Abstract
Costimulation blockade (CoB) via belatacept is a lower morbidity alternative to calcineurin inhibitor (CNI)-based immunosuppression. However, it has higher rates of early acute rejection. These early rejections are mediated in part by memory T cells, which have reduced dependence on the pathway targeted by belatacept, and increased adhesion molecule expression. One such molecule is Leukocyte Function Associated Antigen (LFA)-1. LFA-1 exists in two forms, a commonly expressed, low-affinity form, and a transient, high-affinity form, expressed only during activation. We have shown that antibodies reactive with LFA-1 irrespective of its configuration are effective in eliminating memory T cells, but at the cost of impaired protective immunity. Here we test two novel agents, Leukotoxin A and AL-579, each of which targets the high affinity form of LFA-1, to determine whether this more precise targeting prevents belatacept-resistant rejection. Despite evidence of ex vivo and in vivo ligand-specific activity, neither agent when combined with belatacept proved superior to belatacept monotherapy. Leukotoxin A approached a ceiling of toxicity prior to efficacy, while AL-579 failed to significantly alter the peripheral immune response. These data, and prior studies, suggest that LFA-1 blockade may not be a suitable adjuvant agent for CoB resistant rejection.
Introduction
Renal transplantation remains the most effective means of treating ESRD, improving morbidity and mortality over the alternative of dialysis (1). The success of transplantation requires long-term immunosuppression largely based on calcineurin inhibitors (CNIs), which can often result in significant side effects including nephrotoxicity. These toxicities from conventional immunosuppression are due to their effects on broader molecular pathways not isolated to lymphocyte specific mechanisms. T-cell costimulation blockade (CoB) provides a lymphocyte specific target for the suppression of alloreactive responses, and is now available for clinical transplantation through the CD28-B7 pathway inhibitor belatacept. The initial clinical studies of belatacept demonstrated efficacy, absent many of the off-target side effects typically seen with CNIs (2–4). However, widespread application of belatacept in the clinic has been hesitant due to increased rates of aggressive early acute rejection (5). The etiology of CoB resistant rejection (CoBRR) is attributed at least in part to memory T cells, which during their development and expansion downregulate CD28 and are thereby indifferent to CD28:B7 blockade (6, 7). In addition, T effector memory (TEM) cells possess an increased potential to proliferate and mediate immune effector functions such as leukocyte adhesion and diapedesis; teleological attributes which are invariably deleterious to the allograft (6, 8). The favorable side effect profile of belatacept has given impetus to better understanding memory T cells and development of adjuvant therapies for use with belatacept.
The functional requirements of TEM cells lead to a unique surface protein phenotype with increased expression of CD2 and adhesion molecules such as VLA-4 and LFA-1 (7, 9). A recent report demonstrated a novel CD4+CD57+PD-1− T cell subset phenotype associated with CoBRR in renal transplant patients, which also expressed these adhesion molecules in greater quantity (10). Indeed, the differential expression of these markers on the suspected T cell subsets in CoBRR offers unique opportunities for adjuvant therapy, supported in proof of concept by several murine studies (11–15). We have previously studied agents targeting memory T cell markers as adjuvant therapy to CoB in nonhuman primate (NHP) models. Alefacept, a depleting agent targeting CD2, effectively reduced TEM cells in circulation (9) and variably prolonged allograft survival in kidney, islet, and vascularized composite allograft models, but at the cost of a significant reduction in protective immunity (16–18). The use of LFA-1 blockade to improve clinical islet allotransplant outcomes (19, 20) and in a NHP model (21) supports the conceptual efficacy in other models of transplantation. We recently reported our experience inhibiting LFA-1 with belatacept in a NHP renal transplant model. This approach did not improve allograft survival over belatacept alone, and like CD2-specific blockade, resulted in diminished protective immunity leading to increased CMV re-activation (22).
Integrins such as LFA-1 require specific control of receptor-ligand affinities based on functional need. In the case of LFA-1, three conformational states are defined, of which only one has high affinity for ligand (23). The generally expressed closed conformations prevent unnecessary binding to intercellular adhesion molecules (ICAMs), while the open, high affinity (HA) form is expressed transiently upon chemokine mediated activation (24). Dynamic change in LFA-1 structure has been demonstrated to be involved in the immunological synapse, chemokine induced activation, and tissue migration (25–27). Our prior study did not so much indicate that there was no effect of LFA-1 blockade, but rather that the effect was so global that it overly impaired protective immunity; the safety signature capped out prior to its efficacy signature. We hypothesize that specifically targeting the HA conformation of LFA-1 would more precisely target acutely activated TEM cells, reduce their access to the allograft, and potentially limit the detrimental effects on protective immunity.
Leukotoxin A (LtxA) is a protein isolated from aggregatibacter actinomycetemcomitans (28) that is more selective for the HA conformation of LFA-1 and induces cell death (29). AL-579 is a ligand mimetic antibody which specifically binds to the HA LFA-1 conformation, but does not induce cell death (30, 31). The fundamental rationale in these experiments is that more precise targeting of high affinity LFA-1 will act only at the site of lymphocyte activation and not globally disrupt all lymphocyte trafficking. This could allow for a focused therapeutic effect, likely within the allograft, without a global effect on established protective immunity. In these studies, we incorporate selective targeting of the activated conformation of LFA-1 either through depletion with LTxA or blockade with AL-579 in combination with belatacept. We demonstrate that despite evidence of in vitro and in vivo efficacy, these agents ultimately do not mitigate CoBRR.
Materials and Methods
In vitro assays
Peripheral blood mononuclear cells (PBMCs) were isolated from cell processing tubes (BD Biosciences, San Jose, CA) from 3 rhesus macaques and suspended in RPMI supplemented with 10% FBS, 1% Hepes Buffer, glutamine and penicillin/streptomycin at a concentration of 106 cells/mL. LtxA was added at concentrations of 1 ng/mL, 10 ng/mL, 100 ng/mL, 1 μg/mL, or 10 μg/mL, and cells were incubated for 1.5 hours at 37°C. Cells were then washed and plated in a 96-well round-bottom plate with 106 cells/well. Cells were resuspended in 100 μL of Annexin V buffer (BD Biosciences) per the manufacturer’s instructions and stained with surface antibodies for 15 minutes at room temperature. The surface staining consisted of antibodies against CD3, CD4, CD8, CD56, CD11a, CD16, CD95, CD28, and CD20. The cells were then washed twice with Annexin V Buffer with addition of 7AAD after the final wash and immediately acquired on the flow cytometer. Heat killed cells were used as a compensation control for 7AAD staining. For experiments using activated cells, surface staining was done prior to incubation with the same varying concentrations of LtxA, with or without addition of phorbol 12-myristate 13-acetate and ionomycin.
Development of LtxA and AL-579
LtxA was purified from culture supernatants of A. actinomycetemcomitans strain NJ4500 as described previously (32, 33). The activity was confirmed against the THP-1 leukemia cell line. AL-579 is a high affinity variant of AL-57 that contains further substitutions to make it more drug-like (Supplemental Figure 1) (31, 34). The heavy and light chains of antibody AL-579 were subcloned into expression vectors and used to co-transduce Chinese hamster ovary cells by electroporation. An oligoclonal pool of transduced cells was grown in serum-free, chemically-defined medium and secreted antibody was purified by protein A affinity chromatography. The purified antibody was diafiltered into citrate buffer; endotoxin levels were confirmed to be less than 1 endotoxin unit/mg.
Rhesus renal transplantation and monitoring
All experiments performed in this study were conducted with the approval of the Emory University or Duke University Institutional Animal Care and Use Committee and in adherence with the principles laid out in The Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, National Research Council, DHHS). Rhesus macaques (Macaca mulatta) were obtained from breeding colonies at AlphaGenesis, Inc. (Yemassee, SC, USA, for both Emory University and Duke University) or Yerkes National Primate Research Center (Lawrenceville, GA, USA for Emory University). Donor-recipient pairs were determined primarily by MHC disparity with a minimum of 3 major antigen mismatch and secondarily by similar size. MHC typing for both class I and class II was performed by 454 pyrosequencing (University of Wisconsin, Madison, WI, USA). Transplantation was performed in a domino fashion to maximize the utility of the available animals, with each animal serving as a kidney donor prior to receiving a transplant as described previously (16, 22, 35). Left donor nephrectomy was performed at least 3 weeks prior to transplantation. A right nephrectomy was simultaneously conducted after implantation to leave each recipient entirely dependent on the allograft. Post-transplant monitoring consisted of daily clinical assessment by veterinary staff, as well as laboratory studies including serum chemistry and complete blood count assessments performed 2–4 times monthly, or more often if dictated by clinical condition. Rhesus CMV (rhCMV) was monitored with every blood draw and when clinically indicated using qPCR. CMV Prophylaxis was initiated at the time of transplant which consisted of valganciclovir 60 mg twice daily, with viral copies >10,000/mL treated with the addition of IM ganciclovir 5–7.5 mg/kg twice daily. Experimental endpoint was determined by significant increase in serum creatinine confirmed over two days. Humane endpoints were implemented when necessary after consultation with veterinary staff.
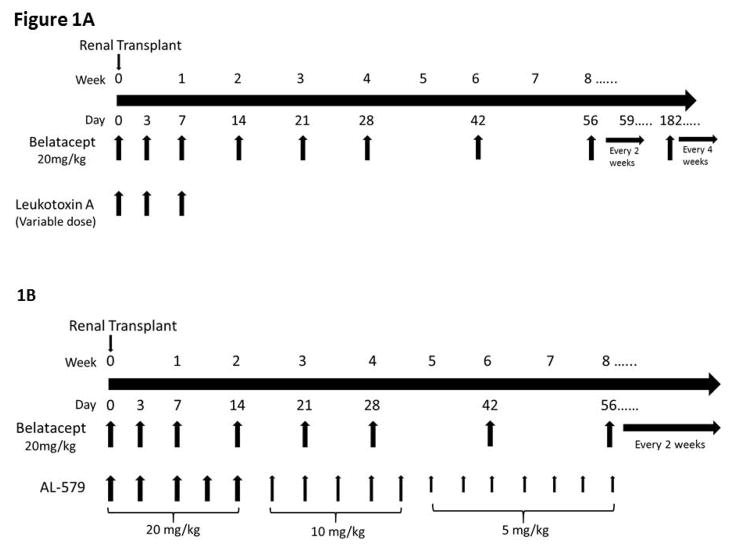
Transplant groups and immunosuppression
Transplants incorporating LtxA based regimens were conducted at Emory University. Three animals received LtxA monotherapy with 15mcg/kg IV given on the day of transplant, and 2.5mcg/kg, 10mcg/kg or 15mcg/kg (each n=1) given on days 3 and 7. Different doses of maintenance drug were used in an effort to identify a potential therapeutic dose. Animals receiving belatacept monotherapy (n=3) were given belatacept 20mg/kg on the day of transplant, post-operative day 3, 7, 14, 21, 28, and then every two weeks until POD 182, then monthly. Eight animals in total received combined LtxA and belatacept. LtxA was given at a high dose of 75mcg/kg on the day of transplant, day 3 and day 7 (n=2), intermediate dose of 25mcg/kg on the day of transplant and 25mcg on day 3 and day 7 (n=3), and low dose of 15mcg/kg on the day of transplant and 15mcg on day 3 and day 7 (n=3). All recipients in this study received a single dose of methylprednisolone 15mg/kg intraoperatively prior to graft implantation.
The cohort for AL-579 based regiments were performed at Duke, with the exception of one AL-579 with belatacept transplant performed at Emory University. The control group of transplant recipients for AL-579 received Belatacept at 20mg/kg on the day of transplant, post-operative day 3, 7, 14, 21, 28, then every two weeks (no change in frequency at POD 182, n=4). The experimental group to this belatacept regimen was given AL-579 as a taper, starting with 20mg/kg on POD 0, 3, 7,10, and 14, then 10mg/kg twice weekly until POD 31, then 5mg/kg twice weekly until POD 56 (n=4). All recipients in this study received a single dose of methylprednisolone 15mg/kg intraoperatively prior to graft implantation.
Polychromatic flow cytometry
Peripheral blood mononuclear cells were isolated from transplant recipients immediately prior to transplantation (POD 0) and immediately prior to every belatacept infusion outlined above. After washing with PBS and lysis of RBC, cells were stained with the following antibodies: CD3, CD4, CD2, CD28, CD95, CD11a, CD16, CD20, CD56, CD45, FoxP3, CD25, CD127, Ki67, and Bcl2. At the time of rejection and recipient euthanasia, PBMCs were also collected in addition to lymph nodes and spleen tissue. Cells were isolated from these lymphoid organs through mechanical disassociation and passage of cells though a 70um filter. The resulting cells were lysed and stained for flow cytometry using the methods described above.
Histology
At the time of recipient endpoint, lymph nodes, spleen, and the allograft were collected in 10% formalin for histological evaluation. These samples were embedded in paraffin with subsequent sectioning and hematoxylin and eosin staining. Staining for high affinity LFA-1 was performed on specimens from our previous NHP transplant studies (35). Samples were identified, then stained after deparaffinization using AL-579 as a primary antibody and visualized using the LSAB+ labeled Streptavidin-Biotin kit (Dako; Carpenteria, CA, USA). Nuclei were counterstained with hematoxylin. Needle-core biopsies were also obtained for animals with potential rejection without meeting the requirements for humane euthanasia. Images were digitally scanned using Aperio Scanscope AT Turbo (Leica Biosystems, Buffalo Grove, IL).
Statistical analysis
Statistical analyses were performed using Prism (GraphPad; La Jolla, CA). Analysis of ex vivo data were performed with two-way ANOVAs. Allograft survival was compared using Kaplan-Meier analysis (Log-rank) of graft rejection. Longitudinal analysis of flow cytometry was performed using two-way ANOVA. Histology scoring was compared using a Chi squared test.
Results
Activated high-affinity LFA-1 is associated with costimulation blockade resistant rejection
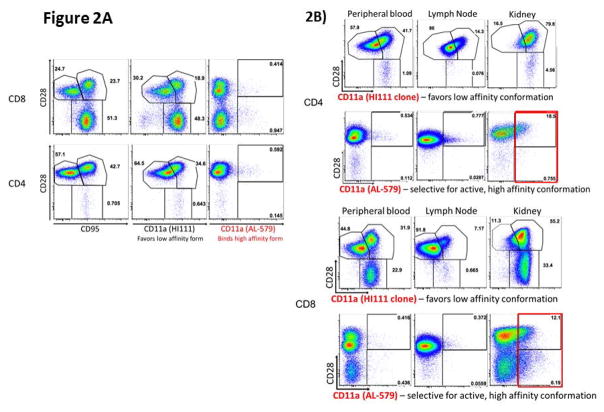
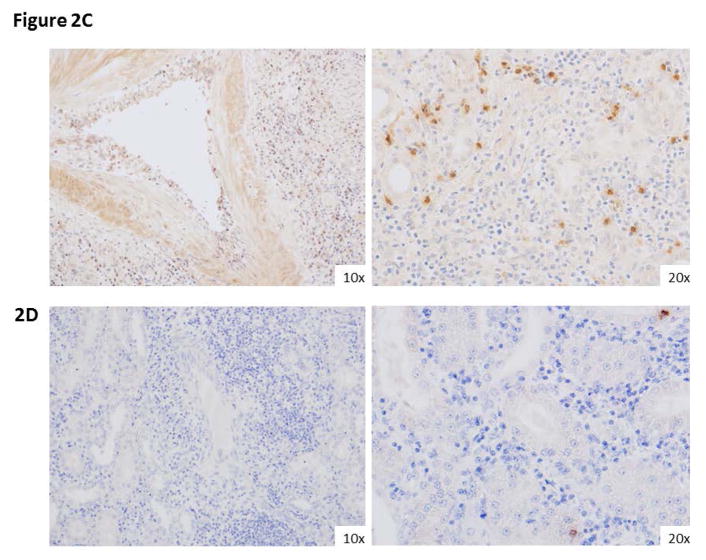
We first identified the specificity of AL-579 in rhesus splenocytes, lymphocytes, and PBMCs from healthy control animals (Figure 2a) and animals with CoBRR (Figure 2b) using flow cytometry. As expected, we found that HA LFA-1 as detected by AL-579 was present at very low levels in healthy animals. Conversely, the frequency of AL-579 positive T cells were markedly higher in animals with CoBRR. As expected, AL-579 positive cells were not detected in peripheral blood or lymph nodes, however a significant proportion of graft infiltrating lymphocytes expressed HA LFA-1. We also compared binding of AL-579 to a selective LFA-1 antibody that favors the low affinity conformation of LFA-1. This confirmed in our model that AL-579 does bind a subset of T cells that differs from low-affinity LFA-1 selective binding. Figure 2c illustrates histology from a rejected NHP renal allograft during CoBRR. Staining using AL-579 demonstrates expression of HA LFA-1 on the vascular endothelium and within the graft parenchyma during rejection. Comparison of this rejection phenotype histology from a rejection during conventional triple therapy (consisting of tacrolimus, mycophenolate mofetil, and steroids), again stained with AL-579, further suggests this to be a accentuated in CoBRR. These data support the involvement of high affinity LFA-1 in CoBRR and the potential for specific adjuvant therapy to CoB.
Figure 2. High affinity LFA-1 is present in costimulation resistant rejection.
A) Flow cytometry using AL-579 demonstrates selective binding of a small subset of lymphocytes (right panels). B) Flow cytometric analysis of PBMCs (left panels), lymphocytes (middle panels), and graft infiltrating cells (right panels) demonstrate the presence of high-affinity LFA-1 intragraft during rejection in both CD8 (above) and CD4 (below). C) Immunohistochemical staining of NHP renal allografts during CoBRR demonstrates endarteritis with leukocytes expressing HA LFA-1 (left) and infiltration (right) of leukocytes expressing HA LFA-1. D) Infiltrates from rejection during conventional triple therapy immunosuppression did not have as many HA LFA-1 expressing cells. CoBRR, costimulation blockade resistant rejection; LFA, leukocyte function antigen; NHP, non-human primate, PBMC, peripheral blood mononuclear cell.
LtxA effectively depletes CD8+ TEM and NK cells
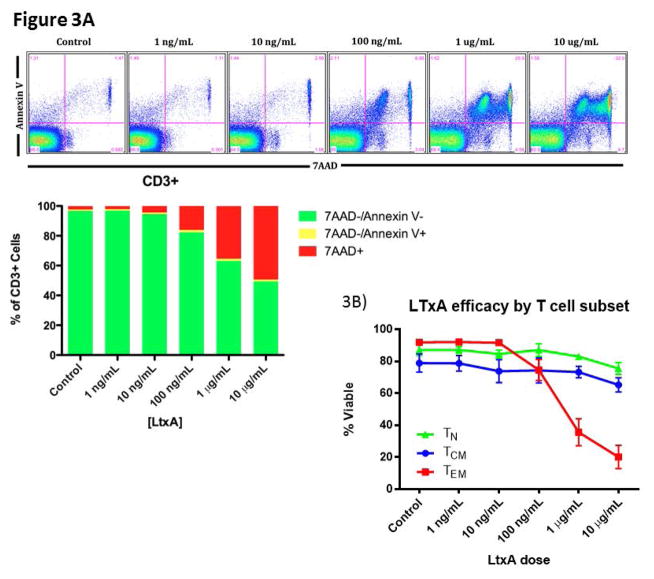
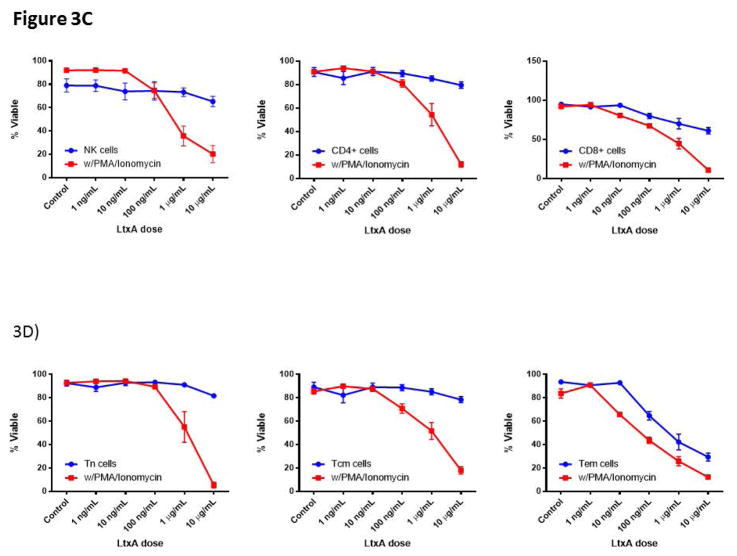
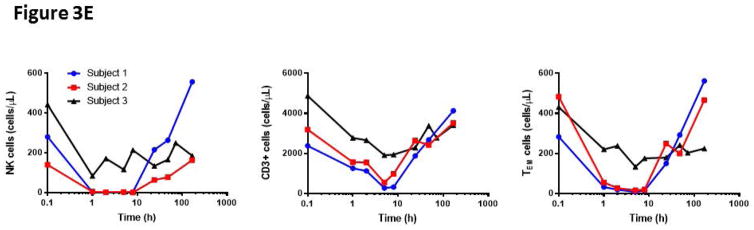
LtxA has been shown to effectively deplete malignant leukocytes with high expression of the activated LFA-1 conformation (36). We therefore sought to examine the efficacy of LtxA mediated leukocyte and lymphocyte toxicity in our NHP model. Using naïve NHP peripheral blood, we introduced increasing doses of LtxA ex vivo and measured cells with flow cytometry for WBC and T cell markers in combination with 7AAD and Annexin V cell death markers (Figure 3a). Initial findings demonstrated a significant dose response effect for CD8 TN or TCM with a greater effect on CD8 TEMs (p<0.01, Figure 3b). We next tested the effect of activation induced LtxA cytotoxicity on PBMCs. We found that NK cells in addition to CD4 and CD8 T cells were more susceptible to LtxA after treatment with PMA/Ionomycin (p<0.01, Figure 3c). Upon closer examination of memory subsets as defined by CD95 and CD28, TEM cells appeared to be depleted in a dose dependent fashion without a requirement for chemically induced activation, although PMA/Ionomycin further increased this effect (p<0.01, Figure 3d). To confirm these effects in vivo, we then administered LtxA to 3 different naïve rhesus macaques and measured the peripheral counts using flow cytometry at set time points up to 168 hours after administration. Figure 3d illustrates similar depletion in vivo of NK cells, T cells, and CD8+ TEMs as compared to ex vivo observations. These data reveal a substantial depletional effect with LtxA administration that favors NK and TEM cells, the latter suggesting this agent as another potential adjuvant therapy for CoB.
Figure 3. Leukotoxin A selectively depletes activated lymphocytes.
A)LtxA depletes lymphocytes in vitro in a dose dependent manner (B) and preferentially depletes TEM at higher doses (p<0.01). C) NK cells and T cells are depleted in a dose dependent manner with increased efficacy during activation with PMA/Ionomycin (p<0.01). This susceptibility to LTxA on activated CD8 TEMs although significant (p<0.01) was already present without stimulation, only marginally increasing afterwards. This suggests an increased relative presence of the high affinity LFA-1 on CD8 TEMs without the need for activation. D) NK cells and T cells are rapidly depleted in vivo after administration of LtxA, with rapid reconstitution after 10 hours. Subset specific depletion of CD8+ TEM cells in vivo parallels ex vivo findings. LFA, leukocyte function antigen; LtxA, leukotoxin A.
Adjuvant depletion or blockade of high-affinity LFA-1 does not improve costimulation blockade therapy in a nonhuman primate model
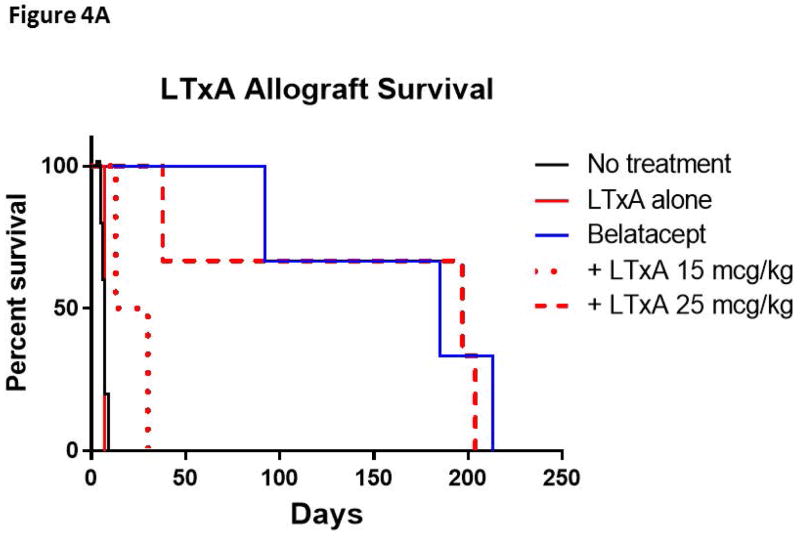
Having demonstrated the effects of both LtxA and AL-579 in vitro and in vivo, we next examined these agents in a well-described NHP model of renal transplantation. We administered these agents in conjunction with belatacept in order to assess the potential synergistic effect of blocking or depleting activated LFA-1 on TEM cells, which can be indifferent to CD28:B7 blockade alone. In first examining the effects of LtxA with COB, we found that LtxA administration after renal transplantation at two different doses did not improve allograft survival over CoB alone (Figure 4a). While LtxA given as a monotherapy did not provide any additional survival benefit over no treatment, LtxA given at 15 mcg/kg doses after transplantation in conjunction with belatacept let to rapid allograft loss with only marginal improvement over LtxA alone (p=0.08). Interestingly, this addition of low dose LtxA significantly worsened allograft survival when compared to belatacept alone (p=0.04). LtxA given at an intermediate dose (25mcg/kg) in conjunction with belatacept did not improve or worsen survival over belatacept alone. When LtxA was given at a higher dose of 75mcg/kg, both recipients experienced significant toxicity requiring removal from the study.
Figure 4. Adjuvant therapies selective for high-affinity LFA-1 do not prolong renal allograft survival with belatacept therapy.
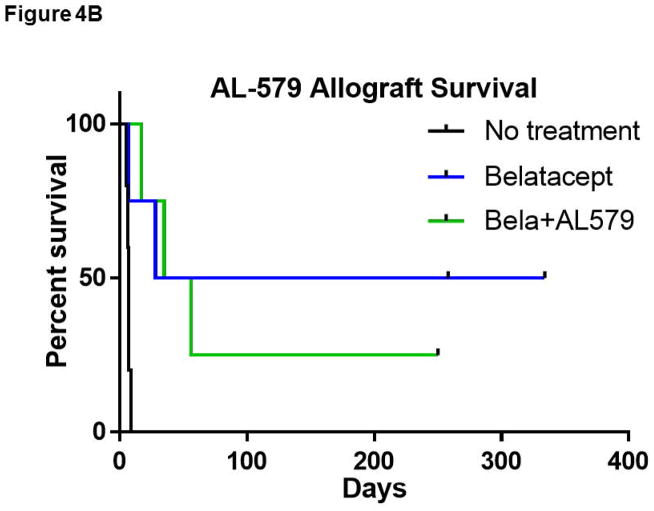
A) LTxA based regimens did not improve belatacept based therapy. LTxA monotherapy did not improve allograft survival compared to no treatment. Low dose LtxA (15mcg/kg) with belatacept appeared to marginally improve survival over LTxA alone (p=0.08). Both belatacept monotherapy and the addition of intermediate dose LTxA (25mcg/kg) significantly improved survival over untreated controls (p<0.05), however neither demonstrated superiority over the other. Two animals that received high dose LTxA (75 mcg/kg) in addition to belatacept were removed from study due to toxicity within a week of transplant (not shown in figure). B) The addition of AL-579 to belatacept did not protect against CoBRR (p=0.86). CoBRR, costimulation blockade resistant rejection; LtxA, leukotoxin A.
Next, we examined AL-579 in conjunction with belatacept based CoB. As illustrated in Figure 4b, the addition of AL-579 to belatacept did not improve allograft survival compared to belatacept monotherapy (p=0.86). The inclusion of targeted HA LFA-1 depletion or blockade in our renal transplant model ultimately did not provide further benefit to CoB based immunosuppression.
Minimal changes in immunophenotype during treatment with AL-579
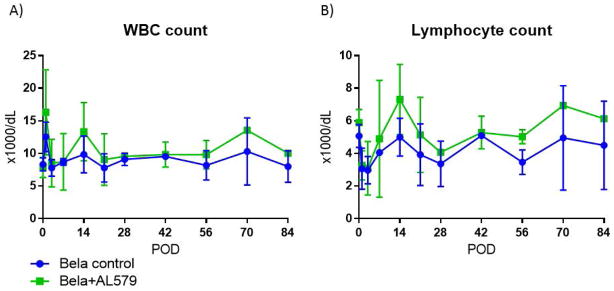
Our prior study inhibiting all conformations of LFA-1 in a renal transplant model demonstrated an increased peripheral lymphocytosis, which diminishes during the tapering of LFA-1 inhibiting agent (22). The short induction course of AL-579 did not affect peripheral WBC counts or lymphocyte counts (Figure 5). Further examination of peripheral T cells using flow cytometry demonstrated similar frequencies of CD8 TEMs (Figure 6). Regulatory T cells defined as CD25+CD127−FoxP3+ CD4+ cells were also similar in frequency in the peripheral blood (data not shown). Banff scoring of rejected allografts in AL-579 treated and control group animals suggested a strong trend that AL-579 treatment prevented vasculopathy and glomerulopathy during rejection (Table 1 and Figure 7, p=0.09). Serum CMV viral copies did not differ greatly between the two treatment arms. However, an increase of CMV viral copies did occur at the peak of AL-579 treatment and reduces to normal levels as this agent was tapered without any additional treatment (data not shown).
Figure 5. The addition of AL-579 to belatacept therapy does not alter peripheral leukocyte counts.
A) The addition of AL-579 to belatacept did not significantly alter WBC counts and (B) lymphocyte counts.
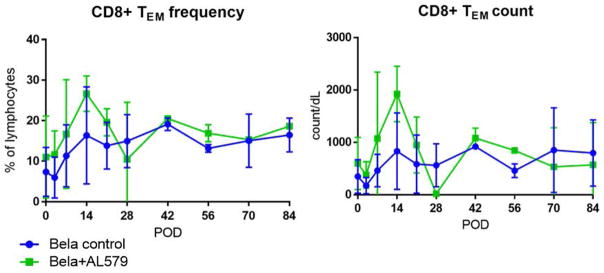
Figure 6. Peripheral effector memory T cell counts are not altered with the addition of AL-579.
Peripheral circulation of effector memory CD8 T cells were similar between both treatment groups in both frequency (left) and counts (right).
Table 1.
| Recipient Treatment | Histology POD | Banff score | Vasculitis score (v) | Glomerulopathy score (cg) |
|---|---|---|---|---|
| Bela | 7 | ACR2B | 2 | 2 |
| Bela | 28 | ACR1A | 0 | 0 |
| Bela | 71 | Borderline | 0 | 0 |
| Bela | 44 | ACR2A | 1 | 1 |
| Bela | 185 | ACR2A | 1 | 2 |
| Bela | 213 | Borderline | 0 | 0 |
| Bela+AL579 | 56 | ACR1B |
|
|
| Bela+AL579 | 17 | ACR1B | ||
| Bela+AL579 | 35 | ACR1A | ||
| Bela/LtxA IV(low) | 30 | ACR2A | 1 | 0 |
| Bela/LtxA IV (low) | 4 | Borderline | 0 | 0 |
| Bela/LtxA IV (low) | 13 | ACR3 | 3 | 0 |
| Bela/LtxA IV (high) | 197 | Borderline | 0 | 0 |
| Bela/LtxA IV (high) | 38 | ACR2A | 1 | 2 |
| Bela/LtxA IV (high) | 7 | ACR2B | 2 | 0 |
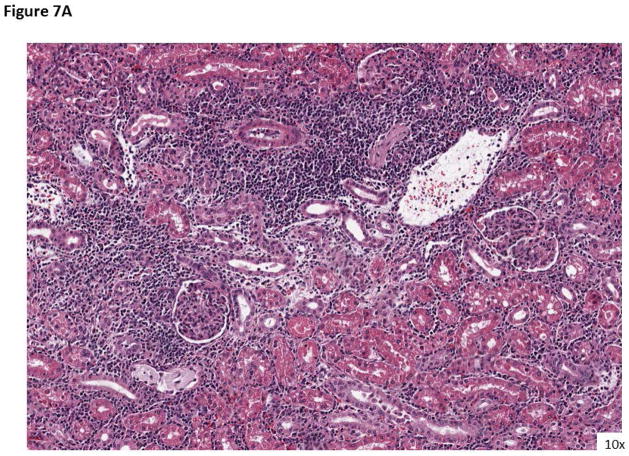
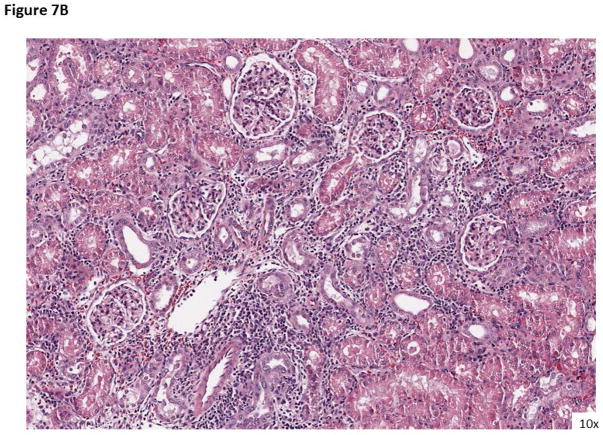
Figure 7. Rejection histology when treated with AL-579.
A) An example of rejection with belatacept monotherapy (ACR IIB) with significant infiltration and glomerulopathy. B) Rejection while under treatment with AL-579 and belatacept (ACR 1A) again with significant infiltration, however without vasculitis.
Discussion
Belatacept has been introduced as an alternative to CNI-based transplantation immunotherapy. The advantages of this biologic include the reduction of off target side effects, which are problematic and common with CNI use. These CNI-associated complications have largely motivated further understanding of CoBRR to optimize the application of belatacept and leverage its benefits for transplant patients. The evasion of TEM cells to the effects of belatacept have led to further studies and trials targeting TEM cells in order to identify adjuvant therapies to facilitate CD28:B7 blockade (7). Data obtained from rejected NHP kidneys in the described model demonstrated CoBRR specific involvement of HA LFA-1 expressing lymphocytes within rejected allografts. The implication of this distinct signature led us to hypothesize that blocking function of the more relevant conformation of LFA-1 would protect against intragraft migration and effector function. In these studies, we applied highly selective agents targeting HA LFA-1 in an NHP renal transplant model in order to further explore this modality of CoB adjuvant therapy. We find, however, that despite evidence of in vitro and in vivo efficacy, neither the addition of LTxA nor AL-579 in these studies were able to improve on belatacept monotherapy alone. These data, in addition to prior and ongoing studies, highlight the difficulty in targeting TEM cell-specific surface markers in relevant preclinical models.
Critical to the function of differentiated T cells are their interactions with lymphoid or vascular endothelium with migration into the lymph nodes or peripheral tissues respectively. This process is dependent on the function of adhesion molecules such as LFA-1 and VLA-4 (37). LFA-1 in particular demonstrates a unique cell surface localization of high and intermediate conformations during endothelial migration (27), with the HA conformation necessary for intercellular adhesion (23). This conformational change is also seen during TCR engagement and T cell activation, serving to stabilize the immunological synapse (38). Earlier studies identified the necessity of LFA-1 in migration via CD2 and TCR dependent mechanisms (39, 40). It is important to note, however, that the current data available have not clarified the function of the various affinity conformations of LFA-1 in TEM cell migration or activation (26). The current understanding of CoBRR and the implication of TEM cells offered a unique opportunity to assess specifically HA LFA-1’s significance in the alloreactive TEM cell response seen in CoBRR.
In this study, an appropriate therapeutic window for LtxA to achieve an immunosuppressive effect while avoiding serious toxicity was not identified. Our initial in vivo study demonstrated that a single dose of IV LtxA in healthy animals was sufficient to transiently deplete lymphocytes from the circulating population. However, in the setting of immune activation via surgical laparotomy and allogenic solid organ transplantation, we hypothesized that of the behavior LtxA might be less predictable. The three initial animals that were given LtxA monotherapy were each given different doses in an effort to determine a therapeutic dose. However, these animals all rejected with the same kinetics as untreated controls. These animals demonstrated allograft pathology consistent with rejection with no clinical evidence of side effects. Therefore, the next cohort of animals combined belatacept with higher doses of LtxA, again seeking a potential therapeutic window for LtxA. The intermediate dose (25 mcg/kg) appeared to have no effect, while interestingly, the low dose LtxA (15mcg/kg) significantly worsened allograft survival. Attempting a higher dose of LTxA (75 mcg/kg) resulted in significant toxicity necessitating the removal of two animals from the study. We hypothesize that high dose LtxA treatment may have precipitated rapid lymphocyte lysis in the setting of surgical trauma and inflammation resulting in physiologic signs analogous to a tumor lysis phenomenon. Therefore, despite desired the ex vivo and in vivo effects, application of HA LFA-1 based depletion may be not efficacious in the setting of a solid organ transplant.
The alternative strategy of HA LFA-1 blockade (without depletion) in conjunction with CoB was also explored in this transplant model. However, inclusion of AL-579 adjuvant therapy to a belatacept based regimen had no obvious benefit over belatacept alone. Study of circulating PBMCs through the transplant period demonstrated no major differences in leukocyte or lymphocyte count, although given the transient expression of high affinity LFA, no peripheral or bulk phenotype was anticipated (30, 34). This is in contrast to our previous finding using agents broadly directed against LFA-1, in which peripheral lymphocytosis paralleled the dose of anti-LFA-1 given at the time (22). This particular finding may illustrate the limited significance of HA LFA-1 in margination and migration in this scenario, or the inability of systemically distributed antibody therapy to sufficiently interrupt the very transient and local expression of HA-LFA-1. Circulating effector TEM cell populations were also unchanged with the addition of AL-579. Histological data during AL-579 treatment suggested that this interaction may be involved in rejection associated vasculopathy, suggesting some level of anti-inflammatory effect. Regardless, this possible lack of vascular involvement did not ultimately preserve allograft function from rejection. These data in combination with the incorporation of LtxA in our transplant model ultimately demonstrate that HA LFA-1 may not be an efficacious target for CD28:B7 blockade adjuvant therapy. Of note, our control group in the AL-579 study appeared to have better outcomes when compared to the control group from the LtxA study; this observation is likely a results of each control and experimental group being performed at two institutions with different belatacept dosing after day 180, a time point irrelevant to this study. Nevertheless, it underscores the value of continued belatacept dosing late after transplantation.
Although our initial data associated a unique phenotype of HA LFA-1 involvement in CoBRR, the lack of any benefits seen with either blockade or depletion of HA LFA-1 may be explained through several possibilities. The conformational changes of LFA-1 are required in the priming and proliferation of naïve T cells (26), but TCR engagement alone does not induce greater expression of HA LFA-1 without the presence of insoluble ICAMs (41). Additionally, the rapid flux of LFA-1 conformational shifts may also significantly narrow the temporal window where an effect may be observed in this model (42). These observations suggest that TEM cell dependence on HA LFA-1 for activation may be limited in the setting of antigen presentation, although further study would be warranted. Mice with genetically modified LFA-1 to maintain a persistent activated state acquire significant immunological defects in regards to T cell activation and migration (43), underlining the complex regulation of LFA-1 affinity required for normal immune function. Examination of ICAM interactions in a murine model demonstrated its necessity for T cell priming and activation, but the lack of ICAM on transplanted allografts did not prevent alloreactive T cell infiltration and subsequent rejection (44). With the redundant presence of adhesion molecules present on immune cells and their target proteins, it is very likely that migration is not dependent solely on LFA-1. Indeed, VLA-4 may play a more significant role for alloreactive CD4 T cell intragraft migration (13, 45). Additionally, prior studies examining adhesion blockade in transplantation may not necessarily distinguish its effects on the immunologic synapse versus memory T cell effector function. These data in addition to prior NHP studies (22) suggest that alloreactive memory T cell function, particularly intragraft migration and subsequent injury, is not dependent on LFA-1 in the either the intermediate or high affinity state.
These studies examining HA-LFA-1 in CoBRR, in addition to prior and ongoing preclinical trials in this domain, further contribute to the evidence against targeting TEM cell-specific surface markers to abrogate CoBRR. Prior studies with demonstrable efficacy against TEM cells through surface marker targeting have also demonstrated some loss of protective immunity. Targeting belatacept resistant TEM cells may either require more creative strategies to redistribute or redefine one’s TEM cell repertoire in order to suppresses alloreactivity and maintain protective immunity. TEM cells possess unique traits acquired along the process of their differentiation outside of their surface protein expression, such as metabolic and functional properties, which may offer these additional opportunities (37). Additionally, further refining the etiology of CoB resistance, whether more specific cell subtype definition or molecular mechanisms, will elucidate potential adjuvant strategies.
Supplementary Material
(A) The AL-579 gamma4 heavy chain is identical to the AL-57 heavy chain except it incorporates F to Y and I to S substitutions (indicated) found in a higher affinity variant, C09, of AL-57. It also contains a M to L substitution to remove an exposed, oxidation-prone methionine, and an S to P substitution (indicated) in the gamma4 hinge to prevent heavy chain exchange among IgG4 molecules. (B) AL-579 kappa light chain is identical to the AL-57 light chain.
Figure 1. Immunosuppression regimen for LTxA and AL-579.
A) LTxA based regimen. Belatacept controls were transplanted with the same timeline for belatacept dosing. B) AL-579 based regimen. Belatacept controls for each group were transplanted with the same timeline for belatacept dosing. LtxA, leukotoxin A.
Acknowledgments
This study was funded by NIH grant #U19-AI051731 (ADK). We would also like to thank the Duke University Substrate Core for their assistance in CMV monitoring. Anti-LFA-1 used in this study was produced by the NIH Nonhuman Primate Reagent Resource (NIAID contract HHSN 2722001300031C).
Abbreviations
- NHPs
Non-human primates
- CoB
costimulation blockade
- CoBRR
costimulation blockade resistant rejection
- LFA
leukocyte function antigen
- HA
high affinity
- LtxA
Leukotoxin A
- PBMC
peripheral blood mononuclear cells
- TEM
T effector memory
Footnotes
Disclosure
The authors of this manuscript have conflicts of interest to disclose as described by the American Journal of Transplantation. SCK is an employee of Actinobac Biomed, which provided LtxA for the study. The other authors have no conflicts of interest to disclose.
Additional Supporting Information may be found in the online version of this article.
References
- 1.Wolfe RA, Ashby VB, Milford EL, Ojo AO, Ettenger RE, Agodoa LY, et al. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. The New England Journal of Medicine. 1999;341(23):1725–30. doi: 10.1056/NEJM199912023412303. [DOI] [PubMed] [Google Scholar]
- 2.Durrbach A, Pestana JM, Pearson T, Vincenti F, Garcia VD, Campistol J, et al. A phase III study of belatacept versus cyclosporine in kidney transplants from extended criteria donors (BENEFIT-EXT study) American Journal of Transplantation. 2010;10(3):547–57. doi: 10.1111/j.1600-6143.2010.03016.x. [DOI] [PubMed] [Google Scholar]
- 3.Vincenti F, Charpentier B, Vanrenterghem Y, Rostaing L, Bresnahan B, Darji P, et al. A phase III study of belatacept-based immunosuppression regimens versus cyclosporine in renal transplant recipients (BENEFIT study) American Journal of Transplantation. 2010;10(3):535–46. doi: 10.1111/j.1600-6143.2009.03005.x. [DOI] [PubMed] [Google Scholar]
- 4.Vincenti F, Rostaing L, Grinyo J, Rice K, Steinberg S, Gaite L, et al. Belatacept and Long-Term Outcomes in Kidney Transplantation. The New England Journal of Medicine. 2016;374(4):333–43. doi: 10.1056/NEJMoa1506027. [DOI] [PubMed] [Google Scholar]
- 5.Masson P, Henderson L, Chapman JR, Craig JC, Webster AC. Belatacept for kidney transplant recipients. The Cochrane database of systematic reviews. 2014;11:Cd010699. doi: 10.1002/14651858.CD010699.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Espinosa JR, Samy KP, Kirk AD. Memory T cells in organ transplantation: progress and challenges. Nature Reviews Nephrology. 2016;12(6):339–47. doi: 10.1038/nrneph.2016.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Page AJ, Ford ML, Kirk AD. Memory T-cell-specific therapeutics in organ transplantation. Current Opinion in Organ Transplantation. 2009;14(6):643–9. doi: 10.1097/MOT.0b013e328332bd4a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Su CA, Fairchild RL. Memory T Cells in Transplantation. Current Transplantation Reports. 2014;1(3):137–46. doi: 10.1007/s40472-014-0018-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lo DJ, Weaver TA, Stempora L, Mehta AK, Ford ML, Larsen CP, et al. Selective targeting of human alloresponsive CD8+ effector memory T cells based on CD2 expression. American Journal of Transplantation. 2011;11(1):22–33. doi: 10.1111/j.1600-6143.2010.03317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Espinosa J, Herr F, Tharp G, Bosinger S, Song M, Farris AB, 3rd, et al. CD57(+) CD4 T Cells Underlie Belatacept-Resistant Allograft Rejection. American Journal of Transplantation. 2016;16(4):1102–12. doi: 10.1111/ajt.13613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beus JM, Hashmi SS, Selvaraj SA, Duan D, Stempora LL, Monday SA, et al. Heterologous immunity triggered by a single, latent virus in Mus musculus: combined costimulation- and adhesion- blockade decrease rejection. PloS one. 2013;8(8):e71221. doi: 10.1371/journal.pone.0071221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Luo L, Sun Z, Cheng H, Luo G. Memory T-cell-specific therapeutics attenuate allograft rejection via mediation of alloreactivity in memory cells. Immunology Letters. 2012;148(1):53–8. doi: 10.1016/j.imlet.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 13.Kitchens WH, Haridas D, Wagener ME, Song M, Ford ML. Combined costimulatory and leukocyte functional antigen-1 blockade prevents transplant rejection mediated by heterologous immune memory alloresponses. Transplantation. 2012;93(10):997–1005. doi: 10.1097/TP.0b013e31824e75d7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kitchens WH, Haridas D, Wagener ME, Song M, Kirk AD, Larsen CP, et al. Integrin antagonists prevent costimulatory blockade-resistant transplant rejection by CD8(+) memory T cells. American Journal of Transplantation. 2012;12(1):69–80. doi: 10.1111/j.1600-6143.2011.03762.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ramsey H, Pilat N, Hock K, Klaus C, Unger L, Schwarz C, et al. Anti-LFA-1 or rapamycin overcome costimulation blockade-resistant rejection in sensitized bone marrow recipients. Transplant international : official journal of the European Society for Organ Transplantation. 2013;26(2):206–18. doi: 10.1111/tri.12021. [DOI] [PubMed] [Google Scholar]
- 16.Lo DJ, Anderson DJ, Weaver TA, Leopardi F, Song M, Farris AB, et al. Belatacept and sirolimus prolong nonhuman primate renal allograft survival without a requirement for memory T cell depletion. American Journal of Transplantation. 2013;13(2):320–8. doi: 10.1111/j.1600-6143.2012.04342.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Freitas AM, Samy KP, Farris AB, Leopardi FV, Song M, Stempora L, et al. Studies Introducing Costimulation Blockade for Vascularized Composite Allografts in Nonhuman Primates. American Journal of Transplantation. 2015 doi: 10.1111/ajt.13379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lowe MC, Badell IR, Turner AP, Thompson PW, Leopardi FV, Strobert EA, et al. Belatacept and sirolimus prolong nonhuman primate islet allograft survival: adverse consequences of concomitant alefacept therapy. American Journal of Transplantation. 2013;13(2):312–9. doi: 10.1111/j.1600-6143.2012.04341.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Posselt AM, Bellin MD, Tavakol M, Szot GL, Frassetto LA, Masharani U, et al. Islet transplantation in type 1 diabetics using an immunosuppressive protocol based on the anti-LFA-1 antibody efalizumab. American Journal of Transplantation. 2010;10(8):1870–80. doi: 10.1111/j.1600-6143.2010.03073.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Turgeon NA, Avila JG, Cano JA, Hutchinson JJ, Badell IR, Page AJ, et al. Experience with a novel efalizumab-based immunosuppressive regimen to facilitate single donor islet cell transplantation. American Journal of Transplantation. 2010;10(9):2082–91. doi: 10.1111/j.1600-6143.2010.03212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Badell IR, Russell MC, Thompson PW, Turner AP, Weaver TA, Robertson JM, et al. LFA-1-specific therapy prolongs allograft survival in rhesus macaques. The Journal of Clinical Investigation. 2010;120(12):4520–31. doi: 10.1172/JCI43895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Anderson DJ, Lo DJ, Leopardi F, Song M, Turgeon NA, Strobert EA, et al. Anti-Leukocyte Function-Associated Antigen 1 Therapy in a Nonhuman Primate Renal Transplant Model of Costimulation Blockade-Resistant Rejection. American Journal of Transplantation. 2016;16(5):1456–64. doi: 10.1111/ajt.13628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schurpf T, Springer TA. Regulation of integrin affinity on cell surfaces. The EMBO Journal. 2011;30(23):4712–27. doi: 10.1038/emboj.2011.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nishida N, Xie C, Shimaoka M, Cheng Y, Walz T, Springer TA. Activation of leukocyte beta2 integrins by conversion from bent to extended conformations. Immunity. 2006;25(4):583–94. doi: 10.1016/j.immuni.2006.07.016. [DOI] [PubMed] [Google Scholar]
- 25.Park EJ, Peixoto A, Imai Y, Goodarzi A, Cheng G, Carman CV, et al. Distinct roles for LFA-1 affinity regulation during T-cell adhesion, diapedesis, and interstitial migration in lymph nodes. Blood. 2010;115(8):1572–81. doi: 10.1182/blood-2009-08-237917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang Y, Li D, Nurieva R, Yang J, Sen M, Carreno R, et al. LFA-1 affinity regulation is necessary for the activation and proliferation of naive T cells. The Journal of Biological Chemistry. 2009;284(19):12645–53. doi: 10.1074/jbc.M807207200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smith A, Stanley P, Jones K, Svensson L, McDowall A, Hogg N. The role of the integrin LFA-1 in T-lymphocyte migration. Immunological Reviews. 2007;218:135–46. doi: 10.1111/j.1600-065X.2007.00537.x. [DOI] [PubMed] [Google Scholar]
- 28.Kachlany SC. Aggregatibacter actinomycetemcomitans leukotoxin: from threat to therapy. Journal of Dental Research. 2010;89(6):561–70. doi: 10.1177/0022034510363682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.DiFranco KM, Gupta A, Galusha LE, Perez J, Nguyen TV, Fineza CD, et al. Leukotoxin (Leukothera(R)) targets active leukocyte function antigen-1 (LFA-1) protein and triggers a lysosomal mediated cell death pathway. The Journal of Biological Chemistry. 2012;287(21):17618–27. doi: 10.1074/jbc.M111.314674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang H, Liu JH, Yang W, Springer T, Shimaoka M, Wang JH. Structural basis of activation-dependent binding of ligand-mimetic antibody AL-57 to integrin LFA-1. PNAS. 2009;106(43):18345–50. doi: 10.1073/pnas.0909301106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang L, Shimaoka M, Rondon IJ, Roy I, Chang Q, Po M, et al. Identification and characterization of a human monoclonal antagonistic antibody AL-57 that preferentially binds the high-affinity form of lymphocyte function-associated antigen-1. Journal of Leukocyte Biology. 2006;80(4):905–14. doi: 10.1189/jlb.1105649.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Diaz R, Ghofaily LA, Patel J, Balashova NV, Freitas AC, Labib I, et al. Characterization of leukotoxin from a clinical strain of Actinobacillus actinomycetemcomitans. Microbial Pathogenesis. 2006;40(2):48–55. doi: 10.1016/j.micpath.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 33.Balashova NV, Crosby JA, Al Ghofaily L, Kachlany SC. Leukotoxin confers beta-hemolytic activity to Actinobacillus actinomycetemcomitans. Infection and Immunity. 2006;74(4):2015–21. doi: 10.1128/IAI.74.4.2015-2021.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shimaoka M, Kim M, Cohen EH, Yang W, Astrof N, Peer D, et al. AL-57, a ligand-mimetic antibody to integrin LFA-1, reveals chemokine-induced affinity up-regulation in lymphocytes. PNAS. 2006;103(38):13991–6. doi: 10.1073/pnas.0605716103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lo DJ, Anderson DJ, Song M, Leopardi F, Farris AB, Strobert E, et al. A pilot trial targeting the ICOS-ICOS-L pathway in nonhuman primate kidney transplantation. American Journal of Transplantation. 2015;15(4):984–92. doi: 10.1111/ajt.13100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kachlany SC, Schwartz AB, Balashova NV, Hioe CE, Tuen M, Le A, et al. Anti-leukemia activity of a bacterial toxin with natural specificity for LFA-1 on white blood cells. Leukemia Research. 2010;34(6):777–85. doi: 10.1016/j.leukres.2009.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang Q, Lakkis FG. Memory T Cell Migration. Frontiers in Immunology. 2015;6:504. doi: 10.3389/fimmu.2015.00504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Springer TA, Dustin ML. Integrin inside-out signaling and the immunological synapse. Current Opinion in Cell Biology. 2012;24(1):107–15. doi: 10.1016/j.ceb.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van Kooyk Y, van de Wiel-van Kemenade P, Weder P, Kuijpers TW, Figdor CG. Enhancement of LFA-1-mediated cell adhesion by triggering through CD2 or CD3 on T lymphocytes. Nature. 1989;342(6251):811–3. doi: 10.1038/342811a0. [DOI] [PubMed] [Google Scholar]
- 40.Dustin ML, Bromley SK, Kan Z, Peterson DA, Unanue ER. Antigen receptor engagement delivers a stop signal to migrating T lymphocytes. PNAS. 1997;94(8):3909–13. doi: 10.1073/pnas.94.8.3909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Feigelson SW, Pasvolsky R, Cemerski S, Shulman Z, Grabovsky V, Ilani T, et al. Occupancy of lymphocyte LFA-1 by surface-immobilized ICAM-1 is critical for TCR- but not for chemokine-triggered LFA-1 conversion to an open headpiece high-affinity state. Journal of Immunology. 2010;185(12):7394–404. doi: 10.4049/jimmunol.1002246. [DOI] [PubMed] [Google Scholar]
- 42.Ishibashi M, Miyanaga Y, Matsuoka S, Kozuka J, Togashi Y, Kinashi T, et al. Integrin LFA-1 regulates cell adhesion via transient clutch formation. Biochemical and Biophysical Research Communications. 2015;464(2):459–66. doi: 10.1016/j.bbrc.2015.06.155. [DOI] [PubMed] [Google Scholar]
- 43.Semmrich M, Smith A, Feterowski C, Beer S, Engelhardt B, Busch DH, et al. Importance of integrin LFA-1 deactivation for the generation of immune responses. The Journal of Experimental Medicine. 2005;201(12):1987–98. doi: 10.1084/jem.20041850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang QW, Kish DD, Fairchild RL. Absence of allograft ICAM-1 attenuates alloantigen-specific T cell priming, but not primed T cell trafficking into the graft, to mediate acute rejection. Journal of Immunology. 2003;170(11):5530–7. doi: 10.4049/jimmunol.170.11.5530. [DOI] [PubMed] [Google Scholar]
- 45.Hammer MH, Zhai Y, Katori M, Ritter T, Volk HD, Coito AJ, et al. Homing of in vitro-generated donor antigen-reactive CD4+ T lymphocytes to renal allografts is alpha 4 beta 1 but not alpha L beta 2 integrin dependent. Journal of Immunology. 2001;166(1):596–601. doi: 10.4049/jimmunol.166.1.596. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) The AL-579 gamma4 heavy chain is identical to the AL-57 heavy chain except it incorporates F to Y and I to S substitutions (indicated) found in a higher affinity variant, C09, of AL-57. It also contains a M to L substitution to remove an exposed, oxidation-prone methionine, and an S to P substitution (indicated) in the gamma4 hinge to prevent heavy chain exchange among IgG4 molecules. (B) AL-579 kappa light chain is identical to the AL-57 light chain.