Abstract
Objective:
To identify the functional and pathologic correlates underlying subjective memory complaints (SMCs) in cognitively normal older adults.
Methods:
Two hundred fifty-one older adults underwent resting-state fluorodeoxyglucose (FDG)-PET and Pittsburg compound B-PET β-amyloid (Aβ) imaging and filled out a questionnaire regarding SMCs. Participants were classified into 2 groups based on their Aβ burden. Age-adjusted voxel-wise correlations were used to examine SMCs, amyloid status (Aβ+ vs Aβ−), and the interaction between SMCs and Aβ status as predictors of metabolism. Region-of-interest (ROI) analyses were performed to confirm the whole-brain analyses and to test for additional covariates.
Results:
Greater SMCs correlated with decreased FDG metabolism in the bilateral precuneus, bilateral inferior parietal lobes, right inferior temporal lobe, right medial frontal gyrus, and right orbitofrontal gyrus. A significant interaction effect between SMCs and amyloid burden was found such that Aβ+ individuals with increased complaints had decreased FDG metabolism in the bilateral medial temporal lobes. ROI analyses confirmed the voxel-wise analyses result in that decreased precuneus metabolism was associated with greater SMCs regardless of Aβ status, age, or thickness, whereas the relationship between hippocampal metabolism and SMCs was a function of Aβ, even after adjustment for age, hippocampal volume, or depressive symptoms.
Conclusions:
These data show the relevant role of posterior and anterior midline regions in SMCs in older individuals. Decreased hippocampal metabolism may be a specific marker of subclinical changes in cognition due to amyloid pathology. However, longitudinal studies are needed to determine whether our findings foreshadow clinical decline.
Subjective memory complaint (SMC) is defined by self-reports of memory worsening and objective memory performance in the normal adjusted range. SMCs are common in the elderly population without dementia, with an estimated prevalence range between 22% and 56%,1 and have been associated with increased risk of incident Alzheimer disease (AD).2 Despite the high prevalence of self-perceived changes in memory among older adults, relatively little is known about its functional and pathologic correlates, especially in the preclinical stages of AD. This information is crucial because SMCs alone may have insufficient sensitivity and specificity to predict the development of dementia.
With regard to SMCs and β-amyloid (Aβ; one hallmark of AD pathology most commonly used to define the preclinical stage), previous studies have found conflicting results. Whereas most studies have reported an association between SMCs and Aβ,3–6 others could not demonstrate this relationship.7 Previous studies with fluorodeoxyglucose (FDG)-PET, a biomarker supposed to reflect neuronal integrity,8 have demonstrated hypometabolism when comparing cognitively normal (CN) individuals with and without SMCs, but the results have been inconsistent as to which brain regions show hypometabolism, e.g., the precuneus,9 parietotemporal and parahippocampal gyrus,10 or periventricular region.11 These inconsistent patterns of FDG-PET hypometabolism could be due to several factors, including the use of different techniques to measure SMCs. In addition, although FDG metabolism has been studied in groups of individuals with and without SMCs, correlation between continuous measures of self-reported complaints and FDG metabolism has never been assessed. Because the degree of memory complaints varies greatly within older adults, investigating the changes in metabolic function that covary with self-appraisal of memory function may shed some additional light on the brain substrate underlying SMCs. Lastly, the contribution of Aβ pathology to the functional metabolic correlates of SMCs in CN elders also remains to be elucidated. This information would improve our ability to understand whether SMCs may have prognostic meaning.
The objective of the present study is to further our understanding of the functional and pathologic substrate underlying SMCs by investigating the relationship between self-reported measures of memory function and functional brain metabolism in a large cohort of CN older adults with known Aβ status. We hypothesized that the metabolic consequences of SMCs might depend on the presence of Aβ pathology and that the occurrence of different FDG patterns might help us better distinguish between SMCs due to decline in cognitive aging from preclinical AD.
METHODS
Subjects.
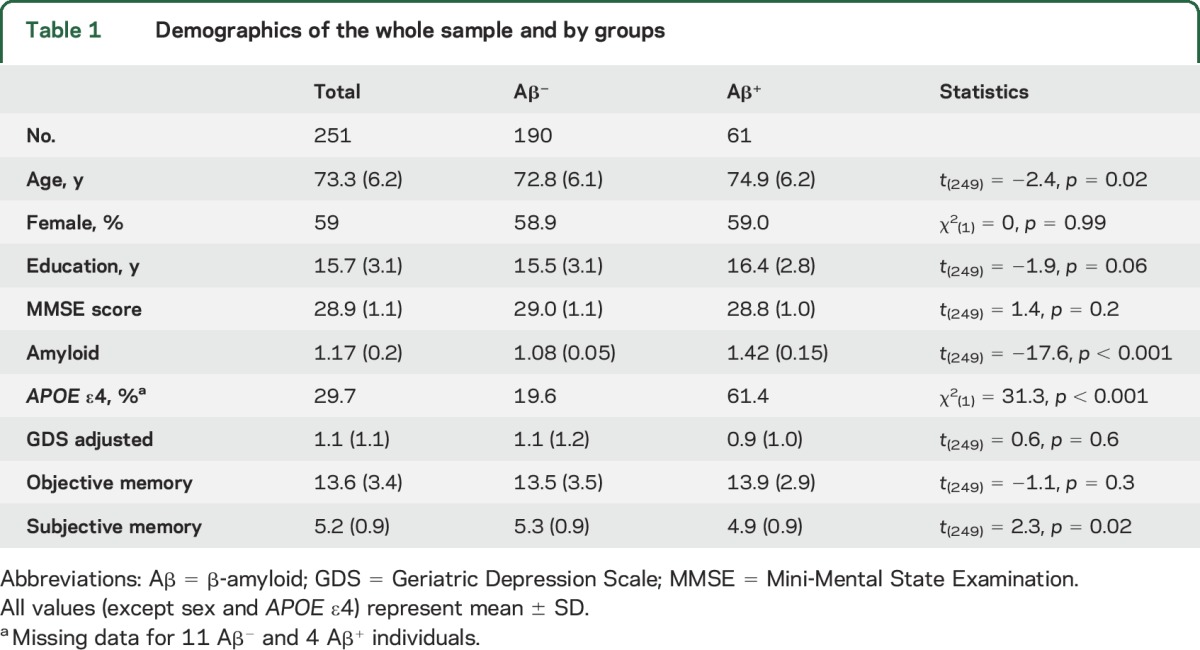
The study included 251 English-speaking individuals (table 1) enrolled in the Harvard Aging Brain Study at the Massachusetts General Hospital (MGH) and the Brigham and Women’s Hospital. Inclusion criteria were a Mini-Mental State Examination12 score of 27 to 30 (inclusive with educational adjustment), a global Clinical Dementia Rating13 score of 0, and a Geriatric Depression Scale (GDS, short form)14 score of <6. Exclusion criteria were a history of neurologic or major psychiatric disorder, history of head trauma with loss of consciousness, contraindications for MRI scanning, use of medications that affect cognitive function, severe cardiovascular disease, alcohol or substance abuse, or known cerebrovascular disease (as determined by a Hachinski Ischemia Score15 >4 or presence of cortical infarct, multiple lacunar strokes, or extensive white matter hyperintensities on structural MRI).
Table 1.
Demographics of the whole sample and by groups
Standard protocol approvals, registrations, and patient consents.
This study was approved by and conducted under the auspices of the Partners Human Research Committee at the Brigham and Women’s Hospital and MGH (Boston, MA). Informed written consent was obtained from every participant before the experimental procedures.
Subjective memory measures.
SMCs were measured with the 18 first questions in the General Frequency of Forgetting Subscale of the Memory Functioning Questionnaire.16 In this subscale, the person is asked, “How often does remembering or doing the following things present a problem for you?” On each item, the person rates his/her subjective experience on a Likert scale ranging from 1 (always) to 7 (never). The scale was then transformed to numbers ranging from 0 to 6, and the raw scores were converted to z scores for each participant with the use of the mean and SD from the whole group. Finally, z scores were then reversed by multiplying each value by −1 so that a higher score indicates increased complaints.
Aβ Pittsburg compound B-PET processing.
Pittsburg compound B (PiB)-PET data were processed with SPM8 (Wellcome Department of Cognitive Neurology, London, UK) using a protocol similar to that described in our previous publication.17 PiB images were realigned, and the first 8 minutes of data were averaged and used to normalize data to the Montreal Neurologic Institute 18F-fluorodeoxyglocuse template. Distribution volume ratio images were created with Logan plotting (40- to 60-minute interval, gray matter cerebellum reference region). An aggregate of cortical regions that typically have elevated PiB burden in patients with AD, including frontal, lateral temporal and parietal, and retrosplenial cortices, was used to extract a mean PiB value for each participant. A gaussian mixture model approach, as described previously,18 was used to classify individuals as having high or low Aβ burden. The model estimated a cutoff of PiB distribution volume ratio >1.2 for the high-Aβ group.
FDG-PET acquisition and processing.
FDG was acquired at the MGH PET department (Boston, MA) according to previously published guidelines.19 FDG was acquired from 45 to 75 minutes after a 5- to 10-mCi bolus injection 6 times in 5-minute frames. To evaluate the anatomy of PET binding, each individual PET data set was rigidly coregistered to the participant's MRI data with SPM8. Additional preprocessing steps included spatial normalization to the Montreal Neurologic Institute space (resampled voxel size 2 × 2 × 2 mm) with the parameters estimated from the corresponding T1-weighted MRI and quantitative scaling with the cerebellum gray matter as reference to obtain standardized uptake volume ratio images. The resulting images were used in the correlation analyses with the SMC score.
Structural data: Acquisition.
T1-weighted structural images were acquired with a multiecho magnetization-prepared rapid gradient echo sagittal-oriented with the following parameters: repetition time = 2,200 milliseconds; inversion time = 1,100 milliseconds; echo time = 1.54, 3.36, 5.18, and 7 milliseconds; flip angle = 7°; field of view = 230 × 230 mm; matrix size = 192 × 192; number of excitations = 1; and 1.2 × 1.2 × 1.2-mm voxels.
Region-of-interest analysis.
Structural images were processed with Freesurfer version 5.1 (http://surfer.nmr.mgh.harvard.edu) to identify gray, white, and pial surfaces to permit region-of-interest (ROI) parcellation based on the Desikan-Killiany atlas. Manual quality control of the automated segmentation was performed on all participants. Three participants, 2 Aβ− and 1 Aβ+, were excluded from ROI analyses because of insufficient MRI quality. The cortical ribbon and subcortical ROIs defined by MRI were transformed into the FDG-PET native space to obtain FDG standardized uptake volume ratio values in the Freesurfer-defined ROI. Data were averaged within each right-left hemisphere ROI pair. ROIs were selected according to the voxel-wise results to control for additional covariates, including structural data.
Statistics.
Differences in demographic and neuropsychological measures between Aβ+ and Aβ− individuals were examined with independent-sample t tests or χ2 tests (sex and APOE ε4) using SPSS 23 (SPSS Inc, Chicago, IL). The general linear model, univariate analysis, was used to examine SMCs (continuous variable), amyloid status (Aβ+ vs Aβ−), and the interaction between SMCs and Aβ status as predictors of cerebral metabolic rate for glucose consumption measures. Age was controlled for in all analyses. Exploratory SPM8 t maps were conducted with a threshold of p < 0.05, corrected for multiple comparisons with a voxel-level false discovery rate (FDR) method. Analyses were done with SPM8 implemented in Matlab R2014b (Mathworks, Natick, MA). Confirmatory ROI analyses, investigating the impact of additional covariates (age thickness, GDS, and amyloid) on the associations between FDG and SMCs, were conducted in selected ROIs following the voxel-wise results. ROI analyses were done offline with general linear model univariate analyses in SPSS version 23 (SPSS Inc, Chicago, IL).
RESULTS
Participants.
There were no significant differences between the Aβ− and Aβ+ individuals on most demographic variables (table 1). Because the Aβ− individuals were slightly younger than the Aβ+ individuals, age was included as covariates in all analyses. In addition, a significant difference between Aβ status and SMCs was found such that individuals with increased amyloid burden had greater SMCs.
SMCs are related to decreased FDG metabolism in associative neocortical regions.
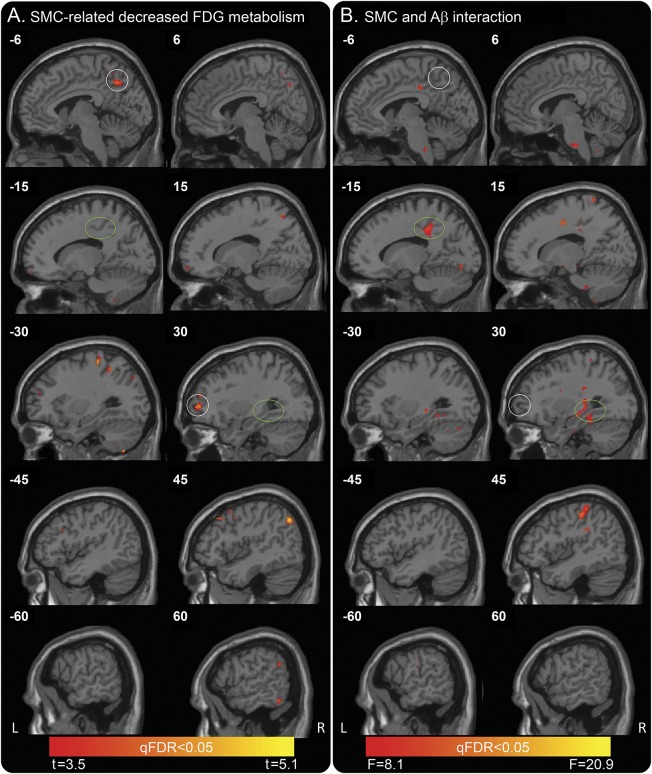
Whole-brain voxel-wise correlations (pFDR <0.05 corrected, equal to an uncorrected p = 0.0003, t = 3.45) with SMCs (continuous variable) used as a predictor of cerebral metabolic rate for glucose consumption measures (controlling for age) demonstrated significant correlations in the bilateral precuneus, bilateral inferior parietal lobes, right inferior temporal lobe, and right middle and inferior frontal gyrus, which extended into orbitofrontal gyrus (figure 1A). Coordinate details are presented in table e-1 at Neurology.org. After GDS was added, the model did not survive correction for multiple comparisons, indicating that part of the effect may be due to depressive symptoms. Post hoc univariate analyses in the left precuneus and right middle frontal gyrus (MFG; see white circles in figure 1A) demonstrated that more complaints (increased z score) were related to decreased FDG metabolism across all participants (figure 2, A and B; left precuneus [F1,246 = 18.4, p < 0.001]; right MFG [F1,246 = 10.7, p = 0.001]). A nonsignificant interaction effect was found between amyloid and SMCs (left precuneus [F1,246 = 0.6, p = 0.45]); right MFG ([F1,246 = 1.7, p = 0.2]). No significant regional hypermetabolism associated with SMCs was found.
Figure 1. Whole-brain voxel-wise correlations to examine SMCs as a predictor of glucose uptake (FDG-PET) and the interaction effect with Aβ.
(A) A statistically significant correlation, using a pFDR <0.05 corrected, equal to an uncorrected p = 0.0003, t = 3.45, of SMCs and metabolism was found in several associative neocortical regions, including bilateral precuneus (white circle), bilateral inferior parietal lobes, and bilateral middle frontal gyrus (white circle). Note the empty green circles. Color bar depicts threshold from minimum t = 3.455 to maximum t = 5.077 in the map. (B) SMC by amyloid status interactions were found bilaterally in the middle temporal lobe (green circle), including parahippocampal gyrus, hippocampus, and entorhinal cortex, and the left mid cingulum (green circle). pFDR <0.05 corrected, equal to an uncorrected p = 0.00002, F = 8.1. Note the empty white circles. Color bar depicts threshold from minimum F = 8.062 to maximum F = 20.895 in the map. Aβ = β-amyloid; FDG = fluorodeoxyglucose; pFDR = positive false discovery rate; qFDR = false discovery rate adjusted p value (q value); SMC = subjective memory complaint.
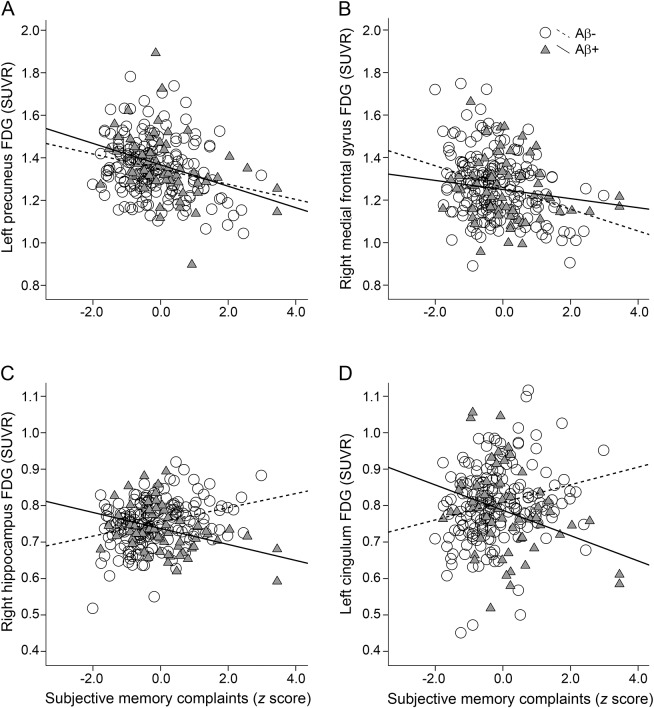
Figure 2. FDG metabolism plots.
Extracted FDG metabolism in (A) left precuneus and (B) right middle frontal gyrus (see white circles from figure 1A) showing the main effect of SMCs. In these regions, decreased glucose metabolism relates to increased SMCs regardless of amyloid status. Extracted FDG metabolism in (C) right hippocampus and (D) left mid cingulum (see green circles in figure 1B) demonstrating the SMCs by amyloid interaction. In Aβ+ individuals (triangles/solid line), having more complaints is related to decreased glucose metabolism. Aβ = β-amyloid; FDG = fluorodeoxyglucose; SMC = subjective memory complaint; SUVR = standardized uptake volume ratio.
SMCs are related to decreased FDG metabolism in hippocampal and limbic regions in CN Aβ+ individuals.
SMCs by amyloid status interactions were found bilaterally in the medial temporal lobe (MTL), including parahippocampal gyrus, hippocampus, and entorhinal cortex, and other limbic areas, including the mid cingulum (figure 1B), with the use of a pFDR <0.05 corrected, equal to an uncorrected p value = 0.00002, F = 8.1. Coordinate details are presented in table e-2. Post hoc univariate analyses in the right hippocampus and left mid cingulum (see green circles in figure 1B) showed a significant interaction effect between amyloid and SMCs (right hippocampus [F1,246 = 23.1, p < 0.001] and left mid cingulum [F1,246 = 16.0, p < 0.001]; figure 2, C and D). In these regions, the Aβ+ individuals demonstrated a significant correlation between SMCs and FDG metabolism such that more complaints were related to decreased FDG metabolism (right hippocampus [F1,58 = 8.3, p = 0.006]; left mid cingulum [F1,58 = 5.9, p < 0.001]). In contrast, Aβ− individuals demonstrated a significant relationship between complaints and FDG metabolism (right hippocampus [F1,187 = 17.9, p < 0.001] and left mid cingulum [F1,187 = 10.6, p = 0.001]) such that more complaints were related to increased FDG metabolism. The results remained the same when GDS was added to the model. Age was controlled for in all analyses.
ROI analysis: Precuneus.
ROI analysis in the bilateral precuneus confirmed a significant main effect of SMCs (F1,246 = 6.38, p = 0.012) such that more complaints were related to decreased FDG metabolism. The results became marginally significant when age, precuneus thickness, and GDS were added to the model (F1,243 = 3.12, p = 0.08). A nonsignificant interaction effect was found between amyloid and SMCs (F1,241 = 0.35, p = 0.56), with age, precuneus thickness, and GDS as covariates in the model.
ROI analysis: Hippocampus.
A nonsignificant main effect of SMCs (F1,247 = 1.61, p = 0.21) on bilateral hippocampal metabolism was observed. The results became marginally significant when age, hippocampal volume, and GDS were added to the model (F1,244 = 3.74, p = 0.054). ROI analysis confirmed a significant interaction effect between amyloid and SMCs in bilateral hippocampus (F1,242 = 4.51, p = 0.035), with age, hippocampal volume, and GDS as covariates in the model.
DISCUSSION
The present study investigated the functional and pathologic correlates underlying SMCs by combining measures of regional brain metabolism (as measured with FDG-PET) and Aβ burden (as measured with PiB-PET). Our results demonstrate that SMCs in CN older adults are related to reduced metabolism in cortical midline regions, which are brain structures known to play a role in self-referential processing.20 The MTL, a region known to be involved in memory performance, related to SMCs only in the participants who had high Aβ, a pattern that suggests that SMCs have different functional correlates according to Aβ status. Specifically, decreased MTL metabolism may be a specific marker of subclinical changes in cognition in preclinical AD.
Consistent with our expectation and prior published results,4 we found that Aβ+ individuals had more SMCs than Aβ− individuals. No difference was found between the groups in regard to sex, education, global cognition, or depression, although the Aβ+ individuals were slightly older than the Aβ− individuals. The current data reinforce the notion that SMCs may be a useful marker to identify individuals who might be in the preclinical stages of AD. In line with this, we recently reported that when multiple biomarkers were combined, those CN older individuals who exhibited either increased Aβ burden or neurodegeneration (smaller hippocampal volume or decreased FDG metabolism in a set of AD-vulnerable regions) had a statistically significant higher report of SMCs when compared to biomarker-negative CN individuals.3 In our previous study, we operationalized Aβ and neurodegeneration groups by using cutoffs of predefined regions. In the current study, we went one step further by trying to characterize the brain substrate underlying SMCs. Specifically, to unveil the brain metabolic correlates of SMCs, we used an exploratory approach by entering the individual SMCs scores and performed a whole-brain voxel-vise correlation in individual FDG-PET maps.
Our findings demonstrate that self-appraisal of memory function, more specifically, increased SMCs, was associated with decreased glucose metabolism in the posterior (precuneus) and anterior (MFG) midline regions, as well as the bilateral inferior parietal lobes and the right inferior temporal lobe. Interestingly, with regard to the midline regions, evidence from several lines of research has converged on the same set of brain regions (including the ventromedial prefrontal, medial parietal, and posterior cingulate cortex and the inferior parietal lobule) implicated in self-referential processing.20,21
With regard to the pathophysiology of AD, mounting evidence has demonstrated the vulnerability of the same cortical midline brain regions to AD-like changes, including early Aβ deposition22 decreased glucose metabolism,23,24 structural changes24,25 and functional disruption.26–29 The convergence of these effects was further pointed out by Buckner et al.,24,30 who proposed that these cortical brain regions, including the MTL, may be part of a network, the disruption of which contributes to memory impairment. Although the relationships between the hippocampus and the cortical midline regions are not yet fully elucidated, the parahippocampal gyrus could play a critical role in linking these regions together.31
With regard to FDG metabolism, previous studies showed that hippocampal metabolism is closely related to memory performance. That is, previous studies have shown that hippocampal metabolism is preserved in preclinical AD but decreased in prodromal AD.32 In contrast and concurrent with the present results, precuneus metabolism has been shown to decrease in the preclinical AD stage, before the onset of cognitive impairment.19 However, the specificity of precuneus FDG in AD dementia has been criticized because it has been shown that Aβ− individuals with mild cognitive impairment also exhibit hypometabolism in this region.33 Interestingly, our main effect of SMCs in the midline regions did not survive when we added depression (GDS) to the model, suggesting that some of the observed changes in the posterior midline regions might be due to depressive symptoms. Indeed, previous findings from our group have indicated a relationship between higher GDS score and FDG hypometabolism in AD-vulnerable regions, including the precuneus in CN older adults.34 In contrast, we found that the interaction effect between SMCs and Aβ pathology remained significant after controlling for age, GDS, and hippocampal volume in the model, supporting the idea that the observed decreased MTL metabolism may be a specific marker of subclinical changes in cognition due to amyloid pathology, above and beyond depressive symptoms. Noteworthy is the finding that the relationship between SMCs and metabolism was affected by amyloid burden in the hippocampus, an area with relatively little amyloid deposition, but not in the precuneus, an area with high amyloid deposition. These findings complement previous functional neuroimaging studies in patients with mild cognitive impairment demonstrating functional abnormalities in the MTL during memory task performance (for review, see reference 35), indicating that this region is a site of very early pathology in AD, e.g., neurofibrillary tangles. Thus, the observed finding of hypometabolism in the MTL is consistent with the expected effects of progressive AD pathology, resulting in increased neurodegenerative processes, e.g., neurofibrillary tangles. The recent advent of tau-PET imaging in conjunction with PiB-PET will enable future studies to provide regionally specific information about the underlying distribution of AD pathology to shed further light on this issue.
The current results are also in concordance with a previous study by Mosconi et al.10 demonstrating FDG hypometabolism in the parahippocampus in CN older individuals with an APOE ε4 allele and SMCs, further supporting the idea that alterations in the MTL may be a specific marker of subclinical changes in cognition in preclinical AD. Although we were not able to unravel the relationships among genotype, amyloid, SMCs, and metabolism in the current study, we did find, in line with the findings of Mosconi et al., a significantly increased percentage of APOE ε4 carriers in the Aβ+ group (61.4%).
We acknowledge that there are several limitations to this study. First, to date, there is no consensus on how to assess SMCs.36 Previous studies have used methods ranging from nonstandardized clinical interviews to well-validated scales, as well as having the complaints corroborated by an informant. In this study, we used the Memory Functioning Questionnaire to create a subjective memory score. Although this test does not include an informant version, future studies will use other subjective memory questionnaires to investigate potential discrepancies between the participant's self-report and the report of the informant to gain insight into the self-awareness of these memory problems in our participants and how that is related to Aβ and FDG metabolism. Second, we are aware that self-reports on cognitive decline could be influenced by confounding factors, for instance, substance use, medication, or other neurologic and medical conditions. In the current study, a history of neurologic or major psychiatric disorder, the use of medications that affect cognitive function, and alcohol and substance abuse were used as exclusion criteria. In addition, none of the participants met criteria for depression. However, other factors that could have influenced the results such as personality traits, anxiety, and hypochondria were not considered in this study.
These findings add to existing literature by demonstrating the importance of obtaining measures of biomarkers in addition to subjective report of memory complaints because they may help distinguish between SMCs due to decline in cognitive aging from preclinical AD dementia.
GLOSSARY
- Aβ
β-amyloid
- AD
Alzheimer disease
- CN
cognitively normal
- FDG
fluorodeoxyglucose
- FDR
false discovery rate
- GDS
Geriatric Depression Scale
- MFG
middle frontal gyrus
- MGH
Massachusetts General Hospital
- MTL
medial temporal lobe
- PiB
Pittsburg compound B
- ROI
region of interest
- SMC
subjective memory complaint
Footnotes
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
P. Vannini and B. Hanseeuw: study concept and design, analysis and interpretation of the data, preparation of the manuscript. C. Munro: analysis and interpretation of the data, review and approval of the manuscript. R. Amariglio, G. Marshall, and D. Rentz: collection and interpretation of the data, review and approval of the manuscript. A. Pascual-Leone: study concept and design, interpretation of the data, review and approval of the manuscript. K. Johnson: interpretation of the data, review and approval of the manuscript. R. Sperling: study concept and design, interpretation of the data, review and approval of the manuscript.
STUDY FUNDING
This research was carried out in whole or in part at the Athinoula A. Martinos Center for Biomedical Imaging at the MGH with resources provided by the Center for Functional Neuroimaging Technologies, P41EB015896, a P41 Regional Resource supported by the National Institute of Biomedical Imaging and Bioengineering, NIH. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
DISCLOSURE
P. Vannini received funding from NIH-National Institute on Aging grant K01AG048287. B. Hanseeuw was supported by Belgian-American Education Foundation. C. Munro reports no disclosures relevant to the manuscript. R. Amariglio was supported by Alzheimer's Association NIRG-12-243012 and NIH grant K23AG044431. She is coinvestigator for Eisai, Eli Lilly, and Merck. G. Marshall receives research support from NIH grant K23 AG033634. G. Marshall has served as paid consultant for Halloran Consulting Group and Grifols. He is a site principal investigator or coinvestigator for Janssen Alzheimer Immunotherapy, Wyeth/Pfizer Pharmaceuticals, Eisai Inc, Eli Lilly and Company, Avid Radiopharmaceuticals, Bristol-Myers-Squibb, Merck, and Navidea clinical trials. These relationships are not related to the content in the manuscript. D. Rentz received research support from the NIH grants P01 AG036694, R01MH090291, U01 AG024904, R01 AG027435, R01 AG037497, and P50 AG005134, a Fidelity investigator-initiated grant, and Alzheimer Association grant SGCOG-13-282201. She is a coinvestigator for Eli Lilly. K. Johnson has served as a paid consultant for Bayer, Biogen Idec, Bristol-Myers Squibb, GE Healthcare, Isis Pharmaceuticals Inc, Janssen Alzheimer's Immunotherapy, Piramal, Siemens Medical Solutions, and Genzyme. He is a site principal investigator or coinvestigator for Lilly/Avid, Biogen Idec, Bristol-Myers Squibb, Eisai, Pfizer, Janssen Alzheimer Immunotherapy, Merck, and Navidea clinical trials. He has spoken at symposia sponsored by Janssen Alzheimer's Immunotherapy, GEHC, Lundbeck, and Pfizer. These relationships are not related to the content in the manuscript. K. Johnson receives research support from NIH grants R01EB014894, R21, AG038994, R01 AG026484, R01 AG034556, P50 AG00513421, U19 AG10483, P01 AG036694, R13 AG042201174210, R01, AG027435, and R01 AG037497. A. Pascual-Leone serves on the scientific advisory boards for Nexstim, Neuronix, Starlab Neuroscience, Neuroelectrics, Axilum Robotics, Magstim Inc, and Neosync and is listed as an inventor on several issued and pending patents on the real-time integration of transcranial magnetic stimulation with electroencephalography and MRI. A. Pascual-Leone receives research support from NIH grants R01MH100186, R01HD069776, R01NS073601, R21 NS082870, R21 MH099196, R21 NS085491, and R21 HD07616, Harvard Catalyst | The Harvard Clinical and Translational Science Center (National Center for Research Resources and National Center for Advancing Translational Sciences, NIH, UL1 RR025758), Sidney Baer Jr. Foundation, Michael J. Fox Foundation, and Football Players Health Study at Harvard University. R. Sperling has served as a paid consultant for Bristol-Myers Squibb, Eisai, Janssen Alzheimer Immunotherapy, Pfizer, Merck, and Roche and as an unpaid consultant to Avid and Eli Lilly. She is a site principal investigator or coinvestigator for Avid, Bristol-Myers Squibb, Pfizer, and Janssen Alzheimer Immunotherapy clinical trials. She has spoken at symposia sponsored by Eli Lilly, Pfizer, and Janssen Alzheimer Immunotherapy. These relationships are not related to the content in the manuscript. R. Sperling receives research support from NIH grants U01 AG032438, U01 AG024904, R01 AG037497, R01 AG034556, K24 AG035007, P50 AG005134, U19 AG010483, R01 AG027435, and P01 AG036694. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Jonker C, Geerlings MI, Schmand B. Are memory complaints predictive for dementia? A review of clinical and population-based studies. Int J Ger Psychiatry 2000;15:983–991. [DOI] [PubMed] [Google Scholar]
- 2.Jessen F, Wiese B, Bachmann C, et al. Prediction of dementia by subjective memory impairment: effects of severity and temporal association with cognitive impairment. Arch Gen Psychiatry 2010;67:414–422. [DOI] [PubMed] [Google Scholar]
- 3.Amariglio RE, Mormino EC, Pietras AC, et al. Subjective cognitive concerns, amyloid-β and neurodegeneration in clinically normal elderly. Neurology 2015;85:56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Perrotin A, Mormino EC, Madison CM, Hayenga AO, Jagust WJ. Subjective cognition and amyloid deposition imaging a Pittsburgh compound B positron emission tomography study in normal elderly individuals. Arch Neurol 2012;69:223–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Snitz BE, Weissfeld LA, Cohen AD, et al. Subjective cognitive complaints, personality and brain amyloid-beta in cognitively normal older adults. Am J Geriatr Psychiatry 2015;23:985–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Snitz BE, Lopez OL, McDade E, et al. Amyloid-β imaging in older adults presenting to a memory clinic with subjective cognitive decline: a pilot study. J Alzheimers Dis 2015;48:S151–S159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buckley RF, Saling MM, Ames D, et al. Factors affecting subjective memory complaints in the AIBL aging study: biomarkers, memory, affect, and age. Int Psychogeriatr 2013;25:1307–1315. [DOI] [PubMed] [Google Scholar]
- 8.Sokoloff L, Reivich M, Kennedy C, et al. The 14-C deoxyglucose method for the measurement of local cerebral glucose utilization, theory, procedure and normal values in the conscious and anasthetized albino rat. J Neurochem 1977;28:897–916. [DOI] [PubMed] [Google Scholar]
- 9.Scheef L, Spottke A, Daerr M, et al. Glucose metabolism, gray matter structure, and memory decline in subjective memory impairment. Neurology 2012;79:1332–1339. [DOI] [PubMed] [Google Scholar]
- 10.Mosconi L, De Santi S, Brys M, et al. Hypometabolism and altered cerebrospinal fluid markers in normal apolipoprotein E E4 carriers with subjective memory complaints. Biol Psychiatry 2008;63:609–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Song IU, Choi EU, Oh JK, Chung YA, Chung SW. Alteration patterns of brain glucose metabolism: comparisons of healthy controls, subjective memory impairment and mild cognitive impairment. Acta Radiol 2016;57:90–97. [DOI] [PubMed] [Google Scholar]
- 12.Folstein MF, Folstein SE, McHough PR. “Mini-Mental State”: a practical method for grading cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189–198. [DOI] [PubMed] [Google Scholar]
- 13.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology 1993;43:2412–2414. [DOI] [PubMed] [Google Scholar]
- 14.Yesavage JA, Brink T, Rose TL, et al. Development and validation of a geriatric depression screening scale. J Psychiatr Res 1983;17:37–49. [DOI] [PubMed] [Google Scholar]
- 15.Rosen WG, Terry RD, Fuld PA, Katzman R, Peck A. Pathological verification of ischemic score in differentiation of dementias. Ann Neurol 1980;7:486–488. [DOI] [PubMed] [Google Scholar]
- 16.Gilewski MJ, Zelinski EM, Schaie KW. The Memory Functioning Questionnaire for assessment of memory complaints in adulthood and old age. Psychol Aging 1990;5:482–490. [DOI] [PubMed] [Google Scholar]
- 17.Hedden T, Mormino EC, Amariglio RE, et al. Cognitive profile of amyloid burden and white matter hyperintensities in cognitively normal older adults. J Neurosci 2012;32:16233–16242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mormino EC, Betensky RA, Hedden T, et al. Contributions of amyloid and APOE4 to cognitive and functional decline in aging. Neurology 2014;82:1760–1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hanseeuw B, Schultz AP, Betensky RA, Sperling RA, Johnson KA. Decreased hippocampal metabolism in high-amyloid mild cognitive impairment. Alzheimers Dement 2016;12:1288–1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Whitfield-Gabrieli S, Moran JM, Nieto-Castañón A, Triantafyllou C, Saxe R, Gabrieli JDE. Associations and dissociations between default and self-reference networks in the human brain. Neuroimage 2011;55:225–232. [DOI] [PubMed] [Google Scholar]
- 21.Andrews-Hanna JR, Reidler JS, Sepulcre J, Poulin R, Buckner RL. Functional-anatomic fractionation of the brain's default network. Neuron 2010;65:550–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mintun MA, LaRossa GN, Sheline YI, et al. [11C]PIB in a nondemented population: potential antecedent marker of Alzheimer disease. Neurology 2006;67:446–452. [DOI] [PubMed] [Google Scholar]
- 23.Reiman EM, Caselli RJ, Yun LS, et al. Preclinical evidence of Alzheimer's disease in persons homozygous for the ε4 allele for apolipoprotein E. N Engl J Med 1996;334:752–758. [DOI] [PubMed] [Google Scholar]
- 24.Buckner RL, Snyder AZ, Shannon BJ, et al. Molecular, structural, and functional characterization of Alzheimer's disease: evidence for a relationship between default activity, amyloid, and memory. J Neurosci 2005;25:7709–7717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saykin AJ, Wishart HA, Rabin LA, et al. Older adults with cognitive complaints show brain atrophy similar to that of amnestic MCI. Neurology 2006;67:834–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hedden T, Van Dijk KR, Becker JA, et al. Disruption of functional connectivity in clinically normal older adults harboring amyloid burden. J Neurosci 2009;29:12686–12694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sheline YI, Raichle ME, Snyder AZ, et al. Amyloid plaques disrupt resting state default mode network connectivity in cognitively normal elderly. Biol Psych 2010;67:584–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vannini P, Hedden T, Huijbers W, Ward A, Johnson KA, Sperling RA. The ups and downs of the posteromedial cortex: age- and amyloid- related functional alterations of the encoding/retrieval flip in cognitively normal older adults. Cereb Cortex 2013;23:1317–1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vannini P, Hedden T, Becker JA, et al. Age and amyloid-related alterations in default network habituation to stimulus repetition. Neurobiol Aging 2012;33:1237–1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Buckner RL, Sepulcre J, Talukdar T, et al. Cortical hubs revealed by intrinsic functional connectivity: mapping, assessment of stability, and relation to Alzheimer's disease. J Neurosci 2009;29:1860–1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ward AM, Schultz AP, Huijbers W, Van Dijk KR, Hedden T, Sperling RA. The parahippocampal gyrus links the default-mode cortical network with the medial temporal lobe memory system. Hum Brain Mapp 2014;35:1061–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hanseeuw B, Dricot L, Lhommel R, Quenon L, Ivanoiu A. Patients with amyloid-negative mild cognitive impairment have cortical hypometabolism but the hippocampus is preserved. J Alzheimers Dis 2016;53:651–660. [DOI] [PubMed] [Google Scholar]
- 33.Tiepolt S, Patt M, Hoffmann KT, Schroeter ML, Sabri O, Barthel H. Alzheimer's disease FDG PET imaging pattern in an amyloid-negative mild cognitive impairment subject. J Alzheimers Dis 2015;47:539–543. [DOI] [PubMed] [Google Scholar]
- 34.Donovan N, Hsu DC, Dagley AS, et al. Depressive symptoms and biomarkers of Alzheimer's disease in cognitively normal older adults. J Alzheimers Dis 2015;46:63–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dickerson BC, Sperling RA. Functional abnormalities of the medial temporal lobe memory system in mild cognitive impairment and Alzheimer's disease: insights from functional MRI studies. Neuropsychologia 2008;46:1624–1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rabin LA, Smart CM, Crane PK, et al. Subjective cognitive decline in older adults: an overview of self-report measures used across 19 international research studies. J Alzheimers Dis 2015;48:S63–S68. [DOI] [PMC free article] [PubMed] [Google Scholar]