ABSTRACT
The putative transfer and gene regulatory activities of diet-derived small RNAs (sRNAs) in ingesting animals are still debated. The existence of natural uptake of diet-derived sRNA by invertebrate species could have significant implication for our understanding of ecological relationships and could synergize with efforts to use RNA interference (RNAi) technology in agriculture. Here, we synthesize information gathered from studies in invertebrates using natural or artificial dietary delivery of sRNA and from studies of sRNA in vertebrate animals and plants to review our current understanding of uptake and impact of natural diet-derived sRNA on invertebrates. Our understanding has been influenced and sometimes confounded by the diversity of invertebrates and ingested plants studied, our limited insights into how gene expression may be modulated by dietary sRNAs at the mechanistic level, and the paucity of studies focusing directly on natural uptake of sRNA. As such, we suggest 2 strategies to investigate this phenomenon more comprehensively and thus facilitate the realization of its potentially broad impact on ecology and agriculture in the future.
KEYWORDS: Agriculture, biotechnology, cross-kingdom, diet, ecology, genetically engineered, invertebrate, miRNA, plant, sRNA
Abbreviations
- GE
genetically engineered
- siRNA
short-interfering RNA
- dsRNA
double-stranded RNA
- miRNA
microRNA
- sRNA
small RNA
Introduction
The putative transfer and gene regulatory activities of diet-derived small RNAs (sRNAs) in ingesting animals are still debated. The natural uptake of diet-derived sRNA by invertebrate species could have significant implications for our understanding of ecological relationships and for the use of RNA interference (RNAi) in agricultural biotechnology. Here, we synthesize information gathered from a number of experimental systems to review our current understanding of uptake and impact of natural diet-derived sRNA in invertebrates. In the context of this review, we will focus on herbivorous invertebrates and how their plant-based diets could be involved in RNAi, the RNA-mediated post-transcriptional regulation of gene expression, in these organisms. We will use sRNA to refer to microRNA (miRNA), short interfering RNA (siRNA) or longer double-stranded RNA (dsRNA) from which siRNAs can be derived.
sRNAs are RNA molecules of <200 nucleotides in length that typically do not code for protein, but are instead involved in regulating other cellular processes. A subset of these, siRNA and miRNA, are involved in the post-transcriptional regulation of gene expression in animals, including invertebrates (reviewed in1). These two RNA biotypes are processed similarly in the cytoplasm and affect gene expression via similar RNAi-mediated mechanisms throughout the plant and animal kingdoms. However, their origin is distinct: miRNAs are encoded by endogenous genes while siRNAs are usually generated from dsRNAs that are introduced into the cell from an exogenous source.1
miRNAs are initially transcribed as a single-stranded RNA precursor of several hundred nucleotides termed a primary miRNA (pri-miRNA) which can contain multiple hairpin structures destined to become miRNA. Pri-miRNAs are subject to a number of nuclear processing steps, including cleavage by a microprocessor complex to release one or more pre-miRNA hairpins. Pre-miRNAs are then exported into the cytoplasm where they are further processed by DICER, an RNase, to the mature form of approximately 22 nucleotide dsRNA molecules. One strand of this is then incorporated into the RNA-induced silencing complex (RISC), which includes the target recognition facilitating protein ARGONAUTE and target mRNA species. miRNAs bind specific complimentary sequences in the 3′UTR of target mRNA transcripts and regulate post-transcriptional gene expression through the repression of translation and/or degradation of the targeted mRNA (reviewed in2). miRNAs were first discovered in Caenorhabditis elegans,3,4 the model organism that has driven much of our understanding in this field. Beginning with the first cross-species studies of miRNA in animals5 and discovery in plants,6 these molecules are now understood to be highly conserved and to regulate widespread cellular and organismal processes in species across all branches of animal and plant life.
By contrast, siRNAs are not highly conserved, as they are generated de novo by DICER from longer dsRNA species usually introduced into the cell from exogenous sources. These RNA molecules are typically 20–25 nucleotides and do not contain hairpin structures in their immature form, but, similar to miRNA, are incorporated into the RISC complex to regulate gene expression of target mRNA via similar mechanisms. They were also first discovered in C. elegans,7 but rapidly found to function in diverse species across kingdoms.8,9
That sRNA found in dietary material could influence gene expression of an ingesting organisms was first shown in C. elegans,10,11 where dsRNAs added to the diet or expressed in bacteria that make up the diet of this organism caused silencing of multiple genes after serving as the template for siRNA formation. Since then, oral exposure of various invertebrate organisms to dietary material containing in vitro synthesized dsRNA12-14 or artificially expressing dsRNA (in plant tissue15-18 or bacteria19) has demonstrated that various invertebrate organisms could take up sRNA molecules from diverse dietary sources. Since these early studies, both methods have been employed to demonstrate uptake of artificially introduced sRNA from the diet in more than 20 species, but failure to efficiently take up dietary sRNA has also been shown in a similar number of species, underscoring the species-dependent variability in this process (reviewed in20-22). For example, among insects, RNAi is typically very efficient in coeleopteran species, while most lepidopteran species appear intractable to such methods.23
The variability in efficacy in uptake between invertebrate species, and the often high doses of sRNA molecules required when successful, suggested that transfer of naturally occurring sRNA from dietary material to ingesting organisms was unlikely.
Therefore, a great deal of excitement was generated when transfer of diet-derived small RNA to ingesting organisms in a natural context was first described in the report by C-Y Zhang and colleagues,24 which showed that dietary plant miRNAs could enter the mammalian bloodstream and regulate cholesterol metabolism through regulation of specific target mRNA in ingesting mice. Concurrently, the presence of sRNA from exogenous sources was discovered in human plasma.25 These studies stimulated much interest,26,27 and in conjunction with studies showing other sources of exogenous sRNA,28 particular emphasis was placed on the paradigm-shifting possibility of cross-kingdom communication mediated through the diet. However, a number of subsequent studies provided considerable evidence that systemic uptake of orally ingested foreign miRNAs, as a class of molecules, is negligible in mammals29-32 and significantly below levels required to be biologically relevant when acting through canonical sequence-specific RNAi-mediated mechanisms. With respect to dietary uptake of miRNA or other sRNA in vertebrates, controversy remains, with a number of groups providing data in support or in opposition (reviewed in33,34). A robust scientific discourse continues to discuss biological and technical reasons for the differential results found in the dietary uptake in vertebrates. Further progress has been impeded in large part by a limited understanding of the mechanisms that could allow dietary uptake of amounts of sRNA necessary to impact the gene expression of distal cells of an ingesting vertebrate organism.
This controversy has overshadowed the potential impact of natural diet-derived sRNA on gene expression in invertebrates. In fact, despite a large body of evidence showing successful uptake of sRNA under artificial conditions in invertebrates (reviewed in20-22), only 6 studies have examined natural sRNA uptake in invertebrate organisms (Table 1). The first group to examine this found uptake, and an impact on gene expression, of 2 Escherichia coli endogenous noncoding RNAs in ingesting C. elegans.35 However, some evidence contradicting this finding has been reported.36 In a different study, Zhang et al found no evidence of miRNAs in a number of invertebrates including pea aphid and silkworm and no uptake of miRNAs in feeding experiments with the corn rootworm.29 By contrast, analysis of uptake of other sRNA types in corn rootworm fed on wild-type plants by another group revealed uptake of plant endogenous long dsRNAs, but not small sRNAs,37 with no apparent effect on the transcriptome. This same group found uptake in another coleopteran species, the Colorado potato beetle, but no uptake was observed in 2 lepidopteran species.37 Finally, our own group found no evidence of biologically relevant delivery of a miRNA species highly expressed in dietary pollen to proximal or distal tissues of recipient honey bees.30,38 Taken together, these data suggest that natural sRNA uptake is variable and possibly dependent on the sRNA biotype and invertebrate species examined. A more complete understanding of the natural uptake of diet-derived sRNA by invertebrate species could have significant implications for our understanding of ecological relationships and our use of RNAi technology in an agricultural setting. Here, we synthesize information gathered from studies in invertebrates using natural or artificial dietary delivery of sRNA and from studies of sRNA in vertebrate animals and plants to review our current understanding of uptake and impact of natural diet-derived sRNA on invertebrates.
Table 1.
Experiments examining uptake of natural diet-derived sRNAs.
| natural sRNA uptake |
||||
|---|---|---|---|---|
| Consuming organism | Competent for oral RNAi | miRNA | dsRNA/siRNA | other |
| Caenorhabditis elegans35 | Y | nd | nd | Y |
| Acyrthosiphon pisum29 | N | N | nd | nd |
| Apis mellifera30,38 | Y | N* | nd | nd |
| Bombyx mori29 | N | N | nd | nd |
| Diabrotica spp.29,37 | Y | N* | Y* | nd |
| Helicoverpa zea37 | N | nd | N* | nd |
| Leptinotarsa decemlineata37 | Y | nd | Y* | nd |
| Spodoptera frugiperda29,.37 | N | N | N* | nd |
nd = not done,
controlled feeding experiments performed.
Critical steps for successful alteration of gene expression of an ingesting organism by dietary sRNA
For dietary sRNA to have the potential to alter the gene expression of ingesting organism, a number of discrete steps must occur efficiently for a given sRNA entity, starting with availability of the sRNA molecule in the diet and ending with successful alteration of gene expression within distal cells of the ingesting organism (Fig. 1). In this review, we will examine these steps separately as 5 questions.
Are there sufficient levels of bioavailable sRNAs in the diet?
Do sRNAs cross the digestive tract barrier
Are sRNAs disseminated systemically?
Is there cellular uptake of sRNA?
Can delivered sRNA alter the post-transcriptional expression of specific target genes?
Figure 1.
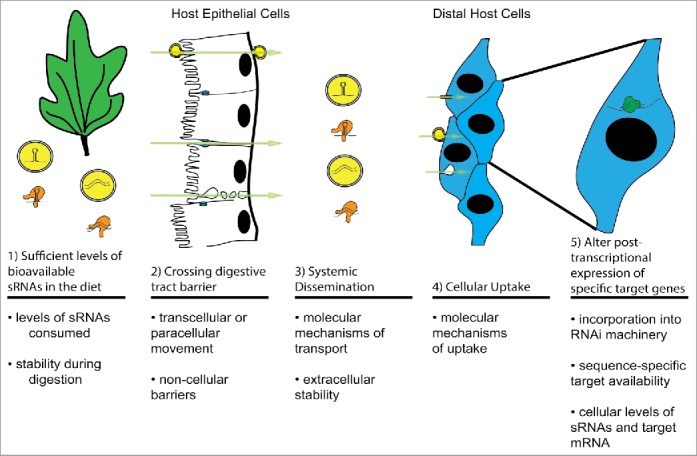
Critical steps for successful alteration of gene expression of an ingesting organism by dietary sRNA (adapted from58). (1) Sufficient levels of bioavailable sRNA in the diet, (2) Crossing the digestive tract barrier, (3) Systemic Dissemination, (4) Cellular Uptake, and (5) Alteration of post-transcriptional expression of specific target genes.
All of these conditions must be met for successful regulation of gene expression in an ingesting organism by dietary sRNAs and some have been discussed as key determinants of the variability in the ability of different species to respond to dietary sRNA.20 Thus, they will provide an intellectual framework for examining our current knowledge drawing from multiple fields. In addition, these steps likely provide the key areas of focus for future mechanistic studies.
Are there sufficient levels of bioavailable sRNAs in the diet?
For there to be sufficient bioavailable sRNA in the diet to impact gene expression in an ingesting organism, 2 conditions must be met. First, an animal must be able to consume enough of the diet to ingest a biologically relevant amount of a given sRNA, which is estimated to be 100–1000 copies per target cell.39,40 For different plant miRNA species, the sRNA type that has been studied most extensively, the levels found in different dietary material vary widely in the relatively small set of plant species examined.41 For example plant MIR156a exists at 5×106 copies per mg of cantaloupe tissue and a representative pollen sample, but 1000 copies per mg of apple tissue.30 In addition, sRNA expression is known to vary significantly between plant tissues in a given species and are highly sensitive to environmental conditions.42 As an example, miRNAs in wheat were found to be expressed in a tissue-specific manner and this was influenced significantly by abiotic stressors.43 Plants also possess a number of other sRNA types, which are generated from longer dsRNA precursors, including hairpin-derived siRNA, natural antisense siRNA, secondary siRNA, and heterochromatic siRNA.42,44 The amounts of these have primarily been examined in model organisms, such as Arabidopsis thaliana, and the agriculturally important rice, maize, and soy, so very few conclusions can currently be made about their typical amounts. In addition, the amount of a given food consumed by an animal also varies considerably45 and both must be considered to meet this first condition. For example, honey bees are thought to consume a maximum of ∼4 mg of pollen per day, suggesting that an adult honey bee might consume approximately 107 copies of MIR156a per day, resulting in a maximum theoretical delivery of 2 copies per cell if 100% transfer of the diet-derived miRNA was achieved and the miRNAs were distributed equally between cells.30 Therefore, a key consideration is the use of diets that would be typically consumed by an organism in a natural environment.46 For example, sucking insects such as aphids would only encounter sRNAs in the phloem.47 This condition may also be dependent on the feeding behavior of the animal, such that an organism that is highly promiscuous in its food choices may be less likely to ingest sufficient quantities of a species-specific sRNA molecule to be biologically relevant. In addition, the presence of an amplification system, as discussed later, could have significant impact on the amount of sRNA required in the diet to result in regulation of gene expression.
Second, the sRNA species must be able to withstand the harsh environment of the digestive tract. Oral bioavailability of intact macromolecules is typically very low due to the action of digestive hydrolases in the lumen and the physical barrier of the digestive tract. dsRNA molecules considered here are known to be relatively stable48 and can be resistant to degradation even in harsh environmental conditions.49 However, dsRNAs are susceptible to breakdown like all macromolecules and nuclease activity has been characterized in multiple regions of the digestive tract of various insects. For example nuclease activity against dsRNA has been found in the saliva of the tarnished plant bug50 and pea aphid51 as well as the midgut of the silk worm,52 the desert locust,53 and others.54 It is important to note that the digestive tract tissue architecture and environment are quite divergent within invertebrates,55,56 leading to differences in potential for sRNA degradation.
Thus, for meaningful bioavailability, dietary sRNAs must be somehow sheltered from such degradation. Possible mechanisms for protection can be found in the research on extracellular sRNAs found in plants (reviewed in57). While the mechanistic understanding of extracellular transport of sRNAs in plants is still incomplete, there is some evidence that strategies may in part be shared with vertebrates, where extracellular sRNA is transported after packaging in exosomes and inclusion in ribonucleoprotein complexes (reviewed in58). Exosomes are a heterogeneous group of membrane-bound vesicles that can be released from the cell as part of a regulated process to allow delivery of diverse macromolecules to other cells within an organism.59 Plants contain exosome-like particles, known as nanoparticles, that can contain sRNAs, lipids, and proteins and are theorized to mediate cell-cell communication in a manner similar to the exosomes secreted by mammalian cells. Such particles are now being explored as a possible method for delivering therapeutics in humans.60 In addition, sRNAs complexed with the protein PHLOEM SMALL RNA BINDING PROTEIN1 have been found in the vascular system of a number of plants.61 A more complete understanding of how plant sRNAs are packaged such that they could be protected from degradation in the digestive tract is essential. These ribonucleoprotein complexes appear to provide stability to sRNA in an extracellular environment, but would seem inadequate to provide protection in the highly protoelytic context of the digestive tract, at least in vertebrates, where only 1–2% of protein remain intact and bioavailable after digestion.62 Additional mechanisms may protect sRNA in the digestive tract. For example, sRNA in eukaryotes can be subject to a number of covalent modifications, including, 3′-uridylation, 3′-adenylation, and deamination.63 All sRNA in plants are modified by 2′O-methlation to protect them from degradation.42 While the significance of this modification on the stability of extracellular sRNA is not known, it could impact nuclease activity in some settings.
In addition to our lack of knowledge about the types of packaging employed in plants, there is an equal paucity of data about the packaging requirements for escaping degradation in the digestive tract of specific invertebrates. Some studies have attempted to directly assay sRNA stability in simulated digestive environments64 or in vivo65 in vertebrates. These studies have identified certain sRNA molecules that appear particularly stable, but the mechanistic cause for the stability has not been defined. For example, MIR2911 appears unique in its ability to withstand degradation in vitro and within the mouse digestive tract.66 MIR2911 was shown to be extra-exosomal and associated with a ribonucleoprotein complex not containing AGO and to be rich in GC sequence, some or all of which may contribute to this stability.66 Some evidence suggests that artificial ‘exosome’ lipoplexes can protection dsRNA from degradation in the digestive tract to allow for successful RNAi in recalcitrant species, including in the fruit fly67 and the German cockroach.68 Such exosome-mediated mechanisms could protect natural sRNAs from degradation as well.
In dietary material, most active packaging, such as inclusion in a ribonucleoprotein complex or exosome, would likely occur in a manner designed to allow intercellular communication within the context of the diet organism. In some cases, such as the inclusion of miRNA into exosomes in breast milk in mammals,69,70 packaging could occur to allow specifically for dietary uptake of sRNA by offspring. Similarly, bee-derived miRNAs may be transferred to larval honey bees via inoculation of royal jelly with miRNAs by adult nurse bees, leading to specific effects on gene expression during larval development.71,72 In theory, packaging could also occur in a manner that has been selected for cross-kingdom communication. In addition, incidental inclusion of miRNA into exosomes or cell-free ribonucleoprotein complexes will occur during the processes of digestion. Some evidence suggests that cellular debris such as apoptotic bodies can transmit sRNA to other cells.73
Another variable is the microbiota of the digestive tract, with actions known to be integral in the digestive process.74 While invertebrate microbiome communities are thought to be less complex than those found in vertebrates,75 they do contribute to metabolism of consumed molecules and could play a role in sRNA availability in the digestive tract as well.
Do sRNAs cross the digestive tract barrier?
The most stringent impediment to uptake is likely the limitation imposed by the highly selective barrier of healthy gut epithelial tissue,76 which in insects is comprised of mucus, peritrophic matrix, and the epithelial cells themselves.77 The importance of the gut as a potent obstacle to dietary uptake of sRNAs in invertebrates is underscored by the significantly higher number of invertebrates for which sRNA injection results in robust RNAi compared to those where oral exposure to sRNA is successful.78,79 Evidence to date from a variety of organisms suggests that there are 2 possible modes of transport across the digestive tract epithelium, either transcellular or paracellular.76 The epithelial cells themselves regulate transcellular permeability via transport pathways through their cytoplasm. Transcytosis involves vesicle-mediated transport of cargo through the endomembrane system for release in internal tissues. Transmembrane protein transporters exist that can allow conveyance of a given molecule across the membrane into the epithelial cell. Alternatively, microvesicles or exosomes could fuse with the epithelial cell membrane. Both of these last 2 mechanisms would need to be coupled with an export mechanism to allow the cargo to reach the body cavity. Paracellular permeability on the other hand requires transport between the epithelial cells and is controlled by septate junctions (tight junctions in vertebrates).
In invertebrates, the relative importance of these 2 routes for a given cargo, such as sRNAs, has rarely been demonstrated.80 The majority of our information about the mechanism for dietary uptake of sRNA comes from nematodes (reviewed in81). C. elegans possess a pathway for sRNA uptake by cells that was identified in a screen for systemic RNAi defective (SID) mutants.82 The role of SID proteins in systemic spread of sRNAs and RNAi will be discussed later, but they also appear to play a key role in the dietary uptake of sRNA in this organism. SID-2 is a gut-specific receptor and mediates dietary uptake of sRNAs in a SID-1-dependent manner83 via endocytosis.84 SID-5 appears to be required for releasing sRNAs from endosomes,85 further supporting the critical role of endocytosis in sRNA uptake. SID-1 homologues and SID-like (SIL) proteins have been found in other insects,86,87 but no SID-2 proteins have been discovered. However, the importance of the endocytic pathway has been shown in the oriental fruit fly88 and both SID-like proteins and endocytosis are important in the dietary uptake of sRNA in in the Colorado potato beetle.89 These data suggest that endocytosis may be a common mechanism for sRNA uptake by the cells of the digestive tract.
Yet, the process of transport across the gut is likely not shared across all invertebrates, given the divergence of the invertebrate digestive tract in general,55,56 which requires further research. A recent study in Drosophila melanogaster suggests that morphological and molecular characterization of invertebrate digestive tracts could reveal important details concerning functional compartmentalization and thus could provide key insights into barrier properties that are salient for sRNA uptake.90 Both normal and pathogenic changes in the barrier properties of the digestive tract could influence sRNA in invertebrates. For example, oral dsRNA delivery appears to vary depending on insect life-stage in many insects91 and natural RNA uptake may similarly change with age. Indeed, the barrier of function of the Drosophila midgut has been shown to decrease with age likely due to increased paracellular permeability.92 Pathogenic changes in barrier function, such as that caused by xenotoxicity or infection could also change the efficiency of sRNA passage across the digestive tract. For example, various toxins work by disrupting epithelial cells in the digestive tract, such as the pore forming peptide pesticides derived from Bacillus thuringiensis.93 In addition, one group found that introduction of a cysteine protease that reduced the peritrophic matrix resulted in increased uptake of dsRNA delivered via transgenic plants to the bollworm,94 indicating that non-cellular barriers are important for inhibiting sRNA uptake as well.
In vertebrates, passage across the gut epithelium is also mediated by transcellular and paracellular transport, but there is limited data in support of either mechanism for sRNA uptake. Some studies have suggested that milk exosomes are endocytosed by mammalian cell lines, perhaps as the first stage for transcytosis.95 Paracellullar transport may occur in vertebrates as well, as evidence has been reported that dietary uptake of some sRNA can be enhanced by intestinal injury that results in increased leakage of molecules between epithelial cells.96 In fact, drugs that modulate tight junction function are now being explored to enhance therapeutic delivery across epithelial barriers.97
One interesting finding that has come out of a large body of studies on uptake of artificial sRNA in invertebrates is the apparent importance of the size of the sRNA molecule. In most invertebrates, uptake of longer dsRNAs is more efficient than shorter sRNA, such as miRNAs or siRNAs.91 This molecular feature has complicated the intent of transgenic plants to facilitate sRNA delivery via oral ingestion to invertebrates, because the plant RNAi machinery usually processes the longer dsRNA to shorter forms. For example, Mao et al found that A. thaliana mutants lacking functional RNAi machinery produced more longform sRNA and resulted in increased knockdown compared to wild-type when fed to cotton bollworm.18 One elegant strategy to circumvent this issue was recently pioneered in genetic engineering-mediated RNAi directed against the Colorado potato beetle, where plastid specific expression of the transgenic sRNA strategy bypassed sRNA exposure to cytoplasmically located RNAi machinery.78 However, uptake of short miRNA mimics have been shown to be taken up in some insects,98 again underscoring the diversity of uptake potential among species.
Are sRNAs disseminated systemically?
Once across the barrier of the digestive tract epithelia, sRNAs must be able to survive the internal environment and must have some mechanisms for spreading to distal tissues. The existence of ‘systemic’ RNAi in a large number of species suggests that mechanisms exist for the dissemination of sRNA from the point of origin to distal cells. In addition, miRNAs have been found in C. elegans interstitial fluid99 and D. melanogaster hemolymph100 suggesting extracellular transport. Furthermore, the existence of endocytotic mechanisms for systemic RNAi spread in both organisms,81,101,102 suggests that extracellular sRNA exists in invertebrates. However, there are no data on the form extracellular sRNA takes in invertebrate species. Exosomes do exist in invertebrates,59 but their cargo is almost completely uncharacterized, and there are no data on the specific packaging of extracellular sRNAs in invertebrate species in exosomes or otherwise. Recent evidence also suggests that intracellular bridges, termed nanotubes, might transfer sRNA and associated complexes between cells in the fruit fly.103
Extracellular sRNAs have been studied most extensively in vertebrates and it is worth discussing the mechanisms observed in those studies. In these organisms, miRNAs, which are secreted to regulate gene expression in a non-cell-autonomous manner, appear to be relatively stable due in part to special processing, including packaging in exosomes and inclusion in ribonucleoprotein complexes.104 First, miRNAs can be delivered by a wide variety of lipid-bound vesicles, including exosomes in a wide range of biological processes.105-112 miRNA sorting into exosomes can be influenced by a number of factors,113 including dose,114 3′ end uridylation,115 nucleotide sequence motifs,116 interaction with RNA-binding proteins,117,118 and localization to GW bodies.119 Second, miRNAs can be incorporated into a variety of ribonucleoprotein complexes, including those containing ARGONAUTE family members,120-122 HDL,123,124 and HuR.125 In addition, 2 other modes of sRNA spread that have been documented in vertebrates may play a role in invertebrates. Gap junctions can allow for transfer of siRNAs126 and miRNAs,127 and some data suggest that specialized connecting structures, such as cell bridges, can also be involved in sRNA transfer between cells in vertebrates as well.128
The stability of sRNA species once across the barrier epithelia appears to be a major determinant of systemic sRNA in invertebrates that differs considerably between species. Non-specific nuclease activity has been found in many insects that appears to degrade sRNA in hemolymph and tissues. For example, the pea aphid possesses potent nuclease activity in its hemolymph.51 In insects studied to date, differences in persistence of dsRNA injected into the hemolymph has correlated well with RNAi sensitivity.54,129,130
When discussing systemic RNAi in invertebrates, it is important to point out that C. elegans and some other invertebrates also possesses a system that amplifies a primary siRNA to give rise to a secondary siRNA. In the worm, this activity is mediated by an RNA-dependent RNA polymerase for which no homologs have been found in most other invertebrates, discounting the universal importance of this particular mechanism on amplification.91 However, other types of amplification are possible, such as described in the fruit fly.131 Because the presence of sRNA amplification in a species would have significant impact the amount of sRNA that must be ingested to have downstream effects, further work is required to understand the prevalence of this phenomenon.
Is there cellular uptake of sRNAs?
The mechanisms responsible for mediating sRNA uptake by cells in distal parts of the organism are overlapping yet distinct from those posited to allow for entry across the barrier epithelia. SID-1, from the C. elegans screen mentioned above, was the first protein to be shown to be involved in sRNA uptake by cells.132 It is a dsRNA channel133 that is not selective in the length of sRNA it imports.134 SID-like proteins can be found in diverse species. Notably, a SID-1 homolog exists in vertebrates and has been implicated in sRNA uptake in humans.135,136 However, it is important to point out that in many insect species SID-like proteins are not involved in sRNA uptake, but instead perform other cellular functions.87,137 A second mechanism for taking sRNA into cells involves clathrin-mediated endocytosis and was first discovered in D. melanogaster.101,102 Here, the scavenger receptor is responsible for recognizing the sRNA and initiating endocytosis.
Examination of other invertebrates that are competent for systemic spread of RNAi has revealed that uptake of sRNAs by internal cells is often mediated by a SID-like protein-dependent pathway or an endocytosis-dependent pathway. However, as pointed out in the recent study by Capelle et al, both pathways have rarely been examined in a given insect species making comprehensive comparison difficult.89 In the tick and the desert locust, the scavenger receptor has been implicated in endocytosis-mediated spread,138,139 which may indicate that this receptor is commonly used for this mechanism; however, other receptors yet to be discovered may also be employed for sRNA recognition. In addition, cells of different tissues within an organism can have different potential for uptake. In the migratory locust, injected dsRNA was taken up by fatbody cells but not by cells of the ovary.140 In addition, lipophilic siRNA molecules can be imported into cells through endocytosis mediated by scavenger and low density lipoprotein (LDL) receptors.141 However, more comprehensive comparisons of human versus invertebrate processes for sRNA uptake await further definition.
However, escape of extracellular sRNA from the endosome to the cytoplasm once internalized may in fact be a more limiting factor than gaining access to the endomembrane system.142,143 Once endocytosed, sRNA can be recycled back to the extracellular space, can be degraded in the lysosome, or can exit the endosome via opaque mechanisms.144,145 Further definition of the processes governing this decision for endogenous extracellular sRNA is warranted to provide a better understanding of the potential handling of diet-derived sRNA.
Can delivered sRNAs alter the post-transcriptional expression of specific target genes?
Three conditions have to be met to initiate canonical post-transcriptional regulation of specific target genes by sRNAs after cellular uptake. First, the sRNA molecules must be recognized by the RNAi machinery of the cell. Core RNAi machinery exists in all invertebrate species examined for which genomes are available.20 Evidence suggests that inclusion of an sRNA in active RISC complexes is highly regulated and may be coupled to processing.1 In addition, association of the RISC with the appropriate cellular compartment, such as the GW bodies and specific membrane-bound structures,146 may be required for function. Thus, it is possible that sRNA molecules from other distant species, such as plants, do not have the requisite characteristics for recognition and efficient use by the RNAi machinery in some ingesting organisms. In one study, dsRNAs were found to be taken up by lepidopteran cells but not processed to siRNAs.130 In addition, both vertebrates147 and invertebrates148 possess a number of pattern-recognition receptors that recognizes dsRNAs associated with viral infection. These pathways may actually inhibit inclusion of exogenous sRNAs into the RNAi pathway by making them targets of antiviral defenses. In one study, sRNA exposure, likely mimicking viral infection, caused a downregulation of endocytic pathway genes limiting subsequent sRNA uptake and function.88 In addition, as RNAi itself is an important component of anti-viral immunity in insects,149 sRNA and viral infection are likely to influence each other and sRNA function may be heavily influenced by the infection status of the studied insects.150 Studies in marine shrimp 151and honey bees152 demonstrate that in these divergent species, exposure to a wide variety of non-specific sRNA can trigger a non-specific antiviral response. Conversely, viruses, such as Cricket paralysis virus in the fruit fly,153 often manipulate RNAi machinery leading to decreased function.
Second, there must be appropriate target mRNA sequences to be regulated via antisense sequence-specific mechanisms. This is a challenging question to answer given the difficulty in identifying the appropriate target mRNAs potentially recognized by a given sRNA in the recipient invertebrate cell. That is, prediction of intended and actual mRNA transcripts that are bound and regulated by a specific sRNA molecule is quite difficult (reviewed in2). In silico methods have been employed to predict such cross-species target mRNA molecules in vertebrates for plant miRNAs using algorithms that take into account the challenges of different kingdom-specific rules for RNAi function154,155 and select targets are often validated using in vitro assays. While compelling, such data should be coupled with transcript analysis in in vivo feeding experiments to be definitive. However, the converse is also true; transcripts observed to change in response to ingestion of a given sRNA species should be characterized in more detail in biochemical assays to demonstrate direct action of an sRNA. Such bioinformatics tools will need to be further developed to allow for inclusion of other sRNA types and mRNA sequences found in more diverse ingesting organisms. In addition, while genomes are now being sequenced at an accelerating pace, many of the plant and ingesting organism genome sequences are not yet available in their entirety. In the only study examining gene expression associated with dietary sRNA uptake, Ivashuta et al used next generation RNA sequencing to circumvent this issue and found no substantial changes in gene expression a the transcript level after dietary uptake of natural sRNA.37
Finally, as mentioned in the first section, it is important to reiterate that functional post-transcriptional gene regulation of mRNA by sRNA requires that a minimum amount of a given sRNA species be taken up by a ‘target’ cell. Based on the range of amounts of typical mRNA species, the amount of sRNA required for biologically relevant effects on gene expression is estimated to be 100–1000 copies per target cell.39,40
Implications
The natural uptake of diet-derived sRNA by invertebrate species, if proven to be robust and common, could have significant implication for our understanding of ecological relationships. In addition, significant overlap exists in the questions that should be answered in this field and the field developing RNAi technology for use in an agricultural setting and mutual benefit could arise in both fields from such investigations.
The impact of sRNA on the ecological interactions between parasites and their hosts is already well appreciated (reviewed in156). The possibility of cross-kingdom regulation of gene expression via ingested sRNAs could also be of particular ecological interest. For example, the mutualistic relationships of plants and their pollinators involves an exchange of nutrients for the reproduction-enabling service of pollination, but is complicated by the antagonistic relationship of the 2 players, which is characterized by a fundamental conflict of interest.157 In addition to offering nutritional rewards for pollinators, plants often try to control pollinators. For example, plants use nicotine158 and caffeine159 to increase pollination. Demonstrating that plants can control gene expression in pollinating insects via small RNA transfer would reveal a new and exciting level of complexity in these plant-pollinator relationships.
Such cross-kingdom interactions might also be expected to impact predator-prey interactions,160 where selection for molecular defenses in prey could include increased production of sRNA with detrimental impacts on predator physiology or behavior.161 In response to predation by herbivores, plants are known to defend themselves with chemical weapons.162,163 For example, coffee plants produce caffeine that can repel slugs and snails.164
There is a large body of evidence that animals employ self-medication or zoopharmocognosy, a concept which spans from invertebrates to great apes165 and has been examined in the use of traditional medicine in human societies.166 This research has led to a model in which animals increase the consumption of specific dietary sources in a manner independent of nutritional parameters to benefit from non-nutritional constituents of the diet. For example, bumble bees self-medicate by ingesting more nectar containing the anti-parasitic compound Anabasine when parasitized by a specific trypanosome.167 Provision of such compounds has positive effects on plant fitness168 and could therefore provide an evolutionary rationale for the production of sRNAs with cross-kingdom effects by plant species.
In agriculture, RNAi-based technologies which take advantage of cross-kingdom small RNA (miRNA, siRNA, dsRNA) transfer, including genetically engineered (GE) plants, will be among the cutting edge developments with the potential to transform food production in the coming years.169 While these technologies will use GE-based delivery of sRNA not normally expressed in the plant, they will presumably utilize the same pathways and processes that could be employed by plants and the invertebrates that consume them for natural uptake of dietary sRNAs. Whether used as pesticides or to combat the diseases of beneficial invertebrates, a solid mechanistic understanding of the steps required for uptake and a framework for assessing uptake potential for a given target species would represent a substantial benefit. In addition, RNAi technologies require rigorous research to assess their safety170,171 and the establishment of safety protocols can be informed by such knowledge.
While most of the foundational initial studies of RNAi were performed in a basic research context, our understanding of the variety of RNAi mechanisms in diverse invertebrates, and dietary uptake in particular, has been significantly advanced in recent years by studies focused on agricultural biotechnology. In fact, while variable, the success of in planta expression of sRNAs for invertebrate modulation is perhaps the best evidence in support of the potential for natural dietary uptake of sRNA. Although agricultural biotechnology is and can continue to be a significant driver of research through studies that focus on the artificial uptake of sRNA, a number of areas will be informed by information garnered through studies of the prevalence and mechanisms of natural uptake of dietary sRNA by invertebrates. Perhaps the most striking will be the potential benefit on our understanding of the selective pressures acting on dietary uptake of sRNA. From recent studies examining the potential for orally delivered RNAi, such as the recent study demonstrating numerous instances of loss of this potential in the Caenorhabditis genus,172 it is evident that this phenomenon is driven by evolutionary processes. In agricultural biotechnology, mechanisms of resistance are a major issue that impact efficacy in crop protection strategies. An understanding of the natural processes governing such resistance at the molecular, organismal, and population levels would be immensely valuable.
Conclusions and future directions
The existence of natural uptake of diet-derived sRNA by invertebrate species could have significant impact on both our understanding of ecological relationships and our efforts to use RNAi technology in agriculture. Moreover, the enormous number and diversity of invertebrate species make this possibility highly likely. However, while the potential impacts are exciting, the diversity of invertebrates and ingested plants studied, the limited mechanistic understanding of the steps required for successful modulation of the gene expression by dietary sRNAs, and the paucity of studies focusing directly on natural uptake of sRNA, make future progress challenging. Below and summarized in Fig. 2 are 2 strategies / steps that could be taken to address these issues:
Invertebrate and plant model selection: It has been suggested that ability to take up artificially fed dsRNA can be used to determine uptake of natural dietary sRNA and vice versa.37 If so, future studies on natural uptake of diet-derived sRNA should focus on invertebrates for which successful orally administered RNAi has been shown. For example, studies of diet-derived RNAi in the control of the corn rootworm93 for agricultural uses has provided one of the best examples of natural uptake of dietary sRNAs.37 Research in this area should focus on species for which genomic tools exist for both the plant species of the diet and the ingesting organism. Omics-based approaches should be used to identify all sRNA species in the diet and ingesting organisms and levels of all mRNA transcripts and proteins to prevent the limitations of candidate sRNA and putative target approaches.
Ongoing emphasis on mechanistic studies: There should continue to be an emphasis on research that investigates the 5 major mechanistic motifs that would govern sRNA delivery, especially as they represent key determinants in the ability of different species to respond to dietary sRNA in an agricultural context.20 Investigations providing foundational mechanistic insight should continue to be encouraged in model plants and invertebrates. In addition, studies to mechanistically define the steps inhibiting dietary uptake in recalcitrant species can also be instructive. However, additional studies in more diverse invertebrates with oral RNAi potential and their dietary plants should also be undertaken, perhaps in parallel to agricultural biotechnology studies. Again using the corn rootworm as an example, recent studies in this organism have established it as a valuable model for elucidating the molecular mechanisms of dietary sRNA uptake and function.173-175 Parallel studies on mechanisms of sRNA packaging and stability of the dietary plants will also be paramount for generating a complete biological picture.
Figure 2.
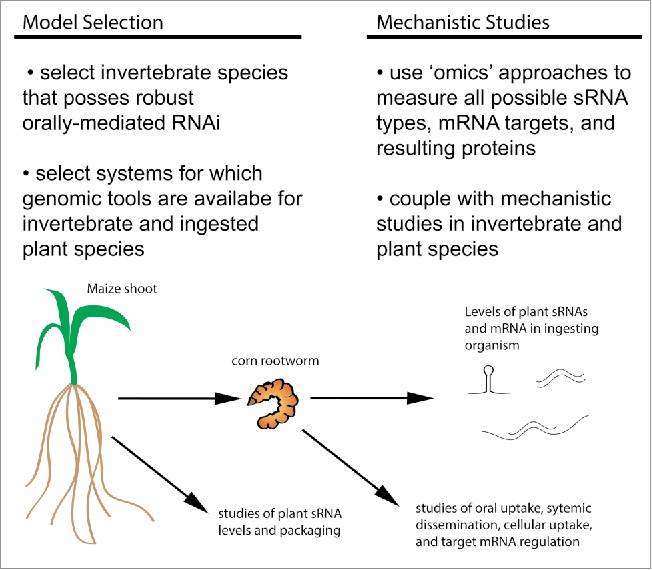
Future strategies for elucidating a biological role of natural dietary sRNA on ingesting invertebrates depicted using the corn rootworm. One) Selection of invertebrate and plant models and 2) Continued emphasis on mechanistic studies.
The number and diversity of invertebrates is staggering, with 1.3 million currently recognized species, a number that is likely to grow by a factor of 5 in the coming years with new species discovery.176 Based on the number of invertebrate species for which oral RNAi has already been shown to be successful, it seems highly likely that natural uptake of dietary uptake will occur in a manner that impacts the biology of the ingesting organisms in a substantial number of these. However, considerable work at the molecular, cellular, and organismal levels remains before any exploration of broader ecological questions can be initiated. In addition, while agricultural biotechnology should continue to have an important role in the field of natural uptake of dietary sRNA in invertebrates, independent studies of the natural uptake of diet-derived sRNA should be undertaken to provide unique viewpoints and knowledge.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
The authors acknowledge Jennifer Mansfield for helpful comments and critical review of the manuscript. The authors acknowledge the influence of many additional references that were not cited in this review due to length limitations and apologize for their omission.
Funding
This work was supported by the National Institutes of Health (HL096834, HL124021 to SYC) and the American Heart Association (14GRNT19600012 to SYC).
References
- 1.Carthew RW, Sontheimer EJ. Origins and mechanisms of miRNAs and siRNAs. Cell 2009; 136:642-55; PMID:19239886; http://dx.doi.org/ 10.1016/j.cell.2009.01.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bartel DP. MicroRNAs: Target recognition and regulatory functions. Cell 2009; 136:215-33; PMID:19167326; http://dx.doi.org/ 10.1016/j.cell.2009.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee RC, Feinbaum RL, Ambros V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell 1993; 75:843-54; PMID:8252621; http://dx.doi.org/ 10.1016/0092-8674(93)90529-Y [DOI] [PubMed] [Google Scholar]
- 4.Wightman B, Ha I, Ruvkun G. Posttranscriptional regulation of the heterochronic gene lin-14 by lin-4 mediates temporal pattern formation in C. elegans. Cell 1993; 75:855-62; PMID:8252622; http://dx.doi.org/ 10.1016/0092-8674(93)90530-4 [DOI] [PubMed] [Google Scholar]
- 5.Pasquinelli AE, Reinhart BJ, Slack F, Martindale MQ, Kuroda MI, Maller B, Hayward DC, Ball EE, Degnan B, Muller P, et al.. Conservation of the sequence and temporal expression of let-7 heterochronic regulatory RNA. Nature 2000; 408:86-9; PMID:11081512; http://dx.doi.org/ 10.1038/35040556 [DOI] [PubMed] [Google Scholar]
- 6.Llave C, Kasschau KD, Rector MA, Carrington JC. Endogenous and silencing-associated small RNAs in plants. Plant Cell 2002; 14:1605-19; PMID:12119378; http://dx.doi.org/ 10.1105/tpc.003210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fire A, Xu S, Montgomery MK, Kostas SA, Driver SE, Mello CC. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature 1998; 391:806-11; PMID:9486653; http://dx.doi.org/ 10.1038/35888 [DOI] [PubMed] [Google Scholar]
- 8.Hamilton AJ, Baulcombe DC. A species of small antisense RNA in posttranscriptional gene silencing in plants. Science 1999; 286:950-2; PMID:10542148; http://dx.doi.org/ 10.1126/science.286.5441.950 [DOI] [PubMed] [Google Scholar]
- 9.Elbashir SM, Harborth J, Lendeckel W, Yalcin A, Weber K, Tuschl T. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature 2001; 411:494-8; PMID:11373684; http://dx.doi.org/ 10.1038/35078107 [DOI] [PubMed] [Google Scholar]
- 10.Timmons L, Fire A. Specific interference by ingested dsRNA. Nature 1998; 395:854; PMID:9804418; http://dx.doi.org/ 10.1038/27579 [DOI] [PubMed] [Google Scholar]
- 11.Timmons L, Fire A. Ingestion of bacterially expressed dsRNAs can produce specific and potent genetic interference in Caenorhabditis elegans. Gene 2001; 263:103-12; PMID:11223248; http://dx.doi.org/ 10.1016/S0378-1119(00)00579-5 [DOI] [PubMed] [Google Scholar]
- 12.Urwin PE, Lilley CJ, Atkinson HJ. Ingestion of double-stranded RNA by preparasitic juvenile cyst nematodes leads to RNA interference. Mol Plant-Microbe 2002; 15(8):747-52; PMID:12182331; http://dx.doi.org/16756557 10.1094/MPMI.2002.15.8.747 [DOI] [PubMed] [Google Scholar]
- 13.Araujo RN, Santos A, Pinto FS, Gontijo NF, Lehane MJ, Pereira MH. RNA interference of the salivary gland nitrophorin 2 in the triatomine bug Rhodnius prolixus (Hemiptera: Reduviidae) by dsRNA ingestion or injection. Insect Biochem Molec 2006; 36:683-93; PMID:16935217; http://dx.doi.org/16756557 10.1016/j.ibmb.2006.05.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Turner CT, Davy MW, MacDiarmid RM, Plummer KM, Birch NP, Newcomb RD. RNA interference in the light brown apple moth, Epiphyas postvittana (Walker) induced by double-stranded RNA feeding. Insect Mol Biol 2006; 15:383-91; PMID:16756557; http://dx.doi.org/ 10.1111/j.1365-2583.2006.00656.x [DOI] [PubMed] [Google Scholar]
- 15.Huang G, Allen R, Davis EL, Baum TJ, Hussey RS. Engineering broad root-knot resistance in transgenic plants by RNAi silencing of a conserved and essential root-knot nematode parasitism gene. Proc Natl Acad Sci USA 2006; 103:14302-6; PMID:16985000; http://dx.doi.org/ 10.1073/pnas.0604698103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yadav BC, Veluthambi K, Subramaniam K. Host-generated double stranded RNA induces RNAi in plant-parasitic nematodes and protects the host from infection. Mol Biochem Parasitol 2006; 148:219-22; PMID:16678282; http://dx.doi.org/ 10.1016/j.molbiopara.2006.03.013 [DOI] [PubMed] [Google Scholar]
- 17.Baum JA, Bogaert T, Clinton W, Heck GR, Feldmann P, Ilagan O, Johnson S, Plaetinck G, Munyikwa T, Pleau M, et al.. Control of coleopteran insect pests through RNA interference. Nat Biotechnol 2007; 25:1322-6; PMID:17982443; http://dx.doi.org/ 10.1038/nbt1359 [DOI] [PubMed] [Google Scholar]
- 18.Mao Y-B, Cai W-J, Wang J-W, Hong G-J, Tao X-Y, Wang L-J, Huang Y-P, Chen XY. Silencing a cotton bollworm P450 monooxygenase gene by plant-mediated RNAi impairs larval tolerance of gossypol. Nat Biotechnol 2007; 25:1307-13; PMID:17982444; http://dx.doi.org/ 10.1038/nbt1352 [DOI] [PubMed] [Google Scholar]
- 19.Tian H, Peng H, Yao Q, Chen H, Xie Q, Tang B, Zhang W. Developmental control of a Lepidopteran pest spodoptera exigua by ingestion of bacteria expressing dsRNA of a non-midgut gene. PLoS One 2009; 4:e6225; PMID:19593438; http://dx.doi.org/ 10.1371/journal.pone.0006225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baum JA, Roberts JK. Progress towards RNAi-mediated insect pest management. Insect Midgut and Insecticidal Proteins 2014; http://dx.doi.org/ 10.1016/B978-0-12-800197-4.00005-1 [DOI] [Google Scholar]
- 21.Dutta TK. The status of RNAi-based transgenic research in plant nematology. Front Microbiol 2015; 5:1-7; PMID:25653648; http://dx.doi.org/ 10.3389/fmicb.2014.00760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sagi A, Manor R, Ventura T. Gene silencing in crustaceans: from basic research to biotechnologies. Genes 2013; 4:620-45; PMID:24705266; http://dx.doi.org/ 10.3390/genes4040620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Terenius O, Papanicolaou A, Garbutt JS, Eleftherianos I, Huvenne H, Kanginakudru S, Albrechtsen M, An C, Aymeric J-L, Barthel A, et al.. Journal of Insect Physiology. J Insect Physiol 2011; 57:231-45; PMID:21078327; http://dx.doi.org/ 10.1016/j.jinsphys.2010.11.006 [DOI] [PubMed] [Google Scholar]
- 24.Zhang L, Hou D, Chen X, Li D, Zhu L, Zhang Y, Li J, Bian Z, Liang X, Cai X, et al.. Exogenous plant MIR168a specifically targets mammalian LDLRAP1: evidence of cross-kingdom regulation by microRNA. Cell Res 2011; 22:107-26; PMID:21931358; http://dx.doi.org/ 10.1038/cr.2011.158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang K, Li H, Yuan Y, Etheridge A, Zhou Y, Huang D, Wilmes P, Galas D. The complex exogenous RNA spectra in human plasma: An interface with human gut biota? PLoS One 2012; 7:e51009; PMID:23251414; http://dx.doi.org/22265093 10.1371/journal.pone.0051009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hirschi KD. New foods for thought. Trends Plant Sci 2012; 17:123-5; PMID:22265093; http://dx.doi.org/ 10.1016/j.tplants.2012.01.004 [DOI] [PubMed] [Google Scholar]
- 27.Witwer KW. XenomiRs and miRNA homeostasis in health and disease: evidence that diet and dietary miRNAs directly and indirectly influence circulating miRNA profiles. RNA Biol 2012; 9:1147-54; PMID:22951590; http://dx.doi.org/ 10.4161/rna.21619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sarkies P, Miska EA. Molecular biology. Is there social RNA? Science 2013; 341:467-8; PMID:23908213; http://dx.doi.org/ 10.1126/science.1243175 [DOI] [PubMed] [Google Scholar]
- 29.Zhang Y, Wiggins BE, Lawrence C, Petrick J, Ivashuta S, Heck G. Analysis of plant-derived miRNAs in animal small RNA datasets. BMC Genomics 2012; 13:1-1; PMID:22214261; http://dx.doi.org/ 10.1186/1471-2164-13-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Snow JW, Hale AE, Isaacs SK, Baggish AL, Chan SY. Ineffective delivery of diet-derived microRNAs to recipient animal organisms. rnabiology 2013; 10:1107-16; PMID:23669076; http://dx.doi.org/23770773 10.4161/rna.24909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Witwer KW, McAlexander MA, Queen SE, Adams RJ. Real-time quantitative PCR and droplet digital PCR for plant miRNAs in mammalian blood provide little evidence for general uptake of dietary miRNAs: Limited evidence for general uptake of dietary plant xenomiRs. RNA Biol 2013; 10:1080-6; PMID:23770773; http://dx.doi.org/ 10.4161/rna.25246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dickinson B, Zhang Y, Petrick JS, Heck G, Ivashuta S, Marshall WS. correspondence. Nat Biotechnol 2013; 31:965-7; PMID:24213763; http://dx.doi.org/ 10.1038/nbt.2737 [DOI] [PubMed] [Google Scholar]
- 33.Hirschi KD, Pruss GJ, Vance V. Dietary delivery: a new avenue for microRNA therapeutics? Trends Biotechnol 2015; 33:431-2; PMID:26113189; http://dx.doi.org/ 10.1016/j.tibtech.2015.06.003 [DOI] [PubMed] [Google Scholar]
- 34.Witwer KW. Hypothetical plant-mammal small RNA Communicationcommunication: Packaging and Stoichiometry. In: Non-coding RNAs Inter-Kingdom Communication Cham: Springer International Publishing; 2016; 161-76; http://dx.doi.org/ 10.1007/978-3-319-39496-1_10 [DOI] [Google Scholar]
- 35.Liu H, Wang X, Wang H-D, Wu J, Ren J, Meng L, Wu Q, Dong H, Wu J, Kao T-Y, et al.. Escherichia coli noncoding RNAs can affect gene expression and physiology of Caenorhabditis elegans. Nat Communications 2012; 3:1073-11; PMID:23011127; http://dx.doi.org/ 10.1038/ncomms2071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Akay A, Sarkies P, Miska EA. E. coli OxyS non-coding RNA does not trigger RNAi in C. elegans. Sci Rep 2015; 5:9597; PMID:25873159; http://dx.doi.org/ 10.1038/srep09597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ivashuta S, Zhang Y, Wiggins BE, Ramaseshadri P, Segers GC, Johnson S, Meyer SE, Kerstetter RA, McNulty BC, Bolognesi R, et al.. Environmental RNAi in herbivorous insects. Rna 2015; 21:840-50; PMID:25802407; http://dx.doi.org/ 10.1261/rna.048116.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Masood M, Everett CP, Chan SY, Snow JW. Negligible uptake and transfer of diet-derived pollen microRNAs in adult honey bees. rnabiology 2016; 13:109-18; PMID:26680555; http://dx.doi.org/19609263 10.1080/15476286.2015.1128063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brown BD, Naldini L. Exploiting and antagonizing microRNA regulation for therapeutic and experimental applications. Nat Rev Genet 2009; 10:578-85; PMID:19609263; http://dx.doi.org/ 10.1038/nrg2628 [DOI] [PubMed] [Google Scholar]
- 40.Mullokandov G, Baccarini A, Ruzo A, Jayaprakash AD, Tung N, Israelow B, Evans MJ, Sachidanandam R, Brown BD. High-throughput assessment of microRNA activity and function using microRNA sensor and decoy libraries. Nat Methods 2012; 9:840-6; PMID:22751203; http://dx.doi.org/ 10.1038/nmeth.2078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wagner AE, Piegholdt S, Ferraro M, Pallauf K, Rimbach G. Food derived microRNAs. Food Funct 2015; 6:714-8; PMID:25644027; http://dx.doi.org/ 10.1039/C4FO01119H [DOI] [PubMed] [Google Scholar]
- 42.Borges F, Martienssen RA. The expanding world of small RNAsin plants. Nat Rev Mol Cell Biol 2015; 16:727-41; PMID:26530390; http://dx.doi.org/ 10.1038/nrm4085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pandey R, Joshi G, Bhardwaj AR, Agarwal M, Katiyar-Agarwal S. A comprehensive genome-wide study on tissue-specific and abiotic stress-specific miRNAs in triticum aestivum. PLoS One 2014; 9:e95800; PMID:24759739; http://dx.doi.org/23330790 10.1371/journal.pone.0095800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Axtell MJ. Classification and comparison of small RNAs from plants. Annu Rev Plant Biol 2013; 64:137-59; PMID:23330790; http://dx.doi.org/ 10.1146/annurev-arplant-050312-120043 [DOI] [PubMed] [Google Scholar]
- 45.Maino JL, Kearney MR. Ontogenetic and interspecific scaling of consumption in insects. Oikos 2015; 124:1564-70; PMID:25438170; http://dx.doi.org/17183358 10.1111/oik.02341 [DOI] [Google Scholar]
- 46.Waqas A, Shan G. Uptake and Reaction of C. elegans to Environmental RNAs. Cham: Springer International Publishing; 2016. pages 117-24; http://dx.doi.org/ 10.1007/978-3-319-39496-1_7 [DOI] [Google Scholar]
- 47.Christiaens O, Smagghe G. The challenge of RNAi-mediated control of hemipterans. Current Opinion in Insect Science 2014; 6:15-21; http://dx.doi.org/ 10.1016/j.cois.2014.09.012 [DOI] [PubMed] [Google Scholar]
- 48.Rana TM. Illuminating the silence: understanding the structure and function of small RNAs. Nat Rev Mol Cell Biol 2007; 8:23-36; PMID:17183358; http://dx.doi.org/ 10.1038/nrm2085 [DOI] [PubMed] [Google Scholar]
- 49.Jung M, Schaefer A, Steiner I, Kempkensteffen C, Stephan C, Erbersdobler A, Jung K. Robust MicroRNA stability in degraded RNA preparations from human tissue and cell samples. Clin Chem 2010; 56:998-1006; PMID:20378769; http://dx.doi.org/ 10.1373/clinchem.2009.141580 [DOI] [PubMed] [Google Scholar]
- 50.Allen ML, Walker WB III. Journal of insect physiology. J Insect Physiol 2012; 58:391-6; PMID:22226823; http://dx.doi.org/ 10.1016/j.jinsphys.2011.12.014 [DOI] [PubMed] [Google Scholar]
- 51.Christiaens O, Swevers L, Smagghe G. DsRNA degradation in the pea aphid (Acyrthosiphon pisum) associated with lack of response in RNAi feeding and injection assay. Peptides 2014; 53:307-14; PMID:24394433; http://dx.doi.org/ 10.1016/j.peptides.2013.12.014 [DOI] [PubMed] [Google Scholar]
- 52.Liu J, Swevers L, Iatrou K, Huvenne H, Smagghe G. Journal of insect physiology. J Insect Physiol 2012; 58:1166-76; PMID:22709524; http://dx.doi.org/ 10.1016/j.jinsphys.2012.05.016 [DOI] [PubMed] [Google Scholar]
- 53.Wynant N, Santos D, Verdonck R, Spit J, Van Wielendaele P, Broeck JV. Identification, functional characterization and phylogenetic analysis of double stranded RNA degrading enzymes present in the gut of the desert locust, Schistocerca gregaria. Insect Biochem Molec 2014; 46:1-8; PMID:24418314; http://dx.doi.org/21314432 10.1016/j.ibmb.2013.12.008 [DOI] [PubMed] [Google Scholar]
- 54.Wang K, Peng Y, Pu J, Fu W, Wang J, Han Z. Variation in RNAi efficacy among insect species is attributable to dsRNA degradation in vivo. Insect Biochem Molec 2016; 77:1-9; PMID:27449967; http://dx.doi.org/21314432 10.1016/j.ibmb.2016.07.007 [DOI] [PubMed] [Google Scholar]
- 55.Karasov WH, Martínez del Rio C, Caviedes-Vidal E. Ecological physiology of diet and digestive systems. Annu Rev Physiol 2011; 73:69-93; PMID:21314432; http://dx.doi.org/ 10.1146/annurev-physiol-012110-142152 [DOI] [PubMed] [Google Scholar]
- 56.Linser PJ, Dinglasan RR. Insect Gut Structure, Function, Development and Target of Biological Toxins. In: Insect Midgut and Insecticidal Proteins. Cambridge; 2014; 1-37; http://dx.doi.org/ 10.1016/B978-0-12-800197-4.00001-4 [DOI] [Google Scholar]
- 57.Pyott DE, Molnar A. Going mobile: Non-cell-autonomous small RNAs shape the genetic landscape of plants. Plant Biotechnol J 2015; 13:306-18; PMID:25756494; http://dx.doi.org/ 10.1111/pbi.12353 [DOI] [PubMed] [Google Scholar]
- 58.Fritz JV, Heintz-Buschart A, Ghosal A, Wampach L, Etheridge A, Galas D, Wilmes P. Sources and functions of extracellular small RNAs in human circulation. Annu Rev Nutr 2016; 36:301-36; PMID:27215587; http://dx.doi.org/ 10.1146/annurev-nutr-071715-050711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Colombo M, Raposo G, Théry C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol 2014; 30:255-89; PMID:25288114; http://dx.doi.org/ 10.1146/annurev-cellbio-101512-122326 [DOI] [PubMed] [Google Scholar]
- 60.Zhang M, Viennois E, Prasad M, Zhang Y, Wang L, Zhang Z, Han MK, Xiao B, Xu C, Srinivasan S, et al.. Biomaterials. Biomaterials 2016; 101:321-40; PMID:27318094; http://dx.doi.org/ 10.1016/j.biomaterials.2016.06.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yoo B-C, Kragler F, Varkonyi-Gasic E, Haywood V, Archer-Evans S, Lee YM, Lough TJ, Lucas WJ. A systemic small RNA signaling system in plants. Plant Cell 2004; 16:1979-2000; PMID:15258266; http://dx.doi.org/ 10.1105/tpc.104.023614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pauletti GM, Gangwar S, Knipp GT, Nerurkar MM, Okumu FW, Tamura K, Siahaan TJ, Borchardt RT. Structural requirements for intestinal absorption of peptide drugs. J Controlled Release 1996; 41:3-17; http://dx.doi.org/ 10.1016/0168-3659(96)01352-1 [DOI] [Google Scholar]
- 63.Kim YK, Heo I, Kim VN. Modifications of small RNAs and their associated proteins. Cell 2010; 143:703-9; PMID:21111232; http://dx.doi.org/ 10.1016/j.cell.2010.11.018 [DOI] [PubMed] [Google Scholar]
- 64.Philip A, Ferro VA, Tate RJ. Determination of the potential bioavailability of plant microRNAs using a simulated human digestion process. Mol Nutr Food Res 2015; 59(10):1962-72; PMID:26147655; http://dx.doi.org/ 10.1002/mnfr.201500137 [DOI] [PubMed] [Google Scholar]
- 65.Yang J, Farmer LM, Agyekum AAA, Elbaz-Younes I, Hirschi KD. Detection of an abundant Plant-Based small RNA in healthy consumers. PLoS One 2015; 10:e0137516; PMID:26335106; http://dx.doi.org/ 10.1371/journal.pone.0137516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yang J, Hotz T, Broadnax L, Yarmarkovich M, Elbaz-Younes I, Hirschi KD. Anomalous uptake and circulatorycharacteristics of the plant-basedsmall RNA MIR2911. Sci Rep 2016:1-9; PMID:27251858; http://dx.doi.org/20548333 10.1038/srep26834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Whyard S, Singh AD, Wong S. Ingested double-stranded RNAs can act as species-specific insecticides. Insect Biochem Molec 2009; 39:824-32; PMID:19815067; http://dx.doi.org/20548333 10.1016/j.ibmb.2009.09.007 [DOI] [PubMed] [Google Scholar]
- 68.Lin Y-H, Huang J-H, Liu Y, Bellés X, Lee H-J. Oral delivery of dsRNA lipoplexes to German cockroach protects dsRNA from degradation and induces RNAi response. Pest Manag Sci 2016; PMID:27470169; http://dx.doi.org/20548333 10.1002/ps.4407 [DOI] [PubMed] [Google Scholar]
- 69.Chen X, Gao C, Li H, Huang L, Sun Q, Dong Y, Tian C, Gao S, Dong H, Guan D, et al.. Identification and characterization of microRNAs in raw milk during different periods of lactation, commercial fluid, and powdered milk products. Cell Res 2010; 20:1128-37; PMID:20548333; http://dx.doi.org/ 10.1038/cr.2010.80 [DOI] [PubMed] [Google Scholar]
- 70.Kosaka N, Izumi H, Sekine K, Ochiya T. microRNA as a new immune-regulatory agent in breast milk. Silence 2010; 1:7; PMID:20226005; http://dx.doi.org/ 10.1186/1758-907X-1-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Guo X, Su S, Skogerboe G, Dai S, Li W, Li Z, Liu F, Ni R, Guo Y, Chen S, et al.. Recipe for a busy bee: microRNAs in Honey Bee caste determination. PLoS One 2013; 8:e81661; PMID:24349106; http://dx.doi.org/ 10.1371/journal.pone.0081661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shi YY, Wu XB, Huang ZY, Wang ZL, Yan WY, Zeng ZJ. Epigenetic modification of gene expression in honey bees by heterospecific gland secretions. PLoS One 2012; 7:e43727; PMID:22928024; http://dx.doi.org/ 10.1371/journal.pone.0043727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zernecke A, Bidzhekov K, Noels H, Shagdarsuren E, Gan L, Denecke B, Hristov M, Köppel T, Jahantigh MN, Lutgens E, et al.. Delivery of microRNA-126 by apoptotic bodies induces CXCL12-dependent vascular protection. Sci Signal 2009; 2:ra81; PMID:19996457; http://dx.doi.org/ 10.1126/scisignal.2000610 [DOI] [PubMed] [Google Scholar]
- 74.Sekirov I, Russell SL, Antunes LCM, Finlay BB. Gut microbiota in health and disease. Physiol Rev 2010; 90:859-904; PMID:20664075; http://dx.doi.org/ 10.1152/physrev.00045.2009 [DOI] [PubMed] [Google Scholar]
- 75.Engel P, Moran NA. The gut microbiota of insects – diversity in structure and function. FEMS Microbiol Rev 2013; 37:699-735; PMID:23692388; http://dx.doi.org/ 10.1111/1574-6976.12025 [DOI] [PubMed] [Google Scholar]
- 76.Peterson LW, Artis D. Intestinal epithelial cells: regulators of barrier function and immune homeostasis. Nat Rev Immunol 2014; 14:141-53; PMID:24566914; http://dx.doi.org/ 10.1038/nri3608 [DOI] [PubMed] [Google Scholar]
- 77.Lemaitre B, Miguel-Aliaga I. The digestive tract of Drosophila melanogaster. Annu Rev Genet 2013; 47:377-404; PMID:24016187; http://dx.doi.org/ 10.1146/annurev-genet-111212-133343 [DOI] [PubMed] [Google Scholar]
- 78.Zhang J, Khan SA, Hasse C, Ruf S, Heckel DG, Bock R. Pest control. Full crop protection from an insect pest by expression of long double-stranded RNAs in plastids. Science 2015; 347:991-4; PMID:25722411; http://dx.doi.org/ 10.1126/science.1261680 [DOI] [PubMed] [Google Scholar]
- 79.Wynant N, Santos D, Vanden Broeck J. Biological mechanisms determining the success of RNA interference in insects. Int Rev Cell Mol Biol 2014; 312:139-67; PMID:25262241; http://dx.doi.org/ 10.1016/B978-0-12-800178-3.00005-1 [DOI] [PubMed] [Google Scholar]
- 80.Huang J-H, Jing X, & Douglas AE. The multi-tasking gut epithelium of insects. Insect Biochem Mol 2015; 67:15-20; PMID:25982023; http://dx.doi.org/26138457 10.1016/j.ibmb.2015.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jose AM. Movement of regulatory RNA between animal cells. Genesis 2015; 53:395-416; PMID:26138457; http://dx.doi.org/ 10.1002/dvg.22871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kamath RS, Fraser AG, Dong Y, Poulin G, Durbin R. Systematic functional analysis of the Caenorhabditis elegans genome using RNAi. Nature 2003; 421(6920):231-7; PMID:12529635; http://dx.doi.org/17563372 10.1038/nature01278 [DOI] [PubMed] [Google Scholar]
- 83.Winston WM, Sutherlin M, Wright AJ, Feinberg EH, Hunter CP. Caenorhabditis elegans SID-2 is required for environmental RNA interference. Proc Natl Acad Sci USA 2007; 104:10565-70; PMID:17563372; http://dx.doi.org/ 10.1073/pnas.0611282104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.McEwan DL, Weisman AS, Hunter CP. Uptake of Extracellular Double-Stranded RNA by SID-2. Mol Cell 2012; 47:746-54; PMID:22902558; http://dx.doi.org/ 10.1016/j.molcel.2012.07.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hinas A, Wright AJ, Hunter CP. SID-5 Is an Endosome-Associated Protein Required for Efficient Systemic RNAi in C. elegans. Current Biol 2012; 22:1938-43; PMID:22981770; http://dx.doi.org/18201385 10.1016/j.cub.2012.08.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Aronstein K, Pankiw T, Saldivar E. SID-I is implicated in systemic gene silencing in the honey bee. J Apicultural Res 2006; 45:20-4; http://dx.doi.org/ 10.1080/00218839.2006.11101307 [DOI] [Google Scholar]
- 87.Tomoyasu Y, Miller SC, Tomita S, Schoppmeier M, Grossmann D, Bucher G. Exploring systemic RNA interference in insects: a genome-wide survey for RNAi genes in Tribolium. Genome Biol 2008; 9:R10; PMID:18201385; http://dx.doi.org/ 10.1186/gb-2008-9-1-r10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Li X, Dong X, Zou C, Zhang H. Endocytic pathway mediates refractoriness of insect Bactrocera dorsalis to RNA interference. Sci Rep 2015; 5:8700; PMID:25731667; http://dx.doi.org/ 10.1038/srep08700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cappelle K, de Oliveira CFR, Van Eynde B, Christiaens O, Smagghe G. The involvement of clathrin-mediated endocytosis and two Sid-1-like transmembrane proteins in double-stranded RNA uptake in the Colorado potato beetle midgut. Insect Mol Biol 2016; 25:315-23; PMID:26959524; http://dx.doi.org/ 10.1111/imb.12222 [DOI] [PubMed] [Google Scholar]
- 90.Buchon N, Osman D, David FPA, Fang HY, Boquete J-P, Deplancke B, Lemaitre B. Morphological and molecular characterizationof adult midgut compartmentalization in drosophila. CellReports 2013; 3:1725-38; PMID:23643535; http://dx.doi.org/19837076 10.1016/j.celrep.2013.04.001 [DOI] [PubMed] [Google Scholar]
- 91.Huvenne H, Smagghe G. Mechanisms of dsRNA uptake in insects and potential of RNAi for pest control: a review. J Insect Physiol 2010; 56:227-35; PMID:19837076; http://dx.doi.org/ 10.1016/j.jinsphys.2009.10.004 [DOI] [PubMed] [Google Scholar]
- 92.Rera M, Clark RI, Walker DW. Intestinal barrier dysfunction links metabolic and inflammatory markers of aging to death in Drosophila. Proc Natl Acad Sci USA 2012; 109:21528-33; PMID:23236133; http://dx.doi.org/ 10.1073/pnas.1215849110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Fishilevich E, Vélez AM, Storer NP, Li H, Bowling AJ, Rangasamy M, Worden SE, Narva KE, Siegfried BD. RNAi as a management tool for the western corn rootworm, Diabrotica virgifera virgifera. Pest Manag Sci 2016; 72:1652-63; PMID:27218412; http://dx.doi.org/ 10.1002/ps.4324 [DOI] [PubMed] [Google Scholar]
- 94.Mao YB, Xue XY, Tao XY, Yang CQ, Wang LJ, Chen XY. Cysteine protease enhances plant-mediated bollworm RNA interference. Plant Mol Biol 2013; 83:119-29; PMID:23460027; http://dx.doi.org/ 10.1007/s11103-013-0030-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wolf T, Baier SR, Zempleni J. The intestinal transport of bovine milk exosomes is mediated by endocytosis in human colon carcinoma caco-2 cells and rat small intestinal IEC-6 cells. J Nutr 2015; 145(10):2201; PMID:26269243; http://dx.doi.org/ 10.3945/jn.115.218586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yang J, Farmer LM, Agyekum AAA, Hirschi KD. Detection of dietary plant-based small RNAs in animals. Cell Res 2015; 25:517-20; PMID:25721324; http://dx.doi.org/ 10.1038/cr.2015.26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Salama NN, Eddington ND, Fasano A. Tight junction modulation and its relationship to drug delivery. Adv Drug Delivery Rev 2006; 58:15-28; PMID:16517003; http://dx.doi.org/ 10.1016/j.addr.2006.01.003 [DOI] [PubMed] [Google Scholar]
- 98.Jayachandran B, Hussain M, Asgari S. An insect trypsin-like serine protease as a target of microRNA: utilization of microRNA mimics and inhibitors by oral feeding. Insect Biochem Mol 2013; 43:398-406; PMID:23108205; http://dx.doi.org/22453516 10.1016/j.ibmb.2012.10.004 [DOI] [PubMed] [Google Scholar]
- 99.Banse SA, Hunter CP. Vampiric isolation of extracellular fluid from caenorhabditis elegans. JoVE 2012; PMID:22453516; http://dx.doi.org/ 10.3791/3647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Iftikhar H, Carney GE. Evidence and potential in vivo functions for biofluid miRNAs: From expression profiling to functional testing. Bioessays 2016; 38:367-78; PMID:26934338; http://dx.doi.org/ 10.1002/bies.201500130 [DOI] [PubMed] [Google Scholar]
- 101.Saleh M, van Rij R, Hekele A, Gillis A, Foley E, O'Farrell P, Andino R. The endocytic pathway mediates cell entry of dsRNA to induce RNAi silencing. Nat Cell Biol 2006; 8:793; PMID:16862146; http://dx.doi.org/ 10.1038/ncb1439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ulvila J, Parikka M, Kleino A, Sormunen R, Ezekowitz RA, Kocks C, Rämet M. Double-stranded RNA is internalized by scavenger receptor-mediated endocytosis in Drosophila S2 cells. J Biol Chem 2006; 281:14370-5; PMID:16531407; http://dx.doi.org/ 10.1074/jbc.M513868200 [DOI] [PubMed] [Google Scholar]
- 103.Karlikow M, Goic B, Mongelli V, Salles A, Schmitt C, Bonne I, Zurzolo C, Saleh MC Drosophila cells use nanotube-like structures to transfer dsRNA and RNAi machinery between cells. Sci. Rep. 6, 27085(2016); 2016:1-9; PMID:27255932; http://dx.doi.org/25027649 10.1038/srep27085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ha M, Kim VN. Regulation of microRNA biogenesis. Nat Rev Mol Cell Biol 2014; 15:509-24; PMID:25027649; http://dx.doi.org/ 10.1038/nrm3838 [DOI] [PubMed] [Google Scholar]
- 105.Valadi H, Ekström K, Bossios A, Sjöstrand M, Lee JJ, Lötvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol 2007; 9:654-9; PMID:17486113; http://dx.doi.org/ 10.1038/ncb1596 [DOI] [PubMed] [Google Scholar]
- 106.Zhang Y, Liu D, Chen X, Li J, Li L, Bian Z, Sun F, Lu J, Yin Y, Cai X, et al.. Secreted Monocytic miR-150 enhances targeted endothelial cell migration. Mol Cell 2010; 39:133-44; PMID:20603081; http://dx.doi.org/ 10.1016/j.molcel.2010.06.010 [DOI] [PubMed] [Google Scholar]
- 107.Kosaka N, Iguchi H, Yoshioka Y, Takeshita F, Matsuki Y, Ochiya T. Secretory mechanisms and intercellular transfer of microRNAs in living cells. J Biol Chem 2010; 285:17442; PMID:20353945; http://dx.doi.org/ 10.1074/jbc.M110.107821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Pegtel DM, Cosmopoulos K, Thorley-Lawson DA, van Eijndhoven MAJ, Hopmans ES, Lindenberg JL, de Gruijl TD, Wurdinger T, Middeldorp JM. Functional delivery of viral miRNAs via exosomes. Proc Natl Acad Sci USA 2010; 107:6328-33; PMID:20304794; http://dx.doi.org/ 10.1073/pnas.0914843107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mittelbrunn MIA, zquez CGER VA, Villarroya-Beltri C, lez SGA, nchez-Cabo FATSA, lez MANGA, Bernad A, nchez-Madrid FSA. Unidirectional transfer of microRnA-loadedexosomes from T cells to antigen-presenting cells. Nat Communications 2011; 2:282-10; PMID:21505438; http://dx.doi.org/ 10.1038/ncomms1285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hergenreider E, Heydt S, Tréguer K, Boettger T, Horrevoets AJG, Zeiher AM, Scheffer MP, Frangakis AS, Yin X, Mayr M, et al.. Atheroprotective communication between endothelial cells and smooth muscle cells through miRNAs. Nat Cell Biol 2012; 14:249-56; PMID:22327366; http://dx.doi.org/ 10.1038/ncb2441 [DOI] [PubMed] [Google Scholar]
- 111.Zhuang G, Wu X, Jiang Z, Kasman I, Yao J, Guan Y, Oeh J, Modrusan Z, Bais C, Sampath D, et al.. Tumour-secreted miR-9 promotes endothelial cell migration and angiogenesis by activating the JAK-STAT pathway. EMBO J 2012; 31:3513-23; PMID:22773185; http://dx.doi.org/ 10.1038/emboj.2012.183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Montecalvo A, Larregina AT, Shufesky WJ, Stolz DB, Sullivan MLG, Karlsson JM, Baty CJ, Gibson GA, Erdos G, Wang Z, et al.. Mechanism of transfer of functional microRNAs between mouse dendritic cells via exosomes. Blood 2012; 119:756-66; PMID:22031862; http://dx.doi.org/ 10.1182/blood-2011-02-338004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Janas T, Janas MM, Sapoń K, Janas T. Mechanisms of RNA loading into exosomes. FEBS Lett 2015; 589:1391-8; PMID:25937124; http://dx.doi.org/ 10.1016/j.febslet.2015.04.036 [DOI] [PubMed] [Google Scholar]
- 114.Squadrito ML, Baer C, Burdet F, Maderna C, Gilfillan GD, Lyle R, Ibberson M, De Palma M. Endogenous RNAs modulate MicroRNA sorting to exosomes and transfer to acceptor cells. CellReports 2014; 8:1432-46; PMID:25159140; http://dx.doi.org/19684575 10.1016/j.celrep.2014.07.035 [DOI] [PubMed] [Google Scholar]
- 115.Koppers-Lalic D, Hackenberg M, Bijnsdorp IV, van Eijndhoven MAJ, Sadek P, Sie D, Zini N, Middeldorp JM, Ylstra B, de Menezes RX, et al.. Nontemplated nucleotide additions distinguish the small RNA composition in cells from exosomes. CellReports 2014; 8:1649-58 [DOI] [PubMed] [Google Scholar]
- 116.Villarroya-Beltri, C., Gutierrez-Vazquez C., Sanchez-Cabo F., Perez-Hernandez D., Vazquez J. et al. Sumoylated hnRNPA2B1 controls the sorting of miRNAs into exosomes through binding to specific motifs. Nature Communications 2013; 4:2980 PMID:24356509; http://dx.doi.org/19684575 10.1038/ncomms3980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Gibbings DJ, Ciaudo C, Erhardt M, Voinnet O. Multivesicular bodies associate with components of miRNA effector complexes and modulate miRNA activity. Nat Cell Biol 2009; 11:1143-9; PMID:19684575; http://dx.doi.org/ 10.1038/ncb1929 [DOI] [PubMed] [Google Scholar]
- 118.Guduric-Fuchs J, O'Connor A, Camp B, O'Neill CL, Medina RJ, Simpson DA. Selective extracellular vesicle-mediated export of an overlapping set of microRNAs from multiple cell types. BMC Genomics 2012; 13:357; PMID:22849433; http://dx.doi.org/ 10.1186/1471-2164-13-357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Yao B, La LB, Chen YC, Chang LJ, Chan EKL. scientific report. EMBO Rep 2012; 13:1102-8; PMID:23090477; http://dx.doi.org/ 10.1038/embor.2012.160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Arroyo JD, Chevillet JR, Kroh EM. Argonaute2 complexes carry a population of circulating microRNAs independent of vesicles in human plasma. Proc Natl Acad Sci U S A 2011; 108(12):5003-8; PMID:21383194; http://dx.doi.org/21609964 10.1073/pnas.1019055108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Turchinovich A, Weiz L, Langheinz A, Burwinkel B. Characterization of extracellular circulating microRNA. Nucleic Acids Res 2011; 39:7223-33; PMID:21609964; http://dx.doi.org/ 10.1093/nar/gkr254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Turchinovich A, Burwinkel B. Distinct AGO1 and AGO2 associated miRNA profiles in human cells and blood plasma. rnabiology 2012; 9:1066-75; PMID:22858679; http://dx.doi.org/21423178 10.4161/rna.21083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Vickers KC, Palmisano BT, Shoucri BM, Shamburek RD, Remaley AT. MicroRNAs are transported in plasma and delivered to recipient cells by high-density lipoproteins. Nat Cell Biol 2011; 13:423-33; PMID:21423178; http://dx.doi.org/ 10.1038/ncb2210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wagner J, Riwanto M, Besler C, Knau A, Fichtlscherer S, Röxe T, Zeiher AM, Landmesser U, Dimmeler S. Characterization of levels and cellular transfer of circulating lipoprotein-bound microRNAs. Arteriosclerosis Thrombosis Vascular Biol 2013; 33:1392-400; PMID:23559634; http://dx.doi.org/ 10.1161/ATVBAHA.112.300741 [DOI] [PubMed] [Google Scholar]
- 125.Mukherjee N, Corcoran DL, Nusbaum JD, Reid DW, Georgiev S, Hafner M, Ascano M Jr, Tuschl T, Ohler U, Keene JD. Integrative Regulatory Mapping Indicates that the RNA-Binding Protein HuR Couples Pre-mRNA Processing and mRNA Stability. Mol Cell 2011; 43:327-39; PMID:21723170; http://dx.doi.org/ 10.1016/j.molcel.2011.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Valiunas V, Polosina YY, Miller H, Potapova IA, Valiuniene L, Doronin S, Mathias RT, Robinson RB, Rosen MR, Cohen IS, et al.. Connexin-specific cell-to-cell transfer of short interfering RNA by gap junctions. J Physiol 2005; 568:459-68; PMID:16037090; http://dx.doi.org/ 10.1113/jphysiol.2005.090985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kizana E, Cingolani E, Marbán E. Non-cell-autonomous effects of vector-expressed regulatory RNAs in mammalian heart cells. Gene Ther 2009; 16:1163-8; PMID:19516277; http://dx.doi.org/ 10.1038/gt.2009.64 [DOI] [PubMed] [Google Scholar]
- 128.Mittelbrunn M, Sánchez-Madrid F. Intercellular communication: diverse structures for exchange of genetic information. Nat Rev Mol Cell Biol 2012; 13: 328-35; PMID:22510790; http://dx.doi.org/ 10.1038/nrm3335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Garbutt JS, Bellés X, Richards EH, Reynolds SE. Journal of Insect Physiology. J Insect Physiol 2013; 59:171-8; PMID:22664137; http://dx.doi.org/ 10.1016/j.jinsphys.2012.05.013 [DOI] [PubMed] [Google Scholar]
- 130.Shukla JN, Kalsi M, Sethi A, Narva KE, Fishilevich E, Singh S, Mogilicherla K, Palli SR. Reduced stability and intracellular transport of dsRNA contribute to poor RNAi response in lepidopteran insects. rnabiology 2016; 13:656-69; PMID:27245473; http://dx.doi.org/23435119 10.1080/15476286.2016.1191728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Goic B, Vodovar N, Mondotte JA, Monot C, Frangeul L, Blanc H, Gausson V, Vera-Otarola J, Cristofari G, Saleh MC. RNA-mediated interference and reverse transcription control the persistence of RNA viruses in the insect model Drosophila. Nat Immunol 2013; 14:396-403; PMID:23435119; http://dx.doi.org/ 10.1038/ni.2542 [DOI] [PubMed] [Google Scholar]
- 132.Feinberg EH, Hunter CP. Transport of dsRNA into cells by the transmembrane protein SID-1. Science 2003; 301:1545-7; PMID:12970568; http://dx.doi.org/ 10.1126/science.1087117 [DOI] [PubMed] [Google Scholar]
- 133.Shih JD, Hunter CP. SID-1 is a dsRNA-selective dsRNA-gated channel. Rna 2011; 17:1057-65; PMID:21474576; http://dx.doi.org/ 10.1261/rna.2596511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Shih JD, Fitzgerald MC, Sutherlin M, Hunter CP. The SID-1 double-stranded RNA transporter is not selective for dsRNA length. Rna 2009; 15:384-90; PMID:19155320; http://dx.doi.org/ 10.1261/rna.1286409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Duxbury MS, Ashley SW, Whang EE. RNA interference: A mammalian SID-1 homologue enhances siRNA uptake and gene silencing efficacy in human cells. Biochem Biophys Res Commun 2005; 331:459-63; PMID:15850781; http://dx.doi.org/ 10.1016/j.bbrc.2005.03.199 [DOI] [PubMed] [Google Scholar]
- 136.Elhassan MO, Christie J, Duxbury MS. Homo sapiens systemic RNA interference-defective-1 transmembrane family member 1 (SIDT1) protein mediates contact-dependent small RNA transfer and microRNA-21-driven chemoresistance. J Biol Chem 2012; 287:5267-77; PMID:22174421; http://dx.doi.org/ 10.1074/jbc.M111.318865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Luo Y, Wang X, Yu D, Kang L. The SID-1 double-stranded RNA transporter is not required for systemic RNAi in the migratory locust. rnabiology 2012; 9:663-71; PMID:22614832; http://dx.doi.org/22145043 10.4161/rna.19986 [DOI] [PubMed] [Google Scholar]
- 138.Aung KM, Boldbaatar D, Umemiya-Shirafuji R, Liao M, Xuenan X, Suzuki H, Linggatong Galay R, Tanaka T, Fujisaki K. Scavenger receptor mediates systemic RNA interference in ticks. PLoS One 2011; 6:e28407; PMID:22145043; http://dx.doi.org/ 10.1371/journal.pone.0028407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Wynant N, Santos D, Van Wielendaele P, Vanden Broeck J. Scavenger receptor-mediated endocytosis facilitates RNA interference in the desert locust, Schistocerca gregaria. Insect Mol Biol 2014; 23(3):320-9; PMID:24528536; http://dx.doi.org/ 10.1111/imb.12083 [DOI] [PubMed] [Google Scholar]
- 140.Ren D, Cai Z, Song J, Wu Z, Zhou S. dsRNA uptake and persistence account for tissue-dependent susceptibility to RNA interference in the migratory locust, Locusta migratoria. Insect Mol Biol 2013; 23:175-84; PMID:24308607; http://dx.doi.org/ 10.1111/imb.12074 [DOI] [PubMed] [Google Scholar]
- 141.Wolfrum C, Shi S, Jayaprakash KN, Jayaraman M, Wang G, Pandey RK, Rajeev KG, Nakayama T, Charrise K, Ndungo EM, et al.. Mechanisms and optimization of in vivo delivery of lipophilic siRNAs. Nat Biotechnol 2007; 25:1149-57; PMID:17873866; http://dx.doi.org/ 10.1038/nbt1339 [DOI] [PubMed] [Google Scholar]
- 142.Dominska M, Dykxhoorn DM. Breaking down the barriers: siRNA delivery and endosome escape. J Cell Sci 2010; 123:1183-9; PMID:20356929; http://dx.doi.org/ 10.1242/jcs.066399 [DOI] [PubMed] [Google Scholar]
- 143.Wang Y, Huang L. A window onto siRNA delivery. Nat Biotechnol 2013; 31:611-2; PMID:23839146; http://dx.doi.org/ 10.1038/nbt.2634 [DOI] [PubMed] [Google Scholar]
- 144.Gilleron J, Querbes W, Zeigerer A, Borodovsky A, Marsico G, Schubert U, Manygoats K, Seifert S, Andree C, ter MSO, et al.. image-based analysis of lipid nanoparticle–mediated sirNA delivery, intracellular trafficking and endosomal escape. Nat Biotechnol 2013; 31:638-46; PMID:23792630; http://dx.doi.org/ 10.1038/nbt.2612 [DOI] [PubMed] [Google Scholar]
- 145.Sahay G, Querbes W, Alabi C, Eltoukhy A, Sarkar S, Zurenko C, Karagiannis E, Love K, Chen D, Zoncu R, et al.. efficiency of sirNA delivery by lipid nanoparticles is limited by endocytic recycling. Nat Biotechnol 2013; 31:653-8; PMID:23792629; http://dx.doi.org/ 10.1038/nbt.2614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Lee YS, Pressman S, Andress AP, Kim K, White JL, Cassidy JJ, Li X, Lubell K, Lim DH, Cho IS, et al.. Silencing by small RNAs is linked to endosomal trafficking. Nat Cell Biol 2009; 11:1150-6; PMID:19684574; http://dx.doi.org/ 10.1038/ncb1930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Yan N, Chen ZJ. Intrinsic antiviral immunity. Nat Immunol 2012; 13:214-22; PMID:22344284; http://dx.doi.org/ 10.1038/ni.2229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Brutscher LM, Daughenbaugh KF, Flenniken ML. ScienceDirectAntiviral defense mechanisms in honey bees. Curr Opin Insect Sci 2015; 10:71-82; PMID:26273564; http://dx.doi.org/ 10.1016/j.cois.2015.04.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Gammon DB, Mello CC. RNA interference-mediated antiviral defense in insects. Curr Opin Insect Sci 2015; 8:111-20; PMID:26034705; http://dx.doi.org/ 10.1016/j.cois.2015.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Swevers L, Broeck JV, Smagghe G. The possible impact of persistent virus infection on the function of the RNAi machinery in insects: a hypothesis. Front Physiol 2013; 4:319; PMID:24204347; http://dx.doi.org/ 10.3389/fphys.2013.00319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Robalino J, Bartlett T, Chapman R, Gross P, Browdy C, Warr G. Double-stranded RNA and antiviral immunity in marine shrimp: inducible host mechanisms and evidence for the evolution of viral counter-responses. Dev Comp Immunol 2007; 31:539-47; PMID:17109960; http://dx.doi.org/ 10.1016/j.dci.2006.08.011 [DOI] [PubMed] [Google Scholar]
- 152.Flenniken ML, Andino R. Non-Specific dsRNA-Mediated antiviral response in the honey bee. PLoS One 2013; 8:e77263; PMID:24130869; http://dx.doi.org/ 10.1371/journal.pone.0077263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Nayak A, Berry B, Tassetto M, Kunitomi M, Acevedo A, Deng C, Krutchinsky A, Gross J, Antoniewski C, Andino R. Cricket paralysis virus antagonizes Argonaute 2 to modulate antiviral defense in Drosophila. Nat Structural Mol Biol 2010; 17(5):547-54; PMID:20400949; http://dx.doi.org/ 10.1038/nsmb.1810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Sharma A, Sahu S, Kumari P, Gopi SR, Malhotra R, Biswas S. Genome wide identification and functional annotation of miRNAs in anti-inflammatory plant and their cross kingdom regulation in Homo sapiens. J Biomol Structure Dynamics 2016:1-12; PMID:27183869; http://dx.doi.org/26123395 10.1080/07391102.2016.1185381 [DOI] [PubMed] [Google Scholar]
- 155.Pirro S, Minutolo A, Galgani A, Potestà M, Colizzi V, Montesano C. Bioinformatics prediction and experimental validation of MicroRNAs involved in cross-kingdom interaction. J Computational Biol 2016; PMID:27428722; http://dx.doi.org/26123395 10.1089/cmb.2016.0059 [DOI] [PubMed] [Google Scholar]
- 156.Weiberg A, Jin H. Small RNAs–the secret agents in the plant-pathogen interactions. Curr Opin Plant Biol 2015; 26:87-94; PMID:26123395; http://dx.doi.org/ 10.1016/j.pbi.2015.05.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Waser NM, Ollerton J, editors. Plant-pollinator interactions: From specialization to generalization. Chicago: University of Chicago Press 2006 [Google Scholar]
- 158.Kessler D, Gase K, Baldwin IT. Field experiments with transformed plants reveal the sense of floral scents. Science 2008; 321:1200-2; PMID:18755975; http://dx.doi.org/ 10.1126/science.1160072 [DOI] [PubMed] [Google Scholar]
- 159.Wright GA, Baker DD, Palmer MJ, Stabler D, Mustard JA, Power EF, Borland AM, Stevenson PC. Caffeine in floral nectar enhances a pollinator's memory of reward. Science 2013; 339:1202-4; PMID:23471406; http://dx.doi.org/ 10.1126/science.1228806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Dawkins R, Krebs JR. Arms races between and within species. Proc R Soc Lond B Biol Sci 1979; 205:489-511; PMID:42057; http://dx.doi.org/ 10.1098/rspb.1979.0081 [DOI] [PubMed] [Google Scholar]
- 161.Chaloner T, van Kan JAL, Grant-Downton RT. RNA “Information Warfare” in Pathogenic and Mutualistic interactions. Trends Plant Sci 2016; 21(738-748); PMID:26686410; http://dx.doi.org/ 10.1016/j.tplants.2016.05.008 [DOI] [PubMed] [Google Scholar]
- 162.Pare P, Tumlinson J. Plant volatiles as a defense against insect herbivores. Plant Physiol 1999; 121:325-32; PMID:10517823; http://dx.doi.org/ 10.1104/pp.121.2.325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Iason G. The role of plant secondary metabolites in mammalian herbivory: ecological perspectives. Proc Nutr Soc 2005; 64:123-31; PMID:15877931; http://dx.doi.org/ 10.1079/PNS2004415 [DOI] [PubMed] [Google Scholar]
- 164.Hollingsworth RG, Armstrong JW, Campbell E. Caffeine as a repellent for slugs and snails. Nature 2002; 417:915-6; PMID:12087394; http://dx.doi.org/ 10.1038/417915a [DOI] [PubMed] [Google Scholar]
- 165.de Roode JC, Lefèvre T, Hunter MD. Self-Medication in Animals. Science 2013; 340:150-1; PMID:23580516; http://dx.doi.org/ 10.1126/science.1235824 [DOI] [PubMed] [Google Scholar]
- 166.Huffman MA. Animal self-medication and ethno-medicine: exploration and exploitation of the medicinal properties of plants. Proc Nutr Soc 2003; 62:371-81; PMID:14506884; http://dx.doi.org/ 10.1079/PNS2003257 [DOI] [PubMed] [Google Scholar]
- 167.Anthony WE, Palmer-Young EC, Leonard AS, Irwin RE, Adler LS. Testing Dose-dependent effects of the nectar alkaloid anabasine on trypanosome parasite loads in adult bumble bees. PLoS One 2015; 10:e0142496; PMID:26545106; http://dx.doi.org/ 10.1371/journal.pone.0142496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Richardson LL, Bowers MD, Irwin RE. Nectar chemistry mediates the behavior of parasitized bees: consequences for plant fitness. Ecology 2016; 97:325-37; PMID:27145608; http://dx.doi.org/ 10.1890/15-0263.1 [DOI] [PubMed] [Google Scholar]
- 169.Koch A, Kogel KH. New wind in the sails: improving the agronomic value of crop plants through RNAi-mediated gene silencing. Plant Biotechnol J 2014; 12:821-31; PMID:25040343; http://dx.doi.org/ 10.1111/pbi.12226 [DOI] [PubMed] [Google Scholar]
- 170.Roberts AF, Devos Y, Lemgo GNY, Zhou X. Biosafety research for non-target organism risk assessment of RNAi-based GE plants. Front Plant Sci 2015; 6:958; PMID:26594220; http://dx.doi.org/ 10.3389/fpls.2015.00958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Sherman JH, Munyikwa T, Chan SY, Petrick JS, Witwer KW, Choudhuri S. Regulatory Toxicology and Pharmacology. Regulatory Toxicology and Pharmacology 2015; 73:671-80; PMID:26361858; http://dx.doi.org/ 10.1016/j.yrtph.2015.09.001 [DOI] [PubMed] [Google Scholar]
- 172.Nuez I, Félix MA. Evolution of susceptibility to ingested double-stranded RNAs in Caenorhabditis nematodes. PLoS One 2012; 7(1):e29811; PMID:22253787; http://dx.doi.org/23071820 10.1371/journal.pone.0029811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Bolognesi R, Ramaseshadri P, Anderson J, Bachman P, Clinton W, Flannagan R, Ilagan O, Lawrence C, Levine S, Moar W, et al.. Characterizing the Mechanism of Action of Double-Stranded RNA Activity against Western Corn Rootworm (Diabrotica virgifera virgifera LeConte). PLoS One 2012; 7:e47534; PMID:23071820; http://dx.doi.org/ 10.1371/journal.pone.0047534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Miyata K, Ramaseshadri P, Zhang Y, Segers G. Establishing an in vivo assay system to identify components involved in environmental RNA interference in the western corn rootworm. PLoS One 2014; 9(7):e101661; PMID:25003334; http://dx.doi.org/21886482 10.1371/journal.pone.0101661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Li H, Bowling AJ, Gandra P, Rangasamy M, Pence HE, McEwan R, Khajuria C, Siegfried B, Narva KE. Systemic RNAi in western corn rootworm, Diabrotica virgifera virgifera LeConte, does not involve transitive pathways. Insect Science 2016; PMID:27520841; http://dx.doi.org/21886482 10.1111/1744-7917.12382 [DOI] [PubMed] [Google Scholar]
- 176.May RM. Why worry about how many species and their loss? PLoS Biol 2011; 9:e1001130; PMID:21886482; http://dx.doi.org/ 10.1371/journal.pbio.1001130 [DOI] [PMC free article] [PubMed] [Google Scholar]


