Abstract
Perinatal hypoxia ischemia (HI) is a significant cause of brain injury in surviving infants. Although hypothermia improves outcomes in some infants, additional therapies are needed since about 40% of infants still have a poor outcome. Acetyl-L-carnitine (ALCAR), an acetylated derivative of L-carnitine, protects against early changes in brain metabolites and mitochondrial function after HI in the postnatal day (PND) 7 rat pup model of near-term HI injury. However, its efficacy in long-term structural and functional outcomes remains unexplored. We determined the efficacy of ALCAR therapy administered to rat pups after HI at PND 7, using both longitudinal in vivo magnetic resonance imaging (MRI) and behavioral tests in male and female rats. HI lead to sex-specific behavioral impairment, with males exhibiting more global functional deficits compared to females. Interestingly, HI reduced volume of the contralateral hemisphere in males only, suggesting that the brain injury is more diffuse in males than females. Treatment with ALCAR improved both morphological and functional outcomes in both male and female rats. These results suggest ALCAR may be a potential therapy for clinical use since treatment attenuated the moderate injury produced under the experimental conditions used and improved functional outcome in preclinical studies.
Keywords: hypoxia ischemia, sex differences, rat, neonatal, acetyl-l-carnitine, magnetic resonance imaging, behavior
Introduction
Perinatal hypoxia-ischemia (HI) which occurs in 1–4/1000 live births is a primary cause of brain injury and long-term neurologic disability [1–3]. Although hypothermia, the current standard clinical intervention, is effective in many cases, 40–50% of infants receiving hypothermia have adverse outcomes [1, 2, 4]. Thus, there is a crucial need for additional therapies to attenuate damage to the developing brain and improve outcome in survivors. HI causes rapid energy failure due to lack of oxygen and glucose delivery to the brain, followed by a transient normalization during reperfusion, and a later secondary energy failure, within 6 hours[5–7]. The early brain injury consisting of metabolic acidosis, excitotoxicity, oxidative stress and inflammation develops over weeks, leading to protracted loss of brain volume [7, 8]. Additional interventions besides hypothermia that stabilize brain metabolism after HI might further reduce injury-induced damage.
The endogenous metabolite and dietary supplement acetyl-L-carnitine (ALCAR) is an acetylated derivative of L-carnitine with known neuroprotective effects [9–17]. Preclinical studies have revealed several modes of action through which ALCAR can be neuroprotective [11, 18–21]. In brain, ALCAR can stabilize membrane composition [22], serve as an energy substrate [21, 23], protect against oxidative stress [10, 18, 19], enhance nerve growth factor activity [24], and promote mitochondrial biogenesis [25]. ALCAR can also serve as a precursor of acetylcholine and protect choline acetyltransferase after injury [26]. In adult rats, multiple protective actions of ALCAR were detected after spinal cord injury, including reduction of injury volume, increased mitochondrial mass and protection of cholinergic neurons [16].
ALCAR rapidly enters the brain and is metabolized in mitochondria to acetyl-CoA and carnitine [22, 23]. The carnitine moiety facilitates transport of fatty acids across mitochondrial membranes for metabolism [22]. Carnitine and its esters prevent accumulation of free fatty acids within the cell and thereby provide acetyl-CoA for energy production in mitochondria [22, 23]. The acetyl moiety of ALCAR is used for energy and is metabolized primarily in astrocytes and GABAergic neurons in developing brain [23]. ALCAR can also serve as an antioxidant, stabilize membranes and enhance cholinergic transmission [22]. ALCAR is therefore a potentially effective treatment for HI induced brain injuries in infants. A recent in vivo magnetic resonance spectroscopy (MRS) study by our group reported that pups treated with ALCAR after HI had lower levels of lactate and maintained creatine levels in hippocampus at 24 hours after injury [10]. Other studies from our group demonstrate improved mitochondrial function and reduced indices of oxidative stress in HI pups treated with ALCAR [27].
Clinical studies reported a higher incidence of HI, increased severity and more long-term cognitive deficits in male infants compared to females [28]. The mechanisms of perinatal brain injury diverge between the sexes. For example, cell death is more likely to occur via caspase-dependent pathways in females, while caspase-independent pathways dominate in the male brain [29]. Manipulations that prevent or reduce HI-induced inhibition of PARP-mediated cell death are protective in male mice subjected to neonatal HI, but has no effect in female mice [30]. Similarly, mechanisms of repair differ between the sexes, also contributing to potential treatment efficacy [31]. Thus, preclinical screening of potential therapies following HI must determine efficacy in both males and females, as treatments that benefit one sex may not protect the other sex [31–34].
In the present study, we evaluated brain development and behavioral outcomes in male and female rat pups after neonatal HI using parameters that typically produce a moderate injury. Our results suggest that ALCAR can effectively reduce the magnitude of injury in the long-term and improve functional outcomes in both male and female rat pups.
Materials and methods
Hypoxia Ischemia
Timed-pregnant female Sprague-Dawley rats were obtained from Charles River (Frederick, MD). On postnatal day 7 (day of birth is postnatal day 1 (PND 1)), pups were subjected to a modified version of the Rice-Vannucci method of HI, or sham surgery (e.g., Rice et al., 1981). Pups weighing between 12.5–15.5 g were anesthetized with isoflurane (3% induction, 1.5% maintenance). The right common carotid artery was ligated twice, and severed between the ligations. Pups were placed in an open jar in a water bath maintained at 37°C for 25 min to recover from surgery, and were then returned to the dam for 1 hour before hypoxia. Transient hypoxia was produced in the pre-warmed 450 ml glass jars (2 pups per jar) in the 37°C water bath. Jars were sealed, flushed with warmed gas mix of 8% oxygen and 92% nitrogen at 1 liter/minute and placed in a 37°C water bath for 75 minutes. After hypoxia the pups were allowed to recover at 37°C in room air for 2 hours prior to return to the dam. Sham treated controls were anesthetized for the same average length of time required for artery ligation (4–4.5 min). Sham operated pups received an incision, without artery manipulation or hypoxia. ALCAR (100 mg/kg in 8% sodium bicarbonate to control pH) or saline was injected subcutaneously at 0, 4, 24 and 48 hours after HI.
Magnetic Resonance Imaging Procedure
The day following HI, pups were transported with the dam and littermates to the MRI facility. No more than one pup from each litter was scanned in each condition. Each condition was balanced by sex. Each group consisted of 6 males and 6 females. Two pups died during imaging, and data from these animals were not used in the analysis presented here. In vivo MRI was performed on a BrukerBioSpec 70/30USR Avance III 7T horizontal boreMR scanner (Bruker BioSpin Corporation, Billerica, MA) at 24 and 72 hours, 7 and 28 days after injury. A Bruker four-element 1H surface coil array was the receiver and a Bruker 72 mm linear-volume coil was the transmitter. Rats were anesthetized with 2.5–3% isoflurane, and maintained at 1 – 1.5 % during scanning. An MR compatible small-animal monitoring and gating system was used to monitor respiration and body temperature. Body temperature was maintained at 36–37°C using a water circulating pad beneath the pup.
A two-dimensional rapid acquisition with relaxation enhancement (RARE) sequence in coronal view (TR/TE = 5500/56.82 ms, FOV = 25mm × 25mm, slice thickness = 0.5 mm, in-plane resolution = 0.1 mm × 0.1 mm, slice number = 20) was used for T2-weighted imaging acquisition. Diffusion weighted imaging (DWI) was acquired on all three orthogonal axes with b values equal to 350 s/mm2, 700 s/mm2, 1050 s/mm2 and along with a non-DWI (b0 = 0 s/mm2) (TR/TE = 4500/26.91 ms, slice thickness = 1 mm, in-plane resolution = 0.208 mm × 0.208 mm, slice number = 10).
Behavioral tests
Behavioral tests were conducted in a separate group of rats than those used for longitudinal MR imaging. Both males and females were tested. No more than one pup of each sex per litter was assigned to the same condition. The number of rats in each group was: Males: Sham+Saline n = 9, Sham+ALCAR n = 8, HI+Saline n = 7, HI+ALCAR n = 10; Females: Sham+Saline n = 9, Sham+ ALCAR n = 7, HI+Saline n = 8, HI+ALCAR n = 8.
Reflex Tests: Righting and Negative Geotaxis
On PND 10, pups were tested on a simple righting reflex test, or the ability to turn from their back upright to their feet. Pups were placed on their backs on a heating pad (36° C). Each pup was gently restrained on its back, and quickly released. The amount of time taken to turn upright was recorded. This was repeated 3 times, with a 3 min interval between each trial. The average righting time for each pup was calculated for statistical analysis. Seven days after HI, pups were placed on a ramp with a 25 degree incline, head pointing downwards. The incline was covered by Versi-dry™ (Thermo Fisher Scientific, Waltham, MA) to enable traction. Though this is interpreted as a motivation to restore postural stability, interpretation of this behavioral tendency has been questioned [35]. We consistently observe that sham operated control pups quickly reorient their position to point their heads up the incline, and use this task because it is sensitive to HI, and detect injury induced deficits in very young male and female rats [31]. The time to turn to face up the slope was measured 3 times, with a 3 min interval between trials and the average was determined for each pup.
Suspension on a dowel
On PND 18, the rats’ front paws were placed on a metal dowel suspended above a padded bin. The trial period was a maximum of 90 seconds. Rats were considered successful if they were able to bring their rear paws to the dowel and hold on [36]. If the rat fell, it was considered to have failed. The percentage of rats in each group to successfully grasp the dowel with their hind limbs was calculated. This test has been used previously to detect motor deficits after neonatal HI [37]. We find that this behavior is transiently disrupted by HI in the developing rat as reported by others [31, 38].
Novel Object Recognition
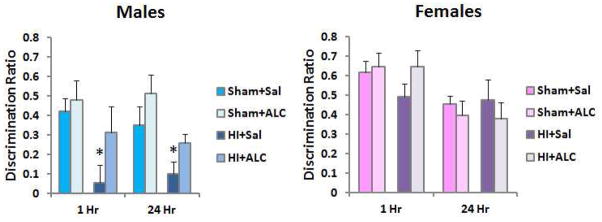
On PND 32–33, rats were placed in a plexiglass arena (49 cm length, 37 cm wide, 24 cm high) with an open top under dim room lighting for 10 min to permit acclimation to the testing chamber. On PND 34, two identical objects were placed in the arena (i.e., ink bottles, flask stoppers). The rat was allowed to explore freely for 5 min and video recorded. Following a 1 hour retention interval, one object was replaced with a novel one in the same location as the object it replaced. All objects were secured to the floor of the chamber with velcro. The rat was again permitted to explore freely for 5 min. The now familiar object was tested with a novel object in the same location 24 hours later. Rats are capable of recognition of the familiar object for at least 24 hours at this age [39]. All test sessions were video recorded and scored offline. The amount of time the rat spent investigating each object was recorded. To be considered object exploration, the rat had to be touching and/or sniffing the object, with its snout within 2 cm of the object, and not looking elsewhere. Data are presented as (Time spent exploring novel object-Time spent exploring familiar object)/Total time spent exploring both objects. Therefore, a ratio of 0 means the rats spent the same amount of time with both the familiar and novel objects and did not express memory of the familiar object. A ratio of 0.5 indicates the rat spent twice as much time with the novel object.
Social Play
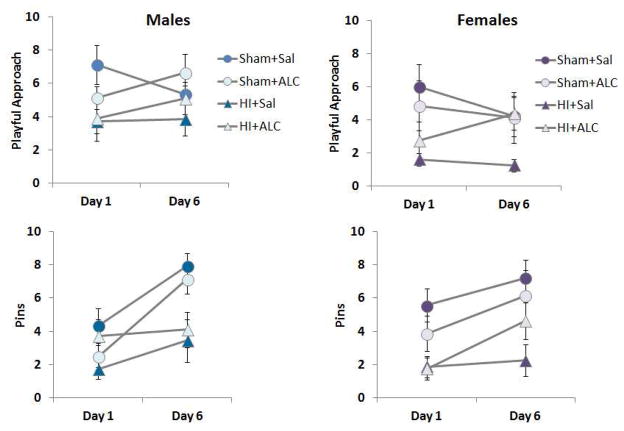
On PND 28–35, rats were separated from their cage mates 1–1.5 hours before onset of the dark cycle. During this time, each rat received a distinct marking/number on the back. At the end of this period of social isolation, same sex groups, consisting of 4–5 rats with at least 1 rat from each experimental condition, were placed in the behavior arena used for novel object recognition testing with the addition of cob bedding. All social play testing was conducted under red lights in an otherwise dark room. After a 30 seconds delay, video recording began. Rats remained in the play arena for 10 min per session. Play behavior was video recorded was scored offline. The number of playful approaches and successful pinnings of another rat were counted for the 5 min of sessions 1 and 6, omitting the first 30 sec of video to permit the rats to acclimate to the arena. These sessions were selected to determine whether play behavior was persistently reduced in HI rats, and the potential of ALCAR to restore play over time. Play behavior was robust at this time, and persisted for at least 5–8 min [40].
Imaging data analysis
Imaging data analysis was conducted by an experimenter unaware of either surgery or post HI treatment condition. Volumetric image processing was conducted using Medical Image Processing, Analysis and Visualization (MIPAV). Brain volumes and lesion sizes were obtained from T2-weighted images. Prior to any image analysis and quantification of lesion magnitude, surface coil induced signal inhomogeneity was corrected using the N3 shading correction within MIPAV on each T2-weighted image. Early appearance of edema and later cyst formation in the neocortex, hippocampus and tissues around ventricle sometimes made delineation of the ventricle from brain parenchyma difficult. We therefore included the ventricle in brain volume estimation for all groups.
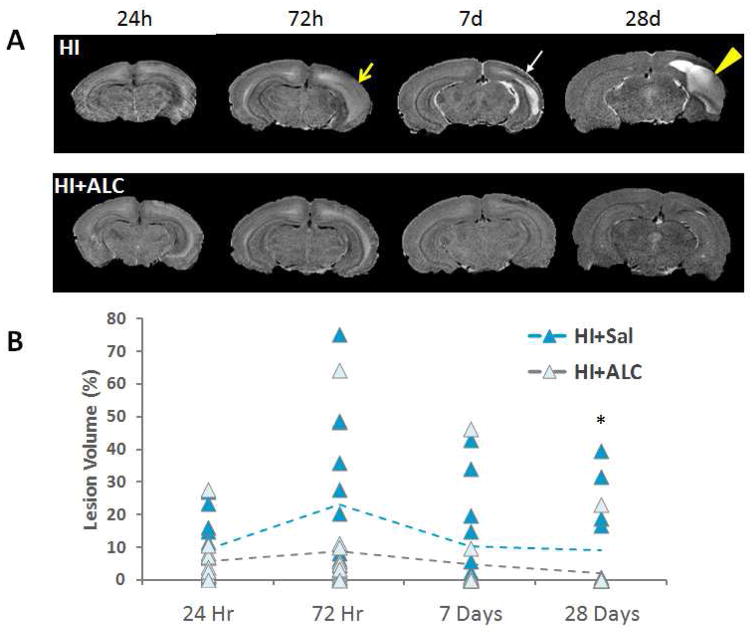
Lesion Volume of Interest (VOI) in the ipsilateral hemisphere was drawn using the contralateral hemisphere as a reference. No pups subjected to HI exhibited obvious injury in the contralateral hemisphere in the T2-weighted images. Our imaging time points captured the development of the injury over time. Injured areas appeared brighter at early post-HI intervals, and these areas became cysts at later time points. Hypointense signals in the ipsilateral hemisphere were observed in both HI and HI+ALCAR rats suggestive of scar tissue (Fig. 1). Lesion size determination was based on the complete lesion and incorporated both these hypo- and hyperintense regions in the brain. For each rat, lesion size was calculated as the percentage of lesion volume over the right (ipsilateral) hemisphere volume.
Figure 1.
A) Representative skull-stripped T2-weighted images showing the lesion over time. T2-weighted image for typically developing brain injury and a rat pup 24 hours, 72 hours, 7 days and 28 days after hypoxia ischemia (top panel) and a rat pup treated with ALCAR after hypoxic ischemia (bottom panel). Yellow arrow: edema; white arrow: scar tissue; yellow arrow head: cyst. B) Scatter plot of lesion volume compared to the ipsilateral hemisphere volume. Treatment with ALCAR reduced the size of the lesion in most animals at the final timepoint (28 days post-HI). * denotes significant Kruskall Wallace, p < 0.038.
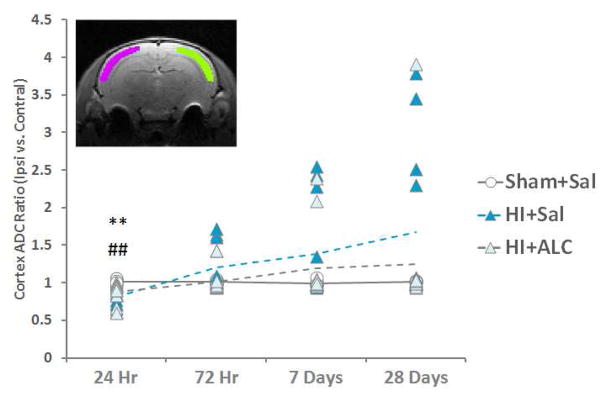
Diffusion weighted images were processed in FSLView (http://fsl.fmrib.ox.ac.uk/fsl/fslview/). Lesions were more consistent in neocortex than other regions in the brain across all four of the time points. For this reason, we examined diffusivity in the neocortex. The location of neocortex exhibiting the most consistent injury was chosen for all animals. ROIs in ipsilateral and contralateral neocortex were drawn on the same slice of each animal to calculate the average diffusion coefficient (ADC). ADC ratio = (ipsilateral ADC)/(contralateral ADC) of those parameters were used for statistical analysis.
Statistical analysis was performed using SPSS (Ver.23.0, SPSS Inc., Chicago, IL, USA). Normality of each measurement was tested with the Shapiro-Wilk test. Measurements with normal distribution were analyzed with repeated-measures two-way ANOVA to test the group × time after injury interaction with the between-subject factors of group and sex and time after injury as the within-subject variable. Multivariate ANOVA was used to determine group differences at specific times after injury. Post hoc analysis was performed with Tukey’s test. Males and females were further analyzed separately with repeated measures ANOVA and one-way ANOVA. Data that failed normality testing were analyzed with Kruskal-Wallis test at each specific time after injury with group as the factor. Post hoc analysis was performed with Dunn-Bonferroni test. Loess fitting was calculated with R package (Version 3.3.2, https://www.r-project.org/) for demonstration purpose.
Behavioral data analysis
Data from male and female rat pups were analyzed separately, yielding sufficient power to detect differences between experimental conditions within each sex. The experimenter was unaware of the experimental condition at the time of data collection. For both novel object recognition and social play analysis, a subset of videos from each task was scored by a second experimenter, also unaware of experimental condition, to generate inter-rater reliability scores, as scoring of these behaviors can be subjective. For both play and object recognition, inter-rater reliability correlation values were r = 0.84, p < 0.001 and r = 0.91, p < 0.001 respectively. Reflex tests were analyzed using 2 × 2 × 2 mixed factor ANOVA, with sex, treatment (ALCAR or saline) and HI (HI or sham) as between subject factors. Group comparisons were then performed using Bonferoni’s post hoc tests when ANOVA revealed significant main effects. Wire hanging was measured as either success or failure, and therefore was analyzed using Pearson’s chi square. Novel object recognition and social play were analyzed using repeated measures ANOVA with session as the within group variable.
Results
Lesion volume detected by T2-weighted imaging
T2-weighted brain images from representative HI and HI+ALCAR rat pups are shown in Figure 1A. At 24 hours, 72 hours, 7 days and 28 days after the HI injury at PND 7, rat pups treated with ALCAR after HI exhibited relatively smaller lesion areas than HI pups without ALCAR treatment. The distribution of lesion size (percentage of lesion volume compared to the ipsilateral hemisphere volume) failed the normality test. Therefore, analysis was performed with Kruskal-Wallis test in each specific time after injury. Treatment with ALCAR reduced the lesion size at 28 days after the HI injury (H = 4.313, p = 0.038) (Fig. 1B).
Effects of HI and ALCAR treatment on brain volume
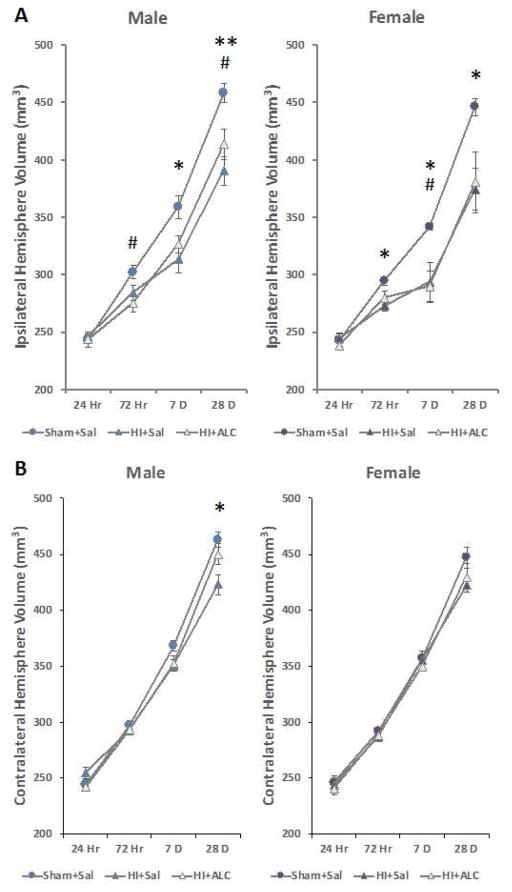
Volumes of ipsilateral and contralateral hemispheres were normally distributed and therefore parametric statistical methods were used. Both the HI and HI+ALCAR pups had smaller ipsilateral hemispheres compared to sham group. A repeated measures two-way ANOVA on ipsilateral hemisphere volume showed a significant overall effect of group (sham, HI and HI+ALCAR), F(2,30) = 12.720, p < 0.001, and group × time post-HI interaction, F(6,58) = 3.226, p = 0.008. Significant reduction of volumes in both the HI and HI+ALCAR groups were observed at 72 hours (F(2,30) = 8.115, p = 0.002), 7 days (F(2,30) = 10.225, p < 0.001) and 28 days (F(2,30) = 11.075, p < 0.001) after injury.
Males and females were then analyzed separately to determine the presence or absence of sex differences in the trajectory of the developing injury and tissue loss. Repeated measures ANOVA confirmed significant effects of group in both males and females, F(2,15) = 5.782, p < 0.014 and F(2,15) = 5.426, p < 0.03. Post hoc analysis further confirmed a significant difference of both HI (p = 0.004) and HI+ALCAR (p = 0.016) groups compared to sham group in males and significant difference of HI (p = 0.029) and HI+ALCAR (p = 0.033) group compared to shams in females. In males, volume of the ipsilateral hemisphere of the HI group was significantly smaller than the sham group at 7 days (p = 0.011) and 28 days (p = 0.003) after injury. The volume of the ipsilateral hemisphere in the HI+ALCAR male group was significantly smaller at 72 hours (p = 0.026) and 28 days (p = 0.043) after HI compared to sham group (Fig. 2A). In females, the HI group exhibited a significantly reduced ipsilateral hemisphere at 72 hours (p = 0.021), 7 days (p = 0.047) and 28 days (p = 0.04) after injury while the HI+ALCAR group only exhibited a significant reduction at 7 days (p = 0.032) but this difference almost reached significance at 28 days (p<0.061) (Fig. 2B). Analysis of between group differences in the volume of the contralateral hemisphere by repeated measures two-way ANOVA revealed a significant difference, F(2,29) = 3.805, p = 0.034. Post hoc tests revealed that only the HI group was significantly reduced compared to sham operated controls (p = 0.037). There was a delayed loss of volume of the contralateral hemisphere detected 28 days after injury only in the HI group compared to sham group (p = 0.002). Further analysis of males and females separately revealed that this delayed volume reduction was evident only in males (p = 0.011; Fig. 2B).
Figure 2.
Ipsilateral and contralateral hemisphere volumes of male and female rats subjected to hypoxia ischemia on PND 7. Values are means ± SEM. A) Ipsilateral hemisphere volumes were smaller than sham operated controls in male and female rats. B) Contralateral hemisphere volume was smaller in HI males only, and this was reversed by administration of ALCAR after HI.
*=p<0.05, **=p<0.01 HI vs. Sham; #=p<0.05 HI+ALCAR vs. Sham.
Effects of HI and ALCAR treatment on cortex diffusional properties
The distribution of apparent diffusion coefficient (ADC) ratio of brain tissue failed the normality test. Therefore, analysis was performed with Kruskal-Wallis test at each specific time after injury. A significant group effect was detected at 24 hours (H = 12.322, p = 0.002). Post-hoc analysis showed lower ADC ratio in both HI (adjusted p = 0.003) and HI+ALCAR (adjusted p=0.008) group compared to the sham group (Fig. 3) suggesting a general reduction of water mobility ipsilaterally at 24 hours. Treatment of pups with ALCAR after HI limited further alterations of diffusional properties, to some extent, in the cortex after HI. Pups without ALCAR treatment showed an increase of ADC at later time points after HI while, although not significant, ALCAR demonstrated a trend of stabilization of the ADC level.
Figure 3.
The cortex ADC ratio (ipsilateral cortex ADC over contralateral cortex ADC) was tested with Kruskal-Wallis test within each time point. Post-hoc analysis was performed with Dunn-Bonferroni test. Decreased diffusivity was observed in HI animals. ALCAR did not prevent this alteration. **=p<0.01 HI vs. Sham; ##=p<0.01 HI+ALCAR vs. Sham. Inset: illustrates the ROIs for ADC measurement (green: ipsilateral cortex; pink: contralateral cortex)
Simple Reflex Tests
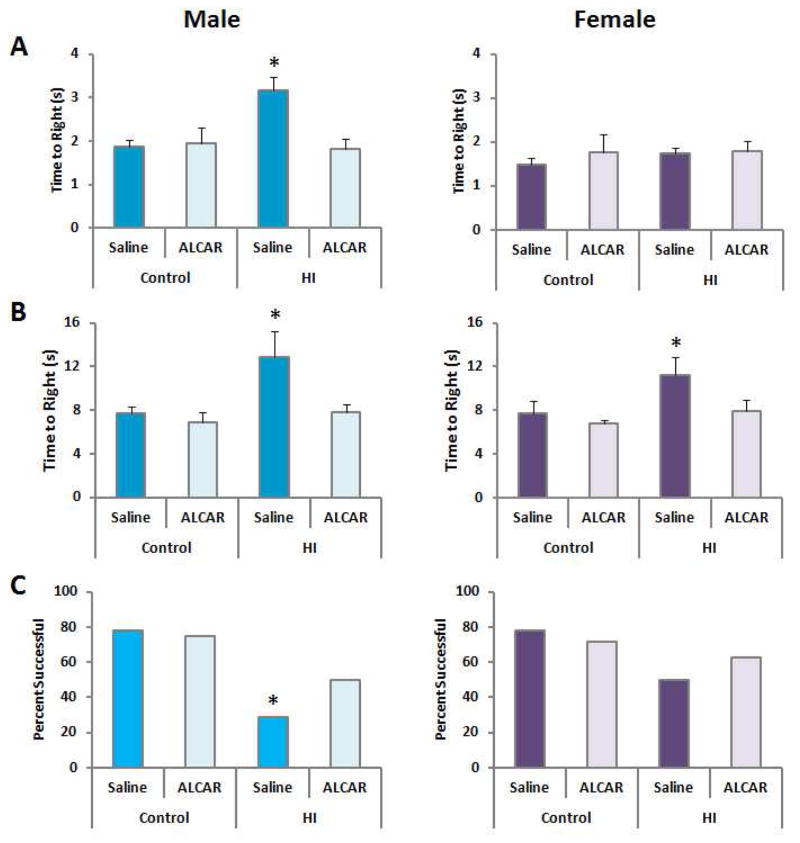
There was a sexually dimorphic response to HI detected in the righting reflex test 72 hours after injury. The effect of HI was significant in males, F(1,30) = 5.05, p < 0.032, as was the effect of ALCAR treatment, F(1,30) = 5.8, p < 0.022. The HI x treatment interaction also reached significance, F(1,30) = 7.15, p < 0.012. Male pups treated with ALCAR after HI required less time to return their paws to the floor compared to HI males, and performed comparable to controls. In contrast to males, the righting reflex was not impaired in female pups after HI. No significant effects of HI, treatment or an interaction were detected in females, largest F(1,30) < 1 (Fig. 4A).
Figure 4.
A) Righting reflexes (72 hours post-HI): Values represent means ± SEM. Only males exhibited a deficit which was reversed by ALCAR. B) Negative geotaxis (7 days post-HI): Both males and females exhibited an impairment which was reversed by ALCAR. C) Suspension on a dowel (11 days post-HI) Males were significantly less likely to grasp the dowel with their hind paws after HI. Females were not significantly affected.
In the negative geotaxis test, HI increased latency to turn and point the head upward on the slope in males, F(1,30) = 6.75, p < 0.014. The effect of ALCAR treatment was significant, F(1,30) = 6.2, p < 0.018. The surgery x treatment interaction did not reach significance, F(1,30) = 3.12, p < 0.08. Bonferroni post hoc comparisons confirmed that only HI pups treated with saline significantly differed from sham operated controls, p = 0.026 or ALCAR treated controls, p = 0.016; whereas HI pups treated with ALCAR did not differ from controls. HI also increased latency to turn around in females, F(1,28) = 4.29, p < 0.04. The effect of ALCAR did not reach significance, p = 0.08. The surgery x treatment interaction also failed to reach significance. Group differences were not as robust in females. Though ALCAR reduced latencies in female pups subjected to HI, this pattern did not reach statistical significance (Fig. 4B).
Suspension on a Dowel
Only males subjected to HI treated with saline were more likely to fall from the wire dowel or fail to grasp the dowel with their hindpaws, χ2 = 4.88, p < 0.05. This analysis did not detect a significant effect in females. Thus, ALCAR increased the number of males capable of grasping the metal dowel with their hindpaws after HI (Fig. 4C). This result, combined with the reflex test results presented above provides evidence that males are more impaired on simple motor tasks at short time points after HI, while females are largely unaffected by HI in performing simple motor tasks.
Social Play
Repeated measures ANOVA with day as the within group variable and surgery (Sham or HI) and treatment (Saline or ALCAR) as between subject variable was conducted for the number of playful approaches each rat made during two 5 minutes play sessions. Analysis of males did not detect significant changes across the 2 days analyzed, largest F = 2.87. The main effect of HI was significant, F(1,30) = 6.722, p < 0.015. ALCAR did not significantly improve this measure of social play in males, F < 1. The pattern of results was the same for females. HI significantly reduced playful approaches, F(1,28) = 6.79, p < 0.014. ALCAR did not significantly increase this aspect of social play (Fig. 5).
Figure 5.
Social play testing was conducted between PND 28–35 and analyzed with repeated measures ANOVA. Values are means ± SEM. HI reduced playful approach and pins in both males and females. ALCAR did not significantly improve the behaviors in either males or females.
The number of times each rat successfully pinned another rat was also tallied and analyzed for day 1 and day 6 of social play. In males, this type of interaction increased as play experience increased F(1,30) = 17.38, p < 0.001. The surgery x day interaction was also significant, F(1,30) = 6.034, p < 0.02. Sham animals increased the number of successful pins as social play continued across days. The main effect of HI was significant. Rats subjected to HI performed fewer successful pins than sham operated controls, F(1,30) = 9.03, p < 0.005. The main effect of ALCAR did not reach significance, F < 1. The number of successful pins increased from day 1 to day 6 in females as well, F(1,28) = 6.57, p < 0.016. No interactions were significant, largest F < 1.23. The main effect of HI was also significant. Like males, HI females had fewer successful pins than controls, F(1,28) = 17.1, p < 0.0001 (Fig. 5). The main effects of treatment or treatment x surgery interaction were not significant, largest F = 2.85. HI appears to disrupt social play similarly in males and females. ALCAR did not significantly restore these measures of social play.
Novel Object Recognition
The effect of HI on short and long-term object recognition memory was determined using the novel object recognition test. Recognition ratios for each group at both the 1 hour and 24 hours retention interval were analyzed using repeated measures two-way ANOVA with surgery (Sham or HI) and treatment (Saline or ALCAR) as between subject factors. In males, this analysis confirmed that HI significantly impaired memory, F(1,30) = 8.95, p < .0001. ALCAR attenuated the retention deficit, indicated by a significant treatment effect, F(1, 30) = 5.57, p < .025. The surgery (HI) x treatment interaction was not significant, F < 1. No significant effects of HI or treatment were detected in females, largest F(1,28) < 1, Fig. 6. The lack of impairment did not permit the detection of a beneficial effect of ALCAR. There were no group differences in the amount of time spent investigating the objects during the initial exposure phases for males or females, all Fs < 1. Similarly, the total time spent exploring objects did not differ between groups in either sex, largest F = 1.84. Therefore, poor retention performance does not appear to be the result of reduced object exploration in males subjected to HI.
Figure 6.
Novel object recognition testing was conducted between PND 32–35. Results were analyzed with repeated measures ANOVA. Values are means ± SEM. Males exhibited poor retention at a short (1 hour) and long (24 hours) retention delay and this was significantly improved by ALCAR. Females were not significantly impaired.
Discussion
Pre-clinical models of HI suggest a protracted development of injury that worsens over time [7, 41, 42]. Between 2 and 8 weeks after HI, volume of the ipsilateral hemisphere continues to decrease [8]. However, relatively few studies have determined whether therapies that are effective in the first 72 hours after HI are protective in the long-term [10]. The present study demonstrates that ALCAR administration during the first 48 hours after HI on postnatal day 7 led to significant long-term protection of the ipsilateral hemisphere in both sexes and improved behavioral outcomes in males.
The loss of blood flow in hypoxic ischemic injury results in an immediate energy failure that is transiently recovered as blood flow returns [43]. However, a secondary energy failure occurs, several hours after HI [5–7]. Metabolic and cerebrovascular fluctuations continue during reperfusion, contributing to the developing injury [44]. Impaired brain metabolism after H/I leads to decreased levels of creatine and choline coupled with increases in lactate, which can accumulate when mitochondrial oxidative metabolism is impaired [45, 46]. Decreased N-acetylaspartate (NAA) after H/I is evidence of impaired function of neuronal mitochondria [45–47]. NAA formed in neurons is an important substrate for the synthesis of myelin lipids in oligodendroglia [48–50]. ALCAR might improve outcomes after HI through stabilization of post-injury brain metabolism [6, 11, 23, 45, 51, 52]. In vivo 1H-magnetic resonance spectroscopy (1H-MRS) in the same animals presented in the MRI experiment presented here revealed decreased levels of glutathione, creatine, and the osmolytes myo-inositol and taurine in the ipsilateral hippocampus of HI rat pups [10]. ALCAR treatment after HI maintained creatine concentration in the hippocampus and improved lactate levels compared to HI pups treated with saline [10].
ALCAR is rapidly taken up by the brain and converted to acetyl-CoA and free carnitine by the mitochondrial enzyme carnitine acetyl transferase [22]. The carnitine moiety helps transfer free fatty acids into the mitochondria for oxidative degradation, and the acetyl CoA from ALCAR can be oxidized for energy via the TCA cycle [22, 23]. An earlier study by our group demonstrated that the acetyl moiety of [2-13C]acetyl-L-carnitine is metabolized for energy and incorporated into neurotransmitters in developing brain [23]. 13C-NMR spectroscopy revealed high enrichment of 13C-ALCAR in glutamine and GABA [23]. 13C-ALCAR was also metabolized via the pyruvate recycling pathway, a pathway considered neuroprotective as it provides pyruvate for oxidative metabolism when the supply of pyruvate from glucose metabolism is limited [23, 50].
Hypothermia, the current clinical standard of care, leads to increases in ATP, phosphocreatine and NAA levels after HI compared to normothermia[53, 54]. Improved ATP, phosphocreatine and NAA levels suggest that hypothermia protects mitochondrial metabolism and prevents secondary energy failure in brain [53]. Hypothermia may also attenuate the magnitude of injury by slowing overall brain metabolism. Takenouchi et al. [53] proposed that modulation of the cellular acetylation status through suppression of acetyl-CoA led to protection after hypothermia. Decreased acetylcholine levels in both the ipsilateral and contralateral hemispheres were detected in hypothermic pups [53]. The concept that decreasing acetyl CoA would be protective is at odds with the neuroprotective effects of ALCAR, since ALCAR, would expand the acetyl CoA pool and support energy metabolism, GABAergic neurotransmission and synthesis of acetylcholine in brain [23]. It is possible that ALCAR can stabilize metabolism when energy demand is not suppressed by hypothermia in the paradigm used in the current study that produces a mild to moderate injury. Future studies should explore the effects of hypothermia in combination with ALCAR administration given that the behavioral results presented here do not suggest that ALCAR is harmful in either males or females, even in instances when it did not improve behavior, such as social play.
Several recent reports showing differences in both the magnitude and mechanism of injury in male and female brain after HI illustrate the need for determining the response to therapy in both sexes after HI[27, 55–57]. Using 13C-NMR Morken et al. [55] found that male pups had lower astrocytic mitochondrial metabolism than females 30 minutes after H/I, but metabolism in females was reduced longer in both neurons and astrocytes. They proposed that astrocytic malfunction may predict severity of injury after HI and contribute to sex differences in outcome [55]. Weis et al. [56] found significantly lower activity of electron transport chain complexes I–III in brains of male rat pups compared to female pups 18 hours after HI on PND 7. However, it should be noted that activity of the electron transport chain was decreased in the ipsilateral cortex and hippocampus of both males and females[56]. Male rat pups exhibited a more extensive impairment of mitochondrial respiration than females at 20 hours after HI [27]. Interestingly, this impairment occurred in both the contralateral and ipsilateral hemisphere of the brain in males [27]. ALCAR administration restored mitochondrial respiration in the contralateral hemisphere of male brain [27]. Female brain had higher basal glutathione levels than male brain [27]. HI increased total glutathione peroxidase activity in the ipsilateral hemisphere of the female brain [27]. Taken together these findings suggest that females have an advantage in reducing oxidative stress after injury compared to males. ALCAR administration promoted mitochondrial glutathione peroxidase activity in male brain, and decreased the massive increase in protein carbonyl formation seen only in the male brain after injury [27]. The efficacy of ALCAR treatment may be due, in part, to anti-inflammatory effects of this compound [15]. Mirza et al. [42] reported increased inflammation in brain of male pups after HI at PND 10 compared to female brain. Such early sex differences in metabolism and inflammation likely contribute to the observed sex differences in histological and functional outcomes after HI. Converging evidence suggests that males are more vulnerable to injury in the cerebral hemisphere contralateral to the carotid artery ligation than females. Earlier studies by our group showed that the volume of the contralateral hippocampus was reduced after HI in males only, measured in young adult rats subjected to HI on PND 10 [31]. Similarly, we observed reduced volume of the contralateral hemisphere here in males on 28 days post HI. Whether this is because males are more vulnerable to hypoxia alone, or undergoing more extensive diaschesis than females is unknown.
In the present study ALCAR administration improved righting reflex latencies and performance on the dowel suspension test in male pups, though no impairment was detected in female pups on these tests. Both males and females subjected to HI exhibited increased righting latencies on the negative geotaxis test, and ALCAR restored this measure to sham level in both sexes. Females did not exhibit a significant impairment on the novel object recognition test at either the 1 or 24hours retention interval. In contrast, male pups showed significant impairment in both short and long-term memory retention at 28–29 days of age. This finding together with a recent study by Waddell et al. [31] in the PND 10 rat pup model of term HI, suggest increased vulnerability of male brain to impairment in learning and memory using parameters that produce a mild to moderate injury. Specifically, the novel object recognition test relies on the perirhinal cortex [58]. It is possible that males perform poorly because this cortical region is injured bilaterally in males, but not in females. In the present study, the male HI pups treated with ALCAR performed significantly better on the novel object recognition test at both 1 hour and 24 hours than HI pups treated with saline. This result is consistent with earlier studies by Scafidi et al. [11] demonstrating that treatment with ALCAR after injury improved motor function and novel object recognition in male rat pups after TBI at PND 21–22. It should be noted that other studies have observed deficits in the novel object recognition task in both sexes when longer hypoxia intervals are used to produce a more severe injury (e.g., [59]). Our moderate injury procedure might spare female performance on this task.
ALCAR did not significantly improve performance in tests of social play in either sex. Both male and female HI rat pups showed impairment in social play compared to sham-operated pups. Normal brain development, evidenced by cognitive ability and affective processing, relies on social play and interaction [60]. The medial prefrontal cortex and striatum are critically involved in the motivational and social aspects of play [61]. Pups that underwent HI made fewer approaches to other pups, and had fewer pins than sham pups. It is possible that more protracted ALCAR administration might further improve performance on this measure. Importantly, social play is disrupted in both males and females subjected to HI, making it a simple and effective way of detecting deficits in males and females [31]. Our results confirm more global deficits in male rats after HI. This pattern has been reported by other groups [28, 31, 34, 62]. An elegant and thourough study has determined that males subjected to HI suffer rapid auditory processing deficits, while females remain unaffected [34]. This sex difference is mediated at least in part by gonadal steroids in the neonatal period [63]. Administration of testosterone to females at birth lead to male-like deficits after HI [63]. These findings along with those presented here suggest that rat models of HI capture sex differences in sensory processing and learning ability after perinatal brain injury often observed in humans.
Our study used parameters that typically produce a mild to moderate injury, based on extensive pilot work in our laboratory. Use of these parameters permits us to detect potential therapeutic efficacy of ALCAR, since an attenuation of injury is more readily detected when the injury is not severe. Studies using longer hypoxia intervals (e.g., 150–160 min) often observe consistent severe injuries, in which the resulting lesion accounts for 50% or more of the ipsilateral hemisphere [64–66]. Interventions such as hypothermia and xenon do not appear protective when the magnitude of injury is this high [64–66]. Therefore, we cannot conclude that ALCAR would benefit infants suffering severe hypoxia ischemic injury. Our results hold promise, however, and future experiments will determine the potential additive benefit of ALCAR and hypothermia. Overall the results of the present study demonstrate that male rat pups exhibited a loss in hemisphere volume in the contralateral hemisphere that was not detected in females. Males also showed more global impairment in behavioral outcomes than female pups. Treatment with ALCAR after HI was particularly effective at normalizing the motor reflexes tested at 3 and 7 days after HI, but did not restore levels of social play that was impaired in male and female pups after HI. ALCAR improved both short term and long-term memory in male pups after HI, and this is particularly promising, indicating that additional preclinical studies are warranted.
Acknowledgments
This work was supported by NIH NICHD P01 HD016596 to MCM
Footnotes
Declaration of Competing Interests: The authors have no competing interests.
Role of each author
Shiyu Tang acquired and analyzed MR data, interpreted MR data, wrote sections of the manuscript and assisted with final editorial changes. Dr. Su Xu assisted with experimental design, MR data acquisition and data analysis. Dr. Xu also wrote sections of the manuscript and assisted with final editorial changes. Dr. Xin Lu also assisted MR data acquisition and analysis. Dr. Rao Gullipalli and Dr. Mary McKenna designed the MRI experiment and contributed to the writing of the manuscript and interpretation of the data. Dr. Jaylyn Waddell conducted the hypoxia ischemia procedure, ALCAR treatment, designed the behavioral experiments and acquired and analyzed behavioral data. Dr. Waddell also contributed significantly to the interpretation of the data, writing and editing of the manuscript.
References
- 1.Shankaran S. Neonatal encephalopathy: treatment with hypothermia. J Neurotrauma. 2009;26(3):437–43. doi: 10.1089/neu.2008.0678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Azzopardi D. Predictive value of the amplitude integrated EEG in infants with hypoxic ischaemic encephalopathy: data from a randomised trial of therapeutic hypothermia. Arch Dis Child Fetal Neonatal Ed. 2014;99(1):F80–82. doi: 10.1136/archdischild-2013-303710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rocha-Ferreira E, Hristova M. Plasticity in the Neonatal Brain following Hypoxic-Ischaemic Injury. Neural Plast. 2016;2016:4901014. doi: 10.1155/2016/4901014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Robertson NJ, Thayyil S, Cady EB, Raivich G. Magnetic resonance spectroscopy biomarkers in term perinatal asphyxial encephalopathy: from neuropathological correlates to future clinical applications. Curr Pediatr Rev. 2014;10(1):37–47. doi: 10.2174/157339631001140408120613. [DOI] [PubMed] [Google Scholar]
- 5.Edwards AD, Yue X, Squier MV, Thoresen M, Cady EB, Penrice J, Cooper CE, Wyatt JS, Reynolds EO, Mehmet H. Specific inhibition of apoptosis after cerebral hypoxia-ischaemia by moderate post-insult hypothermia. Biochem Biophys Res Commun. 1995;217(3):1193–9. doi: 10.1006/bbrc.1995.2895. [DOI] [PubMed] [Google Scholar]
- 6.Perlman JM. Intervention strategies for neonatal hypoxic-ischemic cerebral injury. Clin Ther. 2006;28(9):1353–65. doi: 10.1016/j.clinthera.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 7.Savman K, Brown KL. Treating neonatal brain injury - promise and inherent research challenges. Recent Pat Inflamm Allergy Drug Discov. 2010;4(1):16–24. doi: 10.2174/187221310789895586. [DOI] [PubMed] [Google Scholar]
- 8.Geddes R, Vannucci RC, Vannucci SJ. Delayed cerebral atrophy following moderate hypoxia-ischemia in the immature rat. Dev Neurosci. 2001;23(3):180–5. doi: 10.1159/000046140. [DOI] [PubMed] [Google Scholar]
- 9.Rosenthal RE, Williams R, Bogaert YE, Getson PR, Fiskum G. Prevention of postischemic canine neurological injury through potentiation of brain energy metabolism by acetyl-L-carnitine. Stroke. 1992;23(9):1312–1317. doi: 10.1161/01.str.23.9.1312. [DOI] [PubMed] [Google Scholar]
- 10.Xu S, Waddell J, Zhu W, Shi D, Marshall AD, McKenna MC, Gullapalli RP. In vivo longitudinal proton magnetic resonance spectroscopy on neonatal hypoxic-ischemic rat brain injury: Neuroprotective effects of acetyl-L-carnitine. Magn Reson Med. 2015;74(6):1530–42. doi: 10.1002/mrm.25537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scafidi S, Racz J, Hazelton J, McKenna MC, Fiskum G. Neuroprotection by acetyl-L-carnitine after traumatic injury to the immature rat brain. Dev Neurosci. 2010;32(5–6):480–7. doi: 10.1159/000323178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ishii T, Shimpo Y, Matsuoka Y, Kinoshita K. Anti-apoptotic effect of acetyl-l-carnitine and I-carnitine in primary cultured neurons. Jpn J Pharmacol. 2000;83(2):119–24. doi: 10.1254/jjp.83.119. [DOI] [PubMed] [Google Scholar]
- 13.Virmani MA, Caso V, Spadoni A, Rossi S, Russo F, Gaetani F. The action of acetyl-L-carnitine on the neurotoxicity evoked by amyloid fragments and peroxide on primary rat cortical neurones. Ann N Y Acad Sci. 2001;939:162–78. doi: 10.1111/j.1749-6632.2001.tb03623.x. [DOI] [PubMed] [Google Scholar]
- 14.Calabrese V, Ravagna A, Colombrita C, Scapagnini G, Guagliano E, Calvani M, Butterfield DA, Giuffrida Stella AM. Acetylcarnitine induces heme oxygenase in rat astrocytes and protects against oxidative stress: involvement of the transcription factor Nrf2. J Neurosci Res. 2005;79(4):509–21. doi: 10.1002/jnr.20386. [DOI] [PubMed] [Google Scholar]
- 15.Zanelli SA, Solenski NJ, Rosenthal RE, Fiskum G. Mechanisms of ischemic neuroprotection by acetyl-L-carnitine. Ann N Y Acad Sci. 2005;1053:153–61. doi: 10.1196/annals.1344.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Patel SP, Sullivan PG, Lyttle TS, Magnuson DS, Rabchevsky AG. Acetyl-L-carnitine treatment following spinal cord injury improves mitochondrial function correlated with remarkable tissue sparing and functional recovery. Neuroscience. 2012;210:296–307. doi: 10.1016/j.neuroscience.2012.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang R, Zhang H, Zhang Z, Wang T, Niu J, Cui D, Xu S. Neuroprotective effects of pre-treatment with l-carnitine and acetyl-L-carnitine on ischemic injury in vivo and in vitro. Int J Mol Sci. 2012;13(2):2078–90. doi: 10.3390/ijms13022078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bogaert YE, Rosenthal RE, Fiskum G. Postischemic inhibition of cerebral cortex pyruvate dehydrogenase. Free Radic Biol Med. 1994;16(6):811–20. doi: 10.1016/0891-5849(94)90197-x. [DOI] [PubMed] [Google Scholar]
- 19.Liu Y, Rosenthal RE, Starke-Reed P, Fiskum G. Inhibition of postcardiac arrest brain protein oxidation by acetyl-L-carnitine. Free Radic Biol Med. 1993;15(6):667–70. doi: 10.1016/0891-5849(93)90171-p. [DOI] [PubMed] [Google Scholar]
- 20.Rosenthal RE, Bogaert YE, Fiskum G. Delayed therapy of experimental global cerebral ischemia with acetyl-L-carnitine in dogs. Neurosci Lett. 2005;378(2):82–7. doi: 10.1016/j.neulet.2004.12.011. [DOI] [PubMed] [Google Scholar]
- 21.Aureli T, Miccheli A, Di Cocco ME, Ghirardi O, Giuliani A, Ramacci MT, Conti F. Effect of acetyl-L-carnitine on recovery of brain phosphorus metabolites and lactic acid level during reperfusion after cerebral ischemia in the rat--study by 13P- and 1H-NMR spectroscopy. Brain Res. 1994;643(1–2):92–9. doi: 10.1016/0006-8993(94)90013-2. [DOI] [PubMed] [Google Scholar]
- 22.Jones LL, McDonald DA, Borum PR. Acylcarnitines: role in brain. Prog Lipid Res. 2010;49(1):61–75. doi: 10.1016/j.plipres.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 23.Scafidi S, Fiskum G, Lindauer SL, Bamford P, Shi D, Hopkins I, McKenna MC. Metabolism of acetyl-L-carnitine for energy and neurotransmitter synthesis in the immature rat brain. J Neurochem. 2010;114(3):820–31. doi: 10.1111/j.1471-4159.2010.06807.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chiechio S, Copani A, Nicoletti F, Gereau RWt. L-acetylcarnitine: a proposed therapeutic agent for painful peripheral neuropathies. Curr Neuropharmacol. 2006;4(3):233–7. doi: 10.2174/157015906778019509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hota KB, Hota SK, Chaurasia OP, Singh SB. Acetyl-L-carnitine-mediated neuroprotection during hypoxia is attributed to ERK1/2-Nrf2-regulated mitochondrial biosynthesis. Hippocampus. 2012;22(4):723–36. doi: 10.1002/hipo.20934. [DOI] [PubMed] [Google Scholar]
- 26.White HL, Scates PW. Acetyl-L-carnitine as a precursor of acetylcholine. Neurochem Res. 1990;15(6):597–601. doi: 10.1007/BF00973749. [DOI] [PubMed] [Google Scholar]
- 27.Demarest TG, Schuh RA, Waddell J, McKenna MC, Fiskum G. Sex dependent mitochondrial respiratory impairment and oxidative stress in a rat model of neonatal hypoxic-ischemic encephalopathy. J Neurochem. 2016;137(5):714–729. doi: 10.1111/jnc.13590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hill CA, Fitch RH. Sex differences in mechanisms and outcome of neonatal hypoxia-ischemia in rodent models: implications for sex-specific neuroprotection in clinical neonatal practice. Neurol Res Int. 2012;2012:867531. doi: 10.1155/2012/867531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhu C, Xu F, Wang X, Shibata M, Uchiyama Y, Blomgren K, Hagberg H. Different apoptotic mechanisms are activated in male and female brains after neonatal hypoxia-ischaemia. J Neurochem. 2006;96(4):1016–27. doi: 10.1111/j.1471-4159.2005.03639.x. [DOI] [PubMed] [Google Scholar]
- 30.Hagberg H, Wilson MA, Matsushita H, Zhu C, Lange M, Gustavsson M, Poitras MF, Dawson TM, Dawson LV, Northington F, Johnston MV. PARP-1 gene disruption in mice preferentially protects males from perinatal brain injury. J Neurochem. 2004;90:1068–1075. doi: 10.1111/j.1471-4159.2004.02547.x. [DOI] [PubMed] [Google Scholar]
- 31.Waddell J, Hanscom M, Shalon Edwards N, McKenna MC, McCarthy MM. Sex differences in cell genesis, hippocampal volume and behavioral outcomes in a rat model of neonatal HI. Exp Neurol. 2016;275(Pt 2):285–95. doi: 10.1016/j.expneurol.2015.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Renolleau S, Fau S, Goyenvalle C, Joly LM, Chauvier D, Jacotot E, Mariani J, Charriaut-Marlangue C. Specific caspase inhibitor Q-VD-OPh prevents neonatal stroke in P7 rat: a role for gender. J Neurochem. 2007;100(4):1062–71. doi: 10.1111/j.1471-4159.2006.04269.x. [DOI] [PubMed] [Google Scholar]
- 33.Northington FJ, Chavez-Valdez R, Graham EM, Razdan S, Gauda EB, Martin LJ. Necrostatin decreases oxidative damage, inflammation, and injury after neonatal HI. J Cereb Blood Flow Metab. 2011;31(1):178–189. doi: 10.1038/jcbfm.2010.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smith AL, Alexander M, Rosenkrantz TS, Sadek ML, Fitch RH. Sex differences in behavioral outcome following neonatal hypoxia ischemia: insights from a clinical meta-analysis and a rodent model of induced hypoxic ischemic brain injury. Exp Neurol. 2014;254:54–67. doi: 10.1016/j.expneurol.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 35.Motz BA, Alberts JR. The validity and utility of geotaxis in young rodents. Neurotoxicol Teratol. 2005;27(4):529–533. doi: 10.1016/j.ntt.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 36.Dean SL, Knutson JF, Krebs-Kraft DL, McCarthy MM. Prostaglandin E2 is an endogenous modulator of cerebellar development and complex behavior during a sensitive postnatal period. Eur J Neurosci. 2012;35(8):1218–29. doi: 10.1111/j.1460-9568.2012.08032.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fan LW, Lin S, Pang Y, Lei M, Zhang F, Rhodes PG, Cai Z. Hypoxia-ischemia induced neurological dysfunction and brain injury in the neonatal rat. Behav Brain Res. 2005;165(1):80–90. doi: 10.1016/j.bbr.2005.06.033. [DOI] [PubMed] [Google Scholar]
- 38.Hermans RH, McGivern RF, Chen W, Longo LD. Altered adult sexual behavior in the male rat following chronic prenatal hypoxia. Neurotoxicol Teratol. 1993;15(6):353–63. doi: 10.1016/0892-0362(93)90051-o. [DOI] [PubMed] [Google Scholar]
- 39.Reger ML, Hovda DA, Giza CC. Ontogeny of Rat Recognition Memory measured by the novel object recognition task. Dev Psychobiol. 2009;51(8):672–8. doi: 10.1002/dev.20402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Panksepp J. The ontogeny of play in rats. Dev Psychobiol. 1981;14(4):327–332. doi: 10.1002/dev.420140405. [DOI] [PubMed] [Google Scholar]
- 41.Chan KC, Xing KK, Cheung MM, Zhou IY, Wu EX. Functional MRI of postnatal visual development in normal and hypoxic-ischemic-injured superior colliculi. Neuroimage. 2010;49(3):2013–20. doi: 10.1016/j.neuroimage.2009.10.069. [DOI] [PubMed] [Google Scholar]
- 42.Mirza MA, Ritzel R, Xu Y, McCullough LD, Liu F. Sexually dimorphic outcomes and inflammatory responses in hypoxic-ischemic encephalopathy. J Neuroinflammation. 2015;12:32. doi: 10.1186/s12974-015-0251-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huguet F, Guerraoui A, Barrier L, Guilloteau D, Tallineau C, Chalon S. Changes in excitatory amino acid levels and tissue energy metabolites of neonate rat brain after hypoxia and hypoxia-ischemia. Neurosci Lett. 1998;240(2):102–6. doi: 10.1016/s0304-3940(97)00907-5. [DOI] [PubMed] [Google Scholar]
- 44.Buckley EM, Patel SD, Miller BF, Franceschini MA, Vannucci SJ. In vivo Monitoring of Cerebral Hemodynamics in the Immature Rat: Effects of Hypoxia-Ischemia and Hypothermia. Dev Neurosci. 2015;37(4–5):407–16. doi: 10.1159/000381704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McKenna MC, Scafidi S, Robertson CL. Metabolic alterations in developing brain after injury: Knowns and unknowns. Neurochem Res. 2015;40:2527–2543. doi: 10.1007/s11064-015-1600-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Davidson JO, Wassink G, van den Heuij LG, Bennet L, Gunn AJ. Therapeutic Hypothermia for Neonatal Hypoxic-Ischemic Encephalopathy - Where to from Here? Front Neurol. 2015;6:198. doi: 10.3389/fneur.2015.00198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Oz G, Alger JR, Barker PB, Bartha R, Bizzi A, Boesch C, Bolan PJ, Brindle KM, Cudalbu C, Dincer A, Dydak U, Emir UE, Frahm J, Gonzalez RG, Gruber S, Gruetter R, Gupta RK, Heerschap A, Henning A, Hetherington HP, Howe FA, Huppi PS, Hurd RE, Kantarci K, Klomp DW, Kreis R, Kruiskamp MJ, Leach MO, Lin AP, Luijten PR, Marjanska M, Maudsley AA, Meyerhoff DJ, Mountford CE, Nelson SJ, Pamir MN, Pan JW, Peet AC, Poptani H, Posse S, Pouwels PJ, Ratai EM, Ross BD, Scheenen TW, Schuster C, Smith IC, Soher BJ, Tkac I, Vigneron DB, Kauppinen RA. Clinical proton MR spectroscopy in central nervous system disorders. Radiology. 2014;270(3):658–79. doi: 10.1148/radiol.13130531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Moffett JR, Arun P, Ariyannur PS, Namboodiri AM. N-Acetylaspartate reductions in brain injury: impact on post-injury neuroenergetics, lipid synthesis, and protein acetylation. Front Neuroenergetics. 2013;5:11. doi: 10.3389/fnene.2013.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Moffett JR, Ross B, Arun P, Madhavarao CN, Namboodiri AM. N-Acetylaspartate in the CNS: from neurodiagnostics to neurobiology. Prog Neurobiol. 2007;81(2):89–131. doi: 10.1016/j.pneurobio.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McKenna MC, Dienel GA, Sonnewald U, Waagepetersen HS, Schousboe A. Energy metabolism of the brain. In: Brady S, et al., editors. Basic Neurochemistry: Principles of Molecular, Cellular and Medical Neurobiology. Academic Press; Burlington: 2012. pp. 200–231. [Google Scholar]
- 51.Cady EB. Magnetic resonance spectroscopy in neonatal hypoxic-ischaemic insults. Childs Nerv Syst. 2001;17(3):145–149. doi: 10.1007/s003810000391. [DOI] [PubMed] [Google Scholar]
- 52.Kasdorf E, Perlman JM. Strategies to prevent reperfusion injury to the brain following intrapartum hypoxia-ischemia. Semin Fetal Neonatal Med. 2013;18(6):379–84. doi: 10.1016/j.siny.2013.08.004. [DOI] [PubMed] [Google Scholar]
- 53.Takenouchi T, Sugiura Y, Morikawa T, Nakanishi T, Nagahata Y, Sugioka T, Honda K, Kubo A, Hishiki T, Matsuura T, Hoshino T, Takahashi T, Suematsu M, Kajimura M. Therapeutic hypothermia achieves neuroprotection via a decrease in acetylcholine with a concurrent increase in carnitine in the neonatal hypoxia-ischemia. J Cereb Blood Flow Metab. 2015;35(5):794–805. doi: 10.1038/jcbfm.2014.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu J, Sheldon RA, Segal MR, Kelly MJ, Pelton JG, Ferriero DM, James TL, Litt L. 1H nuclear magnetic resonance brain metabolomics in neonatal mice after hypoxia-ischemia distinguished normothermic recovery from mild hypothermia recoveries. Pediatr Res. 2013;74(2):170–9. doi: 10.1038/pr.2013.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Morken TS, Brekke E, Haberg A, Wideroe M, Brubakk AM, Sonnewald U. Altered astrocyte-neuronal interactions after hypoxia-ischemia in the neonatal brain in female and male rats. Stroke. 2014;45(9):2777–85. doi: 10.1161/STROKEAHA.114.005341. [DOI] [PubMed] [Google Scholar]
- 56.Weis SN, Pettenuzzo LF, Krolow R, Valentim LM, Mota CS, Dalmaz C, Wyse AT, Netto CA. Neonatal hypoxia-ischemia induces sex-related changes in rat brain mitochondria. Mitochondrion. 2012;12(2):271–9. doi: 10.1016/j.mito.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 57.McCullough LD, Zeng Z, Blizzard KK, Debchoudhury I, Hurn PD. Ischemic nitric oxide and poly (ADP-ribose) polymerase-1 in cerebral ischemia: male toxicity, female protection. J Cereb Blood Flow Metab. 2005;25(4):502–12. doi: 10.1038/sj.jcbfm.9600059. [DOI] [PubMed] [Google Scholar]
- 58.Barker GR, Warburton EC. When is the hippocampus involved in recognition memory? J Neurosci. 2011;31(29):10721–31. doi: 10.1523/JNEUROSCI.6413-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pereira LO, Strapasson AC, Nabinger PM, Achaval M, Netto CA. Early enriched housing results in partial recovery of memory deficits in female, but not in male, rats after neonatal hypoxia-ischemia. Brain Res. 2008;1218:257–66. doi: 10.1016/j.brainres.2008.04.010. [DOI] [PubMed] [Google Scholar]
- 60.Trezza V, Campolongo P, Vanderschuren LJ. Evaluating the rewarding nature of social interactions in laboratory animals. Dev Cogn Neurosci. 2011;1(4):444–458. doi: 10.1016/j.dcn.2011.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.van Kerkhof LW, Damsteegt R, Trezza V, Voorn P, Vanderschuren LJ. Social play behavior in adolescent rats is mediated by functional activity in medial prefrontal cortex and striatum. Neuropsychopharmacology. 2013;38(10):1899–1909. doi: 10.1038/npp.2013.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tsuji M, Aoo N, Harada K, Sakamoto Y, Akitake Y, Irie K, Mishima K, Ikeda T, Fujiwara M. Sex differences in the benefits of rehabilitative training during adolescence following neonatal hypoxia-ischemia in rats. Exp Neurol. 2010;226(2):285–92. doi: 10.1016/j.expneurol.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 63.Hill CA, Threlkeld SW, Fitch RH. Early testosterone modulated sex differences in behavioral outcome following neonatal hypoxia ischemia in rats. Int J Dev Neurosci. 2011;29(4):381–8. doi: 10.1016/j.ijdevneu.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Park WS, Sung SI, Ahn SY, Yoo HS, Sung DK, Im GH, Choi SJ, Chang YS. Hypothermia augments neuroprotective activity of mesenchymal stem cells for neonatal hypoxic-ischemic encephalopathy. PLoS One. 2015;10(3):e0120893. doi: 10.1371/journal.pone.0120893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sabir H, Scull-Brown E, Liu X, Thoresen M. Immediate hypothermia is not neuroprotective after severe hypoxia-ischemia and is deleterious when delayed by 12 hours in neonatal rats. Stroke. 2012;43(12):3364–70. doi: 10.1161/STROKEAHA.112.674481. [DOI] [PubMed] [Google Scholar]
- 66.Sabir H, Osredkar D, Maes E, Wood T, Thoresen M. Xenon Combined with Therapeutic Hypothermia Is Not Neuroprotective after Severe Hypoxia-Ischemia in Neonatal Rats. PLoS One. 2016;11(6):e0156759. doi: 10.1371/journal.pone.0156759. [DOI] [PMC free article] [PubMed] [Google Scholar]