOI] [PubMed] [Google Scholar]
- 10.Santos A., Navascués E., Bravo E., Marquina D. Ustilago maydis killer toxin as a new tool for the biocontrol of the wine spoilage yeast Brettanomyces bruxellensis. Int. J. Food Microbiol. 2011;145:147–154. doi: 10.1016/j.ijfoodmicro.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 11.Chang S.L., Leu J.Y., Chang T.H. A population study of killer viruses reveals different evolutionary histories of two closely related Saccharomyces sensu stricto yeasts. Mol. Ecol. 2015;24:4312–4322. doi: 10.1111/mec.13310. [DOI] [PubMed] [Google Scholar]
- 12.Breinig F., Sendzik T., Eisfeld K., Schmitt M.J. Dissecting toxin immunity in virus-infected killer yeast uncovers an intrinsic strategy of self-protection. Proc. Natl. Acad. Sci. USA. 2006;103:3810–3815. doi: 10.1073/pnas.0510070103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kast A., Voges R., Schroth M., Schaffrath R., Klassen R., Meinhardt F. Autoselection of cytoplasmic yeast virus like elements encoding toxin/antitoxin systems involves a nuclear barrier for immunity gene expression. PLoS Genet. 2015;11:e1005005. doi: 10.1371/journal.pgen.1005005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wickner R.B., Edskes H.K. Yeast killer elements hold their hosts hostage. PLoS Genet. 2015;11:e1005139. doi: 10.1371/journal.pgen.1005139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fuentefria A.M., Faganello J., Pazzini F., Schrank A., Valente P., Vainstein M. Typing and patterns of cellular morphological alterations in Cryptococcus neoformans and Cryptococcus gattii isolates exposed to a panel of killer yeasts. Med. Mycol. 2007;45:503–512. doi: 10.1080/13693780701416580. [DOI] [PubMed] [Google Scholar]
- 16.Keszthelyi A., Ohkusu M., Takeo K., Pfeiffer I., Litter J., Kucsera J. Characterisation of the anticryptococcal effect of the Fc-1 toxin produced by Filobasidium capsuligenum. Mycoses. 2006;49:176–183. doi: 10.1111/j.1439-0507.2006.01227.x. [DOI] [PubMed] [Google Scholar]
- 17.Pérez M.F., Contreras L., Garnica N.M., Fernández-Zenoff M.V., Farías M.E., Sepulveda M., Ramallo J., Dib J.R. Native killer yeasts as biocontrol agents of postharvest fungal diseases in lemons. PLoS ONE. 2016;11:e0165590. doi: 10.1371/journal.pone.0165590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ramírez M., Velázquez R., Maqueda M., Zamora E., López-Piñeiro A., Hernández L.M. Influence of the dominance of must fermentation by Torulaspora delbrueckii on the malolactic fermentation and organoleptic quality of red table wine. Int. J. Food Microbiol. 2016;238:311–319. doi: 10.1016/j.ijfoodmicro.2016.09.029. [DOI] [PubMed] [Google Scholar]
- 19.Santos A., Marquina D. Killer toxin of Pichia membranifaciens and its possible use as a biocontrol agent against grey mould disease of grapevine. Microbiology. 2004;150:2527–2534. doi: 10.1099/mic.0.27071-0. [DOI] [PubMed] [Google Scholar]
- 20.Santos A., San Mauro M., Bravo E., Marquina D. PMKT2, a new killer toxin from Pichia membranifaciens, and its promising biotechnological properties for control of the spoilage yeast Brettanomyces bruxellensis. Microbiology. 2009;155:624–634. doi: 10.1099/mic.0.023663-0. [DOI] [PubMed] [Google Scholar]
- 21.Schmitt M.J., Breinig F. Yeast viral killer toxins: Lethality and self-protection. Nat. Rev. Microbiol. 2006;4:212–221. doi: 10.1038/nrmicro1347. [DOI] [PubMed] [Google Scholar]
- 22.Kurtzman C., Fell J.W., Boekhout T. The Yeasts: A Taxonomic Study. 5th ed. Elsevier; Amsterdam, The Netherland: 2011. [Google Scholar]
- 23.Hansen E.C. Grundlinien zur Systematik der Saccharomyceten. Zentralbl. Bakteriol. Parasitenk. Abt. II. 1904;12:529–538. [Google Scholar]
- 24.Noronha-da-Costa P., Rodrigues C., Spencer-Martins I., Loureiro V. Fatty acid patterns of film-forming yeasts and new evidence for the heterogeneity of Pichia membranaefaciens. Lett. Appl. Microbiol. 1996;23:79–84. doi: 10.1111/j.1472-765X.1996.tb00035.x. [DOI] [PubMed] [Google Scholar]
- 25.Villa-Carvajal M., Querol A., Belloch C. Identification of species in the genus Pichia by restriction of the internal transcribed spacers (ITS1 and ITS2) and the 5.8 S ribosomal DNA gene. Antonie Leeuwenhoek. 2006;90:171–181. doi: 10.1007/s10482-006-9071-0. [DOI] [PubMed] [Google Scholar]
- 26.Deak T., Beuchat L. Handbook of Food Spoilage Yeasts, Crc Series in Contemporary Food Science. 2nd ed. CRC Press; Boca Raton, FL, USA: 1996. [Google Scholar]
- 27.Boekhout T., Robert V. Yeasts in Food. 1st ed. Elsevier; Amsterdam, The Netherland: 2003. [Google Scholar]
- 28.Bakır M., Cerikcioğlu N., Tırtır A., Berrak S., Özek E., Canpolat C. Pichia anomala fungaemia in immunocompromised children. Mycoses. 2004;47:231–235. doi: 10.1111/j.1439-0507.2004.00962.x. [DOI] [PubMed] [Google Scholar]
- 29.Otag F., Kuyucu N., Erturan Z., Sen S., Emekdas G., Sugita T. An outbreak of Pichia ohmeri infection in the paediatric intensive care unit: Case reports and review of the literature. Mycoses. 2005;48:265–269. doi: 10.1111/j.1439-0507.2005.01126.x. [DOI] [PubMed] [Google Scholar]
- 30.Kast A., Klassen R., Meinhardt F. RNA fragmentation induced by a yeast killer toxin. Mol. Microbiol. 2014;91:606–617. doi: 10.1111/mmi.12481. [DOI] [PubMed] [Google Scholar]
- 31.Satwika D., Klassen R., Meinhardt F. Anticodon nuclease encoding virus-like elements in yeast. Appl. Microbiol. Biotechnol. 2012;96:345–356. doi: 10.1007/s00253-012-4349-9. [DOI] [PubMed] [Google Scholar]
- 32.Wemhoff S., Klassen R., Meinhardt F. DNA damage induced by the anticodon nuclease from a Pichia acaciae killer strain is linked to ribonucleotide reductase depletion. Cell. Microbiol. 2016;18:211–222. doi: 10.1111/cmi.12496. [DOI] [PubMed] [Google Scholar]
- 33.Coelho A.R., Tachi M., Pagnocca F.C., Nobrega G.M.A., Hoffmann F.L., Harada K.-I., Hirooka E.Y. Purification of Candida guilliermondii and Pichia ohmeri killer toxin as an active agent against Penicillium expansum. Food Addit. Contam. 2009;26:73–81. doi: 10.1080/02652030802227227. [DOI] [PubMed] [Google Scholar]
- 34.Worsham P., Bolen P. Killer toxin production in Pichia acaciae is associated with linear DNA plasmids. Curr. Genet. 1990;18:77–80. doi: 10.1007/BF00321119. [DOI] [PubMed] [Google Scholar]
- 35.Bevan E., Herring A., Mitchell D.J. Preliminary characterization of two species of dsRNA in yeast and their relationship to the “killer“ character. Nature. 1973;245:81–86. doi: 10.1038/245081b0. [DOI] [PubMed] [Google Scholar]
- 36.Chen W.-B., Han Y.-F., Jong S.-C., Chang S.-C. Isolation, purification, and characterization of a killer protein from Schwanniomyces occidentalis. Appl. Environ. Microbiol. 2000;66:5348–5352. doi: 10.1128/AEM.66.12.5348-5352.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Middelbeek E., Hermans J., Stumm C. Production, purification and properties of a Pichia kluyveri killer toxin. Antonie Leeuwenhoek. 1979;45:437–450. doi: 10.1007/BF00443282. [DOI] [PubMed] [Google Scholar]
- 38.Pfeiffer P., Radler F. Comparison of the killer toxin of several yeasts and the purification of a toxin of type K2. Arch. Microbiol. 1984;137:357–361. doi: 10.1007/BF00410734. [DOI] [PubMed] [Google Scholar]
- 39.İzgü F., Altınbay D., Türeli A.E. In vitro susceptibilities of Candida spp. To Panomycocin, a novel exo-β-1,3-glucanase isolated from Pichia anomala NCYC 434. Microbiol. Immunol. 2007;51:797–803. doi: 10.1111/j.1348-0421.2007.tb03975.x. [DOI] [PubMed] [Google Scholar]
- 40.Klassen R., Paluszynski J.P., Wemhoff S., Pfeiffer A., Fricke J., Meinhardt F. The primary target of the killer toxin from Pichia acaciae is tRNAgln. Mol. Microbiol. 2008;69:681–697. doi: 10.1111/j.1365-2958.2008.06319.x. [DOI] [PubMed] [Google Scholar]
- 41.Santos A., Marquina D. Ion channel activity by Pichia membranifaciens killer toxin. Yeast. 2004;21:151–162. doi: 10.1002/yea.1069. [DOI] [PubMed] [Google Scholar]
- 42.Santos A., Alonso A., Belda I., Marquina D. Cell cycle arrest and apoptosis, two alternative mechanisms for PmKT2 killer activity. Fungal Genet. Biol. 2013;50:44–54. doi: 10.1016/j.fgb.2012.10.006. [DOI] [PubMed] [Google Scholar]
- 43.Marquina D., Peres C., Caldas F., Marques J., Peinado J., Spencer-Martins I. Characterization of the yeast population in olive brines. Lett. Appl. Microbiol. 1992;14:279–283. doi: 10.1111/j.1472-765X.1992.tb00705.x. [DOI] [Google Scholar]
- 44.Llorente P., Marquina D., Santos A., Peinado J., Spencer-Martins I. Effect of salt on the killer phenotype of yeasts from olive brines. Appl. Environ. Microbiol. 1997;63:1165–1167. doi: 10.1128/aem.63.3.1165-1167.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Da Silva S., Calado S., Lucas C., Aguiar C. Unusual properties of the halotolerant yeast Candida nodaensis killer toxin, CnKT. Microbiol. Res. 2008;163:243–251. doi: 10.1016/j.micres.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 46.Klassen R., Meinhardt F. Linear plasmids pWR1A and pWR1B of the yeast Wingea robertsiae are associated with a killer phenotype. Plasmid. 2002;48:142–148. doi: 10.1016/S0147-619X(02)00101-4. [DOI] [PubMed] [Google Scholar]
- 47.Schmitt M.J., Neuhausen F. Killer toxin-secreting double-stranded RNA mycoviruses in the yeasts Hanseniaspora uvarum and Zygosaccharomyces bailii. J. Virol. 1994;68:1765–1772. doi: 10.1128/jvi.68.3.1765-1772.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gunge N., Tamaru A., Ozawa F., Sakaguchi K. Isolation and characterization of linear deoxyribonucleic acid plasmids from Kluyveromyces lactis and the plasmid-associated killer character. J. Bacteriol. 1981;145:382–390. doi: 10.1128/jb.145.1.382-390.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Comitini F., Di Pietro N., Zacchi L., Mannazzu I., Ciani M. Kluyveromyces phaffii killer toxin active against wine spoilage yeasts: Purification and characterization. Microbiology. 2004;150:2535–2541. doi: 10.1099/mic.0.27145-0. [DOI] [PubMed] [Google Scholar]
- 50.Young T., Yagiu M. A comparison of the killer character in different yeasts and its classification. Antonie Leeuwenhoek. 1978;44:59–77. doi: 10.1007/BF00400077. [DOI] [PubMed] [Google Scholar]
- 51.Dignard D., Whiteway M., Germain D., Tessier D., Thomas D.Y. Expression in yeast of a cDNA copy of the K2 killer toxin gene. Mol. Gen. Genet. 1991;227:127–136. doi: 10.1007/BF00260717. [DOI] [PubMed] [Google Scholar]
- 52.Lukša J., Podoliankaitė M., Vepštaitė I., Strazdaitė-Žielienė Ž., Urbonavičius J., Servienė E. Yeast β-1,6-glucan is a primary target for the Saccharomyces cerevisiae K2 toxin. Eukaryot. Cell. 2015;14:406–414. doi: 10.1128/EC.00287-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schmitt M.J., Tipper D.J. K28, a unique double-stranded RNA killer virus of Saccharomyces cerevisiae. Mol. Cell. Biol. 1990;10:4807–4815. doi: 10.1128/MCB.10.9.4807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rodríguez-Cousiño N., Maqueda M., Ambrona J., Zamora E., Esteban R., Ramírez M. A new wine Saccharomyces cerevisiae killer toxin (Klus), encoded by a double-stranded RNA virus, with broad antifungal activity is evolutionarily related to a chromosomal host gene. Appl. Environ. Microbiol. 2011;77:1822–1832. doi: 10.1128/AEM.02501-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Goto K., Iwatuki Y., Kitano K., Obata T., Hara S. Cloning and nucleotide sequence of the KHR killer gene of Saccharomyces cerevisiae. Agric. Biol. Chem. 1990;54:979–984. doi: 10.1271/bbb1961.54.979. [DOI] [PubMed] [Google Scholar]
- 56.Park C.M., Banerjee N., Koltin Y., Bruenn J.A. The Ustilago maydis virally encoded KP1 killer toxin. Mol. Microbiol. 1996;20:957–963. doi: 10.1111/j.1365-2958.1996.tb02537.x. [DOI] [PubMed] [Google Scholar]
- 57.Park C.M., Bruenn J.A., Ganesa C., Flurkey W.F., Bozarth R.F., Koltin Y. Structure and heterologous expression of the Ustilago maydis viral toxin KP4. Mol. Microbiol. 1994;11:155–164. doi: 10.1111/j.1365-2958.1994.tb00297.x. [DOI] [PubMed] [Google Scholar]
- 58.Tao J., Ginsberg I., Banerjee N., Held W., Koltin Y., Bruenn J. Ustilago maydis KP6 killer toxin: Structure, expression in Saccharomyces cerevisiae, and relationship to other cellular toxins. Mol. Cell. Biol. 1990;10:1373–1381. doi: 10.1128/MCB.10.4.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ashida S., Shimazaki T., Kitano K., Hara S. New killer toxin of Hansenula mrakii. Agric. Biol. Chem. 1983;47:2953–2955. doi: 10.1271/bbb1961.47.2953. [DOI] [Google Scholar]
- 60.Kasahara S., Inoue S.B., Mio T., Yamada T., Nakajima T., Ichishima E., Furuichi Y., Yamada H. Involvement of cell wall β-glucan in the action of HM-1 killer toxin. FEBS Lett. 1994;348:27–32. doi: 10.1016/0014-5793(94)00575-3. [DOI] [PubMed] [Google Scholar]
- 61.Komiyama T., Furuichi Y., Tsukada Y. Structure and activity of HYI killer toxin from Hansenula saturnus. Biol. Pharm. Bull. 1995;18:1057–1059. doi: 10.1248/bpb.18.1057. [DOI] [PubMed] [Google Scholar]
- 62.Komiyama T., Shirai T., Urakami H., Furuichi Y., Tsukada Y. Action properties of HYI killer toxin from Williopsis saturnus var. Saturnus, and antibiotics, Aculeacin a and Papulacandin b. Biol. Pharm. Bull. 1998;21:1013–1019. doi: 10.1248/bpb.21.1013. [DOI] [PubMed] [Google Scholar]
- 63.Radler F., Herzberger S., Schönig I., Schwarz P. Investigation of a killer strain of Zygosaccharomyces bailii. Microbiology. 1993;139:495–500. doi: 10.1099/00221287-139-3-495. [DOI] [PubMed] [Google Scholar]
- 64.Ramírez M., Velázquez R., Maqueda M., López-Piñeiro A., Ribas J.C. A new wine Torulaspora delbrueckii killer strain with broad antifungal activity and its toxin-encoding double-stranded RNA virus. Front. Microbiol. 2015;6:983. doi: 10.3389/fmicb.2015.00983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Izgü F., Altinbay D. Isolation and characterization of the k5-type yeast killer protein and its homology with an exo-β-1,3-glucanase. Biosci. Biotechnol. Biochem. 2004;68:685–693. doi: 10.1271/bbb.68.685. [DOI] [PubMed] [Google Scholar]
- 66.Guyard C., Seguy N., Lange M., Ricard I., Polonelli L., Cailliez J. First steps in the purification and characterization of a Pichia anomala killer toxin. J. Eukaryot. Microbiol. 1998;46:144S. [PubMed] [Google Scholar]
- 67.De Ingeniis J., Raffaelli N., Ciani M., Mannazzu I. Pichia anomala DBVPG 3003 secretes a ubiquitin-like protein that has antimicrobial activity. Appl. Environ. Microbiol. 2009;75:1129–1134. doi: 10.1128/AEM.01837-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang X., Chi Z., Yue L., Li J. Purification and characterization of killer toxin from a marine yeast Pichia anomala YF07B against the pathogenic yeast in crab. Curr. Microbiol. 2007;55:396–401. doi: 10.1007/s00284-007-9010-y. [DOI] [PubMed] [Google Scholar]
- 69.Farkas Z., Márki-Zay J., Kucsera J., Vágvölgyi C., Golubev W., Pfeiffer I. Characterization of two different toxins of Wickerhamomyces anomalus (Pichia anomala) VKM Y-159. Acta Biol. Hung. 2012;63:277–287. doi: 10.1556/ABiol.63.2012.2.9. [DOI] [PubMed] [Google Scholar]
- 70.Suzuki C., Nikkuni S. The primary and subunit structure of a novel type killer toxin produced by a halotolerant yeast, Pichia farinosa. J. Biol. Chem. 1994;269:3041–3046. [PubMed] [Google Scholar]
- 71.Klassen R., Meinhardt F. Structural and functional analysis of the killer element pPin1—3 from Pichia inositovora. Mol. Genet. Genom. 2003;270:190–199. doi: 10.1007/s00438-003-0920-5. [DOI] [PubMed] [Google Scholar]
- 72.Bajaj B.K., Raina S., Singh S. Killer toxin from a novel killer yeast Pichia kudriavzevii RY55 with idiosyncratic antibacterial activity. J. Basic Microbiol. 2013;53:645–656. doi: 10.1002/jobm.201200187. [DOI] [PubMed] [Google Scholar]
- 73.Santos A., del Mar Álvarez M., San Mauro M., Abrusci C., Marquina D. The transcriptional response of Saccharomyces cerevisiae to Pichia membranifaciens killer toxin. J. Biol. Chem. 2005;280:41881–41892. doi: 10.1074/jbc.M507014200. [DOI] [PubMed] [Google Scholar]
- 74.Santos A., Marquina D. The transcriptional response of Saccharomyces cerevisiae to proapoptotic concentrations of Pichia membranifaciens killer toxin. Fungal. Genet. Biol. 2011;48:979–989. doi: 10.1016/j.fgb.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 75.Santos A., Marquina D., Leal J., Peinado J. (1→6)-β-d-glucan as cell wall receptor for Pichia membranifaciens killer toxin. Appl. Environ. Microbiol. 2000;66:1809–1813. doi: 10.1128/AEM.66.5.1809-1813.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Santos A., San Mauro M., Abrusci C., Marquina D. Cwp2p, the plasma membrane receptor for Pichia membranifaciens killer toxin. Mol. Microbiol. 2007;64:831–843. doi: 10.1111/j.1365-2958.2007.05702.x. [DOI] [PubMed] [Google Scholar]
- 77.Arroyo-López F.N., Medina E., Ruiz-Bellido M.Á., Romero-Gil V., Montes-Borrego M., Landa B.B. Enhancement of the knowledge on fungal communities in directly brined Aloreña de Málaga green olive fermentations by metabarcoding analysis. PLoS ONE. 2016;11:e0163135. doi: 10.1371/journal.pone.0163135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Leventdurur S., Sert-Aydın S., Boyaci-Gunduz C.P., Agirman B., Ben Ghorbal A., Francesca N., Martorana A., Erten H. Yeast biota of naturally fermented black olives in different brines made from cv. Gemlik grown in various districts of the Cukurova region of Turkey. Yeast. 2016;33:289–301. doi: 10.1002/yea.3170. [DOI] [PubMed] [Google Scholar]
- 79.Arroyo-López F., Romero-Gil V., Bautista-Gallego J., Rodríguez-Gómez F., Jiménez-Díaz R., García-García P., Querol A., Garrido-Fernández A. Yeasts in table olive processing: Desirable or spoilage microorganisms? Int. J. Food Microbiol. 2012;160:42–49. doi: 10.1016/j.ijfoodmicro.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 80.Vaughn R.H., Jakubczyk T., MacMillan J.D., Higgins T.E., Davé B.A., Crampton V.M. Some pink yeasts associated with softening of olives. Appl. Microbiol. 1969;18:771–775. doi: 10.1128/am.18.5.771-775.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Vaughn R.H., Stevenson K.E., Davé B.A., Park H.C. Fermenting yeasts associated with softening and gas-pocket formation in olives. Appl. Microbiol. 1972;23:316–320. doi: 10.1128/am.23.2.316-320.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Arroyo-López F., Querol A., Bautista-Gallego J., Garrido-Fernández A. Role of yeasts in table olive production. Int. J. Food Microbiol. 2008;128:189–196. doi: 10.1016/j.ijfoodmicro.2008.08.018. [DOI] [PubMed] [Google Scholar]
- 83.Hernández A., Martín A., Córdoba M.G., Benito M.J., Aranda E., Pérez-Nevado F. Determination of killer activity in yeasts isolated from the elaboration of seasoned green table olives. Int. J. Food Microbiol. 2008;121:178–188. doi: 10.1016/j.ijfoodmicro.2007.11.044. [DOI] [PubMed] [Google Scholar]
- 84.Barandica J., Santos A., Marquina D., López F., Acosta F., Peinado J. A mathematical model for toxin accumulation by killer yeasts based on the yeast population growth. J. Appl. Microbiol. 1999;86:805–811. doi: 10.1046/j.1365-2672.1999.00729.x. [DOI] [PubMed] [Google Scholar]
- 85.Palfree R.G., Bussey H. Yeast killer toxin: Purification and characterisation of the protein toxin from Saccharomyces cerevisiae. Eur. J. Biochem. 1979;93:487–493. doi: 10.1111/j.1432-1033.1979.tb12847.x. [DOI] [PubMed] [Google Scholar]
- 86.Woods D., Bevan E. Studies on the nature of the killer factor produced by Saccharomyces cerevisiae. Microbiology. 1968;51:115–126. doi: 10.1099/00221287-51-1-115. [DOI] [PubMed] [Google Scholar]
- 87.Hutchins K., Bussey H. Cell wall receptor for yeast killer toxin: Involvement of (1→6)-β-d-glucan. J. Bacteriol. 1983;154:161–169. doi: 10.1128/jb.154.1.161-169.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Brown J.L., Kossaczka Z., Jiang B., Bussey H. A mutational analysis of killer toxin resistance in Saccharomyces cerevisiae identifies new genes involved in cell wall (1→6)-beta-glucan synthesis. Genetics. 1993;133:837–849. doi: 10.1093/genetics/133.4.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ohta Y., Tsukada Y., Sugimori T. Production, purification and characterization of HYI, an antiyeast substance, produced by Hansenula saturnus. Agric. Biol. Chem. 1984;48:903–908. doi: 10.1271/bbb1961.48.903. [DOI] [Google Scholar]
- 90.Lowes K., Shearman C., Payne J., MacKenzie D., Archer D., Merry R., Gasson M. Prevention of yeast spoilage in feed and food by the yeast mycocin HMK. Appl. Environ. Microbiol. 2000;66:1066–1076. doi: 10.1128/AEM.66.3.1066-1076.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yamamoto T., Hiratani T., Hirata H., Imai M., Yamaguchi H. Killer toxin from Hansenula mrakii selectively inhibits cell wall synthesis in a sensitive yeast. FEBS Lett. 1986;197:50–54. doi: 10.1016/0014-5793(86)80296-4. [DOI] [PubMed] [Google Scholar]
- 92.Yamamoto T., Imai M., Tachibana K., Mayumi M. Application of monoclonal antibodies to the isolation and characterization of a killer toxin secreted by Hansenula mrakii. FEBS Lett. 1986;195:253–257. doi: 10.1016/0014-5793(86)80170-3. [DOI] [PubMed] [Google Scholar]
- 93.Golubev W. Killer activity of Tilletiopsis albescens gokhale: Taxonomic and phylogenetic implication. Syst. Appl. Microbiol. Title. 1998;21:429–432. doi: 10.1016/S0723-2020(98)80052-0. [DOI] [PubMed] [Google Scholar]
- 94.De S., Bubnys A., Alonzo F., Hyun J., Lary J.W., Cole J.L., Torres V.J., Olson R. The relationship between glycan binding and direct membrane interactions in Vibrio cholerae Cytolysin, a channel-forming toxin. J. Biol. Chem. 2015;290:28402–28415. doi: 10.1074/jbc.M115.675967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Al-Aidroos K., Bussey H. Chromosomal mutants of Saccharomyces cerevisiae affecting the cell wall binding site for killer factor. Can. J. Microbiol. 1978;24:228–237. doi: 10.1139/m78-041. [DOI] [PubMed] [Google Scholar]
- 96.Schmitt M., Radler F. Mannoprotein of the yeast cell wall as primary receptor for the killer toxin of Saccharomyces cerevisiae strain 28. Microbiology. 1987;133:3347–3354. doi: 10.1099/00221287-133-12-3347. [DOI] [PubMed] [Google Scholar]
- 97.Takita M.A., Castilho-Valavicius B. Absence of cell wall chitin in Saccharomyces cerevisiae leads to resistance to Kluyveromyces lactis killer toxin. Yeast. 1993;9:589–598. doi: 10.1002/yea.320090605. [DOI] [PubMed] [Google Scholar]
- 98.Breinig F., Tipper D.J., Schmitt M.J. Kre1p, the plasma membrane receptor for the yeast K1 viral toxin. Cell. 2002;108:395–405. doi: 10.1016/S0092-8674(02)00634-7. [DOI] [PubMed] [Google Scholar]
- 99.Novotná D., Flegelová H., Janderová B. Different action of killer toxins K1 and K2 on the plasma membrane and the cell wall of Saccharomyces cerevisiae. FEMS Yeast Res. 2004;4:803–813. doi: 10.1016/j.femsyr.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 100.Lesage G., Bussey H. Cell wall assembly in Saccharomyces cerevisiae. Microbiol. Mol. Biol. Rev. 2006;70:317–343. doi: 10.1128/MMBR.00038-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kollár R., Petráková E., Ashwell G., Robbins P.W., Cabib E. Architecture of the yeast cell wall the linkage between chitin and β-(1,3)-glucan. J. Biol. Chem. 1995;270:1170–1178. doi: 10.1074/jbc.270.3.1170. [DOI] [PubMed] [Google Scholar]
- 102.Radler F., Schmitt M., Meyer B. Killer toxin of Hanseniaspora uvarum. Arch. Microbiol. 1990;154:175–178. doi: 10.1007/BF00423329. [DOI] [PubMed] [Google Scholar]
- 103.Schmitt M., Radler F. Molecular structure of the cell wall receptor for killer toxin KT28 in Saccharomyces cerevisiae. J. Bacteriol. 1988;170:2192–2196. doi: 10.1128/jb.170.5.2192-2196.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Weiler F., Schmitt M.J. Zygocin, a secreted antifungal toxin of the yeast Zygosaccharomyces bailii, and its effect on sensitive fungal cells. FEMS Yeast Res. 2003;3:69–76. doi: 10.1111/j.1567-1364.2003.tb00140.x. [DOI] [PubMed] [Google Scholar]
- 105.Boone C., Sommer S., Hensel A., Bussey H. Yeast KRE genes provide evidence for a pathway of cell wall-glucan assembly. J. Cell. Biol. 1990;110:1833–1843. doi: 10.1083/jcb.110.5.1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Schmitt M.J., Radler F. Blockage of cell wall receptors for yeast killer toxin KT28 with antimannoprotein antibodies. Antimicrob. Agents Chemother. 1990;34:1615–1618. doi: 10.1128/aac.34.8.1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Bussey H. K1 killer toxin, a pore-forming protein from yeast. Mol. Microbiol. 1991;5:2339–2343. doi: 10.1111/j.1365-2958.1991.tb02079.x. [DOI] [PubMed] [Google Scholar]
- 108.Zhu H., Bussey H. Mutational analysis of the functional domains of yeast K1 killer toxin. Mol. Cell. Biol. 1991;11:175–181. doi: 10.1128/MCB.11.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Jones G.H., Ballou C.E. Studies on the structure of yeast mannan. I. Purification and some properties of an α-mannosidase from an arthrobacter species. J. Biol. Chem. 1969;244:1043–1051. [PubMed] [Google Scholar]
- 110.Lipke P.N., Ovalle R. Cell wall architecture in yeast: New structure and new challenges. J. Bacteriol. 1998;180:3735–3740. doi: 10.1128/jb.180.15.3735-3740.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Bussey H., Sherman D., Somers J. Action of yeast killer factor: A resistant mutant with sensitive spheroplasts. J. Bacteriol. 1973;113:1193–1197. doi: 10.1128/jb.113.3.1193-1197.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Breinig F., Schleinkofer K., Schmitt M.J. Yeast Kre1p is gpi-anchored and involved in both cell wall assembly and architecture. Microbiology. 2004;150:3209–3218. doi: 10.1099/mic.0.27175-0. [DOI] [PubMed] [Google Scholar]
- 113.Becker B., Blum A., Gießelmann E., Dausend J., Rammo D., Müller N.C., Tschacksch E., Steimer M., Spindler J., Becherer U. H/KDEL receptors mediate host cell intoxication by a viral A/B toxin in yeast. Sci. Rep. 2016;6 doi: 10.1038/srep31105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zink S., Mehlgarten C., Kitamoto H.K., Nagase J., Jablonowski D., Dickson R.C., Stark M.J., Schaffrath R. Mannosyl-diinositolphospho-ceramide, the major yeast plasma membrane sphingolipid, governs toxicity of Kluyveromyces lactis zymocin. Eukaryot. Cell. 2005;4:879–889. doi: 10.1128/EC.4.5.879-889.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sawant A., Abdelal A., Ahearn D. Purification and characterization of the anti-candida toxin of Pichia anomala WC 65. Antimicrob. Agents Chemother. 1989;33:48–52. doi: 10.1128/AAC.33.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sawant A., Ahearn D. Involvement of a cell wall receptor in the mode of action of an anti-candida toxin of Pichia anomala. Antimicrob. Agents Chemother. 1990;34:1331–1335. doi: 10.1128/AAC.34.7.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Diep D.B., Nelson K.L., Raja S.M., Pleshak E.N., Buckley J.T. Glycosylphosphatidylinositol anchors of membrane glycoproteins are binding determinants for the channel-forming toxin aerolysin. J. Biol. Chem. 1998;273:2355–2360. doi: 10.1074/jbc.273.4.2355. [DOI] [PubMed] [Google Scholar]
- 118.Zhang F., Schmidt W.G., Hou Y., Williams A.F., Jacobson K. Spontaneous incorporation of the glycosyl-phosphatidylinositol-linked protein Thy-1 into cell membranes. Proc. Natl. Acad. Sci. USA. 1992;89:5231–5235. doi: 10.1073/pnas.89.12.5231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hua M.-X., Chi Z., Liu G.-L., Buzdar M.A., Chi Z.-M. Production of a novel and cold-active killer toxin by Mrakia frigida 2E00797 isolated from sea sediment in Antarctica. Extremophiles. 2010;14:515–521. doi: 10.1007/s00792-010-0331-6. [DOI] [PubMed] [Google Scholar]
- 120.Wang X.-X., Chi Z., Peng Y., Wang X.-H., Ru S.-G., Chi Z.-M. Purification, characterization and gene cloning of the killer toxin produced by the marine-derived yeast Williopsis saturnus WC91–2. Microbiol. Res. 2012;167:558–563. doi: 10.1016/j.micres.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 121.De la Pena P., Barros F., Gascon S., Lazo P., Ramos S. Effect of yeast killer toxin on sensitive cells of Saccharomyces cerevisiae. J. Biol. Chem. 1981;256:10420–10425. [PubMed] [Google Scholar]
- 122.Martinac B., Zhu H., Kubalski A., Zhou X., Culbertson M., Bussey H., Kung C. Yeast K1 killer toxin forms ion channels in sensitive yeast spheroplasts and in artificial liposomes. Proc. Natl. Acad. Sci. USA. 1990;87:6228–6232. doi: 10.1073/pnas.87.16.6228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ahmed A., Sesti F., Ilan N., Shih T.M., Sturley S.L., Goldstein S.A. A molecular target for viral killer toxin: Tok1 potassium channels. Cell. 1999;99:283–291. doi: 10.1016/S0092-8674(00)81659-1. [DOI] [PubMed] [Google Scholar]
- 124.Kurzweilová H., Sigler K. Comparison of three different methods for determining yeast killer toxin K1 activity and standardisation of units. Experientia. 1995;51:26–28. [PubMed] [Google Scholar]
- 125.Kagan B.L. Mode of action of yeast killer toxins: Channel formation in lipid bilayer membranes. Nature. 1983;302:709–711. doi: 10.1038/302709a0. [DOI] [PubMed] [Google Scholar]
- 126.Kapteyn J., Ter Riet B., Vink E., Blad S., De Nobel H., Van Den Ende H., Klis F. Low external pH induces HOG1-dependent changes in the organization of the Saccharomyces cerevisiae cell wall. Mol. Microbiol. 2001;39:469–480. doi: 10.1046/j.1365-2958.2001.02242.x. [DOI] [PubMed] [Google Scholar]
- 127.García-Marqués S., Randez-Gil F., Prieto J.A. Nuclear versus cytosolic activity of the yeast Hog1 MAP kinase in response to osmotic and tunicamycin-induced ER stress. FEBS Lett. 2015;589:2163–2168. doi: 10.1016/j.febslet.2015.06.021. [DOI] [PubMed] [Google Scholar]
- 128.Nadal E., Posas F. Osmostress-induced gene expression—A model to understand how stress-activated protein kinases (SAPKs) regulate transcription. FEBS J. 2015;282:3275–3285. doi: 10.1111/febs.13323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Rep M., Krantz M., Thevelein J.M., Hohmann S. The transcriptional response of Saccharomyces cerevisiae to osmotic shock. Hot1p and Msn2p/Msn4p are required for the induction of subsets of high osmolarity glycerol pathway-dependent genes. J. Biol. Chem. 2000;275:8290–8300. doi: 10.1074/jbc.275.12.8290. [DOI] [PubMed] [Google Scholar]
- 130.Smith D.A., Nicholls S., Morgan B.A., Brown A.J., Quinn J. A conserved stress-activated protein kinase regulates a core stress response in the human pathogen Candida albicans. Mol. Biol. Cell. 2004;15:4179–4190. doi: 10.1091/mbc.E04-03-0181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Alonso-Monge R., Navarro-García F., Román E., Negredo A.I., Eisman B., Nombela C., Pla J. The Hog1 mitogen-activated protein kinase is essential in the oxidative stress response and chlamydospore formation in Candida albicans. Eukaryot. Cell. 2003;2:351–361. doi: 10.1128/EC.2.2.351-361.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Alonso-Monge R., Román E., Arana D.M., Prieto D., Urrialde V., Nombela C., Pla J. The Sko1 protein represses the yeast-to-hypha transition and regulates the oxidative stress response in Candida albicans. Fungal Genet. Biol. 2010;47:587–601. doi: 10.1016/j.fgb.2010.03.009. [DOI] [PubMed] [Google Scholar]
- 133.Enjalbert B., Smith D.A., Cornell M.J., Alam I., Nicholls S., Brown A.J., Quinn J. Role of the Hog1 stress-activated protein kinase in the global transcriptional response to stress in the fungal pathogen Candida albicans. Mol. Biol. Cell. 2006;17:1018–1032. doi: 10.1091/mbc.E05-06-0501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Pagé N., Gérard-Vincent M., Ménard P., Beaulieu M., Azuma M., Dijkgraaf G.J., Li H., Marcoux J., Nguyen T., Dowse T. A Saccharomyces cerevisiae genome-wide mutant screen for altered sensitivity to K1 killer toxin. Genetics. 2003;163:875–894. doi: 10.1093/genetics/163.3.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Schmitt M.J., Reiter J. Viral induced yeast apoptosis. Biochim. Biophys. Acta Mol. Cell. Res. 2008;1783:1413–1417. doi: 10.1016/j.bbamcr.2008.01.017. [DOI] [PubMed] [Google Scholar]
- 136.Somers J., Bevan E. The inheritance of the killer character in yeast. Genet. Res. 1969;13:71–83. doi: 10.1017/S0016672300002743. [DOI] [PubMed] [Google Scholar]
- 137.Cailliez J., Séguy N., Aliouat E., Polonelli L., Camus D., Del-Cas E. The yeast killer phenomenon: A hypothetical way to control Pneumocystis carinii pneumonia. Med. Hypotheses. 1994;43:167–171. doi: 10.1016/0306-9877(94)90147-3. [DOI] [PubMed] [Google Scholar]
- 138.Polonelli L., Conti S., Gerloni M., Magliani W., Castagnola M., Morace G., Chezzi C. Antibiobodies‘: Antibiotic-like anti-idiotypic antibodies. J. Med. Vet. Mycol. 1991;29:235–242. doi: 10.1080/02681219180000351. [DOI] [PubMed] [Google Scholar]
- 139.Séguy N., Cailliez J.-C., Polonelli L., Dei-Cas E., Camus D. Inhibitory effect of a Pichia anomala killer toxin on Pneumocystis carinii infectivity to the scid mouse. Parasitol. Res. 1996;82:114–116. doi: 10.1007/s004360050080. [DOI] [PubMed] [Google Scholar]
- 140.Javadekar V., SivaRaman H., Gokhale D. Industrial yeast strain improvement: Construction of a highly flocculent yeast with a killer character by protoplast fusion. J. Ind. Microbiol. 1995;15:94–102. doi: 10.1007/BF01569806. [DOI] [PubMed] [Google Scholar]
- 141.Palpacelli V., Ciani M., Rosini G. Activity of different ‘killer‘yeasts on strains of yeast species undesirable in the food industry. FEMS Microbiol. Lett. 1991;84:75–78. doi: 10.1111/j.1574-6968.1991.tb04572.x. [DOI] [PubMed] [Google Scholar]
- 142.Petering J., Symons M., Langridge P., Henschke P. Determination of killer yeast activity in fermenting grape juice by using a marked Saccharomyces wine yeast strain. Appl. Environ. Microbiol. 1991;57:3232–3236. doi: 10.1128/aem.57.11.3232-3236.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Seki T., Choi E.-H., Ryu D. Construction of killer wine yeast strain. Appl. Environ. Microbiol. 1985;49:1211–1215. doi: 10.1128/aem.49.5.1211-1215.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Kitamoto H., Hasebe A., Ohmomo S., Suto E., Muraki M., Iimura Y. Prevention of aerobic spoilage of maize silage by a genetically modified killer yeast, Kluyveromyces lactis, defective in the ability to grow on lactic acid. Appl. Environ. Microbiol. 1999;65:4697–4700. doi: 10.1128/aem.65.10.4697-4700.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kitamoto H.K., Ohmomo S., Nakahara T. Selection of killer yeasts (Kluyveromyces lactis) to prevent aerobic deterioration in silage making. J. Dairy res. 1993;76:803–811. doi: 10.3168/jds.S0022-0302(93)77404-4. [DOI] [PubMed] [Google Scholar]
- 146.Walker G.M., Mcleod A.H., Hodgson V.J. Interactions between killer yeasts and pathogenic fungi. FEMS Microbiol. Lett. 1995;127:213–222. doi: 10.1111/j.1574-6968.1995.tb07476.x. [DOI] [PubMed] [Google Scholar]
- 147.Yamamoto T., Uchida K., Hiratani T., Miyazaki T., Yagiu J., Yamaguchi H. In vitro activity of the killer toxin from yeast Hansenula mrakii against yeasts and molds. J. Antibiot. 1988;41:398–403. doi: 10.7164/antibiotics.41.398. [DOI] [PubMed] [Google Scholar]
- 148.Magliani W., Conti S., Gerloni M., Bertolotti D., Polonelli L. Yeast killer systems. Clin. Microbiol. Rev. 1997;10:369–400. doi: 10.1128/cmr.10.3.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Magliani W., Conti S., Salati A., Vaccari S., Ravanetti L., Maffei D.L., Polonelli L. Therapeutic potential of yeast killer toxin-like antibodies and mimotopes. FEMS Yeast Res. 2004;5:11–18. doi: 10.1016/j.femsyr.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 150.Theisen S., Molkenau E., Schmitt M.J. Wicaltin, a new protein toxin secreted by the yeast Williopsis californica and its broad-spectrum antimycotic potential. J. Microbiol. Biotechnol. Res. 2000;10:547–550. [Google Scholar]
- 151.Buzzini P., Corazzi L., Turchetti B., Buratta M., Martini A. Characterization of the in vitro antimycotic activity of a novel killer protein from Williopsis saturnus DBVPG 4561 against emerging pathogenic yeasts. FEMS Microbiol. Lett. 2004;238:359–365. doi: 10.1016/j.femsle.2004.07.060. [DOI] [PubMed] [Google Scholar]
- 152.Masih E., Slezack-Deschaumes S., Marmaras I., Barka E.A., Vernet G., Charpentier C., Adholeya A., Paul B. Characterisation of the yeast Pichia membranifaciens and its possible use in the biological control of Botrytis cinerea, causing the grey mould disease of grapevine. FEMS Microbiol. Lett. 2001;202:227–232. doi: 10.1111/j.1574-6968.2001.tb10808.x. [DOI] [PubMed] [Google Scholar]
- 153.Masih E.I., Paul B. Secretion of β-1,3-glucanases by the yeast Pichia membranifaciens and its possible role in the biocontrol of Botrytis cinerea causing grey mold disease of the grapevine. Curr. Microbiol. 2002;44:391–395. doi: 10.1007/s00284-001-0011-y. [DOI] [PubMed] [Google Scholar]
- 154.Santos A., Sanchez A., Marquina D. Yeasts as biological agents to control Botrytis cinerea. Microbiol. Res. 2004;159:331–338. doi: 10.1016/j.micres.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 155.Lutz M.C., Lopes C.A., Rodriguez M.E., Sosa M.C., Sangorrín M.P. Efficacy and putative mode of action of native and commercial antagonistic yeasts against postharvest pathogens of pear. Int J. Food Microbiol. 2013;164:166–172. doi: 10.1016/j.ijfoodmicro.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 156.Parafati L., Cirvilleri G., Restuccia C., Wisniewski M. Potential role of exoglucanase genes (WaEXG1 and WaEXG2) in the biocontrol activity of Wickerhamomyces anomalus. Microb. Ecol. 2016:1–9. doi: 10.1007/s00248-016-0887-5. [DOI] [PubMed] [Google Scholar]
- 157.Hua S.S.T., Beck J.J., Sarreal S.B.L., Gee W. The major volatile compound 2-phenylethanol from the biocontrol yeast, Pichia anomala, inhibits growth and expression of aflatoxin biosynthetic genes of Aspergillus flavus. Mycotoxin Res. 2014;30:71–78. doi: 10.1007/s12550-014-0189-z. [DOI] [PubMed] [Google Scholar]
- 158.Huang N., Enkegaard A., Osborne L.S., Ramakers P.M., Messelink G.J., Pijnakker J., Murphy G. The banker plant method in biological control. Crit. Rev. Plant Sci. 2011;30:259–278. doi: 10.1080/07352689.2011.572055. [DOI] [Google Scholar]
- 159.Huang X., Zhang N., Yong X., Yang X., Shen Q. Biocontrol of Rhizoctonia solani damping-off disease in cucumber with Bacillus pumilus SQR-N43. Microbiol. Res. 2012;167:135–143. doi: 10.1016/j.micres.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 160.Druvefors U.Ä., Schnürer J. Mold-inhibitory activity of different yeast species during airtight storage of wheat grain. FEMS Yeast Res. 2005;5:373–378. doi: 10.1016/j.femsyr.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 161.Lemos Junior W.J.F., Bovo B., Nadai C., Crosato G., Carlot M., Favaron F., Giacomini A., Corich V. Biocontrol ability and action mechanism of Starmerella bacillaris (synonym Candida zemplinina) isolated from wine musts against gray mold disease agent Botrytis cinerea on grape and their effects on alcoholic fermentation. Front. Microbiol. 2016;7:1249. doi: 10.3389/fmicb.2016.01499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Nally M., Pesce V., Maturano Y., Assaf L.R., Toro M., de Figueroa L.C., Vazquez F. Antifungal modes of action of Saccharomyces and other biocontrol yeasts against fungi isolated from sour and grey rots. Int. J. Food Microbiol. 2015;204:91–100. doi: 10.1016/j.ijfoodmicro.2015.03.024. [DOI] [PubMed] [Google Scholar]
- 163.Cao S., Yuan Y., Hu Z., Zheng Y. Combination of Pichia membranifaciens and ammonium molybdate for controlling blue mould caused by Penicillium expansum in peach fruit. Int. J. Food Microbiol. 2010;141:173–176. doi: 10.1016/j.ijfoodmicro.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 164.Cao S., Zheng Y., Tang S., Wang K. Improved control of anthracnose rot in loquat fruit by a combination treatment of Pichia membranifaciens with CaCl2. Int. J. Food Microbiol. 2008;126:216–220. doi: 10.1016/j.ijfoodmicro.2008.05.026. [DOI] [PubMed] [Google Scholar]
- 165.Niu X., Deng L., Zhou Y., Wang W., Yao S., Zeng K. Optimization of a protective medium for freeze-dried Pichia membranifaciens and application of this biocontrol agent on citrus fruit. J. Appl. Microbiol. 2016;121:234–243. doi: 10.1111/jam.13129. [DOI] [PubMed] [Google Scholar]
- 166.Carroll S.Y., Stirling P.C., Stimpson H.E., Gießelmann E., Schmitt M.J., Drubin D.G. A yeast killer toxin screen provides insights into A/B toxin entry, trafficking, and killing mechanisms. Dev. Cell. 2009;17:552–560. doi: 10.1016/j.devcel.2009.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Buzzini P., Martini A. Utilisation of differential killer toxin sensitivity patterns for fingerprinting and clustering yeast strains belonging to different genera. Syst. Appl. Microbiol. 2000;23:450–457. doi: 10.1016/S0723-2020(00)80077-6. [DOI] [PubMed] [Google Scholar]
- 168.Corte L., Lattanzi M., Buzzini P., Bolano A., Fatichenti F., Cardinali G. Use of RAPD and killer toxin sensitivity in Saccharomyces cerevisiae strain typing. J. Appl. Microbiol. 2005;99:609–617. doi: 10.1111/j.1365-2672.2005.02631.x. [DOI] [PubMed] [Google Scholar]
- 169.Boekhout T., Van Belkum A., Leenders A.C., Verbrugh H.A., Mukamurangwa P., Swinne D., Scheffers W.A. Molecular typing of Cryptococcus neoformans: Taxonomic and epidemiological aspects. Int. J. Syst. Evol. Microbiol. 1997;47:432–442. doi: 10.1099/00207713-47-2-432. [DOI] [PubMed] [Google Scholar]
- 170.Lopes C.A., Lavalle T.L., Querol A., Caballero A.C. Combined use of killer biotype and mtDNA-RFLP patterns in a patagonian wine Saccharomyces cerevisiae diversity study. Antonie Leeuwenhoek. 2006;89:147–156. doi: 10.1007/s10482-005-9017-y. [DOI] [PubMed] [Google Scholar]
- 171.Vaughan-Martini A., Cardinali G., Martini A. Differential killer sensitivity as a tool for fingerprinting wine-yeast strains of Saccharomyces cerevisiae. J. Ind. Microbiol. Biotechnol. 1996;17:124–127. doi: 10.1007/BF01570055. [DOI] [PubMed] [Google Scholar]
- 172.Provost F., Polonelli L., Conti S., Fisicaro P., Gerloni M., Boiron P. Use of yeast killer system to identify species of the Nocardia asteroides complex. J. Clin. Microbiol. 1995;33:8–10. doi: 10.1128/jcm.33.1.8-10.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Polonelli L., Conti S. Biotyping of Candida albicans and other fungi by yeast killer toxins sensitivity. Candida Albicans Methods Mol. Biol. 2009:97–115. doi: 10.1007/978-1-60327-151-6_11. [DOI] [PubMed] [Google Scholar]
- 174.Séguy N., Polonelli L., Dei-Cas E., Cailliez J.-C. Effect of a killer toxin of Pichia anomala to pneumocystis. Perspectives in the control of pneumocystosis. FEMS Immunol. Med. Microbiol. 1998;22:145–149. doi: 10.1016/S0928-8244(98)00072-8. [DOI] [PubMed] [Google Scholar]
- 175.Fuentefria A.M., Franskoviaki I.M., Mercado L.W., Ramos J.P., Vainstein M.H., Valente P. Inhibition of clinical and environmental Cryptococcus neoformans isolates by two brazilian killer yeasts. J. Basic Microbiol. 2006;46:87–93. doi: 10.1002/jobm.200510018. [DOI] [PubMed] [Google Scholar]
- 176.Keszthelyi A. Characterisation of anticryptococcal Fc-1 toxin of Filobasidium capsuligenum. Acta Biol. Szeged. 2006;50:155. doi: 10.1111/j.1439-0507.2006.01227.x. [DOI] [PubMed] [Google Scholar]
- 177.Cenci E., Mencacci A., Spreca A., Montagnoli C., Bacci A., Perruccio K., Velardi A., Magliani W., Conti S., Polonelli L. Protection of killer antiidiotypic antibodies against early invasive aspergillosis in a murine model of allogeneic T-cell-depleted bone marrow transplantation. Infect. Immun. 2002;70:2375–2382. doi: 10.1128/IAI.70.5.2375-2382.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Guyard C., Séguy N., Cailliez J.-C., Drobecq H., Polonelli L., Dei-Cas E., Mercenier A., Menozzi F.D. Characterization of a Williopsis saturnus var. mrakii high molecular weight secreted killer toxin with broad-spectrum antimicrobial activity. J. Antimicrob. Chemother. 2002;49:961–971. doi: 10.1093/jac/dkf040. [DOI] [PubMed] [Google Scholar]
- 179.Buzdar M.A., Chi Z., Wang Q., Hua M.-X., Chi Z.-M. Production, purification, and characterization of a novel killer toxin from Kluyveromyces siamensis against a pathogenic yeast in crab. Appl. Microbiol. Biotechnol. 2011;91:1571–1579. doi: 10.1007/s00253-011-3220-8. [DOI] [PubMed] [Google Scholar]
- 180.Pérez M.A., Gallego F.J., Hidalgo P. Evaluation of molecular techniques for the genetic characterization of Saccharomyces cerevisiae strains. FEMS Microbiol. Lett. 2001;205:375–378. doi: 10.1111/j.1574-6968.2001.tb10975.x. [DOI] [PubMed] [Google Scholar]
- 181.Velázquez R., Zamora E., Álvarez M., Álvarez M.L., Ramírez M. Using mixed inocula of Saccharomyces cerevisiae killer strains to improve the quality of traditional sparkling-wine. Food Microbiol. 2016;59:150–160. doi: 10.1016/j.fm.2016.06.006. [DOI] [PubMed] [Google Scholar]
- 182.Ciani M., Fatichenti F. Killer toxin of Kluyveromyces phaffii DBVPG 6076 as a biopreservative agent to control apiculate wine yeasts. Appl. Environ. Microbiol. 2001;67:3058–3063. doi: 10.1128/AEM.67.7.3058-3063.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Liu S.-Q., Tsao M. Inhibition of spoilage yeasts in cheese by killer yeast Williopsis saturnus var. saturnus . Int. J. Food Microbiol. 2009;131:280–282. doi: 10.1016/j.ijfoodmicro.2009.03.009. [DOI] [PubMed] [Google Scholar]
- 184.Oro L., Ciani M., Bizzaro D., Comitini F. Evaluation of damage induced by KwKT and PiKT zymocins against Brettanomyces/Dekkera spoilage yeast, as compared to sulphur dioxide. J. Appl. Microbiol. 2016;121:207–214. doi: 10.1111/jam.13121. [DOI] [PubMed] [Google Scholar]
- 185.Mehlomakulu N., Prior K., Setati M., Divol B. Candida pyralidae killer toxin disrupts the cell wall of Brettanomyces bruxellensis in red grape juice. J. Appl. Microbiol. 2016;121:207–214. doi: 10.1111/jam.13383. [DOI] [PubMed] [Google Scholar]
- 186.Alonso A., Belda I., Santos A., Navascués E., Marquina D. Advances in the control of the spoilage caused by Zygosaccharomyces species on sweet wines and concentrated grape musts. Food Control. 2015;51:129–134. doi: 10.1016/j.foodcont.2014.11.019. [DOI] [Google Scholar]
- 187.Todd B.E., Fleet G.H., Henschke P.A. Promotion of autolysis through the interaction of killer and sensitive yeasts: Potential application in sparkling wine production. Am. J. Enol. Vitic. 2000;51:65–72. [Google Scholar]
- 188.Satora P., Tarko T., Sroka P., Blaszczyk U. The influence of Wickerhamomyces anomalus killer yeast on the fermentation and chemical composition of apple wines. FEMS Yeast Res. 2014;14:729–740. doi: 10.1111/1567-1364.12159. [DOI] [PubMed] [Google Scholar]
- 189.Yap N., de Barros Lopes M., Langridge P., Henschke P. The incidence of killer activity of non-Saccharomyces yeasts towards indigenous yeast species of grape must: Potential application in wine fermentation. J. Appl. Microbiol. 2000;89:381–389. doi: 10.1046/j.1365-2672.2000.01124.x. [DOI] [PubMed] [Google Scholar]
- 190.Oro L., Ciani M., Comitini F. Antimicrobial activity of Metschnikowia pulcherrima on wine yeasts. J. Appl. Microbiol. 2014;116:1209–1217. doi: 10.1111/jam.12446. [DOI] [PubMed] [Google Scholar]
- 191.Steensels J., Snoek T., Meersman E., Nicolino M.P., Voordeckers K., Verstrepen K.J. Improving industrial yeast strains: Exploiting natural and artificial diversity. FEMS Microbiol. Rev. 2014;38:947–995. doi: 10.1111/1574-6976.12073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Polonelli L., Magliani W., Ciociola T., Giovati L., Conti S. From Pichia anomala killer toxin through killer antibodies to killer peptides for a comprehensive anti-infective strategy. Antonie. Leeuwenhoek. 2011;99:35–41. doi: 10.1007/s10482-010-9496-3. [DOI] [PubMed] [Google Scholar]