Abstract
Hsp70 chaperone machineries play pivotal roles in a wide array of fundamental biological processes, through their facilitation of protein folding, disaggregation and remodeling. Hsp70’s obligate J-protein co-chaperones drive much of this remarkable multi-functionality, with most Hsp70s having multiple J-protein partners. Recent data suggest that J-protein-driven versatility is substantially due to precise localization within the cell and specificity of substrate protein binding. However, this relatively simple view belies the intricacy of J-protein function. Examples are emerging of J-protein interactions with Hsp70 and other chaperones, as well as integration into broader cellular networks. These interactions fine-tune, in critical ways, the ability of Hsp70 to participate in diverse cellular processes.
Keywords: molecular chaperone, Hsp40, DnaJ, Hsp70 chaperone, protein-interaction network
Hsp70 machinery overview
Hsp70-based machines are arguably the most versatile of molecular chaperones, interacting with a wide variety of substrate polypeptides, thereby playing critical roles in a myriad of cellular processes (Fig 1). Though folding of proteins is usually the first mentioned, Hsp70 systems function in many other processes, both in the course of normal growth and development and during periods of environmental stress. They do so not only by suppressing aggregation of unfolded proteins but by, for example, disassembling multimeric protein complexes and providing the driving force for translocation of proteins across membranes. Hsp70s are the heart of the machinery, but J-proteins, the focus of this review, and nucleotide exchange factors (Fig. 2) drive much of this versatility through their action as co-chaperones. [1, 2]. The mechanistic key is the profound effect of nucleotide (i.e. ATP and ADP) on Hsp70 conformation, and thereby the kinetics of its interactions with substrates, which are principally short, hydrophobic stretches of residues in full-length proteins. J-proteins, via the action of their conserved J-domains, control the rate of hydrolysis of Hsp70-bound ATP (Fig. 2) [3].
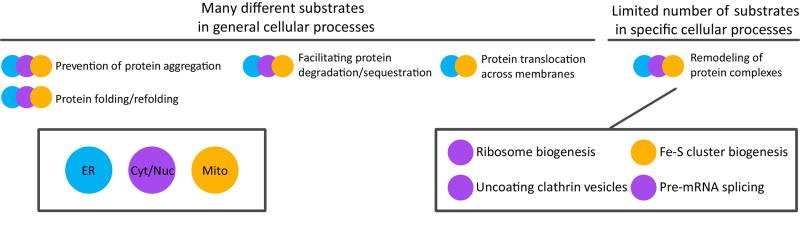
Figure 1. J-proteins function in many cellular processes.
J-proteins are found in major cellular compartments: endoplasmic reticulum (ER, blue), cytosol/nucleus (Cyt/Nuc, purple) and mitochondria (Mito, orange), as indicated by color-coded key at bottom-left. (Top) An overview of cellular processes in which J-protein/Hsp70 chaperone systems function, with the adjacent small circles indicating the compartments in which the process occurs. Those processes listed on the left half are, in general, carried out by J-proteins referred to in the text as “general binders”, those that interact with a wide range of substrates. The ER and mitochondrial process of “Protein translocation across membranes” (middle right) is driven by J-proteins that are localized to a specific site of action where many different substrates emerge from the membrane. Remodeling of protein complexes (far right, top and bottom), generally involve “specific binders”, which interact with one or a few substrates and function in a specific cellular process, as discussed in the text. However, it should be kept in mind that all these generalizations have exceptions and there are cases that are not easily classified. For example, amorphous protein aggregates and yeast prions can be considered protein complexes, but both are disassembled, in part, through the action of the “broad binder” β-barrel J-proteins.
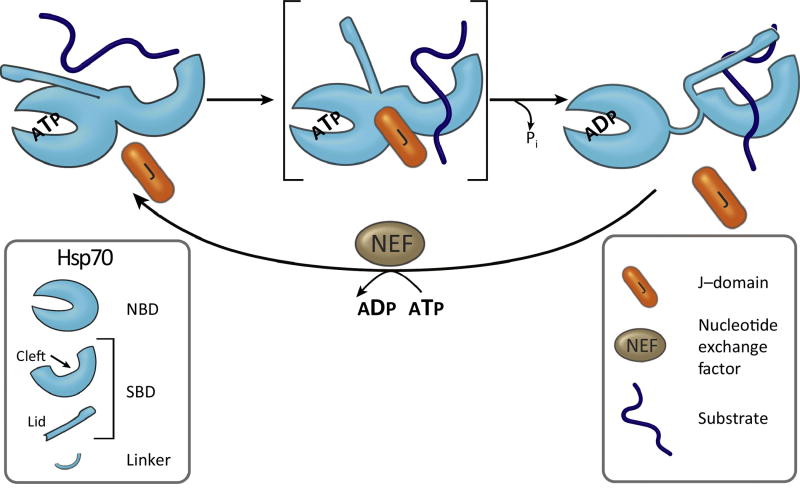
Figure 2. Hsp70 machinery fundamentals.
Hsp70’s two domain architecture [N-terminal nucleotide binding domain (NBD) and C-terminal substrate binding domain (SBD)] is key to its function, as is interaction of J-protein and nucleotide exchange factor (NEF) co-chaperones, which, as indicated, stimulate ATPase activity and exchange of nucleotide, respectively. This figure also serves to illustrate four functional keys to the interaction of Hsp70 with substrate: (1) Dramatic difference between the ATP- and ADP-bound conformations are key to substrate interaction on a biologically relevant timescale. When ATP is bound, the SBD is docked onto the NBD (left). When ADP is bound the two domains do not interact, tethered to each other only by a linker (right). The SBD contains two subdomains: one containing the substrate-binding cleft, the other a lid. Both subdomains, as well as the linker, interact with the NBD in the docked, ATP state. The lid is restrained by this interaction, giving substrate easy, rapid access to the cleft. In the ADP-state, when closed, the lid limits access of substrate to the cleft, but once bound stabilizes it. (2) The two conformational states are not static, stable states, but are dynamic, with intermediates, such as the one shown in brackets with the lid undocked, but linker and cleft subdomain docked (center). (3) NBD’s ATPase activity serves as a switch between conformations. ATPase stimulation is the core activity of J-proteins. The J-domain (J) forms a finger-like structure that interacts at the interface between the NBD and SBD. Substrate interaction in the peptide binding cleft also stimulates Hsp70s ATPase activity, with coordinated timing of stimulation with the J-domain likely being key to productive binding of substrate (center and right). (4) NEFs are also key to regulating the cycle by stimulating release of ADP, allowing binding of ATP, which is typically more abundant (bottom).
Hsp70s often have more than one J-protein partner. Indeed, cellular compartments in some species have a single Hsp70 that partners with 3 or more J-proteins to carry out a multitude of functions. J-protein driven diversity of Hsp70 functions is due to regions outside the conserved J-domain [4]. These regions can vary considerably among J-proteins and, in many cases, have no sequence relationship at all. The number of J-protein genes in an organism varies across the tree of life, positively correlating with overall genomic complexity (Box 1), and leads to challenges in defining a consistent, informing nomenclature for this family (Box 2). Below we summarize recent work that collectively suggests that divergent J-proteins have evolved interactions with other proteins, some of which are surprisingly sophisticated. These interactions serve to not only direct Hsp70 to particular substrate proteins, but also to integrate Hsp70 machinery into both chaperone-mediated protein homeostasis networks and cellular developmental and regulatory pathways.
Box 1. J-protein evolution - How did we get here?
The copy number variation of J-proteins, as well as their sequence/structure divergence, across the tree of life implies that they are the product of dynamic and complex evolutionary events (Fig. I). In general, gene duplications and rearrangements (e.g. domain fusion, expansion, and loss), which generate protein diversity, are most frequent in the complex genomes of plants and metazoans [72]. Overall, J-proteins diversity follows this general pattern, correlating with the overall increase in genomic complexity [73].
In many cases, the copy number variation amongst J-proteins can be explained by specific gene duplications that occurred along the branches of the tree of life [74]. For example, a recent study of three double β-barrel J-proteins from Saccharomyces cerevisiae, revealed that two, Xdj1 and Apj1, emerged in the fungi branch by sequential duplication of Ydj1, the most abundant cytosolic J-protein [75]. Despite partnering with the same Hsp70, the duplicates evolved specialized functions - Xdj1 in translocation of proteins into mitochondria and Apj1 in sumoylation-dependent proteolysis. However, even in cases as well characterized as that of these three J-proteins, the mechanisms behind duplicate retention remain unclear; that is, whether such specialized functions are new (neofunctionalization), or whether they existed in a parental gene (i.e. Ydj1) and then partitioned among duplicates (subfunctionalization), making retention crucial.
The origin of structural divergence among J-proteins is even more difficult to explain. Yet, gene duplications are likely also behind the more extreme differences, as they create a situation in which one copy can maintain its original functions, leaving the other “free” to evolve [72, 74]. For example, a gene duplication is likely behind the highly conserved 60S subunit interaction domain (ZHD) present in two, otherwise structurally unrelated, J-proteins that bind to the ribosome (e.g. S. cerevisiae Zuo1 and Jjj1; Fig. 4A). In some cases, proteins that function in a particular cellular process may have gained a J-domain, enabling the Hsp70 system to participate more intimately in it. Perhaps this explains the fact that the human J-protein ERdj5 (Fig. 3B) is the only identified disulfide reductase in the lumen of the ER. Regardless of the exact evolutionary history of ERdj5, that the J-domain is able to function in various structural contexts (i.e. at either end of the protein or internally) undoubtedly facilitated J-protein structural and functional diversity.
In sum, the structural and functional diversity of J-proteins presents challenges for cross species comparisons and generalizations. However, more in depth evolutionary analyses should lead to functional insights and, hopefully, eventually to a more informed J-protein classification system.
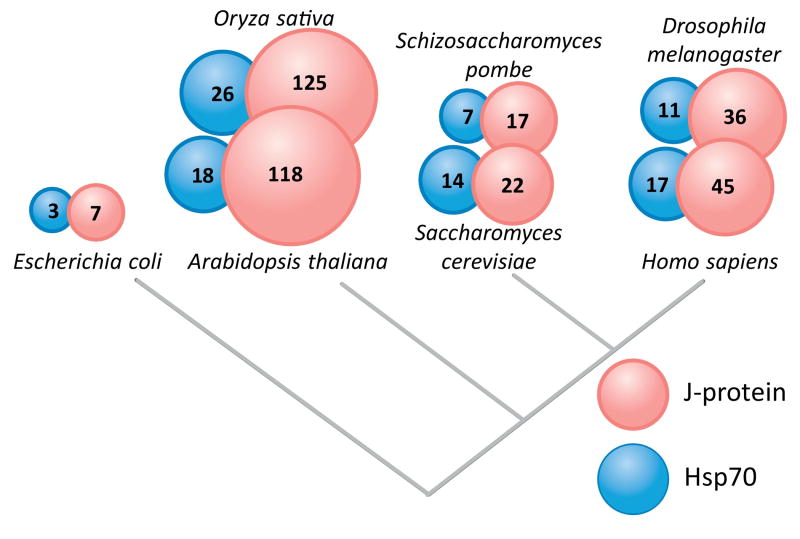
Figure I. Variation of J-protein and Hsp70 gene number across model organisms.
Best estimates of gene numbers for each organism are given, and represented by the size of the circle [78, 79].
Box 2. J-protein Classification Woes.
Classification of J-proteins is very challenging because of their complex evolutionary history (Box 1) and highly variable structure outside of the defining J-domain. The original J-protein classification scheme was put forth when analysis of J-proteins was in its infancy. As more information about J-protein structure and function has become available, this scheme has caused confusion. The β-barrel J-proteins are a case in point. When the scheme was established, the structure of the β-barrel domain was not known, nor were the similarities amongst these proteins appreciated, because the β-barrel domain has relatively weak sequence conservation. Those double β-barrel J-proteins, having an N-terminal J-domain, followed by a region rich in glycines and phenylalanines (i.e. G/F region) and 4 CxxCxGxG motifs, that we now know form a Zinc binding domain (ZnBD), were termed Class I (or Class A). Now we know that double β-barrel J-proteins having a ZnBD were the only ones to fall into this class. All J-proteins having a G/F region adjacent to an N-terminal J-domain, but lacking the CxxCxGxG motifs, were termed Class II (or Class B). Thus, all β-barrel J-proteins without a ZnBD fell into this class. Unfortunately, by these criteria, a number of otherwise structurally unrelated J-proteins fell into Class II as well, simply because they have a glycine-rich stretch adjacent to an N-terminal J-domain. These include the polyQ binding proteins DNAJB6 and DNAJB8, as well as ERdj4 (Fig. 3C), and unfortunately led them to be, at times, equated with β-barrel J-proteins lacking the ZnBD (e.g. Sis1) in the literature.
Moreover, the original classification scheme grouped all J-proteins that did not belong to class I or class II, that is any J-protein that did not have an N-terminal J-domain with an adjacent region rich in glycines, fell into Class III. As such, the classification has no biological meaning and little structural relevance, as exemplified by the fact that most J-proteins discussed in this review fall into Class III. Obviously, it would be advantageous to have a more informative categorization of J-proteins. Unfortunately, there is not yet sufficient information available to do so. Hopefully, in the future, a combination of structure/function data available for J-proteins from model organisms, extensive survey of genomic data and phylogenetic analysis will, in time, lead to a more informed J-protein classification system. In the meantime, we suggest that using the term “β-barrel J-proteins” plus/minus ZnBD, as we have done here, will help minimize confusion.
Getting substrate protein and Hsp70 together
Stimulation of Hsp70’s ATPase activity by the J-domain is the central, universal function of J-proteins. But the role of the J-domain in recruiting Hsp70s to substrates is nearly as fundamental. This interaction serves to give a J-protein-bound polypeptide a “leg up” in becoming a substrate of Hsp70. Less appreciated is that localization of a J-protein to a specific site within a compartment, in close proximity to a potential substrate protein, can serve an analogous purpose.
J-protein-substrate binding
Many J-proteins bind substrates. Some interactions are exquisitely specific; a few J-proteins bind only one. At the other extreme, some J-proteins have a broad substrate specificity, with a breath similar to that of typical Hsp70-substrate interactions. Two of the “specific binders” are amongst the best studied J-proteins. One, eukaryotic-specific auxilin, functions in removal of the coat of clathrin from vesicles, allowing release of the cargo molecules they transport between membrane-bound compartments (Fig. 3A) [5]. The other, the ubiquitous J-protein Hsc20, functions in the biogenesis of iron-sulfur (Fe-S) clusters by destabilizing the scaffold protein on which clusters are built, enabling cluster transfer to recipient proteins [6, 7] (Fig. 3A). These systems have served as models for understanding the fundamentals of Hsp70 machinery activity and how it functions in cellular processes. This is because they are more biochemically tractable than systems in which the substrates, such as protein folding intermediates, have a variety of conformational states.
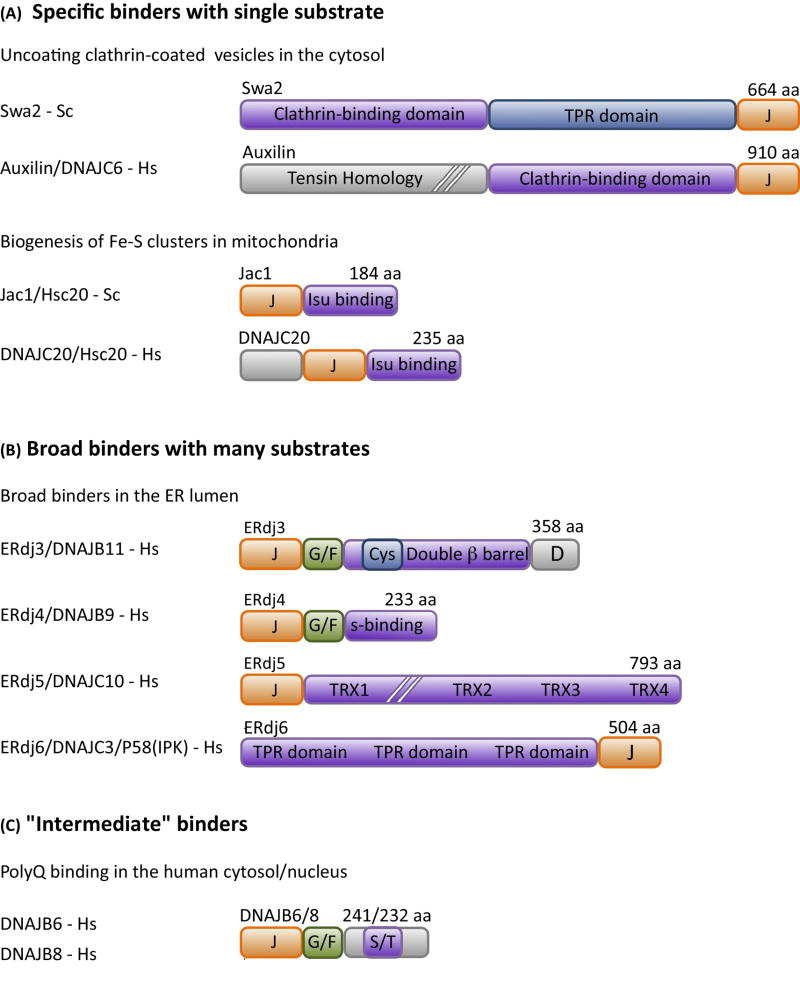
Figure 3. Diverse modes of binding of substrate by J-proteins.
J-proteins have structurally diverse substrate binding domains, which range in binding specificity from (A) very selective (i.e. in some cases a single substrate), that is “specific binders” to (B) quite promiscuous, binding to most any short hydrophobic stretch of amino acids, that is “broad binders”. The 4 “broad binders” of the human ER lumen are shown. ERdj3 is a double β-barrel protein (see Box 3). ERdj4 has an ill-defined substrate binding domain (S-binding). Erdj5 and ERdj6 have thioredoxin repeats (TRX) with reductase activity and tetratricopeptide repeats (TPR), respectively, but exact sites of substrate binding are yet to be well defined. (C) Many J-proteins do not fall neatly into these two categories. The DNAJB6/8 J-proteins that bind polyglutamine (polyQ) stretches are an example of the less well-defined intermediate class; the serine/threonine-rich (S/T) region is the experimentally defined region critical for polyQ binding, but it is dispensable for binding of another substrate, as described in the text. As the J-protein family as a whole becomes better characterized, well defined classes of J-proteins based on binding specificity will likely be difficult to delineate, as the binding specificity spectrum is likely a continuous one. Most common names of J-proteins S. cerevisiae (Sc) and H. sapiens (Hs) used in the literature are listed. Domain organization of representative J-proteins is drawn to scale, except where indicated by hatch (//) marks; J- J-domain; G/F- glycine/phenylalanine rich region; Cys-cysteine-rich region.
Examples of “broad binders” are those having a double β-barrel substrate binding domain (Box 3; Fig. 3B), such as Escherichia coli DnaJ. Such J-proteins are ubiquitous, found not only in prokaryotes, but in the cytosol, nucleus, endoplasmic reticulum (ER) and mitochondria of eukaryotes. Other J-protein substrate binding domain that has broad substrate-binding specificity include the substrate binding domains of three mammalian J-proteins (ERdj4, ERdj5, and ERdj6) (Fig. 3B) of the ER lumen. Each are structurally different from each other and from the ER double β-barrel domain J-protein Erdj3. Yet, each binds a variety of unfolded/misfolded protein substrates [8–10].
Box 3. J-protein class having a double β-barrel substrate binding domain.
Not only are J-proteins of the double β-barrel type found in every cellular compartment having an Hsp70 (i.e. cytosol/nucleus, ER and mitochondria of eukaryotes and cytosol of prokaryotes), they are typically the most abundant J-protein present. In all cases, the double β-barrel segment has two structurally similar, interacting “subdomains” (often called C-terminal domain I and II, or CTD I and CTD II, Fig. I). The identified peptide binding site is in CTD1 (see Fig. I for Ydj1-substrate peptide (red) co-crystal structure). There are two general types of double β-barrel J-proteins (see Box 2 for discussion of nomenclature issues). One has a cysteine-rich zinc-binding domain (ZnBD) extruding from CTD I; Escherichia coli DnaJ, S. cerevisiae Ydj1 and human DNAJA1 are examples of this type of J-protein. The second type of double β-barrel J-protein does not have a ZnBD. Sis1 of S. cerevisiae and human DNAJB1 are of this type. ERdj3/DNAJB1 that resides in the ER lumen of metazoans is an interesting case. It has a Cys-rich domain at the same position in CTD1. However, it has fewer cysteines (i.e. 4 rather than 8), and apparently does not bind Zn. In fungi, however, the ER lumen double β-barrel J-protein in the ER lumen has a canonical ZnBD.
All double β-barrel proteins also have a glycine-rich region (often called the G/F region as it is typically rich in phenylalanine, as well as glycine) between the N-terminal J-domain and the β-barrels. The function of the glycine-rich region remains unclear. Although referred to as a linker in the past, its function(s) is clearly more sophisticated than that. An extreme example is the case of the essential Sis1, which is also required for maintenance of yeast prions. A Sis1 fragment consisting of only the J-domain and glycine-rich region is able to rescue viability, as well as the prion maintenance defect [76, 77]. Possible functions of the glycine-rich region range from regulation of the J-domain’s ability to stimulate Hsp70’s ATPase activity to direct binding to substrate protein. As discussed in the text, both types of β-barrel proteins have evolved interactions with proteins that are components of cellular quality control pathways. Two such interaction regions are indicated in Fig I: Ydj1, interaction of site of ubiquitin ligase Rsp5 is located at the junction between CTDI and CTDII (see highlighted prolines between CTDI and CTDII); Sis1, the C-terminal dimerization domain of Sis1 (in gray in Fig. I) is important for binding of Btn2.
Figure I. Double β-barrel domain J-proteins.
Structures of double β-barrel domains of S. cerevisiae J-proteins Ydj1 (left; PDB: 1NLT), with yellow balls indicting Zinc ions) and Sis1 (right, PDB: 1C3G); generated using PyMOL software (http://pymol.sourceforge.net/). Examples of members of the two classes from Escherichia coli (Ec), Saccharomyces cerevisiae (Sc) and Homo sapiens (Hs), ZnBD present or absent, left and right, respectively, are given in boxes.
However, J-proteins do not fall neatly into “specific” and “broad” binder classes. The related metazoan J-proteins DNAJB6 and DNAJB8 (Fig. 3C) serve as an example. Both bind stretches of glutamine (polyQ) in a number of different proteins, suppressing their aggregation [11]. A serine/threonine (S/T) rich region within these proteins is crucial for this binding ability [12, 13]. This is a particularly relevant example, as aggregation of proteins having polyQ stretches is responsible for a number of neurological disorders, including Huntington’s disease [14]. However, binding to an S/T rich region is not always a prerequisite for DNAJB6 and DNAJB8 function. The S/T rich region is not required for DNAJB6 and DNAJB8 to bind a disease variant of parkin, a ubiquitin ligase linked to Parkinson disease, which prevents its aggregation [15].
Positioning of J-proteins near substrates
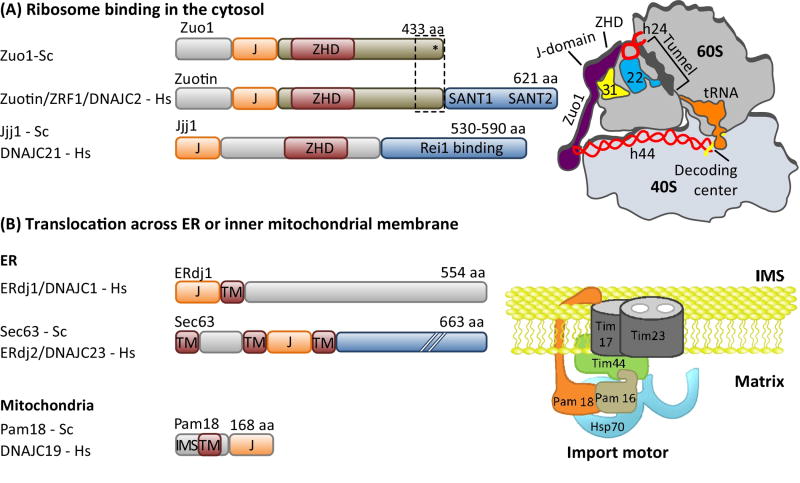
The inner mitochondrial and ER membranes and the eukaryotic ribosome are examples of sub-compartment locations where J-proteins help recruit Hsp70 to substrates [16, 17]. Although not apparent at first glance, these systems have fundamental similarities. In all, a wide variety of unfolded polypeptide chains emerge from a narrow channel (i.e. the translocating polypeptide from the membrane--embedded import channel; the nascent polypeptide from the exit tunnel of the ribosome) (Fig. 4). Translocation of proteins across the mitochondrial membrane is absolutely dependent on Hsp70 machinery activity, often described as the “import motor”, as it provides the driving force (Fig. 4B) [16]. Translocation of proteins across ER membrane does not required chaperone activity, but J-protein/Hsp70 systems make the predominantly co-translational process more efficient [17]. Similarly, binding of Hsp70 to the nascent chain is also not essential for its advancement through the ribosomal tunnel; yet, data indicate that binding does facilitate the process [18], as well as subsequent folding of the protein [19]. In neither case does the J-protein itself bind the emerging polypeptides, but it is positioned very close by; in the case of the import channel, it is positioned close to the import channel; in the case of the ribosome, it is close to the exit site in the 60S subunit. In this way, J-protein binding to Hsp70 via its J-domain brings Hsp70 close to the emerging substrate.
Figure 4. Localization of J-proteins to specific sites within a compartment.
J-proteins that localize (A) near the opening ribosome exit tunnel and (B) at the import channel (translocon) in the ER and inner mitochondrial membranes do not bind substrates, but help recruit and orient their partner Hsp70 for effective substrate binding. (A) One ribosome-associated J-protein, called Zuo1/Zuotin, interacts with both subunits via indicated protein (eL31, yellow) and rRNAs (red) (right). Interaction with the 60S subunit is via the Zuotin homology domain (ZHD). Regions of Zuo1/Zuotin towards the C-terminus (boxed) are important for interaction with the 30S subunit; ribosomal protein ul22 (22) interacts both with helix 44 (h24) and the interior of the tunnel, serving as a potential site for monitoring the nascent chain. As discussed in the text, the architecture and function of Zuo1/Zuotin illustrate how some J-proteins have evolved complex activities; C-terminal SANT domains of human and the 13 C-terminal residues of Zuo1 (*) are both involved in cellular regulation. A second J-protein (Jjj1/DNAJC21) also interacts with eukaryotic 60S ribosome particles via its ZHD (bottom). Jjj1 serves as an example of the difficulties in rigorously categorizing J-proteins by their structure and/or client binding properties. While localized to its site of action, it also binds a specific substrate protein, Rei1, as discussed in the text. (B) J-protein functioning in protein translocation are integral membrane proteins and also interaction with other proteins of the translocation apparatus. In the case of the import motor of the mitochondrial inner membrane (right), Pam18/DNAJC19 interacts with translocon subunit Tim17 on the intermembrane space (IMS) side and indirectly on the matrix side (with the import motor component Pam16 directly; Pam16 directly interacts with Tim44, which directly interacts with the import channel). J-protein names from S. cerevisiae (Sc) and H. sapiens (Hs) are listed. Domain organization of J-proteins is drawn to scale except where indicated by hatch marks (//); J- J-domain; TM- transmembrane region.
How important is precise positioning of J-proteins?
It is apparent that either J-protein substrate binding or localization near a substrate fosters Hsp70-substrate interaction. But, is positioning of J-proteins important beyond increasing local concentrations? There are hints that at least in some cases it is critical. This issue has been experimentally addressed for the J-protein auxilin, a “specific” binder. Both auxilin and Hsp70 bind the clathrin heavy chain. Auxilin binds, via its “clathrin binding domain”, underneath the apex of the three-legged clathrin trimeric complex (triskelion), the basic unit that assembles into the lattice-like coat [20]. Subsequently, auxilin’s J-domain recruits Hsp70, positioning it on the inside of the coat, where it binds the exposed QLMLT pentapeptide in the heavy chain’s C-terminal unstructured region. The exact biochemical mechanism by which Hsp70 binding causes lattice disassembly into triskelia remains unresolved [20–22]. However, the importance of the juxtaposition of auxilin and Hsp70 binding sites is clear, as the efficiency of uncoating decreased significantly when QLMLT was moved further away from the triskelion apex [22].
Whether spatial constraints are as important for other systems has not been experimentally tested. In some cases, such as the J-protein and Hsp70 involved in biogenesis of Fe-S clusters (Fig. 3A), one would predict that they are. Here, the substrate for both chaperones is only 14 kDa and has well-defined binding sites in close proximity. However, the importance for broad binders is less apparent. Because of the rather promiscuous binding by both components, the general availability of sites may make precise positioning unnecessary.
Complex interactions beyond Hsp70 recruitment
The concept of J-protein localization serving to recruit Hsp70 to a substrate is straightforward. But this simple description belies the complexity of some J-protein-Hsp70 systems. J-proteins are indeed localized to sites of action, but complex interactions that play additional roles have also evolved. Because of advances in electron cryo-microscopy (cryo-EM), ribosome-associated J-proteins serve to illustrate this idea. For many years the J-protein Zuotin (Fig. 4A) has been known to bind near the polypeptide exit site on the 60S ribosomal subunit. However, recent cryo-EM studies revealed that Zuotin spans the ribosomal subunits, interacting with the 40S subunit, as well [23]. Intriguingly, Zuotin’s 40S binding domain interacts with the surface-exposed end of a long RNA segment that originates at the decoding center of the 40S. This interaction is important for Zuotin’s role in translational fidelity [24, 25]. Additionally, the importance of 28S RNA for the association of Zuotin’s 60S binding domain raises the possibility that Zuotin monitors the nascent chain within the tunnel [24]. The critical rRNA helix, h24, of the 60S is in direct contact with a ribosomal protein, uL22, which extends into the subunit interior forming a patch on the exit tunnel wall. This patch is known to serve as a monitoring point for arresting or pausing translation [26]. So, a picture is unfolding that suggests Zuotin’s dual interaction with ribosomal subunits may coordinate the rate of translation and protein folding, not “just” facilitate the movement and folding of the nascent chain.
A second, Zuotin-related 60S ribosomal subunit-associated J-protein, called Jjj1 in Saccharomyces cerevisiae (Fig. 4A), is also ubiquitous in eukaryotes. However, Jjj1-based Hsp70 machinery is not involved in nascent chain folding; rather, it functions in quality control of 60S subunit maturation. Jjj1 contains a region closely related to Zuotin’s 60S-interaction domain (i.e., a Zuotin Homology Domain (ZHD)) [27, 28]. But, other regions of Jjj1 that are unrelated to Zuotin interact with a 60S maturation factor, Rei1, which in turn interacts with a second maturation factor, Arx1 [29]. The Jjj1-Hsp70 machinery facilitates release of Arx1 from the pre60S subunit. A segment of Rei1 extends far into the tunnel, while Arx1 is positioned such that it interacts with much of the ribosome surface around the tunnel exit that binds other chaperones and nascent chain targeting factors [30]. Thus, in addition to performing the typical type of Hsp70 machinery function of disassembly of a protein complex, Jjj1 and associated proteins may well serve as a dual quality control check during biogenesis – monitoring correct assembly of both the interior of the ribosome tunnel through which nascent chains travel and the binding sites of proteins that facilitate protein folding and targeting.
Keeping it in the family: J-protein-Hsp70 interaction networks
It has long been appreciated that J-protein-Hsp70 systems do not act in isolation; substrates are transferred from one chaperone system to another [1]. In folding or refolding pathways, Hsp70s typically interact with substantially unfolded proteins, passing them off to the chamber-type chaperones (e.g. GroE), which provide an insulated environment for folding [31], or to Hsp90, which is involved in the final stages of folding or in activation [32]. Less appreciated are “J-protein-Hsp70 pathways”; that is, the sequential transit of substrate polypeptide from one J-protein-Hsp70 system to another, thereby facilitating movement of newly synthesized polypeptides to a final cellular destination. A recent study of the J-protein requirement for the life cycle of dengue virus in human cells illustrates this idea [33]. At least 9 J-proteins are required, functioning with their compartment-specific Hsp70 partners for viral entry, RNA synthesis, virion production and host processes required for the life cycle.
In general, folding of proteins exiting the ribosome or translocating across a membrane involves the action of several J-proteins, in addition to those localized to the ribosome or import channels. Recent work on the lumen of the mammalian ER, which has a single Hsp70 and 7 J-proteins, exemplifies this concept. Two transmembrane J-proteins (ERdj1 and ERdj2; Fig. 4B) are intimately associated with the import channel complex (called the translocon), and function in translocation of the polypeptide and gating of the channel [17]. ERdj3, which is loosely associated with the translocon, binds polypeptides emerging from the import channel and targets them to Hsp70 (Fig. 3B) [8, 34]. As folding proceeds, properly matured protein is transported to the Golgi. However, if folding fails, another set of J-proteins (ERdj4 and ERdj5), which are structurally unrelated to either each other or to ERdj3, bind aggregation-prone regions that are exposed only in improperly folded polypeptides and are not recognized by ERdj3 [8]. This sequence recognition pattern enables ERdj4 and ERdj5 to target unfolded polypeptides to the endoplasmic reticulum associated degradation (ERAD) pathway for export and degradation in the cytosol [35].
Chaperone contacts that make the system work
A picture of physical connections between cooperating chaperone systems is emerging. These interactions are more intricate and pervasive than previously envisioned.
Interaction of J-proteins with other J-proteins
That metazoans do not have an obvious homolog of the potent AAA+ Hsp104/ClpB disaggregase found in prokaryotes and fungi has been a conundrum [36]. A recent report indicates that metazoans have evolved a different way to deal with disassembly and refolding of aggregated proteins, dependent on physical interaction between two different types of double β-barrel domain J-proteins (Box 3) [37]. One is a double β-barrel J-protein with a zinc binding domain (ZnBD) (e.g. human DNAJA1, A2); the other is one lacking the ZnBD (e.g. human DNAJB1, B4). Both are homo-dimers. Their mixed complex consists of two homo-dimers interacting with each other, with a J-domain of one binding the β-barrel domain of another, forming a platform on which Hsp70s and nucleotide exchange factors interact with each other and with aggregated protein substrates. It will be interesting to see whether mixed J-protein complexes are restricted to the β-barrel class or if other classes form similar interactions.
J-protein-Hsp70 interactions
At least some J-proteins have functionally important interactions with Hsp70s beyond that of the J-domain. The class of double β-barrel J-proteins having a ZnBD domain is one example (Box 3). Evidence suggests that interaction between the ZnBD and Hsp70 is important for the transfer of bound substrate to Hsp70 [38]. Eukaryotic cytosolic J-proteins of the double β-barrel class that lack a ZnBD have a different interaction with their partner Hsp70. Cytosolic Hsp70s, unlike those of the ER and mitochondria, have a C-terminal EEVD tetrapeptide. The β-barrel domain specifically interacts with this tetrapeptide. Cytosolic systems of both yeast and humans require this interaction for robust in vitro protein refolding activity [39, 40]. However, this activity of an Hsp70 lacking its EEVD is restored if an intramolecular salt bridge between the J-domain and the adjacent glycine-rich region of its J-protein partner is disrupted, suggesting that intramolecular interactions regulate activity.
Hsp70-Hsp70 interactions
Several lines of evidence indicate that Hsp70 forms homodimers. In the case of E. coli DnaK, transient dimers, involving interaction between nucleotide binding domains, formed spontaneously in the presence of ATP [41]. Mutational analysis indicated that the dimerization interface was needed for efficient binding of the double β-barrel J-protein DnaJ (Fig. 4C). Studies of eukaryotic proteins also detected Hsp70 dimers [42]. But these were antiparallel. Dimer formation was stimulated by a eukaryotic DnaJ homolog and showed dependence on post-translational modifications, particularly phosphorylation. It remains to be resolved why these two systems appear to be so fundamentally different. Regardless, both sets of results underscore the idea that intermolecular interactions of Hsp70 with each other are more complex and pervasive than anticipated.
Connecting chaperone systems
Besides contacts within the Hsp70 system itself, direct physical interactions that coordinate function with other chaperone systems, such as the cytosolic AAA+ disaggregases [43, 44] and Hsp90s [45], also occur. Hsp90, highly conserved chaperone involved in the final stages of folding of many proteins [32], is a particularly interesting case. In bacteria, the interaction is direct [46]; in eukaryotes, the co-chaperone Hop serves as a bridge, interacting with both Hsp70 and Hsp90 [42, 47]. In both, a double β-barrel J-protein targets substrate to its Hsp70 partner, then Hsp70 recruits Hsp90. Hop interactions also serve to illustrate the unique cytosolic Hsp70 system interaction network. The Hop-Hsp70 interaction occurs via Hsp70’s C-terminal EEVD tetrapeptide [45]. Intriguingly, additional proteins bind Hsp70’s EEVD, including a receptor on the mitochondrial outer membrane [48] and a ubiquitin ligase [49]. In this way, priority of binding to Hsp70’s EEVD serves to funnel Hsp70 substrates to different fates. Likely the extent of physical interactions amongst different chaperone systems is underappreciated, as indicated by recent proteomic data [50].
Broadening J-protein influence: integration into biological pathways
Recent data suggest that J-proteins functionally intertwine into cellular pathways. In the case of the Fe-S cluster biogenesis pathway, binding of the J-protein Hsc20 (Fig. 3A) to its substrate, the scaffold on which the cluster is built, has multiple effects. Acting as an Hsp70 co-chaperone, its primary role is facilitating cluster transfer to recipient proteins. However, Hsc20 binding to the scaffold also affects the regulation of the progression of the cluster biogenesis pathway more generally. Binding sites for Hsc20 and the cysteine desulfurase responsible for donation of the sulfur needed for cluster formation are overlapping, and thus their binding is mutually exclusive [51, 52]. Furthermore, this mutual exclusivity of binding promotes an orderly progression of cluster formation and transfer, as Hsc20 has a higher affinity for the more structured, cluster-containing holo-form of the scaffold, than the less structured, apo-form [53]. In addition, both J-protein and cysteine desulfurase binding protect the scaffold from degradation [54]. This protection serves as a post-translational regulatory mechanism to modulate the cell’s capacity for cluster biogenesis.
Quality control pathways illustrate how J-proteins drive the fate of the substrates that they bind through their interaction with other proteins. Such pathways exist in all cells, controlling protein refolding, protein degradation and sequestration of misfolded proteins into large inclusions [55, 56]. In some pathways, J-proteins serve important roles. For example, the two abundant double β-barrel type J-proteins of the S. cerevisiae cytosol/nucleus, Ydj1 and Sis1 (Box 3), have been linked to proteolysis and sequestration of misfolded proteins, respectively. Both J-proteins interact directly with a component of the quality control pathway in which they function. Furthermore, the site of interaction of the component on the J-protein is distinct from the J-protein’s substrate binding site [57–59]. Ydj1 binds the ubiquitin ligase Rsp5/Nedd4. Both Ydj1 and this ligase play key roles in substrate protein degradation after heat stress, leading to the hypothesis that binding between Rps5 and Ydj1 facilitates the transfer of Ydj1’s substrates to the ubiquitin system and thus degradation by the proteasome [60]. By contrast, Sis1 interacts with Btn2, a protein important for both transporting misfolded proteins to the nucleus [59] and the formation of misfolded protein inclusion sites near or in the nucleus [61].
There are also hints that J-protein influence may extend beyond the specific metabolic or quality control pathway in which they function. For example, the ribosome-associated J-protein Zuotin (Fig. 4A), discussed above, has been linked to cellular regulatory pathways associated with growth control in yeast and metazoans. In S. cerevisiae, the 13 residues at the C terminus are sufficient for activation of the Pdr1 transcription factor, which has been linked to quorum sensing [62, 63]. In metazoans, Zuotin (Fig. 4A) has SANT domains, protein segments known to foster interaction of many proteins with histones. Consistent with this, metazoan Zuotin has been shown to be involved in repair of DNA damage and activation of transcription in the nucleus [64, 65]. It is possible that the function of these sequences are unrelated to the ribosome associated functions of Zuotin; instead, these roles in the nucleus may be moonlighting functions [66]. However, the idea that these functions may be linked to their roles on the ribosome, and thereby integrate protein translation with other cellular pathways, is also intriguing.
Do J-proteins sometimes go it alone?
It is clear that some J-proteins can function independently of Hsp70; when overexpressed, they suppress aggregation of their substrate proteins [11, 67]. While such manipulated expression potentially has applications, the more fundamental question is whether certain J-proteins “normally”, either during growth and development or under conditions of stress, perform Hsp70-independent functions. Several studies point to a “yes” answer, at least in some circumstances.
Two particularly intriguing cases involve the double β-barrel J-proteins DnaJ and ERdj3. DnaJ is exploited by a virus; interacting with a phage encoded protein, DnaJ assists in excision of a prophage, allowing its “escape” from the E. coli genome [68]. Remarkably, this DnaJ function does not require assistance of an Hsp70 partner. The ERdj3 case involves a response to stress. ERdj3 normally resides in the ER lumen, participating with Hsp70 in protein folding. Upon ER stress, a good portion of ERdj3 is transported outside the cell via the secretory pathway, either with a substrate in tow or not [69]. ERdj3 may play a role in alleviating toxic effects of aggregation-prone proteins, either by removing such proteins from the ER or by binding them in the extracellular environment. This might be an Hsp70-independent function, as unlike most ER proteins, including ERdj5 and the ER luminal Hsp70, ERdj3 does not have an ER-retention sequence.
Possible examples are not restricted to double β-barrel J-proteins. Cwc23 plays an essential role in pre-mRNA splicing, acting in spliceosome disassembly. A variant without a functional J-domain can carry out this vital role [70]. J-domain independent functioning of ERdj5 (Fig. 3B) has also been reported. ERdj5 not only recognizes misfolded proteins in the ER lumen, it activates an ER membrane calcium pump, regulating Ca2+ uptake and, thus, cellular calcium homeostasis. It does so by reduction of critical disulfide bonds in the pump using its thioredoxin-like domains, independent of J-domain activity [71]. It remains to be resolved whether in the course of the J-protein evolution these J-domain independent functions emerged early or late. (Box 1).
Concluding Remarks
Recent advances have provided a glimpse of the stunning complexity and diversity of J-protein-Hsp70 systems. The challenge now is two-fold. On one hand, there is much more to learn about interactions of J-proteins (and nucleotide exchange factors) with other cellular components that help drive these diverse biological functions. On the other hand, although the fundamental basis of the Hsp70-substrate interaction cycle has been appreciated for many years, the critical mechanistic details that drive biological functions remain elusive - from the allostery of Hsp70-substrate interactions driven by J-proteins to the role of J-protein regions of which we have little knowledge (e.g. the glycine-rich region present in all double β-barrel J-proteins). A multitude of experimental approaches will be required to reach a fuller understanding of this fascinating multi-functional system.
Outstanding Questions.
What drives priority of interaction amongst different J-proteins with their common Hsp70 partner?
What are the biochemical functions of conserved, yet under-studied, regions of J-proteins?
How extensive, and functionally important, is the participation of J-proteins in physical interaction networks of molecular chaperones?
Does the intertwining of J-protein function in cellular pathways in which they work extend beyond the few known examples?
Can a J-protein classification scheme be developed based on structure-function features, combined with evolutionary history.
Can the specificity of J-protein interactions be exploited for therapeutic purposes?
Trends.
J-proteins drive the ability of Hsp70 to function in a wide variety of cellular processes.
Both substrate binding and sub-compartment localization of J-proteins serve to target Hsp70s to particular substrate polypeptides.
Some J-proteins function in cellular processes on a scale far beyond their basic, universal function of stimulating hydrolysis of ATP bound to Hsp70.
Physical interactions amongst components of different chaperone systems are emerging as an important factor in function.
Some J-proteins likely have functions independent of Hsp70s.
Acknowledgments
We thank Bogumila Paterkiewicz, Bartlomiej Tomiczek and Igor Grochowina for help with preparing figures. Work was supported by NIH grant GM27870 (E.A.C.) and by Polish National Science Center Grant DEC- 2012/06/A/NZ1/00002 and Foundation for Polish Science Master- 6/2014 programme (J.M.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Balchin D, et al. In vivo aspects of protein folding and quality control. Science. 2016;353(6294):aac4354. doi: 10.1126/science.aac4354. [DOI] [PubMed] [Google Scholar]
- 2.Clerico EM, et al. How hsp70 molecular machines interact with their substrates to mediate diverse physiological functions. J Mol Biol. 2015;427(7):1575–88. doi: 10.1016/j.jmb.2015.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mayer MP. Hsp70 chaperone dynamics and molecular mechanism. Trends Biochem Sci. 2013;38(10):507–14. doi: 10.1016/j.tibs.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 4.Kampinga HH, Craig EA. The HSP70 chaperone machinery: J proteins as drivers of functional specificity. Nat Rev Mol Cell Biol. 2010;11(8):579–92. doi: 10.1038/nrm2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fotin A, et al. Structure of an auxilin-bound clathrin coat and its implications for the mechanism of uncoating. Nature. 2004;432(7017):649–53. doi: 10.1038/nature03078. [DOI] [PubMed] [Google Scholar]
- 6.Vickery LE, Cupp-Vickery JR. Molecular chaperones HscA/Ssq1 and HscB/Jac1 and their roles in iron-sulfur protein maturation. Crit Rev Biochem Mol Biol. 2007;42(2):95–111. doi: 10.1080/10409230701322298. [DOI] [PubMed] [Google Scholar]
- 7.Lill R, et al. The role of mitochondria and the CIA machinery in the maturation of cytosolic and nuclear iron-sulfur proteins. Eur J Cell Biol. 2015;94(7–9):280–91. doi: 10.1016/j.ejcb.2015.05.002. [DOI] [PubMed] [Google Scholar]
- 8.Behnke J, et al. Members of the Hsp70 Family Recognize Distinct Types of Sequences to Execute ER Quality Control. Mol Cell. 2016;63(5):739–52. doi: 10.1016/j.molcel.2016.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tao J, et al. Crystal structure of P58(IPK) TPR fragment reveals the mechanism for its molecular chaperone activity in UPR. J Mol Biol. 2010;397(5):1307–15. doi: 10.1016/j.jmb.2010.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ushioda R, et al. Glycosylation-independent ERAD pathway serves as a backup system under ER stress. Mol Biol Cell. 2013;24(20):3155–63. doi: 10.1091/mbc.E13-03-0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hageman J, et al. A DNAJB chaperone subfamily with HDAC-dependent activities suppresses toxic protein aggregation. Mol Cell. 2010;37(3):355–69. doi: 10.1016/j.molcel.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 12.Gillis J, et al. The DNAJB6 and DNAJB8 protein chaperones prevent intracellular aggregation of polyglutamine peptides. J Biol Chem. 2013;288(24):17225–37. doi: 10.1074/jbc.M112.421685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kakkar V, et al. The S/T-Rich Motif in the DNAJB6 Chaperone Delays Polyglutamine Aggregation and the Onset of Disease in a Mouse Model. Mol Cell. 2016 doi: 10.1016/j.molcel.2016.03.017. [DOI] [PubMed] [Google Scholar]
- 14.Finkbeiner S. Huntington’s Disease. Cold Spring Harb Perspect Biol. 2011;3(6) doi: 10.1101/cshperspect.a007476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kakkar V, et al. Versatile members of the DNAJ family show Hsp70 dependent anti-aggregation activity on RING1 mutant parkin C289G. Sci Rep. 2016;6:34830. doi: 10.1038/srep34830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schulz C, et al. Unlocking the presequence import pathway. Trends Cell Biol. 2015;25(5):265–75. doi: 10.1016/j.tcb.2014.12.001. [DOI] [PubMed] [Google Scholar]
- 17.Dudek J, et al. Protein transport into the human endoplasmic reticulum. J Mol Biol. 2015;427(6 Pt A):1159–75. doi: 10.1016/j.jmb.2014.06.011. [DOI] [PubMed] [Google Scholar]
- 18.Liu B, et al. Cotranslational response to proteotoxic stress by elongation pausing of ribosomes. Mol Cell. 2013;49(3):453–63. doi: 10.1016/j.molcel.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Willmund F, et al. The cotranslational function of ribosome-associated Hsp70 in eukaryotic protein homeostasis. Cell. 2013;152(1–2):196–209. doi: 10.1016/j.cell.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bocking T, et al. Key interactions for clathrin coat stability. Structure. 2014;22(6):819–29. doi: 10.1016/j.str.2014.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krantz KC, et al. Clathrin coat disassembly by the yeast Hsc70/Ssa1p and auxilin/Swa2p proteins observed by single-particle burst analysis spectroscopy. J Biol Chem. 2013;288(37):26721–30. doi: 10.1074/jbc.M113.491753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sousa R, et al. Clathrin-coat disassembly illuminates the mechanisms of Hsp70 force generation. Nat Struct Mol Biol. 2016;23(9):821–9. doi: 10.1038/nsmb.3272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang Y, et al. Structural basis for interaction of a cotranslational chaperone with the eukaryotic ribosome. Nat Struct Mol Biol. 2014;21(12):1042–6. doi: 10.1038/nsmb.2908. [DOI] [PubMed] [Google Scholar]
- 24.Lee K, et al. Dual interaction of the Hsp70 J-protein cochaperone Zuotin with the 40S and 60S ribosomal subunits. Nat Struct Mol Biol. 2016 doi: 10.1038/nsmb.3299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rakwalska M, Rospert S. The ribosome-bound chaperones RAC and Ssb1/2p are required for accurate translation in Saccharomyces cerevisiae. Mol Cell Biol. 2004;24(20):9186–97. doi: 10.1128/MCB.24.20.9186-9197.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pechmann S, et al. The ribosome as a hub for protein quality control. Mol Cell. 2013;49(3):411–21. doi: 10.1016/j.molcel.2013.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Greber BJ, et al. Cryo-EM structures of Arx1 and maturation factors Rei1 and Jjj1 bound to the 60S ribosomal subunit. Nat Struct Mol Biol. 2012;19(12):1228–33. doi: 10.1038/nsmb.2425. [DOI] [PubMed] [Google Scholar]
- 28.Kaschner LA, et al. A conserved domain important for association of eukaryotic J-protein co-chaperones Jjj1 and Zuo1 with the ribosome. Biochim Biophys Acta. 2015;1853(5):1035–45. doi: 10.1016/j.bbamcr.2015.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Woolford JL, Jr, Baserga SJ. Ribosome biogenesis in the yeast Saccharomyces cerevisiae. Genetics. 2013;195(3):643–81. doi: 10.1534/genetics.113.153197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Greber BJ, et al. Insertion of the Biogenesis Factor Rei1 Probes the Ribosomal Tunnel during 60S Maturation. Cell. 2016;164(1–2):91–102. doi: 10.1016/j.cell.2015.11.027. [DOI] [PubMed] [Google Scholar]
- 31.Hayer-Hartl M, et al. The GroEL-GroES Chaperonin Machine: A Nano-Cage for Protein Folding. Trends Biochem Sci. 2016;41(1):62–76. doi: 10.1016/j.tibs.2015.07.009. [DOI] [PubMed] [Google Scholar]
- 32.Karagoz GE, Rudiger SG. Hsp90 interaction with clients. Trends Biochem Sci. 2015;40(2):117–25. doi: 10.1016/j.tibs.2014.12.002. [DOI] [PubMed] [Google Scholar]
- 33.Taguwa S, et al. Defining Hsp70 Subnetworks in Dengue Virus Replication Reveals Key Vulnerability in Flavivirus Infection. Cell. 2015;163(5):1108–23. doi: 10.1016/j.cell.2015.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tan YL, et al. ERdj3 is an endoplasmic reticulum degradation factor for mutant glucocerebrosidase variants linked to Gaucher’s disease. Chem Biol. 2014;21(8):967–76. doi: 10.1016/j.chembiol.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Christianson JC, Ye Y. Cleaning up in the endoplasmic reticulum: ubiquitin in charge. Nat Struct Mol Biol. 2014;21(4):325–35. doi: 10.1038/nsmb.2793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aguado A, et al. Chaperone-assisted protein aggregate reactivation: Different solutions for the same problem. Arch Biochem Biophys. 2015;580:121–34. doi: 10.1016/j.abb.2015.07.006. [DOI] [PubMed] [Google Scholar]
- 37.Nillegoda NB, et al. Crucial HSP70 co-chaperone complex unlocks metazoan protein disaggregation. Nature. 2015;524(7564):247–51. doi: 10.1038/nature14884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Linke K, et al. The roles of the two zinc binding sites in DnaJ. J Biol Chem. 2003;278(45):44457–66. doi: 10.1074/jbc.M307491200. [DOI] [PubMed] [Google Scholar]
- 39.Yu HY, et al. Functionality of Class A and Class B J-protein co-chaperones with Hsp70. FEBS Lett. 2015;589(19 Pt B):2825–30. doi: 10.1016/j.febslet.2015.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yu HY, et al. Roles of intramolecular and intermolecular interactions in functional regulation of the Hsp70 J-protein co-chaperone Sis1. J Mol Biol. 2015;427(7):1632–43. doi: 10.1016/j.jmb.2015.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sarbeng EB, et al. A functional DnaK dimer is essential for the efficient interaction with Hsp40 heat shock protein. J Biol Chem. 2015;290(14):8849–62. doi: 10.1074/jbc.M114.596288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Morgner N, et al. Hsp70 forms antiparallel dimers stabilized by post-translational modifications to position clients for transfer to Hsp90. Cell Rep. 2015;11(5):759–69. doi: 10.1016/j.celrep.2015.03.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Seyffer F, et al. Hsp70 proteins bind Hsp100 regulatory M domains to activate AAA+ disaggregase at aggregate surfaces. Nat Struct Mol Biol. 2012;19(12):1347–55. doi: 10.1038/nsmb.2442. [DOI] [PubMed] [Google Scholar]
- 44.Lee J, et al. Heat shock protein (Hsp) 70 is an activator of the Hsp104 motor. Proc Natl Acad Sci U S A. 2013;110(21):8513–8. doi: 10.1073/pnas.1217988110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mayer MP, Le Breton L. Hsp90: breaking the symmetry. Mol Cell. 2015;58(1):8–20. doi: 10.1016/j.molcel.2015.02.022. [DOI] [PubMed] [Google Scholar]
- 46.Genest O, et al. Hsp70 and Hsp90 of E. coli Directly Interact for Collaboration in Protein Remodeling. J Mol Biol. 2015;427(24):3877–89. doi: 10.1016/j.jmb.2015.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rohl A, et al. Hsp90 regulates the dynamics of its cochaperone Sti1 and the transfer of Hsp70 between modules. Nat Commun. 2015;6:6655. doi: 10.1038/ncomms7655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wu Y, Sha B. Crystal structure of yeast mitochondrial outer membrane translocon member Tom70p. Nat Struct Mol Biol. 2006;13(7):589–93. doi: 10.1038/nsmb1106. [DOI] [PubMed] [Google Scholar]
- 49.Matsumura Y, et al. Endoplasmic reticulum protein quality control is determined by cooperative interactions between Hsp/c70 protein and the CHIP E3 ligase. J Biol Chem. 2013;288(43):31069–79. doi: 10.1074/jbc.M113.479345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rodina A, et al. The epichaperome is an integrated chaperome network that facilitates tumour survival. Nature. 2016;538(7625):397–401. doi: 10.1038/nature19807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Majewska J, et al. Binding of the chaperone Jac1 protein and cysteine desulfurase Nfs1 to the iron-sulfur cluster scaffold Isu protein is mutually exclusive. J Biol Chem. 2013;288(40):29134–42. doi: 10.1074/jbc.M113.503524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Manicki M, et al. Overlapping binding sites of the frataxin homologue assembly factor and the heat shock protein 70 transfer factor on the Isu iron-sulfur cluster scaffold protein. J Biol Chem. 2014;289(44):30268–78. doi: 10.1074/jbc.M114.596726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Markley JL, et al. Metamorphic protein IscU alternates conformations in the course of its role as the scaffold protein for iron-sulfur cluster biosynthesis and delivery. FEBS Lett. 2013;587(8):1172–9. doi: 10.1016/j.febslet.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ciesielski SJ, et al. Protection of scaffold protein Isu from degradation by the Lon protease Pim1 as a component of Fe-S cluster biogenesis regulation. Mol Biol Cell. 2016;27(7):1060–8. doi: 10.1091/mbc.E15-12-0815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Miller SB, et al. Spatially organized aggregation of misfolded proteins as cellular stress defense strategy. J Mol Biol. 2015;427(7):1564–74. doi: 10.1016/j.jmb.2015.02.006. [DOI] [PubMed] [Google Scholar]
- 56.Kleiger G, Mayor T. Perilous journey: a tour of the ubiquitin-proteasome system. Trends Cell Biol. 2014;24(6):352–9. doi: 10.1016/j.tcb.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shiber A, et al. Ubiquitin conjugation triggers misfolded protein sequestration into quality control foci when Hsp70 chaperone levels are limiting. Mol Biol Cell. 2013;24(13):2076–87. doi: 10.1091/mbc.E13-01-0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Guerriero CJ, et al. Hsp70 targets a cytoplasmic quality control substrate to the San1p ubiquitin ligase. J Biol Chem. 2013;288(25):18506–20. doi: 10.1074/jbc.M113.475905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Park SH, et al. PolyQ proteins interfere with nuclear degradation of cytosolic proteins by sequestering the Sis1p chaperone. Cell. 2013;154(1):134–45. doi: 10.1016/j.cell.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 60.Fang NN, et al. Rsp5/Nedd4 is the main ubiquitin ligase that targets cytosolic misfolded proteins following heat stress. Nat Cell Biol. 2014;16(12):1227–37. doi: 10.1038/ncb3054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Malinovska L, et al. Molecular chaperones and stress-inducible protein-sorting factors coordinate the spatiotemporal distribution of protein aggregates. Mol Biol Cell. 2012;23(16):3041–56. doi: 10.1091/mbc.E12-03-0194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ducett JK, et al. Unfolding of the C-terminal domain of the J-protein Zuo1 releases autoinhibition and activates Pdr1-dependent transcription. J Mol Biol. 2013;425(1):19–31. doi: 10.1016/j.jmb.2012.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Prunuske AJ, et al. Role for the molecular chaperones Zuo1 and Ssz1 in quorum sensing via activation of the transcription factor Pdr1. Proc Natl Acad Sci U S A. 2012;109(2):472–7. doi: 10.1073/pnas.1119184109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Papadopoulou T, Richly H. On-site remodeling at chromatin: How multiprotein complexes are rebuilt during DNA repair and transcriptional activation. Bioessays. 2016 doi: 10.1002/bies.201600094. [DOI] [PubMed] [Google Scholar]
- 65.Aloia L, et al. ZRF1: a novel epigenetic regulator of stem cell identity and cancer. Cell Cycle. 2015;14(4):510–5. doi: 10.4161/15384101.2014.988022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Copley SD. An evolutionary perspective on protein moonlighting. Biochem Soc Trans. 2014;42(6):1684–91. doi: 10.1042/BST20140245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shen Y, Hendershot LM. ERdj3, a stress-inducible endoplasmic reticulum DnaJ homologue, serves as a cofactor for BiP’s interactions with unfolded substrates. Mol Biol Cell. 2005;16(1):40–50. doi: 10.1091/mbc.E04-05-0434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Champ S, et al. Chaperone-assisted excisive recombination, a solitary role for DnaJ (Hsp40) chaperone in lysogeny escape. J Biol Chem. 2011;286(45):38876–85. doi: 10.1074/jbc.M111.281865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Genereux JC, et al. Unfolded protein response-induced ERdj3 secretion links ER stress to extracellular proteostasis. EMBO J. 2015;34(1):4–19. doi: 10.15252/embj.201488896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sahi C, et al. Cwc23, an essential J protein critical for pre-mRNA splicing with a dispensable J domain. Mol Cell Biol. 2010;30(1):33–42. doi: 10.1128/MCB.00842-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ushioda R, et al. Redox-assisted regulation of Ca2+ homeostasis in the endoplasmic reticulum by disulfide reductase ERdj5. Proc Natl Acad Sci U S A. 2016;113(41):E6055–E6063. doi: 10.1073/pnas.1605818113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bornberg-Bauer E, Alba MM. Dynamics and adaptive benefits of modular protein evolution. Curr Opin Struct Biol. 2013;23(3):459–66. doi: 10.1016/j.sbi.2013.02.012. [DOI] [PubMed] [Google Scholar]
- 73.Lynch M, Conery JS. The origins of genome complexity. Science. 2003;302(5649):1401–4. doi: 10.1126/science.1089370. [DOI] [PubMed] [Google Scholar]
- 74.Andersson DI, et al. Evolution of new functions de novo and from preexisting genes. Cold Spring Harb Perspect Biol. 2015;7(6) doi: 10.1101/cshperspect.a017996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sahi C, et al. Sequential duplications of an ancient member of the DnaJ-family expanded the functional chaperone network in the eukaryotic cytosol. Mol Biol Evol. 2013;30(5):985–98. doi: 10.1093/molbev/mst008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yan W, Craig EA. The glycine-phenylalanine-rich region determines the specificity of the yeast Hsp40 Sis1. Mol Cell Biol. 1999;19(11):7751–8. doi: 10.1128/mcb.19.11.7751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Harris JM, et al. Functional diversification of hsp40: distinct j-protein functional requirements for two prions allow for chaperone-dependent prion selection. PLoS Genet. 2014;10(7):e1004510. doi: 10.1371/journal.pgen.1004510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Powers ET, Balch WE. Diversity in the origins of proteostasis networks--a driver for protein function in evolution. Nat Rev Mol Cell Biol. 2013;14(4):237–48. doi: 10.1038/nrm3542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kominek J, et al. The complex evolutionary dynamics of Hsp70s: a genomic and functional perspective. Genome Biol Evol. 2013;5(12):2460–77. doi: 10.1093/gbe/evt192. [DOI] [PMC free article] [PubMed] [Google Scholar]