Abstract
Objective:
To characterize the effect of white matter microstructural integrity on cerebral tissue and long-term functional outcomes after acute ischemic stroke (AIS).
Methods:
Consecutive AIS patients with brain MRI acquired within 48 hours of symptom onset and 90-day modified Rankin Scale (mRS) score were included. Acute infarct volume on diffusion-weighted imaging (DWIv) and white matter hyperintensity volume (WMHv) on T2 fluid-attenuated inversion recovery MRI were measured. Median fractional anisotropy (FA), mean diffusivity, radial diffusivity, and axial diffusivity values were calculated within normal-appearing white matter (NAWM) in the hemisphere contralateral to the acute lesion. Regression models were used to assess the association between diffusivity metrics and acute cerebral tissue and long-term functional outcomes in AIS. Level of significance was set at p < 0.05 for all analyses.
Results:
Among 305 AIS patients with DWIv and mRS score, mean age was 64.4 ± 15.9 years, and 183 participants (60%) were male. Median NIH Stroke Scale (NIHSS) score was 3 (interquartile range [IQR] 1–8), and median normalized WMHv was 6.19 cm3 (IQR 3.0–12.6 cm3). Admission stroke severity (β = 0.16, p < 0.0001) and small vessel stroke subtype (β = −1.53, p < 0.0001), but not diffusivity metrics, were independently associated with DWIv. However, median FA in contralesional NAWM was independently associated with mRS score (β = −9.74, p = 0.02), along with age, female sex, NIHSS score, and DWIv.
Conclusions:
FA decrease in NAWM contralateral to the acute infarct is associated with worse mRS category at 90 days after stroke. These data suggest that white matter integrity may contribute to functional recovery after stroke.
The role of white matter hyperintensity (WMH), or leukoaraiosis, in outcomes after acute ischemic stroke (AIS) is increasingly recognized through its link to larger acute infarct lesions,1 likelihood of infarct expansion,2 and association with worse functional poststroke outcomes.3–5
However, a growing body of evidence suggests that macrostructural white matter (WM) disease burden, quantified as WMH on T2-weighted fluid-attenuated inversion recovery (FLAIR) MRI, may not adequately reflect the total extent of WM injury and that microstructural injury to normal-appearing WM (NAWM) can be associated with clinical phenotypes.6,7 We hypothesized that, in patients with AIS, WM microstructural integrity can be assessed globally on diffusion tensor imaging (DTI) of NAWM in the hemisphere contralateral to the AIS (i.e., contralesional NAWM) and that the metrics of diffusivity will demonstrate association with acute ischemic tissue and long-term functional outcomes. We tested this hypothesis in a large, hospital-based cohort of patients with AIS and brain MRI acquired during the acute-stage evaluation. In this study, we sought to assess the relationship between the diffusivity metrics in the contralesional NAWM and acute infarct volume measured on diffusion-weighted imaging (DWIv) and functional outcomes measured as a modified Rankin Scale (mRS) score at 3 to 6 months after stroke.
METHODS
Standard protocol approvals, registrations, and patient consent.
Informed written consent was obtained from all participants or their surrogates at the time of enrollment in this study. The Partners Institutional Review Board approved the use of human participants in this study.
Study design, setting, and patient population.
Between 2003 and 2011, all participants >18 years old presenting to the Massachusetts General Hospital emergency department with signs and symptoms of AIS were eligible for enrollment in the institutional ischemic stroke cohort. Patients with confirmed acute DWI lesions on brain MRI scans performed within 48 hours of symptom onset and FLAIR sequences available for volumetric WMH quantification were included in this analysis. Of 481 initial participants, we excluded participants with DWI-confirmed infratentorial (brainstem or cerebellum) or bilateral supratentorial acute cerebral infarcts, motion artifact precluding MRI assessment, or a confirmed diagnosis of ischemia secondary to a systemic illness (vasculitis, subacute bacterial endocarditis, etc). Patients were also excluded if the contralesional hemisphere had a chronic infarct occupying more than one-third of the hemisphere territory. Baseline clinical characteristics and laboratory values were obtained through review of the medical records or direct interview of patients or their surrogates.
Clinical variables.
Age was recorded at the time of enrollment in the study. Antecedent atrial fibrillation, coronary artery disease, diabetes mellitus, hyperlipidemia, hypertension, prior stroke/TIA, and tobacco use were determined from medical records or patient/surrogate interview. All patients were evaluated in the emergent setting by a neurologist, at which point stroke severity was assessed with the NIH Stroke Scale (NIHSS) score. Treatment with intravenous tissue plasminogen activator (tPA) or intra-arterial therapy was also recorded. Clinical and laboratory data were abstracted from the medical record, including first documented admission systolic blood pressure and diastolic blood pressure. AIS subtypes were assigned by neurologists (K.L.F., P.C., G.B., H.H.K., A.L.) according to the Trial of Org 10172 in Acute Stroke Treatment (TOAST) classification and for analysis dichotomized into small vessel (lacunar) and non–small vessel (nonlacunar) categories.8
Clinical outcome assessment.
Patients and their caregivers were interviewed in person or by telephone at 3 to 6 months after stroke to assess functional outcome with the mRS. Recurrent cerebrovascular events, newly diagnosed medical conditions, and medication use were specifically assessed in this interview. If the patient (or surrogate) was not available in person/by phone at 3 to 6 months after AIS hospitalization, his or her chart was reviewed and mRS score was abstracted from all the clinical data available contemporaneously. Only those patients for whom mRS score could be determined directly or from chart review (n = 305) were included in this analysis.
Neuroimaging analysis.
Image acquisition.
MRI was performed within 48 hours of admission with a 1.5T Signa scanner (General Electric, Fairfield, CT) except for 4 cases performed on a 1.5T or 3T Siemens (Malvern, PA) scanner. In the majority of cases, diagnostic MRI included axial FLAIR (repetition time 5000 milliseconds, minimum echo time 62–116 milliseconds, inversion time 2200 milliseconds, 220- to 240-mm field of view 128 × 128 acquisition matrix upsampled to 256 × 256, 25–26 × 5–mm slices, 1-mm slice gap), DWI, and DTI (single-shot echo-planar imaging with 1 to 5 b = 0 acquisitions, 6–30 diffusion directions [b = 1000 s/mm2], and 1 to 3 averages) sequences. DWI datasets were corrected for eddy current distortions and motion.9 All DWI and FLAIR datasets were coregistered to one another with nonlinear coregistration techniques (Montreal Neurological Institute [MNI] Autoreg10,11) and to the MNI 452 1-mm atlas.12
WMH volumetric analysis.
The semiautomated approach to calculation of total WMH volume (WMHv) and derivation of WMH masks has been described previously.13,14 In brief, WMH and contralateral chronic infarct masks were constructed with MRIcro (www.mricro.com). Total WMHv was normalized for differences in head size (nWMHv) with a validated method for calculating intracranial area.15
NAWM mask acquisition and analysis.
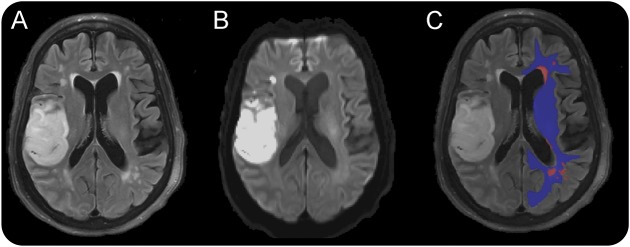
WMH and chronic infarct masks in the hemisphere contralateral to the AIS (i.e., contralesional) were constructed in MRIcro on FLAIR images by a trained expert blinded to the clinical outcomes. Each apparent diffusion coefficient and fractional anisotropy (FA) sequence was coregistered to the individual FLAIR image and WMH and chronic infarct masks, in addition to the MNI 452 1-mm atlas, with nonlinear coregistration techniques (MNI Autoreg).10,12,16 The probabilistic NAWM mask for the contralesional hemisphere was then determined, with a 90% probability for being within WM, by subtracting WMH (after morphometric dilation operation) and chronic infarct masks from the coregistered anatomic atlas (figure 1).
Figure 1. Image processing steps (here shown on 3T MRI of an acute stroke patient) of deriving NAWM masks in the contralesional hemisphere.
Sample FLAIR image (A), acute DWI scan depicting ischemic stroke in the right frontal operculum (B), and representative WMH mask (red) and NAWM mask (blue) overlaid on FLAIR image (C). DWI = diffusion-weighted imaging; FLAIR = fluid-attenuated inversion recovery; NAWM = normal-appearing white matter; WM = white matter; WMH = white matter hyperintensity.
The median voxel values for FA, axial diffusivity (AD), mean diffusivity (MD), and radial diffusivity (RD) were obtained from the contralesional NAWM with the 90% probability NAWM mask.17 Because WM was defined on the basis of probability masks, no threshold values were used for extraction of the diffusivity anisotropy metrics in this analysis.
Statistical analysis.
Variables were reported as mean ± SD (for data with a normal distribution), median with an interquartile range (IQR; for data with a nonnormal distribution), or proportion/percentage of total participants. DWIv was normalized for head size using intracranial area15 and subsequently natural log–transformed for linear regression analyses. Ordinal logistic regression analysis was performed to assess the association between clinical characteristics and 90-day mRS score. Variables that demonstrated a nominal value of p < 0.10 in univariable analyses were subsequently included in the multivariable regression models of acute tissue and long-term clinical outcomes. Follow-up mRS score was treated as an ordinal variable and lnDWIv as a continuous variable in all models. Variance inflation factor estimates were calculated to assess the influence of collinearity on variables in regression analysis. Statistical significance was set at a value of p < 0.05 in all analyses. Statistical analysis was conducted in SAS 9.3 statistical package (SAS Institute Inc, Cary, NC).
RESULTS
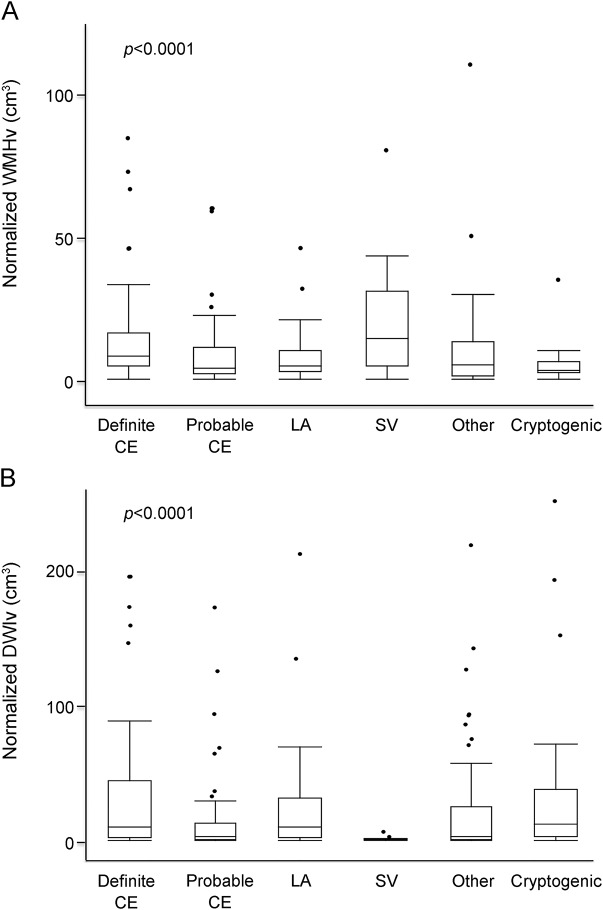
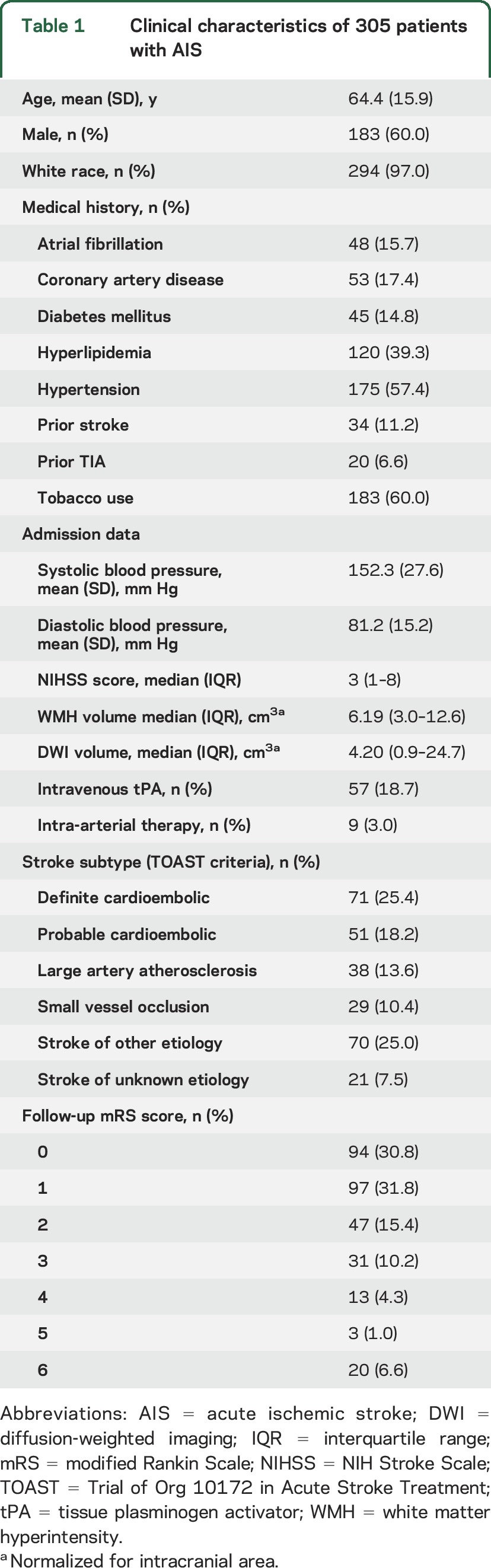
We included 305 AIS patients with follow-up mRS score available for this analysis. Mean age was 64.4 years (SD 15.9 years), and 183 of the participants (60%) were men. The participants were predominantly white (97.0%) and free of baseline disability (93.7% had a premorbid mRS score ≤1). Median nWMHv was 6.19 cm3 (IQR 3.0–12.6 cm3) and normalized DWIv (nDWIv) was 4.20 cm3 (IQR 0.9–24.7 cm3). Median admission NIHSS score was 3 (IQR 1–9). Fifty-seven participants (18.7%) received intravenous tPA, and 9 participants (3.0%) underwent intra-arterial therapy. On the basis of the TOAST classification, 29 patients (10.4%) had strokes secondary to small vessel occlusive disease (figure 2). Table 1 summarizes baseline patient characteristics. Compared to the larger institutional dataset of AIS patients with brain MRI on admission, there was no significant difference in age, admission stroke severity, distribution of the TOAST subtypes, nWMHv, or poststroke mRS score (data not shown).18
Figure 2. WMH and infarct volume as a function of stroke subtype.
Box plots of normalized WMHv (A) and normalized DWIv (B) as a function of TOAST subtype. Plots include outliers for each subtype depicted as solid circles. CE = cardioembolic; DWI = diffusion-weighted imaging; DWIv = diffusion-weighted imaging volume; LA = large artery; SV = small vessel; TOAST = Trial of Org 10172 in Acute Stroke Treatment; WMH = white matter hyperintensity; WMHv = white matter hyperintensity volume.
Table 1.
Clinical characteristics of 305 patients with AIS
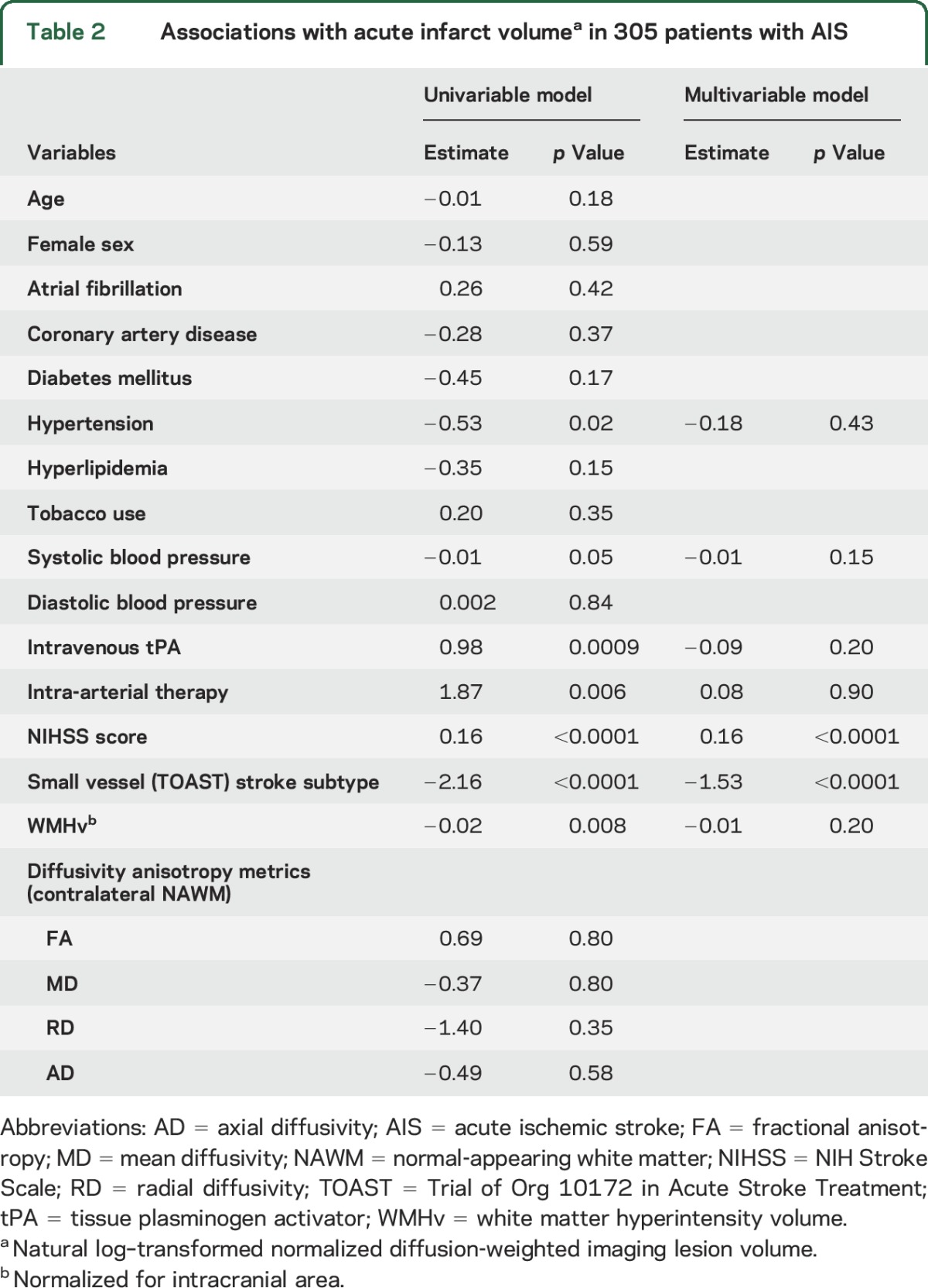
In univariable analysis, hypertension (β = −0.53, p = 0.02), systolic blood pressure (β = −0.01, p = 0.05), admission NIHSS score (β = 0.16, p < 0.0001), small vessel stroke subtype (β = −2.16, p < 0.0001), treatment with intravenous tPA (β = 0.98, p = 0.0009), intra-arterial therapy (β = 1.87, p = 0.006), and nWMHv (β = −0.02, p = 0.008) were associated with lnDWIv (table 2). In the multivariable linear regression model, admission NIHSS score (β = 0.16, p < 0.0001) and small vessel TOAST subtype (β = −1.53, p < 0.0001) were independent predictors of lnDWIv (table 2). There was no association between the metrics of diffusivity anisotropy and acute infarct volume.
Table 2.
Associations with acute infarct volumea in 305 patients with AIS
In the univariable analysis of poststroke functional outcomes, age (β = 0.03, p < 0.0001), female sex (β = 0.73, p = 0.0006), atrial fibrillation (β = 0.82, p = 0.004), hypertension (β = 0.42, p = 0.043), admission NIHSS score (β = 0.16, p < 0.0001), treatment with intravenous tPA (β = 0.62, p = 0.02), intra-arterial therapy (β = 1.47, p = 0.01), nDWIv (β = 0.01, p < 0.0001), MD (β = 5.42, p = 0.0003), RD (β = 4.83, p = 0.001), and AD (β = 2.53, p = 0.005) were associated with follow-up mRS score (table 3). In addition, systolic blood pressure (β = 0.007, p = 0.09) and FA (β = −4.45, p = 0.06) demonstrated an association with mRS score at a nominal value of p < 0.1 in univariable analysis and were included in the multivariable model. In a multivariable ordinal regression model (R2 = 36%), FA (β = −9.74, p = 0.02), DWIv (β = 0.01, p = 0.002), admission NIHSS score (β = 0.13, p < 0.0001), female sex (β = 0.76, p = 0.003), and age (β = 0.03, p = 0.006) were independently associated with follow-up mRS score (table 3). In additional analysis, introducing nWMHv into the multivariable model above, FA remained independently associated with mRS score (β = −8.60, p = 0.04).
Table 3.
Associations with follow-up mRS score in 305 patients with AIS
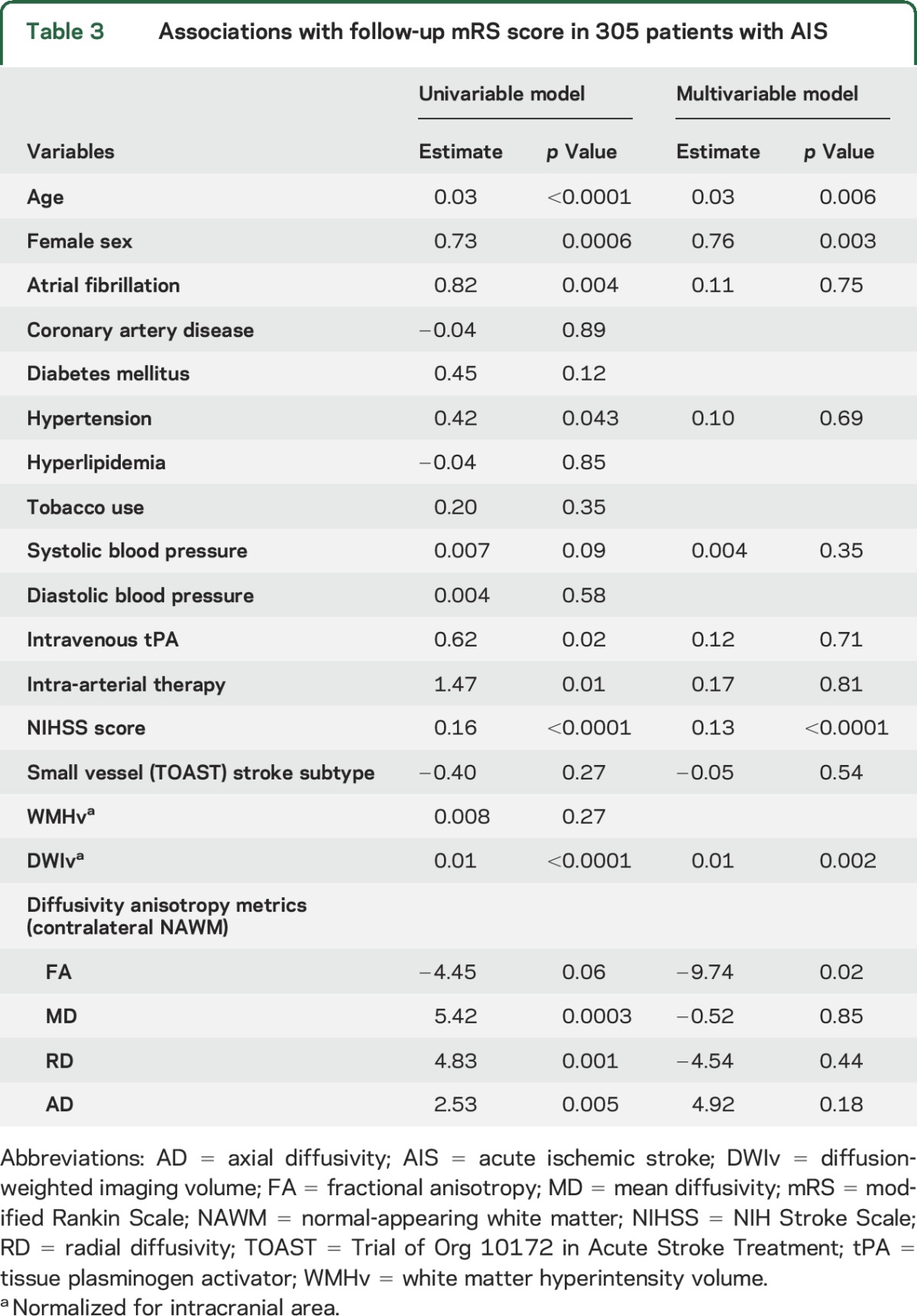
Lastly, to address potential collinearity, we built a stepwise forward-selection regression for mRS score. Similarly, age (β = 0.03, p = 0.0004), female sex (β = 0.76, p = 0.0016), admission NIHSS score (β = 0.14, p < 0.0001), nDWIv (β = 0.01, p = 0.004), and FA (β = −7.16, p = 0.013) remained independently associated with mRS score.
DISCUSSION
In this analysis, we demonstrated that metrics of diffusivity extracted from the NAWM in the hemisphere contralateral to the acute ischemic lesion are associated with a clinical measure of functional poststroke recovery. As a surrogate of microstructural WM integrity, lower values of FA detected on the admission MRI were associated with higher mRS scores collected at 3 to 6 months after stroke. These data suggest that the preexisting burden of microstructural WM disease may affect functional recovery after stroke and that diffusivity anisotropy measurements of NAWM may serve as a novel imaging marker of this relationship in patients with ischemic stroke. In the future, because FA is believed to reflect in vivo axonal microstructure,19,20 loss of axonal integrity detected in NAWM with validated FA thresholds may improve prediction models of poststroke recovery.
Prior studies demonstrate that global DTI-based measurements of WM provide a more accurate account of microstructural changes associated with cognitive and functional performance in general populations.7,21 Our data demonstrate that global assessment of the microstructural WM integrity is feasible in a hospital-based AIS cohort and that diffusivity metrics within the NAWM of contralesional hemisphere may serve as markers of functional outcome in this patient population when included in the models adjusted for traditional clinical variables and measurement of macrostructural assessment of WMH.
At the macrostructural level, WMH burden has been previously linked to infarcted cerebral tissue volume and likelihood of expansion,1,2 as well as admission NIHSS score.22 In addition, WMH burden deleteriously affects functional outcomes at 1 to 12 months after ischemic stroke and is associated with greater likelihood of long-term disability.3–5,23–25 There is a growing body of evidence, however, that clinically significant WM injury may exist that is subthreshold of T2 FLAIR detection and that represents a form of microstructural injury.6,7,21 DTI-based techniques, which are predicated on the quantification of the randomness of water molecule diffusion, are informative on the microstructural integrity of WM.9,26,27
With respect to NAWM, several studies have used a DTI-based approach to emphasize that the detection of microstructural injury of NAWM precedes the genesis of overt WMH.6,28 A recent study of patients with mild ischemic stroke demonstrated that MD was the most sensitive metric for distinguishing WMH from NAWM and that the accumulation of interstitial fluid is involved in WMH pathogenesis.17 Therefore, it is reasonable to assume that DTI-based anisotropy metrics of NAWM provide insights into WM composition and, as a result, are informative of functional connectivity and overall cognitive performance.7,27,29–31 Thus, it seems intuitive that WM integrity would influence poststroke recovery.3,4,32–35 The exact mechanisms by which prestroke WM integrity might influence poststroke functional recovery are yet to be determined; however, data that underscore the role of structural connectivity in functional performance in general populations point in the direction of potential underlying pathology.7,21 Therefore, more severe total pre-AIS burden of WM disease (including overt WMH and microstructural injury in NAWM) may signify a degree of subclinical baseline cognitive and functional decline that predisposes these individuals for less favorable recovery after AIS.
In our analysis, FA was associated with functional poststroke outcome at a nominal level of significance in univariate analysis but emerged as an independent DTI measurement in multivariable analysis, as opposed to MD, RD, and AD. There are several possible explanations for this observation. First, there is a degree of collinearity between MD, RD, AD, and FA that is predicated on how these variables are calculated. The principal eigenvalues that contribute to the calculation of diffusivity anisotropy metrics are shared between MD and AD (λ1) and MD and RD (λ2 and λ3), whereas FA represents the normalized variance of λ1, λ2, and λ3.26 However, the final model, which accounted for potential collinearity among these variables by using a forward-selection stepwise regression technique, was not significantly altered. The observation that reduced FA alone was not an independent predictor of follow-up mRS score in univariable analysis is likely due to known FA heterogeneity. Alternatively, the effect size of each individual DTI metric is small, and the statistical power in this analysis might be insufficient to demonstrate an independent association between each individual variable and functional outcome. Therefore, we hypothesize that, on the basis of the results of the univariable analysis, FA, MD, RD, and AD are to some extent associated with poststroke functional outcome; however, their contributions to the multivariable analysis may be offset somewhat by collinearity.
In this analysis, we used tissue segmentation results for NAWM and WMH in combination with diffusion maps to assess global metrics of NAWM microstructural integrity. A similar approach was recently used to demonstrate that global NAWM microstructural integrity improved stroke risk prediction.36 Global WM integrity may also play a role in compensatory mechanisms of early and long-term poststroke recovery, as has been suggested by studies on overt WMH burden.4,23,37 In line with this hypothesis, our findings of reduced FA in the contralateral NAWM being associated with functional recovery implicate a contribution from WM integrity to poststroke recovery. Supporting our findings, in patients in the chronic state of recovery from left hemisphere ischemic stroke, FA values in specific regions of interest in the contralateral hemisphere (middle temporal gyrus, precentral gyrus, and pars opercularis) were predictive of speech fluency.38 Similarly, in a cohort of patients with right hemispheric ischemic stroke, with the use of Tract Based Spatial Statistics, FA values in the specific regions of the contralateral hemisphere correlated with performance on neuropsychological testing.33 Our data now offer new evidence to demonstrate the utility of global measures of WM integrity in this population.
The effect of WM integrity on functional outcome after AIS appears to be independent of its effect on acute infarct size. In this analysis, we demonstrated that diffusivity anisotropy metrics in the contralateral NAWM were not associated with acute DWI lesion volume. However, both NIHSS score and small vessel (TOAST) stroke subtype were independently associated with acute infarct volume, consistent with prior reports.1,22 Interestingly, small vessel stroke subtype was not associated with mRS score, which is likely a consequence of insufficient power of this portion of the analysis (n = 29 patients with stroke attributed to small vessel occlusion), or alternatively, it is suggestive of a complex relationship between clinical outcome and stroke subtype, which may involve additional variables such as interaction with stroke lesion topography.18 Overall, the role of WM microstructural integrity in the acute ischemic tissue outcomes warrants further investigation.
Several limitations of this study warrant consideration in the interpretation and general applicability of our results. First, this retrospective analysis of a prospectively collected, cross-sectional hospital-based cohort is subject to the important limitations inherent to such design. In particular, an inherent selection bias might have contributed to the inclusion of AIS patients with milder stroke severity, small DWIv, and consequently, prevalence of favorable long-term outcomes. Despite the fact that 57% of patients had strokes attributed to either large artery or cardioembolic etiologies with the use of the TOAST classification, more severe syndromes often seen in association with the large artery atherosclerosis and cardioembolism are underrepresented in our cohort. The impact of severe stroke syndromes on poststroke outcomes is expected to be significant, making it difficult to discern the effect of microstructural WM changes in models of clinical outcome. To overcome these challenges and to differentiate the full effect of WM injury on functional outcomes in etiologically various stroke subtypes, future studies that are systematic and adequately powered are required. Furthermore, we were not able to demonstrate the well-established effect of intravenous tPA and intra-arterial therapy on long-term functional outcomes in this cohort,39,40 which is also most likely related to the mild stroke severity of the cohort, resulting in low treatment rates (18.7% intravenous tPA and 3.0% intra-arterial therapy) and insufficient statistical power to demonstrate the effect of acute interventions.
Second, this analysis was restricted to AIS patients who had DWI and FLAIR MRI within 48 hours from symptom onset, which could potentially confer bias by capturing evolving infarcts that underestimate final infarct volume. In addition, we excluded participants with bilateral supratentorial DWI lesions, patients with infratentorial lesions, and those with MRI scans unsuitable for volumetric and diffusivity metrics analysis. While possibly contributing to a potential selection bias (as above) in this analysis, there were no significant differences in age, median NIHSS score, DWIv, WMHv, TOAST stroke subtype distribution, or follow-up mRS score between our study subset and the larger institutional dataset of AIS patients with admission brain MRI (data not shown). Third, we were not able to demonstrate a statistically significant association between WMHv and outcomes, possibly a result of the deliberate approach to this analysis that is not limited to a specific stroke subtype, as opposed to prior studies. In this study, we prioritized the considerations of statistical power to discover the effect of DTI metrics on mRS score, which required the greatest sample size available. Fourth, we elected to assess median values of the selected diffusivity metrics within contralesional NAWM rather than targeting a specific region of interest, as has been done in some prior studies.41,42 While region-of-interest analysis is important for tract-specific changes, we draw from the established methodology of a global assessment of NAWM integrity that proved to be more informative with regard to the complex functional assessments in the general population and is likely to have a broader role in the studies of poststroke recovery.7,21,41 The variability in the number of diffusion-encoding gradient directions and number of excitations could potentially introduce error in the DTI acquisition; however, this was previously reported to not significantly affect mean FA or MD measurements despite slight differences in the individual eigenvalues.43 Lastly, our approach was predicated on clinical scans (e.g., 1.5T MRI in the acute setting of hospitalization) as opposed to high-resolution neuroimaging. This resulted in nonisotropic voxel for FA analysis, which might have led to some bias due to partial volume averaging.44 However, high-resolution isotropic whole-brain DTI requires several minutes to acquire, which is not feasible for acute stroke patients. In addition, we consider it to be a strength of our study that our findings are based on standard clinical DTI scans and therefore could be practically useful in the immediate clinical setting.
Additional strengths of our study include a large hospital-based cohort of AIS patients, rigorous quality control applied to the MRI analysis pipeline, assessment of MRI variables by investigators blinded to clinical outcomes, volumetric analysis of WMH and acute infarct lesion burden, and use of the mRS score as an ordinal variable to prevent loss of information that occurs as a result of dichotomization.
We report herein a role for diffusivity anisotropy metrics of the contralesional NAWM obtained in the acute stage to predict long-term functional outcomes in acute cerebral ischemia. Our findings highlight that in the acute stages of ischemic stroke, diffusivity anisotropy metrics of contralesional NAWM, while having no influence on DWI volume, are strongly linked to long-term functional outcomes. These data suggest that DTI analysis in patients with AIS could contribute to early and improved prognostication of poststroke recovery and warrant future studies of WM structural integrity in stroke outcomes.
GLOSSARY
- AD
axial diffusivity
- AIS
acute ischemic stroke
- DTI
diffusion tensor imaging
- DWIv
diffusion-weighted imaging volume
- FA
fractional anisotropy
- FLAIR
fluid-attenuated inversion recovery
- IQR
interquartile range
- MD
mean diffusivity
- MNI
Montreal Neurological Institute
- mRS
modified Rankin Scale
- nDWIv
normalized diffusion-weighted imaging volume
- NAWM
normal-appearing white matter
- NIHSS
NIH Stroke Scale
- nWMHv
normalized white matter hyperintensity volume
- RD
radial diffusivity
- TOAST
Trial of Org 10172 in Acute Stroke Treatment
- tPA
tissue plasminogen activator
- WM
white matter
- WMH
white matter hyperintensity
- WMHv
white matter hyperintensity volume
AUTHOR CONTRIBUTIONS
Mark R. Etherton: study concept and design, statistical analysis/interpretation of data, drafting/revising the manuscript for intellectual content. Ona Wu: study concept and design, analysis and interpretation of data, revising the manuscript for intellectual content. Pedro Cougo: study design, data acquisition, revising the manuscript for intellectual content. Anne-Katrin Giese, Lisa Cloonan, Kaitlin Fitzpatrick, and Allison S. Kanakis: data acquisition, revising the manuscript for intellectual content. Gregoire Boulouis: data acquisition, interpretation of data, revising the manuscript for intellectual content. Hasan Karadeli: data acquisition, revising the manuscript for intellectual content. Arne Lauer: data acquisition, interpretation of data, revising the manuscript for intellectual content. Jonathan Rosand: interpretation of data, revising the. manuscript for intellectual content. Karen L. Furie: data acquisition, interpretation of data, revising the manuscript for intellectual content. Natalia S. Rost: study concept/design, study supervision, data acquisition, interpretation of data, drafting/revising the manuscript for intellectual content.
STUDY FUNDING
This study was in part supported by the NIH–National Institute of Neurological Disorders and Stroke K23NS064052, R01NS082285, and R01NS086905 (N.S.R.), and American Heart Association/Bugher Foundation Centers for Stroke Prevention Research (0775010N) and Deane Institute for Integrative Study of Atrial Fibrillation and Stroke (K.L.F.).
DISCLOSURE
M. Etherton reports no disclosures relevant to the manuscript. O. Wu is in part supported by NIH Health (P50NS051343, R01NS082285, R01NS086905) and National Institute of Biomedical Imaging and Bioengineering (P41EB015896). P. Cougo, A. Giese, L. Cloonan, K. Fitzpatrick, A. Kanakis, G. Boulouis, H. Karadeli, A. Lauer, and J. Rosand report no disclosures relevant to the manuscript. K. Furie is supported in part by NIH-NINDS 5P50NS051343-04, American Heart Association/Bugher Foundation Centers for Stroke Prevention Research (0775010N), and Deane Institute for Integrative Study of Atrial Fibrillation and Stroke. N. Rost is in part supported by the NIH–National Institute of Neurological Disorders and Stroke K23NS064052, R01NS082285, and R01NS086905 and American Heart Association/Bugher Foundation Centers for Stroke Prevention Research (0775010N). Go to Neurology.org for full disclosures.
REFERENCES
- 1.Henninger N, Lin E, Haussen DC, et al. Leukoaraiosis and sex predict the hyperacute ischemic core volume. Stroke 2013;44:61–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ay H, Arsava EM, Rosand J, et al. Severity of leukoaraiosis and susceptibility to infarct growth in acute stroke. Stroke 2008;39:1409–1413. [DOI] [PubMed] [Google Scholar]
- 3.Arsava EM, Rahman R, Rosand J, et al. Severity of leukoaraiosis correlates with clinical outcome after ischemic stroke. Neurology 2009;72:1403–1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Henninger N, Lin E, Baker SP, Wakhloo AK, Takhtani D, Moonis M. Leukoaraiosis predicts poor 90-day outcome after acute large cerebral artery occlusion. Cerebrovasc Dis 2012;33:525–531. [DOI] [PubMed] [Google Scholar]
- 5.Kissela B, Lindsell CJ, Kleindorfer D, et al. Clinical prediction of functional outcome after ischemic stroke: the surprising importance of periventricular white matter disease and race. Stroke 2009;40:530–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Groot M, Verhaaren BF, de Boer R, et al. Changes in normal-appearing white matter precede development of white matter lesions. Stroke 2013;44:1037–1042. [DOI] [PubMed] [Google Scholar]
- 7.Vernooij MW, Ikram MA, Vrooman HA, et al. White matter microstructural integrity and cognitive function in a general elderly population. Arch Gen Psychiatry 2009;66:545–553. [DOI] [PubMed] [Google Scholar]
- 8.Adams HP Jr, Bendixen BH, Kappelle LJ, et al. Classification of subtype of acute ischemic stroke: definitions for use in a multicenter clinical trial: TOAST: Trial of Org 10172 in Acute Stroke Treatment. Stroke 1993;24:35–41. [DOI] [PubMed] [Google Scholar]
- 9.Sorensen AG, Wu O, Copen WA, et al. Human acute cerebral ischemia: detection of changes in water diffusion anisotropy by using MR imaging. Radiology 1999;212:785–792. [DOI] [PubMed] [Google Scholar]
- 10.Collins DL, Neelin P, Peters TM, Evans AC. Automatic 3D intersubject registration of MR volumetric data in standardized Talairach space. J Comput Assist Tomogr 1994;18:192–205. [PubMed] [Google Scholar]
- 11.UCLA Laboratory of Neuro Imaging. ICBM 452 T1 Atlas. Los Angeles, CA: UCLA Laboratory of Neuro Imaging; 2008. [Google Scholar]
- 12.Grabner G, Janke AL, Budge MM, Smith D, Pruessner J, Collins DL. Symmetric atlasing and model based segmentation: an application to the hippocampus in older adults. Med Image Comput Comput Assist Interv 2006;9:58–66. [DOI] [PubMed] [Google Scholar]
- 13.Cloonan L, Fitzpatrick KM, Kanakis AS, Furie KL, Rosand J, Rost NS. Metabolic determinants of white matter hyperintensity burden in patients with ischemic stroke. Atherosclerosis 2015;240:149–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rost NS, Rahman RM, Biffi A, et al. White matter hyperintensity volume is increased in small vessel stroke subtypes. Neurology 2010;75:1670–1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferguson KJ, Wardlaw JM, Edmond CL, Deary IJ, Maclullich AM. Intracranial area: a validated method for estimating intracranial volume. J Neuroimaging 2005;15:76–78. [DOI] [PubMed] [Google Scholar]
- 16.Rex DE, Ma JQ, Toga AW. The LONI pipeline processing environment. NeuroImage 2003;19:1033–1048. [DOI] [PubMed] [Google Scholar]
- 17.Munoz Maniega S, Chappell FM, Valdes Hernandez MC, et al. Integrity of normal-appearing white matter: influence of age, visible lesion burden and hypertension in patients with small-vessel disease. J Cereb Blood Flow Metab 2017;37:644–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu O, Cloonan L, Mocking SJ, et al. Role of acute lesion topography in initial ischemic stroke severity and long-term functional outcomes. Stroke 2015;46:2438–2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wardlaw JM, Smith C, Dichgans M. Mechanisms of sporadic cerebral small vessel disease: insights from neuroimaging. Lancet Neurol 2013;12:483–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maillard P, Fletcher E, Harvey D, et al. White matter hyperintensity penumbra. Stroke 2011;42:1917–1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Verlinden VJ, van der Geest JN, de Groot M, et al. Structural and microstructural brain changes predict impairment in daily functioning. Am J Med 2014;127:1089–1096.e2. [DOI] [PubMed] [Google Scholar]
- 22.Helenius J, Henninger N. Leukoaraiosis burden significantly modulates the association between infarct volume and National Institutes of Health Stroke Scale in ischemic stroke. Stroke 2015;46:1857–1863. [DOI] [PubMed] [Google Scholar]
- 23.Onteddu SR, Goddeau RP Jr, Minaeian A, Henninger N. Clinical impact of leukoaraiosis burden and chronological age on neurological deficit recovery and 90-day outcome after minor ischemic stroke. J Neurol Sci 2015;359:418–423. [DOI] [PubMed] [Google Scholar]
- 24.Dhamoon MS, McClure LA, White CL, et al. Long-term disability after lacunar stroke: secondary prevention of small subcortical strokes. Neurology 2015;84:1002–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koton S, Schwammenthal Y, Merzeliak O, et al. Cerebral leukoaraiosis in patients with stroke or TIA: clinical correlates and 1-year outcome. Eur J Neurol 2009;16:218–225. [DOI] [PubMed] [Google Scholar]
- 26.Basser PJ, Jones DK. Diffusion-tensor MRI: theory, experimental design and data analysis: a technical review. NMR Biomed 2002;15:456–467. [DOI] [PubMed] [Google Scholar]
- 27.Pasi M, van Uden IW, Tuladhar AM, de Leeuw FE, Pantoni L. White matter microstructural damage on diffusion tensor imaging in cerebral small vessel disease: clinical consequences. Stroke 2016;47:1679–1684. [DOI] [PubMed] [Google Scholar]
- 28.Maillard P, Carmichael O, Harvey D, et al. FLAIR and diffusion MRI signals are independent predictors of white matter hyperintensities. AJNR Am J Neuroradiol 2013;34:54–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wagner M, Helfrich M, Volz S, et al. Quantitative T2, T2*, and T2' MR imaging in patients with ischemic leukoaraiosis might detect microstructural changes and cortical hypoxia. Neuroradiology 2015;57:1023–1030. [DOI] [PubMed] [Google Scholar]
- 30.Wang C, Qin W, Zhang J, et al. Altered functional organization within and between resting-state networks in chronic subcortical infarction. J Cereb Blood Flow Metab 2014;34:597–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sun YW, Qin LD, Zhou Y, et al. Abnormal functional connectivity in patients with vascular cognitive impairment, no dementia: a resting-state functional magnetic resonance imaging study. Behav Brain Res 2011;223:388–394. [DOI] [PubMed] [Google Scholar]
- 32.Caprio FZ, Maas MB, Rosenberg NF, et al. Leukoaraiosis on magnetic resonance imaging correlates with worse outcomes after spontaneous intracerebral hemorrhage. Stroke 2013;44:642–646. [DOI] [PubMed] [Google Scholar]
- 33.Dacosta-Aguayo R, Grana M, Fernandez-Andujar M, et al. Structural integrity of the contralesional hemisphere predicts cognitive impairment in ischemic stroke at three months. PLoS One 2014;9:e86119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dufouil C, Godin O, Chalmers J, et al. Severe cerebral white matter hyperintensities predict severe cognitive decline in patients with cerebrovascular disease history. Stroke 2009;40:2219–2221. [DOI] [PubMed] [Google Scholar]
- 35.Jokinen H, Schmidt R, Ropele S, et al. Diffusion changes predict cognitive and functional outcome: the LADIS study. Ann Neurol 2013;73:576–583. [DOI] [PubMed] [Google Scholar]
- 36.Evans TE, O'Sullivan MJ, de Groot M, et al. White matter microstructure improves stroke risk prediction in the general population. Stroke 2016;47:2756–2762. [DOI] [PubMed] [Google Scholar]
- 37.Senda J, Ito K, Kotake T, et al. Association of leukoaraiosis with convalescent rehabilitation outcome in patients with ischemic stroke. Stroke 2016;47:160–166. [DOI] [PubMed] [Google Scholar]
- 38.Pani E, Zheng X, Wang J, Norton A, Schlaug G. Right hemisphere structures predict poststroke speech fluency. Neurology 2016;86:1574–1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Goyal M, Menon BK, van Zwam WH, et al. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet 2016;387:1723–1731. [DOI] [PubMed] [Google Scholar]
- 40.Tissue plasminogen activator for acute ischemic stroke: the National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. N Engl J Med 1995;333:1581–1587. [DOI] [PubMed] [Google Scholar]
- 41.Charlton RA, Barrick TR, McIntyre DJ, et al. White matter damage on diffusion tensor imaging correlates with age-related cognitive decline. Neurology 2006;66:217–222. [DOI] [PubMed] [Google Scholar]
- 42.Borich MR, Mang C, Boyd LA. Both projection and commissural pathways are disrupted in individuals with chronic stroke: investigating microstructural white matter correlates of motor recovery. BMC Neurosci 2012;13:107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ni H, Kavcic V, Zhu T, Ekholm S, Zhong J. Effects of number of diffusion gradient directions on derived diffusion tensor imaging indices in human brain. AJNR Am J Neuroradiol 2006;27:1776–1781. [PMC free article] [PubMed] [Google Scholar]
- 44.Vos SB, Jones DK, Viergever MA, Leemans A. Partial volume effect as a hidden covariate in DTI analyses. NeuroImage 2011;55:1566–1576. [DOI] [PubMed] [Google Scholar]