Abstract
Objective
To examine ethnic disparities in maternal prepregnancy obesity and gestational weight gain, and to examine to which extent these differences can be explained by socio-demographic, lifestyle and pregnancy related characteristics.
Methods
In a multi-ethnic population-based prospective cohort study among 6,444 pregnant women in Rotterdam, the Netherlands, maternal anthropometrics were repeatedly measured throughout pregnancy. Ethnicity, socio-demographic, lifestyle and pregnancy related characteristics were assessed by physical examinations and questionnaires.
Results
The prevalence of prepregnancy overweight and obesity was 23.1% among Dutch-origin women. Statistically higher prevalences were observed among Dutch Antillean-origin (40.8%), Moroccan-origin (49.9%), Surinamese-Creole-origin (38.6%) and Turkish-origin (41.1%) women (all p-values <0.05). Only Dutch Antillean-origin, Moroccan-origin, Surinamese-Creole-origin and Turkish-origin women had higher risks of maternal prepregnancy overweight and obesity as compared to Dutch-origin women (p-values <0.05). Socio-demographic and lifestyle related characteristics explained up to 45% of the ethnic differences in body mass index. Compared to Dutch-origin women, total gestational weight gain was lower in all ethnic minority groups, except for Cape Verdean-origin and Surinamese-Creole-origin women (p-values <0.05). Lifestyle and pregnancy related characteristics explained up to 33% and 40% of these associations, respectively. The largest ethnic differences in gestational weight gain were observed in late pregnancy.
Conclusion
We observed moderate ethnic differences in maternal prepregnancy overweight, obesity and gestational weight gain. Socio-demographic, lifestyle and pregnancy related characteristics partly explained these differences. Whether these differences also lead to ethnic differences in maternal and childhood outcomes should be further studied.
Keywords: maternal body mass index, maternal obesity, gestational weight gain, ethnicity, pregnancy outcomes
Introduction
Maternal obesity and excessive weight gain during pregnancy are associated with increased risks of preeclampsia and gestational diabetes, stillbirth and premature delivery (1–6). Also, recent studies suggested that offspring of mothers who were obese during pregnancy or gained excessive weight during pregnancy are at increased risk of an adverse cardiovascular risk profile in later life (2). The prevalence of maternal obesity is described to differ between ethnic groups (7, 8). In the United States (US), the highest prevalence of maternal prepregnancy obesity has been reported among black women (8). US studies have also shown that, as compared to white women, black and Hispanic women have a decreased risk of gaining excessive gestational weight according to the Institute of Medicine (IOM) criteria (9, 10).
Thus far, most studies on ethnic differences in maternal overweight and obesity have been conducted in the US or the United Kingdom (UK) and were focused on white, black, Asian and Hispanic women (9–11). Less is known about ethnic differences in prepregnancy obesity prevalences and weight gain during pregnancy in other European countries than the UK, which is mainly due to the large variety of ethnic groups in these countries. In the Netherlands, due to colonial and immigrant worker history, the major ethnic groups are Cape Verdean-origin, Dutch Antillean-origin, Moroccan-origin, Turkish-origin, Surinamese-Creole-origin and Surinamese-Hindustani-origin (12, 13).
Previously, we have shown that as compared to Dutch-origin women, Cape Verdean-origin and Surinamese-Creole-origin women have an increased risk of gestational hypertensive disorders (14). Also, mean birth weight was lower in the offspring of Turkish-origin, Cape Verdean-origin, Dutch-Antillean-origin, Surinamese-Creole-origin, Surinamese-Hindustani-origin and Surinamese-other-origin women, as compared to Dutch-origin women (15). Differences in maternal prepregnancy obesity and excessive gestational weight gain may partly explain these differences in pregnancy outcomes among women from different ethnic minority groups.
Obtaining insight into ethnic differences in maternal prepregnancy obesity and excessive gestational weight gain prevalences is important for the development of future tailored preventive strategies that aim to improve both maternal and fetal pregnancy outcomes.
Therefore, we examined in a multi-ethnic population-based prospective cohort study among 6444 mothers in the city of Rotterdam, the Netherlands, ethnic differences in the risk of maternal prepregnancy overweight and obesity and excessive weight gain during pregnancy. We further explored to what extent these differences can be explained by socio-demographic, lifestyle and pregnancy related characteristics.
Methods
Study design
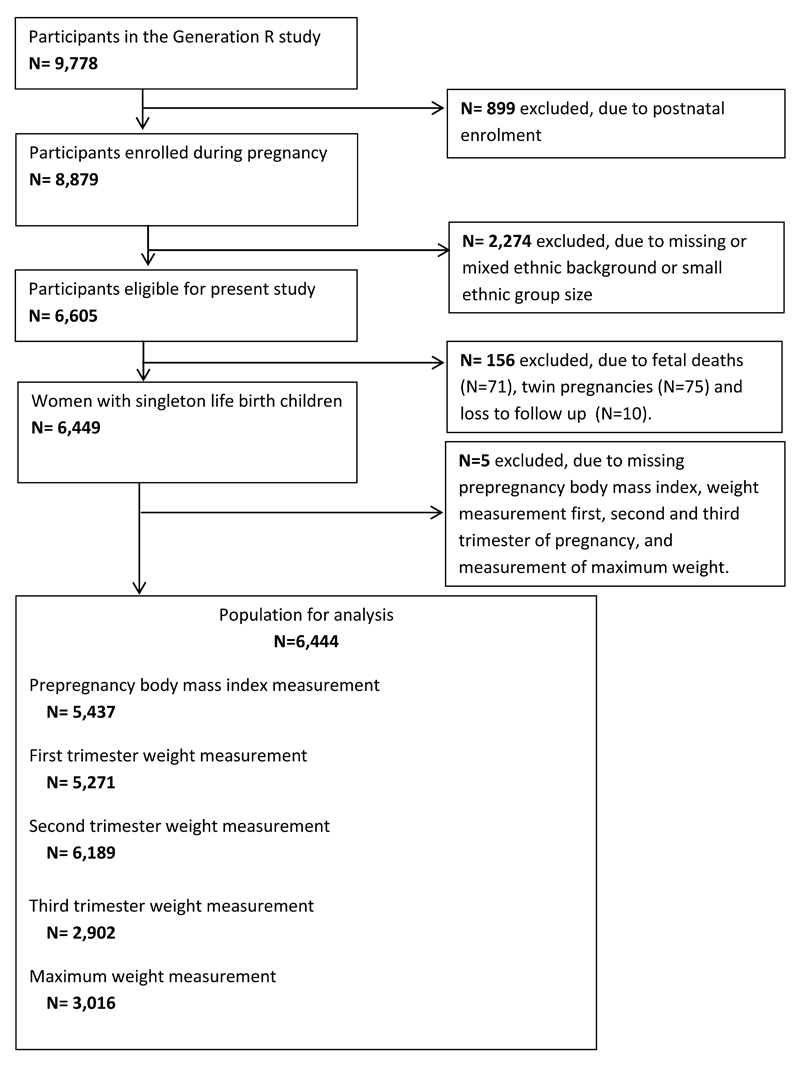
This study was embedded in the Generation R Study, a population-based prospective cohort study from early pregnancy onwards in Rotterdam, the Netherlands. The Medical Ethical Committee of the Erasmus Medical Center, Rotterdam, approved the study (MEC 198.782/2001/31). Written informed consent was obtained from all pregnant women. Pregnant women were enrolled between 2001 and 2005 (12). In total, 8,879 pregnant women were enrolled during pregnancy. For the present study, we excluded pregnant women without information on ethnic background, because of the small country specific sample sizes (<100 per group) or mixed ethnicity (n=2,274), leading to inclusion of a total of 6,605 mothers belonging to one of the major ethnic groups. Furthermore, we excluded pregnant women with pregnancies leading to fetal death, twin pregnancies, and loss to follow-up, because our main interest was in low-risk pregnancies (n=156). Of the remaining 6,449 pregnant women, those without any maternal weight measurement available (n=5), were excluded. Thus, the population for analysis included 6,444 pregnant women. A participant flowchart is given in Figure 1.
Figure 1.
Flow chart of the study population
Ethnic Background
Ethnicity was assessed by country of birth of the participating women and their parents and was obtained by questionnaires (12). Ethnicity was defined according to the classification of Statistics Netherlands. The participant was of non-Dutch origin if one of her parents was born in another country than the Netherlands (16). If both parents were born abroad, the country of the participant’s mother decided on her ethnic background. Next, a distinction was made between women of Dutch ethnic background and the non-Dutch minority groups in this study, Turkish-origin, Moroccan-origin, Dutch Antillean-origin, Surinamese-origin, and Cape Verdean-origin. Since the Surinamese population consists of persons who originate from Africa (Creoles) and India (Hindustani), women with a Surinamese ethnicity were further classified into Surinamese-Hindustani-origin and Surinamese-Creole-origin, based on the ethnic origin of the Surinamese participant (15).
Maternal anthropometrics
Anthropometrics in pregnant women were measured in the first, second, and third trimester of pregnancy at one of the research centres (2). Height (cm) and weight (kg) were measured without shoes and heavy clothing, and body mass index (kg/m2) was calculated for each pregnancy period. Information about weight just before pregnancy was obtained by questionnaire. As enrolment in our study was in pregnancy, we were not able to measure maternal weight before pregnancy. In our population for analysis, 46.2% of all women were enrolled before a gestational age of 14 weeks. Correlation between prepregnancy weight, obtained by questionnaire, and weight measured at enrolment was 0.95 (P < 0.001). No differences in results were found when we used weight measured at enrolment instead of prepregnancy weight obtained by questionnaire. Prepregnancy body mass index was categorised in 4 categories (underweight (<20 kg/m2), normal weight (20-24.9 kg/m2), overweight (25-29.9 kg/m2) and obese (≥30 kg/m2)) (2). Weight gain until a gestational age of 30 weeks was measured and available for 6,148 mothers. Information about weight just before delivery, described as maximum weight during pregnancy in this study, was available in a subgroup of 3,016 mothers and was assessed by questionnaire 2 months after delivery. Maximum weight from questionnaire and weight measured at 30 weeks were strongly correlated (r = 0.97 [P <0.001]). According to IOM guidelines, we defined excessive gestational weight gain in relation to maternal prepregnancy body mass index (for underweight and normal weight mothers: total weight gain >16 kg; for overweight mothers: total weight gain >11.5 kg; for obese mothers: total weight gain >9 kg (17). Weight gain was further analysed in each trimester of pregnancy.
Covariates
We obtained information about maternal age, educational level, household income, marital status, parity and folic acid supplement use at enrolment by questionnaire. Information about maternal diet during pregnancy (total energy intake [kcal]) was obtained by a food frequency questionnaire in the first trimester (2, 18). Information about maternal smoking and alcohol consumption was obtained repeatedly in each trimester of pregnancy. To define maternal smoking and alcohol consumption throughout pregnancy we used information obtained from all three questionnaires. Information on sex, gestational age and weight at birth and pregnancy complications (preeclampsia, gestational hypertension, gestational diabetes, Caesarian delivery) was obtained from medical records. Preterm birth was defined as a gestational age of <37 weeks at birth. Small size for gestational age at birth and large size for gestational age at birth were defined as a gestational age-adjusted birth weight below the 10th percentile and above the 90th percentile in the study cohort.
Statistical analysis
First, we compared maternal characteristics between different ethnic groups, using One-Way ANOVA and Chi-square tests. Second, we used logistic regression models to examine the ethnic differences in the risks of prepregnancy overweight and obesity, and excessive weight gain during pregnancy. Next, we examined the association of maternal ethnicity with prepregnancy body mass index in further detail using linear regression analyses in different models: a basic model (unadjusted), which was subsequently additionally adjusted for (1) socio-demographic characteristics (maternal age, education, income, marital status, parity) and (2) life-style related characteristics (smoking, alcohol consumption, diet, folic acid supplementation). Similarly, we examined the associations of maternal ethnicity with total gestational weight gain and gestational weight gain in first, second and third trimester of pregnancy in further detail using linear regression analyses in different models: a basic model (unadjusted), which subsequently additionally adjusted for (1) socio-demographic characteristics (maternal age, education, income, marital status, parity) and (2) life-style related characteristics (prepregnancy body mass index, smoking, alcohol consumption, diet, folic acid supplementation) and (3) pregnancy related characteristics (pregnancy complications, gestational age and weight at birth). In order to reduce potential bias due to missing data, we performed multiple imputations of missing covariates (percentage of missing values for each covariate are shown in Supplemental Table S1) by generating five independent datasets using the Markov Chain Monte Carlo method, and the pooled effect estimates (95% Confidence Interval (CI)) are presented. Imputations were based on the relationships between covariates, determinants and outcomes (19). All analyses were performed using the Statistical Package for the Social Sciences version 21.0 for Windows (SPSS Inc, Chicago, IL).
Results
Subject characteristics
Table 1 shows characteristics of the included women for different ethnic groups. Compared with Dutch-origin women, women of ethnic minority groups were younger, more frequently multiparous, more frequently lower educated, consumed alcohol less frequently, used folic acid supplementation less often, and had a lower total energy intake (all p-values <0.05). As compared to Dutch-origin women, prepregnancy body mass index was higher among all ethnic minority groups, except among Surinamese-Hindustani-origin women. Mean total gestational weight gain was higher among Dutch-origin women than among non-Dutch-origin women. As compared to Dutch-origin women, prevalences of gestational hypertensive disorders were higher among women of Cape Verdean-origin and Surinamese-Creole-origin, whereas the prevalences of gestational diabetes were higher among women of Moroccan-origin and Surinamese-Hindustani-origin. Women from ethnic minority groups, except for Moroccan-origin women, more often delivered preterm born infants, infants with a lower birth weight and small for gestational age infants, as compared to Dutch-origin women.
Table 1. Characteristics of mothers (N = 6,444)1.
| Dutch-origin N=3,992 |
Cape Verdean-origin N=350 |
Dutch Antillean-origin N=282 |
Moroccan-origin N=574 |
Surinamese-Creole-origin N=239 |
Surinamese-Hindustani-origin N=251 |
Turkish-origin N=756 |
P-value | |
|---|---|---|---|---|---|---|---|---|
| Maternal Characteristics | ||||||||
| Age, y | 31.2 (4.5) | 27.3 (6.0) | 26.3 (5.5) | 28.1 (5.3) | 27.9 (6.5) | 27.6 (4.9) | 27.2 (4.9) | P<0.001 |
| Gestational age at intake, wks | 14.5 (3.8) | 15.7 (4.0) | 16.2 (4.5) | 16.7 (4.9) | 15.9 (4.6) | 15.0 (3.5) | 16.0 (4.4) | P<0.001 |
| Height, cm | 170.8 (6.4) | 164.8 (6.5) | 164.8 (6.2) | 162.7 (5.6) | 166.3 (6.7) | 160.0 (5.4) | 161.5 (5.8) | P<0.001 |
| Weight, kg | 67.8 (12.3) | 64.7 (12.1) | 68.1 (15.1) | 67.6 (12.4) | 69.2 (16.8) | 59.5 (12.4) | 65.3 (13.5) | P<0.001 |
| Body mass index, kg/m2 | 23.2 (4.0) | 23.7 (3.9) | 25.0 (5.5) | 25.5 (4.5) | 25.0 (5.6) | 23.2 (4.7) | 25.0 (5.0) | P<0.001 |
| Maternal overweight and obesity, % | 23.1 | 28.0 | 40.8 | 49.9 | 38.6 | 24.9 | 41.1 | P<0.001 |
| Total gestational weight gain, kg | 15.4 (5.4) | 14.3 (6.5) | 13.3 (7.2) | 11.3 (6.6) | 14.1 (7.3) | 12.6 (6.3) | 14.3 (6.1) | P<0.001 |
| First trimester weight gain, kg/wk | 0.18 (0.22) | 0.19 (0.30) | 0.19 (0.35) | 0.13 (0.38) | 0.18 (0.32) | 0.14 (0.28) | 0.17 (0.30) | P=0.009 |
| Second trimester weight gain, kg/wk | 0.50 (0.23) | 0.47 (0.29) | 0.44 (0.28) | 0.42 (0.30) | 0.45 (0.24) | 0.42 (0.24) | 0.52 (0.23) | P<0.001 |
| Third trimester weight gain, kg/wk | 0.57 (0.37) | 0.45 (0.44) | 0.50 (0.38) | 0.46 (0.51) | 0.54 (0.44) | 0.46 (0.43) | 0.53 (0.34) | P<0.001 |
| Parity, % Nulliparous | 59.5 | 55.1 | 57.1 | 38.9 | 55.2 | 57.4 | 45.5 | P<0.001 |
| Educational level, % | P<0.001 | |||||||
| Primary | 4.0 | 24.2 | 17.5 | 28.8 | 11.5 | 14.1 | 34.0 | |
| Secondary | 38.6 | 66.1 | 67.5 | 58.8 | 74.0 | 71.8 | 53.3 | |
| Higher | 57.4 | 9.6 | 14.9 | 12.4 | 14.5 | 14.1 | 12.7 | |
| Income, % | P<0.001 | |||||||
| <2000 euro | 19.7 | 84.2 | 78.2 | 84.7 | 69.2 | 65.1 | 81.7 | |
| 2000-2200 euro | 6.9 | 5.4 | 5.7 | 5.9 | 5.1 | 9.7 | 5.0 | |
| >2200 euro | 73.3 | 10.4 | 16.1 | 9.3 | 25.6 | 25.1 | 13.3 | |
| Marital status, % Married/living together | 92.2 | 49.1 | 48.1 | 96.1 | 44.5 | 77.2 | 94.3 | P<0.001 |
| Smoking habits, % | P<0.001 | |||||||
| None | 72.9 | 67.3 | 71.3 | 92.8 | 65.1 | 77.1 | 60.4 | |
| First trimester only | 9.1 | 10.7 | 8.4 | 1.6 | 14.0 | 7.1 | 6.9 | |
| Continued | 18.0 | 22.0 | 20.3 | 5.5 | 21.0 | 15.8 | 32.7 | |
| Alcohol consumption, % | P<0.001 | |||||||
| None | 34.7 | 52.3 | 56.3 | 96.3 | 47.8 | 70.5 | 90.4 | |
| First trimester only | 16.2 | 18.3 | 14.6 | 0.6 | 15.6 | 13.9 | 2.5 | |
| Continued | 49.1 | 29.3 | 29.1 | 3.0 | 36.6 | 15.6 | 7.0 | |
| Folic acid supplement use, % | P<0.001 | |||||||
| No use | 11.8 | 65.4 | 52.7 | 69.5 | 49.2 | 46.7 | 57.3 | |
| First 10-wk use | 33.3 | 23.6 | 26.3 | 17.9 | 33.2 | 33.0 | 25.3 | |
| Periconceptional use | 54.9 | 11.0 | 21.0 | 12.6 | 17.6 | 20.3 | 17.3 | |
| Diet, total energy intake (kcal) | 2145 (511) | 1870 (624) | 1921 (616) | 1911.4 (623) | 1776 (569) | 1781 (626) | 1851 (630) | P<0.001 |
| Pregnancy complications, % | ||||||||
| Gestational hypertensive disorders | 7.0 | 7.1 | 6.1 | 2.5 | 8.0 | 7.0 | 3.3 | P<0.001 |
| Gestational diabetes | 1.0 | 1.2 | 0.8 | 2.0 | 1.3 | 2.5 | 1.1 | P=0.231 |
| Delivery and birth characteristics | ||||||||
| Gestational age at birth, wks | 39.9 (1.8) | 39.7 (2.1) | 39.4 (2.2) | 40.2 (1.6) | 39.4 (2.5) | 39.2 (1.7) | 39.8 (1.7) | P<0.001 |
| Birth weight, g | 3481 (559) | 3243 (535) | 3212 (568) | 3524 (497) | 3202 (617) | 3051 (527) | 3402 (514) | P<0.001 |
| Sex, % Boy | 50.6 | 48.3 | 48.9 | 52.1 | 55.2 | 49.4 | 52.6 | P=0.563 |
| Caesarian section, % | 12.7 | 14.1 | 11.9 | 8.0 | 14.2 | 12.2 | 8.5 | P=0.003 |
| Preterm birth2, % | 5.6 | 6.7 | 10.3 | 3.6 | 8.4 | 8.2 | 6.0 | P=0.002 |
| Small for gestational age2, % | 7.8 | 17.9 | 15.9 | 5.8 | 18.7 | 24.6 | 9.2 | P<0.001 |
| Large for gestational age2, % | 12.1 | 1.1 | 2.1 | 0.9 | 1.7 | 1.2 | 0.9 | P<0.001 |
Values are mean (SD), median (95% range) or percentages. P-value was estimated by using One-Way ANOVA test for continuous variables and Chi-square tests for proportions.
SGA is defined as <10th percentile of age-and sex-adjusted birth weight; LGA is defined as 90th percentile of age-and sex-adjusted birth weight; preterm birth is defined as <37 weeks of gestation.
Ethnicity and prepregnancy body mass index
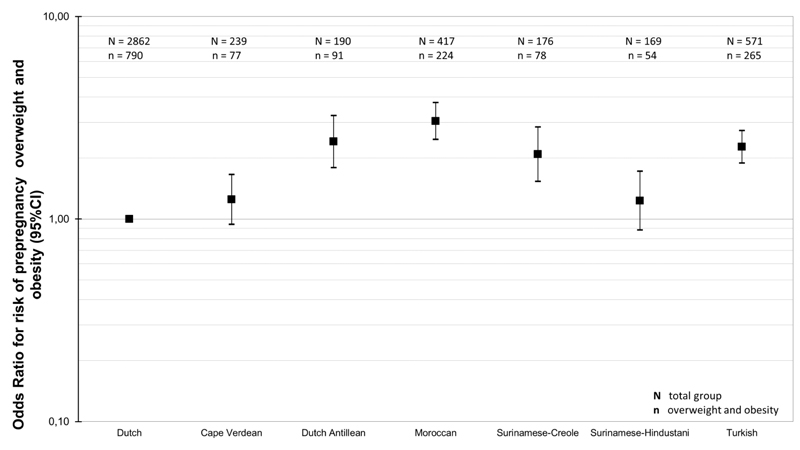
Figure 2 shows that as compared to Dutch-origin women, Dutch Antillean-origin, Surinamese-Creole-origin, Moroccan-origin and Turkish-origin women had a higher risk of prepregnancy overweight or obesity (all p-values <0.05). The highest risk was present among Moroccan-origin women (Odds ratio (OR): 3.04 (95% Confidence Interval (CI) 2.47 to 3.75)). Table 2 shows the associations of ethnicity with prepregnancy body mass index in different models. In the crude model, Dutch Antillean-origin, Moroccan-origin, Surinamese-Creole-origin and Turkish-origin women had a higher prepregnancy body mass index compared to Dutch-origin women, with the strongest association present for Moroccan-origin women (difference: 2.31 kg/m2 (95% CI: 1.88 to 2.73)). Socio-demographic and lifestyle related characteristics explained up to 42% and 45% of these differences, respectively. In the fully adjusted model, Dutch Antillean-origin, Moroccan-origin, Surinamese-Creole-origin and Turkish-origin women still had a higher prepregnancy body mass index as compared to Dutch-origin women (all p-values <0.05).
Figure 2. Risks of prepregnancy overweight or obesity among different ethnic groups.
Abbreviations: BMI; body mass index; OR; odds ratios; CI; confidence interval; Values are odds ratios (95% CI) that reflect the risks of maternal prepregnancy overweight and obesity for each ethnicity group as compared to Dutch-origin women, obtained from logistic regression analyses.
Table 2. Associations of maternal ethnicity with prepregnancy body mass index (kg/m2) (N=5,437).
| Ethnicity groups | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dutch-origin N = 3427 |
Cape Verdean-origin N = 275 |
Dutch Antillean-origin N = 223 |
Moroccan-origin N = 449 |
Surinamese-Creole-origin N = 202 |
Surinamese-Hindustani-origin N = 217 |
Turkish-origin N = 644 |
|||||||
| β (95% CI ) | %-C | β (95% CI ) | %-C | β (95% CI ) | %-C | β (95% CI ) | %-C | β (95% CI ) | %-C | β (95% CI ) | %-C | ||
| Crude model1 | Reference | 0.50 (-0.04,1.03) |
- | 1.83 (1.25,2.42)* |
- | 2.31 (1.88,2.73)* |
- | 1.75 (1.14,2.37)* |
- | -0.06 (-0.65,0.54) |
- | 1.80 (1.44,2.17)* |
- |
| +Socio-demographic characteristics2 | Reference | 0.13 (-0.44,0.70) |
NA | 1.65 (1.03,2.27)* |
-9.84 | 1.44 (0.97,1.92)* |
-37.7 | 1.57 (0.93,2.21)* |
-10.3 | -0.50 (-1.10,0.10) |
NA | 1.04 (0.62,1.47)* |
-42.2 |
| + Lifestyle characteristics3 | Reference | -0.20 (-0.78,0.38) |
NA | 1.34 (0.71,1.97)* |
-18.8 | 0.85 (0.34,1.36)* |
-40.9 | 1.30 (0.64,1.95)* |
-17.2 | -0.93 (-1.54,-0.31)* |
NA | 0.57 (0.12,1.02)* |
-45.2 |
Values are linear regression coefficients (95% confidence interval) that reflect the difference in prepregnancy body mass index per ethnic background compared with the Dutch-origin (reference) group.
These models were subsequently additionally adjusted for socio-demographic characteristics during pregnancy (maternal age, education, income, marital status and parity).
Finally, these models were subsequently additionally adjusted for life-style related characteristics during pregnancy (maternal smoking during pregnancy, alcohol consumption during pregnancy, folic acid supplement use and diet).
P-value for the associations <0.05. Percentage change (%-C); we calculated the exact percentages of change in the effect estimates after adjustment for different factors, using the formula (% change = (β2 – β1)/ β1* 100).
Ethnicity and gestational weight gain
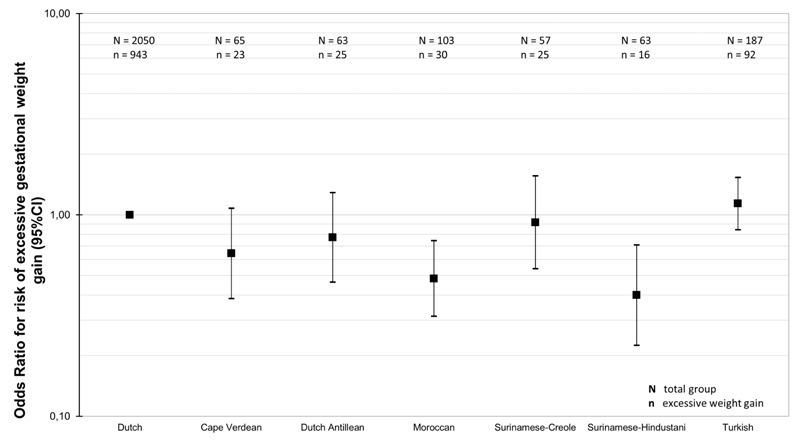
Figure 3 shows that as compared to Dutch-origin women, Surinamese-Hindustani-origin women (OR: 0.40 (95% CI 0.23 to 0.71)) and Moroccan-origin women (OR: 0.48 (95% CI 0.31 to 0.74)) had lower risks of excessive gestational weight gain. Table 3 shows that in the crude model, Dutch Antillean-origin, Moroccan-origin, Surinamese-Hindustani-origin and Turkish-origin women gained significantly lower total amount of gestational weight than Dutch-origin women, with the strongest association present for Moroccan-origin mothers (difference: -0.10 kg/wk (95% CI: -0.13 to -0.08)). Lifestyle related characteristics and pregnancy related characteristics explained up to 33% and 50% of ethnic differences in total gestational weight gain, respectively. In the fully adjusted models, Moroccan-origin and Surinamese-Hindustani-origin women still had a lower weight gain as compared to Dutch-origin women (all p-values <0.05).
Figure 3. Risks of excessive gestational weight gain among different ethnic groups.
Abbreviations: BMI; body mass index; OR; odds ratios; CI; confidence interval; Values are odds ratios (95% CI) that reflect the risks of excessive gestational weight gain for each ethnicity group as compared to Dutch-origin women, obtained from logistic regression analyses.
Table 3. Associations of maternal ethnicity with total gestational weight gain (kg/wk) (N=2,588).
| Ethnicity groups | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dutch-origin N = 2,050 |
Cape Verdean-origin N = 65 |
Dutch Antillean-origin N = 63 |
Moroccan-origin N =103 |
Surinamese-Creole-origin N = 57 |
Surinamese-Hindustani-origin N = 63 |
Turkish-origin N = 187 |
|||||||
| β (95% CI ) | %-C | β (95% CI ) | %-C | β (95% CI ) | %-C | β (95% CI ) | %-C | β (95% CI ) | %-C | β (95% CI ) | %-C | ||
| Crude model1 | Reference | -0.03 (-0.06,0.01) |
- | -0.05 (-0.09,-0.01)* |
- | -0.10 (-0.13,-0.08)* |
- | -0.03 (-0.07,0.01) |
- | -0.07 (-0.10,-0.03)* |
- | -0.03 (-0.05,-0.01)* |
|
| + Socio-demographic characteristics2 | Reference | -0.04 (-0.08,0)* |
33.3 | -0.07 (-0.10,-0.03)* |
40.0 | -0.10 (-0.13,-0.07)* |
0 | -0.04 (-0.08,0)* |
33.3 | -0.08 (-0.11,-0.04)* |
14.3 | -0.03 (-0.05,0)* |
0 |
| + Lifestyle related characteristics3 | Reference | -0.03 (-0.06,0.01) |
-25.0 | -0.05 (-0.09,-0.01)* |
-28.6 | -0.07 (-0.10,-0.04)* |
-30.0 | -0.03 (-0.07,0.01) |
-25.0 | -0.07 (-0.11,-0.04)* |
-12.5 | -0.02 (-0.04,0.01) |
-33.3 |
| + Pregnancy related characteristics4 | Reference | -0.02 (-0.05,0.02) |
-33.3 | -0.03 (-0.07,0) |
-40.0 | -0.07 (-0.10,-0.04)* |
0 | -0.02 (-0.06,0.01) |
-33.3 | -0.05 (-0.09,-0.02)* |
-28.6 | -0.01 (-0.03,0.02) |
-50.0 |
Values are linear regression coefficients (95% confidence interval) that reflect the difference in total gestational weight gain (kg/wk) per ethnic background compared with the Dutch-origin (reference) group.
These models were subsequently additionally adjusted for socio-demographic characteristics during pregnancy (maternal age, education, income, marital status and parity).
Third, these models were subsequently additionally adjusted for life-style related characteristics during pregnancy (body mass index (kg/m2) at the start of pregnancy, maternal smoking during pregnancy, alcohol consumption during pregnancy, folic acid supplement use and diet).
Finally, these models were subsequently additionally adjusted for pregnancy related characteristics (pregnancy complications, gestational age at birth, birth weight).
P-value for the associations <0.05. Percentage change (%-C); we calculated the exact percentages of change in the effect estimates after adjustment for different factors, using the formula (% change = (β2 – β1)/ β1* 100).
Table 4 shows the ethnic differences in trimester specific weight gain. In the crude model, Moroccan-origin and Surinamese-Hindustani-origin women, but not women from other ethnic backgrounds, gained less weight in early pregnancy than Dutch-origin women (p-values <0.05). Additional adjustment for lifestyle related characteristics, but not socio-demographic characteristics, fully explained the differences for Moroccan-origin women. Dutch Antillean-origin, Moroccan-origin, Surinamese-Creole-origin and Surinamese-Hindustani-origin women gained significantly less weight in mid-pregnancy as compared to Dutch-origin women (p-values <0.05). Adjustment for lifestyle related characteristics fully explained the differences for Dutch Antillean-origin and Surinamese-Creole-origin women. In late pregnancy, only Cape Verdean-origin, Moroccan-origin and Surinamese-Hindustani-origin women had significantly lower gestational weight gain as compared to Dutch-origin women (p-values in the crude models <0.05). Adjustment for lifestyle related characteristics did not explain these ethnic differences in late pregnancy weight gain. Adjustment for pregnancy related characteristics fully explained the ethnic differences for Surinamese-Hindustani-origin women, but not for Cape Verdean-origin and Moroccan-origin women.
Table 4. Associations of maternal ethnicity with critical periods of gestational weight gain (kg/wk) (N=4,474)1.
| Ethnicity groups | |||||||
|---|---|---|---|---|---|---|---|
| Early pregnancy | Dutch-origin N = 2,948 |
Cape Verdean-origin N = 213 |
Dutch Antillean-origin N = 167 |
Moroccan-origin N = 326 |
Surinamese-Creole-origin N = 149 |
Surinamese-Hindustani-origin N = 186 |
Turkish-origin N = 485 |
| β (95% CI ) | β (95% CI ) | β (95% CI ) | β (95% CI ) | β (95% CI ) | β (95% CI ) | β (95% CI ) | |
| Crude model1 | Reference | 0.01 (-0.03,0.05) | 0.01 (-0.03,0.05) | -0.05 (-0.08,-0.02)* | 0.00 (-0.04,0.04) | -0.05 (-0.08,-0 01)* | -0.01 (-0.04,0.01) |
| + Socio-demographic | Reference | -0.02 (-0.06,0.02) | -0.01 (-0.05,0.03) | -0.06 (-0.09,-0.02)* | -0.03 (-0.07,0.02) | -0.06 (-0.10,-0.02)* | -0.01 (-0.04,0.02) |
| + Lifestyle related characteristics3 | Reference | 0 (-0.04,0.04) | 0.01 (-0.03,0.06) | -0.03 (-0.07,0) | -0.02 (-0.06,0.03) | -0.05 (-0.09,-0.01)* | -0.01 (-0.04,0.02) |
| + Pregnancy related characteristics4 | Reference | 0.01 (-0.03,0.05) | 0.02 (-0.02,0.07) | -0.03 (-0.07,0.01) | -0.01 (-0.05,0.04) | -0.03 (-0.07,0.01) | -0.01 (-0.04,0.02) |
| Ethnicity groups | |||||||
| Mid pregnancy |
Dutch-origin N = 3,312 |
Cape Verdean-origin N = 269 |
Dutch Antillean-origin N =196 |
Moroccan-origin N = 381 |
Surinamese-Creole-origin N =168 |
Surinamese-Hindustani-origin N = 203 |
Turkish-origin N = 537 |
| β (95% CI ) | β (95% CI ) | β (95% CI ) | β (95% CI ) | β (95% CI ) | β (95% CI ) | β (95% CI ) | |
| Crude model1 | Reference | -0.03 (-0.06,0) | -0.06 (-0.10,-0.03)* | -0.08 (-0.11,-0.05)* | -0.05 (0.09,-0.01)* | -0.08 (-0.12,-0 05)* | 0.01 (-0.01,0.04) |
| + Socio-demographic | Reference | -0.02 (-0.05,0.01) | -0.06 (-0.10,-0.02)* | -0.06 (-0.09,-0.03)* | -0.04 (-0.08,0)* | -0.08 (-0.11,-0.04)* | 0.03 (0,0.05)* |
| + Lifestyle related characteristics3 | Reference | 0 (-0.04,0.04) | -0.04 (-0.08,0) | -0.04 (-0.07,0)* | -0.02 (-0.06,0.02) | -0.08 (-0.12,-0.04)* | 0.03 (0,0.05) |
| + Pregnancy related characteristics4 | Reference | 0.01 (-0.02,0.05) | -0.02 (-0.06,0.02) | -0.03 (-0.07,0) | -0.01 (-0.05,0.04) | -0.05 (-0.09,-0.01) | 0.03 (0,0.06)* |
| Ethnicity groups | |||||||
| Late pregnancy |
Dutch-origin N = 3,312 |
Cape Verdean-origin N = 269 |
Dutch Antillean-origin N =196 |
Moroccan-origin N = 381 |
Surinamese-Creole-origin N =168 |
Surinamese-Hindustani-origin |
Turkish-origin N = 537 |
| β (95% CI ) | β (95% CI ) | β (95% CI ) | β (95% CI ) | β (95% CI ) | β (95% CI ) | β (95% CI ) | |
| Crude model1 | Reference | -0.13 (-0.21,-0 04)* | -0.07 (-0.16,0.02) | -0.11 (-0.18,-0.04)* | -0.04 (-0.14,0.07) | -0.11 (-0.20,-0 02)* | -0.04 (-0.10,0.01) |
| + Socio-demographic | Reference | -0.17 (-0.26,-0.08)* | -0.13 (-0.23,-0.04)* | -0.13 (-0.21,-0.06)* | -0.07 (-0.18,0.04) | -0.16 (-0.25,-0.07)* | -0.08 (-0.14,-0.02)* |
| + Lifestyle related characteristics3 | Reference | -0.15 (-0.24,-0.05)* | -0.11 (-0.21,-0.01)* | -0.15 (-0.24,-0.06)* | 0.05 (-0.17,0.06) | -0.11 (-0.21,-0.02)* | -0.07 (-0.14,0) |
| + Pregnancy related characteristics4 | Reference | -0.12 (-0.22,-0.03)* | -0.10 (-0.20,0) | -0.13 (-0.21,-0.04)* | -0.04 (-0.15,0.07) | -0.09 (-0.18,0.01) | -0.05 (-0.12,0.02) |
Values are linear regression coefficients (95% confidence interval) that reflect the difference in weight gain (kg/wk) in critical periods of pregnancy per ethnic background compared with the Dutch-origin (reference) group.
These models were subsequently additionally adjusted for socio-demographic characteristics at the start of pregnancy (maternal age, education, income, marital status and parity).
Third, these models were subsequently additionally adjusted for life-style related characteristics during pregnancy (body mass index (kg/m2) at the start of pregnancy, maternal smoking during pregnancy, alcohol consumption during pregnancy, folic acid supplement use and diet).
Finally, these models were subsequently additionally adjusted for pregnancy related characteristics (pregnancy complications, gestational age at birth, birth weight).
P-value for the associations <0.05.
Comments
This multi-ethnic population-based prospective cohort study in the Netherlands showed that Dutch Antillean-origin, Surinamese-Creole-origin, Moroccan-origin and Turkish-origin women had higher risks of prepregnancy overweight and obesity, as compared to Dutch-origin women. Moroccan-origin and Surinamese-Hindustani-origin women had a decreased risk of gaining excessive gestational weight as compared to Dutch-origin women. The strongest differences in gestational weight gain were present in late pregnancy. Socio-demographic, lifestyle and pregnancy related characteristics only partly explained the observed ethnic differences in maternal prepregnancy overweight and obesity and gestational weight gain.
Methodological considerations
We used a multi-ethnic population-based prospective cohort design including a large number of women who were followed from early pregnancy onwards. We had detailed maternal weight measurements throughout pregnancy available. The response at baseline for participation in the Generation R cohort was 61%. The non-response at baseline would lead to biased effect estimates if associations would be different between women included and not included in the analyses, which seems unlikely (20). Pregnant women who participated were higher educated, healthier and more frequently of Dutch origin than were those who did not participate (12, 21). This selection to a more healthy population might have affected the generalizability of our results. Information about ethnic background was obtained by questionnaires. Questionnaires in different languages were available for mothers who did not understand the Dutch language (12). We classified ethnic background by country of birth of the parents, according to Statistics Netherlands (16). The advantage of this classification is that it is objective and stable over time. However, this approach does not distinguish first, second and third generation migrants and it does not take into account the heterogeneity within ethnic groups. Moreover it does not differentiate between ethnic subgroups. Information about maternal prepregnancy weight and maximum weight during pregnancy was collected by questionnaires. Self-reported weight tends to be underestimated. However, self-reported prepregnancy weight and weight measured at intake, and self-reported maximum weight and weight measured at 30 weeks were strongly correlated. This suggests that our measures were less affected by recall bias. Finally, although adjustment was performed for a large number of maternal socio-demographic, lifestyle and pregnancy related characteristics, residual confounding due to other characteristics, including maternal physical activity and dietary factors, might still be an issue, as in any observational study.
Interpretation of main findings
An accumulating body of evidence suggests that maternal prepregnancy overweight and obesity and excessive gestational weight gain are important risk factors for adverse maternal and fetal pregnancy outcomes, including infertility, gestational hypertension, preeclampsia, gestational diabetes, stillbirth, prematurity, Caesarean delivery, and large size for gestational age infants (1, 3, 22, 23). Maternal prepregnancy overweight and obesity and excessive gestational weight gain may also have long-term maternal and offspring consequences. Multiple studies have shown that these factors are strongly associated with higher levels of maternal postpartum weight retention and an adverse cardio metabolic risk profile in the offspring (24–27). Previous studies reported that prevalences of maternal overweight and obesity and gestational weight gain differ between ethnic groups. These studies have mainly been performed among white, black, Asian and Hispanic women. A large study in the US among 14,613 white, African American, Hispanic and Asian women reported that prepregnancy body mass index was higher in the women of these minority groups, except for Asian women, as compared to white women (28). These ethnic populations do not reflect the major ethnic groups in Western-European countries, and less is known about ethnic differences in these European countries. Due to colonial and immigrant worker history the major ethnic groups in the Netherlands are Cape Verdean-origin, Dutch Antillean-origin, Moroccan-origin, Turkish-origin, Surinamese-Creole-origin and Surinamese-Hindustani-origin.
In this study, we observed that prepregnancy body mass index was higher among women of Dutch Antillean-origin, Moroccan-origin, Surinamese-Creole-origin and Turkish-origin, as compared to Dutch-origin women. The strongest association was present for Moroccan-origin women. Our findings are in line with another Dutch study among 7,871 women that showed that maternal prepregnancy obesity was more prevalent among Turkish-origin women, Moroccan-origin women and women of African descent, as compared to Dutch-origin women (29). Other European studies have also shown increased prepregnancy overweight and obesity prevalences among women from a variety of ethnic minority groups (11, 28, 30–34). A large study from the UK among 619,323 women reported that black descent is associated with higher risks of maternal overweight and obesity during pregnancy (11). A study among 456 women in Spain showed that Moroccan-origin women had a significantly higher prepregnancy body mass index, as compared to Caucasian women (32). A study performed in Switzerland among 1,432 women showed that Asian women had a lower prepregnancy body mass index as compared to Caucasian women. No significant differences in prepregnancy body mass index for black women were present in this study (33).
Other studies have also suggested ethnic differences in the risk of excessive weight gain during pregnancy (10, 35–41). Two large studies from the US among 230,698 women and 52,988 women showed that Hispanic and black women had a decreased risk of excessive gestational weight gain as compared to white women (10, 41). To the best of our knowledge no previous studies among multi-ethnic European populations have been performed. We observed that women from Dutch Antillean-origin, Moroccan-origin, Surinamese-Hindustani-origin and Turkish-origin gained less weight during pregnancy as compared to Dutch-origin women. Gestational weight gain is a complex trait, which reflects multiple components, which may differ according to the timing of gestational weight gain. Maternal first-trimester gestational weight gain largely reflects maternal fat deposition, whereas second- and third-trimester gestational weight gain largely reflects maternal and amniotic fluid expansion, and growth of fetus, placenta and uterus (17, 42). We observed the strongest ethnic differences in late pregnancy weight gain. This may suggest that differences in pregnancy related hemodynamic adaptations and fetal growth partly explain these observed associations.
In line with this hypothesis, we have previously shown within our study cohort that mean birth weight was lower in offspring of Turkish-origin, Cape Verdean-origin, Dutch-Antillean-origin, Surinamese-Creole-origin, Surinamese-Hindustani-origin, as compared to Dutch-origin women (15). Also, differences in blood pressure development during pregnancy and risks of gestational hypertensive disorders were present between women from different ethnic groups (14).
The socio-demographic, lifestyle and pregnancy related characteristics underlying the ethnic differences in maternal prepregnancy overweight and obesity and excessive gestational weight gain are not well-known. Several risk factors, such as maternal lower educational level, multiparity and a lower household income, are associated with an increased risk of prepregnancy overweight and obesity, whereas nulliparity, higher total energy intake and high early prepregnancy body mass index are associated with an increased risk of excessive gestational weight gain (2, 26, 43, 44). Several previous studies have assessed the influence of maternal socio-demographic and lifestyle related characteristics as potential risk factors that might explain ethnic disparities in maternal overweight and obesity during pregnancy and gestational weight gain. The study among 619,323 UK women showed that increasing age, parity, deprivation and unemployment influenced the risk of maternal obesity during pregnancy (11). Two studies performed in the US among 52,988 and 230,698 women showed that age, parity, education, and prepregnancy body mass index were significantly associated with ethnic differences in gestational weight gain (10, 41). In the current study, we observed that socio-demographic characteristics did not explain ethnic differences in the risk of prepregnancy overweight and obesity and excessive gestational weight gain. Lifestyle and pregnancy related characteristics explained up to 33% and 40% of the ethnic differences in maternal weight outcomes. We used a stepwise approach adding socio-demographic, lifestyle and pregnancy related risk factors to the models in additive fashion to identify critical groups of risk factors for development of preventive strategies. However, even after full adjustment for a large number of potential confounders, ethnicity was still associated with differences in maternal prepregnancy body mass index and gestational weight gain. Other environmental factors, genetic variants and gene-environment interactions may explain part of the observed associations. Further studies are needed to obtain insight into these complex underlying mechanisms.
Conclusion
This population based prospective cohort study in the Netherlands showed ethnic disparities in maternal prepregnancy overweight and obesity and gestational weight gain. Although we included a wide range of known risk factors, these ethnic disparities were not fully explained by maternal socio-demographic, lifestyle and pregnancy related characteristics. Whether the ethnic differences in maternal prepregnancy overweight and obesity and gestational weight gain also lead to ethnic difference in maternal and childhood outcomes should be further studied. Based on our findings, preventive strategies focused on reducing maternal prepregnancy overweight and obesity should target women from ethnic minority groups, whereas preventive strategies focused on reducing excessive gestational weight gain should target Dutch-origin women.
Supplementary Material
Acknowledgements
The Generation R Study is conducted by the Erasmus Medical Center in close collaboration with the School of Law and Faculty of Social Sciences of the Erasmus University Rotterdam, the Municipal Health Service Rotterdam area, Rotterdam, the Rotterdam Homecare Foundation, Rotterdam and the Stichting Trombosedienst and Artsenlaboratorium Rijnmond (STAR), Rotterdam.
We gratefully acknowledge the contribution of participating mothers, general practitioners, hospitals, midwives and pharmacies in Rotterdam.
Sources of Funding
The general design of Generation R Study is made possible by financial support from the Erasmus Medical Center, Rotterdam, the Erasmus University Rotterdam, the Netherlands Organization for Health Research and Development (ZonMw), the Netherlands Organisation for Scientific Research (NWO), the Ministry of Health, Welfare and Sport and the Ministry of Youth and Families. The research leading to these results has received funding from the European Union's Seventh Framework Programme (FP7/2007-2013), project EarlyNutrition under grant agreement n°289346. Vincent Jaddoe received an additional grant from the Netherlands Organization for Health Research and Development (VIDI 016.136.361).
Role of the Sponsor: The funding agencies had no role in the design and conduct of the study; nor in the collection, management, analysis and interpretation of the data; nor in the preparation, review, and approval of the manuscript.
Footnotes
Conflict of Interest Statement: The authors declare no conflict of interest.
Disclosures: none
References
- 1.Aune D, Saugstad OD, Henriksen T, Tonstad S. Maternal body mass index and the risk of fetal death, stillbirth, and infant death: a systematic review and meta-analysis. JAMA. 2014 Apr 16;311(15):1536–46. doi: 10.1001/jama.2014.2269. [DOI] [PubMed] [Google Scholar]
- 2.Gaillard R, Durmus B, Hofman A, Mackenbach JP, Steegers EA, Jaddoe VW. Risk factors and outcomes of maternal obesity and excessive weight gain during pregnancy. Obesity (Silver Spring) 2013 May;21(5):1046–55. doi: 10.1002/oby.20088. [DOI] [PubMed] [Google Scholar]
- 3.Ruager-Martin R, Hyde MJ, Modi N. Maternal obesity and infant outcomes. Early Hum Dev. 2010 Nov;86(11):715–22. doi: 10.1016/j.earlhumdev.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 4.Bhattacharya S, Campbell DM, Liston WA, Bhattacharya S. Effect of Body Mass Index on pregnancy outcomes in nulliparous women delivering singleton babies. BMC Public Health. 2007;7:168. doi: 10.1186/1471-2458-7-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Athukorala C, Rumbold AR, Willson KJ, Crowther CA. The risk of adverse pregnancy outcomes in women who are overweight or obese. BMC Pregnancy Childbirth. 2010;10:56. doi: 10.1186/1471-2393-10-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weiss JL, Malone FD, Emig D, Ball RH, Nyberg DA, Comstock CH, et al. Obesity, obstetric complications and cesarean delivery rate--a population-based screening study. Am J Obstet Gynecol. 2004 Apr;190(4):1091–7. doi: 10.1016/j.ajog.2003.09.058. [DOI] [PubMed] [Google Scholar]
- 7.Ramos GA, Caughey AB. The interrelationship between ethnicity and obesity on obstetric outcomes. Am J Obstet Gynecol. 2005 Sep;193(3 Pt 2):1089–93. doi: 10.1016/j.ajog.2005.06.040. [DOI] [PubMed] [Google Scholar]
- 8.Ehrenberg HM, Dierker L, Milluzzi C, Mercer BM. Prevalence of maternal obesity in an urban center. Am J Obstet Gynecol. 2002 Nov;187(5):1189–93. doi: 10.1067/mob.2002.127125. [DOI] [PubMed] [Google Scholar]
- 9.Fontaine PL, Hellerstedt WL, Dayman CE, Wall MM, Sherwood NE. Evaluating body mass index-specific trimester weight gain recommendations: differences between black and white women. J Midwifery Womens Health. 2012 Jul-Aug;57(4):327–35. doi: 10.1111/j.1542-2011.2011.00139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pawlak MT, Alvarez BT, Jones DM, Lezotte DC. The Effect of Race/Ethnicity on Gestational Weight Gain. J Immigr Minor Health. 2013 Aug 10; doi: 10.1007/s10903-013-9886-5. [DOI] [PubMed] [Google Scholar]
- 11.Heslehurst N, Rankin J, Wilkinson JR, Summerbell CD. A nationally representative study of maternal obesity in England, UK: trends in incidence and demographic inequalities in 619 323 births, 1989-2007. Int J Obes (Lond) 2010 Mar;34(3):420–8. doi: 10.1038/ijo.2009.250. [DOI] [PubMed] [Google Scholar]
- 12.Jaddoe VW, van Duijn CM, Franco OH, van der Heijden AJ, van Iizendoorn MH, de Jongste JC, et al. The Generation R Study: design and cohort update 2012. Eur J Epidemiol. 2012 Sep;27(9):739–56. doi: 10.1007/s10654-012-9735-1. [DOI] [PubMed] [Google Scholar]
- 13.Demografie van (niet-westerse) allochtonen in Nederland. Centraal Bureau voor de Statistiek; 2010. [database on the Internet]. Available from: http://www.cbs.nl/NR/rdonlyres/240E5858-D04D-47EA-9C22-AB8E9297E2D2/0/2010k4b15p22art.pdf. [Google Scholar]
- 14.Bouthoorn SH, Gaillard R, Steegers EA, Hofman A, Jaddoe VW, van Lenthe FJ, et al. Ethnic differences in blood pressure and hypertensive complications during pregnancy: the Generation R study. Hypertension. 2012 Jul;60(1):198–205. doi: 10.1161/HYPERTENSIONAHA.112.194365. [DOI] [PubMed] [Google Scholar]
- 15.Troe EJ, Raat H, Jaddoe VW, Hofman A, Looman CW, Moll HA, et al. Explaining differences in birthweight between ethnic populations. The Generation R Study. BJOG. 2007 Dec;114(12):1557–65. doi: 10.1111/j.1471-0528.2007.01508.x. [DOI] [PubMed] [Google Scholar]
- 16.Allochtonen in Nederland 2004. Centraal Bureau voor de Statistiek; 2004. [database on the Internet]. Available from: http://www.cbs.nl/NR/rdonlyres/BD11B2EE-83DE-47FB-9ABC-F8FFFE1F3C07/0/2004b52pub.pdf. [Google Scholar]
- 17.Rasmussen KM, Y A, editors. The Institute of Medicine (US) and National Research Council (US) Committee to Reexamine IOM Pregnancy Weight Guidelines. Weight Gain During Pregnancy: Reexamining the Guidelines. Washington (DC): National Academies Press (US); 2009. pp. 1–4. [PubMed] [Google Scholar]
- 18.Klipstein-Grobusch K, den Breeijen JH, Goldbohm RA, Geleijnse JM, Hofman A, Grobbee DE, et al. Dietary assessment in the elderly: validation of a semiquantitative food frequency questionnaire. Eur J Clin Nutr. 1998 Aug;52(8):588–96. doi: 10.1038/sj.ejcn.1600611. [DOI] [PubMed] [Google Scholar]
- 19.Sterne JA, White IR, Carlin JB, Spratt M, Royston P, Kenward MG, et al. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ. 2009;338:b2393. doi: 10.1136/bmj.b2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nohr EA, Frydenberg M, Henriksen TB, Olsen J. Does low participation in cohort studies induce bias? Epidemiology. 2006 Jul;17(4):413–8. doi: 10.1097/01.ede.0000220549.14177.60. [DOI] [PubMed] [Google Scholar]
- 21.Rotterdam G. Rotterdam Onderzoek. [website]; Available from: http://www.rotterdam.nl/onderzoek.
- 22.Poston L, Harthoorn LF, Van Der Beek EM, Contributors to the IEW Obesity in pregnancy: implications for the mother and lifelong health of the child. A consensus statement. Pediatr Res. 2011 Feb;69(2):175–80. doi: 10.1203/PDR.0b013e3182055ede. [DOI] [PubMed] [Google Scholar]
- 23.McDonald SD, Han Z, Mulla S, Beyene J, Knowledge Synthesis G Overweight and obesity in mothers and risk of preterm birth and low birth weight infants: systematic review and meta-analyses. BMJ. 2010;341:c3428. doi: 10.1136/bmj.c3428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gaillard R, Steegers EA, Duijts L, Felix JF, Hofman A, Franco OH, et al. Childhood cardiometabolic outcomes of maternal obesity during pregnancy: the generation R study. Hypertension. 2014 Apr;63(4):683–91. doi: 10.1161/HYPERTENSIONAHA.113.02671. [DOI] [PubMed] [Google Scholar]
- 25.Reynolds RM, Allan KM, Raja EA, Bhattacharya S, McNeill G, Hannaford PC, et al. Maternal obesity during pregnancy and premature mortality from cardiovascular event in adult offspring: follow-up of 1 323 275 person years. BMJ. 2013;347:f4539. doi: 10.1136/bmj.f4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nohr EA, Vaeth M, Baker JL, Sorensen T, Olsen J, Rasmussen KM. Combined associations of prepregnancy body mass index and gestational weight gain with the outcome of pregnancy. Am J Clin Nutr. 2008 Jun;87(6):1750–9. doi: 10.1093/ajcn/87.6.1750. [DOI] [PubMed] [Google Scholar]
- 27.Dyer JS, Rosenfeld CR. Metabolic imprinting by prenatal, perinatal, and postnatal overnutrition: a review. Semin Reprod Med. 2011 May;29(3):266–76. doi: 10.1055/s-0031-1275521. [DOI] [PubMed] [Google Scholar]
- 28.Solomon CG, Willett WC, Carey VJ, Rich-Edwards J, Hunter DJ, Colditz GA, et al. A prospective study of pregravid determinants of gestational diabetes mellitus. JAMA. 1997 Oct 1;278(13):1078–83. [PubMed] [Google Scholar]
- 29.Djelantik AA, Kunst AE, van der Wal MF, Smit HA, Vrijkotte TG. Contribution of overweight and obesity to the occurrence of adverse pregnancy outcomes in a multi-ethnic cohort: population attributive fractions for Amsterdam. BJOG. 2012 Feb;119(3):283–90. doi: 10.1111/j.1471-0528.2011.03205.x. [DOI] [PubMed] [Google Scholar]
- 30.Oteng-Ntim E, Kopeika J, Seed P, Wandiembe S, Doyle P. Impact of obesity on pregnancy outcome in different ethnic groups: calculating population attributable fractions. PLoS One. 2013;8(1):e53749. doi: 10.1371/journal.pone.0053749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bryant M, Santorelli G, Lawlor DA, Farrar D, Tuffnell D, Bhopal R, et al. A comparison of South Asian specific and established BMI thresholds for determining obesity prevalence in pregnancy and predicting pregnancy complications: findings from the Born in Bradford cohort. Int J Obes (Lond) 2014 Mar;38(3):444–50. doi: 10.1038/ijo.2013.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hernandez-Rivas E, Flores-Le Roux JA, Benaiges D, Sagarra E, Chillaron JJ, Paya A, et al. Gestational diabetes in a multiethnic population of Spain: clinical characteristics and perinatal outcomes. Diabetes Res Clin Pract. 2013 May;100(2):215–21. doi: 10.1016/j.diabres.2013.01.030. [DOI] [PubMed] [Google Scholar]
- 33.Ochsenbein-Kolble N, Roos M, Gasser T, Zimmermann R. Cross-sectional study of weight gain and increase in BMI throughout pregnancy. Eur J Obstet Gynecol Reprod Biol. 2007 Feb;130(2):180–6. doi: 10.1016/j.ejogrb.2006.03.024. [DOI] [PubMed] [Google Scholar]
- 34.Steinfeld JD, Valentine S, Lerer T, Ingardia CJ, Wax JR, Curry SL. Obesity-related complications of pregnancy vary by race. J Matern Fetal Med. 2000 Jul-Aug;9(4):238–41. doi: 10.1002/1520-6661(200007/08)9:4<238::AID-MFM10>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 35.Sridhar SB, Darbinian J, Ehrlich SF, Markman MA, Gunderson EP, Ferrara A, et al. Maternal gestational weight gain and offspring risk for childhood overweight or obesity. Am J Obstet Gynecol. 2014 Apr 12; doi: 10.1016/j.ajog.2014.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu J, Gallagher AE, Carta CM, Torres ME, Moran R, Wilcox S. Racial differences in gestational weight gain and pregnancy-related hypertension. Ann Epidemiol. 2014 Mar 3; doi: 10.1016/j.annepidem.2014.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bowers K, Laughon SK, Kiely M, Brite J, Chen Z, Zhang C. Gestational diabetes, pre-pregnancy obesity and pregnancy weight gain in relation to excess fetal growth: variations by race/ethnicity. Diabetologia. 2013 Jun;56(6):1263–71. doi: 10.1007/s00125-013-2881-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Krukowski RA, Bursac Z, McGehee MA, West D. Exploring potential health disparities in excessive gestational weight gain. J Womens Health (Larchmt) 2013 Jun;22(6):494–500. doi: 10.1089/jwh.2012.3998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huynh M, Borrell LN, Chambers EC. Maternal education and excessive gestational weight gain in New York city, 1999-2001: the effect of race/ethnicity and neighborhood socioeconomic status. Matern Child Health J. 2014 Jan;18(1):138–45. doi: 10.1007/s10995-013-1246-5. [DOI] [PubMed] [Google Scholar]
- 40.Caulfield LE, Witter FR, Stoltzfus RJ. Determinants of gestational weight gain outside the recommended ranges among black and white women. Obstet Gynecol. 1996 May;87(5 Pt 1):760–6. doi: 10.1016/0029-7844(96)00023-3. [DOI] [PubMed] [Google Scholar]
- 41.Chu SY, Callaghan WM, Bish CL, D'Angelo D. Gestational weight gain by body mass index among US women delivering live births, 2004-2005: fueling future obesity. Am J Obstet Gynecol. 2009 Mar;200(3):271 e1–7. doi: 10.1016/j.ajog.2008.09.879. [DOI] [PubMed] [Google Scholar]
- 42.Ay L, Kruithof CJ, Bakker R, Steegers EA, Witteman JC, Moll HA, et al. Maternal anthropometrics are associated with fetal size in different periods of pregnancy and at birth. The Generation R Study. BJOG. 2009 Jun;116(7):953–63. doi: 10.1111/j.1471-0528.2009.02143.x. [DOI] [PubMed] [Google Scholar]
- 43.Stuebe AM, Oken E, Gillman MW. Associations of diet and physical activity during pregnancy with risk for excessive gestational weight gain. Am J Obstet Gynecol. 2009 Jul;201(1):58 e1–8. doi: 10.1016/j.ajog.2009.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Herring SJ, Nelson DB, Davey A, Klotz AA, Dibble LV, Oken E, et al. Determinants of excessive gestational weight gain in urban, low-income women. Womens Health Issues. 2012 Sep;22(5):e439–46. doi: 10.1016/j.whi.2012.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.