Abstract
Aims
Population surveys of Type 2 diabetes mellitus and obesity conducted in Samoa over three decades have used varying methodologies and definitions. This study standardizes measures, and trends of Type 2 diabetes mellitus and obesity for 1978–2013 are projected to 2020 for adults aged 25–64 years.
Methods
Unit records from eight surveys (n = 12 516) were adjusted to the previous census for Division of residence, sex and age to improve national representativeness. Type 2 diabetes mellitus is defined as a fasting plasma glucose ≥ 7.0 mmol/l and/or on medication. Obesity is defined as BMI ≥ 30 kg/m2. Random effects meta‐regression was employed to assess time trends following logit transformation. Poisson regression from strata was used to assess the effects of mean BMI changes on Type 2 diabetes mellitus period trends.
Results
Over 1978–2013, Type 2 diabetes mellitus prevalence increased from 1.2% to 19.6% in men (2.3% per 5 years), and from 2.2% to 19.5% in women (2.2% per 5 years). Obesity prevalence increased from 27.7% to 53.1% in men (3.6% per 5 years) and from 44.4% to 76.7% (4.5% per 5 years) in women. Type 2 diabetes mellitus and obesity prevalences increased in all age groups. From period trends, Type 2 diabetes mellitus prevalence in 2020 is projected to be 26% in men and women. Projected obesity prevalence is projected to be 59% in men and 81% in women. Type 2 diabetes mellitus period trends attributable to BMI increase are estimated as 31% (men) and 16% (women), after adjusting for age.
Conclusion
This is the first study to produce trends of Type 2 diabetes mellitus and obesity in Samoa based on standardized data from population surveys. Type 2 diabetes mellitus is equally prevalent in both sexes, and obesity is widespread. Type 2 diabetes mellitus prevalence in Samoa is likely to continue to increase in the near future.
What's new?
This is the first study to standardize previously conducted, disparate population‐based measures of Type 2 diabetes mellitus and obesity from unit records of empirical surveys to produce period trends in Samoa.
Prevalences of Type 2 diabetes mellitus and obesity have increased over 35 years (1978–2013), and are projected to continue to increase in the near future.
The increase in Type 2 diabetes mellitus is partially attributed to the rise in obesity and effective interventions to reduce obesity are urgently required.
What's new?
This is the first study to standardize previously conducted, disparate population‐based measures of Type 2 diabetes mellitus and obesity from unit records of empirical surveys to produce period trends in Samoa.
Prevalences of Type 2 diabetes mellitus and obesity have increased over 35 years (1978–2013), and are projected to continue to increase in the near future.
The increase in Type 2 diabetes mellitus is partially attributed to the rise in obesity and effective interventions to reduce obesity are urgently required.
Introduction
The Global Burden of Disease (GBD) Study 2010 estimated that global age‐standardized diabetes mortality increased from 16.3 to 19.5/100 000 over 1990–2010 1. Although this includes all forms of diabetes, it can be inferred that mortality rates for Type 2 diabetes mellitus have increased because ~ 5–10% of all diabetes cases are Type 1 2. Obesity, assessed as BMI ≥ 30 kg/m2 3, is the most significant risk factor for Type 2 diabetes mellitus 3.
Over the past three decades, non‐communicable disease (NCD) surveys have been conducted in Samoa, each with somewhat different methods and definitions of Type 2 diabetes mellitus and obesity, which has prevented accurate estimations of period trends 4. In the 2002 World Health Organization (WHO) STEPS survey, Type 2 diabetes mellitus prevalence was estimated as 21.5% and obesity prevalence 54.8% 5. Type 2 diabetes mellitus prevalence from the 2013 STEPS survey was reported to be 45.8% 6.
This study calculates comparable population‐based Type 2 diabetes mellitus and obesity measures from empirical surveys to produce prevalence period trends (1978–2013), and projects Type 2 diabetes mellitus and obesity prevalence in Samoa to 2020.
Methods
Study design
Survey inclusion criteria were: (a) contain blood glucose and Type 2 diabetes mellitus information, and anthropometric measurements; (b) can be disaggregated by age, sex and Division of residence; and (c) designed to be nationally representative, or could be adjusted to reflect national demographic characteristics. Eight studies were included: the 1978 Non‐Communicable Disease Risk Factor (NCDRF) survey (n = 1079) 7, repeated in 1991 (n = 1532) 8, 9; the Samoan Adiposity and Cardiovascular Disease Risk Factor (SACRF) longitudinal study in 1991 (n = 748) and 1995 (n = 719) 10; the 2002 Samoa STEPS (n = 2554) 5; the 2003 Samoan Family Study of Overweight and Diabetes (SFSOD) (n = 684) 11; the 2010 Genome‐Wide Association Study (GWAS, n = 3468) 12; and the Samoa 2013 STEPS (n = 1725) 6.
Excluded are two single‐village studies: the 1979 (n = 336) and 1982 (n = 661) surveys from the Samoan Studies Project 13 and a 2009 obesity prevalence survey 14 (n = 85, aged ≥ 40 years).
Population and samples
On the main island of Upolu (76% of the population), the administrative Divisions are: Apia (capital), officially the only urban area; North West Upolu, adjacent to Apia and with the international airport and inter‐island ferry terminal, it can be considered peri‐urban or semi‐rural; the rural areas of Rest of Upolu, and the smaller island of Savai'i although Tuasivi, with the ferry terminal and government centre, could be considered semi‐rural 15.
The Samoan population structure is largely unchanged over the past 30 years due to high out‐migration counterbalanced by high total fertility rates of 4.7/woman over 1981–2011 15, 16. Population growth was relatively stable at 0.1% (1981–1985) and 0.8% (2006–2011) 16. Over the period 1981–2011, adults aged 55–64 years (proportion of 25–64 years) made up 14–15% of the population, and the urban proportion was between 21% and 20% of the total 15, 16. The male‐to‐female ratio remained almost unchanged over three decades at 1.08 in 1981 and 1.07 in 2011 15, 16. Only non‐pregnant participants aged 25–64 years were included in the analyses. Surveys were adjusted by Division and age for each sex to the nearest previous census to reduce selection bias, increase national representativeness and minimize heterogeneity between surveys. Response rates for randomly selected eligible participants were: 76% (1978 NCVDS) 7, 73% (1991 NCVDS) 8, 9, > 90% (1991 and 1995 SACRF) 10, 97% (2002 STEPS) 5, 64% (2013 STEPS, due to cyclone after‐effects) 6, and 85% (2010 GWAS) 12; the 2003 SFSOD response rates were not reported 11.
Except for the 1991 and 1995 SACRF surveys, case weights by Division and 10‐year age groups for each sex were calculated by dividing the subgroup proportion in the nearest previous census by the proportion of the same subgroup in each survey. Case weights were applied and Type 2 diabetes mellitus and obesity prevalence, and mean fasting plasma glucose and BMI (and their standard errors, se) were then calculated from each survey. For the 1991 and 1995 SACRF surveys, sex‐specific prevalences and means (and se) were derived as aggregate data, because of the need to adjust Type 2 diabetes mellitus case enumeration (Appendix S1), and then directly standardized for each sex by age and Division to the nearest previous census.
The 1978 and 1991 NCDRF surveys were designed to examine NCD risk factor differences between indicative samples of urban and rural areas. These surveys had equal numbers of urban and rural participants, but did not sample in the North West Division. Tuasivi in Savai'i was included, which is likely less rural than the rest of Savai'i. Thus, participants from Savai'i in the NCDRF surveys could be considered as semi‐rural, and somewhat similar in economic circumstances to North West Upolu.
Participant selection in the 2003 SFSOD was conducted informally and non‐randomly (Appendix S2). The 2010 GWAS aimed to recruit a nationally representative sample, but was over‐represented by the Rest of Upolu and Savai'i Divisions, and older age groups. These surveys were adjusted accordingly using case weights described above.
The 2002 STEPS survey did not include participants from North West Upolu. Case weights were applied to reduce selection bias. Participants in the 2013 STEPS survey were selected to be nationally representative based on the 2011 census, but case weights were applied to minimize residual selection bias from differential response.
Measurement, data collection and adjustments
Individual record data were available from all surveys, and were used to calculate prevalences and adjust for measurement and selection biases. The 2002 and 2011 STEPS surveys measured glucose from capillary (whole) blood specimens using a point‐of‐care device. All other surveys collected venous blood samples, which were centrifuged and frozen before transportation to Australia (plasma from the 1978 and 1991 NCDRF) or the USA (serum from 1991 and 1995 SACRF, 2003 SFSOD, 2010 GWAS) for analysis. For each survey, glucose measurements were standardized to one diagnostic criterion to reduce measurement bias.
Diabetes
The current WHO Type 2 diabetes mellitus definition is a fasting plasma glucose ≥ 7.0 mmol/l and/or on medication for Type 2 diabetes mellitus 17. In all surveys, only those positively identified as fasting were included in analyses. For further details on Type 2 diabetes mellitus designation see Appendix S3.
The WHO definition 17 was applied to the 1978 and 1991 NCDRF. The 2003 SFSOD and 2010 GWAS surveys used fasting venous serum instead of plasma. These values were converted to plasma‐equivalent using: –0.137 + 1.047x, where x is serum glucose (mmol/l) 18. Type 2 diabetes mellitus for the 2003 and 2010 surveys was defined as (converted) fasting plasma glucose ≥ 7.0 mmol/l and/or on medication. For the 1991 and 1995 SACRF, which excluded known Type 2 diabetes mellitus or hypertension, a corrected Type 2 diabetes mellitus prevalence (after conversion from serum to plasma glucose) for each sex and age group was estimated by applying a ratio of known‐to‐new Type 2 diabetes mellitus cases (Appendix S1). The 2002 STEPS surveys measured capillary (whole) blood with a Roche Accutrend® GCT glucose meter which tested whole‐blood specimens and produced whole‐blood glucose concentrations, requiring a cut‐point for Type 2 diabetes mellitus as fasting whole blood ≥ 6.1 mmol/l (and/or on medication), instead of using fasting plasma glucose ≥ 7.0 mmol/l, which is appropriate for glucose meters that produce glucose in plasma concentrations 17. For mean fasting plasma glucose analyses, individual glucose readings in the 2002 STEPS survey were multiplied by 1.11 for conversion to a plasma‐equivalent measure to calculate mean values, in accordance with recommendations from the International Federation of Clinical Chemistry (IFCC) in 2006 19. For 2013 STEPS, Type 2 diabetes mellitus was derived from unit record data using a cut‐off point of ≥ 7.0 mmol/l (and/or on Type 2 diabetes mellitus medication) because the Roche Accutrend® plus glucose meter was manufactured after the IFCC recommendations of 2006 for point‐of‐care devices using whole blood to calibrate results to plasma equivalents 19; the upper limit given in Roche Accutrend® plus documentation for impaired fasting glucose of 6.9 mmol/l 20 corresponds to the WHO definition based on fasting plasma glucose 17 (Appendix S3).
Obesity
BMI was calculated as weight/height2 (kg/m2). In this study, obesity prevalence was defined using the WHO (standard) guideline 3 ≥ 30 kg/m2, and the suggested ethnic‐specific cut‐off points (BMI > 32 kg/m2) 21 because Polynesians have higher muscle‐to‐fat ratios than Europids.
The 1991 and 1995 SACRF were adjusted to correct for obesity under‐enumeration from exclusion of known Type 2 diabetes mellitus and known hypertension (on medication). The numerator and denominator were adjusted using ratios of known: new Type 2 diabetes mellitus and/or hypertensive cases in obese populations derived from the 1991 NCDRF survey (Appendix S4).
Trend analysis
Period trends in prevalence (following logit transformation) were analysed using random effects meta‐regression for each sex, and prevalence of Type 2 diabetes mellitus and obesity with 95% prediction intervals (PI) were projected for 2014–2020. Period trends for mean fasting plasma glucose and mean BMI were analysed using linear meta‐regression.
A sensitivity analysis of Type 2 diabetes mellitus and obesity trends from meta‐regression was for all eight surveys compared with six surveys that excluded the 1991 and 1995 SACRF surveys – from which prevalence was adjusted to incorporate estimates of known Type 2 diabetes mellitus and hypertensive cases that were omitted at the participant selection stage.
Effects of changes of BMI on period trends of Type 2 diabetes mellitus
Because population subgroup Type 2 diabetes mellitus and obesity were adjusted using ratios in the 1991 and 1995 SACRF yielding count data, strata analysis (using Poisson regression) of the combined surveys was undertaken. The strata consisted of: survey (8), Division (4), sex (2) and age groups (4), totalling 256 strata (n = 12 516). Each stratum contained Type 2 diabetes mellitus and population counts, mean BMI, and were weighted by Division and age for each period and sex to the nearest previous census. Poisson regression, using log of the stratum population as an offset, was used to analyse the effects of increasing BMI on Type 2 diabetes mellitus prevalence increase over 1978–2013. Type 2 diabetes mellitus counts were modelled with period (model 1), period and age (model 2), and period, age and BMI (model 3). The relative risk (RR) for Type 2 diabetes mellitus in 2013 was compared with 1978 (referent) for each model. The difference in RR between models 1 and 2 indicated the contribution of age to Type 2 diabetes mellitus and obesity trends over 30 years. The difference in RR between models 2 and 3 indicated the contribution of BMI to Type 2 diabetes mellitus trend over 30 years, adjusting for age.
Results
Diabetes
From meta‐regression estimates, Type 2 diabetes mellitus prevalence increased from 1.2% in 1978 to 19.6% in 2013 in men (2.9% per 5 years, P < 0.0001), and from 2.2% to 19.5% in women (2.2% per 5 years, P < 0.0001) (Table 1, Fig. 1). Empirical estimates from surveys are presented in Table 2. Type 2 diabetes mellitus prevalence increased in all age groups over the period, with higher increases at older ages: 25–34 years, 1.2% per 5 years; 35–44 years, 1.4% per 5 years; 45–54 years, 3.2% per 5 years; and 55–64 years, 3.5% per 5 years (Fig. 2). Based on current period trends, Type 2 diabetes mellitus prevalence projected to 2020 is estimated as 26% in men (95% PI, 14–39%) and women (95% PI, 15–38%).
Table 1.
Trends from meta‐regression in diabetes and obesity in Samoan adults, 1978–2013
| Diabetes prevalence (%) | Mean fasting plasma glucose (mmol/l) | Obesity prevalence (standard cut‐off point) (%) | Obesity prevalence (ethnic cut‐off point) (%) | Mean BMI (kg/m2) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Rate per 5 years | 95% CI | Rate per 5 years | 95% CI | Rate per 5 years | 95% CI | Rate per 5 years | 95% CI | Rate per 5 years | 95% CI | |
| Men | 2.91 | 1.89 to 3.94 | 0.17 | 0.14 to 0.20 | 3.61 | 2.59 to 4.62 | 2.84 | 1.81 to 3.87 | 0.54 | 0.38 to 0.70 |
| Women | 2.23 | 1.20 to 3.25 | 0.20 | 0.17 to 0.22 | 4.47 | 3.46 to 5.48 | 4.84 | 3.83 to 5.84 | 0.87 | 0.75 to 1.00 |
| National | 2.57 | 1.55 to 3.59 | 0.18 | 0.16 to 0.21 | 4.21 | 3.21 to 5.21 | 3.99 | 2.97 to 5.00 | 0.73 | 0.64 to 0.83 |
Diabetes = fasting plasma glucose ≥ 7.0 mmol/l and/or on medication.
Cut‐off points for obesity: standard, BMI ≥ 30 kg/m2; ethnic‐specific, BMI > 32 kg/m2.
Sex‐specific estimates adjusted for Division of residence and age to most recent previous census. National estimates adjusted for Division of residence, sex and age. Test for trend from random effects meta‐regression after logit transformation of prevalences, or linear trends (means). All trends are statistically significant at P < 0.05, except mean fasting plasma glucose in men (P = 0.07).
Figure 1.
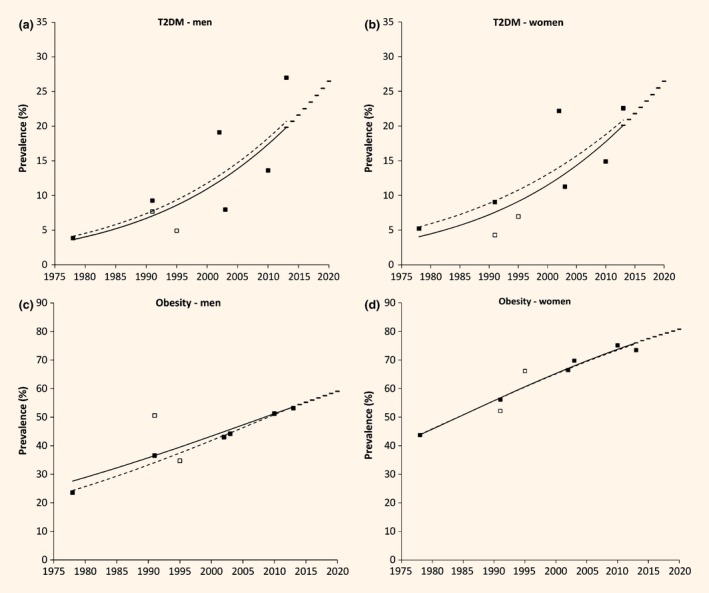
Type 2 diabetes mellitus (T2DM) (a,b) and obesity (c,d) trends in Samoan men and women aged 25–64 years, 1978–2013. Markers are survey years. Trend lines were derived from random effects meta‐regression, 1978–2013. Solid line is the regression using all included surveys. Broke line is a sensitivity analysis excluding the 1991 and 1995 SACRF surveys (indicated by the open markers) due to these surveys requiring significant adjustment for exclusion of known T2DM cases. Bars indicate projected prevalences to 2020.
Table 2.
Diabetes and obesity from empirical surveys in Samoan adults, 1978–2013
| Year | Diabetes prevalence | Mean fasting plasma glucose (mmol/l) | Obesity prevalence (standard cut‐point) | Obesity prevalence (ethnic cut‐point) | Mean BMI (kg/m2) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| % | N | 95% CI | Mean | N | 95% CI | N | % | 95% CI | % | 95% CI | Mean | N | 95% CI | |
| Men | ||||||||||||||
| 1978 | 3.9 | 334 | 1.8 to 5.9 | 5.1 | 421 | 5.0 to 5.2 | 381 | 23.5 | 19.3 to 27.8 | 13.6 | 10.1 to 17.0 | 27.1 | 491 | 26.8 to 27.5 |
| 1991a | 9.3 | 689 | 7.1 to 11.4 | 6.1 | 689 | 6.0 to 6.2 | 246 | 36.5 | 30.5 to 42.6 | 25.1 | 19.7 to 30.5 | 29.0 | 696 | 28.8 to 29.2 |
| 1991b | 7.7 | 336 | 4.8 to 10.5 | 5.8 | 315 | 5.6 to 5.9 | 340 | 50.6 | 45.3 to 55.9 | 49.9 | 44.5 to 55.2 | 30.6 | 181 | 29.8 to 31.5 |
| 1995 | 4.9 | 264 | 2.3 to 7.5 | 4.8 | 346 | 4.7 to 4.9 | 201 | 34.7 | 28.1 to 41.3 | 45.1 | 38.2 to 51.9 | 28.1 | 341 | 27.1 to 29.0 |
| 2002 | 19.1 | 826 | 16.4 to 21.8 | 6.4 | 1136 | 6.3 to 6.5 | 866 | 43.0 | 39.7 to 46.3 | 31.5 | 28.3 to 34.5 | 29.7 | 1185 | 29.4 to 30.0 |
| 2003 | 8.0 | 293 | 4.8 to 11.1 | 5.5 | 295 | 5.2 to 5.7 | 314 | 44.2 | 38.7 to 49.7 | 30.5 | 25.4 to 35.6 | 29.9 | 317 | 29.3 to 30.5 |
| 2010 | 13.6 | 1154 | 11.6 to 15.6 | 5.8 | 1165 | 5.7 to 6.0 | 1381 | 51.2 | 48.6 to 53.9 | 38.4 | 35.8 to 40.9 | 30.9 | 1394 | 30.6 to 31.2 |
| 2013 | 27.0 | 656 | 23.6 to 30.4 | 6.8 | 653 | 6.6 to 7.0 | 582 | 53.1 | 49.1 to 57.2 | 39.2 | 35.2 to 43.1 | 31.5 | 579 | 30.9 to 32.1 |
| Women | ||||||||||||||
| 1978 | 5.2 | 403 | 3.0 to 7.4 | 5.1 | 520 | 5.0 to 5.3 | 447 | 43.7 | 39.1 to 48.3 | 30.0 | 25.8 to 34.2 | 29.2 | 587 | 28.8 to 29.6 |
| 1991a | 9.0 | 826 | 7.1 to 11.0 | 5.9 | 826 | 5.8 to 6.0 | 287 | 56.1 | 50.3 to 61.8 | 42.9 | 37.1 to 48.6 | 31.2 | 836 | 30.9 to 31.4 |
| 1991b | 4.3 | 398 | 2.3 to 6.3 | 5.2 | 383 | 5.1 to 5.3 | 389 | 52.2 | 47.2 to 57.1 | 43.1 | 38.1 to 48.0 | 30.1 | 222 | 29.4 to 30.8 |
| 1995 | 7.0 | 286 | 4.0 to 9.9 | 5.2 | 403 | 5.1 to 5.4 | 221 | 66.1 | 59.9 to 72.4 | 45.4 | 38.8 to 51.9 | 31.2 | 348 | 30.6 to 31.9 |
| 2002 | 22.2 | 906 | 19.5 to 24.9 | 6.5 | 1284 | 6.4 to 6.6 | 947 | 66.4 | 63.4 to 69.4 | 52.7 | 49.5 to 55.9 | 33.2 | 1337 | 32.9 to 33.5 |
| 2003 | 11.2 | 352 | 7.9 to 14.5 | 5.8 | 349 | 5.5 to 6.0 | 370 | 69.7 | 65.0 to 74.4 | 58.6 | 53.6 to 63.6 | 33.8 | 367 | 33.1 to 34.5 |
| 2010 | 14.9 | 1789 | 13.2 to 16.5 | 5.9 | 1769 | 5.7 to 6.0 | 2072 | 75.1 | 73.3 to 77.0 | 63.6 | 61.5 to 65.7 | 34.7 | 2052 | 34.4 to 35.0 |
| 2013 | 22.6 | 966 | 19.9 to 25.2 | 6.7 | 963 | 6.6 to 6.9 | 885 | 73.4 | 70.5 to 76.3 | 63.0 | 59.8 to 66.1 | 34.7 | 889 | 34.2 to 35.2 |
| National | ||||||||||||||
| 1978 | 4.6 | 738 | 3.1 to 6.2 | 5.1 | 941 | 5.0 to 5.2 | 829 | 34.7 | 31.4 to 37.9 | 22.6 | 19.7 to 25.4 | 28.3 | 1078 | 28.0 to 28.6 |
| 1991a | 8.9 | 1515 | 7.5 to 10.4 | 6.0 | 1515 | 5.9 to 6.1 | 1167 | 47.0 | 44.1 to 49.9 | 34.6 | 31.9 to 37.3 | 30.2 | 1532 | 29.9 to 30.4 |
| 1991b | 6.0 | 734 | 4.3 to 7.7 | 5.5 | 698 | 5.4 to 5.6 | 729 | 51.4 | 47.7 to 55.0 | 46.5 | 42.8 to 50.1 | 30.5 | 716 | 30.2 to 30.9 |
| 1995 | 6.0 | 550 | 4.0 to 7.9 | 5.0 | 749 | 4.9 to 5.1 | 422 | 50.4 | 45.6 to 55.2 | 45.2 | 40.5 to 50.0 | 29.8 | 705 | 29.2 to 30.5 |
| 2002 | 20.7 | 1738 | 18.8 to 22.6 | 6.5 | 2420 | 6.4 to 6.5 | 1812 | 55.6 | 53.3 to 57.9 | 42.6 | 40.4 to 44.9 | 31.5 | 2522 | 31.3 to 31.7 |
| 2003 | 9.7 | 644 | 7.4 to 12.0 | 5.6 | 644 | 5.4 to 5.8 | 684 | 57.7 | 54.0 to 61.4 | 45.7 | 41.9 to 49.4 | 32.0 | 684 | 31.5 to 32.5 |
| 2010 | 14.4 | 2942 | 13.1 to 15.7 | 5.9 | 2934 | 5.7 to 6.0 | 3447 | 65.5 | 63.9 to 67.1 | 53.5 | 51.9 to 55.2 | 33.2 | 3446 | 32.9 to 33.4 |
| 2013 | 24.3 | 1622 | 22.2 to 26.4 | 6.8 | 1616 | 6.7 to 6.9 | 1466 | 65.2 | 62.8 to 67.6 | 53.4 | 50.8 to 55.9 | 33.4 | 1468 | 33.0 to 33.8 |
1978 Non‐Communicable Disease Risk Factor (NCDRF) Survey n = 1079 7; 1991a NCDRF Survey (n = 1532) 8, 9; 1991b (n = 748) and 1995 (n = 719) Samoan Adiposity and Cardiovascular Disease Risk Factor (SACRF) study 10; 2002 Samoa STEPS (n = 2554) 5; 2003 Samoan Family Study of Overweight and Diabetes (SFSOD) n = 684 11; 2010 Genome‐Wide Association Study (GWAS) n = 3468 12; and 2013 Samoa STEPS (n = 1725) 6. Estimates weighted to the most recent previous census to improve representativeness.
Sex‐specific estimates adjusted for Division of residence and age. National estimates adjusted for Division of residence, sex and age.
Diabetes = fasting plasma glucose ≥ 7.0 mmol/l and/or on medication. Mean fasting plasma glucose includes those on Type 2 diabetes mellitus medication.
Cut‐off points for obesity: standard, BMI ≥ 30 kg/m2; ethnic‐specific, BMI > 32 kg/m2.
Figure 2.
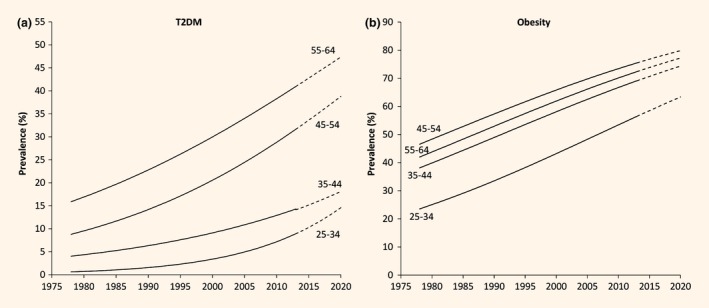
Age‐specific Type 2 diabetes mellitus (T2DM) (a) and obesity (b) prevalence trends in Samoan adults aged 25–64 years, national, 1978–2013. Solid line is meta regression trend for 1978–2013. Broke line is projected estimates to 2020. Survey markers not shown for presentation clarity.
Obesity
From meta‐regression estimates, obesity prevalence (using BMI ≥ 30 kg/m2) increased from 27.7% in 1978 to 53.1% in 2013 (3.6% per 5 years, P < 0.0001) in men, and from 44.4% in 1978 to 76.7% in 2013 (4.5% per 5 years, P < 0.0001) in women (Table 1, Fig. 1). Using BMI > 32 kg/m2, obesity prevalence increased from 24.7% in 1978 to 41.2% in 2013 in men (2.8% per 5 years, P = 0.07), and from 30.0% in 1978 to 65.1% in 2013 in women (4.8% per 5 years, P < 0.0001). Empirical estimates from the surveys are presented in Table 2. Increases in obesity prevalence were highest in the youngest age group: 25–34 years at 4.6% per 5 years compared with 35–44 years at 4.3% per 5 years, 55–64 years at 4.2% per 5 years and 45–54 years at 4.2% per 5 years (Fig. 2). Period projection of obesity prevalence in 2020 is 59% (95% PI, 45–73%) in men and 81% (95% PI, 71–91%) in women using standard cut‐off points; or 47% (95% PI, 23–72%) in men and 71% (95% PI, 61–82%) in women using ethnic‐specific cut‐off points.
Sensitivity analyses
When the 1991 and 1995 SACRF surveys were excluded from meta‐regression, there were small differences in the projections of Type 2 diabetes mellitus or obesity (BMI ≥ 30 kg/m2) in 2020 (Fig. 1). Projected 2020 Type 2 diabetes mellitus prevalences, based on meta‐regression of six surveys, are 27% (men) and 26% (women). Projected 2020 obesity prevalences are 60% in men and 80% in women.
Effects of changes in age adjusted BMI on Type 2 diabetes mellitus period trends
After adjusting for age and BMI, the Type 2 diabetes mellitus RR in men (compared with 1978) was 2.2 [95% confidence interval (CI), 1.6 to 3.2, P < 0.0001), a decrease of 31% between the age‐ and age‐ and BMI‐adjusted RR. In women, after adjusting for age and BMI, Type 2 diabetes mellitus RR was 2.1 (95% CI, 1.6 to 2.8, P < 0.0001), a decrease of 16% compared with age‐only adjustment.
Discussion
Type 2 diabetes mellitus and obesity in Samoa increased in both sexes and across all age groups between 1978 and 2013. The prevalence of Type 2 diabetes mellitus is similar between sexes, but obesity is more prevalent in women than men. Based on current trends using meta‐regression, we predict that more than one in four Samoan adults will have Type 2 diabetes mellitus in 2020 based on period trends.
The increase in Type 2 diabetes mellitus is partially influenced by the rise in obesity. Similar results are seen in other Pacific Island countries where increases in mean BMI in Fiji Melanesians explained 27% (men) and 25% (women) of increases in Type 2 diabetes mellitus prevalence (1980–2011), after age adjustment 22. In Tonga, BMI increases explained 76% (men) and 73% (women) of Type 2 diabetes mellitus prevalence increases over 1973–2012 23. BMI increases in Samoa, Fiji 22 and Tonga 23 have been attributed to changes in way of life, including a shift away from farming and fishing towards more sedentary occupations; and increased consumption of energy‐dense imported foods 24.
The increase in Type 2 diabetes mellitus prevalence may be influenced by birth cohort effects, observed in the USA 25. Over the period 1988–2010, US Type 2 diabetes mellitus prevalence rose with each successive birth cohort (1910–1989) in conjunction with increases in population obesity. Obesity rates in Samoa are projected to continue increasing in the near future, and birth cohort influences may occur in this population, but these would need to be carefully separated from period effects in further analyses.
It is expected that Type 2 diabetes mellitus prevalence would be variable from multiple surveys in Samoa. All studies were included that met stated criteria – considered a desirable approach in meta‐analyses 26. Inclusion criteria were: measured blood glucose and BMI, and that the data could be adjusted to be nationally representative. Measurement bias was minimized by estimating known Type 2 diabetes mellitus when absent, converting blood or serum glucose concentrations to plasma equivalents, and using correct cut‐offs for Type 2 diabetes mellitus. Selection bias was minimized by weighting samples to the closest previous census by age group and division of residence for each sex.
The use of meta‐analysis to produce trends in Type 2 diabetes mellitus prevalence by meta‐regression is preferable to selecting point estimates from empirical surveys because it smooths variations from unmeasured and unadjusted biases and confounding, augments participant numbers for analysis (greater statistical significance), weights studies by their sample size (se), combines statistically heterogeneous studies through random effects analysis, and increases generalizability by including multiple studies from different periods and sites. A sensitivity analysis comparing meta‐regression of all surveys, with that excluding the two surveys in which known cases of Type 2 diabetes mellitus were estimated (SACRF 1991, 1995), revealed minimal changes in slope. Period projections of Type 2 diabetes mellitus and obesity prevalences to 2020 based on meta‐regression (following logit transformation) are appropriate for short‐term forecasting (2014–2020). Type 2 diabetes mellitus prevalence is expected to plateau at some time in the future.
The differences in Type 2 diabetes mellitus estimates between the two surveys in 1991 are likely to be a consequence of the selection effects from variations in modernization, development and urbanization within officially designated urban and rural areas based on large administrative Divisions. In the 1991 SACRF survey, the proportion of participants by Division was similar to the 1991 census: 21% Apia, 25% North West Upolu, 26% Rest of Upolu and 28% Savai'i 27. However, the villages selected were the least developed, even within rural areas (personal communication, STM, 2014). The 1991 NCDRF survey did not recruit participants from North West Upolu Division, leading to a higher proportion from Apia (29%) 8, 9, consistent with the purpose of the survey, which was to compare NCD risk factors between urban and rural populations. After adjusting for differences in sampling across Divisions, Type 2 diabetes mellitus prevalence remains higher in the 1991 NCDRF than the 1991 SACRF survey. This may partly be a consequence of selection of Tuasivi as the survey site in the rural Division of Savai'i in the 1978 and 1991 NCDRF surveys, which, as the ferry port and government centre, is less rural than the rest of island which was sampled in the 1991–1995 SACRF surveys.
The published 2002 STEPS Type 2 diabetes mellitus prevalence, weighted to the previous census, was 21.5% 5 and in the present study was 20.7%. In the 2010 GWAS, published age‐standardized Type 2 diabetes mellitus prevalence was 12.4% for men and 11.8% for women 12, similar to our findings of 13.0% (men) and 14.0% (women).
The 2013 STEPS Type 2 diabetes mellitus is reported as 45.8% (age, sex and Division weighted to the 2011 census) 6, This prevalence (twice that of the 2002 STEPS) was likely calculated from a whole‐blood glucose cut‐off point of 6.1 mmol/l (as used in 2002). We reproduced a similar Type 2 diabetes mellitus prevalence (49.7%) from the 2013 STEPS unit record data using this cut‐off point (age, sex and Division adjusted to the 2011 census). The 2013 STEPS survey used a Roche Accutrend® plus glucose meter that produces readings in plasma equivalents [20], and when using the appropriate plasma cut‐off point of ≥ 7.0 mmol/l, yields a Type 2 diabetes mellitus prevalence of 24.3% – similar to 2002 STEPS (Appendix S3).
The 2013 IDF Diabetes Atlas estimated Type 2 diabetes mellitus in Samoa, from modelled projections, as 7.7% 28, significantly lower than our estimates of 19.5% from empirical surveys. The GBD reported that obesity prevalence in Samoan adults > 20 years increased from 37% to 46% in men and from 62% to 69% in women over 1980–2013 29 compared with our estimates of 23.5% to 53.1% in men and 43.7% to 73.4% in women (25–64 years, 1978–2013). Differences likely stem from the Diabetes Atlas and GBD analysis each using a single survey (1991 NCDRF for Type 2 diabetes mellitus; 2002 Samoa STEPS for obesity), compared with this study in which eight surveys are included. The Diabetes Atlas and the GBD also used extensive projection, whereas in this study interpolation is employed and extrapolation is limited and based on several surveys.
This study is the first to assess Type 2 diabetes mellitus and obesity prevalence trends using multiple cross‐sectional health surveys in Samoa, adjusted to minimize differences in selection, measurement and case definition. Type 2 diabetes mellitus and other obesity‐related conditions will continue to increase due to the large proportion of obese people, especially in women and the young. Continued aging would further increase Type 2 diabetes mellitus prevalence.
Funding sources
This study was funded by the Australian Department of Foreign Affairs and Trade through the Australian Development Research Awards Scheme (ADRAS), grant number 66886.
Competing interests
None declared.
Supporting information
Appendix S1. Adjustment for exclusion of known Type 2 diabetes mellitus 1991 and 1995 SACRF surveys.
Appendix S2. Selection biases in survey participants.
Appendix S3. Designation of Type 2 diabetes mellitus.
Appendix S4. Adjustment for obesity from exclusion of known Type 2 diabetes mellitus and/or hypertension in 1991 and 1995 SACRF surveys.
Diabet. Med. 34, 654–661 (2017)
References
- 1. Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V et al Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012; 380: 2095–2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. American Diabetes Association (ADA) . Diagnosis and classification of diabetes mellitus. Diabetes Care 2009; 32(Suppl. 1): S62–S67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. World Health Organization (WHO), International Association for the Study of Obesity (IASO), International Obesity Task Force (IOTF) . The Asia–Pacific Perspective: Redefining Obesity and its Treatment. Sydney: Health Communications, 2000. [Google Scholar]
- 4. Win Tin ST, Lee CMY, Colaguiri R. A profile of diabetes in Pacific Island Countries and Territories. Diabetes Res Clin Pr 2015; 107: 233–246. [DOI] [PubMed] [Google Scholar]
- 5. World Health Organization (WHO) . Samoa NCD Risk Factors STEPS report 2002. Apia: WHO, 2008. [Google Scholar]
- 6. World Health Organization (WHO). Samoa NCD Risk Factors STEPS report 2013. Apia: WHO, 2014.
- 7. Zimmet P, Faaiuso S, Ainuu J, Whitehouse S, Milne B, Deboer W. The prevalence of diabetes in the rural and urban Polynesian population of Western Samoa. Diabetes 1981; 30: 45–51. [DOI] [PubMed] [Google Scholar]
- 8. Collins VR, Dowse GK, Toelupe PM, Imo TT, Aloaina FL, Spark RA et al Increasing prevalence of NIDDM in the Pacific island population of Western Samoa over a 13‐year period. Diabetes Care 1994; 17: 288–296. [DOI] [PubMed] [Google Scholar]
- 9. Hodge AM, Dowse GK, Toelupe P, Collins VR, Imo T, Zimmet PZ. Dramatic increase in the prevalence of obesity in Western Samoa over the 13 year period 1978–1991. Int J Obesity 1994; 18: 419–428. [PubMed] [Google Scholar]
- 10. McGarvey ST. Cardiovascular disease (CVD) risk factors in Samoa and American Samoa, 1990–1995. Pac Health Dialog 2001; 8: 157–162. [PubMed] [Google Scholar]
- 11. DiBello JR, Baylin A, Viali S, Tuitele J, Bausserman L, McGarvey ST. Adiponectin and Type 2 diabetes in Samoan adults. Am J Hum Biol 2009; 21: 389–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hawley NL, Minster RL, Weeks DE, Viali S, Reupena MS, Sun G et al Prevalence of adiposity and associated cardiometabolic risk factors in the Samoan Genome‐Wide Association Study. Am J Hum Biol 2014; 26: 491–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bindon JR, Baker PT. Modernization, migration and obesity among Samoan adults. Ann Hum Biol 1985; 12: 67–76. [DOI] [PubMed] [Google Scholar]
- 14. Barnes SS, O'Carroll DC, Sumida L, Shafer LA, Yamada S. Evidence for a continuing gap in rural/urban adult obesity in the Samoan archipelago. J Commun Health 2011; 36: 534–537. [DOI] [PubMed] [Google Scholar]
- 15. Samoa Bureau of Statistics . Population and Housing 2011 Analytical Report. Apia: Government of Samoa, 2011. [Google Scholar]
- 16. Hartmann M. Mortality and Fertility in Western Samoa. Apia: South Pacific Commission, 1985. [Google Scholar]
- 17. World Health Organization (WHO) . NCD Global Monitoring Framework, 2013. (full‐fledged text). Available at http://www.who.int/nmh/global_monitoring_framework/2013-11-06-who-dc-c268-whp-gap-ncds-techdoc-def3.pdf?ua=1 Last accessed 10 May 2016.
- 18. Rydén L, Standl E, Bartnik M, Van den Berghe G, Betteridge J, de Boer MJ et al Guidelines on diabetes, pre‐diabetes, and cardiovascular diseases: executive summary. The Task Force on Diabetes and Cardiovascular Diseases of the European Society of Cardiology (ESC) and of the European Association for the Study of Diabetes (EASD). Eur Heart J 2007; 28: 88–136. [DOI] [PubMed] [Google Scholar]
- 19. D'Orazio P, Burnett RW, Fogh‐Andersen N, Jacobs E, Kuwa K, Külpmann WR et al Approved IFCC recommendation on reporting results for blood glucose: International Federation of Clinical Chemistry and Laboratory Medicine Scientific Division, Working Group on Selective Electrodes and Point‐of‐Care Testing (IFCC‐SD‐WG‐SEPOCT). Clin Chem Lab Med 2006; 44: 1486–1490. [DOI] [PubMed] [Google Scholar]
- 20. Roche Diagnostics Ltd . Quick! Who needs to be screened for cardiovascular disease? Accutrend Plus: Spot on. On the spot. Mannheim: Roche Diagnostics GmbH, 2010 [brochure]. Available at http://www.cobas.com/content/dam/cobas_com/pdf/product/Accutrend-Plus-system/Accutrend%20Plus%20brochure.pdf Last accessed 10 February 2016.
- 21. Swinburn BA, Ley SJ, Carmichael HE, Plank LD. Body size and composition in Polynesians. Int J Obesity 1999; 23: 1178–1183. [DOI] [PubMed] [Google Scholar]
- 22. Lin S, Tukana I, Linhart C, Morrell S, Taylor R, Vatucawaqa P et al Diabetes and obesity trends in Fiji over 30 years. J Diabetes 2016; 8: 533–543. [DOI] [PubMed] [Google Scholar]
- 23. Lin S, Hufanga S, Linhart C, Morrell S, Taylor R, Magliano DJ et al Diabetes and obesity trends in Tonga over 40 years. Asia Pac J Pub Health 2016; 28: 475–485. [DOI] [PubMed] [Google Scholar]
- 24. Keighley ED, McGarvey ST, Quested C, McCuddin C, Viali S, Maga U. Nutrition and health in modernizing Samoans: temporal trends and adaptive perspectives In: Ohtsuka R, Ulijaszek SJ. eds. Health Change in the Asia‐Pacific Region: Biocultural and Epidemiological Approaches. New York: Cambridge University Press, 2007. [Google Scholar]
- 25. Fishman EI, Stokes A, Preston SH. The dynamics of diabetes among birth cohorts in the U.S. Diabetes Care 2014; 37: 1052–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Higgins JPT. Commentary: Heterogeneity in meta‐analysis should be expected and appropriately quantified. Int J Epidemiol 2008; 37: 1158–1160. [DOI] [PubMed] [Google Scholar]
- 27. Samoa Department of Statistics . Census of Population and Housing 1991. General Report of the Population Census. Apia: Government of Western Samoa, 1991. [Google Scholar]
- 28. International Diabetes Federation (IDF) . IDF Diabetes Atlas, 6th edn Brussels, Belgium: IDF, 2013. [Google Scholar]
- 29. Ng M, Fleming T, Robinson M, Thomson B, Graetz N, Margono C et al Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 2014; 384: 766–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Adjustment for exclusion of known Type 2 diabetes mellitus 1991 and 1995 SACRF surveys.
Appendix S2. Selection biases in survey participants.
Appendix S3. Designation of Type 2 diabetes mellitus.
Appendix S4. Adjustment for obesity from exclusion of known Type 2 diabetes mellitus and/or hypertension in 1991 and 1995 SACRF surveys.


