Phospholipase D increases the production of triacylglycerol in Camelina sativa seeds.
Abstract
Plant seeds are the primary source of triacylglycerols (TAG) for food, feed, fuel, and industrial applications. As TAG is produced from diacylglycerol (DAG), successful engineering strategies to enhance TAG levels have focused on the conversion of DAG to TAG. However, the production of TAG can be limited by flux through the enzymatic reactions that supply DAG. In this study, two Arabidopsis phospholipase Dζ genes (AtPLDζ1 and AtPLDζ2) were coexpressed in Camelina sativa to test whether the conversion of phosphatidylcholine to DAG impacts TAG levels in seeds. The resulting transgenic plants produced 2% to 3% more TAG as a component of total seed biomass and had increased 18:3 and 20:1 fatty acid levels relative to wild type. Increased DAG and decreased PC levels were examined through the kinetics of lipid assembly by [14C]acetate and [14C]glycerol incorporation into glycerolipids. [14C]acetate was rapidly incorporated into TAG in both wild-type and overexpression lines, indicating a significant flux of nascent and elongated acyl-CoAs into the sn-3 position of TAG. Stereochemical analysis revealed that newly synthesized fatty acids were preferentially incorporated into the sn-2 position of PC, but the sn-1 position of de novo DAG and indicated similar rates of nascent acyl groups into the Kennedy pathway and acyl editing. [14C]glycerol studies demonstrated PC-derived DAG is the major source of DAG for TAG synthesis in both tissues. The results emphasize that the interconversions of DAG and PC pools can impact oil production and composition.
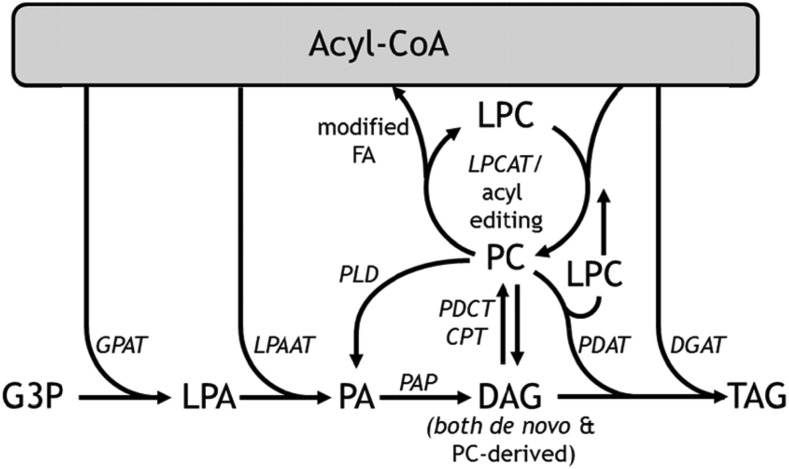
Plant oils are an important source of energy for human and animal diets and are regularly used in production of biofuels and industrial products (Lu et al., 2011). Therefore, there is considerable effort to increase the oil quality and yield in plants through breeding and application of biotechnology (Tan et al., 2011; Shi et al., 2012; Haslam et al., 2013; Vanhercke et al., 2013; van Erp et al., 2014; Li et al., 2015a). Though many of the involved genes have been cataloged and enzyme activities measured, a better understanding of the coordinated metabolic network (Fig. 1) that produces both membrane lipids and storage oils is necessary. Plant oil (triacylglycerol, TAG) biosynthesis begins with generation of 16 and 18 carbon fatty acids (FA) in the plastid (Li-Beisson et al., 2013; Allen et al., 2015). Acyl chains are transported across the plastid envelope (Koo et al., 2004; Li et al., 2015b) and activated to acyl-CoAs (Shockey et al., 2002). Then they can be elongated to lengths of 20 carbons or more (Kunst et al., 1992), shuttled directly into the endoplasmic reticulum (ER) for desaturation on phosphatidylcholine (PC; Stymne and Glad, 1981; Bates et al., 2009), or used by Kennedy pathway enzymes to generate lysophosphatidic acid (LPA) and subsequently phosphatidic acid (PA; Kornberg and Pricer, 1953; Weiss et al., 1960; Kennedy, 1961). PA is converted to diacylglycerol (DAG) by PA phosphatase (Eastmond et al., 2010; Mietkiewska et al., 2011; Pascual and Carman, 2013; Craddock et al., 2015). This de novo synthesized DAG can then be utilized for membrane lipid synthesis or converted to TAG in plants by a further acylation at sn-3 using either the acyl-CoA-dependent diacylglycerol acyltransferase (DGAT; Barron and Stumpf, 1962; Griffiths et al., 1985; Katavic et al., 1995; Zou et al., 1999), or by the transfer of the sn-2 FA from PC to DAG by phosphatidylcholine:diacylglycerol acyltransferase (PDAT; Dahlqvist et al., 2000). These mechanisms of plant TAG biosynthesis summarized in Figure 1 have recently been reviewed in extensive detail (Weselake et al., 2009; Zhang et al., 2009; Chapman and Ohlrogge, 2012; Li-Beisson et al., 2013; Allen et al., 2015, 2016; Bates, 2016).
Figure 1.
Simplified metabolic network description of acyl chain incorporation into TAG in oilseeds. Enzymes are labeled in italics. LPAAT, lyso-PA acyltransferase.
The DAG for TAG synthesis can also be derived from the ER lipid, PC (Bates, 2016). FA desaturation reactions modify acyl chains that are esterified to PC, thus the flux through PC leads to an enhanced degree of unsaturated FA in the PC-derived DAG pool relative to de novo DAG and can provide an increased source of polyunsaturated fatty acid (PUFA) for TAG biosynthesis (Griffiths et al., 1988; Bates et al., 2009; Bates and Browse, 2012). PC is produced from de novo DAG by CDP-choline:1,2-diacylglycerol cholinephosphotransferase (CDP-choline), which may be reversible and consequently produce PC-derived DAG (Slack et al., 1983, 1985). Alternatively, flux from de novo DAG through PC and back to PC-derived DAG may occur through a phosphocholine headgroup exchange mechanism with phosphatidylcholine:diacylglycerol cholinephosphotransferase (PDCT; Lu et al., 2009). In Arabidopsis (Arabidopsis thaliana), the PDCT mutant (rod1) reduces the PUFA content of TAG by 40%, emphasizing the important role of DAG flux through PC for acyl desaturation before incorporation into TAG (Lu et al., 2009).
Other mechanisms also participate in shuttling acyl chains through PC to promote DAG and TAG formation with greater concentrations of desaturates. For example, PC can be produced through esterification of FA to lysophosphatidylcholine (LPC) with lysophosphatidylcholine acyltransferase (LPCAT; Stymne and Stobart, 1984; Bates et al., 2012; Wang et al., 2012) and after modification the FA is released for reentry into the acyl-CoA pool, regenerating LPC and completing the cycle in a process coined acyl editing (Williams et al., 2000; Bates et al., 2009; Bates and Browse, 2012; Tjellström et al., 2012). In soybean (Glycine max) and Arabidopsis it is estimated that >90% of nascent FAs flux through PC by acyl editing or as DAG components before incorporation into TAG (Bates et al., 2009; Bates and Browse, 2011, 2012). However, the mechanisms involved in this acyl flux have not been thoroughly explored. For example, knockouts of the major enzymes involved in Arabidopsis PC acyl editing (LPCAT1/2) and PC-DAG exchange (PDCT) only reduced the TAG desaturate content by 2/3 (Bates et al., 2012). The suppression of phospholipase D (PLD) in soybeans partially reduced the PUFA content of TAG (Lee et al., 2011), indicating an alternative mechanism to PDCT based PC-derived DAG production. Possibly the flux of de novo DAG into PC by CPT, followed by PLD and PA phosphatase activity, is necessary to produce the DAG substrate for TAG synthesis. In Arabidopsis, two PLDζ genes, AtPLDζ1 and AtPLDζ2, have been identified that hydrolyze PC to produce PA (Qin and Wang, 2002; Cruz-Ramírez et al., 2006). Because altered levels of PA have been shown to stimulate production of PC (Eastmond et al., 2010; Craddock et al., 2015); we hypothesized that increased PLD activity in oilseeds would enhance both the flux of de novo DAG into PC and the flux of PC-derived DAG into TAG, leading to enhanced levels of TAG and the accumulation of PUFA in TAG.
Here we show that overexpression of PLDζs in Camelina (Camelina sativa), a close relative of Arabidopsis, had enhanced acyl flux to TAG through an increase in the levels of DAG. Camelina was chosen for obvious advantages—it can be floral-dip transformed (Lu et al., 2011); it has a short lifecycle; and it has oil-rich seeds (30% to 40% of seed weight) that contain high levels of PUFAs (>50%), especially α-linolenic acid (18:3, >30% of total fatty acids; Campbell et al., 2013; Iskandarov et al., 2013). Recently phospholipase A overexpression in Camelina resulted in an increase in total oil (Li et al., 2015a), and other transgenics have resulted in TAG containing nonnative FAs (Lu and Kang, 2008; Petrie et al., 2014; Ruiz-Lopez et al., 2014; Liu et al., 2015; Nguyen et al., 2015). However, mechanisms that control acyl flux through PC into TAG have not been studied as extensively in Camelina as in other species.
In the described work, the heterologous coexpression of two Arabidopsis PLDζ’s (AtPLDζ1 and AtPLDζ2) in Camelina seeds resulted in a 3% increase in TAG and fatty acid composition that was elevated in linolenic and eicosenoic acids. Production of active forms of AtPLDζ1 and AtPLDζ2 was confirmed by RT-PCR and enzyme activity assays with microsomes. Differences in lipid and acyl chain composition from the wild type were consistently observed throughout seed development. [14C]acetate pulse labeling indicated newly synthesized acyl groups were rapidly incorporated into TAG. The rate of [14C]acetate incorporation as fatty acids into PC and DAG were similar, but stereochemically involved different sites of attachment on the glycerol backbone, indicating acyl flux through different branches of lipid metabolism (Fig. 1). [14C]glycerol labeling indicated a prominent role of the PC-derived DAG pathway in Camelina, but significantly more flux through the Kennedy pathway relative to other oilseeds such as Arabidopsis (Bates et al., 2012) or soybean (Bates et al., 2009).
RESULTS
AtPLDζ Overexpression Results in a High Oil Phenotype
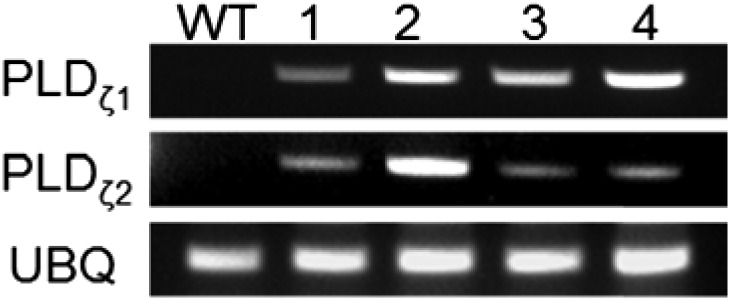
Two PLDζ genes, AtPLDζ1 and AtPLDζ2, have been identified in the Arabidopsis genome (Qin and Wang, 2002; Li et al., 2006). In vitro assays indicated that AtPLDζ1 specifically hydrolyzes PC to produce PA and choline (Qin and Wang, 2002). In this study, AtPLDζ1 and AtPLDζ2 cDNA ORFs were placed behind the seed-specific glycinin and β-conglycinin promoters, respectively. Camelina plants were transformed with Agrobacterium containing a binary vector for coexpression of AtPLDζ1 and AtPLDζ2, and DsRed selection marker. Homozygosity was inspected by screening with DsRed and the expression of both AtPLDζ genes was confirmed in developing seeds through RT-PCR (Fig. 2). No visual differences in plant phenotypes were observed between wild-type and transgenic plants. In vitro assays of PLDζ activity within microsomes prepared from developing seeds indicated increased PLD activity in the transgenic lines (86 to 269 pmol/h/g) relative to wild-type controls (65.74 pmol/h/g; Table I). Measurable activity in total protein extracts was not detected, indicating that AtPLDζ is highly enriched in microsomes and is likely associated with the endoplasmic reticulum in developing Camelina seeds; however, the mechanism of ER association is unclear due to the lack of a predicted transmembrane domain in either AtPLDζ isoform. Four transgenic lines overexpressing both PLDζ isoforms (subsequently referred to as “PLDζ 1–4”) contained 1.9% to 3.5% more oil than wild type on a dry-weight basis across seed development, and 1.9% to 3% in mature seeds (Table I). This is equivalent to an average 12.7% increase in total oil content relative to wild type at 12 days after flowering (DAF), and 9.5% increase in mature seeds.
Figure 2.
Transcript level of AtPLDζ1 and AtPLDζ2 in developing seeds at 22 DAF of Camelina wild-type and PLDζ overexpression lines.
Table I. Oil content in developing and mature seeds of wild-type and PLDζ lines, and PLDζ activity measured in developing seeds of wild type and PLDζ lines at 20 DAF.
| Description/line | Wild type | 1 | 2 | 3 | 4 |
|---|---|---|---|---|---|
| Oil level (% dry weight) | |||||
| 12 DAF | 15.76 ± 0.91 | 17.67 ± 0.48* | 17.00 ± 0.13* | 17.21 ± 0.08* | 19.30 ± 0.11* |
| 20 DAF | 22.64 ± 0.71 | 25.84 ± 0.42* | 24.61 ± 0.35* | 27.03 ± 0.66* | 25.92 ± 0.65* |
| Mature | 24.90 ± 0.60 | 27.96 ± 0.60* | 26.86 ± 1.00* | 27.47 ± 1.86* | 26.80 ± 0.68* |
| PLDζ activity (pmol/h/g) | |||||
| 20 DAF | 65.74 ± 12.58 | 104.67 ± 19.92* | 86.01 ± 10.30* | 269.13 ± 41.97* | 101.24 ± 20.59* |
Values are means ± SD (n = 3). The asterisk indicates a value that was statistically significantly different from the wild type at P < 0.05 based on Student’s T test.
PLDζs Alter Acyl Lipid and Fatty Acid Profiles
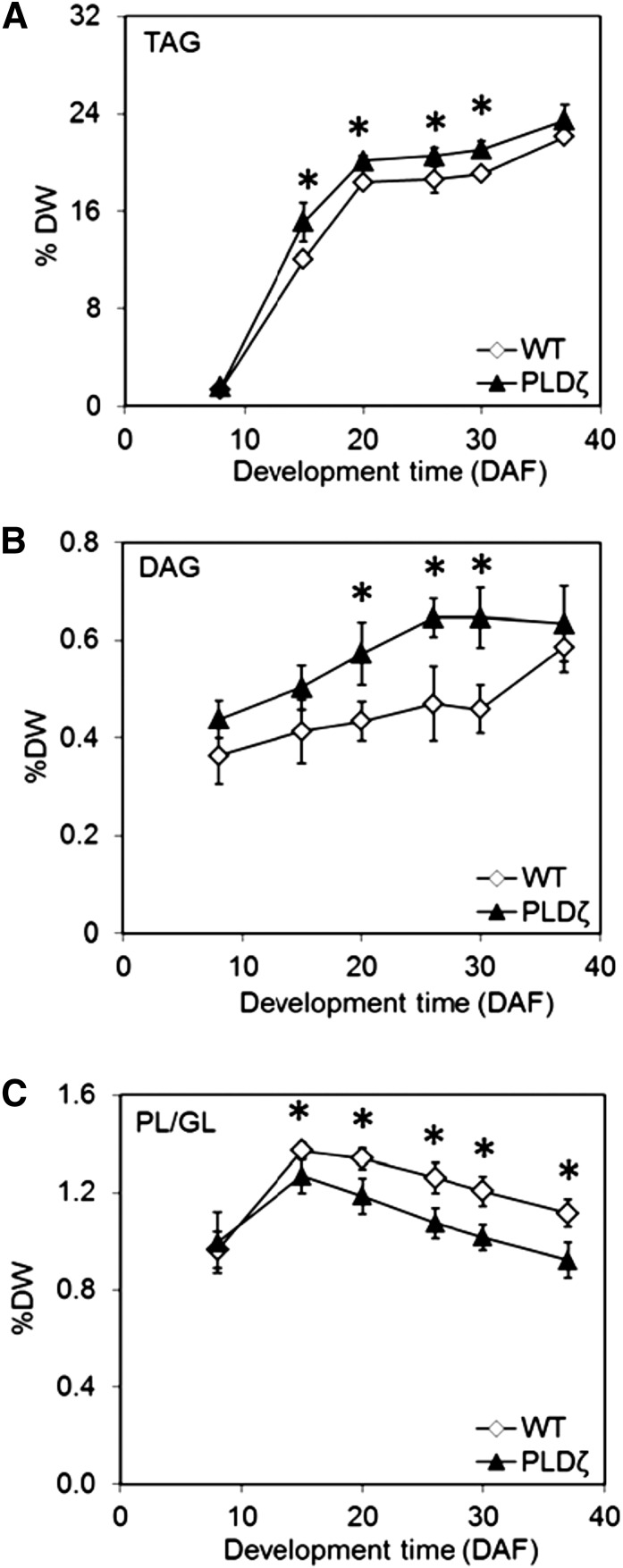
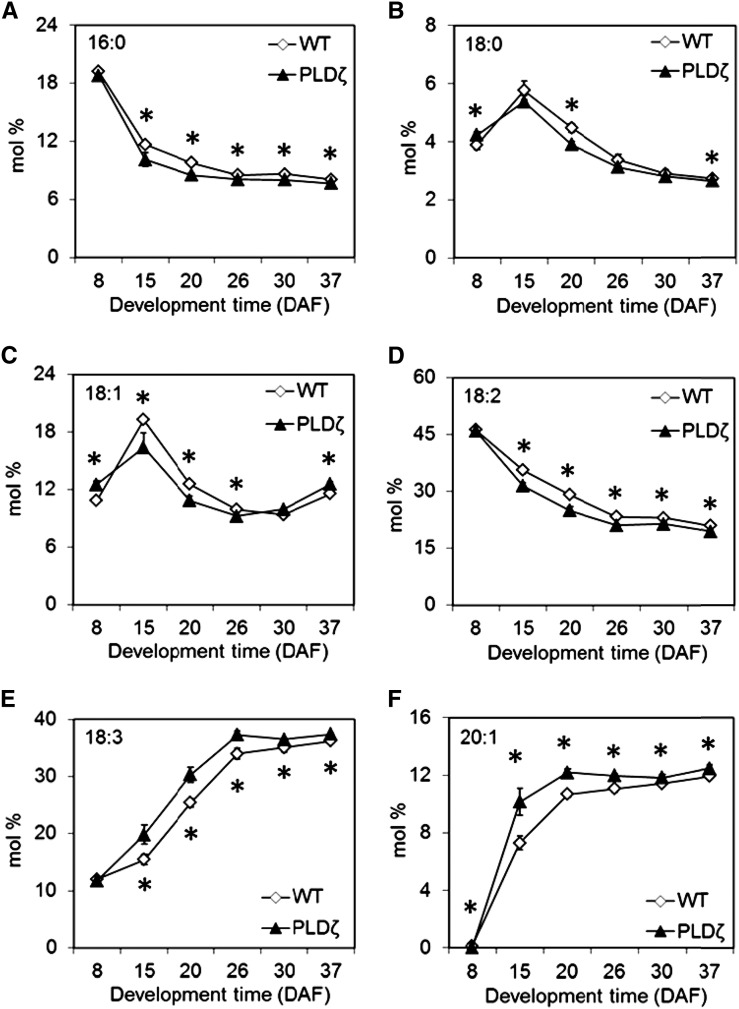
Total lipid extracted from the developing seeds was analyzed for changes in glycerolipid distribution and FA composition. Polar lipids (phospholipids and galactolipids, PL/GL) and neutral lipids including DAG and TAG were separated using silica TLC and the fatty acid profiles were analyzed in wild type and the transgenic PLDζ line 1 (subsequently referred to as “PLDζ”). TAG and DAG levels increased throughout development in the transgenic lines (Fig. 3, A and B) whereas polar lipids decreased (Fig. 3C). The results indicated that PLDζ altered the equilibrium between steady-state pool levels to favor the production of DAG and TAG relative to polar lipids such as PC, which is the primary phospholipid in seeds. In addition, the composition of FAs in total lipid extracts was altered in PLDζ throughout seed development (Fig. 4). Palmitic (16:0), stearic (18:0), and linoleic acids (18:2) were reduced in the transgenic line whereas linolenic (18:3) and eicosenoic acids (20:1) increased. Oleic acid (18:1; Fig. 4C) and minor fatty acids including arachidic acid (20:0), eicosadienoic acid (20:2), eicosatrienoic acid (20:3), docosanoic acid (22:0), and erucic acid (22:1; Supplemental Fig. S1) showed smaller differences between the lines.
Figure 3.
Lipid composition in Camelina seeds. Relative lipid composition including: TAG (A), DAG (B), and PL/GL (C) in seeds of wild type and PLDζ line 1 (henceforth referred to as “PLDζ”) during development. TAG, DAG, and PL/GL were quantified by GC-FID using an internal standard after they were eluted from a TLC plate of total lipid separation (SD, n = 3). Significant differences (T test, P < 0.05) between PLDζ and wild type are denoted with an asterisk.
Figure 4.
Changes in fatty acid composition of major fatty acids in seed lipid during seed development of wild type and a PLDζ. A, Palmitic acid (16:0). B, Stearic acid (18:0). C, Oleic acid (18:1). D, Linoleic acid (18:2). E, Linolenic acid (18:3). F, Eicosenoic acid (20:1; SD, n = 3). Significant differences (T test, P < 0.05) between PLDζ and wild type are denoted with an asterisk.
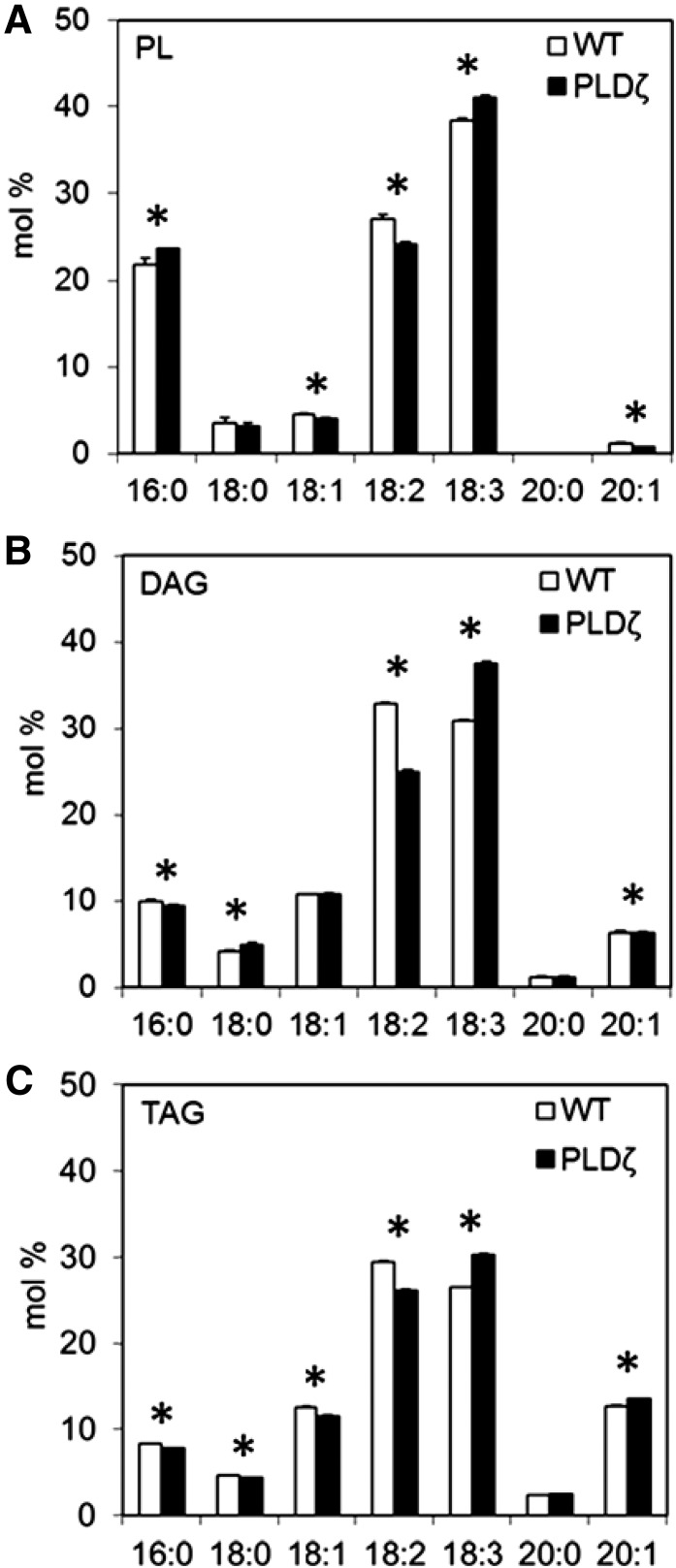
The fatty acid compositions of individual acyl lipids were also evaluated during development to establish the relationship between glycerolipid intermediates. The acyl lipid FA profiles of TAG, DAG, and phospholipids between developing wild-type and PLDζ seeds (Fig. 5) were qualitatively similar to the total lipid fatty acid changes (Fig. 4), though showed some statistical differences (P < 0.05) with the most notable changes in 18:3 and 18:2 lipid fractions of each lipid class.
Figure 5.
Fatty acid composition in PL, DAG, and TAG of wild type and PLDζ developing seeds at 20 DAF. A, Fatty acid profile of PL in developing seeds of wild type and PLDζ at 20 DAF. B, Fatty acid profile of DAG in developing seeds of wild type and PLDζ at 20 DAF. C, Fatty acid profile of TAG in developing seeds of wild type and PLDζ at 20 DAF (SD, n = 3). Significant differences (T test, P < 0.05) between PLDζ and wild type are denoted with an asterisk.
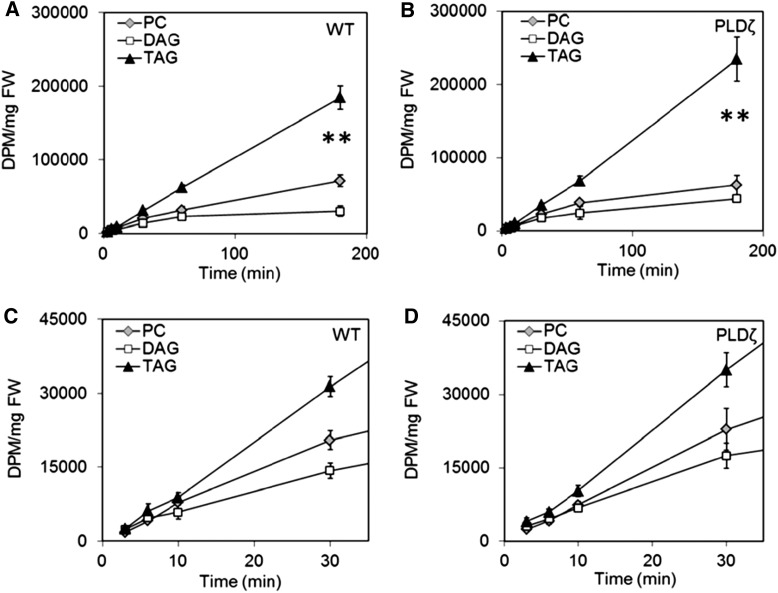
In Vivo [14C]acetate Labeling of Camelina Wild-type and PLDζ Embryos Indicates Significant PC Acyl Editing and DGAT Activity Utilizing a PC-derived DAG Pool
Production of plant oil containing PUFAs requires acyl flux through PC, the site for ER localized fatty acid desaturation. Based on the PUFA amounts in TAG harvested from developing seeds (20 DAF), approximately 56% of acyl groups flux through PC in both wild-type and PLDζ Camelina. However, not all fatty acids are further desaturated while on PC, thus the calculated flux through this metabolic pool is an underestimate (Bates and Browse, 2012; Bates, 2016). To determine the effect of PLDζ on acyl flux through PC, eukaryotic glycerolipid assembly was investigated with [14C]acetate labeling (Allen et al., 2015). Developing embryos of wild type and PLDζ incorporated [14C]acetate-derived fatty acids linearly into glycerolipids over a 3 h period (Supplemental Fig. S2) including PC, DAG, and TAG (Fig. 6, A and B). The initial rate of nascent [14C]fatty acid incorporation into TAG was the same or greater than for PC and DAG in both wild type and the PLDζ, indicating that labeled PC or DAG was not the initially labeled precursor for TAG biosynthesis in Camelina seeds (Fig. 6, C and D). Thus, the TAG was likely enriched by direct incorporation of [14C]acyl chains into the sn-3 position of unlabeled DAG with DGAT. TAG and DAG labeling was greater in the transgenics by the conclusion of the experiment and may reflect the larger pool of DAG (Fig. 3) in the transgenic line that is available for sn-3 acylation with nascent FAs whereas PC enrichment was not statistically different between lines, and likely represents the intermediate role of PC within FA flux into TAG. Other minor phospholipids including PA, phosphatidylinositol (PI), PE/PG, and the galactolipid monogalactosyldiacylglycerol (MGDG) were also labeled in both lines though generally less enriched in PLDζ (Supplemental Fig. S3).
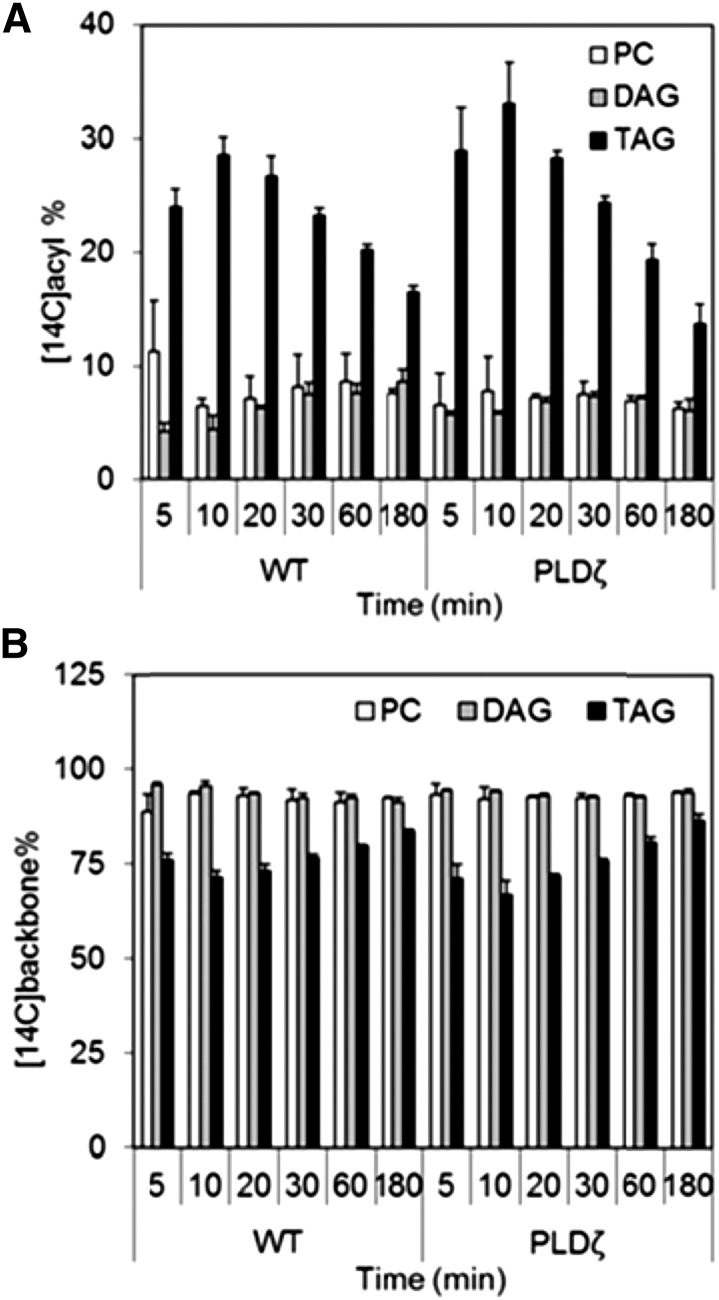
Figure 6.
Incorporation of [14C]fatty acid into glycerolipids during [14C]acetate labeling of wild-type and PLDζ developing embryos. A and C, [14C]fatty acid into TAG, DAG, and PC in wild-type embryos. B and D, [14C]fatty acid into TAG, DAG, and PC in PLDζ embryos (SD, n = 3, time points: 3, 6, 10, 30, 60, 180 min). Significant differences (T test, P < 0.05) between PLDζ (A) and wild type (B) were limited to differences in TAG and differences in DAG at 180 min, as indicated with two asterisks.
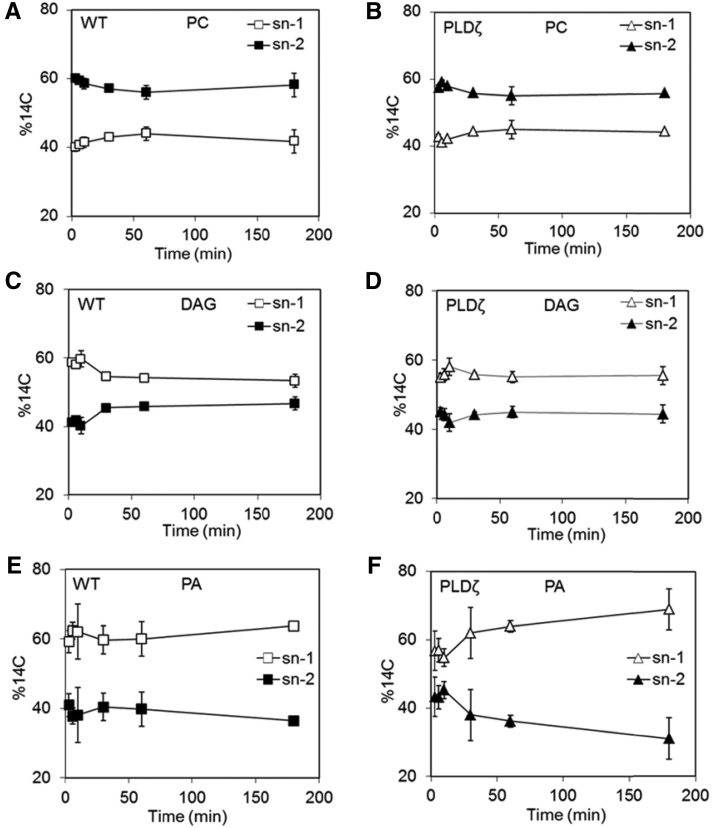
[14C]fatty acids were preferentially incorporated into the sn-2 position of PC, which is consistent with initial incorporation through acyl editing rather than through the Kennedy pathway as has been previously described in pea leaves, soybean developing embryos, and Arabidopsis developing seeds and cell suspensions (Bates et al., 2007, 2009, 2012; Tjellström et al., 2012). Stereochemical analyses indicated similar values for wild type and PLDζ, initially approximately 60% of nascent fatty acids at the sn-2 position of PC at 5 min, which equilibrated to approximately 55% by 60 min (Fig. 7, A and B). This regiospecific incorporation of acyl chains on the glycerol backbone is more balanced between sn-1 and sn-2 in Camelina relative to soybeans or Arabidopsis seeds that initially label the PC sn-2 position at over 70 to 80% but then equilibrate to 50 to 60% with time (Bates et al., 2009, 2012). DAG showed a different trend; unlike PC, the stereochemical incorporation of [14C]fatty acids into DAG (Fig. 7, C and D) indicated a greater preference for the sn-1 position. A quantity of 55 to 60% of nascent [14C]fatty acids was incorporated into the sn-1 position of DAG in wild-type and PLDζ lines. The preference for sn-1 labeling in Camelina DAG is comparable with observations in Arabidopsis, where sn-1 is >60% labeled (Taylor et al., 1995; Bates et al., 2012) but distinct from developing soybean embryos that incorporate nearly equal amounts of nascent [14C]fatty acids into sn-1 and sn-2 positions (Bates et al., 2009). PA labeling was qualitatively similar to DAG, suggesting that at these time points the labeled PA and DAG measured is likely produced consecutively within the Kennedy pathway (Fig. 7, E and F). The contrast in labeling between PC and PA suggests that PC contributes less to overall PA labeling probably because the pool of PA derived from PC is quite small and is mixed with the de novo pool derived directly from LPA or from other parts of membranes.
Figure 7.
Stereochemical incorporation of [14C]fatty acids into PC, PA, and DAG of wild-type and PLDζ developing embryos. A, PC of wild type. B, PC of AtPLDζ-OE1-12. C, DAG of wild type. D, DAG of PLDζ. E, PA of wild type. F, PA of PLDζ (SD, n = 3, time points: 3, 6, 10, 30, 60, 180 min).
[14C]labeled TAG was analyzed regiochemically (Supplemental Fig. S4) to establish whether the rapid initial labeling (Fig. 6) was due to direct incorporation of nascent [14C]fatty acids into sn-3 position of nonlabeled DAG or from labeled de novo DAG produced by the Kennedy pathway (Fig. 7). Cleavage of sn-1 and sn-3 positions of TAG with Rhizomucor miehei lipase produced sn-2 monoacylglycerol, which was labeled at low levels relative to the released fatty acids (Supplemental Fig. S4). When considered with the stereochemical analyses of labeled DAG that indicated 55/45 sn-1/sn-2 ratio of labeling (Fig. 7, C and D), these results suggest that most labeling in TAG is due to incorporation of a [14C]fatty acid at the sn-3 position of an unlabeled DAG. Camelina seeds contain approximately 12% eicosenoic acid (Fig. 4F), and very long chain FAs such as C20:1 have higher specific activities from labeling experiments because they are elongated in the cytosol that contains an acyl-CoA with a higher 14C specific activity than in the plastid (Bao et al., 2000). The rapid sn-3 TAG labeling is qualitatively consistent with previous studies in Camelina (Pollard et al., 2015) and Arabidopsis developing seeds (Bates et al., 2012) that also contain highly labeled 20:1 in the sn-3 position of TAG (Taylor et al., 1995; Bates et al., 2012). Very long chain FAs are predominantly incorporated into TAG by DGAT rather than PDAT (Katavic et al., 1995; Zhang et al., 2009; Xu et al., 2012).
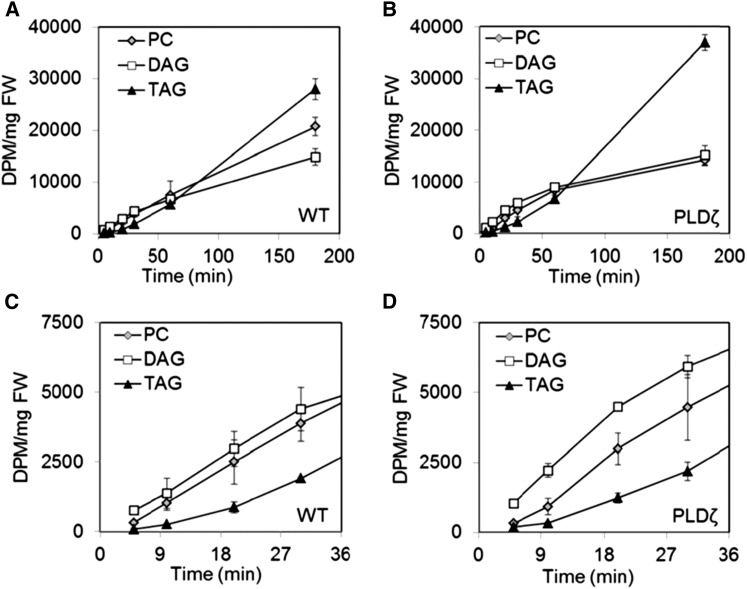
In Vivo Glycerolipid Backbone Labeling Indicates PLDζ Enhances Flux through the PC-derived DAG Pathway of TAG Biosynthesis
The initial steps in eukaryotic glycerolipid assembly in wild-type and PLDζ developing embryos were further investigated with [14C]glycerol kinetic labeling. Developing embryos of wild type and PLDζ incorporated [14C]glycerol linearly into glycerolipids at similar rates over a 3-h time course (Supplemental Fig. S5). The major labeled lipids were DAG, PC, and TAG (Supplemental Fig. S6), and other phospholipids including PA/PI, PE/PG, and MGDG were labeled to low levels in both lines (Supplemental Fig. S7). [14C]glycerol metabolized by the developing embryos was incorporated into the backbone of lipids through carbons in glycerol-3-P (G3P) and to a lesser extent into the acyl chains by glycolytic reactions that convert glycerol to acetyl-CoA. In both wild type and PLDζ, DAG was backbone labeled from [14C]glycerol at a faster rate than PC or TAG (Fig. 8). This description is consistent with the role of de novo DAG for the production of both PC and TAG in developing Camelina seeds [Fig. 1; Bates and Browse (2012)]. DAG labeling was more rapid in PLDζ, possibly reflecting the increased Kennedy pathway flux into de novo DAG that is necessary to produce more TAG. The initial labeling of PC was linear, indicating the de novo DAG precursor pool to PC synthesis was rapidly filled, and that the continual increase in DAG accumulation represents filling of a DAG pool other than that required for PC synthesis. TAG labeling followed an exponential pattern with slow labeling initially because precursor supplies of labeled DAG had not been turned over sufficiently to label TAG. After 60 min, TAG labeling approached a level that was similar to DAG and by 3 h, TAG labeling was twice that of DAG. The delay in TAG labeling relative to PC indicates a larger precursor pool for the flux of enriched backbone into TAG. Such a pattern is consistent with PC synthesis directly from initially labeled de novo DAG and TAG labeling at a reduced rate as the [14C]glycerol-backbone transitions through PC, and PC-derived DAG pools (Allen et al., 2015).
Figure 8.
Labeling of backbones in PC, DAG, or TAG in [14C]glycerol labeled wild-type and PLDζ developing embryos. A and C, [14C]backbone incorporation into TAG, DAT, and PC in wild-type embryos. B and D, [14C]backbone incorporation into TAG, DAG, and PC in PLDζ embryos (SD, n = 3, time points: 5, 10, 20, 30, 60, 180). Significant differences (T test, P < 0.05) between PC and DAG (wild-type time points: 5, 180; PLDζ time points: 5, 10, 20) and between PC and TAG (wild-type time points: 5, 10, 20, 30, 180; PLDζ time points: 10, 20, 30, 180) were observed within, but not between, lines.
[14C]glycerol was also incorporated into fatty acids by conversion to [14C]acetyl-CoA through glycolysis and pyruvate dehydrogenase (Figs. 9, S8). The [14C]acyl chains obtained from lipids were analyzed independent of the glycerol-backbone. Relative to the total label incorporation, approximately 5 to 35% was incorporated into fatty acids in a lipid class-specific manner during the time course experiments with the remainder going into glycerolipid backbone metabolism involving the Kennedy pathway (Fig. 9). The labeling in the acyl chain relative to the glycerol backbone was similar when PC was compared to DAG (approximately 5% to 10% across the time course) and indicated that the precursor pools for these intermediates (i.e. G3P, LPA, PA) are rapidly equilibrated with both forms of label (Bates et al., 2009). However, the fraction of acyl labeling in TAG (relative to TAG backbone labeling) changed more dramatically (decreasing from approximately 30% to approximately 15% during the time course), indicating that over time the glycerol backbone represented more of the total labeling. The distinct labeling in TAG reflects the initial incorporation of sn-3 labeled FAs onto an unlabeled DAG backbone as previously described. Over time, this contribution was diminished as the [14C]glycerol-backbone labeling increased in precursor pools for TAG synthesis. These observed changes in TAG labeling relative to PC are consistent with the presence of a larger precursor pool for backbone labeling of TAG than that of PC, and are thus in agreement with both the [14C]acetate acyl labeling (Fig. 6) and rate of [14C]glycerol incorporation into the backbone of TAG (Fig. 8).
Figure 9.
Incorporation of [14C]glycerol into glycerolipids during labeling of wild-type and PLDζ developing embryos. A, [14C]glycerol into acyl chains of TAG, DAG, and PC in wild-type and PLDζ embryos. B, [14C]glycerol into backbone of TAG, DAG, and PC in wild type and PLDζ embryos (SD, n = 3, time points: 5, 10, 20, 30, 60, 180).
Both PC and TAG can be synthesized from de novo DAG, yet TAG can also be produced from PC-derived DAG (Fig. 1). The relative initial rates of PC and TAG [14C]glycerol backbone labeling (i.e. without acyl labeling) at short time points (e.g. ≤ 10 min) predominantly represent the competition for initially synthesized de novo DAG, whereas at longer time points the labeling in TAG also includes contributions from PC-derived DAG. The initial relative rate of PC/TAG synthesis from de novo DAG was approximately 3.9/1 and 4.3/1, in wild-type and PLDζ, respectively (Supplemental Table S1). Considering that PLDζ may be rapidly turning over the labeled PC, the actual ratio of PC/TAG synthesis in PLDζ line could be an underestimate. Developing embryos predominantly accumulate TAG not PC, thus the higher rate of PC synthesis suggests that most of the PC will eventually turn over for production of PC-derived DAG, and then TAG. The higher ratio of initial PC/TAG synthesis rates from de novo DAG in PLDζ suggests that turnover of PC by PLDζ activity may induce flux through the PC-derived DAG pathway, leading to the higher overall oil levels.
DISCUSSION
The objective of this study was to investigate the endogenous pathways of acyl flux into TAG of Camelina seeds, and to examine how TAG accumulation is affected in seeds of transgenic Camelina expressing Arabidopsis AtPLDζ1 and AtPLDζ2 to alter acyl flux through PC. In oilseeds, two families of enzymes are mainly responsible for TAG assembly: (1) DGATs that catalyze production of TAG by using DAG and acyl-CoAs, and (2) PDATs that produce TAG using DAG with an additional acyl group donated by PC. How much each of these two groups of enzymes contributes to TAG assembly varies with plant species and tissues (Stymne and Stobart, 1987; Zhang et al., 2009; Banaś et al., 2013). Because both enzymes require a supply of DAG as a common precursor for TAG synthesis, de novo DAG must be produced by the Kennedy pathway through sequential acylation of G3P. However, given the high percentage of unsaturated acyl chains in TAG, additional steps that move fatty acids through PC are necessary. Acyl editing and the interconversion of DAG with PC results in a second pool of DAG that is PC-derived and is the direct source for TAG production (Bates and Browse, 2012). The production of higher levels of TAG in oilseeds requires a balance of: (1) enhanced synthesis of nascent fatty acids, (2) increased production of DAG, and (3) enough flux through PC to produce a viable combination of saturated and unsaturated chains.
PLDζ Alters the Steady-state Pool Sizes of PC and DAG Leading to Enhanced DAG Accumulation
This study indicated that coexpression of two phospholipases, PLDζ1 and PLDζ2, which convert PC to PA (Table I), has the consequence of altering the steady-state pool concentrations of DAG and PC in transgenic lines (Fig. 3). The enhanced DAG pool size leads to an increase in TAG accumulation throughout development and results in 3% more TAG (as a percent of total biomass) in mature Camelina seeds (Table I). In addition, the FA profile was altered with increased 18:3 and 20:1 and reduced levels of other acyl groups. Time-course labeling experiments with [14C]acetate resulted in a labeled PC pool that was approaching an asymptotic maximum in the transgenic line, indicating the active PC pool was nearly completely labeled within the time period (Fig. 6). [14C]glycerol kinetic incorporation (Fig. 8) indicated that DAG was more labeled than PC at early time points and was only surpassed by PC in the wild type later in time due to the larger active PC pool size. PC in the transgenics became maximally labeled more quickly and corresponded to a more rapid accumulation of glycerol-labeled TAG. Taken together with the measured changes in DAG and PC pool sizes, and [14C]acetate acyl labeling, the results indicate that PC was being turned over more rapidly to produce DAG. Thus the role of the PLDζ enzyme may be to increase the available substrate for TAG production. Along with a limited number of descriptions of significantly enhanced TAG in oilseeds (Lardizabal et al., 2008; Weselake et al., 2009; Oakes et al., 2011), this study suggests that the last steps in TAG biosynthesis are bottlenecks to its production. By converting PC to DAG more effectively, PLDζ may enhance DGAT activity for TAG production in Camelina seeds. It is unclear from these experiments if the increase in DAG concentration observed in PLDζ is a consequence of DGAT activity that may now be limiting further increases in oil production. Possibly the coexpression of a DGAT with PLDζ could lead to more significant oil levels in the future.
Carbon Flux between DAG and PC Are Highly Interconnected in Camelina, But Have Distinct Differences from Other Oilseeds and Are Enhanced by PLD
Interconversion of the DAG moiety between PC and DAG pools is a central process in TAG assembly of many oilseeds and provides a pool of DAG for TAG synthesis that contains more unsaturated fatty acids than de novo DAG (Bates and Browse, 2012); however, the quantitative roles of enzymes that provide this connection have been difficult to assess and cannot be determined based on TAG fatty acid composition alone. The rapid incorporation of [14C]acetate labeled FAs predominantly into the sn-3 position of TAG, the PC-TAG precursor-product kinetics of [14C]glycerol labeling, and the ratio of glycerol backbone to acyl group labeling from [14C]glycerol all support a predominantly PC-derived DAG pathway of TAG synthesis. Quantitatively, the initial relative rate of PC/TAG synthesis from [14C]glycerol backbone labeled de novo DAG was approximately 3.9:1 and 4.3:1, in wild-type and PLDζ, respectively (Supplemental Table S1). Based on the initial rates of [14C]glycerol labeling into PC/TAG, this would suggest that approximately 80% of TAG in wild-type Camelina is produced from PC-derived DAG with the remaining flux directly through the Kennedy pathway using de novo DAG for TAG biosynthesis. For comparison, the relative initial rates of PC/TAG synthesis are approximately 14:1 in Arabidopsis, indicating >93% of TAG is synthesized from PC-derived DAG (Bates and Browse, 2011). Considering that the membrane lipid PC typically does not accumulate unusual FAs (Millar et al., 2000), and the flux of unusual FAs through PC has been indicated as a bottleneck in oilseed engineering (Bates and Browse, 2011; Bates et al., 2014), the relatively larger proportion of acyl flux through the Kennedy pathway in Camelina may explain the enhanced accumulation of some unusual FAs in TAG of transgenic Camelina (Ruiz-Lopez et al., 2014) relative to the transgenic Arabidopsis (Ruiz-Lopez et al., 2013) engineered with the same genes.
Fluxes between DAG and PC are achieved reversibly through DAG-CPT and PDCT and unidirectionally from PC to DAG by phospholipase C or through a combination of PLD and phosphatidic acid phosphatase (PAP). In Arabidopsis, PDCT is responsible for approximately 40% of the flux of PUFAs from PC into PC-derived DAG for TAG synthesis (Lu et al., 2009), but the Arabidopsis lpcat1 lpcat2 rod1 triple mutant that abolishes both acyl editing and PDCT activity still contains 1/3 of wild-type PUFA levels in TAG (Bates et al., 2012). The remaining flux of PUFA from PC to TAG may involve PC associated with phospholipase-based production of PC-derived DAG. Widespread evidence for the concerted action of PLD and PAP includes reports in mammalian literature that demonstrate DAG production from endothelial-derived PC (Martin, 1988) and endogenous DAG generation in human polymorphonuclear leukocytes where DAG stimulated 5-lipoxygenase enzyme activity and function (Albert et al., 2008). In plants, isolated protein bodies from seedlings show both activities of PLD and PAP (Herman and Chrispeels, 1980), and studies of AtPLDζ1 and AtPLDζ2 suggest the supply of DAG for galactolipid synthesis is dependent on this pathway and also subverts phosphorus starvation (Cruz-Ramírez et al., 2006; Li et al., 2006). It has been shown that knock-down of PLDα in soybean leads to reduced PUFAs in TAG (Lee et al., 2011), implicating a flux from PC to TAG through PLD-PAP without a requirement for PDCT involvement. Thus a number of mechanisms exist that contribute DAG derived from PC that is rich in PUFA, which accumulates in TAG. Our results cannot conclusively distinguish between the mechanistic differences in wild-type Camelina and Arabidopsis or other well-studied oilseeds, but together the studies suggest a combination of PLD and possibly PDCT enzyme activities may be responsible.
Here, we demonstrated that PLDζ enhances steady-state levels of DAG from PC, thus apparently homeostatic mechanisms involving PAP favors conversion of the produced PA to DAG. The increase in PC-derived DAG (Fig. 8) considerably altered TAG accumulation (up to 3% oil increase), supporting that acyl flux through PC may be a regulator of total FA synthesis (Bates et al., 2014; Bates, 2016). However, it was surprising that no significant difference was observed in the stereochemical labeling of PC, which is a marker of acyl flux through acyl editing versus the Kennedy pathway. Stereospecific analyses of the acyl chains from [14C]acetate labeling revealed that DAG (as well as PA) was more enriched at the sn-1 position (Fig. 7) whereas PC favored sn-2 incorporation. A lack of significant changes in regiochemical lipid labeling between the lines indicates that the distribution of nascent FAs into different branches of the lipid metabolic network (Fig. 1) may not be significantly altered by PLDζ activity. In this case, an increased flux of acyl groups into de novo DAG for subsequent PC, and PC-derived DAG synthesis must be balanced by an increased flux of acyl groups into PC by acyl editing. Then the regiochemical labeling of PC would not change significantly between lines. This result is consistent with previous hypotheses that suggest PC is a central intermediate, and a fatty acid or DAG carrier through the ER membrane before TAG synthesis (Allen et al., 2015; Shockey et al., 2016). Together these acyl lipid flux experiments provide novel results that Camelina utilizes a higher proportion of direct Kennedy pathway than other related plants, and that the flux through the PC-derived DAG pathway can be enhanced through modulation of PLD activity.
Increased PLDζ Activity May Enhance DGAT over PDAT Activity for TAG Synthesis
The acylation of DAG to make TAG involves one of two enzymatic routes. PDAT uses the sn-2 acyl chain of PC along with DAG to make TAG, whereas DGAT produces TAG from acyl-CoA and DAG substrates. PDAT activity can lead to synthesis of TAG rich in unsaturated fatty acids at sn-3 position (Dahlqvist et al., 2000; Stähl et al., 2004; Xu et al., 2012) though the differences in substrate specificities for DGAT and PDAT enzymes may not be significant and have not been characterized in a number of oilseeds. Our results indicate changes in 18:3 and 20:1 invoked by PLDζ activity. DGAT1 is apparently responsible for much of the 20:1 incorporation into sn-3 of TAG in Arabidopsis, whereas DGAT2 has been implicated in the incorporation of other modified fatty acids into TAG (Kroon et al., 2006; Shockey et al., 2006). When Camelina embryos were labeled with [14C]acetate, the most rapid labeling was seen in TAG (Fig. 6). This is consistent with studies of Arabidopsis seeds (Bates et al., 2012) that also contain a significant fraction of elongated FAs. It is well-known that the elongation process that occurs outside of the plastid results in higher specific activities for these FAs relative to those of 18 carbons or shorter chains (Bao et al., 2000). In contrast, soybean seeds have very low levels of elongated fatty acids, and in combination with a high degree of acyl editing, result in more rapid PC labeling with nascent FA than TAG (Bates et al., 2009). When TAG labeled from [14C]acetate was regiochemically analyzed through enzymatic cleavage of the sn-1 and sn-3 positions (Supplemental Fig. S4) and compared to the sn-1 and sn-2 positions of DAG, the labeling difference was attributable to sn-3, consistent with both the incorporation of elongated fatty acids at this position and the greater level of 20:1 in TAG relative to PC or DAG (Fig. 5). If the sn-3 labeling was due primarily to PDAT, we would expect to see a precursor-product relationship with sn-2 PC to sn-3 TAG, which was not evident.
A significant challenge to producing increased levels of lipids in plants is that many of the genes putatively assigned by genome studies as being involved in lipid metabolism have not been characterized. Furthermore, the operation of an enzyme within a metabolic network is context-specific (Allen, 2016b) and may differ among species, tissues, environments, or when introduced transgenically. Therefore, the operation of a cellular network in planta requires dynamic analyses of flux with isotopes to assess the underlying changes in metabolism responsible for an altered phenotype. Given the central role of PC in lipid metabolism (Allen, 2016a; Bates, 2016), we hypothesized that overexpressing a phospholipase that acts specifically on PC (PLDζ) would influence the exchange and flux between PC and DAG and potentially alter TAG production. Through combined overexpression of two Arabidopsis PLDζ genes in Camelina, the TAG levels, PUFA concentration, and elongated fatty acid content were all increased. PC levels were reduced and DAG levels were increased presumably due to the altered interchange of these lipids by the PLDζ activity. Labeling with [14C]acetate and [14C]glycerol provided new insights into lipid metabolism in Camelina specifically indicating a higher flux through the Kennedy pathway as compared to Arabidopsis, but still predominantly composed of a PC-derived DAG pathway of TAG synthesis, which was further enhanced by PLDζ expression. When combined with other changes that could further alter the FA profile of TAG (e.g. suppression of FAD2/3 for accumulation of monosaturates), the overexpression of PLDζ may be part of an engineering strategy to enhance seed oil content for biofuels or industrial chemicals.
MATERIALS AND METHODS
Plant Materials and Chemicals
Camelina (Camelina sativa) wild-type “Suneson” (MT5) and AtPLDζ overexpressing lines were grown in greenhouses at 20°C/21°C under supplemental light to ensure a consistent 16-h/8-h d/n cycle at >500 μmoles/cm2/s in St. Louis, MO (38.63°N, 90.20°W). Developing siliques were harvested from plants at various times throughout development for lipid analysis or radioactive labeling.
Organic solvents, primuline, phospholipase A2 from Naja mossambica mossambica, and Rhizomucor miehei lipase, were purchased from Sigma-Aldrich; TLC plates, coated with silica gel 60 Å, were from EMD Millipore; the Hionic Fluor liquid scintillation cocktail was from PerkinElmer; the butylated hydroxytoluene was from MP Biochemicals; and [1-14C]acetate (specific activity, 59 mCi mmol−1) and [1,3-14C] glycerol (specific activity 56 mCi/mmol) were purchased from American Radiolabeled Chemicals.
Vector Construction and Plant Transformation
The full-length cDNA coding regions of AtPLDζ1 and AtPLDζ2 were amplified by PCR using the cDNA library prepared from Arabidopsis (Arabidopsis thaliana) Col-1. The AtPLDζ1 coding region was placed behind a glycinin promoter on vector pGly-DsRed (generating pGly-DsRed-ζ1). The AtPLDζ2 coding region was inserted behind a β-conglycinin promoter on a separate cloning vector. Then, the AtPLDζ2 expression cassette including the β-conglycinin promoter, the coding region of AtPLDζ2, and the terminator was amplified with PCR, and the product was then cloned into the binary vector pGly-DsRed-ζ1 containing DsRed and hygromycin selection markers (generating pGly-DsRed-ζ1-ζ2). The binary vector containing both AtPLDζ1 and AtPLDζ2 cassettes and pGly-DsRed-ζ1-ζ2 was introduced into Agrobacterium strain GV3101 by a freezing and thawing method. Camelina sativa “Suneson” was transformed with the above Agrobacterium GV3101 by floral dipping (Lu and Kang, 2008). Transgenic plants were selected on 10 mg/L hygromycin growth media and confirmed by digital imaging of DsRed expression.
RNA Expression Analysis
Total RNA was isolated from developing seeds using TriPure Isolation Reagent (Roche). cDNA was obtained using Transcriptor First Strand cDNA Synthesis Kit (Roche). PCR reactions were performed using Ex Taq Premix polymerase (Clontech Laboratories) with 30 cycles of amplification. Primers used for AtPLDζ1, AtPLDζ2, and ubiquitin (UBQ) were as follows: AtPLDζ1, forward 5′-ATG GCA TCT GAG CAG TTG ATG TCT CCC-3′, reverse 5′-CTG GTG AGA ATG ACA TCG AAA CCT CC-3′; AtPLDζ2, forward 5′-TAA CGG CGT TAA GTC AGA CGG AGT CAT C-3′, reverse 5′-GGA ACT TGC AGA CCT CTT TGG AGT T-3′; ubiquitin (homolog of Arabidopsis UBQ 10 gene), forward: 5′-AAG ATG GCC GCA CCT TGG CTG ATT AC-3′, reverse 5′-TCT CAA CCT CCA AAG TGA TGG TTT TAC-3′.
AtPLDζ1 Activity Assay
Developing seeds were ground in liquid N, and then extracted with buffer containing 50 mm Tris-HCl, pH 8.0, 10 mm KCl, 1 mm EDTA, 2 mm DTT, and 0.5 mm PMSF. After centrifugation at 12,000 g for 10 min at 4°C, the supernatant containing the soluble protein fraction was centrifuged at 100,000 g for 30 min at 4°C twice to obtain the microsomal protein fraction. AtPLDζ1 activity was assayed using radiolabeled PC, l-a-dipalmitoyl [2-palmitoyl-9,10-3H(N)] (1 mCi/mL and 60 Ci/mmol; American Radiolabeled Chemicals) as described in Qin and Wang (2000). After 2-h reactions at 30°C, lipids were extracted from the reaction mixture with 1 mL chloroform/methanol (2:1, v/v), and then separated on silica gel 60 Å TLC plates with a developing solvent system of chloroform/methanol/30% ammonium hydroxide (65:25:4, v/v/v). Lipid spots for PA were scraped from the plate and counted by scintillation.
[14C]Acetate and [14C]Glycerol Labeling
Camelina seeds were removed from developing pods (20 d after flowering) and embryos were dissected from the seed coats and cultured in medium containing 5 mm MES, pH 5.8, 0.5% Suc, and 0.5× Murashige & Skoog salts (Bates and Browse, 2012). After preincubation of embryos for 20 min under light of 35 μmoles/cm2/s, temperature at 24°C, and relative humidity of 35% with constant shaking, the labeling of 26 embryos per sample was started by replacing the 1 mL preincubation medium with 1 mL fresh culture medium containing [1-14C]-acetate (0.5 mM) or [1,3-14C] glycerol (0.2 mM) in a 2-mL Eppendorf tube (Eppendorf North America). For each time point, the labeling reaction was stopped by removing the 1.0 mL culture medium containing radioactive substrate and freezing the embryos in the tube immediately in liquid N. For [14C]glycerol-labeled lipid classes including PC, DAG, and TAG, the proportion of label in the acyl chains versus the backbone was determined by base-catalyzed transmethylation of TLC-separated and purified lipids and scintillation counting of the separated organic and aqueous phases (Ichihara et al., 1996).
Lipid Analysis
Total lipids of developing seeds or embryos were extracted using a modified method based on a protocol by KS Lipidomics Research Center (http://www.k-state.edu/lipid/lipidomics/protocols.htm). Briefly, seeds or embryos were quickly transferred to an 8-mL glass tube containing hot isopropanol with 0.01% butylated hydroxytoluene (at 75°C), and incubated at 75°C for 15 min. Then, seeds or embryos in isopropanol were homogenized thoroughly with a glass rod before adding 1.0 mL chloroform and 1.0 mL methanol and 0.8 mL water. After vortexing, 1.0 mL chloroform and 1.0 mL water were added. Then the mixture was partitioned into two phases by centrifugation. The chloroform phase with lipids was moved to a separate glass tube, and the remaining mixture was twice extracted by adding 1.0 mL chloroform, shaking, centrifugation, and combining the chloroform phases. The total lipid extract was washed once by adding a small amount (0.5 mL) of 1 m KCl. Lipids were dried under N gas flow, and suspended to a small volume in chloroform. Fatty acid methyl esters were prepared by the acid transmethylation procedure as described in Cahoon et al. (2002), and quantified by gas chromatography (GC) using flame ionization detection (FAD; Focus GC; Thermo Scientific) and a HP-INNOWAX capillary column (30 m length × 0.25 mm i.d., 0.25 μm film thickness, Agilent J&W GC Columns; Agilent Technologies).
Radioactivity in the total lipid samples, and individually purified lipids, were quantified by liquid scintillation counting. Radioactivity on TLC plates was visualized and imaged by electronic radiography (Packard A20240 Instant Imager; Packard Instrument). Polar lipid separation by TLC, recovery of polar lipids from TLC plates, and positional analysis of PC and PA acyl groups using phospholipase A2, were conducted as described in Bates et al. (2007). For acyl chain FA composition in DAG and TAG, Rhizomucor miehei lipase was used for digestion as described similar to the protocol given for porcine pancreatic lipase hydrolysis of TAG (Christie and Han, 2003; Cahoon et al., 2006). Briefly, an aliquot of DAG or TAG was dried under N gas flow, and suspended in 1.0 mL diethyl ether. Then, 0.60 mL of a reaction buffer containing 50 mm Tris-HCl, pH 8.0, 5 mm CaCl2, and 200 μL R. miehei lipase were added to the lipid in diethyl ether. Reactions were incubated at 37°C for 1 ∼ 3 h with shaking. Reactions were stopped by adding 0.3 mL 6N hydrochloric acid and the diethyl ether phase was evaporated under N gas flow. Lipids in the reaction were extracted with chloroform/methanol (2:1, v/v). The lipid extract was dried under N gas flow, and suspended in small volume of chloroform before being separated by TLC plate Silica gel 60 Å together with standards. TLC plates were developed with hexane/diethyl ether/acetic acid (70:30:1, v/v/v) to separate neutral lipid classes and individual neutral lipids were eluted from TLC silica with chloroform/methanol (2:1, v/v) and back-extracted with chloroform. All TLC solvents contained 0.01% (w/v) butylated hydroxytoluene. Phospholipids from the TLC plate were eluted with chloroform/methanol/0.9% KCl (2:1:1, v/v/v), and then the aqueous phase was reextracted with chloroform and lipids combined before further analysis.
Polar lipid classes were separated by a solvent system of chloroform/methanol/30% ammonium hydroxide (65:25:4, v/v/v) that was used to establish the fraction of labeling in PC relative to other polar components. Lipid spots on the TLC plate were visualized under UV light after staining with primuline. Each lipid was eluted from the silica plate as described in Bates et al. (2007). PC and PA stereochemistry was determined by a modified method of phospholipase A2 digestion based on methods described in Bates et al. (2007). Briefly, PC or PA in 1 mL diethyl ether was incubated with 0.5 mL of reaction buffer containing 50 mm borate, pH 7.5, 4 mm CaCl2, and 5 U PLA2 at 25°C for 20 min. Products were extracted and separated by TLC using a developing system of chloroform/methanol/30% ammonium hydroxide (65:25:4, v/v/v). DAG and TAG regiochemistry was determined by R. miehei lipase digestion and TLC (Cahoon et al., 2006). GraphPad Prism (version 6; https://www.graphpad.com/scientific-software/prism/) was used to perform linear regression analysis on initial rate data (Supplemental Table S1).
Seed oil content was determined by fatty acid methylation analysis. The developing seeds were lyophilized then transmethylated by the acid transmethylation procedure as described in Cahoon et al. (2002). Briefly, methanol containing 2.5% sulfuric acid, 0.01% (w/v) butylated hydroxytoluene, 20% toluene, and TAG-17:0 internal standard were added to a glass vial containing seeds. The seeds were crushed with a glass rod and then heated at 90 C for 1 h. Transmethylation was quenched through addition of 1 m NaCl, before extraction with hexane. The hexane fraction was transferred to a GC vial and the fatty acid methyl esters were analyzed by GC-FID. The oil content was determined by comparison of the detector response from seed-derived fatty acid methyl esters relative to methyl heptadecanoate from the triheptadecanoin internal standard.
Accession Numbers
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers At3g16785 for PLDζ1 and PLDζ2 At3g05630 for PLDζ2.
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. Minor fatty acid composition in wild type and PLDζ
Supplemental Figure S2. Total [14C] incorporation from labeled acetate
Supplemental Figure S3. [14C] incorporation into minor polar lipids from labeled acetate
Supplemental Figure S4. [14C] regiochemical analysis of TAG from acetate
Supplemental Figure S5. Total [14C] incorporation from labeled glycerol
Supplemental Figure S6. [14C] incorporation into PC, DAG, and TAG from labeled glycerol
Supplemental Figure S7. [14C] incorporation into minor polar lipids from labeled glycerol
Supplemental Figure S8. Incorporation of [14C]glycerol into acyl chains of glycerolipids
Supplemental Table S1. Initial labeling of glycerol backbone
Supplementary Material
Acknowledgments
Any product or trademark mentioned here does not imply a warranty, guarantee, or endorsement by the authors or their affiliations over other suitable products.
Footnotes
Articles can be viewed without a subscription.
References
- Albert D, Pergola C, Koeberle A, Dodt G, Steinhilber D, Werz O (2008) The role of diacylglyceride generation by phospholipase D and phosphatidic acid phosphatase in the activation of 5-lipoxygenase in polymorphonuclear leukocytes. J Leukoc Biol 83: 1019–1027 [DOI] [PubMed] [Google Scholar]
- Allen DK. (2016a) Assessing compartmentalized flux in lipid metabolism with isotopes. Biochim Biophys Acta 1861(9 Pt B): 1226–1242 [DOI] [PubMed] [Google Scholar]
- Allen DK. (2016b) Quantifying plant phenotypes with isotopic labeling and metabolic flux analysis. Curr Opin Biotechnol 37: 45–52 [DOI] [PubMed] [Google Scholar]
- Allen DK, Bates PD, Tjellström H (2015) Tracking the metabolic pulse of plant lipid production with isotopic labeling and flux analyses: past, present and future. Prog Lipid Res 58: 97–120 [DOI] [PubMed] [Google Scholar]
- Banaś W, Sanchez Garcia A, Banaś A, Stymne S (2013) Activities of acyl-CoA:diacylglycerol acyltransferase (DGAT) and phospholipid:diacylglycerol acyltransferase (PDAT) in microsomal preparations of developing sunflower and safflower seeds. Planta 237: 1627–1636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao X, Focke M, Pollard M, Ohlrogge J (2000) Understanding in vivo carbon precursor supply for fatty acid synthesis in leaf tissue. Plant J 22: 39–50 [DOI] [PubMed] [Google Scholar]
- Barron EJ, Stumpf PK (1962) Fat metabolism in higher plants. XIX. The biosynthesis of triglycerides by avocado-mesocarp enzymes. Biochim Biophys Acta 60: 329–337 [DOI] [PubMed] [Google Scholar]
- Bates PD. (2016) Understanding the control of acyl flux through the lipid metabolic network of plant oil biosynthesis. Biochim Biophys Acta 1861(9 Pt B): 1214–1225 [DOI] [PubMed] [Google Scholar]
- Bates PD, Browse J (2011) The pathway of triacylglycerol synthesis through phosphatidylcholine in Arabidopsis produces a bottleneck for the accumulation of unusual fatty acids in transgenic seeds. Plant J 68: 387–399 [DOI] [PubMed] [Google Scholar]
- Bates PD, Browse J (2012) The significance of different diacylglycerol synthesis pathways on plant oil composition and bioengineering. Front Plant Sci 3: 147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates PD, Durrett TP, Ohlrogge JB, Pollard M (2009) Analysis of acyl fluxes through multiple pathways of triacylglycerol synthesis in developing soybean embryos. Plant Physiol 150: 55–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates PD, Fatihi A, Snapp AR, Carlsson AS, Browse J, Lu C (2012) Acyl editing and headgroup exchange are the major mechanisms that direct polyunsaturated fatty acid flux into triacylglycerols. Plant Physiol 160: 1530–1539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates PD, Johnson SR, Cao X, Li J, Nam JW, Jaworski JG, Ohlrogge JB, Browse J (2014) Fatty acid synthesis is inhibited by inefficient utilization of unusual fatty acids for glycerolipid assembly. Proc Natl Acad Sci USA 111: 1204–1209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates PD, Ohlrogge JB, Pollard M (2007) Incorporation of newly synthesized fatty acids into cytosolic glycerolipids in pea leaves occurs via acyl editing. J Biol Chem 282: 31206–31216 [DOI] [PubMed] [Google Scholar]
- Cahoon EB, Dietrich CR, Meyer K, Damude HG, Dyer JM, Kinney AJ (2006) Conjugated fatty acids accumulate to high levels in phospholipids of metabolically engineered soybean and Arabidopsis seeds. Phytochemistry 67: 1166–1176 [DOI] [PubMed] [Google Scholar]
- Cahoon EB, Ripp KG, Hall SE, McGonigle B (2002) Transgenic production of epoxy fatty acids by expression of a cytochrome P450 enzyme from Euphorbia lagascae seed. Plant Physiol 128: 615–624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell MC, Rossi AF, Erskine W (2013) Camelina (Camelina sativa (L.) Crantz): agronomic potential in Mediterranean environments and diversity for biofuel and food uses. Crop Pasture Sci 64: 388–398 [Google Scholar]
- Chapman KD, Ohlrogge JB (2012) Compartmentation of triacylglycerol accumulation in plants. J Biol Chem 287: 2288–2294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie WW, Han X (2003) Lipid Analysis, 3rd Ed. The Oily Press, Bridgewater, UK [Google Scholar]
- Craddock CP, Adams N, Bryant FM, Kurup S, Eastmond PJ (2015) PHOSPHATIDIC ACID PHOSPHOHYDROLASE regulates phosphatidylcholine biosynthesis in Arabidopsis by phosphatidic acid-mediated activation of CTP:PHOSPHOCHOLINE CYTIDYLYLTRANSFERASE activity. Plant Cell 27: 1251–1264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz-Ramírez A, Oropeza-Aburto A, Razo-Hernández F, Ramírez-Chávez E, Herrera-Estrella L (2006) Phospholipase DZ2 plays an important role in extraplastidic galactolipid biosynthesis and phosphate recycling in Arabidopsis roots. Proc Natl Acad Sci USA 103: 6765–6770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlqvist A, Stahl U, Lenman M, Banas A, Lee M, Sandager L, Ronne H, Stymne S (2000) Phospholipid:diacylglycerol acyltransferase: an enzyme that catalyzes the acyl-CoA-independent formation of triacylglycerol in yeast and plants. Proc Natl Acad Sci USA 97: 6487–6492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eastmond PJ, Quettier AL, Kroon JT, Craddock C, Adams N, Slabas AR (2010) Phosphatidic acid phosphohydrolase 1 and 2 regulate phospholipid synthesis at the endoplasmic reticulum in Arabidopsis. Plant Cell 22: 2796–2811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths G, Stobart AK, Stymne S (1985) The acylation of sn-glycerol 3-phosphate and the metabolism of phosphatidate in microsomal preparations from the developing cotyledons of safflower (Carthamus tinctorius L.) seed. Biochem J 230: 379–388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths G, Stymne S, Stobart AK (1988) Phosphatidylcholine and its relationship to triacylglycerol biosynthesis in oil-tissues. Phytochemistry 27: 2089–2093 [Google Scholar]
- Haslam RP, Ruiz-Lopez N, Eastmond P, Moloney M, Sayanova O, Napier JA (2013) The modification of plant oil composition via metabolic engineering—better nutrition by design. Plant Biotechnol J 11: 157–168 [DOI] [PubMed] [Google Scholar]
- Herman EM, Chrispeels MJ (1980) Characteristics and subcellular localization of phospholipase d and phosphatidic acid phosphatase in mung bean cotyledons. Plant Physiol 66: 1001–1007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichihara K, Shibahara A, Yamamoto K, Nakayama T (1996) An improved method for rapid analysis of the fatty acids of glycerolipids. Lipids 31: 535–539 [DOI] [PubMed] [Google Scholar]
- Iskandarov U, Jin Kim HJ, Chaoon EB (2013) Camelina: An Emerging Oilseed Platform for Advanced Biofuels and Bio-based Materials, Vol 4 Springer, New York [Google Scholar]
- Katavic V, Reed DW, Taylor DC, Giblin EM, Barton DL, Zou J, Mackenzie SL, Covello PS, Kunst L (1995) Alteration of seed fatty acid composition by an ethyl methanesulfonate-induced mutation in Arabidopsis thaliana affecting diacylglycerol acyltransferase activity. Plant Physiol 108: 399–409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy EP. (1961) Biosynthesis of complex lipids. Fed Proc 20: 934–940 [PubMed] [Google Scholar]
- Koo AJ, Ohlrogge JB, Pollard M (2004) On the export of fatty acids from the chloroplast. J Biol Chem 279: 16101–16110 [DOI] [PubMed] [Google Scholar]
- Kornberg A, Pricer WE Jr (1953) Enzymatic esterification of α-glycerophosphate by long chain fatty acids. J Biol Chem 204: 345–357 [PubMed] [Google Scholar]
- Kroon JT, Wei W, Simon WJ, Slabas AR (2006) Identification and functional expression of a type 2 acyl-CoA:diacylglycerol acyltransferase (DGAT2) in developing castor bean seeds which has high homology to the major triglyceride biosynthetic enzyme of fungi and animals. Phytochemistry 67: 2541–2549 [DOI] [PubMed] [Google Scholar]
- Kunst L, Taylor DC, Underhill EW (1992) Fatty acid elongation in developing seeds of Arabidopsis thaliana. Plant Physiol Biochem 30: 425–434 [Google Scholar]
- Lardizabal K, Effertz R, Levering C, Mai J, Pedroso MC, Jury T, Aasen E, Gruys K, Bennett K (2008) Expression of Umbelopsis ramanniana DGAT2A in seed increases oil in soybean. Plant Physiol 148: 89–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Welti R, Schapaugh WT, Trick HN (2011) Phospholipid and triacylglycerol profiles modified by PLD suppression in soybean seed. Plant Biotechnol J 9: 359–372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Qin C, Welti R, Wang X (2006) Double knockouts of phospholipases Dζ1 and Dζ2 in Arabidopsis affect root elongation during phosphate-limited growth but do not affect root hair patterning. Plant Physiol 140: 761–770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Wei F, Tawfall A, Tang M, Saettele A, Wang X (2015a) Overexpression of patatin-related phospholipase AIIIδ altered plant growth and increased seed oil content in camelina. Plant Biotechnol J 13: 766–778 [DOI] [PubMed] [Google Scholar]
- Li N, Gügel IL, Giavalisco P, Zeisler V, Schreiber L, Soll J, Philippar K (2015b) FAX1, a novel membrane protein mediating plastid fatty acid export. PLoS Biol 13: e1002053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li-Beisson Y, Shorrosh B, Beisson F, Andersson MX, Arondel V, Bates PD, Baud S, Bird D, Debono A, Durrett TP, Franke RB, Graham IA, et al. (2013) Acyl-lipid metabolism. Arabidopsis Book 11: e0161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Rice A, McGlew K, Shaw V, Park H, Clemente T, Pollard M, Ohlrogge J, Durrett TP (2015) Metabolic engineering of oilseed crops to produce high levels of novel acetyl glyceride oils with reduced viscosity, freezing point and calorific value. Plant Biotechnol J 13: 858–865 [DOI] [PubMed] [Google Scholar]
- Lu C, Kang J (2008) Generation of transgenic plants of a potential oilseed crop Camelina sativa by Agrobacterium-mediated transformation. Plant Cell Rep 27: 273–278 [DOI] [PubMed] [Google Scholar]
- Lu C, Napier JA, Clemente TE, Cahoon EB (2011) New frontiers in oilseed biotechnology: meeting the global demand for vegetable oils for food, feed, biofuel, and industrial applications. Curr Opin Biotechnol 22: 252–259 [DOI] [PubMed] [Google Scholar]
- Lu C, Xin Z, Ren Z, Miquel M, Browse J (2009) An enzyme regulating triacylglycerol composition is encoded by the ROD1 gene of Arabidopsis. Proc Natl Acad Sci USA 106: 18837–18842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin TW. (1988) Formation of diacylglycerol by a phospholipase D-phosphatidate phosphatase pathway specific for phosphatidylcholine in endothelial cells. Biochim Biophys Acta 962: 282–296 [DOI] [PubMed] [Google Scholar]
- Mietkiewska E, Siloto RM, Dewald J, Shah S, Brindley DN, Weselake RJ (2011) Lipins from plants are phosphatidate phosphatases that restore lipid synthesis in a pah1Δ mutant strain of Saccharomyces cerevisiae. FEBS J 278: 764–775 [DOI] [PubMed] [Google Scholar]
- Millar AA, Smith MA, Kunst L (2000) All fatty acids are not equal: discrimination in plant membrane lipids. Trends Plant Sci 5: 95–101 [DOI] [PubMed] [Google Scholar]
- Nguyen HT, Park H, Koster KL, Cahoon RE, Nguyen HT, Shanklin J, Clemente TE, Cahoon EB (2015) Redirection of metabolic flux for high levels of omega-7 monounsaturated fatty acid accumulation in camelina seeds. Plant Biotechnol J 13: 38–50 [DOI] [PubMed] [Google Scholar]
- Oakes J, Brackenridge D, Colletti R, Daley M, Hawkins DJ, Xiong H, Mai J, Screen SE, Val D, Lardizabal K, Gruys K, Deikman J (2011) Expression of fungal diacylglycerol acyltransferase2 genes to increase kernel oil in maize. Plant Physiol 155: 1146–1157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual F, Carman GM (2013) Phosphatidate phosphatase, a key regulator of lipid homeostasis. Biochim Biophys Acta 1831: 514–522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrie JR, Shrestha P, Belide S, Kennedy Y, Lester G, Liu Q, Divi UK, Mulder RJ, Mansour MP, Nichols PD, Singh SP (2014) Metabolic engineering Camelina sativa with fish oil-like levels of DHA. PLoS One 9: e85061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard M, Delamarter D, Martin TM, Shachar-Hill Y (2015) Lipid labeling from acetate or glycerol in cultured embryos of Camelina sativa seeds: a tale of two substrates. Phytochemistry 118: 192–203 [DOI] [PubMed] [Google Scholar]
- Qin C, Wang X (2002) The Arabidopsis phospholipase D family. Characterization of a calcium-independent and phosphatidylcholine-selective PLD ζ1 with distinct regulatory domains. Plant Physiol 128: 1057–1068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Lopez N, Haslam RP, Napier JA, Sayanova O (2014) Successful high-level accumulation of fish oil omega-3 long-chain polyunsaturated fatty acids in a transgenic oilseed crop. Plant J 77: 198–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Lopez N, Haslam RP, Usher SL, Napier JA, Sayanova O (2013) Reconstitution of EPA and DHA biosynthesis in Arabidopsis: iterative metabolic engineering for the synthesis of n-3 LC-PUFAs in transgenic plants. Metab Eng 17: 30–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi L, Katavic V, Yu Y, Kunst L, Haughn G (2012) Arabidopsis glabra2 mutant seeds deficient in mucilage biosynthesis produce more oil. Plant J 69: 37–46 [DOI] [PubMed] [Google Scholar]
- Shockey JM, Fulda MS, Browse JA (2002) Arabidopsis contains nine long-chain acyl-coenzyme a synthetase genes that participate in fatty acid and glycerolipid metabolism. Plant Physiol 129: 1710–1722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shockey JM, Gidda SK, Chapital DC, Kuan JC, Dhanoa PK, Bland JM, Rothstein SJ, Mullen RT, Dyer JM (2006) Tung tree DGAT1 and DGAT2 have nonredundant functions in triacylglycerol biosynthesis and are localized to different subdomains of the endoplasmic reticulum. Plant Cell 18: 2294–2313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shockey JM, Regmi A, Cotton K, Adhikari N, Browse JA, Bates PD (2016) Identification of Arabidopsis GPAT9 (At5g60620) as an essential gene involved in triacylglycerol biosynthesis. Plant Physiol 170: 163–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slack CR, Campbell LC, Browse JA, Roughan PG (1983) Some evidence for the reversibility of the cholinephosphotransferase-catalysed reaction in developing linseed cotyledons in vivo. Biochim Biophys Acta 754: 10–20 [Google Scholar]
- Slack CR, Roughan PG, Browse JA, Gardiner S (1985) Some properties of cholinephosphotransferase from developing safflower cotyledons. Biochim Biophys Acta 833: 438–448 [Google Scholar]
- Ståhl U, Carlsson AS, Lenman M, Dahlqvist A, Huang B, Banas W, Banas A, Stymne S (2004) Cloning and functional characterization of a phospholipid:diacylglycerol acyltransferase from Arabidopsis. Plant Physiol 135: 1324–1335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stymne S, Glad G (1981) Acyl exchange between oleoyl-CoA and phosphatidylcholine in microsomes of developing soya bean cotyledons and its role in fatty acid desaturation. Lipids 16: 298–305 [Google Scholar]
- Stymne S, Stobart AK (1984) Evidence for the reversibility of the acyl-CoA:lysophosphatidylcholine acyltransferase in microsomal preparations from developing safflower (Carthamus tinctorius L.) cotyledons and rat liver. Biochem J 223: 305–314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stymne S, Stobart AK (1987) Triacylglycerol Biosynthesis, Vol 9 Academic Press, New York [Google Scholar]
- Tan H, Yang X, Zhang F, Zheng X, Qu C, Mu J, Fu F, Li J, Guan R, Zhang H, Wang G, Zuo J (2011) Enhanced seed oil production in canola by conditional expression of Brassica napus LEAFY COTYLEDON1 and LEC1-LIKE in developing seeds. Plant Physiol 156: 1577–1588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor DC, Giblin EM, Reed DW, Hogge LR (1995) Stereospecific analysis and mass spectrometry of triacylglycerols from Arabidopsis thaliana (L.) heynh. Columbia seed. J Am Oil Chem Soc 72: 305–308 [Google Scholar]
- Tjellström H, Yang Z, Allen DK, Ohlrogge JB (2012) Rapid kinetic labeling of Arabidopsis cell suspension cultures: implications for models of lipid export from plastids. Plant Physiol 158: 601–611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Erp H, Kelly AA, Menard G, Eastmond PJ (2014) Multigene engineering of triacylglycerol metabolism boosts seed oil content in Arabidopsis. Plant Physiol 165: 30–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanhercke T, Wood CC, Stymne S, Singh SP, Green AG (2013) Metabolic engineering of plant oils and waxes for use as industrial feedstocks. Plant Biotechnol J 11: 197–210 [DOI] [PubMed] [Google Scholar]
- Wang L, Shen W, Kazachkov M, Chen G, Chen Q, Carlsson AS, Stymne S, Weselake RJ, Zou J (2012) Metabolic interactions between the Lands cycle and the Kennedy pathway of glycerolipid synthesis in Arabidopsis developing seeds. Plant Cell 24: 4652–4669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss SB, Kennedy EP, Kiyasu JY (1960) The enzymatic synthesis of triglycerides. J Biol Chem 235: 40–44 [PubMed] [Google Scholar]
- Weselake RJ, Taylor DC, Rahman MH, Shah S, Laroche A, McVetty PB, Harwood JL (2009) Increasing the flow of carbon into seed oil. Biotechnol Adv 27: 866–878 [DOI] [PubMed] [Google Scholar]
- Williams JP, Imperial V, Khan MU, Hodson JN (2000) The role of phosphatidylcholine in fatty acid exchange and desaturation in Brassica napus L. leaves. Biochem J 349: 127–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Carlsson AS, Francis T, Zhang M, Hoffman T, Giblin ME, Taylor DC (2012) Triacylglycerol synthesis by PDAT1 in the absence of DGAT1 activity is dependent on re-acylation of LPC by LPCAT2. BMC Plant Biol 12: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Fan J, Taylor DC, Ohlrogge JB (2009) DGAT1 and PDAT1 acyltransferases have overlapping functions in Arabidopsis triacylglycerol biosynthesis and are essential for normal pollen and seed development. Plant Cell 21: 3885–3901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou J, Wei Y, Jako C, Kumar A, Selvaraj G, Taylor DC (1999) The Arabidopsis thaliana TAG1 mutant has a mutation in a diacylglycerol acyltransferase gene. Plant J 19: 645–653 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.