EXO70C2, from the family of exocyst subunits, is a novel factor regulating pollen tube tip growth in Arabidopsis, and its paralog EXO70C1 has a partially redundant function.
Abstract
The exocyst, a eukaryotic tethering complex, coregulates targeted exocytosis as an effector of small GTPases in polarized cell growth. In land plants, several exocyst subunits are encoded by double or triple paralogs, culminating in tens of EXO70 paralogs. Out of 23 Arabidopsis thaliana EXO70 isoforms, we analyzed seven isoforms expressed in pollen. Genetic and microscopic analyses of single mutants in EXO70A2, EXO70C1, EXO70C2, EXO70F1, EXO70H3, EXO70H5, and EXO70H6 genes revealed that only a loss-of-function EXO70C2 allele resulted in a significant male-specific transmission defect (segregation 40%:51%:9%) due to aberrant pollen tube growth. Mutant pollen tubes grown in vitro exhibited an enhanced growth rate and a decreased thickness of the tip cell wall, causing tip bursts. However, exo70C2 pollen tubes could frequently recover and restart their speedy elongation, resulting in a repetitive stop-and-go growth dynamics. A pollen-specific depletion of the closest paralog, EXO70C1, using artificial microRNA in the exo70C2 mutant background, resulted in a complete pollen-specific transmission defect, suggesting redundant functions of EXO70C1 and EXO70C2. Both EXO70C1 and EXO70C2, GFP tagged and expressed under the control of their native promoters, localized in the cytoplasm of pollen grains, pollen tubes, and also root trichoblast cells. The expression of EXO70C2-GFP complemented the aberrant growth of exo70C2 pollen tubes. The absent EXO70C2 interactions with core exocyst subunits in the yeast two-hybrid assay, cytoplasmic localization, and genetic effect suggest an unconventional EXO70 function possibly as a regulator of exocytosis outside the exocyst complex. In conclusion, EXO70C2 is a novel factor contributing to the regulation of optimal tip growth of Arabidopsis pollen tubes.
Pollen tubes transporting sperm cells elongate via tip growth within the intercellular space of the transmitting tract in pistils to double fertilize ovules. Pollen tubes growing exclusively at their tips can achieve a rapid growth rate that is dependent on polarized exocytosis (McKenna et al., 2009; Chebli et al., 2013). Once the polarity of a nascent germinating pollen tube is established, the endomembrane secretory machinery supported by the actin cytoskeleton and the tip-focused calcium gradient delivers cell wall and plasma membrane (PM) materials along with cell wall modification enzymes to the growing apex (for review, see Cole and Fowler, 2006; Cheung and Wu, 2008; Hepler et al., 2013; Fu, 2015). Any perturbation in tip growth reduces the chance for an affected pollen tube to fertilize an ovule compared with more fit ones (MacAlister et al., 2016).
At the pollen tube apex, we recognize the clear zone characterized by a network of highly dynamic short F-actin filaments and intensive trafficking of both exocytic and endocytic vesicles that form the inverted cone (for review, see Hepler and Winship, 2015). The exocytic domain is located at the very tip or close to it (Sanati Nezhad et al., 2014; Bloch et al., 2016), then is followed with some partial overlap by the endocytic domain, which helps to remove the excess of membrane added by intensive exocytosis (Zonia and Munnik, 2008, 2009; Moscatelli and Idilli, 2009). Therefore, the balance between polarized exocytosis and endocytic recycling is a critical factor for proper tip growth. In growing pollen tubes, the cell wall composition changes in a gradient from mostly pectinaceous wall at the apex, followed by a cellulose layer generated later, and finally callose (Chebli et al., 2012).
Cell wall components or PM enzymes for their production are delivered to the cell surface in secretory vesicles (for review, see Bashline et al., 2014). Tethering and docking of secretory vesicles to the PM at sites of intensive secretion is mediated by the exocyst, a tethering complex present in most eukaryotic lineages (for review, see Heider and Munson, 2012; Vukašinović and Žárský, 2016) that is regulated by Rab and Rho small GTPases to achieve effective and spatially regulated exocytosis (for review, see Wu et al., 2008; Žárský and Potocký, 2010; Pfeffer, 2013). Therefore, the exocyst generally accumulates at PM domains characterized by intensive secretion, such as the growing bud in yeast (TerBush and Novick, 1995), tips of growing neurites (Vega and Hsu, 2001), lateral membranes of root epidermal cells (Fendrych et al., 2013; Zhang et al., 2016), or pollen tube tips (Hála et al., 2008; Bloch et al., 2016). In plants, the exocyst has been implicated in cell elongation (Synek et al., 2006; Hála et al., 2008; Cole et al., 2014), xylem development (Li et al., 2013; Tu et al., 2015; Vukašinović et al., 2016), pollen-stigma interactions (Samuel et al., 2009; Kitashiba et al., 2011; Safavian et al., 2015), pectin deposition in seed coat development (Kulich et al., 2010), cell wall maturation in trichomes (Kulich et al., 2015), cytokinesis (Fendrych et al., 2010; Rybak et al., 2014), endosomal recycling (Drdová et al., 2013), and response to pathogens (Pečenková et al., 2011; Stegmann et al., 2012).
Structurally, the exocyst is an octameric complex composed stoichiometrically of Sec3, Sec5, Sec6, Sec8, Sec10, Sec15, Exo70, and Exo84 subunits that was discovered originally in budding yeast (TerBush et al., 1996; Guo et al., 1999). Plants encode all exocyst subunits (Cvrčková et al., 2001; Eliáš et al., 2003) that act together as a functional complex (Hála et al., 2008; Fendrych et al., 2010). Although exocyst subunits are typically encoded by one gene in opisthokonts, they are often duplicated or even multiplicated in plants (Cvrčková et al., 2001, 2012; Vukašinović et al., 2014). The EXO70 gene in particular has undergone a dramatic evolutionary expansion: 23 paralogs in Arabidopsis (Arabidopsis thaliana) and 47 in rice (Oryza sativa; Synek et al., 2006; Cvrčková et al., 2012). Together with their differential expression (Synek et al., 2006; Li et al., 2010), this variety allows for a functional and tissue specialization and is probably linked to the transition of plants from water to the challenging land conditions of their evolution (Žárský et al., 2013). The multiple EXO70s may act as exchangeable components of the exocyst complex that confer specific properties to the exocyst; therefore, each cell type might be endowed with a set of functionally distinct exocyst complexes directing different cargos to particular exocytic domains (Žárský et al., 2009, 2013). Indeed, the subfunctionalization of particular EXO70s implicated in autophagy-related transport to vacuoles (Kulich et al., 2013), light-induced stomatal opening (Hong et al., 2016), and plant periarbuscular membrane formation (Zhang et al., 2015) has been described.
In contrast, EXO70A1, a highly abundant isoform in the Arabidopsis sporophyte and a core exocyst subunit, plays a general housekeeping role in polarized exocytosis in multiple tissues, since its depletion cannot be fully compensated by other paralogs and has a dramatic effect on the entire plant growth and morphogenesis (Synek et al., 2006).
Although knockout mutants in several exocyst subunits (SEC5a/b, SEC6, SEC8, and SEC15a) in Arabidopsis exhibit severe defects in pollen tube germination or growth, typically resulting in very short and depolarized pollen tubes and zero transmission of respective mutant alleles (Cole et al., 2005; Hála et al., 2008; Bloch et al., 2016), no EXO70 isoform analyzed so far displayed a comparable phenotype. Only a weak pollen-specific transmission defect was reported for the exo70C1 mutant allele (Li et al., 2010). Recently, the PM localization of SEC3-GFP in Arabidopsis pollen tube tips was shown to predict the pollen tube growth direction (Bloch et al., 2016).
In this work, we aimed to identify EXO70 isoforms expressed in pollen and involved in pollen tube growth. We found that a single mutant in EXO70C2 exhibits a significant male-specific transmission defect due to aberrant pollen tube growth characterized by inefficient cell wall deposition and an increased rate of pollen tube elongation, causing transient growth arrest and frequent partial or lethal pollen tube collapse. Furthermore, we found that EXO70C1, the closest paralog to EXO70C2, and to a minor extent also EXO70H3 play partially redundant roles. Surprisingly, our localization and interaction studies indicate that EXO70C2 and EXO70C1 may not act as stable subunits of the exocyst complex but rather acquired an unconventional function as regulators of tip growth.
RESULTS
Several EXO70 Isoforms, But Not EXO70A1, Are Expressed in Pollen
While several exocyst subunits are essential for pollen tube growth, the EXO70A1 subunit, essential in the sporophyte, is dispensable for pollen functions (Synek et al., 2006; Hála et al., 2008; Drdová et al., 2013). Indeed, EXO70A1 remained undetected in pollen RNA sequencing (RNA-Seq) and proteome analyses (Grobei et al., 2009; Loraine et al., 2013). These facts raised a question: which member(s) of the EXO70 family acquired the role of EXO70A1 in pollen tube tip growth?
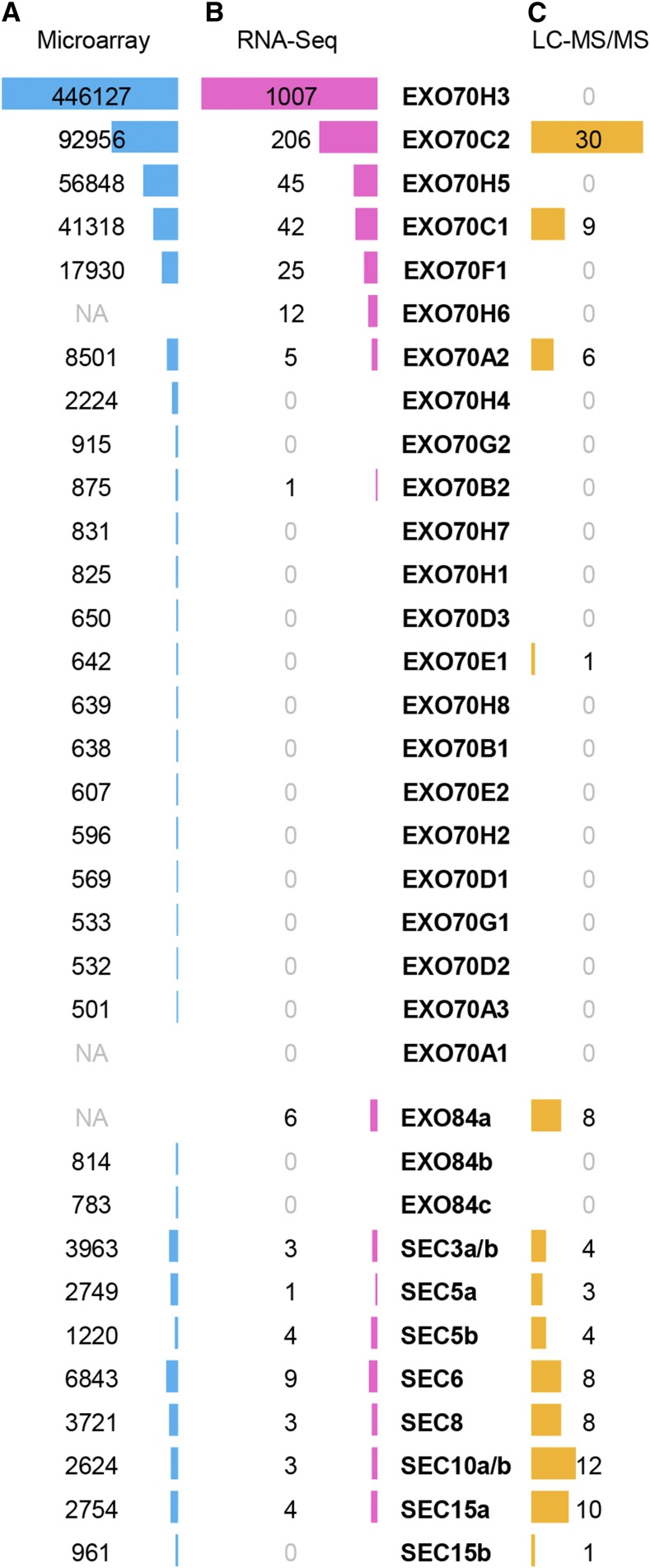
Microarray data (www.genevestigator.com [Zimmermann et al., 2004] and bar.utoronto.ca/efp [Winter et al., 2007]) fit recent RNA-Seq data on the pollen transcriptome in Arabidopsis (Loraine et al., 2013) and provided hints that EXO70C1, EXO70C2, EXO70H3, and EXO70H5 are highly abundant EXO70 isoforms in pollen, while EXO70A2, EXO70F1, and EXO70H6 are detected at lower levels in at least one stage of pollen development (Fig. 1, A and B; Supplemental Fig. S1). Interestingly, significant expression of EXO70C1 and EXO70C2 also has been detected in root trichoblast cells (Supplemental Fig. S2). This dual (pollen-trichoblast) specificity points to their possible general involvement in tip growth.
Figure 1.
Expression of exocyst genes in Arabidopsis pollen. A, Mean expression values in linear scale based on the ATH1 (Affymetrix) microarray data in Genevestigator. NA, Not available on the ATH1 DNA chip. B, Quantification of mRNA based on RNA-Seq data on mature pollen (Loraine et al., 2013). C, Number of peptides detected in the proteome of mature pollen using mass spectroscopy (LC-MS/MS; Grobei et al., 2009).
Given the multiple levels of mRNA posttranscriptional regulation, we attribute a high importance to proteomic data that confirmed EXO70C1, EXO70C2, and EXO70A2 in the mature Arabidopsis pollen, with EXO70C2 being the most abundant EXO70 isoform (Fig. 1C; Grobei et al., 2009; Mayank et al., 2012). EXO70C1 and EXO70C2 also were identified as phosphoproteins, pointing to new regulatory possibilities (Mayank et al., 2012). Surprisingly, EXO70H3 was not detected at the protein level despite its enormous transcript abundance, indicating a strong regulation of its expression at the posttranscriptional or translational level. All core exocyst subunits also were found in the mature pollen, with the numbers of peptides detected in remarkable agreement with the stoichiometry of the exocyst complex (Fig. 1C).
Taken together, several EXO70 isoforms can play some role in the male gametophyte. Therefore, we started experimental analyses of reasonably supported candidates: EXO70A2 (At5g52340), EXO70C1 (At5g13150), EXO70C2 (At5g13990), EXO70F1 (At5g50380), EXO70H3 (At3g09530), EXO70H5 (At2g28640), and EXO70H6 (At1g07725).
The EXO70C2 Disruption Results in a Pollen-Specific Transmission Defect
We obtained and characterized available T-DNA/transposon insertional mutants in EXO70A2, EXO70C1, EXO70C2, EXO70F1, EXO70H3, EXO70H5, and EXO70H6 (Supplemental Fig. S3). All homozygous mutant lines exhibited no obvious phenotypic changes in the sporophyte. Heterozygotes showed a normal segregation ratio based on PCR genotyping, except for exo70C2-1, suggesting a significant transmission defect of this mutant allele (Table I). Based on the segregation ratio 39.8%:50.9%:9.3%, we calculated the efficiency of the exo70C2 pollen tubes in fertilization to be about 23% compared with wild-type pollen tubes (see an interactive model in Supplemental File S1). Reciprocal crosses followed by PCR genotyping of the offspring revealed that the exo70C2-1 transmission defect is male specific (Table II).
Table I. Segregation of heterozygous mutants in putative pollen EXO70 isoforms.
+/+, Wild-type plants; +/−, heterozygous mutants; −/−, homozygous mutants; OE, overexpressor line.
| Allele | +/+ | +/− | −/− | n | χ2 | P | Line |
|---|---|---|---|---|---|---|---|
| Expected | 25.0% | 50.0% | 25.0% | ||||
| exo70A2-OE | 26.7% | 51.7% | 21.7% | 120 | 0.733 | 0.693 | GABI_824D06 |
| exo70C1-1a | 24.0% | 50.0% | 26.0% | 150 | 0.120 | 0.942 | GABI_100A02 |
| exo70C1-2 | 24.6% | 49.1% | 26.3% | 118 | 0.102 | 0.950 | GABI_334D05 |
| exo70C2-1a | 39.8% | 50.9% | 9.3% | 159 | 30.037 | <0.001 | RATM16-1469-1 |
| exo70C2-2b | 24.5% | 48.1% | 27.4% | 106 | 0.321 | 0.853 | SALK_045767 |
| exo70F1 | 21.9% | 53.3% | 24.8% | 105 | 0.638 | 0.727 | SALK_036927 |
| exo70H3 | 23.0% | 56.0% | 21.0% | 100 | 1.520 | 0.468 | GABI_651C10.03 |
| exo70H5 | 23.2% | 48.2% | 28.6% | 112 | 0.786 | 0.675 | SALK_007810 |
| exo70H6 | 26.0% | 44.8% | 29.2% | 154 | 1.987 | 0.370 | SALK_016535 |
These alleles were used for the next analyses. bT-DNA insertion in the promoter; transcripts were present at the wild-type level.
Table II. Reciprocal crossing of exo70C2 and exo70C1 mutants to the wild-type Columbia-0 (Col-0).
+/+, Wild-type plants; +/−, heterozygous mutants.
| Allele | Pollen Donor, +/−; Pollen Recipient, +/+ | Pollen Donor, +/+; Pollen Recipient, +/− | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| +/+ |
+/− |
χ2 |
P |
na |
+/+ |
+/− |
χ2 |
P |
na |
|
| Expected | 50.0% | 50.0% | 50.0% | 50.0% | ||||||
| exo70C2-1 | 87.5% | 12.5% | 63.000 | <0.001 | 112 | 49.1% | 50.9% | 0.035 | 0.851 | 114 |
| exo70C1-1 | 49.1% | 50.9% | 0.034 | 0.853 | 116 | 51.8% | 48.2% | 0.145 | 0.703 | 110 |
Seeds from at least six independent crosses.
In contrast with the two independent exo70C1 knockout lines (in the Col-0 background) with normal transmission analyzed here, the Ds transposon allele CW841908 (i.e. CSHL_ET11742) in the Landsberg erecta background exhibited slightly reduced transmission efficiency (78%) via pollen (Li et al., 2010), albeit this insertion is located at a very similar position to the insertions above in the middle of the single EXO70C1 exon. Furthermore, weak mutant alleles of SEC8, another exocyst subunit, showed normal transmission after self-crossing but decreased significantly after manual reciprocal crossing (Cole et al., 2005). For these two reasons, we performed reciprocal crossing of exo70C1-1, although only to confirm the normal exo70C1-1 transmission in the Col-0 background (Table II). The discrepancy between CSHL_ET11742 and exo70C1-1 mutant alleles may be explained by the specific behavior of EXO70C1 in different ecotypes.
Although the segregation ratio of exo70H3 heterozygous mutants was not statistically different from normal segregation, the enormous abundance of EXO70H3 transcripts in pollen led us to analyze exo70H3 exo70C2-1 double mutants. The exo70C2-1 transmission defect was more pronounced in the homozygous exo70H3 background as documented by the 47.2%:47.3%:5.5% segregation ratio (Table III), suggesting slightly redundant and/or synergistic functions of EXO70C2 and EXO70H3. Additional comprehensive proteomic analyses will probably elucidate why no hits were detected for EXO70H3 in the mature pollen (Grobei et al., 2009). Possibly, the abundant transcripts could be translated only after the germination of pollen tubes or the protein is active at very low quantities.
Table III. Segregation of exo70C2 heterozygotes in the homozygous exo70H3 mutant background.
+/+, Wild-type plants; +/−, heterozygous mutants; −/−, homozygous mutants.
| Allele | +/+ | +/− | −/− | n | χ2 | P |
|---|---|---|---|---|---|---|
| Normal | 25.0% | 50.0% | 25.0% | |||
| exo70C2-1 | 39.8% | 50.9% | 9.3% | |||
| exo70C2-1 | 47.2% | 47.3% | 5.5% | 146 | 51.411a | <0.001a |
| exo70H3 | 4.713b | 0.095b |
χ2 test related to normal segregation. bχ2 test related to exo70C2-1 single mutant segregation.
In conclusion, EXO70C2 seems to play a prominent role among the candidate pollen EXO70s from the genetic point of view. Therefore, in the following work, we focused on the EXO70C2 isoform and its closely related paralog EXO70C1. (For the next analyses, we used exclusively exo70C2-1 and exo70C1-1 mutant lines; hereafter, these alleles are designated exo70C2 and exo70C1, respectively.)
The EXO70C1 Activity Is Partially Redundant with EXO70C2
As EXO70C2 is evolutionarily very closely related to EXO70C1 (Synek et al., 2006; Cvrčková et al., 2012), we investigated whether these paralogs share redundant functions. Since crossing of exo70C1 and exo70C2 failed due to a strong genetic linkage of the two genes (the distance between At5g13150 and At5g13990 is 341 kb, corresponding to ∼2.2 centimorgan), we attempted to inactivate both genes simultaneously using an artificial microRNA (amiRNA) against one gene in the knockout mutant for the complementary gene.
We designed amiRNAs (Schwab et al., 2006) targeted specifically against EXO70C1 or EXO70C2 and cloned them under the control of the pollen-specific LAT52 promoter. We introduced an amiRNA expression cassette against EXO70C1 (hereafter designated as amiRNAxC1) into exo70C2 heterozygous mutants and an amiRNA expression cassette against EXO70C2 (hereafter designated as amiRNAxC2) into exo70C1 heterozygous mutants using Agrobacterium tumefaciens-mediated transformation. T1 plants resistant to Basta (carried by the amiRNA cassette) and being wild type or homozygous for the exo70C1 or exo70C2 mutant allele were used for subsequent analyses. The transmission efficiency of amiRNA cassettes was then determined as percentage of Basta-resistant seedlings in the T2 population.
Our working hypothesis assumes that at least one of the EXO70C1 and EXO70C2 genes must be active to allow for the amiRNA cassette transmission via pollen. T1 transformants that are heterozygous for the amiRNA cassette will then provide differential segregation ratios in their offspring based on the combination of genotypes. For example, in the case of amiRNAxC2 in the exo70C1 background, we can expect 50% to 75% of amiRNA-positive plants in the offspring, where 50% would indicate a complete pollen defect (amiRNA inherited only from the female), while 75% is wild-type-like pollen.
Indeed, we recorded that amiRNAxC2 generated 44% to 56% resistant seedlings in the exo70C1 background and amiRNAxC1 generated 49% to 52% resistant seedlings in the homozygous exo70C2 background (Table IV), suggesting minimal amiRNA transmission via pollen. An identical result was obtained when the presence of the amiRNAxC2 cassette was evaluated for one line by PCR genotyping on a parallel population, justifying the simple chemical selection approach (Table IV). The transmission of amiRNAxC1 or amiRNAxC2 in the wild-type background resembled the transmission of the exo70C1 or exo70C2 mutant allele, respectively, thus excluding that either amiRNA construct blocks the pollen function by itself (Table IV).
Table IV. Transmission of amiRNA cassettes targeting EXO70C1 or EXO70C2.
| Plant | amiRNA | Background Genotype | Plants Bearing amiRNA Cassette in the Offspring | n | Comments |
|---|---|---|---|---|---|
| xC2 | exo70C1 | 50% | Expected for a functional construct | ||
| xC2 | exo70C1 | 75% | Expected for a nonfunctional construct | ||
| #1 | xC2 | exo70C1 | 53% | 128 | PCR genotyping |
| #1 | xC2 | exo70C1 | 52% | 195 | Basta selection |
| #2 | xC2 | exo70C1 | 44% | 116 | Basta selection |
| #3 | xC2 | exo70C1 | 56% | 81 | Basta selection |
| xC2 | Wild type | 60%a | Expected for a functional construct | ||
| xC2 | Wild type | 75% | Expected for a nonfunctional construct | ||
| #7 | xC2 | Wild type | 63% | 230 | Basta selection |
| #8 | xC2 | Wild type | 65% | 249 | Basta selection |
| xC1 | exo70C2 | 50% | Expected for a functional construct | ||
| xC1 | exo70C2 | 75% | Expected for a nonfunctional construct | ||
| #4 | xC1 | exo70C2 | 49% | 174 | Basta selection |
| #5 | xC1 | exo70C2 | 52% | 107 | Basta selection |
| #6 | xC1 | exo70C2 | 52% | 109 | Basta selection |
| xC1 | Wild type | 75% | Expected for a functional construct | ||
| xC1 | Wild type | 75% | Expected for a nonfunctional construct | ||
| #9 | xC1 | Wild type | 79% | 100 | Basta selection |
| #10 | xC1 | Wild type | 76% | 382 | Basta selection |
Estimated based on the transmission efficiency of the exo70C2-1 allele.
Furthermore, we performed reciprocal crossings of homozygous exo70C1 mutants bearing amiRNAxC2 (heterozygous) with exo70C1 homozygous plants without amiRNA and evaluated the amiRNA cassette transmission by PCR genotyping of harvested crosses (Table V). As a result, amiRNAxC2 could be transmitted only via the female gametophyte, indicating that the defects described above were pollen specific.
Table V. Transmission of the amiRNA targeting EXO70C2 in the exo70C1 homozygous mutant background via male or female gametophyte.
| Pollen Donor, amiRNAxC2 in exo70C1; Pollen Recipient, exo70C1 | ||||
| + | − | χ2 | P | n |
| 0% | 100% | 142.00 | <0.001 | 142 |
| Pollen Donor, exo70C1; Pollen Recipient, amiRNAxC2 in exo70C1 | ||||
| + | − | χ2 | P | n |
| 44.9% | 55.1% | 1.44 | 0.23 | 136 |
We conclude that the function of EXO70C1 is redundant to EXO70C2, because inactivation of both genes simultaneously led to a complete pollen-specific transmission defect, in contrast to the partial defect in the case of the exo70C2 single mutant.
Pollen Tube Growth Is Impaired in the exo70C2 Mutant
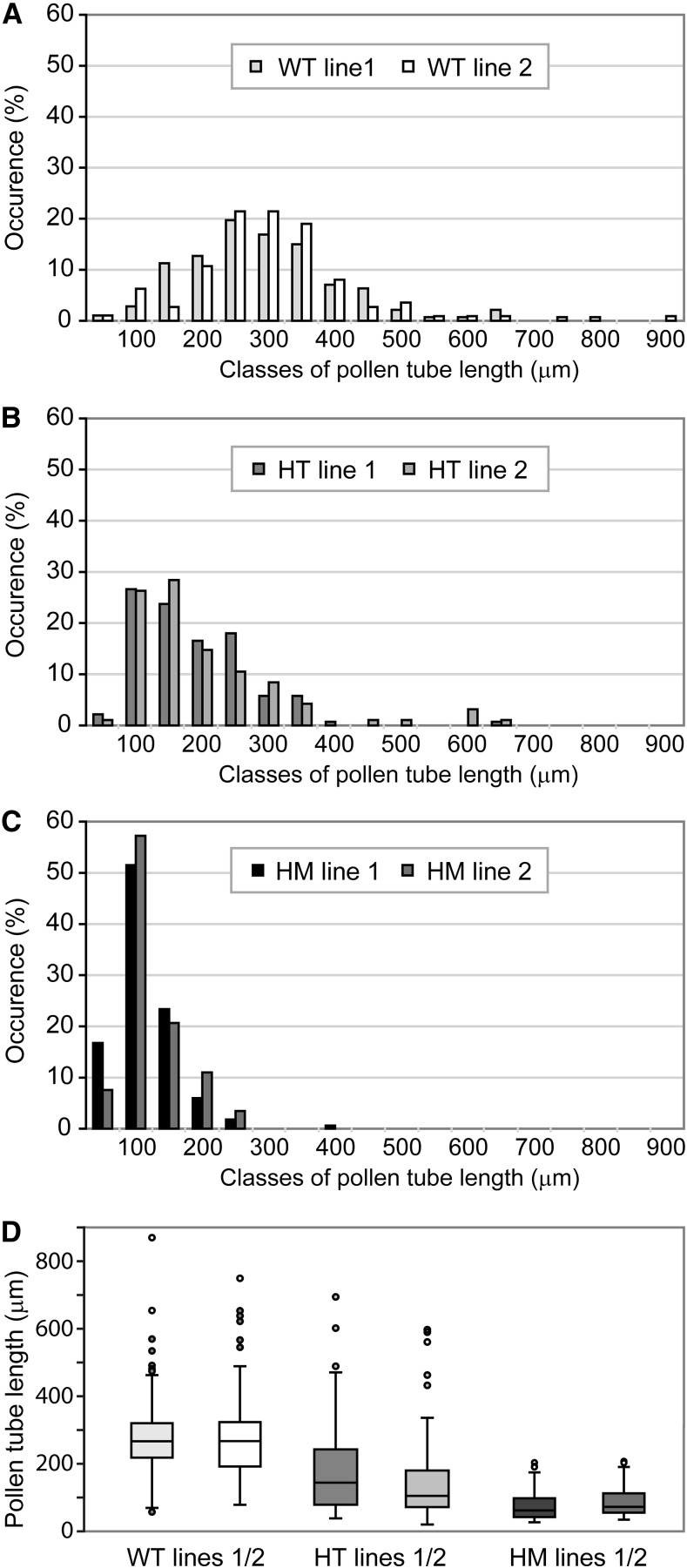
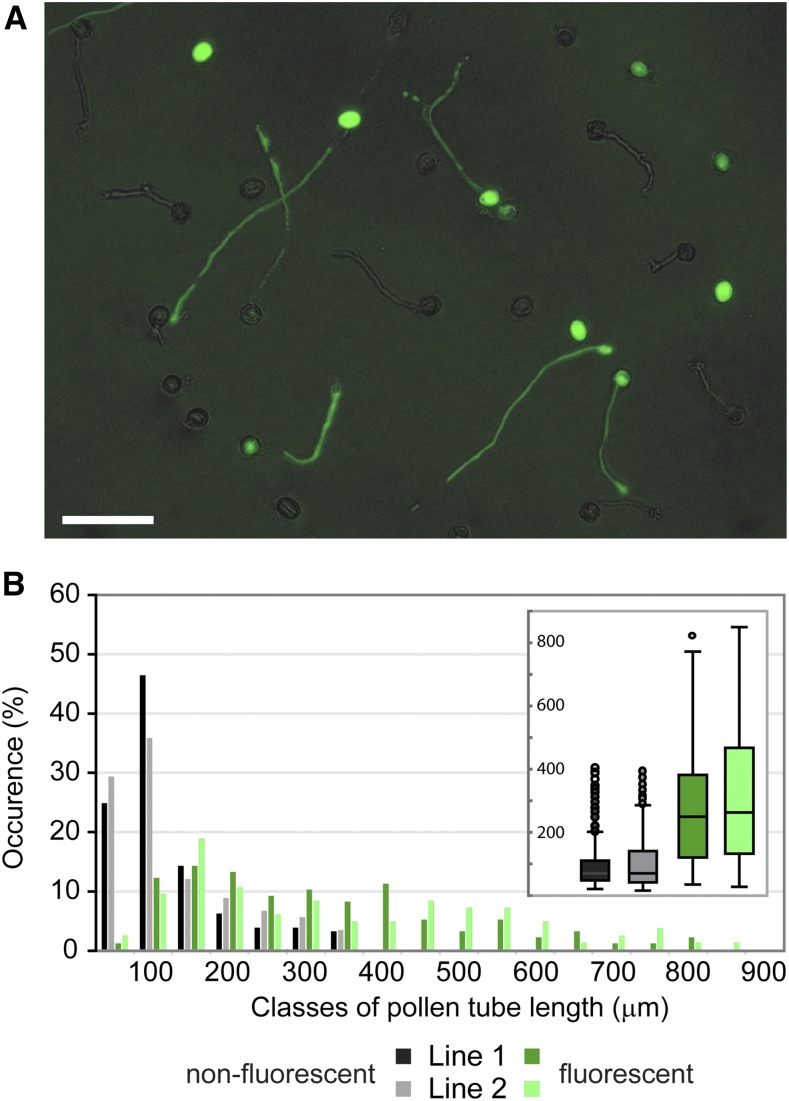
In order to address the cause of the pollen-specific transmission defect of the exo70C2 allele, we examined pollen germination efficiency and pollen tube growth in vitro after 14 h of germination for homozygous and heterozygous exo70C2 mutants and their wild-type siblings. Maximal pollen germination efficiency was comparable among all three genotypes (+/+, 91.7%; +/−, 88.3%; and −/−, 90.1%; pollen of 20 plants for each genotype was evaluated; and more than 150 pollen grains per plant were counted). However, pollen grains from homozygous mutants produced mostly shorter pollen tubes than wild-type pollen with variable morphology (Fig. 2).
Figure 2.
Pollen tube lengths of exo70C2 mutants. A to C, Distribution of pollen tube lengths in samples of in vitro-germinated pollen from the wild type (WT; A), exo70C2 heterozygotes (HT; B), and exo70C2 homozygotes (HM; C). Two independent lines of the same genotype are displayed in each graph. At least 120 pollen tubes were measured for each line. D, Box plots comparing the data from A to C.
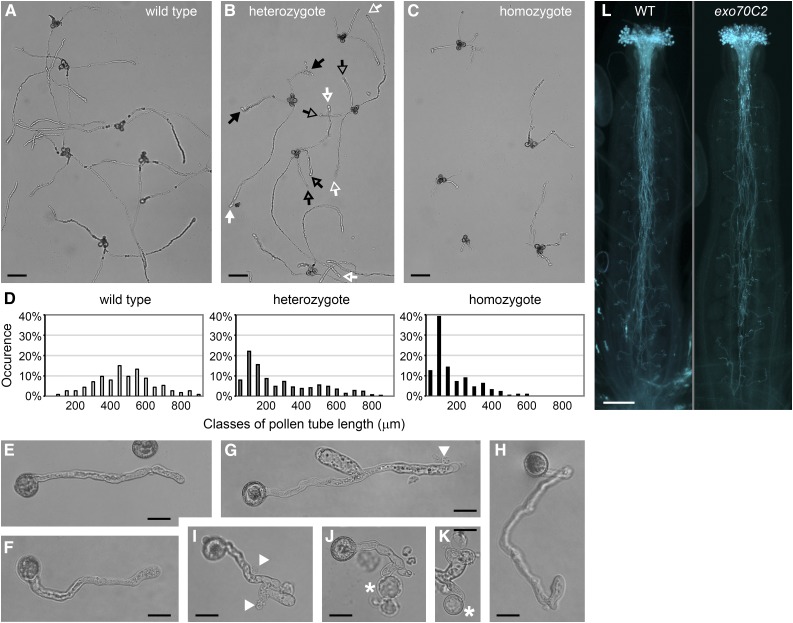
The mutant and wild-type identity of short and long pollen tubes, respectively, was confirmed by crossing exo70C2 to the quartet1 background, where tetrads of sister pollen grains do not separate (Preuss et al., 1994; Johnson-Brousseau and McCormick, 2004). Indeed, two long and two short pollen tubes typically emerged from each tetrad of pollen from exo70C2 heterozygotes germinated in vitro (Fig. 3, A–D).
Figure 3.
Morphology of exo70C2 pollen tubes germinated in vitro. A to C, Germinated pollen of a wild-type plant (A), an exo70C2 heterozygous plant (B), and an exo70C2 homozygous plant (C) in the quartet-1 background. Putative wild-type or mutant pollen tubes are marked by white or black arrows, respectively; arrows of the same design correspond to the same tetrad. Bars = 100 μm. D, Distribution of pollen tube lengths in the samples above (A–C). E, A typical wild-type pollen tube. Bar = 20 μm. F to K, Mutant pollen tubes showing relatively normal morphology (F), branching (G and I), sharp bending (H), effusion of cytoplasm (G and I; marked by arrowheads), or the production of protoplast-like structures emerging from tube tips (J and K; marked by asterisks). Bars = 20 μm. L, Aniline Blue staining of pollinated pistils of the wild type (WT) and an exo70C2 homozygote visualizing callose in pollen tubes. Bar = 200 μm.
While the initial polarity establishment of mutant pollen tubes was unaffected, they regularly exhibited branching and sharp bending after germination in vitro (Fig. 3, G–I). Furthermore, their tips were sensitive to bursting (especially during microscopic manipulation), frequently causing effusions of cytoplasm and collapses (Fig. 3, G–K). Occasionally, they produced protoplast-like structures emerging from the apex (Fig. 3, J–K) with indistinct propidium iodide labeling around these structures (Supplemental Fig. S4). The morphology of exo70C2 pollen tubes is different from the pollen tube phenotype of mutants in core exocyst subunits (sec5a/b, sec6, sec8, and sec15a) that generate short, wide, and intact pollen tubes (Cole et al., 2005; Hála et al., 2008).
In contrast, exo70C2 pollen tubes grown in vivo were much longer than those grown in vitro, often reaching basal ovules (Fig. 3L). This discrepancy may be explained by mechanical support of the transmitting tract, compensating for the mutant pollen tube sensitivity (see below).
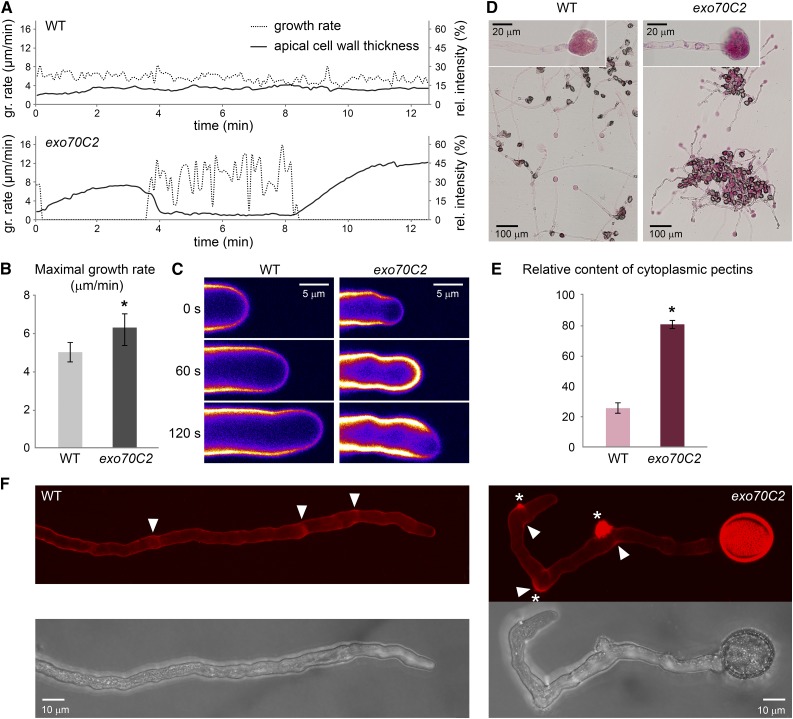
Mutant exo70C2 Pollen Tubes Display Higher Growth Rate and Insufficient Cell Wall Deposition Resulting in Repetitive Transient Stops and Bursts of the Growing Tip
The cytoplasm effusions and bursts of exo70C2 pollen tube tips led us to inspect cell wall morphology and growth dynamics. While wild-type pollen tubes displayed steady growth, accompanied by known regular low-amplitude oscillations, exo70C2 pollen tubes grew with extreme fluctuations in their growth rate in a stop-and-go manner (Fig. 4A). Importantly, the growth rate measurement in 90-s intervals during a growing phase revealed that the averaged maximal growth rate in a population of exo70C2 pollen tubes was 126% of the wild-type average (Fig. 4B). Detailed inspection of individual pollen tubes in 5-s intervals showed that exo70C2 pollen tubes reached in peaks as much as 180% of the maximal wild-type growth rate (Fig. 4A). At the end of the high-speed growth period, the mutant pollen tubes burst, visibly extruding the cytoplasm (Supplemental Movie S1). This was most likely due to an insufficient cell wall formation, since the growth rate was inversely correlated with the cellulose and pectin deposition, as visualized by Calcofluor White staining (Fig. 4, A and C). When an exo70C2 pollen tube elongated rapidly for a longer period, the apical Calcofluor White signal decreased by 75% with respect to the wild-type control (Supplemental Movie S2).
Figure 4.
Growth rate and cell wall characteristics of exo70C2 and wild-type (WT) pollen tubes. A, Growth rate of typical wild-type and exo70C2 pollen tubes correlated with cell wall thickness (Calcofluor White fluorescence) at the tube apex. Images for measurement were captured at intervals of 5 s. B, The averaged maximal growth rate of exo70C2 is significantly higher than that of the wild type (sd is displayed: *, P < 0.00001 by Student’s t test). Measurements were performed on 15 tubes per genotype at multiple time points every 90 s. C, Calcofluor White fluorescence represented as an intensity color scale (purple to white) shows differential cell wall deposition at the tube apex during the highly fluctuating growth rate of the exo70C2 pollen tube compared with the wild type characterized by low oscillations. D, Pollen tubes of the wild type and exo70C2 germinated and stained on the same slide with Ruthenium Red diluted in distilled water to cause the extrusion of cytoplasm. Details of burst tips that were used for quantification are shown in the insets. E, Quantification of the Ruthenium Red staining in extruded cytoplasm as relative intensity in the red channel subtracted from the background. sd is displayed: *, P < 10−10 by Student’s t test; n = 50 for each genotype. F, Propidium iodide staining of growing pollen tubes. Maximum intensity projections over a confocal Z-stack are shown. Asterisks mark sites of collapse; arrowheads point to sites of stopped growth.
Furthermore, the insufficiency of cell wall materials at the cell surface corresponded to its retention in the cytoplasm, as visualized by Ruthenium Red, commonly used for staining of methylesterified pectins (Hou et al., 1999). Ruthenium Red staining of pollen tubes in hypotonic conditions, causing extrusions of the cytoplasmic content, showed significantly stronger cytoplasmic pectin staining in exo70C2 pollen tubes (Fig. 4, D and E). However, live staining of external pectins in the cell wall using propidium iodide (proposed to bind the demethoxylated homogalacturonan component of pectin; Rounds et al., 2011) was comparable to the wild type, except for more frequent patches of thicker pectin deposition, corresponding to events when a pollen tube stopped its growth or collapsed (Fig. 4F).
Surprisingly, the exo70C2 pollen tubes grown in vitro could frequently recover after their burst and, with certain delay, restart their tip growth at the same apex or a new apex established some distance back from the old one. We observed multiple cycles of burst and recovery, although ultimately terminated with growth arrest or total collapse of the handicapped mutant pollen tube (Supplemental Movie S3).
We conclude that exo70C2 mutant pollen tubes exhibit a significantly enhanced growth rate and compromised cell wall deposition at the growing apex compared with the wild type, causing subsequently repetitive bursts of growing tips. This behavior explains why exo70C2 pollen tubes obtain the branched morphology and are ultimately significantly shorter than wild-type siblings in suspension in vitro cultures.
EXO70C2:GFP Complements the exo70C2 Mutation
To prove that the defects observed in pollen tubes are caused by dysfunction of the EXO70C2 gene, we tested exo70C2 mutants for complementation with EXO70C2:GFP expressed under the control of the native pEXO70C2 promoter (for localization of this fusion protein, see next section).
After selection of fluorescent plants heterozygous for the exo70C2 allele and homozygous for the pEXO70C2::EXO70C2:GFP expression cassette, we conducted segregation analysis of the mutant allele in the offspring. As a result, the segregation ratio of the exo70C2 allele was statistically indistinguishable from the Mendelian ratio (+/+:+/−:+/−, 27.1%:49.2%:23.7%; n = 118, χ2 = 0.305, P = 0.859), indicating that pEXO70C2::EXO70C2:GFP can complement the exo70C2 transmission defect. It also demonstrates the functionality of the EXO70C2:GFP fusion protein.
Furthermore, we used plants homozygous for the exo70C2 allele and heterozygous for the pEXO70C2::EXO70C2:GFP expression cassette to characterize pollen tube growth of their pollen germinated in vitro. We measured pollen tube lengths of fluorescent and nonfluorescent pollen tubes separately within each sample (Fig. 5A). In agreement with the previous observation, the distribution of fluorescent pollen tube lengths resembled wild-type characteristics, while the distribution of nonfluorescent pollen tube lengths was similar to that of exo70C2 mutant pollen tubes (Fig. 5B; compare with Fig. 2, A and C). In addition, we used plants homozygous for both the exo70C2 allele and the pEXO70C2::EXO70C2:GFP cassette, where all pollen tubes displayed fluorescence. In this combination, the distribution of fluorescent pollen tube lengths resembled wild-type characteristics as expected (Supplemental Fig. S5; compare with Fig. 2A).
Figure 5.
Pollen tube lengths of exo70C2 lines complemented with pEXO70C2::EXO70C2:GFP. A, A representative microscopic image from the series used for pollen tube length analysis below. Longer pollen tubes emitting pEXO70C2::EXO70C2:GFP fluorescence represent complemented exo70C2 mutant pollen tubes. GFP fluorescence was mixed with bright field. Bar = 100 μm. B, Distribution of pollen tube lengths in samples of in vitro-germinated pollen from homozygous exo70C2 mutants in which the introduced pEXO70C2::EXO70C2:GFP was in a heterozygous state. Fluorescent and nonfluorescent pollen tubes were measured separately within each sample. Two independent lines (1 and 2) were analyzed. Box plots in the inset are another presentation of the same data.
In conclusion, the pEXO70C2::EXO70C2:GFP expression complemented the exo70C2 disruption in pollen tube growth.
EXO70C2 as Well as EXO70C1 Are Localized in the Cytoplasm of Pollen Tubes and Trichoblast Cells
In order to characterize the tissue/cell specificity and subcellular localization of EXO70C2 and EXO70C1, we cloned each gene including its native promoter and fused it to GFP at its C terminus. The localization of EXO70C2:GFP or EXO70C1:GFP was analyzed microscopically in their respective homozygous mutant backgrounds.
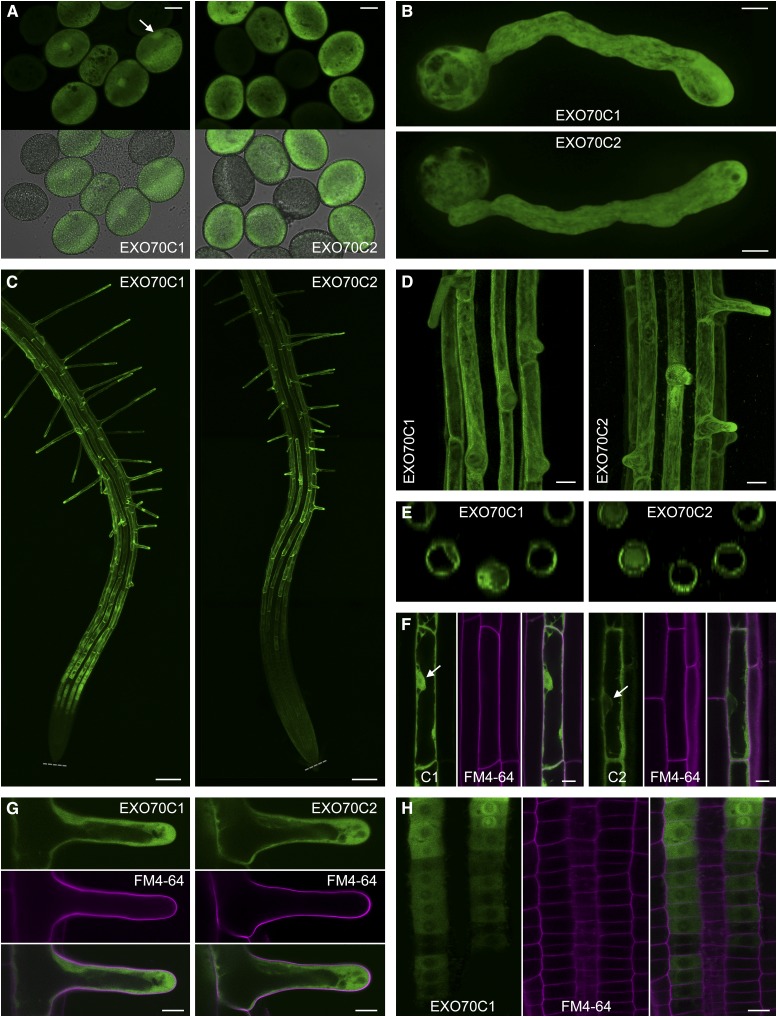
Both pEXO70C2::EXO70C2:GFP and pEXO70C1::EXO70C1:GFP were found expressed specifically in mature pollen grains, pollen tubes, and trichoblast cells in roots (Fig. 6), which is in agreement with available microarray data that do not indicate their expression in any other cell type/tissue (Genevestigator and Arabidopsis eFP Browser). The expression patterns of EXO70C2:GFP and EXO70C1:GFP were similar with a notable difference: EXO70C1:GFP expression in roots started already in trichoblast precursors in the late meristematic zone, while EXO70C2:GFP expression was first detectable in the elongation zone (Fig. 6, C and H).
Figure 6.
Localization of EXO70C1:GFP and EXO70C2:GFP expressed under the control of their native promoters in cells of exo70C1 and exo70C2 homozygotes, respectively. A, EXO70C1:GFP and EXO70C2:GFP in mature pollen grains (the fluorescence intensity in both samples is not to scale). In addition, EXO70C1:GFP accumulates in the vegetative nucleus (marked by the arrow). Expression cassettes were segregating in the samples; nonfluorescent pollen grains provide reference for the background fluorescence. Bars = 10 μm. B, EXO70C1:GFP and EXO70C2:GFP in the cytoplasm of pollen tubes. Bars = 10 μm. C, EXO70C1:GFP and EXO70C2:GFP in roots. The expression of EXO70C1 starts already in the late meristem. Gray dotted lines mark root tips. Bars = 100 μm. D, EXO70C1:GFP and EXO70C2:GFP are expressed specifically in trichoblast cells in roots (maximum intensity projection of confocal Z-stacks). Bars = 20 μm. E, Top views of three-dimensional reconstructions calculated from the Z-stacks in D. F, While EXO70C1:GFP localizes to the cytoplasm and nucleus in elongated trichoblast cells, EXO70C2:GFP is localized exclusively in the cytoplasm. Nuclei are marked by arrows. Cell walls were stained with propidium iodide (in magenta). Bars = 10 μm. G, Cytoplasmic localization of EXO70C1:GFP and EXO70C1:GFP in growing root hairs. Cell walls were stained with propidium iodide (in magenta). Bars = 10 μm. H, The onset of EXO70C1:GFP expression in the root meristem. EXO70C1:GFP accumulates in the perinuclear region and/or nucleolus at certain stages. Cell walls were stained with propidium iodide (in magenta). Bar = 10 μm.
Both EXO70C2:GFP and EXO70C1:GFP localized to the cytoplasm without association with any distinct structures (Fig. 6, B, F, G, and H). In contrast to EXO70C2:GFP, EXO70C1:GFP exhibited a capacity to enter the nucleus, transiently after cytokinesis in trichoblast precursors in the root meristem (Fig. 6H) or permanently in developed trichoblast cells and pollen grains (Fig. 6, A and F), pointing to a potential nuclear regulatory function. Surprisingly, no PM signal was detectable for EXO70C1:GFP or EXO70C2:GFP, albeit all core exocyst subunits typically decorate the PM in various cell types with varying minor fractions in the cytoplasm (Fendrych et al., 2010, 2013; Drdová et al., 2013; Bloch et al., 2016; Zhang et al., 2016).
To evaluate the possible impact of the GFP position on the subcellular localization of EXO70C2, an N-terminal GFP fusion (pEXO70C2::GFP:EXO70C2) was generated and showed an identical localization pattern to EXO70C2:GFP, excluding a negative effect of the GFP position (Supplemental Fig. S6).
EXO70C2 Does Not Colocalize or Interact with Core Exocyst Subunits
The undetectable EXO70C2 PM localization, unexpected for a putative exocyst subunit, led us to perform three comparative experiments with core exocyst subunits.
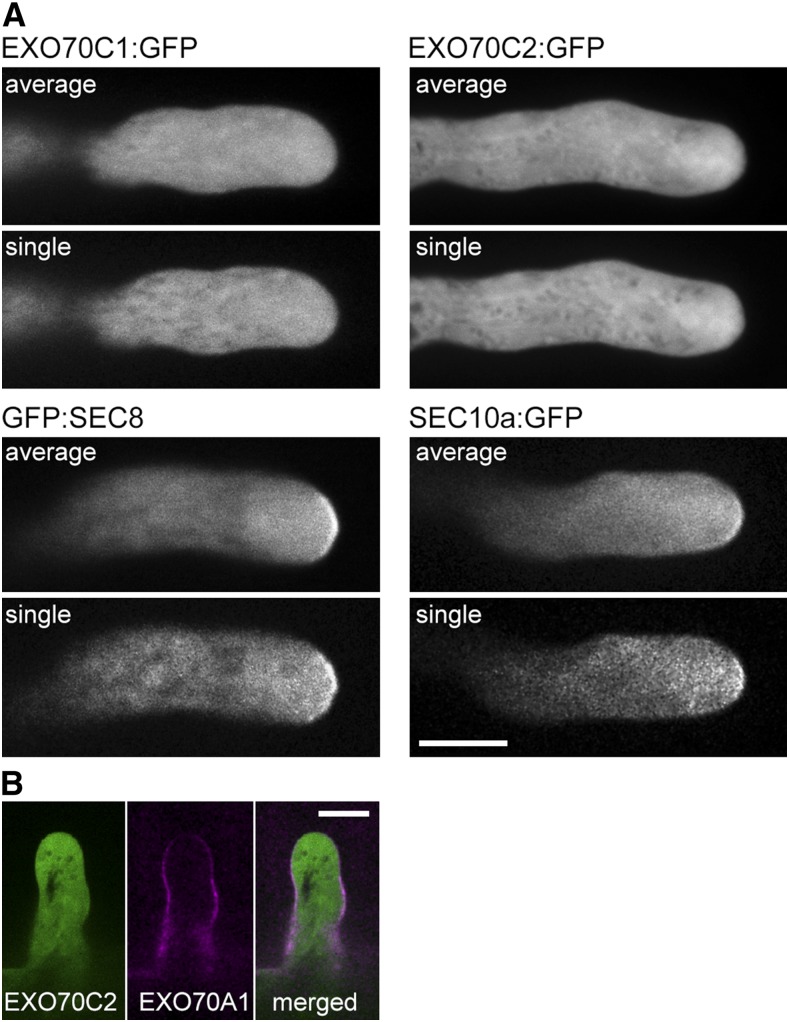
First, we localized two exocyst subunits, pSEC8::GFP:SEC8 and pSEC10a::SEC10a:GFP (Fendrych et al., 2010; Vukašinović et al., 2016), which are active in pollen and were cloned with their native promoters, in comparison with pEXO70C2::EXO70C2:GFP in growing Arabidopsis pollen tubes, using a spinning-disk confocal microscope under identical settings for all three fusion proteins. The imaging was performed in time series to prove that analyzed pollen tubes are growing normally and to document possible localization dynamics. GFP:SEC8 and SEC10a:GFP showed an enrichment in the inverted cone and, importantly, accumulation in distinct patches along the PM at the pollen tube apex, in contrast to the cytoplasmic distribution of pEXO70C2::EXO70C2:GFP in the inverted cone and pollen tube shank without any decoration of the PM (Fig. 7A).
Figure 7.
Localization of EXO70C1 and EXO70C2 in comparison with core exocyst subunits in Arabidopsis pollen tubes and root hairs. A, GFP:SEC8 and SEC10a:GFP exhibit a PM association in the apex of growing pollen tubes, in contrast to EXO70C1:GFP and EXO70C2:GFP, which show cytoplasmic localization. Averaged time series (five sequential images taken at 4-s intervals) and one representative single image out of the series are displayed. Bar = 10 μm. B, Colocalization of EXO70C2:GFP (green) and RFP:EXO70A1 (magenta) in growing root hairs shows no overlap. Bar = 10 μm.
Second, we performed a direct comparison with another EXO70 isoform, EXO70A1, which is a sporophytic experimentally proven exocyst subunit with described PM localization (Drdová et al., 2013; Fendrych et al., 2013; Zhang et al., 2016). Imaging in root hairs showed a PM decoration of developing root hairs by pUBQ10::RFP:EXO70A1, while pEXO70C2::EXO70C2:GFP was again found only in the cytoplasm in the same cell (Fig. 7B).
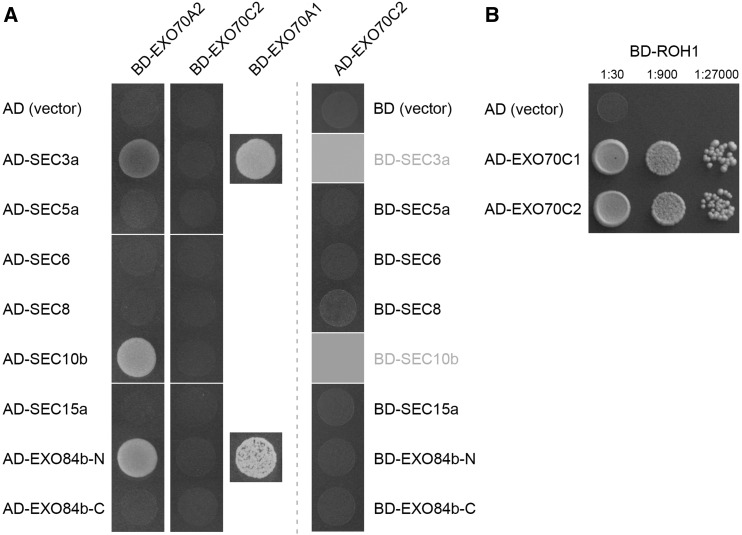
Third, since EXO70A1 interacts with SEC3a and the N-terminal half of EXO84b (EXO84b-N) in the yeast two-hybrid system (Hála et al., 2008; Fendrych et al., 2010), we tested EXO70C2 and also EXO70A2, another isoform highly abundant in the pollen proteome, for pairwise interactions with exocyst subunits. While EXO70A2 did interact with SEC3a and EXO84b-N, and additionally with SEC10b, EXO70C2 showed no positive interactions either as bait or as prey (Fig. 8A). However, we found EXO70C2 interacting with ROH1 in the yeast two-hybrid system, a putative negative regulator of secretion, similar to the EXO70C1-ROH1 interaction published earlier (Kulich et al., 2010; Fig. 8B).
Figure 8.
EXO70C2 and EXO70A2 interactions in the yeast two-hybrid assay. A, Pairwise interactions with core exocyst subunits. The EXO70A1-SEC3a and EXO70A1-EXO84b-N interactions described by Hála et al. (2008) were used as positive controls. Empty vectors pGADT7 (AD) and pGBKT7 (BD) were used as negative controls. BD-SEC3a and BD-SEC10b were omitted in this assay, since they show high autoactivation capacity (Hála et al., 2008). B, AD-EXO70C2 interacts with BD-ROH1, a putative negative regulator of secretion, similar to AD-EXO70C1 published by Kulich et al. (2010).
Taken together, in spite of clear indications that EXO70C2 is involved in the tip growth of pollen tubes, these observations favor a hypothesis that it probably does not serve as a stable functional subunit of the exocyst complex. On the other hand, EXO70A2 is likely incorporated into the exocyst complex, as indicated by physical interactions with three core exocyst subunits.
DISCUSSION
EXO70C2 Is a Key Factor for Efficient Pollen Tube Growth
Very limited experimental data on the EXO70 family in pollen provoked our interest to explore what EXO70 isoforms are expressed in pollen and engaged in tip growth of pollen tubes in Arabidopsis. We anticipated that another EXO70 isoform(s) adopted the essential role of sporophytic EXO70A1 in the male gametophyte. Our list of potential pollen EXO70 isoforms (EXO70A2, EXO70C1, EXO70C2, EXO70F1, EXO70H3, EXO70H5, and EXO70H6) is consistent, except for EXO70A2, with a comprehensive study on the expression of all EXO70 family members in various tissues based on semiquantitative reverse transcription PCR and promoter activity by Li et al. (2010). EXO70C2 expression in later stages of pollen development also was proved by real-time PCR and RNA in situ hybridization (Lai, 2016).
Since only the exo70C2 mutant allele showed a significant pollen-specific transmission defect resulting from pollen tube growth phenotypes, we propose that EXO70C2 is a key factor for efficient pollen tube growth. As the pollen transmission defect is not absolute, an additional EXO70 isoform(s) with redundant or synergistic functions must be responsible for the limited pollen tube growth in exo70C2 mutants. Closely related EXO70C1 most likely provides such a function, because the simultaneous disruption of both EXO70C1 and EXO70C2 leads to nonfunctional pollen. EXO70C1 and EXO70C2 are the closest paralogs, sharing 38% sequence identity at the protein level (which varies 14%–72% within the EXO70 family). On the other hand, the activity of EXO70C1 is not sufficient to compensate for the EXO70C2 loss of function; therefore, we assume that its function is only partially redundant to EXO70C2 despite their identical spatiotemporal distributions in pollen. Alternatively, this insufficiency could be related to the lower EXO70C1 expression level. It remains to be investigated if EXO70C1 has some extra function or whether is serves only as a backup to EXO70C2. Limited contributing activity to the EXO70C1/2 function also can be attributed to EXO70H3.
Importantly, our analyses of the pollen-expressed EXO70 paralogs indicate that the multiplicity of the EXO70 family in land plants could not be explained based only on the tissue/cell-specific expression, as proposed by Li et al. (2010); probably, several versions of the exocyst complex are active in land plant cell types (Žárský et al., 2009, 2013).
EXO70C2 Involvement in Cell Wall Deposition
In comparison with wild-type pollen tubes, exo70C2 pollen tubes exhibited significantly faster growth, which was interrupted by periods of growth arrest at very irregular frequency that were often a consequence of a pollen tube burst of various extents. Indeed, too high a growth rate has been documented in loss-of-function mutants in NADPH oxidases to precede a pollen tube collapse (Lassig et al., 2014). The documented weakened cell wall at the tube apex during the rapid growth is most likely the primary cause of the exo70C2 phenotype. Defects in cell wall deposition in seed coats, trichomes, cell plates, and xylem development are well described for several Arabidopsis exocyst mutants (Fendrych et al., 2010; Kulich et al., 2010, 2015; Li et al., 2013; Vukašinović et al., 2016). The delivery of methylesterified pectins, microfibrillar polysaccharides, and fucosylated xyloglucans as possible exocyst cargo is most likely affected because they represent dominant components at the growing pollen tube tip (Chebli et al., 2012). Also, mutants in Hyp O‐arabinosyltransferases (HPAT1 and HPAT3), enzymes important for modifications of cell wall extensins, exhibited a similar pollen tube phenotype to exo70C2 (MacAlister et al., 2016). Whether the EXO70C2 (and EXO70C1) involvement in the regulation of secretion is direct or indirect remains to be elucidated; nevertheless, we suggest below two major possible and opposing interpretations of the observed phenomenon.
First, since exocytosis of the PM material greatly exceeds the need for cell expansion in growing pollen tubes (Picton and Steer, 1983), exo70C2 could render a mild secretory defect, resulting in insufficient cell wall deposition but still delivering enough membrane to support the rapid growth. This would lead to a rapid turgor-driven expansion, sensitive to bursts. The ratio of cellulose to other components of the cell wall possibly would be shifted. In agreement, an abnormal concentration of retained pectins in the cytoplasm was observed in the exo70C2 pollen tubes. This interpretation also could be supported by the observation that in vivo-grown pollen tubes elongated more efficiently in comparison with in vitro conditions, probably due to stabilizing conditions in the transmitting tract, where the sensitive mutant pollen tube tips are less vulnerable.
Another interpretation is that EXO70C2 acquired a negative regulatory function, negatively affecting cell expansion under normal circumstances by, for example, interfering with putative exocyst regulatory molecules such as small GTPases or lipids. EXO70C2 and EXO70C1 then would probably contribute to the optimal balance between rates of vesicle delivery, exocytosis, and cellulose formation. This interpretation is in full agreement with previous experiments on NADPH oxidases in tip growth. When two NADPH oxidases, RBOHH and RBOHJ, were knocked out, the mutant pollen tubes exhibited higher expansion rates than the wild type, causing their burst (Lassig et al., 2014), similar to the exo70C2 mutant. This indicates that NADPH oxidases negatively regulate pollen tube growth to coordinate the rate of cell expansion with the rate of exocytosis. Such a regulation also is a plausible explanation for the exo70C2 mutant.
Is EXO70C2 Part of the Exocyst Complex?
In spite of clear indications that EXO70C2 is involved in the regulation of secretion in pollen tube tip growth, and bearing in mind both hypotheses suggested above, we assume that EXO70C2 (and EXO70C1) may not serve as a stable functional subunit of the exocyst complex, as it shows features distinct from conventional exocyst subunits concerning the morphology of mutant pollen tubes, PM localization, and physical interactions with exocyst subunits. We reason that EXO70C1/C2 act as transient or indirect modulators of the exocyst, resulting in optimal balance between cell wall biogenesis and surface growth.
Outside of the exocyst complex, EXO70C1/C2 very likely could affect secretion via ROH1, whose up-regulation results in a decreased seed coat mucilage layer (Kulich et al., 2010) and that is highly up-regulated in germinating pollen and root trichoblast cells (Arabidopsis eFP Browser). ROH1 is a putative negative regulator of secretion that is known to bind EXO70C1 and EXO70A1 (Kulich et al., 2010), and here, we report binding to EXO70C2 as well. The absence of EXO70C2 and the insufficient activity of EXO70C1 in the exo70C2 knockout would lead to the release of an interfering or negative regulation of exocytosis, and subsequently to the increased pollen tube growth rate, rendering the unstable growth with stop-and-go dynamics accompanied by a series of tip burst and recovery, finally resulting in a pollen tube collapse. The identified EXO70C2 and EXO70C1 phosphorylation (Mayank et al., 2012) also could play a role in this regulation.
EXO70C1/C2 also might gain novel functions in the regulation of polarized exocytosis unrelated to the exocyst. As postulated earlier (Žárský et al., 2013), it is highly probable that the diversified plant EXO70 isoforms acquired specific functions (even unrelated to exocytosis), since, in yeast and animal cells, the single EXO70 destabilizes the tubulin cytoskeleton (Wang et al., 2004) and influences actin dynamics (Zuo et al., 2006; Liu and Guo, 2012), lamellipodia formation by membrane deformation (Zhao et al., 2013), or pre-mRNA splicing (Dellago et al., 2011). The first examples of EXO70 subfunctionalization in Arabidopsis and Medicago spp. already have been published (see introduction).
If EXO70C2 and EXO70C1 are indeed not stable subunits of the exocyst complex, EXO70A2 is a good candidate for the core EXO70 subunit of the pollen exocyst, as the closest paralog to the prevalent sporophytic EXO70A1 exocyst subunit (72% sequence identity) and because its yeast two-hybrid interactions with other exocyst subunits also were similar to those of EXO70A1. Sekereš et al. (2017) documented the tobacco (Nicotiana tabacum) EXO70A paralog to be positively involved in exocytosis during tobacco pollen tube tip growth as a part of the exocyst complex.
EXO70C1 and EXO70C2 as Common Factors for Tip Growth in Both Male Gametophyte and Sporophyte
The localization pattern of GFP-tagged EXO70C1/C2 in Arabidopsis tissues fully matches the microarray data that indicate their pollen and trichoblast expression (Arabidopsis eFP Browser). Interestingly, this dual tissue specificity was suggested earlier based on a computational comparison of a translated Arabidopsis transcriptome with a soybean (Glycine max) proteome (Cvrčková et al., 2010). Since root hairs produced by trichoblast cells and pollen tubes represent prime examples of tip growth in plants, it is likely that the EXO70C1/C2 regulatory module is specifically recruited to modulate this mode of cell expansion based on highly polarized tip-focused secretion. A number of regulators specific to polar growth are logically strongly correlated with EXO70C1/C2, including three RAB GTPases, ROP-GEF3/11/12, and transcription factors, all representing their potential interacting partners (ATTED-II and Genevestigator). Especially, cooperation with ROP GTPases, major regulators of cell polarity, is highly probable, because their interaction with the exocyst subunits EXO70B1 and SEC3 already has been documented (Lavy et al., 2007; Hong et al., 2016). Future experimental work will likely reveal additional functional interactions of EXO70C2 and EXO70C1 with other players involved in tip growth and elucidate the precise mechanism of their action.
Although well-curated transcriptomic data for pollen-specific gene expression in other angiosperm species apart from Arabidopsis and rice are scarce (Rutley and Twell, 2015), a mere comparison of these two species indicates that the EXO70C class is probably related to pollen tube development in all angiosperms, suggesting conservation of EXO70C expression across monocots and dicots (Wei et al., 2010). This notion is supported by the tight clustering and relatively low divergence of EXO70C representatives in the EXO70 phylogenetic tree (Cvrčková et al., 2012).
CONCLUSION
Pollen tubes characterized by their specific mode of elongation (tip growth) represent an established model system for studies of cell expansion and its regulation in plant cell biology. Although the exact molecular mechanism of EXO70C1/C2 functions and the roles of other pollen EXO70 isoforms remain to be elucidated, we clearly demonstrated the importance of EXO70C1/C2 for optimal pollen tube growth via exocytosis moderation and, thus, revealed novel players in the complex network of tip growth regulation.
MATERIALS AND METHODS
Plant Material
Mutant plants of Arabidopsis (Arabidopsis thaliana) were obtained from the Nottingham Arabidopsis Stock Centre (SALK T-DNA lines in the Col-0 ecotype; Alonso et al., 2003), GABI-Kat (GABI T-DNA lines in the Col-0 ecotype; Kleinboelting et al., 2012), and RIKEN (RATM transposon lines in the Nossen-0 ecotype; Ito et al., 2002; Kuromori et al., 2004). The following lines were used in this study: exo70A2 (GABI_824D06), exo70C1-1 (GABI_100A02), exo70C1-2 (GABI_334D05), exo70C2-1 (RATM16-1469-1), exo70C2-2 (SALK_045767), exo70F1 (SALK_036927), exo70H3 (GABI_651C10), exo70H5 (SALK_007810), exo70H6 (SALK_016535), and qrt1-1 (CS8050; Preuss et al., 1994). Mutant plants were identified by selection when possible (GABI and RATM lines) and confirmed by PCR genotyping, then backcrossed to the wild type (Col-0).
The positions of insertions were verified by sequencing of PCR products obtained from ends of T-DNA/transposon insertions: exo70A2 (−160 bp), exo70C1-1 (+985 bp), exo70C1-2 (+1,030 bp), exo70C2-1 (+788 bp), exo70C2-2 (−405 bp), exo70F1 (+179 bp), exo70H3 (+31 bp), exo70H5 (−63 bp), and exo70H6 (−28 bp), where positive or negative numbers refer to nucleotide positions downstream or upstream, respectively, of the start codon in the genomic sequence.
Genotype Analysis and Semiquantitative RT-PCR
DNA from mutant plants was extracted from 20 mg of fresh leaves as described by Edwards et al. (1991). Plants were genotyped using PCR with T-DNA-specific (LBb1 and o8760) or transposon-specific (Ds5-2a) primers and gene-specific primers, all listed in Supplemental Table S1.
To test for the presence of gene transcripts in mutant plants, total RNA was isolated from 100 mg of flowers using the RNeasy kit (Qiagen). cDNA was synthesized using the Transcriptor High Fidelity cDNA synthesis kit (Roche). Levels of transcript abundance were analyzed by semiquantitative PCR with gene-specific pairs of primers (Supplemental Table S1). ACTIN7 was amplified as a quantitative control. Lines exo70C1-1, exo70C1-2, exo70C2-1, exo70F1, exo70H3, exo70H5, and exo70H6 were shown to be knockouts; exo70C2-2 with a promoter insertion produced EXO70C2 transcripts at the wild-type level; exo70A2 with a promoter insertion displayed overexpression of EXO70A2 (Supplemental Fig. S3).
Plant Cultivation
Seeds were surface sterilized (70% [v/v] ethanol for 3 min, 10% [v/v] commercial bleach for 10 min, and rinsed three times in sterile distilled water) and stratified for 3 d at 4°C. Seeds were then germinated on vertical 1/2 MS1 agar plates (one-half-strength Murashige and Skoog medium [Duchefa Biochemie] supplemented with 1% Suc, vitamin mixture, and 1.6% plant agar [Duchefa Biochemie]) at 21°C and 16 h of light per day. The RIKEN RATM16-1469-1 1ine, GABI lines, and qrt1-1 mutants were selected (when segregation was not of interest) on 1/2 MS1 agar plates with hygromycin (20 μg mL−1), sulfadiazine (7.5 μg mL−1), or Basta (50 μg mL−1), respectively. Eight-day-old seedlings were transferred to turf pellets (Jiffy Products International) and grown again at 22°C and 16 h of light per day.
Preparation of amiRNA-Driven Knockdown Lines
The amiRNAs against EXO70C1 and EXO70C2 (At5g13150 and At5g13990) were designed using the online microRNA designer (http://wmd3.weigelworld.org/cgi-bin/webapp.cgi) and then PCR amplified from the pRS300 vector as described by Schwab et al. (2006). Resulting amiRNA cassettes were cloned using XhoI and NcoI into pWEN240 under the control of the LAT52 promoter. Finally, amiRNA cassettes including the LAT52 promoter were further PCR amplified and subcloned to the pBAR1 vector using XmaI and XbaI. Sequences and primers are listed in Supplemental Table S1.
Resulting constructs with amiRNAxC1 or amiRNAxC2 were transformed by Agrobacterium tumefaciens-mediated transformation (Clough and Bent, 1998) into heterozygous mutants in EXO70C2 or EXO70C1, respectively. Primary transformants (T1) were selected for Basta (phosphinothricin) resistance, and the presence of amiRNA cassettes was verified by PCR genotyping. The transmission of amiRNA cassettes was further analyzed based on the Basta resistance of T2 seedlings. Primer sequences are listed in Supplemental Table S1.
In Vitro Arabidopsis Pollen Germination
Pollen was germinated on microscopic slides in 40-μL droplets of fresh germination medium [10% Suc, 1.6 mm 0.01% H3BO3, 1 mm CaCl2, 1 mm MgSO4, and 1 mm Ca(NO3)2, pH adjusted to 7.5]. Pollen grains from fully open flowers were spread onto each droplet, and one extracted pistil was dipped into each droplet. For confocal microscopy and kinetics, germination medium solidified with 1% low-melting-point agarose (Duchefa) was applied in a thin layer onto a chambered Lab-Tek II coverglass (Thermo Scientific). Slides or chambers were enclosed in a humid chamber and incubated in a plant growth room at 22°C for 4 h (samples for pollen tube morphology and live staining) or 14 h (samples for pollen tube length), if not indicated differently.
Germination Efficiency and Pollen Tube Morphology
Images of in vitro-germinated pollen (14 h) were taken using Olympus BX-51 with UPlanFL N 10×/0.3 (long working distance objective), differential interference contrast optics, epifluorescence filter sets, and a DP50 camera (Olympus). The field aperture was nearly closed to enhance the depth of focus.
Pollen grains were considered germinated when pollen tube length reached at least one-half of pollen grain diameter. Pollen tube length was measured with the AnalySIS software (Olympus). Detailed images of pollen tube morphology in bright field were taken using UPlanFL 20×/0.5 or UPlanFL 40×/0.75 and differential interference contrast optics on Olympus BX-51.
Callose Staining in Pistils
Self-pollinated pistils were harvested ∼12 h after the opening of anthers. Callose staining with Aniline Blue in the pistils was done according to Mori et al. (2006) and imaged using a Nikon 90i microscope with a PlanApo 4×/0.2 objective and a Clara camera (Andor).
Live Staining of Pollen Tubes with Fluorescent Dyes
Propidium iodide, Calcofluor White, FM4-64, or Aniline Blue was diluted in liquid germination medium and applied gently onto in vitro-germinated pollen before imaging. Images were captured using a Zeiss LSM 880 confocal laser scanning microscope with Plan-Apochromat 10×/0.45, Plan-Apochromat 20×/0.8, C-Apochromat 40×/1.2 WI, and C-Apochromat 63×/1.2 WI objectives. Working concentrations, excitations, and the range of recorded emissions were as follows: propidium iodide, 30 μm, 514 nm, and 566 to 719 nm; Calcofluor White, 1 µg mL−1, 405 nm, and 400 to 500 nm.
Measurement of Pollen Tube Growth Kinetics
For evaluation of the maximal growth rate, pollen tubes were germinated on solidified germination medium. Then, four positions with exo70C2 pollen and four with wild-type pollen were imaged for 5 h, each position every 90 s, using a Nikon 90i microscope with a PlanApo 10×/0.45 objective and a Clara camera (Andor), generating 1,392- × 1,040-pixel images. The growth rate was then calculated by measuring the difference in pollen tube length between frames using Fiji ImageJ (Schindelin et al., 2012). For the maximal pollen tube growth rate, values lower than 0.1 µm min−1 were excluded. The experiment was done in a triplicate with very similar results.
For the pollen tube kinetics, germinated pollen was stained with Calcofluor White (1 µg mL−1) diluted in liquid germination medium. Each pollen tube was imaged continuously every 5.3 s using a Zeiss LSM 880 confocal laser scanning microscope with C-Apochromat 40×/1.2 WI with excitation at 405 nm and emission recorded at 400 to 500 nm. The growth rate was then determined as above, and the Calcofluor White signal was measured as a mean intensity in a region of 20 × 30 pixels at the pollen tube apex through images with 14 pixels μm−1 resolution (cutouts of 300 × 140 pixels are presented in Fig. 4C).
Ruthenium Red Staining
Pollen tubes germinated on solid medium were covered in 0.0001% Ruthenium Red solution in distilled water to induce pollen tube burst and stain cytoplasmic pectins. The wild-type and mutant pollen was always applied side by side on one glass slide. Images (1,392 × 1,040 pixels) were taken using a Nikon 90i microscope with a PlanApo 10×/0.45 or PlanApo 40×/0.95 objective and a Clara camera (Andor). Staining intensity was quantified only in the extruded cytoplasm as relative intensity in the red channel after subtraction of the background.
Cloning of Gene Constructs with GFP and RFP
pEXO70C1::EXO70C1:GFP was prepared by PCR amplification of a promoter region 1,507 bp upstream from the start codon together with the EXO70C1 coding sequence (CDS; At5g13150) without stop codon from genomic DNA, bordered by SalI and NotI restriction sites (primer sequences are given in Supplemental Table S1), and cloned into SalI and NotI sites in pENTR3C Gateway vector (Invitrogen). This sequence was further transferred using LR Clonase II (Invitrogen) to pGWB4 Gateway vector (Nakagawa et al., 2007). The construct was then transformed by A. tumefaciens-mediated transformation (Clough and Bent, 1998) to exo70C1-1 homozygous mutants.
pEXO70C2::EXO70C2:GFP was generated analogously but with the addition of BglII and NotI restriction sites to the ends of the CDS (At5g13990) and cloning into BamHI and NotI sites in the pENTR3C vector (Invitrogen). The construct was then introduced to wild-type plants by A. tumefaciens-mediated transformation and, after selection, crossed to homozygous exo70C2-1 mutants (the pGWB4 vector and Tn insertion share the same hygromycin resistance, excluding a direct transformation of mutants). The presence of both the mutant allele and the introduced expression cassette was checked by PCR genotyping (primer sequences are given in Supplemental Table S1).
To prepare pEXO70C2::GFP:EXO70C2, the amplified EXO70C2 CDS bordered by XbaI sites was first cloned into pBAR1-GFP vector using the XbaI site. GFP:EXO70C2 was then reamplified from pBAR1 with the addition of attB2 sequence at the 3′ end and fused in subsequent overlapping PCR to the amplified promoter bordered by attB1 sequence at its 5′ end and a fragment of GFP at its 3′ end. This product was then cloned into pDONR201 (Invitrogen) using BP Clonase II (Invitrogen) and finally transferred to pGWB1 (Nakagawa et al., 2007) using LR Clonase II (Invitrogen). All primer sequences are listed in Supplemental Table S1. The construct was transformed to heterozygous exo70C2-1 mutants.
To prepare pUBQ10::RFP:EXO70A1, the PCR-amplified EXO70A1 CDS (At5g03540) was inserted into pENTR3C (Invitrogen) using EcoRI and NotI (primer sequences are given in Supplemental Table S1) and subsequently transferred to pUBN-RFP using LR Clonase II (Invitrogen). The construct was introduced by A. tumefaciens-mediated transformation into exo70A1-2 heterozygous mutants (Synek et al., 2006) and selected on Basta.
Imaging of GFP-Tagged EXO70C2, EXO70C1, and Exocyst Subunits
For complementation assays, EXO70C2:GFP was imaged using the Olympus BX-51 with an eGFP-specific narrow-pass filter set and mixed with bright field. At least 120 pollen tubes were then measured for each line in the AnalySIS software (Olympus).
Cellular localization of GFP-tagged EXO70C1/C2 was performed in a chambered Lab-Tek II coverglass (Thermo Scientific) using a Zeiss LSM 880 confocal laser scanning microscope equipped with Plan-Apochromat 10×/0.45, Plan-Apochromat 20×/0.8, and C-Apochromat 40×/1.2 WI objectives. FM4-64 (5 μm) was applied before imaging to visualize the PM. After excitation with a 488-nm laser, emitted fluorescence of GFP and FM4-64 was recorded at 493 to 535 nm and 575 to 650 nm, respectively.
Dynamic localization of EXO70C2, EXO70C1, SEC8, and SEC10a in pollen tube tips was performed using a spinning-disk confocal microscope (Yokogawa CSU-X1 on a Nikon Ti-E platform, Agilent MLC400 laser box, Zyla sCMOS camera [Andor], and NIS Elements 4.1 software). Exposure time was 300 ms, with 4× averaging, 488-nm laser power of 75%, and the images were taken every 4 s using a PlanApo 100×/1.4 lens. Five neighboring images were aligned and averaged for the final figures.
Yeast Two-Hybrid Assay
To test the interactions of EXO70C2 with exocyst subunits, we prepared two different fusions of EXO70C2 with DNA-binding domain (BD) or activating domain (AD). The full-length EXO70C2 CDS was PCR amplified with the stop codon and bordered with NdeI and SmaI restriction sites and then digested and cloned into the pGBKT7 vector or pGADT7, respectively (Clontech). The EXO70A2 CDS (At5g52340) was cloned analogously using EcoRI and SalI sites in pGBKT7. The SEC15a CDS (At3g56640) was bordered with BamHI and SalI sites and cloned into BamHI and XhoI in pGADT7. Primers for cloning are listed in Supplemental Table S1. Exocyst genes fused to AD or BD as well as BD-ROH1 and AD-EXO70C1 were cloned previously (Hála et al., 2008; Kulich et al., 2010).
Yeast two-hybrid screening employed the Matchmaker GAL4 Two-Hybrid System 3 (Clontech) following the manufacturer’s protocols. Yeast strain AH109 was stepwise transformed. Prior to the second step, expression of the fusion proteins was verified by western blotting using a rabbit polyclonal anti-GAL4-BD or anti-GAL4-AD antibody (1:1,000; Sigma) according to the manufacturer’s recommendations. Double transformed cells were selected on -Leu-Trp medium. Single colonies were then scale diluted in sterile water and dropped at 10 μL onto -Ade-His-Leu-Trp selective medium.
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. Expression of candidate pollen EXO70 genes during pollen development in Arabidopsis.
Supplemental Figure S2. EXO70C1 and EXO70C2 expression in Arabidopsis roots.
Supplemental Figure S3. Positions of insertions in exo70 mutant lines and semiquantitative RT-PCR analysis of gene expression of the affected EXO70 isoforms.
Supplemental Figure S4. Propidium iodide staining of protoplast-like structures emerging from the exo70C2 pollen tube apex.
Supplemental Figure S5. Pollen tube lengths of complemented exo70C2 lines nonsegregating for the pEXO70C2::EXO70C2:GFP expression cassette.
Supplemental Figure S6. The localization of pEXO70C2::GFP:EXO70C2 is identical to that of pEXO70C2::EXO70C2:GFP.
Supplemental Table S1. List of primers used in this study.
Supplemental Movie S1. Growth dynamics of exo70C2 and wild-type pollen tubes stained with Calcofluor White.
Supplemental Movie S2. Growth dynamics of exo70C2 and wild-type pollen tubes stained with Calcofluor White.
Supplemental Movie S3. Multiple exo70C2 pollen tube bursts and recovery.
Supplemental File S1. Interactive model of genetic segregation.
Supplementary Material
Acknowledgments
We thank Martin Potocký (IEB ASCR) for helpful discussions on this project, on data presentation, and for box plot rendering; Roman Pleskot (IEB ASCR) for the sequence identity calculation; David E. Jarvis (KAUST) for language corrections; and Tsuyoshi Nakagawa (Research Institute of Molecular Genetics, Shimane University) for providing pGWB vectors. The T-DNA GABI lines analyzed here were generated in the context of the GABI-Kat program and provided by B. Weisshaar (Max Planck Institute for Plant Breeding Research).
Glossary
- PM
plasma membrane
- RNA-Seq
RNA sequencing
- Col-0
Columbia-0
- amiRNA
artificial microRNA
- RT
reverse transcription
- CDS
coding sequence
Footnotes
This work was supported by the Czech Science Foundation (grant no. 15-24711S). The income of V.Ž. and I.K. was partly covered by the Ministry of Education of the Czech Republic (grant no. NPUI LO1417).
References
- Alonso JM, Stepanova AN, Leisse TJ, Kim CJ, Chen H, Shinn P, Stevenson DK, Zimmerman J, Barajas P, Cheuk R, et al. (2003) Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science 301: 653–657 [DOI] [PubMed] [Google Scholar]
- Bashline L, Lei L, Li S, Gu Y (2014) Cell wall, cytoskeleton, and cell expansion in higher plants. Mol Plant 7: 586–600 [DOI] [PubMed] [Google Scholar]
- Bloch D, Pleskot R, Pejchar P, Potocký M, Trpkošová P, Cwiklik L, Vukašinović N, Sternberg H, Yalovsky S, Žárský V (2016) Exocyst SEC3 and phosphoinositides define sites of exocytosis in pollen tube initiation and growth. Plant Physiol 172: 980–1002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chebli Y, Kaneda M, Zerzour R, Geitmann A (2012) The cell wall of the Arabidopsis pollen tube: spatial distribution, recycling, and network formation of polysaccharides. Plant Physiol 160: 1940–1955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chebli Y, Kroeger J, Geitmann A (2013) Transport logistics in pollen tubes. Mol Plant 6: 1037–1052 [DOI] [PubMed] [Google Scholar]
- Cheung AY, Wu HM (2008) Structural and signaling networks for the polar cell growth machinery in pollen tubes. Annu Rev Plant Biol 59: 547–572 [DOI] [PubMed] [Google Scholar]
- Clough SJ, Bent AF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16: 735–743 [DOI] [PubMed] [Google Scholar]
- Cole RA, Fowler JE (2006) Polarized growth: maintaining focus on the tip. Curr Opin Plant Biol 9: 579–588 [DOI] [PubMed] [Google Scholar]
- Cole RA, McInally SA, Fowler JE (2014) Developmentally distinct activities of the exocyst enable rapid cell elongation and determine meristem size during primary root growth in Arabidopsis. BMC Plant Biol 14: 386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole RA, Synek L, Žárský V, Fowler JE (2005) SEC8, a subunit of the putative Arabidopsis exocyst complex, facilitates pollen germination and competitive pollen tube growth. Plant Physiol 138: 2005–2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cvrčková F, Bezvoda R, Zárský V (2010) Computational identification of root hair-specific genes in Arabidopsis. Plant Signal Behav 5: 1407–1418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cvrčková F, Eliáš M, Hála M, Obermeyer G, Žárský V (2001) Small GTPases and conserved signalling pathways in plant cell morphogenesis: from exocytosis to exocyst. In Geitmann A, Cresti M, eds, Cell Biology of Plant and Fungal Tip Growth. IOS Press, Amsterdam, pp 105–122 [Google Scholar]
- Cvrčková F, Grunt M, Bezvoda R, Hála M, Kulich I, Rawat A, Zárský V (2012) Evolution of the land plant exocyst complexes. Front Plant Sci 3: 159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dellago H, Löscher M, Ajuh P, Ryder U, Kaisermayer C, Grillari-Voglauer R, Fortschegger K, Gross S, Gstraunthaler A, Borth N, et al. (2011) Exo70, a subunit of the exocyst complex, interacts with SNEV(hPrp19/hPso4) and is involved in pre-mRNA splicing. Biochem J 438: 81–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drdová EJ, Synek L, Pečenková T, Hála M, Kulich I, Fowler JE, Murphy AS, Zárský V (2013) The exocyst complex contributes to PIN auxin efflux carrier recycling and polar auxin transport in Arabidopsis. Plant J 73: 709–719 [DOI] [PubMed] [Google Scholar]
- Edwards K, Johnstone C, Thompson C (1991) A simple and rapid method for the preparation of plant genomic DNA for PCR analysis. Nucleic Acids Res 19: 1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliáš M, Drdová E, Ziak D, Bavlnka B, Hála M, Cvrčková F, Soukupová H, Žárský V (2003) The exocyst complex in plants. Cell Biol Int 27: 199–201 [DOI] [PubMed] [Google Scholar]
- Fendrych M, Synek L, Pecenková T, Drdová EJ, Sekereš J, de Rycke R, Nowack MK, Zársky V (2013) Visualization of the exocyst complex dynamics at the plasma membrane of Arabidopsis thaliana. Mol Biol Cell 24: 510–520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fendrych M, Synek L, Pecenková T, Toupalová H, Cole R, Drdová E, Nebesárová J, Sedinová M, Hála M, Fowler JE, et al. (2010) The Arabidopsis exocyst complex is involved in cytokinesis and cell plate maturation. Plant Cell 22: 3053–3065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y. (2015) The cytoskeleton in the pollen tube. Curr Opin Plant Biol 28: 111–119 [DOI] [PubMed] [Google Scholar]
- Grobei MA, Qeli E, Brunner E, Rehrauer H, Zhang R, Roschitzki B, Basler K, Ahrens CH, Grossniklaus U (2009) Deterministic protein inference for shotgun proteomics data provides new insights into Arabidopsis pollen development and function. Genome Res 19: 1786–1800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W, Grant A, Novick P (1999) Exo84p is an exocyst protein essential for secretion. J Biol Chem 274: 23558–23564 [DOI] [PubMed] [Google Scholar]
- Hála M, Cole R, Synek L, Drdová E, Pecenková T, Nordheim A, Lamkemeyer T, Madlung J, Hochholdinger F, Fowler JE, et al. (2008) An exocyst complex functions in plant cell growth in Arabidopsis and tobacco. Plant Cell 20: 1330–1345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heider MR, Munson M (2012) Exorcising the exocyst complex. Traffic 13: 898–907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hepler PK, Rounds CM, Winship LJ (2013) Control of cell wall extensibility during pollen tube growth. Mol Plant 6: 998–1017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hepler PK, Winship LJ (2015) The pollen tube clear zone: clues to the mechanism of polarized growth. J Integr Plant Biol 57: 79–92 [DOI] [PubMed] [Google Scholar]
- Hong D, Jeon BW, Kim SY, Hwang JU, Lee Y (2016) The ROP2-RIC7 pathway negatively regulates light-induced stomatal opening by inhibiting exocyst subunit Exo70B1 in Arabidopsis. New Phytol 209: 624–635 [DOI] [PubMed] [Google Scholar]
- Hou WC, Chang WH, Jiang CM (1999) Qualitative distinction of carboxyl group distributions in pectins with ruthenium red. Bot Bull Acad Sin 40: 115–119 [Google Scholar]
- Ito T, Motohashi R, Kuromori T, Mizukado S, Sakurai T, Kanahara H, Seki M, Shinozaki K (2002) A new resource of locally transposed Dissociation elements for screening gene-knockout lines in silico on the Arabidopsis genome. Plant Physiol 129: 1695–1699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson-Brousseau SA, McCormick S (2004) A compendium of methods useful for characterizing Arabidopsis pollen mutants and gametophytically-expressed genes. Plant J 39: 761–775 [DOI] [PubMed] [Google Scholar]
- Kitashiba H, Liu P, Nishio T, Nasrallah JB, Nasrallah ME (2011) Functional test of Brassica self-incompatibility modifiers in Arabidopsis thaliana. Proc Natl Acad Sci USA 108: 18173–18178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinboelting N, Huep G, Kloetgen A, Viehoever P, Weisshaar B (2012) GABI-Kat SimpleSearch: new features of the Arabidopsis thaliana T-DNA mutant database. Nucleic Acids Res 40: D1211–D1215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulich I, Cole R, Drdová E, Cvrcková F, Soukup A, Fowler J, Zárský V (2010) Arabidopsis exocyst subunits SEC8 and EXO70A1 and exocyst interactor ROH1 are involved in the localized deposition of seed coat pectin. New Phytol 188: 615–625 [DOI] [PubMed] [Google Scholar]
- Kulich I, Pečenková T, Sekereš J, Smetana O, Fendrych M, Foissner I, Höftberger M, Zárský V (2013) Arabidopsis exocyst subcomplex containing subunit EXO70B1 is involved in autophagy-related transport to the vacuole. Traffic 14: 1155–1165 [DOI] [PubMed] [Google Scholar]
- Kulich I, Vojtíková Z, Glanc M, Ortmannová J, Rasmann S, Žárský V (2015) Cell wall maturation of Arabidopsis trichomes is dependent on exocyst subunit EXO70H4 and involves callose deposition. Plant Physiol 168: 120–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuromori T, Hirayama T, Kiyosue Y, Takabe H, Mizukado S, Sakurai T, Akiyama K, Kamiya A, Ito T, Shinozaki K (2004) A collection of 11 800 single-copy Ds transposon insertion lines in Arabidopsis. Plant J 37: 897–905 [DOI] [PubMed] [Google Scholar]
- Lai KS. (2016) Analysis of EXO70C2 expression revealed its specific association with late stages of pollen development. Plant Cell Tissue Organ Cult 124: 209–215 [Google Scholar]
- Lassig R, Gutermuth T, Bey TD, Konrad KR, Romeis T (2014) Pollen tube NAD(P)H oxidases act as a speed control to dampen growth rate oscillations during polarized cell growth. Plant J 78: 94–106 [DOI] [PubMed] [Google Scholar]
- Lavy M, Bloch D, Hazak O, Gutman I, Poraty L, Sorek N, Sternberg H, Yalovsky S (2007) A novel ROP/RAC effector links cell polarity, root-meristem maintenance, and vesicle trafficking. Curr Biol 17: 947–952 [DOI] [PubMed] [Google Scholar]
- Li S, Chen M, Yu D, Ren S, Sun S, Liu L, Ketelaar T, Emons AMC, Liu CM (2013) EXO70A1-mediated vesicle trafficking is critical for tracheary element development in Arabidopsis. Plant Cell 25: 1774–1786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, van Os GM, Ren S, Yu D, Ketelaar T, Emons AMC, Liu CM (2010) Expression and functional analyses of EXO70 genes in Arabidopsis implicate their roles in regulating cell type-specific exocytosis. Plant Physiol 154: 1819–1830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Guo W (2012) The exocyst complex in exocytosis and cell migration. Protoplasma 249: 587–597 [DOI] [PubMed] [Google Scholar]
- Loraine AE, McCormick S, Estrada A, Patel K, Qin P (2013) RNA-seq of Arabidopsis pollen uncovers novel transcription and alternative splicing. Plant Physiol 162: 1092–1109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacAlister CA, Ortiz-Ramírez C, Becker JD, Feijó JA, Lippman ZB (2016) Hydroxyproline O-arabinosyltransferase mutants oppositely alter tip growth in Arabidopsis thaliana and Physcomitrella patens. Plant J 85: 193–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayank P, Grossman J, Wuest S, Boisson-Dernier A, Roschitzki B, Nanni P, Nühse T, Grossniklaus U (2012) Characterization of the phosphoproteome of mature Arabidopsis pollen. Plant J 72: 89–101 [DOI] [PubMed] [Google Scholar]
- McKenna ST, Kunkel JG, Bosch M, Rounds CM, Vidali L, Winship LJ, Hepler PK (2009) Exocytosis precedes and predicts the increase in growth in oscillating pollen tubes. Plant Cell 21: 3026–3040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori T, Kuroiwa H, Higashiyama T, Kuroiwa T (2006) GENERATIVE CELL SPECIFIC 1 is essential for angiosperm fertilization. Nat Cell Biol 8: 64–71 [DOI] [PubMed] [Google Scholar]
- Moscatelli A, Idilli AI (2009) Pollen tube growth: a delicate equilibrium between secretory and endocytic pathways. J Integr Plant Biol 51: 727–739 [DOI] [PubMed] [Google Scholar]
- Nakagawa T, Kurose T, Hino T, Tanaka K, Kawamukai M, Niwa Y, Toyooka K, Matsuoka K, Jinbo T, Kimura T (2007) Development of series of gateway binary vectors, pGWBs, for realizing efficient construction of fusion genes for plant transformation. J Biosci Bioeng 104: 34–41 [DOI] [PubMed] [Google Scholar]
- Pečenková T, Hála M, Kulich I, Kocourková D, Drdová E, Fendrych M, Toupalová H, Zársky V (2011) The role for the exocyst complex subunits Exo70B2 and Exo70H1 in the plant-pathogen interaction. J Exp Bot 62: 2107–2116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeffer SR. (2013) Rab GTPase regulation of membrane identity. Curr Opin Cell Biol 25: 414–419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picton JM, Steer MW (1983) Membrane recycling and the control of secretory activity in pollen tubes. J Cell Sci 63: 303–310 [DOI] [PubMed] [Google Scholar]
- Preuss D, Rhee SY, Davis RW (1994) Tetrad analysis possible in Arabidopsis with mutation of the QUARTET (QRT) genes. Science 264: 1458–1460 [DOI] [PubMed] [Google Scholar]
- Rounds CM, Lubeck E, Hepler PK, Winship LJ (2011) Propidium iodide competes with Ca2+ to label pectin in pollen tubes and Arabidopsis root hairs. Plant Physiol 157: 175–187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutley N, Twell D (2015) A decade of pollen transcriptomics. Plant Reprod 28: 73–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rybak K, Steiner A, Synek L, Klaeger S, Kulich I, Facher E, Wanner G, Kuster B, Žárský V, Persson S, et al. (2014) Plant cytokinesis is orchestrated by the sequential action of the TRAPPII and exocyst tethering complexes. Dev Cell 29: 607–620 [DOI] [PubMed] [Google Scholar]
- Safavian D, Zayed Y, Indriolo E, Chapman L, Ahmed A, Goring DR (2015) RNA silencing of exocyst genes in the stigma impairs the acceptance of compatible pollen in Arabidopsis. Plant Physiol 169: 2526–2538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel MA, Chong YT, Haasen KE, Aldea-Brydges MG, Stone SL, Goring DR (2009) Cellular pathways regulating responses to compatible and self-incompatible pollen in Brassica and Arabidopsis stigmas intersect at Exo70A1, a putative component of the exocyst complex. Plant Cell 21: 2655–2671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanati Nezhad A, Packirisamy M, Geitmann A (2014) Dynamic, high precision targeting of growth modulating agents is able to trigger pollen tube growth reorientation. Plant J 80: 185–195 [DOI] [PubMed] [Google Scholar]
- Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, et al. (2012) Fiji: an open-source platform for biological-image analysis. Nat Methods 9: 676–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab R, Ossowski S, Riester M, Warthmann N, Weigel D (2006) Highly specific gene silencing by artificial microRNAs in Arabidopsis. Plant Cell 18: 1121–1133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekereš J, Pejchar P, Šantrůček J, Vukašinović N, Žárský V, Potocký M (2017) Analysis of exocyst subunit EXO70 family reveals distinct membrane domains in tobacco pollen tubes. Plant Physiol 173: 1659–1675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stegmann M, Anderson RG, Ichimura K, Pečenková T, Reuter P, Žársky V, McDowell JM, Shirasu K, Trujillo M (2012) The ubiquitin ligase PUB22 targets a subunit of the exocyst complex required for PAMP-triggered responses in Arabidopsis. Plant Cell 24: 4703–4716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Synek L, Schlager N, Eliás M, Quentin M, Hauser MT, Zárský V (2006) AtEXO70A1, a member of a family of putative exocyst subunits specifically expanded in land plants, is important for polar growth and plant development. Plant J 48: 54–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- TerBush DR, Maurice T, Roth D, Novick P (1996) The exocyst is a multiprotein complex required for exocytosis in Saccharomyces cerevisiae. EMBO J 15: 6483–6494 [PMC free article] [PubMed] [Google Scholar]
- TerBush DR, Novick P (1995) Sec6, Sec8, and Sec15 are components of a multisubunit complex which localizes to small bud tips in Saccharomyces cerevisiae. J Cell Biol 130: 299–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu B, Hu L, Chen W, Li T, Hu B, Zheng L, Lv Z, You S, Wang Y, Ma B, et al. (2015) Disruption of OsEXO70A1 causes irregular vascular bundles and perturbs mineral nutrient assimilation in rice. Sci Rep 5: 18609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vega IE, Hsu SC (2001) The exocyst complex associates with microtubules to mediate vesicle targeting and neurite outgrowth. J Neurosci 21: 3839–3848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vukašinović N, Cvrčková F, Eliáš M, Cole R, Fowler JE, Žárský V, Synek L (2014) Dissecting a hidden gene duplication: the Arabidopsis thaliana SEC10 locus. PLoS ONE 9: e94077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vukašinović N, Oda Y, Pejchar P, Synek L, Pečenková T, Rawat A, Sekereš J, Potocký M, Žárský V (2016) Microtubule-dependent targeting of the exocyst complex is necessary for the xylem development in Arabidopsis. New Phytol 213: 1052–1067 [DOI] [PubMed] [Google Scholar]
- Vukašinović N, Žárský V (2016) Tethering complexes in the Arabidopsis endomembrane system. Front Cell Dev Biol 4: 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Liu Y, Adamson CL, Valdez G, Guo W, Hsu SC (2004) The mammalian exocyst, a complex required for exocytosis, inhibits tubulin polymerization. J Biol Chem 279: 35958–35966 [DOI] [PubMed] [Google Scholar]
- Wei LQ, Xu WY, Deng ZY, Su Z, Xue Y, Wang T (2010) Genome-scale analysis and comparison of gene expression profiles in developing and germinated pollen in Oryza sativa. BMC Genomics 11: 338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter D, Vinegar B, Nahal H, Ammar R, Wilson GV, Provart NJ (2007) An “Electronic Fluorescent Pictograph” browser for exploring and analyzing large-scale biological data sets. PLoS ONE 2: e718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H, Rossi G, Brennwald P (2008) The ghost in the machine: small GTPases as spatial regulators of exocytosis. Trends Cell Biol 18: 397–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Žárský V, Cvrcková F, Potocký M, Hála M (2009) Exocytosis and cell polarity in plants: exocyst and recycling domains. New Phytol 183: 255–272 [DOI] [PubMed] [Google Scholar]
- Žárský V, Kulich I, Fendrych M, Pečenková T (2013) Exocyst complexes multiple functions in plant cells secretory pathways. Curr Opin Plant Biol 16: 726–733 [DOI] [PubMed] [Google Scholar]
- Žárský V, Potocký M (2010) Recycling domains in plant cell morphogenesis: small GTPase effectors, plasma membrane signalling and the exocyst. Biochem Soc Trans 38: 723–728 [DOI] [PubMed] [Google Scholar]
- Zhang C, Brown MQ, van de Ven W, Zhang ZM, Wu B, Young MC, Synek L, Borchardt D, Harrison R, Pan S, et al. (2016) Endosidin2 targets conserved exocyst complex subunit EXO70 to inhibit exocytosis. Proc Natl Acad Sci USA 113: E41–E50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Pumplin N, Ivanov S, Harrison MJ (2015) EXO70I is required for development of a sub-domain of the periarbuscular membrane during arbuscular mycorrhizal symbiosis. Curr Biol 25: 2189–2195 [DOI] [PubMed] [Google Scholar]
- Zhao Y, Liu J, Yang C, Capraro BR, Baumgart T, Bradley RP, Ramakrishnan N, Xu X, Radhakrishnan R, Svitkina T, et al. (2013) Exo70 generates membrane curvature for morphogenesis and cell migration. Dev Cell 26: 266–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann P, Hirsch-Hoffmann M, Hennig L, Gruissem W (2004) GENEVESTIGATOR: Arabidopsis microarray database and analysis toolbox. Plant Physiol 136: 2621–2632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zonia L, Munnik T (2008) Vesicle trafficking dynamics and visualization of zones of exocytosis and endocytosis in tobacco pollen tubes. J Exp Bot 59: 861–873 [DOI] [PubMed] [Google Scholar]
- Zonia L, Munnik T (2009) Uncovering hidden treasures in pollen tube growth mechanics. Trends Plant Sci 14: 318–327 [DOI] [PubMed] [Google Scholar]
- Zuo X, Zhang J, Zhang Y, Hsu SC, Zhou D, Guo W (2006) Exo70 interacts with the Arp2/3 complex and regulates cell migration. Nat Cell Biol 8: 1383–1388 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.