Abstract
We have cloned a murine cDNA encoding a tyrosine kinase receptor with about 90% similarity to the chicken fibroblast growth factor (FGF) receptor and the human fms-like gene (FLG) tyrosine kinase. This mouse receptor lacks 88 amino acids in the extracellular portion, leaving only two immunoglobulin-like domains compared to three in the chicken FGF receptor. The cDNA was cloned into an expression vector and transfected into receptor-negative CHO cells. We show that cells expressing the receptor can bind both basic FGF and Kaposi FGF. Although the receptor binds basic FGF with a 15- to 20-fold higher affinity, Kaposi FGF is able to induce down-regulation of the receptor to the same extent as basic FGF. The receptor is phosphorylated upon stimulation with both FGFs, DNA synthesis is stimulated, and a proliferative response is produced in cells expressing the receptor, whereas cells expressing the cDNA in the antisense orientation show none of these responses to basic FGF or Kaposi FGF. Thus this receptor can functionally interact with two growth factors of the FGF family.
Full text
PDF


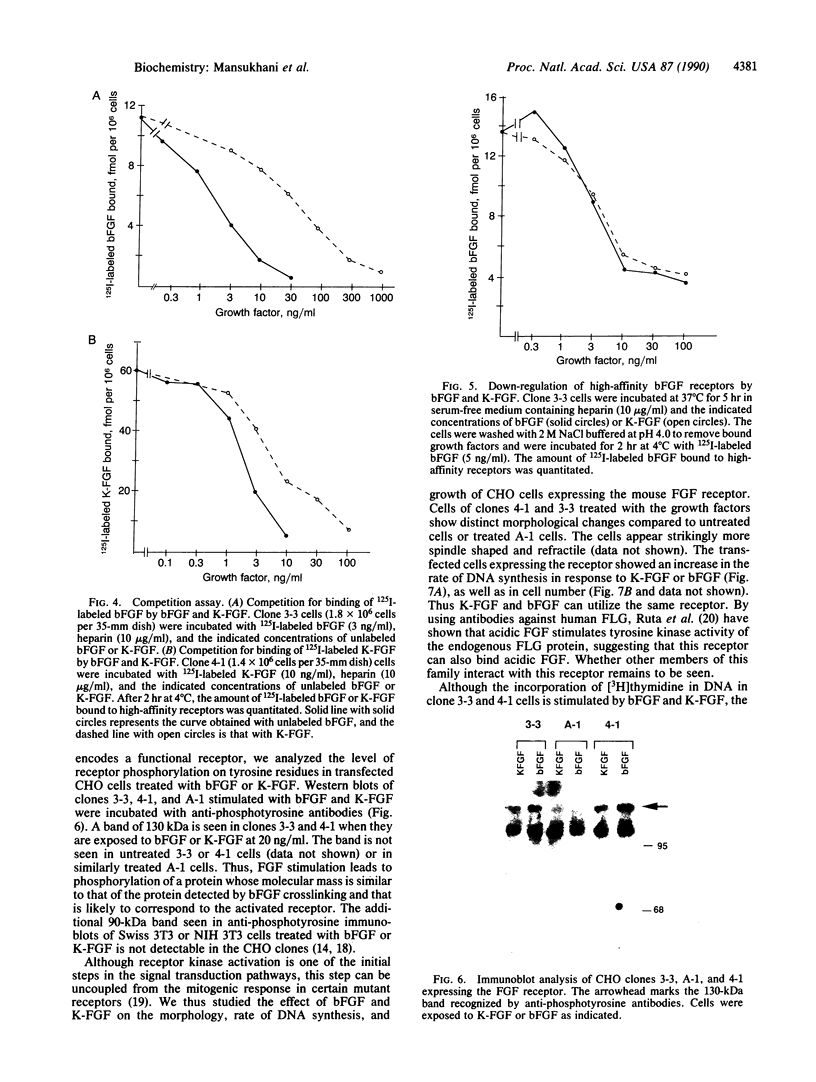
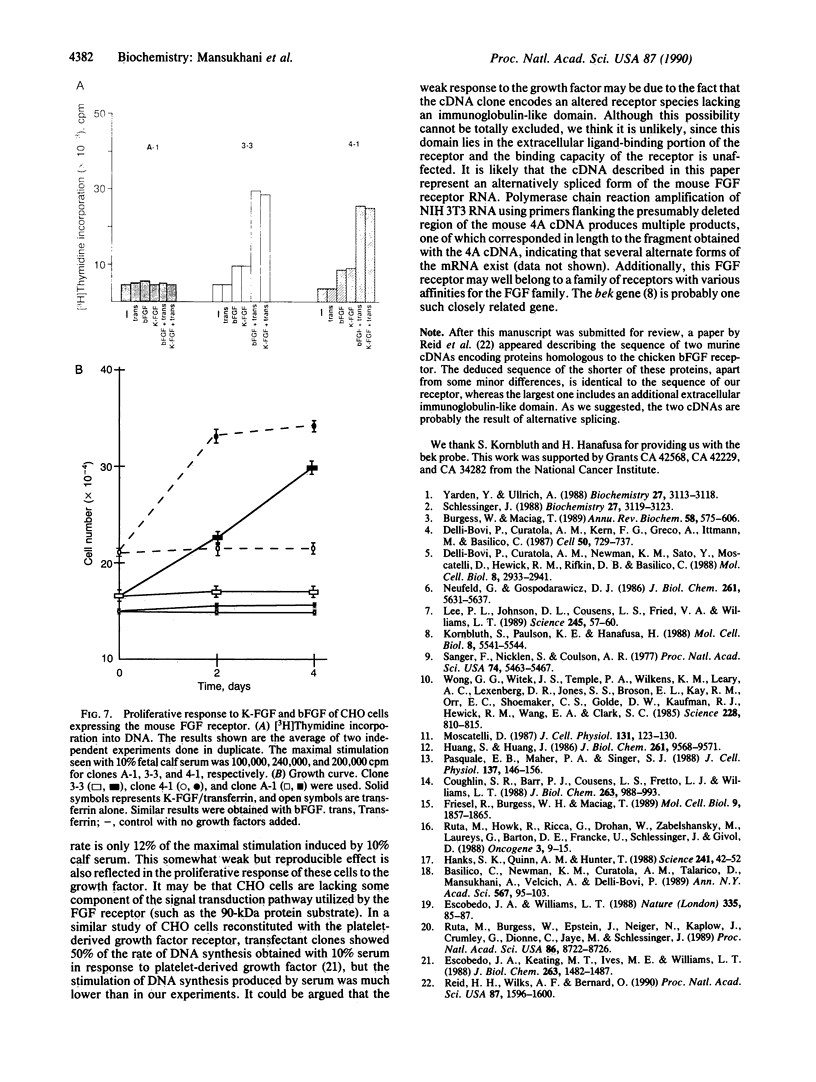
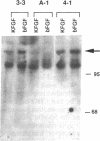
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Basilico C., Newman K. M., Curatola A. M., Talarico D., Mansukhani A., Velcich A., Delli-Bovi P. Expression and activation of the K-fgf oncogene. Ann N Y Acad Sci. 1989;567:95–103. doi: 10.1111/j.1749-6632.1989.tb16462.x. [DOI] [PubMed] [Google Scholar]
- Burgess W. H., Maciag T. The heparin-binding (fibroblast) growth factor family of proteins. Annu Rev Biochem. 1989;58:575–606. doi: 10.1146/annurev.bi.58.070189.003043. [DOI] [PubMed] [Google Scholar]
- Coughlin S. R., Barr P. J., Cousens L. S., Fretto L. J., Williams L. T. Acidic and basic fibroblast growth factors stimulate tyrosine kinase activity in vivo. J Biol Chem. 1988 Jan 15;263(2):988–993. [PubMed] [Google Scholar]
- Delli Bovi P., Curatola A. M., Kern F. G., Greco A., Ittmann M., Basilico C. An oncogene isolated by transfection of Kaposi's sarcoma DNA encodes a growth factor that is a member of the FGF family. Cell. 1987 Aug 28;50(5):729–737. doi: 10.1016/0092-8674(87)90331-x. [DOI] [PubMed] [Google Scholar]
- Delli-Bovi P., Curatola A. M., Newman K. M., Sato Y., Moscatelli D., Hewick R. M., Rifkin D. B., Basilico C. Processing, secretion, and biological properties of a novel growth factor of the fibroblast growth factor family with oncogenic potential. Mol Cell Biol. 1988 Jul;8(7):2933–2941. doi: 10.1128/mcb.8.7.2933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escobedo J. A., Keating M. T., Ives H. E., Williams L. T. Platelet-derived growth factor receptors expressed by cDNA transfection couple to a diverse group of cellular responses associated with cell proliferation. J Biol Chem. 1988 Jan 25;263(3):1482–1487. [PubMed] [Google Scholar]
- Escobedo J. A., Williams L. T. A PDGF receptor domain essential for mitogenesis but not for many other responses to PDGF. Nature. 1988 Sep 1;335(6185):85–87. doi: 10.1038/335085a0. [DOI] [PubMed] [Google Scholar]
- Friesel R., Burgess W. H., Maciag T. Heparin-binding growth factor 1 stimulates tyrosine phosphorylation in NIH 3T3 cells. Mol Cell Biol. 1989 May;9(5):1857–1865. doi: 10.1128/mcb.9.5.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanks S. K., Quinn A. M., Hunter T. The protein kinase family: conserved features and deduced phylogeny of the catalytic domains. Science. 1988 Jul 1;241(4861):42–52. doi: 10.1126/science.3291115. [DOI] [PubMed] [Google Scholar]
- Huang S. S., Huang J. S. Association of bovine brain-derived growth factor receptor with protein tyrosine kinase activity. J Biol Chem. 1986 Jul 25;261(21):9568–9571. [PubMed] [Google Scholar]
- Kornbluth S., Paulson K. E., Hanafusa H. Novel tyrosine kinase identified by phosphotyrosine antibody screening of cDNA libraries. Mol Cell Biol. 1988 Dec;8(12):5541–5544. doi: 10.1128/mcb.8.12.5541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee P. L., Johnson D. E., Cousens L. S., Fried V. A., Williams L. T. Purification and complementary DNA cloning of a receptor for basic fibroblast growth factor. Science. 1989 Jul 7;245(4913):57–60. doi: 10.1126/science.2544996. [DOI] [PubMed] [Google Scholar]
- Moscatelli D. High and low affinity binding sites for basic fibroblast growth factor on cultured cells: absence of a role for low affinity binding in the stimulation of plasminogen activator production by bovine capillary endothelial cells. J Cell Physiol. 1987 Apr;131(1):123–130. doi: 10.1002/jcp.1041310118. [DOI] [PubMed] [Google Scholar]
- Neufeld G., Gospodarowicz D. Basic and acidic fibroblast growth factors interact with the same cell surface receptors. J Biol Chem. 1986 Apr 25;261(12):5631–5637. [PubMed] [Google Scholar]
- Pasquale E. B., Maher P. A., Singer S. J. Comparative study of the tyrosine phosphorylation of proteins in Swiss 3T3 fibroblasts stimulated by a variety of mitogenic agents. J Cell Physiol. 1988 Oct;137(1):146–156. doi: 10.1002/jcp.1041370118. [DOI] [PubMed] [Google Scholar]
- Reid H. H., Wilks A. F., Bernard O. Two forms of the basic fibroblast growth factor receptor-like mRNA are expressed in the developing mouse brain. Proc Natl Acad Sci U S A. 1990 Feb;87(4):1596–1600. doi: 10.1073/pnas.87.4.1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruta M., Burgess W., Givol D., Epstein J., Neiger N., Kaplow J., Crumley G., Dionne C., Jaye M., Schlessinger J. Receptor for acidic fibroblast growth factor is related to the tyrosine kinase encoded by the fms-like gene (FLG). Proc Natl Acad Sci U S A. 1989 Nov;86(22):8722–8726. doi: 10.1073/pnas.86.22.8722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlessinger J. The epidermal growth factor receptor as a multifunctional allosteric protein. Biochemistry. 1988 May 3;27(9):3119–3123. doi: 10.1021/bi00409a002. [DOI] [PubMed] [Google Scholar]
- Wong G. G., Witek J. S., Temple P. A., Wilkens K. M., Leary A. C., Luxenberg D. P., Jones S. S., Brown E. L., Kay R. M., Orr E. C. Human GM-CSF: molecular cloning of the complementary DNA and purification of the natural and recombinant proteins. Science. 1985 May 17;228(4701):810–815. doi: 10.1126/science.3923623. [DOI] [PubMed] [Google Scholar]
- Yarden Y., Ullrich A. Molecular analysis of signal transduction by growth factors. Biochemistry. 1988 May 3;27(9):3113–3119. doi: 10.1021/bi00409a001. [DOI] [PubMed] [Google Scholar]