Abstract
During the early postpartum period, mothers exhibit increased amygdala responses to positive infant expressions, which are important for positive mother-infant relationships. Socioeconomic disadvantage is associated with altered amygdala response to emotional stimuli as well as more negative mother-infant relationships. However, little is known about the role of socioeconomic disadvantage in neural responses specifically to infants. Thus, we examined whether socioeconomic disadvantage (indexed by lower income-to-needs ratio) is associated with neural responses to infant emotions and parenting behaviors among new mothers. Using fMRI, neural responses to infants’ emotional expressions (positive, negative, and neutral faces) were assessed among 39 low- and middle-income first-time mothers during 0–6 postpartum months. Lower income-to-needs ratio was associated with dampened amygdala responses to positive infant faces, but increased amygdala responses to negative infant faces. An indirect effect of socioeconomic disadvantage on emotional availability via amygdala activation suggests that socioeconomic disadvantage is associated with heightened neural sensitivity to infants’ negative emotions, which is further associated with mothers’ intrusiveness observed during interactions with their own infant. The findings suggest that low-income mothers may be more vulnerable to altered neural processing of infants’ emotional expressions which may further influence mothers’ emotional availability during interactions with their own infants.
Keywords: Parenting, Amygdala, Infant Faces, Emotional Expressions, Maternal Behaviors, Neuroimaging
1. Introduction
During the early postpartum period, both structural and functional adaptation in the brain play an important role in parenting among new mothers (Kim, in press; Kim, Strathearn, & Swain, 2016b; Lonstein, Lévy, & Fleming, 2015). Among neural changes associated with parenting, researchers have identified that heightened amygdala sensitivity to infant emotional expressions, particularly positive expressions, play an important role in secure mother-infant attachment and sensitive parenting (Atzil, Hendler, & Feldman, 2011; Barrett & Fleming, 2011; Feldman, 2015; Swain et al., 2014). However, little is known about factors that may influence individual variations in neural responses to infant emotions among new mothers. Socioeconomic disadvantage increases exposure to unstable and stressful environments, which can hinder mothers’ ability to provide optimal care for their own infants (Belsky & Jaffee, 2006; Coldwell, Pike, & Dunn, 2006; Conger & Donnellan, 2007; Whitesell, Teti, Crosby, & Kim, 2015). Thus, in the current study, we examined whether maternal socioeconomic disadvantage is associated with altered neural processing of infants’ emotional expressions. We then examined whether the altered neural responses to infants’ emotional expressions were associated with mothers’ parenting behaviors during the early postpartum period.
The quality of maternal care during the early months of the first year has a significant long-term impact on infants’ cognitive and emotional development (Bornstein, 2002). The concept of mothers’ emotional availability has been used to describe and assess the quality of maternal care (Biringen & Easterbrooks, 2012; Bretherton, 1992; Emde, 1980). Emotional availability is based on Ainsworth’s maternal sensitivity scale with a more explicit emphasis on the dyadic nature of emotions (both positive and negative) during mother-infant interactions. High emotional availability is characterized by mothers’ own emotional expressions that are attuned to the child’s emotional expressions. Among aspects of emotional availability to young children, maternal sensitivity (appropriate emotional expression and response to the child’s expressions) and nonintrusiveness (emotional presence without overriding the child’s needs) are highly relevant to mothers’ appropriate understanding of emotional cues from their infants (Ainsworth, 1979). Difficulties in understanding infant emotions can be associated with low emotional availability behaviors such as intrusive or insensitive parenting, which can lead to negative mother-infant relationships and insecure infant-mother attachment (Belsky, 1993; Cicchetti & Toth, 2005; Killeen & Teti, 2012; Timmer, Thompson, Culver, Urquiza, & Altenhofen, 2012).
Given the importance of sensitive mothering during the first year, the neurobiological mechanisms of parenting have been studied using both animal and human models. Among the many neural regions involved in responding to infant emotional cues, the amygdala has been highlighted for its significant role in understanding emotional expressions of others (Adolphs, Tranel, & Damasio, 1998). The role of the amygdala in processing other’s emotional cues is critically involved in parenting. In rodents, lesions in the amygdala impair maternal sensitivity (Numan et al., 2010), and mother-pup interactions increase c-fos changes in the amygdala (Fleming & Korsmit, 1996). In human mothers, the amygdala is activated by infant faces, indicating the role of the amygdala in detecting the salience of infant cues for parenting (Barrett & Fleming, 2011; Kim et al., 2016b; Seifritz et al., 2003).
However, postpartum factors that influence individual variations in amygdala responses to infant faces are still not well understood. A previous study suggests that lower levels of parental distress are associated with heightened amygdala responses to positive infant faces among new mothers (Barrett et al., 2012). In the current study, we focused on the potential role of socioeconomic disadvantage in maternal neural responses to infants. Socioeconomic disadvantage has been linked to mothers’ more limited emotional availability for their own infants (Little & Carter, 2005; Oyen, Landy, & Hilburn-Cobb, 2000). Socioeconomic disadvantage was also associated with increased maternal distress, which was then linked to reduced neural responses to infant cry in medial and middle prefrontal regions among first-time mothers (Kim, Capistrano, & Congleton, 2016a). However, this study did not examine the relations between neural activation and mothers’ own parental behaviors nor include different types of infants’ emotion cues. Therefore, the current study aims to examine whether neural processing of positive and negative infant cues, particularly in the amygdala, may be a pathway by which socioeconomic disadvantage influences observed parenting behaviors among new mothers.
Currently, there is converging evidence that socioeconomic disadvantage is associated with an individual’s heightened amygdala reactivity to negative emotional cues such as negative adult faces (Gianaros et al., 2008; Javanbakht et al., 2015; Muscatell et al., 2012). Increased levels of stress have been suggested as one of the key pathways by which socioeconomic disadvantage is associated with elevated neural and behavioral reactivity to negative emotion (Hackman, Farah, & Meaney, 2010; Kim, Evans, Chen, Miller, & Seeman, in press; McEwen & Gianaros, 2010). Low-income mothers report higher levels of psychological distress during the postpartum period (Goyal, Gay, & Lee, 2010). Thus, socioeconomic disadvantage and associated emotional distress among new mothers may be associated with altered neural processing of infant emotional expressions, such as elevated neural responses to infants’ negative expressions rather than positive expressions.
The current cross-sectional study examined whether socioeconomic disadvantage (assessed by lower income-to-needs ratio) was associated with neural responses to positive and negative infant faces, and further with parenting behaviors among low- and middle-income first-time new mothers. In the amygdala, we hypothesized that the interaction effects of income-toneeds ratio and infant emotion would be significant, and that lower income-to-needs ratio would be associated with altered amygdala activation, particularly reduced amygdala response to positive infant faces and increased amygdala response to negative infant faces. Increased levels of emotional distress among lower-income mothers may further be associated with variations in neural responses to infant faces. Moreover, we examined the indirect effect of socioeconomic disadvantage on parenting behaviors via the amygdala responses to infant emotions. We hypothesized that dampened amygdala response to positive infant faces and elevated amygdala response to negative infant faces would be associated with mothers’ sub-optimal parenting behaviors, i.e. lower maternal sensitivity and higher maternal intrusiveness.
2. Materials and methods
2.1.Participants
First-time mothers with their biological infants at age 0–6 months were recruited through fliers and brochures in metro Denver areas, including midwifery clinics as well as the WIC (Women, Infant, and Children) and Colorado state Prenatal Plus programs to ensure socioeconomic diversity in the sample. Eligible mothers were English-speaking and free from the following: pregnancy-related or infant medical illnesses involving more than a one-night stay in the neonatal intensive-care unit (NICU), current or historical psychiatric/neurological illness other than depression or anxiety diagnoses (to keep a controlled but ecologically valid community sampling approach), psychoactive drug use (except antidepressants), and magnetic metal in the body. Lower-income women were oversampled to be nearly half of the sample (see Family Income of the Measures section for more details). Of the 47 participants who were recruited, 8 mothers were excluded from analyses because they did not have complete fMRI data. Five mothers completed only home visits, two mothers were claustrophobic, and one mother had excessive motion above criteria (see fMRI data preprocessing for details). Thus, a total of 39 mothers were included in the analyses presented here. Among them, 27 participants were overlapping with the sample of a previously published paper using a different fMRI task (Kim et al., 2016a). Their demographic characteristics are described in Table 1.
Table 1.
Characteristics of the participants and main variables
| N(%) | Mean ± SD | Range | |
|---|---|---|---|
| Maternal age (years) | -- | 24.41±5.22 | 18–36 |
| Maternal Race/Ethnicity | |||
| Caucasian | 17 (43.6) | -- | -- |
| Hispanic | 16 (41) | -- | -- |
| African-American | 2 (5.1) | -- | -- |
| Others | 4 (10.3) | -- | -- |
| Maternal education (years) | -- | 13.49±2.38 | 7–18 |
| Infant sex (female) | 24 (61.5) | -- | -- |
| Postpartum month at the time of fMRI scans | -- | 3.97±1.58 | 0.89–6.96 |
| Income-to-needs ratio | -- | 2.47±1.48 | 0.44–6.34 |
| Maternal behaviors | |||
| Sensitivity | -- | 5.24±1.26 | 3–7 |
| Nonintrusiveness | -- | 5.55±1.26 | 3–7 |
| Beck Depression Inventory (BDI) | -- | 6.97±5.64 | 0–26 |
| Perceived Stress Scale (PSS) | -- | 5.03±2.89 | 0–11 |
| History of depression or anxiety diagnosis (Yes) | 4 (10.3) | -- | -- |
| Anxiety and depression medication use (Yes) | 1 (2.6) | -- | -- |
| Interval between home and fMRI visits (months) | -- | 0.85±0.69 | 0.07–3.42 |
| Breastfeeding (and/or daily pump; but not exclusive formula-feeding) | 38 (97.4) | -- | -- |
| Right handedness | 33 (86.8) | -- | -- |
| Time away from own infant per week (hours) | -- | 11.92±13.31 | 0–40 |
Four participants reported a history of depression or anxiety disorder diagnosis. Participants were largely psychoactive drug free except one participant who reported using an antidepressant. Participants were relatively diverse in terms of their ethnicity/race with 43.6% reporting Caucasian and 41% reporting Hispanic background (Table 1). No demographic variable (i.e. maternal age, race/ethnicity, handedness, mood disorder status, breastfeeding status, postpartum month at the time of scan, mothers’ time spent away from infants, infant’s sex, or interval between home and fMRI visits) was associated with income-to-needs ratio, rs(37) < 0.24 or > −0.27, ps > 0.10.
2.2. Procedures
Mothers were initially contacted by phone to assess their eligibility for the study. If eligible, a home visit was scheduled. During the home visit, mothers completed questionnaires and interviews on income and maternal mood. Mother-infant interactions were also video-recorded during the home visit. In a subsequent visit for the fMRI portion of the study, mothers visited the Intermountain Neuroimaging Center at the University of Colorado – Boulder. The average interval between home and fMRI visits was around 3 weeks (see Table 1). Participants received monetary compensation for their participation at the end of each visit, and child care and transportation support were provided to participants when needed. 2.3.Measures
2.3.1. Family income
Family income was assessed based on an income-to-needs ratio, calculated by dividing total family income by the poverty threshold specified by the U.S. Census Bureau. The range of the income-to-needs ratio was 0.44 to 6.34 (see Table 1), and 46% of the sample lived in poverty (income-to-needs ratio ≤ 1) or near poverty (income-to-needs ratio ≤ 2).
2.3.2. Maternal distress
Maternal distress was assessed by depressed mood and perceived stress. Maternal depressive mood was assessed by the Beck Depression Inventory (BDI) (Beck, Steer, & Garbin, 1988). The BDI includes 21 items which participants rated using a 4-point scale (from “absent” to “severe”). Perceived stress was measured using a self-report measure of the Perceived Stress Scale (Cohen & Williamson, 1988). A total of 4 items (e.g. In the past month, how often have you felt it was difficult to control the important things in your life?) were rated using a 5-point scale (0=never, 4=very often).
2.3.3. Maternal behaviors
Maternal behaviors during interactions with own infant were coded using the Emotional Availability Scales 4th Edition (Biringen, 2008). At the home visit, mother-infant naturalistic interactions were video-recorded for 15 minutes. Mothers were instructed to interact with the infant as they typically do without using any toys to maximize direct interactions between mothers and infants. The unstructured and natural play setting is a common setting used to observe maternal behaviors during interactions with infants younger than 12 months, including studies using the Emotional Availability Scales (Biringen et al., 1999; Bornstein et al., 2006; Bornstein, Hahn, Suwalsky, & Haynes, 2011; Easterbrooks & Biringen, 2005; Wan et al., 2012)
The Emotional Availability Scales include scores on four maternal dimensions: sensitivity, structuring, non-intrusiveness, and non-hostility. In the current study, we focused on maternal sensitivity and non-intrusiveness, two dimensions that are key early elements of synchronous maternal behaviors during the first year of infancy that predict later secure infant-mother attachment (Feldman et al., 2009; Isabella, Belsky, & von Eye, 1989; Smith & Pederson, 1988). The sensitivity scale measures the ability of the mother to be appropriately emotionally connected to her infant and responsive to the infant’s needs. A high score on the sensitivity scale reflects a mother who is affectively appropriate and responds to her infant’s needs optimally. This mother would likely be positive and accepting while attending to the shifting demands of the infant as the dyadic relationship unfolds. The non-intrusiveness scale assesses the extent to which the mother follows her infant’s lead in the interaction and interrupts the flow of play in a smooth manner. A high score on this scale would be seen in a mother who is available to the infant but does not overpower the infant’s will or impose her own sense of how the interaction should go. Each scale ranged from 1 to 7, and the certified rater who coded the behaviors was blinded to the participants’ socioeconomic background. To assess inter-rater reliability, two coders who were trained and certified by Dr. Biringen coded 25% of the sample, and intraclass correlations were performed, (ICC) = 0.713. The correlations between sensitivity and non-intrusiveness were moderate (r = 0.38, p = 0.02).
2.4. fMRI Paradigm
Participants viewed color images of positive, negative, and neutral expressions of two Caucasian infants. Each infant had 10 images of each expression. Many of the infants in the study were too young (< 3 months old) to provide positive expressions, so the paradigm utilized well-established positive, negative, and neutral infant stimuli instead of including images of each mother’s own baby. The images have been included in several other published works (Strathearn, Fonagy, Amico, & Montague, 2009; Strathearn & Kim, 2015; Strathearn, Li, Fonagy, & Montague, 2008).
Faces were presented for 2000 ms followed by an average 1250-ms fixation cross (ranging from 500ms to 5350ms). A run included 90 face trials (30 trials of each expression). The trial order was randomized within a run. Total task duration was 310.10 seconds. Mothers were asked to pay attention to each picture and experience their feelings and thoughts as they naturally occurred. Consistent with other studies using infant images (Strathearn & Kim, 2015; Strathearn et al., 2008), the passive viewing instruction was applied to avoid altering mothers’ natural emotions and parenting-related thoughts in response to the images.
Upon completion of the scan, participants completed a computer task to rate each picture from the fMRI task (Strathearn et al., 2008). For each picture, participants were asked to respond to two questions, “How pleasant or unpleasant does each picture make you feel?” and “How do you think the baby in the picture was feeling?” on the scale of 1 = negative, 5 = neutral, and 9 = positive. The pictures participants saw were grouped into positive, negative, and neutral faces. The ratings of the two questions were positively correlated in each grouping of emotional pictures [rs(37) ≥ 0.69, ps < 0.001].
2.5. fMRI data acquisition and processing
Scanning took place in a 3.0 Tesla Siemens magnet scanner using a standard 32-channel head coil. Functional data was acquired (540 T2*-weighted echo-planar-imaging (EPI) volumes; TR = 2,300 ms; TE = 27 ms; flip angle = 73; field of view = 192 mm; matrix size, 64 × 64; 36 axial slices; voxels = 3mm3). High-resolution anatomical T1-weighted images using the 3D magnetisation-prepared rapid gradient-echo (MPRAGE) protocol were also acquired.
Analysis of Functional Neuroimages software (AFNI) (Cox, 1996) was used for preprocessing and statistical analysis. The first four pre-steady-state volumes in each run were discarded. Preprocessing included slice timing correction, motion correction, affine alignment, and normalization to the Talairach template. Spatial smoothing was applied using 4mm full width at half maximum blur estimates and intensity scaling.
2.6. Analysis
2.6.1.Behavioral ratings
We explored whether behavioral ratings of infant emotions are associated with income-toneeds ratio. Using SPSS (SPSS, Inc., Chicago, IL), an ANOVA analysis with (1) income-toneeds ratio as a between-subjects variable and (2) condition (positive, negative, neutral) as a within-subjects variable, was conducted for two behavioral rating outcomes: (1) emotional response to an infant’s emotion and (2) perception of infant feelings. The interaction effects of income-to-needs ratio and infant emotion and the main effect of income-to-needs ratio were examined.
2.6.2.fMRI data
At the individual-subject level, regressors for each emotion were created by convolving stimulus trains with a gamma-variate hemodynamic-response function. Linear regression modeling was performed per voxel with the following regressors: three condition regressors, fourth degree polynomials modeling low-frequency drift, and six motion parameter regressors. Images with motion greater than 0.5 mm in any direction were censored. Data from a participant with excessive motion (above 20% of TRs removed) was excluded from analysis (see Participants section). In the data included in the analysis, the range of number of volumes censored was 0–21 (M= 1.79±4.51; median=0; below 16% of the total volumes). Thus, at least 101 volumes for each condition were included in the analysis. Beta coefficients from the individual subject level were used in the group level analysis.
At the group level, a whole-brain linear mixed effects model with income-to-needs ratio (a continuous variable) as a between-subject factor, and condition (positive, negative, neutral) as a within-subject factor was conducted with 3dLME in AFNI (Chen, Saad, Britton, Pine, & Cox, 2013; Cox, 1996). To identify brain regions associated with income-to-needs ratio, we examined interaction effects and main effects with income-to-needs ratio. Based on previous studies and our a priori hypothesis, an ROI (region of interest) approach was used in the left and right amygdala. Small-volume correction for multiple comparisons was performed using an independently-defined mask of a 7-mm radius sphere [centered at x,y,z=−23,5, −15 for the right amygdala and x,y,z,=23,5, −15 for the left amygdala (defined by AFNI)], and using the AFNI version 16.2.16 3dClustSim to establish a cluster-wise false positive probability of P < 0.05 (the cluster extent threshold of k ≥ 12 at a height threshold of p < 0.05).
Other neural regions including the insula and orbitofrontal cortex have been shown to respond to infant faces (Lenzi et al., 2009; Nitschke et al., 2004; Strathearn et al., 2008). Thus, for completeness, we explored the interaction and main effect of income-to-needs ratio in the whole-brain analysis. Non-a-priori regions outside of the amygdala were corrected for multiple comparisons within the whole brain using the cluster extent threshold of k ≥ 45 with a height threshold of p < 0.005, equivalent to a whole brain corrected false positive probability of P < 0.05, as calculated by 3dClustSim using the spatial autocorrelation function (acf) option within AFNI version 16.2.16. To characterize significant interactions, posthoc analyses were performed in SPSS using values extracted and averaged from the clusters.
The associations among income-to-needs ratio, emotional distress, neural activation in the suprathreshold clusters, and parenting behaviors were further examined using bivariate Pearson correlations in SPSS. Last, the indirect effect of income-to-needs ratio on mothers’ emotional availability via amygdala response to infant emotion was examined using 95% bias-corrected Confidence Intervals with bootstrapping procedures (10,000 bootstrap resamples) of the PROCESS program (Preacher & Hayes, 2008).
3. Results
3.1.Behavioral rating analysis of infant faces
No interaction term or main effect of income-to-needs ratio was significant for either item. However, consistent with previous studies using similar stimuli (Strathearn et al., 2008), a main effect of condition was significant both in emotional response to an infant’s emotion [F(2,74)=86.01, p < 0.001, η2p = 0.70] and perception of infant feelings [F(2,74)=212.38, p < 0.001, η2p = 0.85]. Mothers reported feeling more positive in response to positive infant faces (M=7.51±1.06) than neutral infant faces (M=6.39±1.33) and negative infant faces (M=3.19±1.26), ps < 0.001. Similarly, after viewing positive infant faces, mothers reported that infants were feeling more positive (M=7.35±1.02) compared with neutral infant faces (M=5.94±1.15) and negative infant faces (M=2.67±0.83), ps < 0.001. These behavioral ratings were not associated with neural findings (described below) and maternal behavior data.
3.2.fMRI analysis of the associations between income and neural activation to infant faces
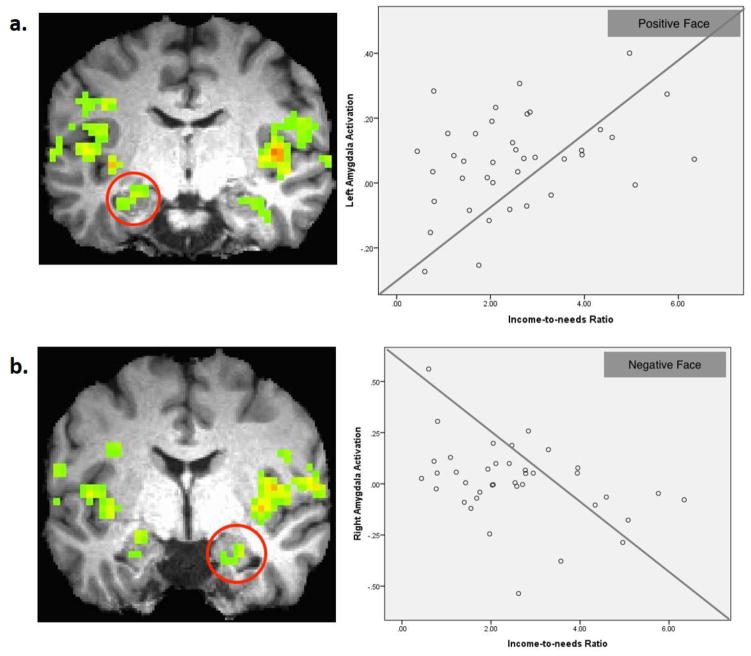
Consistent with the hypothesis that there would be significant interaction effects of income-to-needs ratio and infant emotion in the amygdala, using an ROI approach, a two-way interaction of income-to-needs ratio × condition (positive, negative, neutral) was identified in bilateral amygdala left parahippocampus/amygdala (x, y, z = −25, −10, −13, k=55) and right amygdala (x, y, z = 26, −4, −19, k=42) at p < 0.05, small volume corrected (Figure 1). Lower income-to-needs ratio was associated with dampened responses to positive infant faces in the left amygdala [r(37)=0.36, p < 0.05] while lower income-to-needs ratio was associated with enhanced neural responses to negative infant faces in the right amygdala [r(37)= −0.37, p < 0.05] (Figure 1).
Figure 1.
(a) Left parahippocampus/amygdala (x, y, z = −25, −10, −13, k=55) in a red circle showing income-to-needs ratio X condition interaction (p < 0.05, small volume corrected), and a scatterplot describing the positive associations between income-to-needs ratio and the neural responses to positive infant faces[r(37)=0.36, p < 0.05]; (b) Right amygdala (x, y, z = 26, −4, −19, k=42) in a red circle showing income-to-needs ratio X condition interaction (p < 0.05, small volume corrected) and a scatterplot describing the negative associations between income-to-needs ratio and the neural responses to negative infant faces [r(37)=−0.37, p < 0.05].
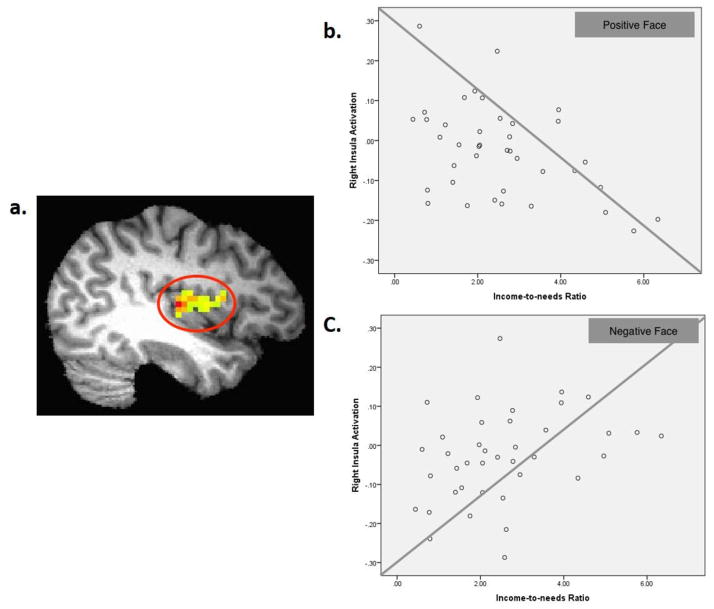
The exploratory whole-brain analysis also revealed a significant two-way interaction of income-to-needs ratio × condition (positive, negative, neutral) in one cluster right insula (BA13; x, y, z = 41, −13, 8, k=88) at p < 0.05, whole-brain cluster-wise corrected (Figure 2). Post-hoc correlation analysis suggests that lower income-to-needs ratio was associated with enhanced neural response to positive infant faces [r(37)= −0.45, p < 0.005], and reduced neural response to negative infant faces [r(37)=0.33, p < 0.05] (Figure 2).
Figure 2.
(a) Right insula (BA13; x, y, z = 41, −13, 8, k=88) in a red circle showing income-toneeds ratio X condition interaction, p < 0.05, whole-brain cluster-wise corrected; (b) and (c) a scatterplot describing the negative associations between income-to-needs ratio and the neural responses to positive infant faces [r(37)=−0.45, p < 0.005] and negative infant faces [r(37)=0.33, p < 0.05].
Income-to-needs ratio was negatively associated with perceived stress levels [r(37) = −0.36, p < 0.05] and, at a trend level, with depressive mood symptoms [r(37) = −0.29, p < 0.10]. However, mothers’ emotional distress levels were not associated with amygdala activation in response to infant emotional faces.
3.3.Indirect effect of income-to-needs ratio on maternal emotional availability via amygdala response to infant emotions
Although no direct associations were found between income-to-needs ratio and emotional availability, the indirect effect of income-to-needs ratio on mothers’ non-intrusiveness via the right amygdala response to negative infant faces was significant (Indirect effect=0.14, 95% CIs =0.01 0.35; Figure 3). Thus, lower income-to-needs ratio was associated with heightened amygdala responses to negative infant faces, which were further associated with lower scores of nonintrusiveness, suggesting high maternal intrusive behaviors. An analysis of maternal sensitivity did not reveal a significant indirect effect of income-to-needs ratio via amygdala responses to infant faces.
Figure 3.
A path diagram shows an indirect effect model with the unstandardized coefficients for each association. The indirect effect of income-to-needs ratio on non-intrusiveness via right amygdala responses to negative infant faces was significant (Indirect effect=0.14, 95% CIs =0.01 0.35), *p < 0.05.
4. Discussion
The current study examined whether socioeconomic disadvantage is associated with neural responses to infant emotional expressions among first-time new mothers during the early postpartum period. As expected, socioeconomic disadvantage was associated with differential neural sensitivity to positive and negative infant emotions. In the amygdala, lower income-to-needs ratio was associated with reduced responses to positive infant faces, but elevated amygdala responses to negative infant faces. Furthermore, the current study examined whether differences in amygdala responses to infant emotions depending on income-to-needs ratio may be associated with parenting behaviors. An indirect effect of income-to-needs ratio on mothers’ emotional availability via amygdala responses suggested that the heightened responses to negative infant faces were associated with mothers’ intrusiveness toward their own infant observed during interactions.
The amygdala plays a critical role in detecting and signaling salience of emotional cues (Adolphs, 2010; Anderson & Phelps, 2001). In past studies, socioeconomic disadvantage was associated with greater amygdalar reactivity to negative emotional stimuli of adult faces compared to positive emotional stimuli of adult faces (Javanbakht et al., 2015; Silverman, Muennig, Liu, Rosen, & Goldstein, 2009), suggesting increased salience and sensitivity to negative emotion among adults with socoeconomic disadvantage (Hackman et al., 2010; Kim et al., in press; Lupien, McEwen, Gunnar, & Heim, 2009; McEwen & Gianaros, 2010). The current study extended the literature by demonstrating the role of socioeconomic disadvantage in differential amygdala responses, specifically to infant emotional cues among new mothers. Lower-income mothers exhibited dampened amygdala response to positive infant faces, but elevated amygdala responses to negative infant faces. The findings suggest decreased salience of positive cues and increased salience of negative cues from infants among first-time new mothers. Such differences in the amygdala responses may further be associated with mothers’ emotional responses to infant emotional cues. In a previous study, low-income mothers also exhibited reduced responses to infant cry sounds in the medial and middle prefrontal regions (Kim et al., 2016a). Together, this may point to altered neural regulation of infant distress cues among new mothers, as heightened amygdala and dampened prefrontal response to negative cues have been markers for altered affective processing and regulation (Etkin, Prater, Schatzberg, Menon, & Greicius, 2009; Ochsner, Silvers, & Buhle, 2012).
Furthermore, the elevated amygdala responses to negative infant faces were associated with mothers’ parenting behaviors during interactions with their own infants. The indirect effect finding suggests that lower income-to-needs ratio was linked to the elevated amygdala response to negative infant faces, which was further associated with lower non-intrusiveness (or higher intrusiveness) expressed by the mothers during interactions with their own infants. There was no effect for maternal sensitivity. The finding is consistent with a previous study showing that elevated right amygdala responses to own infant cues was involved in high maternal intrusiveness, but not in maternal sensitivity (Atzil et al., 2011). The increased amygdala sensitivity to infants’ distress cues and more intrusive maternal behaviors may be part of maternal expression of threat vigilance and protectiveness, and can be considered adaptive maternal responses to highly stressful and unpredictable environments such as living in poverty. Maternal intrusiveness, however, is a concern because it may be a risk factor for insecure attachment and suboptimal infant socioemotional development (Feldman, 2007; Feldman et al., 2009; Isabella et al., 1989; Smith & Pederson, 1988). Thus, follow-up work is needed to examine whether the neural responses to negative infant faces in new mothers prospectively predict infants’ developmental outcomes as well as insecure infant-mother attachment at a later age.
In the exploratory whole-brain analysis, we found that the insula exhibited opposite associations between socioeconomic disadvantage and neural response to infant emotions than observed in the amygdala. Lower income-to-needs ratio was associated with increased insular responses to positive infant faces, but reduced insular responses to negative infant faces. Together with the amygdala, the insula is involved in social and emotional information processing, but it is particularly important for considering the affectively charged states of others (Zaki, Davis, & Ochsner, 2012). Increased insula responses among new mothers are more often observed in response to infants’ distress cues such as negative faces or crying sounds (Kim et al., 2011; Laurent, Stevens, & Ablow, 2011; Lenzi et al., 2009; Riem et al., 2011). Postpartum factors have been associated with altered insula response to infants. Postpartum depression is associated with reduced amygdala-insula connectivity in response to own infant images (Wonch et al., 2015). The current study expanded the literature by providing evidence that socioeconomic disadvantage is associated with altered insular responses reduced insular responses to negative infant faces in new mothers. Further research on the neural connectivity between the amygdala and insula and its relation to parenting behaviors is needed to better understand the role of the insula in processing infant emotions among low- and middle-income new mothers.
We tested a stress pathway of the associations between socioeconomic disadvantage and maternal amygdalar responses to infant emotions. As expected, low-income mothers reported higher levels of emotional distress, indicated by increased perceived levels of stress and depressed symptoms. However, maternal distress was not associated with amygdalar responses to infant emotions. This was a surprising finding. Low-income new mothers are vulnerable for a range of concurrent and/or chronic exposure to stressors including psychosocial stressors (e.g. violence, interpersonal conflict, discrimination, trauma), environmental stressors (e.g. substandard housing, crowding, noise), and childhood adversity (e.g. own parents’ harsh parenting, emotional neglect) (Evans & Kim, 2010; Johnson, Riis, & Noble, 2016; Kim et al., in press; McEwen & Gianaros, 2010). These stressors influence amygdalar responses to emotional cues in adults (Dannlowski et al., 2013; Davidson & McEwen, 2012; Kim et al., 2013), thus, we speculate that emotional distress may fail to reflect the multiple stress pathways by which socioeconomic disadvantage influence amygdala responses to infant emotional cues. It should also be noted that our finding is inconsistent with previous studies showing that heightened levels of maternal distress were associated with new mothers’ dampened amygdala responses to positive infant faces (Barrett et al., 2012) and reduced prefrontal responses to infant cry sounds (Kim et al., 2016a). The differences may be due to the fact that these two studies included own baby stimuli while the current study did not. Therefore, a future study including own and standard infant images is needed to examine the role of multiple domains of stressors in maternal amygdala activation.
The findings should be considered in light of limitations. First, the study paradigm did not include own infant images. Because many infants of the participants were too young to provide positive faces, we instead used standard infant images of positive, neutral, and negative expressions used in previous studies (Strathearn et al., 2009; Strathearn et al., 2008). However, the literature provides evidence of increased amygdala responses to own infant faces compared to other infant faces across different infant expressions (Leibenluft, Gobbini, Harrison, & Haxby, 2004; Ranote et al., 2004; Strathearn & Kim, 2015). Our findings suggest that the neural responses to these infant images may contribute to mothers’ parenting behaviors for their own infants. However, as noted above, future studies should examine specific links among socioeconomic disadvantage and maternal neural responses to own infants’ emotional faces in later postpartum periods. Second, all images in the current study were Caucasian infants although participants had different racial and ethnic backgrounds. However, because income-to-needs ratio did not differ by race/ethnic backgrounds, there is not clear evidence that mothers’ racial/ethnic background significantly influenced the findings related to income-to-needs ratio. It is important to note that individuals with racial and ethnic minority backgrounds are typically more likely to be low-income, thus it would be important for future work, using a larger sample, to match the participant’s race/ethnicity with the race/ethnicity of the infants.
Third, the current study included a relatively small sample size (N=39), particularly compared to behavioral research examining the effects of family income on parenting. Lack of associations between income and demographic variables (e.g. race/ethnicity, a history of depression or anxiety disorder) in the current study is likely to be due to the small sample size. The small sample size also increases influences of each data point on statistical results. For instance, the associations between the income-to-needs ratio and the right amygdala responses to negative faces were no longer significant after removing the two highest values. While the two values were not detected as outliers by the Grubbs test (Grubbs, 1969), the result of the right amygdala require a caution until it is replicated in future studies with a larger sample size. Fourth, participants in the current study have a relatively wide range of infant age (0–6 months). This may have lowered the sensitivity of the data to detect specific associations among maternal variables included in the study, particularly the associations between income-to-needs ratio and mothers’ emotional availability. However, it has been suggested that there can be a meaningful indirect effect via a mediator without a direct effect of the independent variable on the dependent variable (Hayes, 2009; MacKinnon, Fairchild, & Fritz, 2007). Nonetheless, future work with a larger sample and a targeted infant age would be needed to further confirm a mediating role of mothers’ neural processes in the links between income and parenting. Fifth, in future studies, it will be important to examine other indicators of socieonomic disadvanatge and stress, including subjective social standing and maternal educational levels, as these have been associated with neural function in adulthood (Gianaros et al., 2007; Noble et al., 2012) and parenting (Brooks-Gunn & Markman, 2005). It will also be important to consider additional risk factors such as postpartum depression (Hipwell, Guo, Phillips, Swain, & Moses-Kolko, 2015), trauma (Kim, Fonagy, Allen, & Strathearn, 2014), substance use (Landi et al., 2011), and insecure attachment (Strathearn et al., 2009) for their interactions with socioeconomic disadvantage.
5. Conclusions
Although socioeconomic disadvantage has been associated with difficulties in adjustment to parenthood, little is known about maternal neural responses to infant emotions as a pathway by which socioeconomic disadvantage influences parenting behaviors among new mothers. The current study provides evidence for the role of socioeconomic disadvantage in differential sensitivity to infant emotional expressions among new mothers during the postpartum period. Socioeconomic disadvantage was associated with dampened amygdala responses to positive infant faces, but elevated amygdala responses to negative infant faces. The elevated amygdala responses to negative infant faces rather than positive infant faces may reflect mothers’ increased sensitivity to infants’ distress cues. Elevated amygdala responses to negative infant faces were further associated with mothers’ intrusiveness during interactions with their own infants. Intrusive parenting reflects mothers’ heightened sense of vigilance, but can also present increased risk for negative parenting and insecure mother-infant attachment. This understanding of the associations among socioeconomic disadvantage, maternal neural function, and parenting behaviors may encourage consideration of the early postpartum period as a window when maternal brain sensitivity to infants may be vulnerable to socioeconomic disadvantage. Thus, the findings may support two-generation interventions that target both low-income mothers and their infants (Kim & Watamura, 2015; Shonkoff & Fisher, 2013; Smith, 1995) in order to provide more stable and safe environments and support positive mother-infant relationships.
Highlights.
Low income was linked to neural processing of infants’ emotions among mothers.
Low income was associated with dampened amygdala response to positive infant faces.
Low income was associated with elevated amygdala response to negative infant faces.
The amygdala response was linked to more intrusive interactions with own infants.
Acknowledgments
This work was supported by the National Institute of Child Health and Human Development [R21HD078797]; the Professional Research Opportunity for Faculty (PROF) and Faculty Research Fund (FRF), University of Denver; and the Victoria S. Levin Award For Early Career Success in Young Children's Mental Health Research, Society for Research in Child Development (SRCD). The authors declare that they have no conflicts of interest in the research. The authors wish to acknowledge Amy Anderson, Lindsay Blanton, Christina Congleton, Tanisha Crosby-Attipoe, Alexander Dufford, Victoria Everts, Claire Jeske, Laura Jeske, Daniel Mason, and Rebekah Tribble for research assistance and Dr. Lane Strathearn (University of Iowa) for his support and inputs.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adolphs R. What does the amygdala contribute to social cognition? Annals of the New York Academy of Sciences. 2010;1191(1):42–61. doi: 10.1111/j.1749-6632.2010.05445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adolphs R, Tranel D, Damasio AR. The human amygdala in social judgment. Nature. 1998;393(6684):470–474. doi: 10.1038/30982. [DOI] [PubMed] [Google Scholar]
- Ainsworth MS. Infant–mother attachment. American Psychologist. 1979;34(10):932. doi: 10.1037//0003-066x.34.10.932. [DOI] [PubMed] [Google Scholar]
- Anderson AK, Phelps EA. Lesions of the human amygdala impair enhanced perception of emotionally salient events. Nature. 2001;411(6835):305–309. doi: 10.1038/35077083. [DOI] [PubMed] [Google Scholar]
- Atzil S, Hendler T, Feldman R. Specifying the neurobiological basis of human attachment: Brain, hormones, and behavior in synchronous and intrusive mothers. Neuropsychopharmacology. 2011;36(13):2603–2615. doi: 10.1038/npp.2011.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett J, Fleming AS. Annual research review: All mothers are not created equal: Neural and psychobiological perspectives on mothering and the importance of individual differences. Journal of Child Psychology and Psychiatry and Allied Disciplines. 2011;52(4):368–397. doi: 10.1111/j.1469-7610.2010.02306.x. [DOI] [PubMed] [Google Scholar]
- Barrett J, Wonch KE, Gonzalez A, Ali N, Steiner M, Hall GB, Fleming AS. Maternal affect and quality of parenting experiences are related to amygdala response to infant faces. Social Neuroscience. 2012;7(3):252–268. doi: 10.1080/17470919.2011.609907. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Garbin MG. Psychometric properties of the Beck Depression Inventory: Twenty-five years of evaluation. Clinical Psychology Review. 1988;8(1):77–100. [Google Scholar]
- Belsky J. Etiology of child maltreatment: A developmental ecological analysis. Psychological Bulletin. 1993;114(3):413. doi: 10.1037/0033-2909.114.3.413. [DOI] [PubMed] [Google Scholar]
- Belsky J, Jaffee S. The multiple determinants of parenting. In: Cohen DJ, Cicchetti D, editors. Developmental psychopathology: Risk, disorder, and adaptation. 2. Vol. 3. Hoboken, NJ: John Wiley & Sons; 2006. pp. 38–85. [Google Scholar]
- Biringen Z. The emotional availability (EA) scales and the emotional attachment & emotional availability (EA2) clinical screener. 4. Boulder, CO: emotionalavailability.com; 2008. [Google Scholar]
- Biringen Z, Easterbrooks MA. Emotional availability: Concept, research, and window on developmental psychopathology. Development and Psychopathology. 2012;24(1):1–8. doi: 10.1017/S0954579411000617. [DOI] [PubMed] [Google Scholar]
- Biringen Z, Emde R, Brown D, Lowe L, Myers S, Nelson D. Emotional availability and emotion communication in naturalistic mother-infant interactions: Evidence for gender relations. Journal of Social Behavior and Personality. 1999;14(4):463. [Google Scholar]
- Bornstein MH. Parenting infants. In: Bornstein MH, editor. Handbook of parenting. Vol. 1. Mahwah, N.J: Erlbaum; 2002. pp. 3–43. [Google Scholar]
- Bornstein MH, Gini M, Putnick DL, Haynes OM, Painter KM, Suwalsky JT. Short-term reliability and continuity of emotional availability in mother–child dyads across contexts of observation. Infancy. 2006;10(1):1–16. doi: 10.1207/s15327078in1001_1. [DOI] [PubMed] [Google Scholar]
- Bornstein MH, Hahn CS, Suwalsky JT, Haynes OM. Maternal and infant behavior and context associations with mutual emotion availability. Infant Mental Health Journal. 2011;32(1):70–94. doi: 10.1002/imhj.20284. [DOI] [PubMed] [Google Scholar]
- Bretherton I. The origins of attachment theory: John Bowlby and Mary Ainsworth. Developmental Psychology. 1992;28(5):759–775. [Google Scholar]
- Brooks-Gunn J, Markman LB. The contribution of parenting to ethnic and racial gaps in school readiness. The Future of Children. 2005:139–168. doi: 10.1353/foc.2005.0001. [DOI] [PubMed] [Google Scholar]
- Chen G, Saad ZS, Britton JC, Pine DS, Cox RW. Linear mixed-effects modeling approach to FMRI group analysis. Neuroimage. 2013;73:176–190. doi: 10.1016/j.neuroimage.2013.01.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicchetti D, Toth SL. Child maltreatment. Annual Review of Clinical Psychology. 2005;1:409–438. doi: 10.1146/annurev.clinpsy.1.102803.144029. [DOI] [PubMed] [Google Scholar]
- Cohen S, Williamson GM. Perceived stress in a probability sample of the United States. In: Oskamp S, Spacapan S, editors. The social psychology of health: Claremont symposium on applied social psychology. Newbury Park, CA: Sage; 1988. pp. 31–67. [Google Scholar]
- Coldwell J, Pike A, Dunn J. Household chaos-links with parenting and child behaviour. Journal of Child Psychology and Psychiatry. 2006;47(11):1116–1122. doi: 10.1111/j.1469-7610.2006.01655.x. [DOI] [PubMed] [Google Scholar]
- Conger RD, Donnellan MB. An interactionist perspective on the socioeconomic context of human development. Annual Review of Psychology. 2007;58:175–199. doi: 10.1146/annurev.psych.58.110405.085551. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research. 1996;29(3):162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Dannlowski U, Kugel H, Huber F, Stuhrmann A, Redlich R, Grotegerd D, … Arolt V. Childhood maltreatment is associated with an automatic negative emotion processing bias in the amygdala. Human Brain Mapping. 2013;34(11):2899–2909. doi: 10.1002/hbm.22112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson RJ, McEwen BS. Social influences on neuroplasticity: Stress and interventions to promote well-being. Nature Neuroscience. 2012;15(5):689–695. doi: 10.1038/nn.3093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Easterbrooks MA, Biringen Z. The Emotional Availability Scales: Methodological refinements of the construct and clinical implications related to gender and at-risk interactions. Infant Mental Health Journal. 2005;26(4):291–294. doi: 10.1002/imhj.20053. [DOI] [PubMed] [Google Scholar]
- Emde RN. Emotional availability: A reciprocal reward system for infants and parents with implications for prevention of psychosocial disorders. Parent-infant relationships. 1980:87–115. [Google Scholar]
- Etkin A, Prater KE, Schatzberg AF, Menon V, Greicius MD. Disrupted amygdalar subregion functional connectivity and evidence of a compensatory network in generalized anxiety disorder. Archives of General Psychiatry. 2009;66(12):1361–1372. doi: 10.1001/archgenpsychiatry.2009.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans GW, Kim P. Multiple risk exposure as a potential explanatory mechanism for the socioeconomic status-health gradient. Annals of the NY Academy of Sciences. 2010;1186(1):174–189. doi: 10.1111/j.1749-6632.2009.05336.x. [DOI] [PubMed] [Google Scholar]
- Feldman R. Parent-infant synchrony and the construction of shared timing; physiological precursors, developmental outcomes, and risk conditions. Journal of Child Psychology and Psychiatry. 2007;48(3–4):329–354. doi: 10.1111/j.1469-7610.2006.01701.x. [DOI] [PubMed] [Google Scholar]
- Feldman R. The adaptive human parental brain: Implications for children's social development. Trends in Neurosciences. 2015;38(6):387–399. doi: 10.1016/j.tins.2015.04.004. [DOI] [PubMed] [Google Scholar]
- Feldman R, Granat A, Pariente C, Kanety H, Kuint J, Gilboa-Schechtman E. Maternal depression and anxiety across the postpartum year and infant social engagement, fear regulation, and stress reactivity. Journal of the American Academy of Child and Adolescent Psychiatry. 2009;48(9):919–927. doi: 10.1097/CHI.0b013e3181b21651. [DOI] [PubMed] [Google Scholar]
- Fleming AS, Korsmit M. Plasticity in the maternal circuit: Effects of maternal experience on Fos-Lir in hypothalamic, limbic, and cortical structures in the postpartum rat. Behavioral Neuroscience. 1996;110(3):567. doi: 10.1037//0735-7044.110.3.567. [DOI] [PubMed] [Google Scholar]
- Gianaros PJ, Horenstein JA, Cohen S, Matthews KA, Brown SM, Flory JD, … Hariri AR. Perigenual anterior cingulate morphology covaries with perceived social standing. Social Cognitive and Affective Neuroscience. 2007;2(3):161–173. doi: 10.1093/scan/nsm013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianaros PJ, Horenstein JA, Hariri AR, Sheu LK, Manuck SB, Matthews KA, Cohen S. Potential neural embedding of parental social standing. Social Cognitive and Affective Neuroscience. 2008;3(2):91–96. doi: 10.1093/scan/nsn003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyal D, Gay C, Lee KA. How much does low socioeconomic status increase the risk of prenatal and postpartum depressive symptoms in first-time mothers? Womens Health Issues. 2010;20(2):96–104. doi: 10.1016/j.whi.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grubbs FE. Procedures for detecting outlying observations in samples. Technometrics. 1969;11(1):1–21. [Google Scholar]
- Hackman DA, Farah MJ, Meaney MJ. Socioeconomic status and the brain: Mechanistic insights from human and animal research. Nature Reviews Neuroscience. 2010;11(9):651–659. doi: 10.1038/nrn2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes AF. Beyond Baron and Kenny: Statistical mediation analysis in the new millennium. Communication Monographs. 2009;76(4):408–420. [Google Scholar]
- Hipwell AE, Guo C, Phillips ML, Swain JE, Moses-Kolko EL. Right frontoinsular cortex and subcortical activity to infant cry is associated with maternal mental state talk. Journal of Neuroscience. 2015;35(37):12725–12732. doi: 10.1523/JNEUROSCI.1286-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isabella RA, Belsky J, von Eye A. Origins of infant-mother attachment: An examination of interactional synchrony during the infant's first year. Developmental Psychology. 1989;25(1):12. [Google Scholar]
- Javanbakht A, King AP, Evans GW, Swain JE, Angstadt M, Phan KL, Liberzon I. Childhood poverty predicts adult amygdala and frontal activity and connectivity in response to emotional faces. Frontiers in Behavioral Neuroscience. 2015;9:154. doi: 10.3389/fnbeh.2015.00154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SB, Riis JL, Noble KG. State of the art review: Poverty and the developing brain. Pediatrics. 2016;137(4):2015–3075. doi: 10.1542/peds.2015-3075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killeen LA, Teti DM. Mothers' frontal EEG asymmetry in response to infant emotion states and mother–infant emotional availability, emotional experience, and internalizing symptoms. Development and Psychopathology. 2012;24(1):9–21. doi: 10.1017/S0954579411000629. [DOI] [PubMed] [Google Scholar]
- Kim P. Human maternal brain plasticity: Adaptation to parenting. New Directions for Child and Adolescent Development. 2016;2016(153):47–58. doi: 10.1002/cad.20168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim P, Capistrano C, Congleton C. Socioeconomic disadvantages and neural sensitivity to infant cry: Role of maternal distress. Social Cognitive and Affective Neuroscience. 2016a;11(10):1597–1607. doi: 10.1093/scan/nsw063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim P, Evans GW, Angstadt M, Ho SS, Sripada CS, Swain JE, … Phan KL. Effects of childhood poverty and chronic stress on emotion regulatory brain function in adulthood. Proceedings of the National Academy of Sciences. 2013;110(46):18442–18447. doi: 10.1073/pnas.1308240110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim P, Evans GW, Chen E, Miller GE, Seeman TE. How socioeconomic disadvantages get under the skin and into the brain across the lifespan. In: Halfon N, Forrest C, Lerner R, Faustman E, editors. The handbook of life course health development. New York, NY: Springer; (in press) [Google Scholar]
- Kim P, Feldman R, Mayes LC, Eicher V, Thompson N, Leckman JF, Swain JE. Breastfeeding, brain activation to own infant cry, and maternal sensitivity. Journal of Child Psychology and Psychiatry and Allied Disciplines. 2011;52(8):907–915. doi: 10.1111/j.1469-7610.2011.02406.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim P, Strathearn L, Swain JE. The maternal brain and its plasticity in humans. Hormones and Behavior. 2016b;77:113–123. doi: 10.1016/j.yhbeh.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim P, Watamura SE. Two open windows: infant and parent neurobiologic change. 2015 Retrieved from http://ascend.aspeninstitute.org/pages/two-open-windows-infant-and-parent-neurobiologic-change.
- Kim S, Fonagy P, Allen J, Strathearn L. Mothers’ unresolved trauma blunts amygdala response to infant distress. Social Neuroscience. 2014;9(4):352–363. doi: 10.1080/17470919.2014.896287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landi N, Montoya J, Kober H, Rutherford HJ, Mencl WE, Worhunsky PD, … Mayes LC. Maternal neural responses to infant cries and faces: relationships with substance use. Frontiers in Psychiatry. 2011;2:32. doi: 10.3389/fpsyt.2011.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent HK, Stevens A, Ablow JC. Neural correlates of hypothalamic-pituitary-adrenal regulation of mothers with their infants. Biological Psychiatry. 2011;70(9):826–832. doi: 10.1016/j.biopsych.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibenluft E, Gobbini MI, Harrison T, Haxby JV. Mothers' neural activation in response to pictures of their children and other children. Biological Psychiatry. 2004;56(4):225–232. doi: 10.1016/j.biopsych.2004.05.017. [DOI] [PubMed] [Google Scholar]
- Lenzi D, Trentini C, Pantano P, Macaluso E, Iacoboni M, Lenzi GL, Ammaniti M. Neural basis of maternal communication and emotional expression processing during infant preverbal stage. Cerebral Cortex. 2009;19(5):1124–1133. doi: 10.1093/cercor/bhn153. [DOI] [PubMed] [Google Scholar]
- Little C, Carter AS. Negative emotional reactivity and regulation in 12-month-olds following emotional challenge: Contributions of maternal–infant emotional availability in a low-income sample. Infant Mental Health Journal. 2005;26(4):354–368. doi: 10.1002/imhj.20055. [DOI] [PubMed] [Google Scholar]
- Lonstein JS, Lévy F, Fleming AS. Common and divergent psychobiological mechanisms underlying maternal behaviors in non-human and human mammals. Hormones and Behavior. 2015;73:156–185. doi: 10.1016/j.yhbeh.2015.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nature Reviews Neuroscience. 2009;10(6):434–445. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- MacKinnon DP, Fairchild AJ, Fritz MS. Mediation analysis. Annual Review of Psychology. 2007;58:593–614. doi: 10.1146/annurev.psych.58.110405.085542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Gianaros PJ. Central role of the brain in stress and adaptation: Links to socioeconomic status, health, and disease. Annals of the New York Academy of Sciences. 2010;1186(1):190–222. doi: 10.1111/j.1749-6632.2009.05331.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muscatell KA, Morelli SA, Falk EB, Way BM, Pfeifer JH, Galinsky AD, … Eisenberger NI. Social status modulates neural activity in the mentalizing network. Neuroimage. 2012;60(3):1771–1777. doi: 10.1016/j.neuroimage.2012.01.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitschke JB, Nelson EE, Rusch BD, Fox AS, Oakes TR, Davidson RJ. Orbitofrontal cortex tracks positive mood in mothers viewing pictures of their newborn infants. Neuroimage. 2004;21(2):583–592. doi: 10.1016/j.neuroimage.2003.10.005. [DOI] [PubMed] [Google Scholar]
- Noble KG, Grieve SM, Korgaonkar MS, Engelhardt LE, Griffith EY, Williams LM, Brickman AM. Hippocampal volume varies with educational attainment across the life-span. Frontiers in Human Neuroscience. 2012;6:307. doi: 10.3389/fnhum.2012.00307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Numan M, Bress JA, Ranker LR, Gary AJ, DeNicola AL, Bettis JK, Knapp SE. The importance of the basolateral/basomedial amygdala for goal-directed maternal responses in postpartum rats. Behavioural Brain Research. 2010;214(2):368–376. doi: 10.1016/j.bbr.2010.06.006. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Silvers JA, Buhle JT. Functional imaging studies of emotion regulation: A synthetic review and evolving model of the cognitive control of emotion. Annals of the New York Academy of Sciences. 2012;1251(1):E1–E24. doi: 10.1111/j.1749-6632.2012.06751.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyen AS, Landy S, Hilburn-Cobb C. Maternal attachment and sensitivity in an at-risk sample. Attachment & Human Development. 2000;2(2):203–217. doi: 10.1080/14616730050085563. [DOI] [PubMed] [Google Scholar]
- Preacher KJ, Hayes AF. Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behavior Research Methods. 2008;40(3):879–891. doi: 10.3758/brm.40.3.879. [DOI] [PubMed] [Google Scholar]
- Ranote S, Elliott R, Abel KM, Mitchell R, Deakin JF, Appleby L. The neural basis of maternal responsiveness to infants: An fMRI study. Neuroreport. 2004;15(11):1825–1829. doi: 10.1097/01.wnr.0000137078.64128.6a. [DOI] [PubMed] [Google Scholar]
- Riem MM, Bakermans-Kranenburg MJ, Pieper S, Tops M, Boksem MA, Vermeiren RR, … Rombouts SA. Oxytocin modulates amygdala, insula, and inferior frontal gyrus responses to infant crying: A randomized controlled trial. Biological Psychiatry. 2011;70(3):291–297. doi: 10.1016/j.biopsych.2011.02.006. [DOI] [PubMed] [Google Scholar]
- Seifritz E, Esposito F, Neuhoff JG, Luthi A, Mustovic H, Dammann G, … Di Salle F. Differential sex-independent amygdala response to infant crying and laughing in parents versus nonparents. Biological Psychiatry. 2003;54(12):1367–1375. doi: 10.1016/s0006-3223(03)00697-8. [DOI] [PubMed] [Google Scholar]
- Shonkoff JP, Fisher PA. Rethinking evidence-based practice and two-generation programs to create the future of early childhood policy. Development and Psychopathology. 2013;25(4Pt 2):1635–1653. doi: 10.1017/S0954579413000813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman ME, Muennig P, Liu X, Rosen Z, Goldstein MA. The impact of socioeconomic status on the neural substrates associated with pleasure. The Open Neuroimaging Journal. 2009;3(1):58–63. doi: 10.2174/1874440000903010058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith PB, Pederson DR. Maternal sensitivity and patterns of infant-mother attachment. Child Development. 1988:1097–1101. doi: 10.1111/j.1467-8624.1988.tb03262.x. [DOI] [PubMed] [Google Scholar]
- Smith S. Two generation programs for families in poverty: A new intervention strategy. Vol. 9. Greenwood Publishing Group; 1995. [Google Scholar]
- Strathearn L, Fonagy P, Amico J, Montague PR. Adult attachment predicts maternal brain and oxytocin response to infant cues. Neuropsychopharmacology. 2009;34:2655–2666. doi: 10.1038/npp.2009.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strathearn L, Kim S. Mothers’ amygdala response to positive or negative infant affect is modulated by personal relevance. Frontiers in Neuroscience. 2015;7(176):21–30. doi: 10.3389/fnins.2013.00176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strathearn L, Li J, Fonagy P, Montague PR. What's in a smile? Maternal brain responses to infant facial cues. Pediatrics. 2008;122:40–51. doi: 10.1542/peds.2007-1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain JE, Kim P, Spicer J, Ho SS, Dayton CJ, Elmadih A, Abel KM. Approaching the biology of human parental attachment: Brain imaging, oxytocin and coordinated assessments of mothers and fathers. Brain Research. 2014;1580:78–101. doi: 10.1016/j.brainres.2014.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmer SG, Thompson D, Culver MA, Urquiza AJ, Altenhofen S. Mothers' physical abusiveness in a context of violence: Effects on the mother–child relationship. Development and Psychopathology. 2012;24(1):79–92. doi: 10.1017/S0954579411000678. [DOI] [PubMed] [Google Scholar]
- Wan MW, Green J, Elsabbagh M, Johnson M, Charman T, Plummer F. Parent–infant interaction in infant siblings at risk of autism. Research in Developmental Disabilities. 2012;33(3):924–932. doi: 10.1016/j.ridd.2011.12.011. [DOI] [PubMed] [Google Scholar]
- Whitesell CJ, Teti DM, Crosby B, Kim BR. Household chaos, sociodemographic risk, coparenting, and parent-infant relations during infants’ first year. Journal of Family Psychology. 2015;29(2):211. doi: 10.1037/fam0000063. [DOI] [PubMed] [Google Scholar]
- Wonch KE, de Medeiros CB, Barrett JA, Dudin A, Cunningham WA, Hall GB, … Fleming AS. Postpartum depression and brain response to infants: Differential amygdala response and connectivity. Social Neuroscience. 2015;11(6):600–617. doi: 10.1080/17470919.2015.1131193. [DOI] [PubMed] [Google Scholar]
- Zaki J, Davis JI, Ochsner KN. Overlapping activity in anterior insula during interoception and emotional experience. Neuroimage. 2012;62(1):493–499. doi: 10.1016/j.neuroimage.2012.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]