Abstract
Background
Near‐sightedness, or myopia, is a condition in which light rays entering the eye along the visual axis focus in front of the retina, resulting in blurred vision. Myopia can be treated with spectacles, contact lenses, or refractive surgery. Options for refractive surgery include laser‐assisted subepithelial keratectomy (LASEK) and laser‐assisted in‐situ keratomileusis (LASIK). Both procedures utilize a laser to shape the corneal tissue (front of the eye) to correct refractive error, and both create flaps before laser treatment of corneal stromal tissue. Whereas the flap in LASEK is more superficial and epithelial, in LASIK it is thicker and also includes some anterior stromal tissue. LASEK is considered a surface ablation procedure, much like its predecessor, photorefractive keratectomy (PRK). LASEK was developed as an alternative to PRK to address the issue of pain associated with epithelial debridement used for PRK. Assessing the relative benefits and risks/side effects of LASEK and LASIK warrants a systematic review.
Objectives
To assess the effects of LASEK versus LASIK for correcting myopia.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL), which contains the Cochrane Eyes and Vision Trials Register (2016, Issue 10); MEDLINE Ovid (1946 to 24 October 2016); Embase.com (1947 to 24 October 2016); PubMed (1948 to 24 October 2016); LILACS (Latin American and Caribbean Health Sciences Literature Database; 1982 to 24 October 2016); the metaRegister of Controlled Trials (mRCT) (www.controlled‐trials.com), last searched 20 June 2014; ClinicalTrials.gov (www.clinicaltrials.gov); searched 24 October 2016; and the WHO International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp/search/en); searched 24 October 2016. We did not use any date or language restrictions in the electronic searches for trials.
Selection criteria
We considered only randomized controlled trials (RCTs) for the purposes of this review. Eligible RCTs were those in which myopic participants were assigned randomly to receive either LASEK or LASIK in one or both eyes. We also included paired‐eye studies in which investigators randomly selected which of the participant's eyes would receive LASEK or LASIK and assigned the other eye to the other procedure. Participants were men or women between the ages of 18 and 60 years with myopia up to 12 diopters (D) and/or myopic astigmatism of severity up to 3 D, who did not have a history of prior refractive surgery.
Data collection and analysis
Two review authors independently screened all reports and assessed the risk of bias in trials included in this review. We extracted data and summarized findings using risk ratios (RRs) for dichotomous outcomes and mean differences (MDs) for continuous outcomes. In the absence of clinical and methodological heterogeneity across trials, we used a random‐effects model to calculate summary effect estimates. We used a fixed‐effect model when including fewer than three trials in a meta‐analysis. When clinical, methodological, or statistical heterogeneity was observed across trials, we reported our findings in a narrative synthesis.
Main results
We identified four eligible trials with 538 eyes of 392 participants for the review, but only three trials (154 participants) provided outcome data for analysis. We found no ongoing trials. Two of four trials were from China, one trial was from Turkey, and the location of one trial was not reported. The risk of bias for most domains was unclear due to poor reporting of trial methods; no trial had a protocol or trial registry record. Three trials enrolled participants with mild to moderate myopia (less than −6.50 D); one trial included only participants with severe myopia (more than −6.00 D).
The evidence showed uncertainty in whether there is a difference between LASEK and LASIK in uncorrected visual acuity (UCVA) at 12 months, the primary outcome in our review. The RR and 95% confidence interval (CI) at 12 months after surgery was 0.96 (95% CI 0.82 to 1.13) for UCVA of 20/20 or better and 0.90 (95% CI 0.67 to 1.21) for UCVA of 20/40 or better based on data from one trial with 57 eyes (very low‐certainty evidence). People receiving LASEK were less likely to achieve a refractive error within 0.5 diopters of the target at 12 months follow‐up (RR 0.69, 95% CI 0.48 to 0.99; 57 eyes; very low‐certainty evidence). One trial reported mild corneal haze at six months in one eye in the LASEK group and none in the LASIK group (RR 2.11, 95% CI 0.57 to 7.82; 76 eyes; very low‐certainty evidence). None of the included trials reported postoperative pain score or loss of visual acuity, spherical equivalent of the refractive error, or quality of life at 12 months.
Refractive regression, an adverse event, was reported only in the LASEK group (8 of 37 eyes) compared with none of 39 eyes in the LASIK group in one trial (low‐certainty evidence). Other adverse events, such as corneal flap striae and refractive over‐correction, were reported only in the LASIK group (5 of 39 eyes) compared with none of 37 eyes in the LASEK group in one trial (low‐certainty evidence).
Authors' conclusions
Overall, from the available RCTs, there is uncertainty in how LASEK compares with LASIK in achieving better refractive and visual results in mildly to moderately myopic participants. Large, well‐designed RCTs would be required to estimate the magnitude of any difference in efficacy or adverse effects between LASEK and LASIK for treating myopia or myopic astigmatism.
Keywords: Adult; Female; Humans; Male; Middle Aged; Keratectomy, Subepithelial, Laser‐Assisted; Keratectomy, Subepithelial, Laser‐Assisted/adverse effects; Keratectomy, Subepithelial, Laser‐Assisted/methods; Keratomileusis, Laser In Situ; Keratomileusis, Laser In Situ/adverse effects; Keratomileusis, Laser In Situ/methods; Myopia; Myopia/surgery; Quality of Life; Randomized Controlled Trials as Topic; Visual Acuity
Plain language summary
Two different surgeries to treat near‐sightedness
What is the aim of this review? The aim of this Cochrane Review was to find out whether laser‐assisted subepithelial keratectomy (LASEK) surgery is better than laser‐assisted in‐situ keratomileusis (LASIK) surgery for treating near‐sightedness (myopia). Cochrane researchers collected and analyzed all relevant studies to answer this question and found four studies.
Key messages It is uncertain whether LASEK or LASIK is better for the treatment of myopia.
What did this review study? Near‐sightedness (known as myopia) is a condition in which it is difficult to see objects in the distance clearly. Myopia is the most common type of refractive error (inaccurate focusing of light on the retina of the eye) worldwide. Myopia can be treated with spectacles or contact lenses. Surgical correction of myopia includes refractive surgery such as laser‐assisted subepithelial keratectomy (LASEK) and laser‐assisted in‐situ keratomileusis (LASIK). Both procedures use a laser to shape the cornea (front part of the eye) to remove refractive error and provide clear vision without spectacles or contact lenses.
What are the main results of the review? Cochrane researchers found four relevant studies. Two of these four studies were from China, one study was from Turkey and for one study it was unclear where it was from. The people taking part in the studies were men and women between the ages of 18 and 60 years with mild to moderate myopia.
These studies provide only very low‐certainty evidence comparing LASEK and LASIK. It is unclear if either of these two methods are better for vision, or quality of life. There was no information on how painful the procedures were. There was some limited information on harmful effects. Serious problems appeared to be rare. In one study, more people in the LASEK group had refractive regression (return of the myopia) and more people in the LASIK group had over‐correction (shift from near‐sighted to far‐sighted).
How up‐to‐date is this review? Cochrane researchers searched for studies that had been published up to 24 October 2016.
Summary of findings
for the main comparison.
| Laser‐assisted subepithelial keratectomy (LASEK) compared with laser‐assisted in‐situ keratomileusis (LASIK) for correcting myopia | ||||||
|
Population: participants aged 18‐60 years with myopia ranging in severity up to 12 diopters (D) Settings: eye clinics Intervention: LASEK Comparison: LASIK | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of eyes (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| LASIK | LASEK | |||||
|
Uncorrected visual acuity (UCVA) of 20/20 or better follow‐up: 1 year |
931 per 1000* | 894 per 1000 (763 to 1000) | RR 0.96 (0.82 to 1.13) | 57 (1 study) | ⊕⊝⊝⊝ Very lowa,b,c | — |
|
Uncorrected visual acuity (UCVA) of 20/40 or better follow‐up: 1 year |
793 per 1,000 |
714 per 1,000 (531 to 960) |
RR 0.90 (0.67 to 1.21) | 57 (1 study) |
⊕⊝⊝⊝ Very lowa,b,c | — |
|
Proportion of participants who lost two or more lines of best‐corrected visual acuity (BCVA) follow‐up: 1 year |
Not reported | — | ||||
|
Eyes within ± 0.5 D of target refraction follow‐up: 1 year |
828 per 1000 | 571 per 1,000 (397 to 819) | RR 0.69 (0.48 to 0.99) | 57 (1 study) | ⊕⊝⊝⊝ Very lowa,b,c | — |
|
Postoperative corneal haze follow‐up: 1 year |
77 per 1000 | 162 per 1,000 (44 to 602) | RR 2.11 (0.57 to 7.82) | 76 (1 study) | ⊕⊝⊝⊝ Very lowa,b,c | — |
| Postoperative pain score: 1 year | Not reported | — | ||||
| Adverse events (flap‐complications) | — | — | — | — | ⊕⊕⊝⊝ Lowb,c | Study investigators of Gui 2008 reported no unusual complications or adverse events, while study investigators of Al‐Fayez 2008 reported a lower complication rate in eyes in the LASIK group compared with LASEK group. Al‐Fayez 2008 and Gui 2008 did not specify which adverse events they collected. Study investigators of He 2006 reported that there were no severe complications that affected the participants' visual acuity. They did report 5 of 39 eyes in the LASIK group compared with none of the eyes in the LASEK group experienced corneal flap striae. Likewise, 5 of 39 eyes in the LASIK group compared with none of the eyes in the LASEK group had refractive over‐correction. None of the participants in the LASIK group compared with 8 of 37 eyes in the LASEK group had refractive regression. Since adverse events only occurred in one group and not the other, we could not estimate the relative effects. One trial reported excluding 3 of 32 eyes in the LASEK group due to flap complications during epithelial flap preparation, resulting in damage to the epithelium (Kaya 2004). |
| *The basis for the assumed risk is the comparison group risk. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). The assumed risk was calculated using the following formula: (number of events/number of eyes in the LASIK group) × 1000. CI: confidence interval; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High‐certainty: further research is very unlikely to change our confidence in the estimate of effect. Moderate‐certainty: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low‐certainty: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low‐certainty: we are very uncertain about the estimate. | ||||||
aDowngraded for imprecision (wide confidence interval). bDowngraded for high risk of attrition bias (38% of eyes in the LASIK group and 56% of eyes in the LASEK group). cDowngraded for indirectness of evidence (all participants with severe myopia of greater than −6.00 D).
Background
Description of the condition
Myopia, commonly known as near‐sightedness, is the most common type of refractive error (Ang 2009). Refractive error is the inaccurate focusing of light by the eye, and it requires an optical correction to obtain clear, focused vision. With myopia, incoming light rays from objects are focused in front of the retina, the light sensitive part of the inner eye, resulting in blurred distant vision. The public health impact of treating myopia and other refractive errors is significant, with estimated costs ranging from USD 3.9 billion to USD 7.2 billion per year in the United States alone to correct distance visual impairment (Vitale 2006). The prevalence of myopia varies widely in different populations. In urban areas of developed Asian countries, the prevalence of myopia among children completing high school is 80% to 90%, which is one of the highest reported prevalence rates internationally (Morgan 2012). A large, population‐based study of over 3000 children in California recently demonstrated the prevalence of myopia among non‐Hispanic white children to be 1.2% compared to 4% among Asian children (Wen 2013).
Myopia and other refractive errors can be corrected through various methods. Non‐surgical correction involves spectacles or contact lenses. More permanent surgical interventions are offered when a person becomes intolerant of contact lenses, encounters visual aberration from high‐powered spectacles, or refuses to wear spectacles because of cosmetic concerns. Surgical interventions can be divided into procedures that alter the cornea (the clear outer surface of the front of the eye) and procedures that affect the natural lens (a transparent, biconvex structure that sits inside the eye behind the pupil). Corneal procedures include laser vision correction (LVC), incisional surgeries, and tissue or synthetic implants. All corneal procedures work to correct refractive error by altering the shape of the cornea. LVC includes laser in situ keratomileusis (LASIK), photorefractive keratectomy (PRK), laser assisted subepithelial keratectomy (LASEK) and epi‐LASIK. Incisional corneal procedures include radial and astigmatic keratotomies, in which a surgical blade makes incisions (cuts) into the cornea. Tissue implants are used with epikeratophakia and keratophakia, where human donor corneal tissue is transplanted onto a patient's native cornea to correct refractive error. Synthetic implants, such as intracorneal rings, can also be inserted into the cornea to alter its shape and thereby correct refractive error. Interventions that affect the natural lens include refractive lens exchange and phakic intraocular lens implantation. With refractive lens exchange, the natural lens is removed and may or may not be replaced with an artificial intraocular lens implant. With phakic intraocular lens implantation, an artificial intraocular lens is implanted over the natural lens. Recently, a new method of refractive correction involving the removal of a small lenticule (disk) of corneal tissue has also been used to treat myopia and myopic astigmatism (Shah 2011).
Description of the intervention
LVC is currently the most common surgical intervention used to correct myopia (Sandoval 2005). LVC gained popularity because it usually eliminates the need for spectacles or contact lens use, at least for a few years or decades. Prior to its use as a tool in refractive surgery, the excimer laser was first used to remove microscopic amounts of material from the surface of silicone microchips (Basting 2001). The excimer laser utilizes an argon‐fluoride gas mixture passed through high‐voltage electricity in a laser chamber to produce high‐energy light, resulting in the emission of discrete ultraviolet pulses that break corneal molecular bonds and the subsequent vaporization of tissue fragments in the corneal stroma (Manche 1998). Both LASIK and LASEK utilize the excimer laser to correct refractive error.
How the intervention might work
LASIK was first performed in a human eye in 1991 (Pallikaris 1991). First, a microkeratome (an oscillating mechanical blade) or a femtosecond laser is used to create a stromal flap, which includes the outermost layers of the cornea, namely, the epithelium, Bowman's membrane, and anterior stroma. The flap is reflected back and the excimer laser is used to remove tissue from the exposed stromal bed, thus reshaping the posterior corneal bed before the flap is replaced (Bower 2001). The alternatives to LASIK are various surface ablation techniques, which include PRK, LASEK, and epi‐LASIK. Surface ablation does not involve the creation of a stromal flap; it may be recommended over LASIK for patients who have lifestyles that predispose them to head or facial trauma (e.g. athletes, police officers), which could result in a traumatic flap dislocation. Also, the creation of a stromal flap during the LASIK procedure leaves less residual stromal bed than surface ablation and can predispose the development of ectasia (Spadea 2012). Ectasia is a thinning disorder of the cornea that causes excessive loss of the innate mechanical and structural strength of the cornea, leading to refractive instability, irregular astigmatism, and recurrence of myopia. Surface ablation is often preferable to LASIK in patients with thin corneas. Additionally, the transient but significant rise in intraocular pressure (IOP) upon microkeratome application during LASIK is a preoperative consideration in people who have or are at risk of glaucoma (Shrivastava 2011). A large rise in IOP also can put susceptible patients at risk for retinal vascular occlusions (Bashford 2005).
LASEK is a modification of PRK. Whereas in PRK the epithelium is intentionally debrided (i.e. it is simply scraped off), in LASEK a thin epithelial flap is created, so there is a more controlled removal of the epithelium at a fixed depth and diameter. In LASEK, alcohol is used to loosen the epithelium and lift the epithelial flap; alcohol is not used in PRK. The mechanical removal of the epithelium is believed to create nicks in Bowman's membrane and to leave some residual epithelium, so using alcohol to loosen the epithelium leaves a smooth Bowman's membrane surface (Campos 1992). Hence, many authors believe that LASEK minimizes pain and haze formation compared with PRK (Hayashida 2006; Shahinian 2002), and since the epithelial flap created during the LASEK procedure does not invade the stroma, the cornea's biomechanical stability is maintained (Medeiros 2011).
Why it is important to do this review
Cochrane Eyes and Vision published a systematic review that compared LASIK with PRK for the correction of myopia in 2006, as well as a subsequent update in 2013 (Shortt 2006; Shortt 2013). Shortt and colleagues concluded that LASIK provided faster visual recovery and less postoperative pain than PRK. Although visual outcomes and safety at one year after surgery were similar between the two techniques, there was a risk of flap‐related complications in LASIK patients, including flap displacement, lamellar keratitis (an inflammation that occurs in the interface between the corneal flap and underlying cornea stroma), flap melt, and epithelial ingrowth (the migration and growth of surface corneal epithelial cells into the flap interface) (Duffey 2003; Knorz 2002; Sridhar 2002). Additionally, studies have reported keratectasia (pathologic thinning of the cornea) to be more frequent following LASIK versus LASEK or PRK (Holland 2000).
Although PRK has been shown to have equivalent long‐term outcomes as LASIK, it is plagued by long postoperative recovery, postoperative pain and corneal haze formation (Shortt 2013). LASEK, as a modification of PRK, has the potential to address some of these disadvantages while avoiding stromal flap‐related complications, decreasing the risk of keratectasia, and eliminating the need for significant IOP elevation from microkeratome application. In addition, reoperations (enhancements) for regressed refractive error are less technically challenging with surface ablation since there is no flap to lift, so it is possible to avoid flap‐related complications that can arise with flap manipulation. This review will focus on comparing LASIK with LASEK as an alternative to PRK.
Objectives
To assess the effects of LASEK versus LASIK for correcting myopia.
Methods
Criteria for considering studies for this review
Types of studies
We considered only randomized controlled trials (RCTs) for the purposes of this review. Eligible RCTs were those in which participants were assigned randomly to receive either LASEK or LASIK in one or both eyes. We included paired‐eye studies in which investigators randomly selected which of the participant's eyes would receive LASEK or LASIK, with the other eye assigned to the other procedure.
Types of participants
We included studies that enrolled men and women between the ages of 18 and 60 years with myopia up to 12 diopters (D) and/or myopic astigmatism of severity up to 3 D. We did not include studies in participants who had prior refractive surgery. We did not restrict studies based on the ethnicity of participants or the region where trials took place.
Types of interventions
For the purposes of this review we focused on the head‐to‐head comparison (i.e. comparative effectiveness) of LASEK and LASIK as described above. We did not include studies that had compared LASEK or LASIK with epi‐LASIK or another refractive procedure.
Types of outcome measures
Primary outcomes
The primary outcomes for comparison of treatments was the proportion of eyes with postoperative uncorrected visual acuity (UCVA) of 20/20 or better 12 months after surgery. We analyzed visual acuity using LogMAR by converting measurements made using Snellen or other charts to equivalent LogMAR values.
Secondary outcomes
The secondary outcomes for comparison of treatments included the following.
Proportion of eyes within 0.5 D under or over target refraction 12 months after surgery.
Proportion of participants who lost two or more lines of best‐corrected visual acuity (BCVA) at six months or more of follow‐up.
Proportion of eyes with UCVA of 20/20 or better at 1, 3, and 6 months after surgery and the proportion of eyes with UCVA 20/40 or better at 1, 3, 6, and 12 months after surgery.
Mean spherical equivalent of the refractive error.
Proportion of eyes that had postoperative corneal haze at 6 and 12 months.
Pain scores (intraoperative and postoperative) assessed by validated instruments as reported by included trials at one day postoperatively.
Quality of life measures as reported using a standardized tool such as the Refractive Status and Vision Profile (RSVP), National Eye Institute Refractive Quality of Life (NEI‐RQL), or other validated questionnaire, at 6 and 12 months. For this outcome, we planned to use data only from participants who had the same procedure (LASIK or LASEK) in both eyes or in only one eye with the contralateral eye not treated.
Adverse events
Only participants who underwent LASIK were at risk of having stroma flap complication. Thus, we recorded the number of flap complications, such as stroma flap displacement, lamellar keratitis, flap melt, or epithelial ingrowth reported in eyes treated with LASIK. Both LASEK‐ and LASIK‐treated eyes were at risk of keratectasia and dry eye; thus, we reported the number of eyes with keratectasia and dry eye for each respective LASEK and LASIK groups. We recorded all adverse events at 6 and 12 months, or at the longest reported follow‐up in the included studies.
Search methods for identification of studies
Electronic searches
The Cochrane Eyes and Vision Information Specialist conducted systematic searches in the following databases for randomized controlled trials and controlled clinical trials. There were no language or publication year restrictions. The date of the search was 24 October 2016.
Cochrane Central Register of Controlled Trials (CENTRAL; 2016, Issue 10), which contains the Cochrane Eyes and Vision Trials Register in the Cochrane Library (searched 30 November 2016) (Appendix 1).
MEDLINE Ovid (1946 to 24 October 2016) (Appendix 2).
Embase.com (1947 to 24 October 2016) (Appendix 3).
PubMed (1948 to 24 October 2016) (Appendix 4).
LILACS (Latin American and Caribbean Health Science Information database (1982 to 24 October 2016) (Appendix 5).
metaRegister of Controlled Trials (mRCT) (www.controlled‐trials.com; last searched 20 June 2014) (Appendix 6).
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (www.clinicaltrials.gov; searched 24 October 2016) (Appendix 7).
World Health Organization International Clinical Trials Registry Platform (www.who.int/ictrp; searched 24 October 2016) (Appendix 8).
Searching other resources
We reviewed the reference list of included trials and used the Web of Science Citation Index‐Expanded database to search for studies that have referenced the included trials. For the specific purposes of this review, we did not handsearch journals, conference proceedings, or abstracts.
Data collection and analysis
Selection of studies
Two review authors independently screened titles and abstracts, classifying each record as 'definitely relevant', 'possibly relevant', or 'definitely not relevant'. We resolved disagreements by discussion. After reaching a consensus, we retrieved full‐text reports for records classified as 'definitely relevant' or 'possibly relevant' by both review authors. JK and AC independently assessed each full‐text report against eligibility criteria for inclusion in the review and labeled them as 'include' or 'exclude'. We documented assessments and reasons for articles labeled as 'exclude' in the 'Characteristics of excluded studies' table. We resolved discrepancies through discussion. A third review author (RC) made the final judgment whenever the original pair could not reach a consensus. For missing or unclear information, we contacted the study investigators. If they did not respond within two weeks, we used the available data to assess the eligibility of the study.
Data extraction and management
Two authors independently extracted data from the included trials using forms developed by Cochrane Eyes and Vision. We extracted the following data from the included trials: pre‐defined treatment outcomes (see Types of outcome measures), methodological characteristics of the trial, and characteristics of the trial participants. We resolved discrepancies through discussion or consultation with a third review author. We contacted study investigators for clarification of trial details and for missing outcome data. Whenever we did not receive a response from trial investigators within three weeks of our request, we proceeded with the available data. One review author entered data into Review Manager 5 (RevMan 2014), and two review authors verified the data entered.
Assessment of risk of bias in included studies
Two review authors assessed the risk of bias in the included studies according to the domain‐based 'Risk of bias' tool described in chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a). We assessed selection bias through the method of random sequence generation and allocation concealment before participant randomization; performance bias through masking (blinding) of study participants and personnel; detection bias through masking of those assessing outcomes; attrition bias by amount of incomplete outcome data; and reporting bias through selective outcome reporting. We also documented other sources of bias. We judged each domain as being at 'low risk', 'high risk' or 'unclear risk' of bias and provided documentation from trial reports to support our judgments. We resolved disagreements through discussion. For missing or unclear information (e.g. allocation methods or use of masking not reported), we contacted the study investigators. When they did not respond within two weeks, we used the available data.
Measures of treatment effect
To estimate the relative effects of the two interventions, we calculated risk ratios (RRs) and the corresponding 95% confidence interval (CI) for dichotomous outcomes (UCVA, BCVA, and target refraction) above and below cut points. We calculated the mean difference (MD) and the corresponding 95% CI of the mean change from baseline at follow‐up time points for continuous measures (mean spherical equivalent and pain scores).
Unit of analysis issues
The eye was the unit of analysis for this review, as two trials randomized each eye of a participant to a separate intervention (i.e. paired‐eye design). Neither paired‐eye trial accounted for the non‐independence of eyes in the analysis and separately analyzed both eyes of a participant as independent units of analysis (Al‐Fayez 2008; Kaya 2004).
Dealing with missing data
We contacted study investigators to clarify descriptions of their study for "Selection of studies" and to assess "Risk of bias in included studies." There were no missing summary data, so we did not contact study investigators for data describing treatment effect estimates (means and proportions) or corresponding variance estimates (standard deviation, standard error, and 95% CIs). Also, we did not impute missing participant data for analysis (Higgins 2011b).
Assessment of heterogeneity
We assessed clinical and methodological heterogeneity of included trials by examining variations in the trial designs and methods, characteristics of the trial participants, interventions (preoperative and postoperative care), and length of follow‐up. We assessed statistical heterogeneity in the reported treatment effect estimates of included trials by examining the overlap of the 95% CIs of individual trials in the forest plots and I2 values. We considered poor overlap in the 95% CIs and an I2 > 50% as indications of substantial statistical heterogeneity.
Assessment of reporting biases
We did not assess publication bias through visual inspection of funnel plots, as there were only four included studies in this review, and meta‐analyses included data from no more than two studies.
Data synthesis
We used a fixed‐effect model, as fewer than three trials contributed to all meta‐analyses. When only one trial reported the review outcome, we reported its findings in a narrative synthesis. If more trials are included in future updates of this review, we plan to report the results as follows.
In the absence of clinical and methodological heterogeneity across trials, we planned to use a random‐effects model to calculate summary of effect estimates.
In the case of clinical, methodological, or statistical heterogeneity, we planned to report our findings in a narrative synthesis.
Subgroup analysis and investigation of heterogeneity
We did not carry out any subgroup analysis due to insufficient or no data. In future updates of the review, when sufficient data are available, we will stratify our analyses based on the degree of myopia in trial participants (mild: < −3.0 D; moderate: −3.0 to −6.0 D; high: > −6.0 D) or the use of intraoperative mitomycin C (MMC) during the LASEK procedure.
Sensitivity analysis
We did not carry out any sensitivity analysis due to an insufficient number of studies in meta‐analysis or no data.
Summary of findings table
We prepared a 'Summary of findings table', which includes relative and absolute risks based on the risks across intervention groups in the included studies. In the table, we present the primary outcome, five secondary outcomes listed in the "Secondary outcomes" section, and adverse events at one year follow‐up. Two authors independently graded the overall certainty of the evidence for all outcomes in this review using the GRADE classification (GRADEpro 2014).
Results
Description of studies
Results of the search
Our search of bibliographic databases and clinical trial registers on 24 October 2016 yielded 3151 records. After removing duplicates, there were 2361 unique records (Figure 1). We searched the Web of Science Citation Index‐Expanded database on 22 November 2016, but none of the cited studies met the review's inclusion criteria. Of 25 full‐text reports assessed for eligibility, we excluded 21 studies and included four trials. We identified no ongoing trials and none of the included trials had been registered.
1.
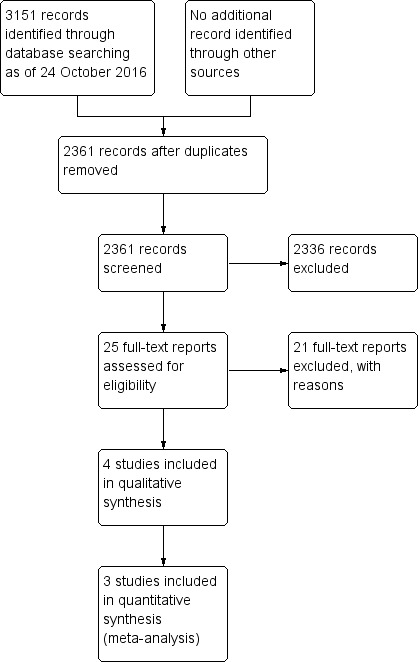
Study flow diagram.
Included studies
We included four trials with a total of 392 participants and 538 eyes with myopia (Al‐Fayez 2008; Gui 2008; He 2006; Kaya 2004). However, investigators of one trial did not report usable outcome data (Al‐Fayez 2008). Each trial included between 32 and 238 participants. These trials took place in China and Turkey (the trial country for Al‐Fayez 2008 was not clear). Preoperative refraction ranged from −1.25 diopters (D) to −10.75 D (note that Gui 2008 reported the range as "low to moderate myopia less than −6 diopters" but did not specify further). The follow‐up ranged from one day to 90 months.
Excluded studies
We excluded 21 studies after reviewing full‐text reports and available abstracts: 18 studies were not RCTs, and 3 studies included participants not eligible for the review. We have provided detailed reasons for exclusions of each trial in the 'Characteristics of included studies' table.
Risk of bias in included studies
We included a graphical display of the risk of bias in included studies (Figure 2).
2.
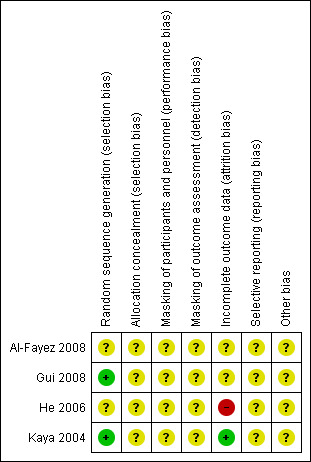
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
There was an unclear risk of selection bias for all four included trials, as the authors did not describe how they concealed allocation from participants before randomization.
Masking (performance bias and detection bias)
There was an unclear risk of performance bias, as none of the included trials described how they masked participants and personnel. There was also an unclear risk of detection bias, as none of the included trials described how they masked the outcome assessment.
Incomplete outcome data
There was an unclear risk of incomplete outcome data for two of the four included trials (Al‐Fayez 2008; Gui 2008). We graded Kaya 2004 as being at low risk of bias, as authors reported outcome data for all randomized participants. He 2006 was at high risk of bias since there was no mention of incomplete data or loss to follow‐up in the study. Outcomes were reported at 12 months for significantly fewer participants than the number enrolled. In addition, in the trial the loss to follow‐up rates differed between treatment groups; 56% of eyes in the LASEK group versus 38% of eyes in the LASIK group were lost to follow‐up.
Selective reporting
We considered the risk of reporting bias to be unclear for all four included trials. Al‐Fayez 2008 did not record enough information to assess for selective reporting. Gui 2008 and He 2006 were not registered trials, and the study protocols were not available, For Kaya 2004, neither the trial protocol nor trial registry information regarding outcomes was available for comparison with the trial report.
Other potential sources of bias
We judged all four trials to be at unclear risk of other potential sources of bias. Investigators in two of the four trials did not comment regarding potential conflicts of interest or their source of funding (Gui 2008; He 2006). Kaya 2004 did not report a source of funding but reported no conflict of interest.
Effects of interventions
See: Table 1
Al‐Fayez 2008 was available only as a published abstract; the investigators did not report any of the review outcomes.
Visual acuity
Regardless of whether we considered UCVA of 20/40 or better or UCVA of 20/20 or better, there was no clinically or statistically important difference between LASEK and LASIK during the first year after surgery.
Uncorrected visual acuity of 20/20 or better
One trial reported UCVA of 20/20 or better at 1, 3, 6, and 12 months (He 2006). Overall, the certainty of the evidence was very low and the risk of bias for most domains was unclear. There was a high risk of attrition bias. At one month, the RCT showed that there was a higher proportion of eyes with UCVA of 20/20 or better in the LASEK group than in the LASIK group. However, from 3 to 12 months follow‐up, the 95% confidence intervals were wide, indicating imprecision in the effect estimate between the two groups.
At one month after surgery, the trial reported that LASEK was better than LASIK at achieving VA of 20/20 or better; 14 of the 37 eyes in the LASEK group compared with 25 of the 39 eyes in the LASIK group had UCVA of 20/20 or better (Analysis 1.1; RR 0.59, 95% CI 0.37 to 0.95; N = 76). We downgraded the evidence one level for indirectness as the trial participants had severe myopia, and one level for high risk of attrition bias (low‐certainty evidence).
1.1. Analysis.

Comparison 1 LASEK versus LASIK, Outcome 1 Eyes with UCVA of 20/20 or better at 1 month.
At three months after surgery, it is uncertain if LASEK was better than LASIK at achieving VA of 20/20 or better; 28 of the 37 eyes in the LASEK group compared with 34 of the 39 eyes in the LASIK group had UCVA of 20/20 or better (Analysis 1.2; RR 0.87, 95% CI 0.70 to 1.08; N = 76). We downgraded the evidence one level for indirectness as the trial participants had severe myopia, one level for imprecision, and one level for high risk of bias (very low‐certainty evidence).
1.2. Analysis.

Comparison 1 LASEK versus LASIK, Outcome 2 Eyes with UCVA of 20/20 or better at 3 months.
At six months after surgery, LASEK maybe slightly better or show no difference than LASIK at achieving VA of 20/20 or better; 25 of the 37 eyes in the LASEK group compared with 34 of the 39 eyes in the LASIK group had UCVA of 20/20 or better (Analysis 1.3; RR 0.78, 95% CI 0.60 to 1.00; N = 76). The evidence was downgraded one level for indirectness as the trial participants had severe myopia, one level for imprecision, and one level for high risk of bias (very low‐certainty evidence).
1.3. Analysis.

Comparison 1 LASEK versus LASIK, Outcome 3 Eyes with UCVA of 20/20 or better at 6 months.
At 12 months after surgery, it is uncertain if LASEK was better than LASIK at achieving VA of 20/20 or better; 25 of the 28 eyes in the LASEK group compared with 27 of the 29 eyes in the LASIK group had UCVA of 20/20 or better (Analysis 1.4; RR 0.96, 95% CI 0.82 to 1.13; N = 57). The evidence was downgraded one level for indirectness as the trial participants had severe myopia, one level for imprecision, and one level for high risk of bias (very low‐certainty evidence).
1.4. Analysis.

Comparison 1 LASEK versus LASIK, Outcome 4 Eyes with UCVA of 20/20 or better at 12 months.
Uncorrected visual acuity of 20/40 or better
One trial reported UCVA of 20/40 or better at 1, 3, 6, and 12 months (He 2006). Overall, the certainty of the evidence was very low and the risk of bias was mostly unclear. There was a high risk of attrition bias. At 1 month, 3 months, and 6 months there was no difference in this outcome between the LASIK and LASEK groups, indicated by wide 95% confidence intervals that included the null value. At 12 months after surgery, the confidence interval was wide, and it was uncertain if LASEK was better than LASIK.
At one month after surgery, there was little or no difference between LASEK and LASIK at achieving VA of 20/40 or better; 37 of the 37 eyes in the LASEK group compared with 39 of the 39 eyes in the LASIK group had UCVA of 20/40 or better (Analysis 1.5; RR 1.00, 95% CI 0.95 to 1.05; N = 76). We downgraded the evidence one level for indirectness as the trial participants had severe myopia, one level for imprecision, and one level for high risk of bias (very low‐certainty evidence).
1.5. Analysis.
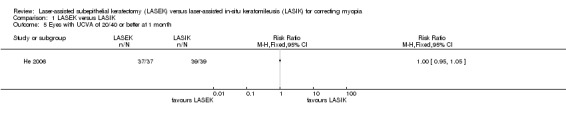
Comparison 1 LASEK versus LASIK, Outcome 5 Eyes with UCVA of 20/40 or better at 1 month.
At three months after surgery, there was little or no difference between LASEK and LASIK at achieving VA of 20/40 or better; 36 of the 37 eyes in the LASEK group compared with 39 of the 39 eyes in the LASIK group had UCVA of 20/40 or better (Analysis 1.6; RR 0.97, 95% CI 0.90 to 1.05; N = 76). We downgraded the evidence one level for indirectness as the trial participants had severe myopia, one level for imprecision, and one level for high risk of bias (very low‐certainty evidence).
1.6. Analysis.
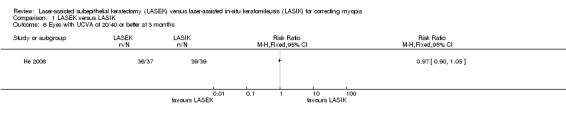
Comparison 1 LASEK versus LASIK, Outcome 6 Eyes with UCVA of 20/40 or better at 3 months.
At six months after surgery, there was little or no difference between LASEK and LASIK at achieving VA of 20/40 or better; 37 of the 37 eyes in the LASEK group compared with 38 of the 39 eyes in the LASIK group had UCVA of 20/40 or better (Analysis 1.7; RR 1.03, 95% CI 0.95 to 1.10; N = 76). We downgraded the evidence one level for indirectness as the trial participants had severe myopia, one level for imprecision, and one level for high risk of bias (very low‐certainty evidence).
1.7. Analysis.
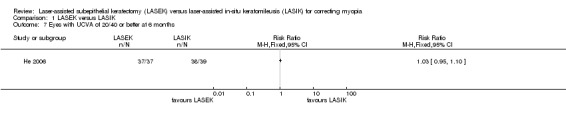
Comparison 1 LASEK versus LASIK, Outcome 7 Eyes with UCVA of 20/40 or better at 6 months.
At 12 months after surgery, it is uncertain if LASEK was better than LASIK at achieving VA of 20/40 or better; 20 of 28 eyes in the LASEK group compared with 23 of the 29 eyes in the LASIK group had UCVA of 20/40 or better (Analysis 1.8; RR 0.90, 95% CI 0.67 to 1.21; N = 57). We downgraded the evidence one level for indirectness as the trial participants had severe myopia, one level for imprecision, and one level for high risk of bias (very low‐certainty evidence).
1.8. Analysis.
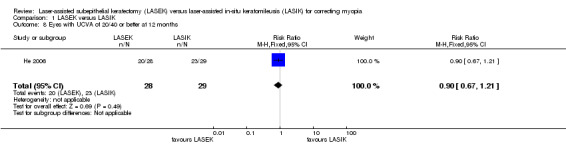
Comparison 1 LASEK versus LASIK, Outcome 8 Eyes with UCVA of 20/40 or better at 12 months.
Proportion of participants who lost two or more lines of best‐corrected visual acuity (BCVA)
One trial reported BCVA from baseline to six months after surgery (Kaya 2004). The investigators reported the mean number of letters lost and not the proportion of participants who lost two or more lines of BCVA. We could not convert the mean number of letters to lines of BCVA lost. We downgraded the evidence one level for indirectness as the trial participants might not be representative of the review population, one level for indirectness as the reported outcome is slightly different than the review outcome (low‐certainty evidence). We report the trial results narratively.
At three months after surgery, the mean number of letters lost (± SD) was 1.02 ± 0.05 and 1.02 ± 0.05 for LASEK and LASIK groups, respectively. At six months after surgery, the mean number of letters lost (± SD) was 1.02 ± 0.06 and 1.0 ± 0.08 for LASEK and LASIK groups, respectively.
Proportion of eyes within 0.5 D of target refraction
At 12 months, one trial reported the proportion of eyes within 0.5 D (more or less) of target refraction (He 2006). The trial investigators reported that the LASEK group attained target refraction less frequently than LASIK group; 16 of 28 eyes in the LASEK group compared with 24 of 29 eyes in the LASIK group had eyes within 0.5 D of target refraction (Analysis 1.9; RR 0.69, 95% CI 0.48 to 0.99; N = 57). We downgraded the evidence three levels for imprecision, indirectness as the trial participants had severe myopia and one level for high risk of bias (very low‐certainty evidence).
1.9. Analysis.
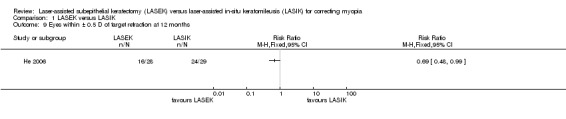
Comparison 1 LASEK versus LASIK, Outcome 9 Eyes within ± 0.5 D of target refraction at 12 months.
Mean spherical equivalent of the refractive error
At six months, two trials reported the mean spherical equivalent of the refractive error (Gui 2008; Kaya 2004). The authors showed that the LASEK and LASIK groups achieved similar outcomes (Analysis 1.10; MD 0.07, 95% CI −0.01 to 0.15; N = 144). We downgraded the evidence one level due to indirectness as the trial participants might not be representative of the review population and one level due to high risk of bias (low‐certainty evidence).
1.10. Analysis.
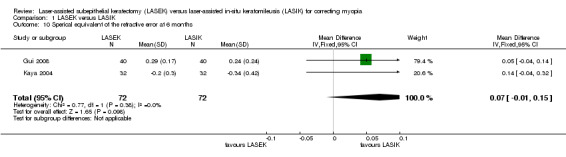
Comparison 1 LASEK versus LASIK, Outcome 10 Sperical equivalent of the refractive error at 6 months.
At 12 months, none of the included studies reported this outcome.
Proportion of eyes that had postoperative corneal haze
At one month, one trial reported the outcome (Kaya 2004). The authors showed that 3 eyes out of 30 in the LASEK group demonstrated 0.5 degrees of haze (defined as barely detectable or trace according to the Corneal Haze Grading Scale from Braunstein 1996. The data were insufficient for further analyses because no measure of variance was reported (e.g., SD). At six months, the haze of those three eyes had healed. The trial investigators reported observing no haze in participants in the LASIK group.
At 12 months, one trial reported this outcome (He 2006); 6 of 38 eyes in the LASEK group compared with 3 of 39 eyes in the LASIK group had postoperative corneal haze (Analysis 1.12; RR 2.11, 95% CI 0.57 to 7.82; N = 76). We downgraded the evidence one level for indirectness as the trial participants might not be representative of the review population, one level for imprecision, and one level for high risk of bias (very low‐certainty evidence).
1.12. Analysis.
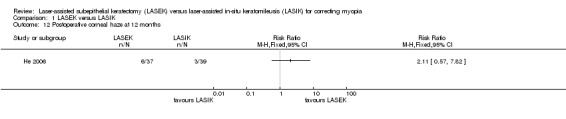
Comparison 1 LASEK versus LASIK, Outcome 12 Postoperative corneal haze at 12 months.
Pain scores (intraoperative and postoperative)
None of the included studies reported on pain at one day postoperatively
Quality of life measures
None of the included studies reported on quality of life.
Adverse events
No two trials provided data on the same outcomes for inclusion in a meta‐analysis and none specified when adverse events occurred. Al‐Fayez 2008 and Gui 2008 did not specify which adverse events they collected; however, Gui 2008 reported no unusual complications or adverse events, while Al‐Fayez 2008 reported a higher complication rate in eyes in the LASEK group compared with LASIK group. We downgraded the evidence one level for indirectness as the trial participants might not be representative of the review population and one level for high risk of bias (very low‐certainty evidence).
He 2006 reported that there were no severe complications that affected the participant's visual acuity; authors did report that none of the eyes in the LASEK group compared with 5 out of 39 eyes in the LASIK group experienced corneal flap striae. Likewise, none of the eyes in the LASEK group compared with 5 out of 39 eyes in the LASIK group had refractive over‐correction. Eight out of 37 eyes in the LASEK group compared with none in the LASIK group had refractive regression. Because adverse events only occurred in one group and not the other, we could not estimate the relative effects.
One trial reported excluding 3 out of 32 eyes in the LASEK group due to flap complications during epithelial flap preparation, resulting in damage to the epithelium (Kaya 2004).
None of the included studies reported keratectasias or dry eye. We downgraded the evidence of adverse events two levels for indirectness of evidence and one level for high risk of bias.
Discussion
Summary of main results
In this review of findings from four RCTs, involving 538 eyes of 392 participants with myopia of severity of up to 10.75 D, we did not find any high‐certainty evidence to precisely estimate differences in efficacy or adverse effects between LASEK and LASIK, if indeed they exist. The primary source of visual acuity outcomes was He 2006. Proportions of eyes that achieved UCVA of 20/40 or better and of 20/20 or better were similar over 12 months. One trial reported the proportion of eyes that achieved refractive correction within 0.5 D of the target (He 2006). In this study, the LASEK group was less likely to achieve the desired target refraction compared with the LASIK group. With regards to safety and adverse effects, one trial noted that 10% of eyes in the LASEK group (but none of in the LASIK group) had barely detectable or trace postoperative corneal haze, but authors did not specify the time of assessment (Kaya 2004). None of the included studies reported mean spherical equivalent of the refractive error at 1 year follow, pain at one day after surgery or quality of life scores. Overall, from the limited data available from the studies relevant to our review, LASEK and LASIK for refractive correction of myopia appear to be similar with regards to efficacy, accuracy, and safety.
Overall completeness and applicability of evidence
One trial consisted only of a published abstract, and the authors did not provide data for any of the review outcomes. Additionally, although none of the trials explicitly reported missing outcome data, we assume that loss to follow‐up explains why there were fewer eyes contributing data at trial completion than at randomization (He 2006). All the trials compared LASEK with LASIK in participants with myopia up to 10.75 D. We do not know whether these results apply to more severe myopia. At least three of the four trials were conducted outside the USA (in China and Turkey), so their applicability to myopic patients in the USA is unknown.
Certainty of the evidence
Overall, the certainty of evidence was very low, with most trials at unclear risk of bias; there was high risk of attrition bias in He 2006. The loss to follow‐up rate in He 2006 was higher in the LASEK group than the LASIK group. We downgraded the certainty of the evidence due to indirectness study populations and outcome measures and wide confidence intervals.
Potential biases in the review process
We followed standard Cochrane procedures to minimize bias in the review process. We are aware of no aspect of our procedures and analysis that had the potential to induce bias in our findings or conclusions.
Agreements and disagreements with other studies or reviews
In this review, we found similar effectiveness for LASEK versus LASIK for the treatment of mild to moderate myopia and myopic astigmatism with regards to UCVA outcomes and adverse effects. These findings are consistent with those of a prior review (Zhao 2014), which reviewed the results of 12 total studies (1 RCT and 11 non‐randomized comparative studies) comparing LASEK and LASIK for the treatment of any degree of myopia in people aged 18 years or older. In that review, there were no significant differences in visual and refractive outcomes for low to moderate myopia. Additionally, the review found that corneal haze was more severe in the LASEK group for moderate to high myopia compared to the LASIK group.
Authors' conclusions
Implications for practice.
Current evidence does not show any large clinically or statistically important differences in efficacy or safety outcomes between LASEK and LASIK for treating mild to moderate myopia. More evidence, in the form of large, high quality RCTs, would be needed to estimate differences in outcomes, if any, between these two interventions.
Implications for research.
Future larger RCTs with rigorous design and attention to all relevant outcomes, including adverse effects, are necessary to reliably assess any difference in treatment outcomes between LASEK versus LASIK for myopia. Additionally, future trials should assess and report pain and quality of life during follow‐up in people undergoing these interventions. There is also a need for evaluating long‐term (greater than one year) adverse events, such as dry eyes and nighttime glare. Given the increasing incidence of myopia worldwide, precise estimation of outcomes and differences between refractive surgical intervention will be important.
Acknowledgements
We would like to acknowledge the US Satellite of the Cochrane Eyes and Vision (CEV) for their assistance. We also acknowledge CEV editors and peer reviewers Barbara Hawkins, George Settas, and Dan Twelker for their insightful comments during the preparation of this review. We acknowledge Lori Rosman, Information Specialist for the US Satellite of CEV, for developing the search strategy and executing the electronic searches.
We thank Andrew Law for his methodological support of this review; Andrew Law screened the search results, assessed trial quality, extracted and entered data, prepared the 'Summary of findings' table, applied GRADE assessment, and edited the main text of the review. We would like to thank Xue Wang, Sueko Ng, Elizabeth Clearfield, Xuan Hui, Shuiqing Liu, Nan Zhang, and Cesar Augusto Ugarte Gil for screening the search results or helping to assess full‐text reports in foreign languages. We would like to thank Colm McAlinden and Luca Buzzonetti for responding to our enquiry about their publications.
Appendices
Appendix 1. CENTRAL search strategy
#1 MeSH descriptor: [Myopia] explode all trees #2 myop* #3 (short near/3 sight*) or ("near" near/3 sight*) #4 nearsighted* #5 #1 or #2 or #3 or #4 #6 MeSH descriptor: [Keratomileusis, Laser In Situ] explode all trees #7 Keratomileus* #8 LASIK #9 MeSH descriptor: [Cornea] explode all trees and with qualifiers: [Surgery ‐ SU] #10 #9 from 1995 to 1999 #11 #6 or #7 or #8 or #10 #12 MeSH descriptor: [Keratectomy, Subepithelial, Laser‐Assisted] explode all trees #13 (laser* near/2 subepithelial keratectom*) #14 (laser* near/2 sub‐epithelial keratectom*) #15 (laser* near/2 subepithelial keratomileus*) #16 (laser* near/2 sub‐epithelial keratomileus*) #17 Subepithelial Photorefractive Keratectom* #18 (laser* near/2 epithelial keratomileus*) #19 (laser* near/2 epithelial keratectom*) #20 LASEK #21 MeSH descriptor: [Epithelium, Corneal] explode all trees and with qualifiers: [Surgery ‐ SU] #22 #21 from 2001 to 2004 #23 #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20 or #22 #24 #11 or #23 #25 #5 and #24
Appendix 2. MEDLINE Ovid search strategy
1. Randomized Controlled Trial.pt. 2. Controlled Clinical Trial.pt. 3. (randomized or randomised).ab,ti. 4. placebo.ab,ti. 5. drug therapy.fs. 6. randomly.ab,ti. 7. trial.ab,ti. 8. groups.ab,ti. 9. 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 10. exp animals/ not humans.sh. 11. 9 not 10 12. exp myopia/ 13. myop*.tw. 14. ((short or near) adj3 sight*).tw. 15. nearsighted*.tw. 16. or/12‐15 17. exp Keratomileusis, Laser In Situ/ 18. Keratomileus*.tw. 19. LASIK.tw. 20. exp Cornea/su [Surgery] 21. 20 22. limit 21 to yr="1995 ‐ 1999" 23. or/17‐19,22 24. exp Keratectomy, Subepithelial, Laser‐Assisted/ 25. (laser* adj2 subepithelial keratectom*).tw. 26. (laser* adj2 sub‐epithelial keratectom*).tw. 27. (laser* adj2 subepithelial keratomileus*).tw. 28. (laser* adj2 sub‐epithelial keratomileus*).tw. 29. Subepithelial Photorefractive Keratectom*.tw. 30. (laser* adj2 epithelial keratomileus*).tw. 31. (laser* adj2 epithelial keratectom*).tw. 32. LASEK.tw. 33. exp Epithelium, Corneal/su [Surgery] 34. 33 35. limit 34 to yr="2001 ‐ 2004" 36. or/24‐32,35 37. 23 or 36 38. 11 and 16 and 37
The search filter for trials at the beginning of the MEDLINE strategy is from the published paper by Glanville 2006.
Appendix 3. Embase.com search strategy
#1 'randomized controlled trial'/exp #2 'randomization'/exp #3 'double blind procedure'/exp #4 'single blind procedure'/exp #5 random*:ab,ti #6 #1 OR #2 OR #3 OR #4 OR #5 #7 'animal'/exp OR 'animal experiment'/exp #8 'human'/exp #9 #7 AND #8 #10 #7 NOT #9 #11 #6 NOT #10 #12 'clinical trial'/exp #13 (clin* NEAR/3 trial*):ab,ti #14 ((singl* OR doubl* OR trebl* OR tripl*) NEAR/3 (blind* OR mask*)):ab,ti #15 'placebo'/exp #16 placebo*:ab,ti #17 random*:ab,ti #18 'experimental design'/exp #19 'crossover procedure'/exp #20 'control group'/exp #21 'latin square design'/exp #22 #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 #23 #22 NOT #10 #24 #23 NOT #11 #25 'comparative study'/exp #26 'evaluation'/exp #27 'prospective study'/exp #28 control*:ab,ti OR prospectiv*:ab,ti OR volunteer*:ab,ti #29 #25 OR #26 OR #27 OR #28 #30 #29 NOT #10 #31 #30 NOT (#11 OR #23) #32 #11 OR #24 OR #31 #33 'myopia'/exp #34 'high myopia'/exp #35 myop*:ab,ti #36 (short NEAR/3 sight*):ab,ti OR (near NEAR/3 sight*):ab,ti #37 nearsighted*:ab,ti #38 #33 OR #34 OR #35 OR #36 OR #37 #39 'keratomileusis'/exp #40 keratomileus*:ab,ti #41 lasik:ab,ti #42 #39 OR #40 OR #41 #43 'laser epithelial keratomileusis'/exp #44 laser*:ab,ti AND (subepithelial NEAR/1 keratectom*):ab,ti #45 laser*:ab,ti AND ('sub‐epithelial' NEAR/1 keratectom*):ab,ti #46 laser*:ab,ti AND (subepithelial NEAR/1 keratomileus*):ab,ti #47 laser*:ab,ti AND ('sub‐epithelial' NEAR/1 keratomileus*):ab,ti #48 ('subepithelial photorefractive' NEAR/1 keratectom*):ab,ti #49 laser*:ab,ti AND (epithelial NEAR/1 keratomileus*):ab,ti #50 laser*:ab,ti AND (epithelial NEAR/1 keratectom*):ab,ti #51 lasek:ab,ti #52 #43 OR #44 OR #45 OR #46 OR #47 OR #48 OR #49 OR #50 OR #51 #53 #42 OR #52 #54 #32 AND #38 AND #53
Appendix 4. PubMed search strategy
#1 ((randomized controlled trial[pt]) OR (controlled clinical trial[pt]) OR (randomised[tiab] OR randomized[tiab]) OR (placebo[tiab]) OR (drug therapy[sh]) OR (randomly[tiab]) OR (trial[tiab]) OR (groups[tiab])) NOT (animals[mh] NOT humans[mh]) #2 myop*[tiab] #3 (short[tiab] OR near[tiab]) AND sight*[tiab] #4 nearsighted*[tiab] #5 #2 OR #3 OR #4 #6 Keratomileus*[tiab] #7 LASIK [tiab] #8 #6 OR #7 #9 (laser*[tiab] AND subepithelial keratectom*[tiab]) #10 (laser*[tiab] AND sub‐epithelial keratectom*[tiab]) #11 (laser*[tiab] AND subepithelial keratomileus*[tiab]) #12 (laser*[tiab] AND sub‐epithelial keratomileus*[tiab]) #13 Subepithelial Photorefractive Keratectom*[tiab] #14 (laser*[tiab] AND epithelial keratomileus*[tiab]) #15 (laser*[tiab] AND epithelial keratectom*[tiab]) #16 LASEK[tiab] #17 #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 #18 #8 OR #17 #19 #1 AND #5 AND #18 #20 Medline[sb] #21 #19 NOT #20
Appendix 5. LILACS search strategy
(Myop* OR Miopía OR Miopia OR MH:C11.744.636 OR ((short or near) AND sight*) OR nearsighted*) AND (Keratomileus* OR "Queratomileusis por Láser In Situ" OR "Ceratomileuse Assistida por Excimer Laser In Situ" OR LASIK OR MH:E02.594.480.750 OR MH:E04.014.520.480.750 OR MH:E04.540.825.437.374 OR (laser* AND (subepithelial OR sub‐epithelial) AND (keratectom* OR keratomileus*)) OR "Queratectomía Subepitelial Asistida por Láser" OR "Ceratectomia Subepitelial Assistida por Laser" OR LASEK OR MH:E02.594.480.500 OR MH:E04.014.520.480.500 OR MH:E04.540.825.437.249)
Appendix 6. metaRegister of Controlled Trials search strategy
Myopia AND (LASIK OR LASEK)
Appendix 7. ClinicalTrials.gov search strategy
Myopia AND (LASIK OR LASEK)
Appendix 8. ICTRP search strategy
Myopia AND LASIK OR Myopia AND LASEK
Data and analyses
Comparison 1. LASEK versus LASIK.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Eyes with UCVA of 20/20 or better at 1 month | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Eyes with UCVA of 20/20 or better at 3 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Eyes with UCVA of 20/20 or better at 6 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4 Eyes with UCVA of 20/20 or better at 12 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5 Eyes with UCVA of 20/40 or better at 1 month | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6 Eyes with UCVA of 20/40 or better at 3 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 7 Eyes with UCVA of 20/40 or better at 6 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 8 Eyes with UCVA of 20/40 or better at 12 months | 1 | 57 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.67, 1.21] |
| 9 Eyes within ± 0.5 D of target refraction at 12 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 10 Sperical equivalent of the refractive error at 6 months | 2 | 144 | Mean Difference (IV, Fixed, 95% CI) | 0.07 [‐0.01, 0.15] |
| 11 Postoperative corneal haze at 6 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 12 Postoperative corneal haze at 12 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
1.11. Analysis.
Comparison 1 LASEK versus LASIK, Outcome 11 Postoperative corneal haze at 6 months.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Al‐Fayez 2008.
| Methods |
Study design: paired‐eye randomized controlled trial Power calculation: none reported Authors did not perform correct paired (matched) analysis |
|
| Participants |
Country: NR Mean age (SD): NR Sex: NR Inclusion criteria: myopia of −1.25 to −6.50 D Exclusion criteria: NR Number randomized: Total: NR eyes (238 participants) LASIK group: NR LASEK group: NR Exclusions after randomization: NR Number analyzed: NR Unit of analysis: individual (1 eye in each participant) Losses to follow‐up: NR Equivalence of baseline characteristics: NR |
|
| Interventions |
Intervention 1: LASIK Intervention 2: LASEK Length of follow‐up: 7.5 years Planned: NR Actual: NR |
|
| Outcomes |
Primary outcome, as defined in study reports: NR
Secondary outcomes, as defined in study reports: NR
Adverse events reported: NR Intervals at which outcomes assessed: NR |
|
| Notes |
Trial registration: NR Type of report: published abstract Funding sources: NR Disclosures of interest: NR Study period: January 2000 to September 2000 Reported subgroup analyses: NR Authors were emailed and did not respond. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | NR |
| Allocation concealment (selection bias) | Unclear risk | NR |
| Masking of participants and personnel (performance bias) | Unclear risk | NR |
| Masking of outcome assessment (detection bias) | Unclear risk | NR |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | NR |
| Selective reporting (reporting bias) | Unclear risk | Study investigators did not report a trial registry number, and the study protocol was not available for comparison. It is unclear whether authors reported outcomes as defined in the study protocol. |
| Other bias | Unclear risk | The study investigators did not explicitly report no conflict of interest, and they did not report the source of funding. |
Gui 2008.
| Methods |
Study design: parallel group randomized controlled trial Power calculation: none reported |
|
| Participants |
Country: China Mean age (SD): Mean age of all participants (SD): NR Mean age of participants in LASIK group (SD): 25.1 years (3.9) Mean age of participants in LASEK group (SD): 24.5 years (3.8) Sex: Total: 34 men (43%) and 46 women (57%) LASIK group: 18 men (45%) and 22 women (55%) LASEK group: 16 men (40%) and 24 women (60%) Inclusion criteria: "Low to moderate myopia (less than −6.00 D), astigmatism of less than 1.50 D, the diameter of pupil is less than 4.5 mm under natural light, higher‐order aberration RMS is larger than 0.2" Exclusion criteria: "Participants with diabetes or systemic connective tissue disease, participants with eye diseases including amblyopia, keratopathy, acute conjunctivitis, cataract, glaucoma or ocular fundus disease" Number randomized (total and per group): Total: 160 eyes (80 participants) LASIK group: 80 eyes (40 participants) LASEK group: 80 eyes (40 participants) Exclusions after randomization: none Number analyzed : Total: 160 eyes (80 participants) LASIK group: 80 eyes (40 participants) LASEK group: 80 eyes (40 participants) Unit of analysis: Individuals (both eyes of each participant) Losses to follow‐up: NR |
|
| Interventions |
Intervention 1: LASIK Intervention 2: LASEK Length of follow‐up: Planned: NR Actual: 1 year |
|
| Outcomes |
Primary outcome, as defined in study reports: uncorrected visual acuity
Secondary outcomes, as defined in study reports: postoperative pain, epithelial healing time, manifest refraction (spherical equivalent; SE), corneal haze, higher order abberations
Adverse events reported: yes, but did not specify which adverse events were reported on. "No adverse events occurred" Intervals at which outcomes assessed: 2 weeks, 1 month, 2 months, 3 months, 6 months, 1 year |
|
| Notes |
Trial registration: NR Type of report: published full‐text Funding sources: 2006 S&T Fund of Health Department Human Province, China (No. B2006‐035) Disclosures of interest: NR Study period: NR Reported subgroup analyses: no Authors were not contacted |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "We divided participants into two groups using a table of random numbers." |
| Allocation concealment (selection bias) | Unclear risk | NR |
| Masking of participants and personnel (performance bias) | Unclear risk | NR |
| Masking of outcome assessment (detection bias) | Unclear risk | NR |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No missing data reported |
| Selective reporting (reporting bias) | Unclear risk | Study investigators did not report a trial registry number, and the study protocol was not available for comparison. It is unclear whether outcomes were reported as defined in the study protocol. |
| Other bias | Unclear risk | The study investigators did not explicitly report no conflict of interest, and they did not report the source of funding. |
He 2006.
| Methods |
Study design: parallel group randomized controlled trial Reported power calculation: no |
|
| Participants |
Country: China Age: Mean age of all participants (SD): NR Mean age of participants in LASIK group (SD): 24.62 years (7.61) Mean age of participants in LASEK group (SD): 22.94 years (3.78) Sex: Total: 16 men (38%) and 26 women (62%) LASIK group: 8 men (35%) and 15 women (65%) LASEK group: 8 men (40%) and 11 women (60%) Severity of myopia: LASIK: 6.00‐9.50 D LASEK: 6.00‐10.75 D Inclusion criteria: corneal thickness between 450‐500 µm, myopia of greater than −6.00 D Exclusion criteria: NR Equivalence of baseline characteristics: yes Number randomized (total and per group): Total: 76 eyes (42 participants) LASIK group: 39 eyes (23 participants) LASEK group: 37 eyes (19 participants) Exclusions after randomization: none Number analyzed : Total: 40 eyes (NR participants) LASIK group: 24 eyes (NR participants) LASEK group: 16 eyes (NR participants) Unit of analysis: mixed (odd number of eyes in either group) Losses to follow‐up: Total: 36 eyes LASIK group: 15 eyes LASEK group: 21 eyes (The study authors did not explicitly report participants lost to follow‐up, but the total number of participants changed for the outcomes reported at 12 months. We assumed that these were lost to follow‐up.) |
|
| Interventions |
Intervention 1: LASIK Intervention 2: LASEK Length of follow‐up: Planned: NR Actual: participants were followed up to 8‐15 months with an average of 12 months |
|
| Outcomes |
Primary outcome, as defined in study reports: uncorrected visual acuity Secondary outcomes, as defined in study reports: postoperative refractive changes, complications, postoperative stability effect, BCVA Adverse events reported: slight haze and small flap streaks Intervals at which outcomes assessed: 1 month, 3 months, 6 months, 12 months |
|
| Notes |
Trial registration: NR
Type of report: published full‐text Funding sources: NR Disclosures of interest: NR Study period: September 2002 to November 2003 Reported subgroup analyses: no Authors were not contacted |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details given. The study authors just say that the groups were randomly assigned. "选择自2002年9月至2003年11月间在门诊检查治疗的近视眼患者,挑选满足下列条件的患者:角膜厚度在450 ‐500 µm,近视度数>‐6. 00D, 随机将患者分为LASEK组或超薄瓣LASIK组." |
| Allocation concealment (selection bias) | Unclear risk | NR |
| Masking of participants and personnel (performance bias) | Unclear risk | NR |
| Masking of outcome assessment (detection bias) | Unclear risk | NR |
| Incomplete outcome data (attrition bias) All outcomes | High risk | There was no mention of incomplete data or loss of follow‐up in the study. However, data outcomes for 12 months had significantly lower total number of participants (> 20% of participants were lost to follow‐up). In addition, the loss to follow‐up rate was different in each group: 38% of eyes in the LASIK group and 56% of eyes in the LASEK group |
| Selective reporting (reporting bias) | Unclear risk | Study investigators did not report a trial registry number, and the study protocol was not available for comparison. It is unclear whether outcomes were reported as defined in the study protocol. |
| Other bias | Unclear risk | The study investigators did not explicitly report no conflict of interest and they did not report the source of funding. |
Kaya 2004.
| Methods |
Study design: paired‐eye randomized controlled trial Reported power calculation: no Authors did not perform correct paired (matched) analysis |
|
| Participants |
Country: Turkey Age: Overall mean age: 26.83 years (5.33) Mean age of participants in LASIK group (SD): NR Mean age of participants in LASEK group (SD): NR Sex: Total: 18 men and 14 women Number of men and women in each group not reported Inclusion criteria: myopia less than −6.00 D Exclusion criteria: "change in refraction during the previous year, history of ocular surgery, keratoconus, dry eye, past or present ocular disease, or a systemic disease that might cause prolongation of wound healing. Three eyes were not included in the study due to complications during epithelial flap preparation for LASEK, which resulted in damage to the integrity of the epithelium" (p 224) Equivalence of baseline characteristics: yes Number randomized: Total: 64 eyes of 32 participants LASIK group: 32 eyes of 32 participants LASEK group: 32 eyes of 32 participants Exclusions after randomization: Total: unclear LASIK group: none LASEK group: unclear Number analyzed : Total: 64 eyes of 32 participants LASIK group: 32 eyes of 32 participants LASEK group: 32 eyes of 32 participants Unit of analysis: individual Losses to follow‐up: Total: none LASIK group: none LASEK group: none |
|
| Interventions |
Intervention 1: LASIK Intervention 2: LASEK Length of follow‐up: Planned: NR Actual: 6 months |
|
| Outcomes |
Primary outcome and secondary outcomes not differentiated
Outcomes reported: UCVA, BCVA, Schirmer test, tear break‐up time, corneal asphericity, corneal uniformity index, and predicted corneal acuity values
Adverse events reported: yes (subepithelial haze) Intervals at which outcomes assessed: 1 day, 3 days, 7 days, 1 month, 3 months, and 6 months |
|
| Notes |
Trial registration: NR
Type of report: published full‐text Funding sources: NR Disclosures of interest: "the authors have no proprietary interest in the materials presented herein" (p 223) Study period: NR Reported subgroup analyses: no Authors were contacted and did not respond |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were randomized (random number table) first for right or left eye, and then for type of surgery; LASIK in one eye and LASEK in their fellow eye" (p 224) |
| Allocation concealment (selection bias) | Unclear risk | NR |
| Masking of participants and personnel (performance bias) | Unclear risk | NR |
| Masking of outcome assessment (detection bias) | Unclear risk | NR |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data |
| Selective reporting (reporting bias) | Unclear risk | Study investigators did not report a trial registry number, and the study protocol was not available for comparison. It is unclear whether outcomes were reported as defined in the study protocol. |
| Other bias | Unclear risk | Source of funding was not reported. Study investigators did reported no conflict of interest. "The authors have no proprietary interest in the materials presented herein" (p 223) |
BCVA: best‐corrected visual acuity; LASEK: laser assisted subepithelial keratectomy; LASIK: laser in situ keratomileusis; NR: not reported; SD: standard deviation; UCVA: uncorrected visual acuity
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Benito‐Llopis 2008 | Not an RCT. "The decision to perform LASEK instead of LASIK was based either on the calculated residual stromal thickness being too thin to perform LASIK or on patient preference after being fully informed about the advantages and disadvantages of the procedure." It was a prospective study comparing participants who opted for LASEK (either poor LASIK candidates or participant preference as stated above) with participants who had LASIK that were matched in terms of preoperative refractive error (≤ 0.50 SE and ≤ 0.75 D cyl) |
| Buzzonetti 2004 | Not an RCT. Authors confirmed. |
| Cabrera Martínez 2009 | Not an RCT |
| Chung 2006 | Not an RCT. Participants were assigned based on their choice |
| Eidt 2003 | Not an RCT |
| Ferguson 2006 | Not an RCT |
| Gao 2005 | Not an RCT |
| Huang 2007 | Not an RCT. Retrospective study and included eyes with > 12 D myopia |
| Kim 2004 | Wrong study participants. Included participants with 12.5 D myopia |
| Kirwan 2009 | Not an RCT |
| Kotb 2004 | Wrong study participants. Included eyes with 17 D myopia |
| Lee 2003 | Not an RCT |
| Li 2005 | Not an RCT |
| McAlinden 2010 | Not an RCT |
| Reilly 2006 | Not an RCT |
| Ruckhofer 2003 | Not an RCT |
| Scerrati 2001 | Not an RCT |
| Sheng 2004 | Not an RCT. Retrospective study that included participants with 13 D myopia |
| Swinger 1981 | Not an RCT. Retrospective study that included participants with lamellar refractive keratoplasty |
| Wu 2005 | Not an RCT |
| Zou 2008 | Wrong study participants. Included participants with > 12D myopia |
D: diopters; RCT: randomized controlled trial.
Differences between protocol and review
We did not dichotomize ordinal outcomes (haze scores) as both trials reported no haze (0) versus any haze (0.5 to 4). We were not able to dichotomize the outcome differently; therefore when future studies that report the distribution of the corneal haze scores are included in this review, we will use the following cut points for grading corneal haze on the 0 to 4 point scale.
Little to no haze (0 to 1) versus mild, moderate, and severe haze (2 to 4).
Clear to mild haze (0 to 2) versus moderate to severe haze (3 to 4).
We did not assess potential for selective outcome reporting within individual studies included in this review in our assessment of risk of bias.
We also included the Summary of findings table to highlight the review primary and secondary outcomes at one year follow‐up, and adverse events at the end of the study follow‐up.
Contributions of authors
JK conceived and designed the review, screened the search results, assessed trial quality, extracted data, checked the data entered into RevMan, analyzed data and wrote the main text of the review. AC assessed trial quality, extracted data, and participated in the adjudication process. RC conceived and designed the review, advised on data interpretations, and provided substantial comments on the content of the review. All authors approved the final draft of this review.
Sources of support
Internal sources
No sources of support supplied
External sources
-
Research to Prevent Blindness, USA.
Unrestricted grant to Montefiore Medical Center's Department of Ophthalmology (outside the scope of this work)
-
National Eye Institute, National Institutes of Health, USA.
Cochrane Eyes and Vision US Project, Grant 1 U01 EY020522
-
National Institute for Health Research (NIHR), UK.
- Richard Wormald, Co‐ordinating Editor for Cochrane Eyes and Vision (CEV) acknowledges financial support for his CEV research sessions from the Department of Health through the award made by the National Institute for Health Research to Moorfields Eye Hospital NHS Foundation Trust and UCL Institute of Ophthalmology for a Specialist Biomedical Research Centre for Ophthalmology.
- The NIHR also funds the CEV Editorial Base in London.
The views expressed in this publication are those of the authors and not necessarily those of the NIHR, NHS, or the Department of Health.
Declarations of interest
JK: none known. AC: none known. RC: none known.
New
References
References to studies included in this review
Al‐Fayez 2008 {published data only}
- Al‐Fayez M. Long‐term results of LASEK vs. LASIK for mild to moderate myopia. American Academy of Ophthalmology; 2008 Nov 8‐11; Atlanta (GA). Atlanta (GA), 2008:247.
Gui 2008 {published data only}
- Gui MY, He SX, Liu W. Comparison of wavefront‐guided laser epithelial keratomileusis and wavefront‐guided laser in situ keratomileusis for low to moderate myopia. International Journal of Ophthalmology 2008;8(5):958‐61. [Google Scholar]
He 2006 {published data only}
- He TG, Shi XR. Clinical study of ultra thin flap LASIK and LASEK for the treatment of high myopia with thin cornea. Chinese Journal of Ophthalmology 2006;42(6):517‐21. [PubMed] [Google Scholar]
Kaya 2004 {published data only}
- Kaya V, Oncel B, Sivrikaya H, Yilmaz OF. Prospective, paired comparison of laser in situ keratomileusis and laser epithelial keratomileusis for myopia less than −6.00 diopters. Journal of Refractive Surgery 2004;20(3):223‐8. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Benito‐Llopis 2008 {published data only}
- Benito‐Llopis L, Teus MA, Sánchez‐Pina JM. Comparison between LASEK with mitomycin C and LASIK for the correction of myopia of −7.00 to −13.75 D. Journal of Refractive Surgery 2008;24(5):516‐23. [DOI] [PubMed] [Google Scholar]
Buzzonetti 2004 {published data only}
- Buzzonetti L, Iarossi G, Valente P, Volpi M, Petrocelli G, Scullica L. Comparison of wavefront aberration changes in the anterior corneal surface after laser‐assisted subepithelial keratectomy and laser in situ keratomileusis: preliminary study. Journal of Cataract and Refractive Surgery 2004;30(9):1929‐33. [DOI] [PubMed] [Google Scholar]
Cabrera Martínez 2009 {published data only}
- Cabrera Martínez A, Cabrera Martínez JA, Tirado Martínez OM. Refractive results in patients operated by LASIK versus LASEK with mitomycin C [Resultados refractivos en pacientes operados por LASIK versus LASEK con mitomicina C]. Revista Cubana de Oftalmología 2009;22(1):1‐10. [Google Scholar]
Chung 2006 {published data only}
- Chung SH, Lee IS, Lee YG, Lee HK, Kim EK, Yoon G, et al. Comparison of higher‐order aberrations after wavefront‐guided laser in situ keratomileusis and laser‐assisted subepithelial keratectomy. Journal of Cataract and Refractive Surgery 2006;32(5):779‐84. [DOI] [PubMed] [Google Scholar]
Eidt 2003 {published data only}
- Eidt SC, Larson B, Bouchard C. LASIK and LASEK: a comparison of starburst phenomenon seen around lights at night. Investigative Ophthalmology & Visual Science 2003;44(13):2645. [Google Scholar]
Ferguson 2006 {published data only}
- Ferguson RA, Reilly CD, Dudenhoefer EJ, Smith RE. The Airforce Warfighter Refractive Surgery Study: laser assisted in‐situ keratomileusis vs. laser assisted subepithelial keratectomy using the VISX star laser platform. Investigative Ophthalmology and Visual Science 2006; Vol. 47, issue 13:56.
Gao 2005 {published data only}
- Gao N, Hu LJ, Xie LX, Guo DQ. Comparison of wavefront aberration after LASIK and LASEK for treatment of myopia. Chinese Journal of Ophthalmology 2005;41(11):966‐71. [PubMed] [Google Scholar]
Huang 2007 {published data only}
- Huang P, Liu J, Xia YJ, Zhong YY, Chen YG. Comparison of laser in situ keratomileusis and laser‐assisted subepithelial keratectomy for myopia more than −10.00 diopters. Journal of Peking University Health Sciences 2007;39(5):498‐502. [PubMed] [Google Scholar]
Kim 2004 {published data only}
- Kim JK, Kim SS, Lee HK, Lee IS, Seong GJ, Kim EK, et al. Laser in situ keratomileusis versus laser‐assisted subepithelial keratectomy for the correction of high myopia. Journal of Cataract and Refractive Surgery 2004;30(7):1405‐11. [DOI] [PubMed] [Google Scholar]
Kirwan 2009 {published data only}
- Kirwan C, O'Keefe M. Comparative study of higher‐order aberrations after conventional laser in situ keratomileusis and laser epithelial keratomileusis for myopia using the technolas 217z laser platform. American Journal of Ophthalmology 2009;147(1):77‐83. [DOI] [PubMed] [Google Scholar]
Kotb 2004 {published data only}
- Kotb AA, Tabbara KF. Laser epithelial keratomileusis (LASEK) compared to laser in situ keratomileusis (LASIK) in the correction of myopia. Investigative Ophthalmology & Visual Science 2004;45(13):1106. [Google Scholar]
Lee 2003 {published data only}
- Lee JH, Snyder RW, Schweigerling J, Nedrud C, Chang M. Longitudinal spherical aberration after LASEK and LASIK. Investigative Ophthalmology & Visual Science 2003;44(13):2644. [Google Scholar]
Li 2005 {published data only}
- Li X, Hu Q, Yang CX, Li QJ. Laser in situ keratomileusis versus laser epithelial keratomileusis for the recovery of high myopia and the repair of corneal epithelium. Chinese Journal of Clinical Rehabilitation 2005;9(18):155‐7. [Google Scholar]
McAlinden 2010 {published data only}
- McAlinden C, Moore JE. Comparison of higher order aberrations after LASIK and LASEK for myopia. Journal of Refractive Surgery 2010;26(1):45‐51. [DOI] [PubMed] [Google Scholar]
Reilly 2006 {published data only}
- Reilly CD, Ferguson R, Dudenhoefer EJ, Smith RE. Advanced surface ablation vs. LASIK for moderate myopia: the Air Force Refractive Surgery Experience. American Academy of Ophthalmology; 2006 Nov 11‐14; Las Vegas (NV). 2006:267.
Ruckhofer 2003 {published data only}
- Ruckhofer J, Dexl A, Stoiber J, Grabner G. LASIK vs LASEK for the correction of low to moderate myopia [LASIK vs LASEK zur Kerrektur einer geringen bis mittelgradigen Mypoie]. Spektrum der Augenheilkunde 2003;17(2):85. [Google Scholar]
Scerrati 2001 {published data only}
- Scerrati E. Laser in situ keratomileusis vs. laser epithelial keratomileusis (LASIK vs. LASEK). Journal of Refractive Surgery 2001;17(2 Suppl):S219‐S221. [DOI] [PubMed] [Google Scholar]
Sheng 2004 {published data only}
- Sheng X, Wu W, Zhuang W, Wang W, Mei H. Comparison of laser epithelial keratomilersis and laser in situ keratomileusis for moderate‐to‐high myopia. Clinical and Surgical Ophthalmology 2004;22(4):92‐5. [Google Scholar]
Swinger 1981 {published data only}
- Swinger CA, Barraquer JI. Keratophakia and keratomileusis ‐ clinical results. Ophthalmology 1981;88(8):709‐15. [DOI] [PubMed] [Google Scholar]
Wu 2005 {published data only}
- Wu Y, Chu RY, Zhou XT, Dai JH, Qu XM. Recovery of corneal sensitivity after laser in situ keratomileusis and laser‐assisted subepithelial keratectomy. Chinese Journal of Ophthalmology 2005;41(11):972‐6. [PubMed] [Google Scholar]
Zou 2008 {published data only}
- Zou J, Chu R, Zhou X. The clinical study on LASIK with ultra‐thin flaps and LASEK for extremely high myopia. Investigative Ophthalmology & Visual Science 2008;49(13):3364. [Google Scholar]
Additional references
Ang 2009
- Ang EK, Couper T, Dirani M, Vajpayee RB, Baird PN. Outcomes of laser refractive surgery for myopia. Journal of Cataract and Refractive Surgery 2009;35(5):921‐33. [DOI] [PubMed] [Google Scholar]
Bashford 2005
- Bashford KP, Shafranov G, Tauber S, Shields MB. Considerations of glaucoma in patients undergoing corneal refractive surgery. Survey of Ophthalmology 2005;50(3):245‐51. [DOI] [PubMed] [Google Scholar]
Basting 2001
- Basting D, Pippert K. Stamm U. History and future prospects of excimer laser technology. aries.ucsd.edu/LMI/TUTORIALS/excimer‐primer.pdf (accessed 1 July 2013).
Bower 2001
- Bower KS, Weichel ED, Kim TJ. Overview of refractive surgery. American Family Physician 2001;64(7):1183‐90. [PubMed] [Google Scholar]
Braunstein 1996
- Braunstein RE, Jain S, McCally RL, Stark WJ, Connolly PJ, Azar DT. Objective measurement of corneal light scattering after excimer laser keratectomy. Ophthalmology 1996;103(3):439‐43. [DOI] [PubMed] [Google Scholar]
Campos 1992
- Campos M, Hertzog L, Wang XW, Fasano AP, McDonnell PJ. Corneal surface after deepithelialization using a sharp and a dull instrument. Ophthalmic Surgery 1992;23(9):618‐21. [PubMed] [Google Scholar]
Duffey 2003
- Duffey RJ, Leaming D. US trends in refractive surgery: 2002 ISRS survey. Journal of Refractive Surgery 2003;19(3):357‐63. [DOI] [PubMed] [Google Scholar]
Glanville 2006
- Glanville JM, Lefebvre C, Miles JN, Camosso‐Stefinovic J. How to identify randomized controlled trials in MEDLINE: ten years on. Journal of the Medical Library Association 2006;94(2):130‐6. [PMC free article] [PubMed] [Google Scholar]
GRADEpro 2014 [Computer program]
- GRADE Working Group, McMaster University. GRADEpro GDT. Version accessed 23 November 2016. Hamilton (ON): GRADE Working Group, McMaster University, 2014.
Hayashida 2006
- Hayashida Y, Nishida K, Yamato M. Transplantation of tissue‐engineered epithelial cell sheets after excimer laser photoablation reduces postoperative corneal haze. Investigative Ophthalmology & Visual Science 2006;47(2):552‐7. [DOI] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JP, Altman DG, Sterne JAC editor(s). Chapter 8: Assessing risk of bias in included studies.In: Higgins JP, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2011b
- Higgins JP, Deeks JJ, Altman DG editor(s). Chapter 16: Special topics in statistics. In: Higgins JP, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Holland 2000
- Holland SP, Srivannaboon S, Reinstein DZ. Avoiding serious corneal complications of laser assisted in situ keratomileusis and photorefractive keratectomy. Ophthalmology 2000;107(4):640‐52. [DOI] [PubMed] [Google Scholar]
Knorz 2002
- Knorz MC. Flap and interface complications in LASIK. Current Opinion in Ophthalmology 2002;13(4):242‐5. [DOI] [PubMed] [Google Scholar]
Manche 1998
- Manche EE, Carr JD, Haw WW, Hersh PS. Excimer laser refractive surgery. Western Journal of Medicine 1998;169(1):30‐8. [PMC free article] [PubMed] [Google Scholar]
Medeiros 2011
- Medeiros FW, Sinha‐Roy A, Alves MR, Dupps WJ Jr. Biomechanical corneal changes induced by different flap thickness created by femtosecond laser. Clinics 2011;66(6):1067‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Morgan 2012
- Morgan IG, Ohno‐Matsui K, Saw SM. Myopia. Lancet 2012;379(9827):1739‐48. [DOI] [PubMed] [Google Scholar]
Pallikaris 1991
- Pallikaris IG, Papatzanaki ME, Siganos DS, Tsilimbaris MK. A corneal flap technique for laser in situ keratomileusis: human studies. Archives of Ophthalmology 1991;109(12):1699‐702. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Sandoval 2005
- Sandoval HP, Castro LE, Vroman DT, Solomon KD. Refractive Surgery Survey 2004. Journal of Cataract and Refractive Surgery 2005;31(1):221‐33. [DOI] [PubMed] [Google Scholar]
Shah 2011
- Shah R, Shah S, Sengupta S. Results of small incision lenticule extraction: All‐in‐one femtosecond laser refractive surgery. Journal of Cataract and Refractive Surgery 2011;37(1):127‐37. [DOI] [PubMed] [Google Scholar]
Shahinian 2002
- Shahinian L Jr. Laser‐assisted subepithelial keratectomy for low to high myopia and astigmatism. Journal of Cataract and Refractive Surgery 2002;28(8):1334‐42. [DOI] [PubMed] [Google Scholar]
Shortt 2006
- Shortt AJ, Allan BDS. Photorefractive keratectomy (PRK) versus laser‐assisted in‐situ keratomileusis (LASIK) for myopia. Cochrane Database of Systematic Reviews 2006, Issue 2. [DOI: 10.1002/14651858.CD005135.pub2] [DOI] [PubMed] [Google Scholar]
Shortt 2013
- Shortt AJ, Allan BDS, Evans JR. Laser‐assisted in‐situ keratomileusis (LASIK) versus photorefractive keratectomy (PRK) for myopia. Cochrane Database of Systematic Reviews 2013, Issue 1. [DOI: 10.1002/14651858.CD005135.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Shrivastava 2011
- Shrivastava A, Madu A, Schultz J. Refractive surgery and the glaucoma patient. Current Opinion in Ophthalmology 2011;22(4):215‐21. [DOI] [PubMed] [Google Scholar]
Spadea 2012
- Spadea L, Cantera E, Cortes M, Conochia NE, Stewar CW. Corneal ectasia after myopic laser in situ keratomileusis: a long‐term study. Clinical Ophthalmology 2012;6(1):1801‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sridhar 2002
- Sridhar MS, Rao SK, Vajpayee RB, Aasuri MK, Hannush S, Sinha R. Complications of laser‐in‐situ‐keratomileusis. Indian Journal of Ophthalmology 2002;50(4):265‐82. [PubMed] [Google Scholar]
Vitale 2006
- Vitale S, Cotch MF, Sperduto R, Ellwein L. Costs of refractive correction of distance vision impairment in the United States, 1999‐2002. Ophthalmology 2006;113(12):2163‐70. [DOI] [PubMed] [Google Scholar]
Wen 2013
- Wen G, Tarczy‐Hornoch K, McKean‐Cowdin R, Cotter SA, Borchert M, Lin J, et al. Prevalence of myopia, hyperopia, and astigmatism in non‐Hispanic white and Asian children: multi‐ethnic pediatric eye disease study. Ophthalmology 2013;120(10):2109‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhao 2014
- Zhao L, Zhu H, Li L. Laser‐assisted subepithelial keratectomy versus laser In situ keratomileusis in myopia: a systematic review and meta‐analysis. ISRN Ophthalmology 2014;2014:672146. [DOI: 10.1155/2014/672146] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Kuryan 2014
- Kuryan J, Cheema A, Chuck RS. Laser‐assisted in‐situ keratomileusis (LASIK) versus laser‐assisted subepithelial keratectomy (LASEK) for the correction of myopia. Cochrane Database of Systematic Reviews 2014, Issue 4. [DOI: 10.1002/14651858.CD011080] [DOI] [PMC free article] [PubMed] [Google Scholar]


