Abstract
Reduced circulating levels of IGF-1 have been proposed as a conserved anti-aging mechanism that contributes to increased lifespan in diverse experimental models. However, IGF-1 has also been shown to be essential for normal development and the maintenance of tissue function late into the lifespan. These disparate findings suggest that IGF-1 may be a pleiotropic modulator of health and aging, as reductions in IGF-1 may be beneficial for one aspect of aging, but detrimental for another. We postulated that the effects of IGF-1 on tissue health and function in advanced age are dependent on the tissue, the sex of the animal, and the age at which IGF-1 is manipulated. In this study, we examined how alterations in IGF-1 levels at multiple stages of development and aging influence overall lifespan, healthspan, and pathology. Specifically, we investigated the effects of perinatal, post-pubertal, and late-adult onset IGF-1 deficiency using genetic and viral approaches in both male and female igf f/f C57Bl/6 mice. Our results support the concept that IGF-1 levels early during lifespan establish the conditions necessary for subsequent healthspan and pathological changes that contribute to aging. Nevertheless, these changes are specific for each sex and tissue. Importantly, late-life IGF-1 deficiency (a time point relevant for human studies) reduces cancer risk but does not increase lifespan. Overall, our results indicate that the levels of IGF-1 during development influence late-life pathology, suggesting that IGF-1 is a developmental driver of healthspan, pathology, and lifespan.
Electronic supplementary material
The online version of this article (doi:10.1007/s11357-017-9971-0) contains supplementary material, which is available to authorized users.
Keywords: Insulin-like growth factor-1, Somatomedin C, Aging, Longevity, Cancer, Pathology
Introduction
One of the central dogmas in the aging field is that the loss of growth signals, including insulin-like growth factor-1 (IGF-1), have beneficial effects on longevity. Decreased growth hormone and IGF-1 levels throughout life have been shown to increase lifespan in several model systems, including Caenorhabditis elegans, Drosophila, and dwarf mice (Bansal et al. 2015; Bartke et al. 2000; Brown-Borg et al. 1996; Richardson et al. 2004; Rincon et al. 2005; Sonntag et al. 1999, 2005). Within the dwarf mice, decreased incidence of cancer and reduced cancer metastasis likely contribute to the observed lifespan extension (Bartke and Brown-Borg 2004; Ikeno et al. 2003, 2009). However, the anti-aging effects of reduced IGF-1 remain controversial since IGF-1 is required for normal tissue development and maintenance of tissue throughout life. The importance of maintaining IGF-1 levels throughout the lifespan is supported by studies that show IGF-1 replacement later in life improves tissue function (Markowska et al. 1998; Ramsey et al. 2004; Sonntag et al. 2000). Moreover, a recent study in C. elegans indicated that while ablation of the IGF-1 homolog (daf2) significantly increased lifespan, it did not proportionally increase the healthspan (e.g., the period of healthy aging). The long-lived mutants exhibited a longer period of functional incapacity, suggesting that reduced IGF-1 levels do not support health in advanced age. Consistent with this concept, we recently reported that IGF-1 deficiency throughout the lifespan resulted in impaired bone structure and function and accelerated the age-related decline in cognitive function. Additionally, deficiency of IGF-1 was shown to impair neurovascular coupling and cognitive function (Toth et al. 2015). Together, these data suggest that the effects of IGF-1 deficiency are highly pleiotropic and may delay or suppress some age-related pathologies but accelerate others.
It is possible that the disparate effects observed with IGF-1 on healthspan and lifespan are related to the period of life in which IGF-1 is manipulated. A majority of the studies suggesting that reduced IGF-1 is beneficial for aging are based on models that exhibit a constitutive deficiency of IGF-1 beginning early in life (Bansal et al. 2015; Bartke et al. 2000; Brown-Borg et al. 1996; Richardson et al. 2004; Rincon et al. 2005; Sonntag et al. 1999, 2005). Based on models that restore IGF-1 levels late in life, other studies have suggested that the age-related loss in IGF-1 is detrimental for aging (Markowska et al. 1998; Ramsey et al. 2004; Sonntag et al. 2000). While the results of these studies present highly conflicting viewpoints of the actions of IGF-1, it is difficult to fully compare the studies since each uses different model systems and time points for the initiation or replacement of IGF-1. With advances in genetic models, we can now test whether the time at which IGF-1 deficiency begins has an impact on lifespan and healthspan by inducing knockdown at specific stages of lifespan.
The present study was designed to assess whether reduction of IGF-1 at unique stages of mammalian development alters healthspan, age-related pathology, and lifespan. We present compelling data that the levels of IGF-1 early during life contribute to age-related disease. IGF-1 actions during the developmental period were found to be critical for the genesis of both age-related pathology and the regulation of lifespan. Importantly, these effects are highly sex specific. Since it is well known that the highest levels of IGF-1 occur during adolescence and these levels are profoundly influenced by nutrition, body weight, and general health, we propose that the effects of IGF-1 (and possibly other hormones) on healthspan, age-related pathology, and lifespan are established during this early stage of life.
Results
Reduced IGF-1 at distinct time points during the lifespan influences body composition
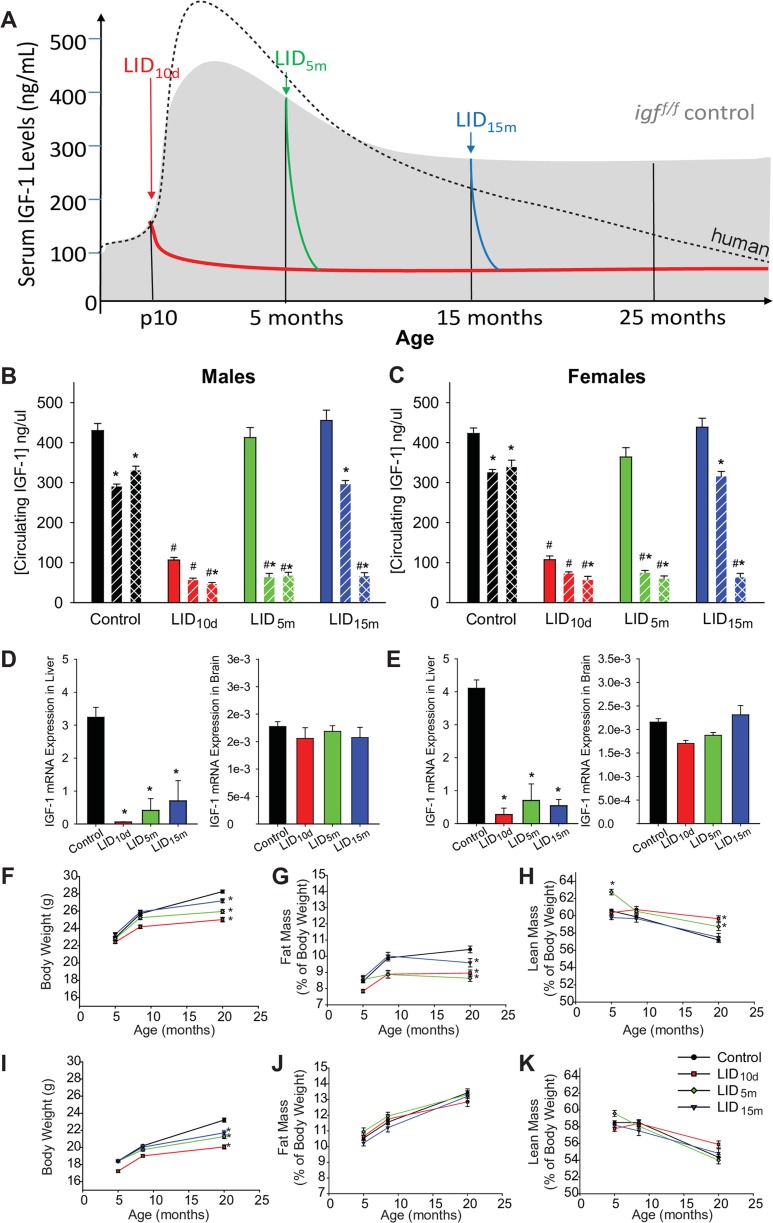
After puberty, circulating levels of IGF-1 in humans continuously decline into advanced age to levels 20–30% of that observed in early adulthood (modeled by dashed line in Fig. 1a). In wild-type mice, we observed an age-related decline in circulating IGF-1 (Fig. 1a (shadowed area), b, c), however not to the degree observed in humans. Thus, to appropriately model IGF-1 deficiency in humans, we utilized genetic and viral approaches that decreased IGF-1 expression specifically in hepatocytes—the cells responsible for producing a majority of circulating IGF-1. Circulating levels of IGF-1 were reduced at three distinct time points in the mouse lifespan: early in life (LID10d), at 5 months of age (LID5m), or at 15 months of age (LID15m) (Fig. 1a) as previously described (Ashpole et al. 2015, 2016a). Following IGF-1 knockdown, circulating levels of IGF-1 remained 20–30% of that observed in the aged control mice (Fig. 1b, c). Analysis of IGF-1 gene expression in the liver and brain at 28 months of age indicated that IGF-1 knockdown was restricted to the liver (Fig. 1d, e), with no significant change in mRNA levels of IGF-1 in the brains of male or female mice (Fig. 1d, e).
Fig. 1.
Inducing IGF-1 deficiency at distinct time periods of life. a Model of circulating IGF-1 levels throughout mouse life in the various treatment groups (LID10d, LID5m, LID15m). The black dotted line represents the average human concentrations of IGF-1 throughout the lifespan (scaled to match our study in mice). Average circulating IGF-1 levels were assessed with an ELISA at multiple time points in male (b) and female (c) mice. The solid bar indicates levels at 4–5 months of age, slanted bars indicate 12–15 months of age, and checkered bars indicate 24–25 months of age. The asterisk indicates significant difference from the 4–5-month blood levels while the pound sign indicates a significant difference from control (WT) mice at that age (n = 37–43 in the treatment groups, 113 male controls, and 123 female controls) (p < 0.05). d, e IGF-1 mRNA expression was assessed in the liver (left panels) and brain (right panels) of male (d) and female (e) mice at 25–27 months of age (n = 6–10). Body weight (f), fat mass normalized to body weight (g), and lean mass normalized to body weight (h) was measured in male mice at multiple time points in life. Body weight (f), fat mass normalized to body weight (g), and lean mass normalized to body weight (h) was measured in female mice at multiple time points in life. In panels d–k, the asterisk indicates significant difference compared to WT control (n = 37–43 in the treatment groups, 113 male controls, and 123 female controls) (p < 0.05)
As an anabolic signaling hormone, IGF-1 has been shown to promote the acquisition of body weight throughout the lifespan. Consistent with this, male mice deficient in IGF-1 starting in early post-natal development (LID10d) demonstrated significant less body weight gain by 8 months of age (Fig. 1f). By 20 months of age, all IGF-1-deficient mice demonstrate an impairment in body weight gain compared to wild-type animals. These impairments were directly related to the time-of-onset of IGF-1 deficiency (Fig. 1f). The reduced body weight in males was accompanied by a significant reduction in body fat (normalized to body weight) (Fig. 1g) and an increase in lean mass (normalized to body weight) (Fig. 1h). Female LID mice showed a similar reduction in body weight by 20 months of age (Fig. 1i). However, no changes in fat or lean mass were evident after correction to body weight (Fig. 1j, k).
IGF-1 deficiency impairs healthspan late in life
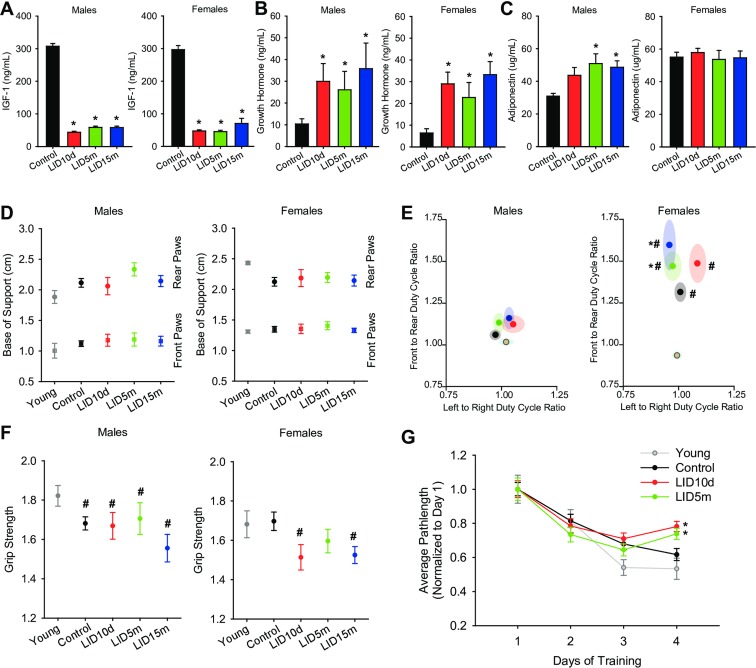
Several measures of healthspan were assessed at 25–27 months of age, a time in which mortality was approaching 50%. Animals (n = 10–20) from each group were removed from the lifespan cohort and analyzed in a cross-sectional study (see Supplemental Table 1 for full description of cohort). Circulating IGF-1 deficiency was verified in this cross-sectional cohort at 25–27 months of age (Fig. 2a). Because of the important negative feedback effects between IGF-1 and GH signaling, the levels of GH in circulation were quantified. Although analysis of pulsatile release of GH was not technically feasible in this cohort, as expected, we observed a significant increase in circulating GH in the presence of IGF-1 deficiency (Fig. 2b). We also examined adiponectin levels, which have been shown to be important in aging. Adiponectin levels were significantly increased in the LID5m and LID15m male mice, with no significant effect in any of the female treatment groups (Fig. 2c).
Fig. 2.
IGF-1 deficiency did not increase healthspan at 25–27 months of age. Average circulating levels of IGF-1 (a), growth hormone (b), and adiponectin (c) in male (left panels) and female (right panels) mice were quantified using ELISAs (n = 12–18 per treatment group, 34–51 for controls). d Base of support and average duty cycle (E, % utilization of each foot) in males (left panels) and females (right panels) was assessed using the CatWalk (n = 13–17 per treatment group, 34–51 for controls). Average grip strength over three trials was assessed using a horizontal force meter at 25–27 months of age in male (left panel) and female (right panel) mice (n = 12–18 per treatment group, 34–51 for controls). Learning and memory was assessed in a subset of male mice using the Barnes Maze. Average path length to the escape box was quantified over 4 days (three trials per day) (n = 8–15 per group). The asterisks indicate a significant difference compared to control animals (p < 0.05)
Gait analysis is a common measurement of human health and aging; thus, we conducted gait analysis within our cross-sectional cohort at 25–27 months of age using the Noldus CatWalk system. Young mice (4–6 months old) purchased from Jackson Laboratories were included as a reference control. Despite the decreased body weight observed in the aged LID mice, there was no change in the base of support in either the front or hind legs in male or female mice (Fig. 2d), suggesting that postural width was not altered in these mice. Additionally, there was no change in the velocity of pace within the LID mice, suggesting overall mobility was not altered. Our initial analyses indicated that many of the gait parameters were associated with body weight. Nevertheless, IGF-1 deficiency decreased stride length (Supplemental Fig. 1A-B), and altered the duty cycle (Fig. 2e) (the amount of time each foot bears weight while walking) in females. Animals that spend equal time on front/back and left/right feet show a duty cycle ratio of 1. As animals spend more time on one foot (or the front or hind legs), the cycle increases or decreases from this value. In male mice, the duty cycle of young, aged, and IGF-1-deficient mice was close to 1 (Fig. 2e, left panel). However, in aged female mice, the use of front feet to bear body weight was favored over hind feet (p < 0.01, Fig. 2e, right panel). Moreover, LID10d and LID5m females exhibited a significant increase in the front to rear duty cycle, when compared to age-matched controls (p < 0.05, Fig. 2e, right panel). This effect may be related to muscle or ligament dysfunction, kyphosis, or the alterations in bone morphology and strength previously reported in this cohort (Ashpole et al. 2016a, b).
Male mice exhibited an age-related decrease in grip strength (Fig. 2f). This effect was not altered by the loss of IGF-1 in the aged LID10d (Fig. 2f, left panel), despite the increased lean mass noted in Fig. 1h. The grip strength of the LID5m and LID15m males was also not statistically different from the aged controls (Fig. 2f, left panel). Additionally, there were no observed differences in the amount of time mice were able to remain on the balance beam (Supplemental Fig. 1C), or their ability to respond to an eye reflex test (Supplemental Fig. 1D). There was no significant effect of age or IGF-1 deficiency on the grip strength of female mice; however, the female LID10d and LID15m mice show a trend for a decrease compared to young controls (p = 0.1 and 0.16, respectively, Fig. 2f, right panel).
Spatial learning and memory in a subset of male mice was assessed in the Barnes Maze. Aged control mice exhibited impairments in learning compared to the young reference controls (Fig. 2g). Furthermore, the LID10d and LID5m mice showed impairments in the acquisition phase of learning compared to the aged controls (Fig. 2g). Due to technical caveats, LID15m mice were omitted from analysis.
Following behavioral assessment, pathology and disease burden were assessed in the cross-sectional cohort (Supplemental Table 2). There were no significant differences in overall disease or cancer burden in any of the male IGF-1-deficient mice (Supplemental Table 2). Furthermore, there were no differences in the incidence of lymphoma, hepatocarcinoma, or hemangiosarcoma in the LID males compared to control. Within females, there was a significant reduction in disease burden in the LID10d group and a trend for a decrease in overall cancer burden (p = 0.1) (Supplemental Table 2). The decreased disease burden in the LID10d females appeared to be related to a significant decrease in pituitary adenomas and reduced glomerular nephritis. IGF-1 deficiency starting in adulthood (LID5m and LID15m) also reduced the incidence of pituitary adenoma, yet there were no global effects on cancer or disease burden in these groups (Supplemental Table 2). Together, these data suggest that IGF-1 deficiency did not lead to robust improvements in cross-sectional pathology or behavioral performance in a majority of treatment groups. Rather, IGF-1 deficiency had detrimental effects on animal gait and cognitive abilities, along with trending decreases in grip strength.
Early IGF-1 deficiency increases lifespan in female mice
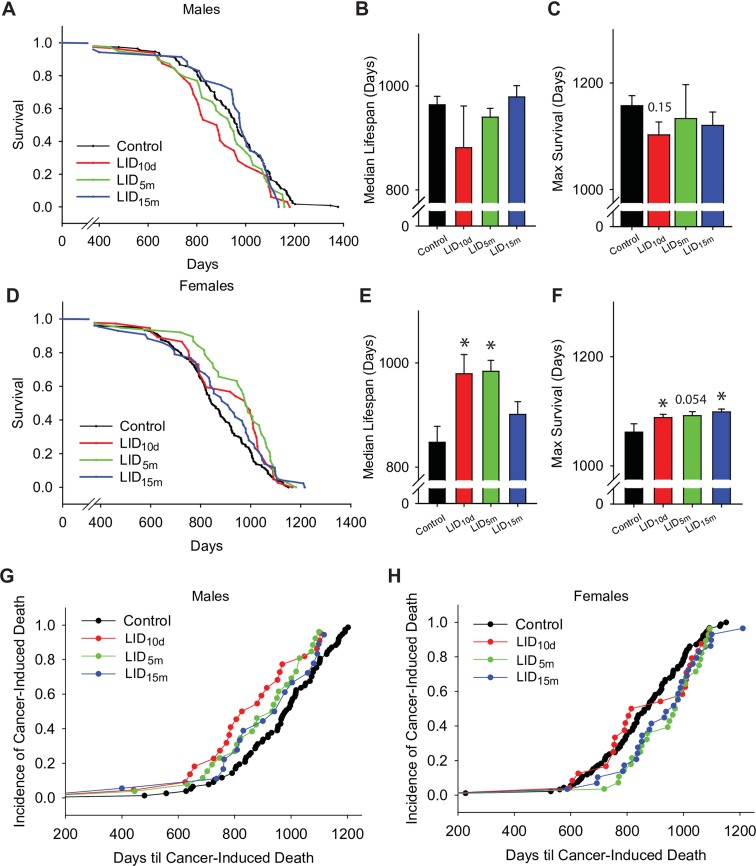
Survival analyses were performed on control and IGF-1-deficient mice, as shown in Fig. 3 and Table 1. The mean survival of control male mice was 944 days (Fig. 3a). IGF-1 deficiency beginning at any age did not significantly affect the mean, median, or maximal lifespan in male mice (Fig. 3a–c, Table 1). Cox regression and log rank analysis indicated that the overall lifespan of the LID10d males trended toward a reduced lifespan; however, this effect did not reach statistical significance (p = 0.052 and p = 0.056, respectively). This may be the result of the significant reduction in survival within the first 30% of the survival curve, as revealed by quantile analysis (p = 0.04, Table 1). After this point, there was no significant difference between LID10d and aged control males.
Fig. 3.
IGF-1 deficiency beginning earlier in life extends female lifespan. a Survival curve analysis of 121 control (black), 39 LID10d (red), 44 LID5m (green), and 38 LID15m (blue) male mice. Lifespan extension was assessed using the average median lifespan (b) and the average maximal lifespan (c, remaining 10% survival) of male mice in the various treatment groups. d Survival curves of 130 control (black), 38 LID10d (red), 43 LID5m (green), and 45 LID15m (blue) female mice. Lifespan extension was assessed using the average median lifespan (e) and the average maximal lifespan (f, remaining 10% survival) of female mice in the various treatment groups. The age of cancer-induced mortality (relative to total cancer-induced deaths) was plotted for male (g) and female (h) mice. Sixty-three percent of all deaths were induced by cancer. The asterisks indicate a significant difference compared to control animals (p < 0.05)
Table 1.
Lifespan statistical analysis
| Group | Cox regression | Quantile analysis | ||||||
|---|---|---|---|---|---|---|---|---|
| Coeff | p value | 30% | p value | 50% | p value | 90% | p value | |
| Males | ||||||||
| WT vs LID10d | 0.39 | 0.052 | −85.9 | 0.04 | −82.6 | 0.25 | −54.8 | 0.15 |
| WT vs LID5m | 0.28 | 0.14 | −50.9 | 0.08 | −23.8 | 0.4 | −24.1 | 0.71 |
| WT vs LID15m | 0.14 | 0.47 | 73.1 | 0.007 | 15.4 | 0.43 | −36.9 | 0.21 |
| Females | ||||||||
| WT vs LID10d | −0.33 | 0.082 | 9.2 | 0.71 | 131.4 | <0.001 | 26.5 | 0.013 |
| WT vs LID5m | −0.52 | 0.005 | 86.5 | 0.009 | 136.1 | <0.001 | 30 | 0.054 |
| WT vs LID15m | −0.37 | 0.043 | 13.4 | 0.81 | 53.6 | 0.29 | 37 | <0.001 |
The mean survival of control female mice was 844 days (Fig. 3d). Early-life IGF-1 deficiency in the LID10d females resulted in a significant increase in the mean and median lifespan of female mice (Fig. 3d–f, Table 1). Cox regression analysis and log rank analysis indicated that the lifespan of LID10d females is trending increased, but this did not reach statistical significance (p = 0.08 and p = 0.07, respectively). Nevertheless, IGF-1 deficiency beginning at 5 months of age (LID5m) resulted in a robust increase in both the mean and median lifespan (p < 0.01 and p < 0.001, respectively, Fig. 3d, e, Table 1). The overall hazard of death is reduced 40% throughout the lifespan of the LID5m females, as indicated by the Cox proportional hazard ratio. Deficiency beginning later in adulthood (LID15m) did not significantly affect the mean or median lifespan (Table 1). The maximal lifespan (the final 10%) were modestly increased (2–3%) in all three female LID groups—WT 1062 days vs LID10d 1088, LID5m 1091, and LID15m 1092 days (Fig. 3f). While several measures of lifespan were increased in the LID10d and LID15m females, the LID5m females were the only group to show consistent lifespan extensions in the Cox regression, log rank, and Boschloo analyses.
IGF-1 deficiency influences disease pathology at end-of-life
End-of-life pathological analyses indicated that cancer was the leading cause of death within this study. There were no differences in the incidence of cancer-induced mortality in the aged control, LID10d, and LID5m males, each of which showed approximately 65% of deaths induced by cancer (Supplemental Table 3). While the overall incidence of cancer-induced mortality was not different between these groups, the LID10d and LID5m male mice died of cancer earlier than controls (Fig. 2g). Interestingly, the LID15m males had a significant reduction in cancer-induced deaths, with approximately 45% of mice in that group dying from cancer pathology (Supplemental Table 3). Despite the decrease in cancer incidence, the LID15m males that died of cancer died significantly earlier than controls (Fig. 2g).
Within the female treatment groups, there were no significant differences in the incidence of cancer-induced mortality (Supplemental Table 3). However, the onset of cancer-induced death was delayed in the LID5m and LID15m female mice (Fig. 3h). Calculation of the rate of death in the LID10d female mice closely matched the wild-type controls for the first half of the lifespan, and the LID5m and LID15m mice thereafter. The reason for this shift is not known, as there was no obvious differences in the cancer pathologies within the short-lived and longer lived LID10d females. Interestingly, the observed lifespan extension in the LID5m females (Fig. 3d) was not associated with reduced cancer incidence or delayed cancer-induced mortality (Supplemental Table 3 and Fig. 3h). This result suggests that IGF-1 deficiency, lifespan, and cancer-induced mortality were not directly correlated in this experimental cohort. This concept is further supported by data from the LID15m females, which did not exhibit increased lifespan, but did show delayed cancer-mortality (Fig. 3d, h).
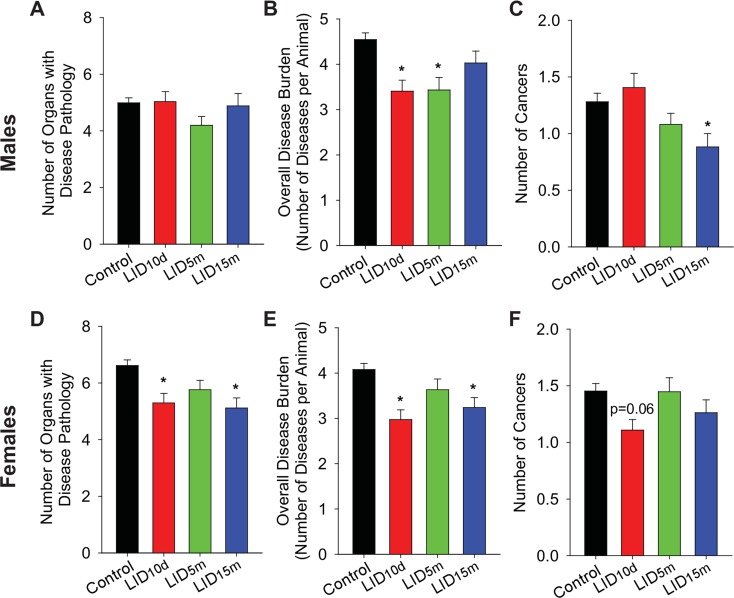
We next examined individual tissue pathology at the end of life (full description found in Supplemental Table 4). Within males, IGF-1 deficiency at any age did not influence the overall number of organs with disease (Fig. 4a). Disease burden (total disease per animal) was significantly reduced in the LID10d and LID5m males (Fig. 4b), suggesting that higher levels of IGF-1 early in life may be a developmental driver of disease in males. Late life loss of IGF-1 (LID15m) did not reduce overall disease load but did lead to a significant reduction in the overall number of cancers observed in each mouse (Fig. 4c). Once again, these data highlight the discontinuity between IGF-1 deficiency, enhanced lifespan, and decreased cancer.
Fig. 4.
IGF-1 deficiency influences disease and cancer burden at the end of life. The average number of organs per animal with pathology (a), number of diseases per animal (b), and number of cancer types per animal (c) in male mice, as assessed with histological pathology. Average number of organs per animal with disease (d), number of diseases per animal (e), and number of cancer types per animal (f) in female mice. The asterisk indicates significant difference compared to control animals (p < 0.05)
Female LID10d and LID15m mice exhibited significant reductions in the number of organs with disease pathology and overall disease burden (Fig. 4d, e). While the LID5m females had the most prominent lifespan extension, no differences in disease burden were observed (Fig. 4e). Additionally, there was no significant difference in the number of cancers per mouse in the female treatment groups. These data once more separate cancer load from lifespan—LID5m females lived longer but did not show decreased cancer risk.
Beneficial and detrimental effects of IGF-1 deficiency on specific tissues
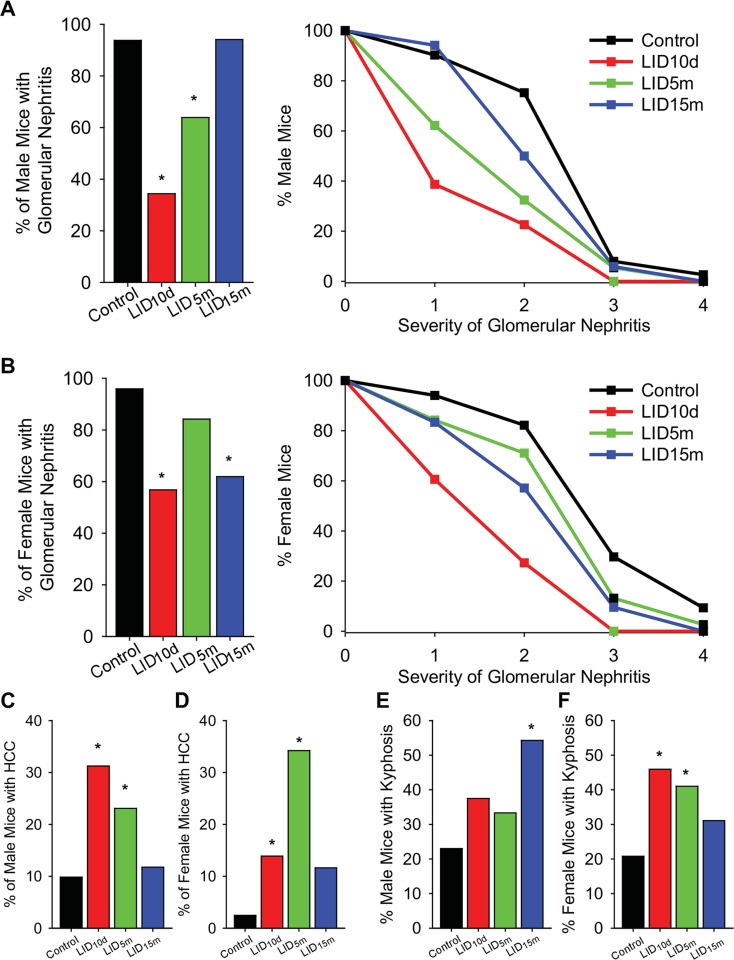
Individual tissues demonstrated pleiotropic effects of IGF-1 deficiency. Early in life (LID10d), IGF-1 deficiency resulted in significant reductions in the incidence and severity of glomerular nephritis in both male (Fig. 5a) and female mice (Fig. 5b). Similarly, LID5m males and LID15m females also showed a decrease in the incidence and severity of nephritis (Fig. 5a, b). Thus, the reduction in IGF-1 was beneficial for kidney-associated pathology in an age-dependent, sex-specific manner.
Fig. 5.
Tissue-specific pathology associated with IGF-1 deficiency. The percent mice with glomerular nephritis (left panels) and the average grade of nephritis (right panels) at the end of life in male (a) and female (b) mice. The percent mice with hepatocellular carcinoma in males (c) and females (d), as assessed with histological pathology. The percent mice with lordokyphosis in males (e) and females (f). The asterisk indicates significant difference as assessed using chi-squared analysis
There were detrimental effects of IGF-1 deficiency as well. Male and female LID10d and LID5m mice showed a significant increase in the incidence of hepatocellular carcinoma (Fig. 5c, d). This is likely associated with the robust increase in GH levels (Fig. 2b) that occurs in response to IGF-1 deficiency, which has been shown to be associated with increased hepatocarcinoma (Gong et al. 2014).
We previously reported impairments in bone structure and strength within this cohort of animals (Ashpole et al. 2016a, b). Male LID15m mice showed a significant increase in the incidence of kyphosis compared to age-matched controls (Fig. 5e). Within females, IGF-1 deficiency beginning earlier in life (LID10d and LID5m) resulted in increased incidence of kyphosis (Fig. 5f). These data are consistent with our previous studies examining the deficits of IGF-1 deficiency on bone structure and function (Ashpole et al. 2016a, b), as well as with the alterations in gait analysis observed in these mice (Fig. 2e). Together, this analysis reveals that the effects of IGF-1 deficiency on tissue pathology are diverse and influenced by sex, tissue, and age-of-onset of IGF-1 deficiency.
Discussion
The levels of circulating IGF-1 undergo dynamic changes throughout the lifespan. IGF-1 concentrations are transiently elevated prenatally but remain low in the early post-natal period. During adolescence, levels of IGF-1 rise dramatically contributing to the growth of the organism and subsequently decrease with advancing age. Despite the profound changes in IGF-1 during the lifespan, there have been no reports that address the importance of IGF-1 during critical periods of life for overall healthspan, age-related pathology, or lifespan. The results presented here represent the first comprehensive analysis of alterations in the regulation of IGF-1 during unique developmental time periods that correspond to the dynamic changes in circulating IGF-1 that occur throughout life. Our results provide compelling evidence that the actions of IGF-1 during development are the primary drivers of lifespan, healthspan, and age-related pathology. Furthermore, our results demonstrate clear sexually dimorphic actions of IGF-1 and indicate that IGF-1 deficiency induced later during the lifespan, a time point that is relevant for intervention in humans, can reduce cancer risk, and delay cancer mortality in females with no effect on lifespan.
It is well known that the function of numerous organ systems decreases with age and contribute to disability. Although the etiology for this decline is likely due to several interacting factors, previous studies indicate that muscle and bone mass, reproductive function, as well as cognitive ability are dependent on the levels of circulating growth factors that decrease with age, including IGF-1 (as reviewed by Sonntag et al. (2012)). Consistent with this concept, work in C. elegans has highlighted that reduced IGF-1/insulin signaling leads to prolonged periods of frailty in aged organisms (Bansal et al. 2015). Additionally, replacement of GH and/or IGF-1 later in life in rodents have been shown to improve microvascular, vascular, and cognitive function (Markowska et al. 1998; Ramsey et al. 2004; Sonntag et al. 2000). Nevertheless, the specific role of GH/IGF-1 during aging has remained controversial since there are reports of improved healthspan and lifespan in response to GH/IGF-1 deficiency (Arum et al. 2014; Coschigano et al. 2000, 2003; Hascup et al. 2016). For example, studies by Holzenberger indicated that haploinsufficiency of igfr increased lifespan by 33% in females and 16% in males (Holzenberger et al. 2003). Although this study has been routinely cited in the literature as evidence that IGF-1 signaling in mammals is detrimental to both healthspan and lifespan, the short lifespan of the control animals in this study (∼750 days) compromise interpretation. Follow-up studies where control animals exhibited a normal lifespan did not result in a dramatic extension of lifespan and females exhibited a modest 5–10% increase in lifespan whereas no increase was observed in response to IGF-1 deficiency in males (Bokov et al. 2011; Xu et al. 2014). The studies detailed here clearly demonstrate that deficiencies in circulating IGF-1 beginning at or before 5 months of age result in a 15% increase in lifespan in females and that decreases in IGF-1 occurring later in life have little effect. Importantly, early-life IGF-1 deficiency in males exhibits a trend for shortened lifespan (similar to previous reports (Gong et al. 2014)) and this effect diminishes with later-onset IGF-1 deficiency. These data provide compelling support for the conclusion that the effects of IGF-1 deficiency on lifespan are manifest early during the lifespan and are sex specific. Although some of the inconsistencies that have been evident in the field for the last 20 years can be attributed to low numbers of animals and atypical mortality data, the inconsistent results also reflect the complexity of studying a dynamic endocrine system that demonstrates profound changes in blood concentrations throughout the lifespan.
It is well established that circulating hormones exhibit both organizational and regulatory roles. IGF-1, acting as a classic anabolic hormone and growth factor, has the capacity to influence cellular growth and tissue organization during development and regulate normal cellular and tissue function in adults. There are three mouse models of GH/IGF-1 deficiency that have consistently shown increased lifespan across multiple studies: Ames (prop1 mutation), Snell (pit1 mutation), and ghr knockout mice (as reviewed by Brown-Borg (2015) and Ladiges et al. (2009)). Each of these models demonstrates a profound reduction in GH and/or IGF-1 initiated early during development. Importantly, more modest reductions in IGF-1 or induction in GH/IGF-1 deficiency later in life has a modest or no effect on lifespan even though there are important changes in age-related pathology (Sonntag et al. 2005). Consistent with the importance of the developmental effects of GH/IGF-1, we found that LID10d and LID5m females exhibited substantially increased lifespan compared to controls but this effect was absent after 15 months of age. In the present study, the longevity phenotype could not have been due to an overall lifetime reduction in IGF-1 levels since deficiency in IGF-1 beginning at 5 months of age in females exhibited a trend for a greater effect than when it was initiated around day 10. In addition, males exhibited a shorter lifespan with an early life reduction in IGF-1. Thus, despite the general consensus in the field that the GH/IGF-1 pathway is a conserved mechanism of aging, a thorough review of the published literature and our current results do not support this conclusion. Rather, the organizational actions of IGF-1 during development and early adulthood likely underlie its effects on pathology and lifespan.
There has been growing evidence to support the concept that the healthspan of the organism and incidence of late-life pathologies are regulated by events occurring during development—a concept collectively known as the “Developmental Origins of Disease.” Although the etiology of this regulation is poorly understood, the dynamic changes in IGF-1, especially during adolescence, profoundly influences growth velocity, tissue development, and cellular function and has been reported to have effects on age-related pathology and lifespan (Podlutsky et al. 2017; Sonntag et al. 2005). In humans, adolescent levels of IGF-1 are highly variable and are influenced by both the environment and nutrition. Interestingly, increasing clinical and experimental evidence suggest that the endocrine milieu present around adolescence activates cellular programs that influence age-related disease, including cerebrovascular disease (Eriksson et al. 1999, 2007; Kajantie et al. 2005; Osmond et al. 2007). In the present study, we found that glomerular nephritis and overall disease burden were reduced in both males and females in response to early-life IGF-1 deficiency. Nevertheless, only females demonstrated an increase in lifespan. Although lymphoma was the major pathology in animals of both sexes, days until cancer-induced death was one of the best pathological correlates of lifespan. Our results indicated that males with early IGF-1 deficiency exhibit an accelerated cancer-induced mortality rate while days until cancer induced death is slowed in IGF-1-deficient females. In addition, females with early life IGF-1 deficiency exhibited a reduction in the number of organs exhibiting disease pathology at the end of life compared to control animals. A similar pattern was observed for the incidence of cancer. Thus, our results provide compelling data that a reduction in IGF-1 during early life in females has a profound effect on the number, distribution, and progression of cancer. The nature of the sexually dimorphic effects are unknown but clearly merit further investigation.
One of the prevailing concepts in the field is that reduced levels of GH/IGF-1 have the potential to increase longevity by delaying the onset of age-related pathological conditions such as cancer and diabetes. In humans, low levels of IGF-1 and mutations in IGFR have been associated with enhanced longevity in females (Milman et al. 2014; Suh et al. 2008; van Heemst et al. 2005). Moreover, patients with Laron’s syndrome (an autosomal recessive disorder characterized by an insensitivity to GH caused by a mutation in the GH receptor) have been shown to be protected from cancer and diabetes, although these individuals do not live significantly longer than their healthy counterparts (as reviewed by Sonntag et al. (2012)). In addition to the human studies, several animal models of GH/IGF-1 deficiency have been shown to be resistant to neoplastic disease. Snell dwarf mice and Ames dwarf mice, which have GH/IGF-1 deficiency and also have low very levels of prolactin, and thyroid-stimulating hormone, exhibit reduced incidence of tumors and cancer mortality (Bartke and Brown-Borg 2004; Ikeno et al. 2003, 2009). Similarly, GHRKO mice also exhibit reduced tumorgenesis as well as delayed onset of tumors (Ikeno et al. 2009). In this study, we also observed a delay in the onset of cancer-induced mortality within our early-life IGF-1-deficient females, although this effect was not present in males. On the contrary, males showed reduced cancer burden when IGF-1 deficiency began in late adulthood, but no difference was observed when IGF-1 was decreased earlier in life. Thus, the developmental time window in which IGF-1 deficiency occurs has a significant impact on long-term cancer risk.
Interestingly, one neoplasm was significantly reduced in all IGF-1-deficient females, independent of the time of onset. Sixteen percent of wild-type female mice exhibited pituitary adenomas, yet no IGF-1-deficient mice exhibited this pathology. This was a sex-specific effect, since pituitary tumors were not observed in male mice. Other studies have highlighted the elevated risk of pituitary tumors in female mice (Radaelli et al. 2009). The majority of pituitary tumors in females are prolactinomas that result from loss of tuberoinfundibular dopamine levels within the hypothalamus. The fact that IGF-1 replacement has been demonstrated to preserve the function of these neurons, restore dopamine levels, and suppress prolactin suggests that this effect is not directly related to IGF-1 deficiency but mediated through other indirect mechanisms (Herenu et al. 2007).
Based on a thorough review of the pleiotropic actions of IGF-1, the reduced cancer risk with IGF-1 deficiency comes with enhanced risk for other non-neoplastic diseases. In humans, GH/IGF-1 deficiency is associated with cardiovascular disease, reduced muscle and bone mass, neurological deficits, increased body fat, and impaired energy metabolism (Amato et al. 1993; De Boer et al. 1992; Johansson et al. 1995; Merola et al. 1993; van Dam et al. 2005). These trade-offs are consistent with the results of our recent studies indicating compromised bone health (Ashpole et al. 2016a, b), reduced lean mass, but no overall reduction in disease burden in our longest-lived IGF-1-deficient females. Other studies have addressed the elevated incidence of cardiovascular disease in IGF-1-deficient rodents (Bailey-Downs et al. 2012a; b; Toth et al. 2014, 2015) and reported a high incidence of neurological disorders when IGF-1 levels are reduced. Thus, despite potential decreases in cancer risk, IGF-1 deficiency results in an increased risk of disease conditions that impair quality of life and are significant causes of mortality in elderly humans.
One of the rapidly growing areas of interest in the aging field has been the sexual dimorphism evident in many lifespan studies. A recent review highlighted differences in lifespan of wild-type male and female mice (Austad and Fischer 2016). In our study, male control mice lived ∼10% longer than control female mice. However, our study also indicates that the beneficial effects of IGF-1 deficiency on lifespan extension is limited to females. While the majority of lifespan studies have historically been performed with male mice, several recent studies have indicated that many genetic and pharmacological interventions result in lifespan extension that are sex specific, with only rapamycin affecting both sexes (Harrison et al. 2014; Miller et al. 2011, 2014, 2007). The Interventions Testing Program, designed to test the reproducibility of lifespan studies at multiple testing sites, has validated eight treatments that extend lifespan; seven are unique to one sex. Our lifespan extension in the IGF-1-deficient female mice mirrors the haploinsufficiency of igfr study performed by the Richardson laboratory, where females showed a modest increase in lifespan that was not evident in males (Bokov et al. 2011). The mechanism(s) underlying the sexual dimorphism of lifespan studies, including those with IGF-1 deficiency, are still largely unknown. It is likely that gonadal hormones are a contributing factor. Age-related reductions in estrogen have been shown to alter many signals in the HPA axis, cardiovascular system, and nervous system. While changes in estrogen and testosterone may contribute to the dichotomy in aging studies, they are likely not the sole cause. This is underscored by recent data from the ITP program indicating that 17α-estradiol supplementation extended lifespan in male rather than female mice (Harrison et al. 2014). Considering these findings, it is imperative that further studies be performed to understand the mechanisms underlying the sex-specificity of lifespan extensions.
Summary
Together, our data reveal the complex relationship between the levels of IGF-1 in circulation and its overall impact on health, age-related pathology, and lifespan. Lifespan and healthspan diverge with IGF-1 deficiency. Reduction of IGF-1 beginning early in life leads to increased lifespan in females, with no substantial improvement in the health of these mice. This study also highlights the divergent effects of decreasing IGF-1 at three distinct stages of life: development, early adulthood, and late adulthood. Benefits on lifespan were only apparent when deficiency started early in life, indicating that decreasing IGF-1 levels in late adulthood is not a viable option for increasing lifespan. These data also suggest that the normal age-related reduction in circulating IGF-1 levels is not a robust contributor to enhanced longevity. Importantly, the effects of IGF-1 deficiency are dependent on the sex of the animal and are tissue-dependent. The data presented in this study emphasize the complexities in interpreting studies that examine the consequences of treatments at a single time point, in a single sex, and in a single tissue. IGF-1 is a critical regulator of a variety of cellular functions. As such, robust deviations from the “normal” concentrations of circulating IGF-1 throughout the lifespan result in substantial changes in several tissues. As studies continue to identify the downstream effectors of IGF-1 signaling and characterize how these effectors change with advanced age, it is imperative to recognize the hormonal fluctuations that occur throughout the lifespan, the pleiotropic nature of IGF-1, and its influence on lifespan and healthspan.
Methods
Animals
All procedures were approved by and followed the guidelines of the Institutional Animal Care and Use Committee of OUHSC. Male and female mice homozygous for a floxed exon 4 of the Igf1 gene (igf1 f/f) in a 129/FVB background were gifted by Shoshanna Yakar and subsequently backcrossed six generations to C57Bl/6 mice in house. A total of 717 mice were enrolled in this study. The lifespan cohort included 508 mice, while an additional 209 mice were included in the cross-sectional cohort. Of the 508 mice in the lifespan study, 4 (0.8%) died prior to the initiation of treatment, while 27 (5.3%) died from unnatural deaths (extensive fighting, cage flooding, complications of whole blood collection, etc.); these animals were omitted from the final analysis.
To target IGF-1 production early in post-natal development, igf1 f/f mice were crossed with mice expressing albumin-driven Cre recombinase (Yakar et al. 1999), which led to decreased hepatic IGF-1 10–15 days after birth (mice termed liver IGF-1-deficient, LID10d). To target IGF-1 production in early and late adulthood, igf1 f/f mice were administered 1.3 × 1010 viral particles of adeno-associated viruses (AAV8-TBG-Cre or AAV8-TBG-eGFP) via retro-orbital injection, as described (Ashpole et al. 2016b; Toth et al. 2014). Similar to albumin-driven Cre, thyroxine-binding globulin (TBG)-driven Cre leads to a hepatocyte-specific knockout of exon 4 (Toth et al. 2014) and a reduction in circulating IGF-1. At 5 or 15 months of age, igf1 f/f mice were randomly assigned to treatment groups, given an intraperitoneal injection of ketamine/xylazine (80 mg/15 m/kg) for anesthesia and administered 100 μl of virus diluted in physiological saline into the retro-orbital sinus. Purified viruses were purchased from the University of Pennsylvania Viral Vector Core. Mice were monitored in their home cage as they recovered from anesthesia. Two mice died from complications associated with viral injection and were not included in the lifespan study. Mice injected with AAV8-TBG-Cre at 5 months of age were termed LID5m, while the mice that received injections at 15 months of age were termed LID15m. Young reference control C57/Bl6J male and female mice (3–4 months of age) were purchased from Jackson Laboratories (Bar Harbor, ME).
Animals were housed (3–4 per cage) in Allentown XJ cages with Anderson’s Enrich-o-cob bedding (Maumee, OH). The Rodent Barrier Facility at OUHSC is specific pathogen free (including helicobacter and parvovirus). Mice were maintained on a 14-h light/10-h dark cycle at 21 °C and were given access to standard irradiated bacteria-free rodent chow (5053 Pico Lab, Purina Mills, Richmond, IN) and reverse osmosis filtered water ad libitum. At 25–27 months of age, the cross-sectional cohort was harvested using a randomized block design.
Sera analysis
Whole blood was isolated from the submandibular vein and centrifuged (2500×g for 20 min at 4 °C), sera removed, and frozen at −80 °C until further analysis. IGF-1, GH, and adiponectin concentrations were quantified using Mouse IGF-1 Quantikine ELISA kit (R&D Systems, Minneapolis, MN), GH ELISA kit (EMD Millipore, Billerica, MA), and adiponectin ELISA kit (EMD). All analytes were measured in duplicate following the manufacturers’ recommendations.
RNA analysis
Gene expression for specific targets was quantified using the 7900HT Fast-Time PCR System and QuantStudio 12k Flex (Applied Biosystems, Life Technologies, Waltham, MA). RNA was purified from the liver and hippocampus using the RNeasy kit, and cDNA was prepared using Superscript III (Life Technologies). Microfluidic cards containing the target primers were purchased from Life Technologies. All data were normalized to the geometric mean of three housekeeping genes, HPRT, YWHAZ, and β-Actin.
Body composition
Lean, fat, and fluid mass were quantified in animals using the Bruker Whole-Body Composition TD-NMR Analyzer (Billerica, MA).
Grip strength
Front- and four-paw grip strength were assessed using a horizontal digital force gauge, Chatillon DFIS2 (Ametek, Largo FL). Mean and maximal performance over three independent trials were compared.
Gait analysis
Gait analysis was measured using the Noldus CatWalk XT (Leesburg, VA). Mice were placed in the 30 × 7-cm arena and were allowed to walk freely on a fluorescently illuminated glass plate. Upon touch, the light is reflected, allowing for visualization of each footprint. Walking parameters were captured using a video camera mounted under the CatWalk. The run was successful when the animal walked across the arena without stopping or turning. A minimum of two successful runs was necessary for the animal to be included in the analysis. All data were acquired, compressed, cleaned, and analyzed using CatWalk XT software.
Barnes Maze
Spatial cognition was assessed using the Barnes Maze. Mice were acclimated to the escape box for 30 s on the day before training and subsequently given three training trials a day for 4 days. Each trial was 90 s long. Animals that did not reach the escape box were directed to the box after the trial ended. Path length, errors, average distance, and success rate were calculated using Noldus Ethovision software.
End-of-life pathology
All mice were inspected at least twice daily (between 0700 and 0900 hours and between 1400 and 1600 hours). Mice that died spontaneously were removed from the cage and necropsied, followed by preservation of tissues in 10% formalin. Approximately 2.9% (distributed equally across groups) of the mice exhibited severe autolysis and tissues from these animals were excluded from histopathological examination.
The following organs and tissues were excised for examination: brain, pituitary gland, heart, lung, thymus, aorta, stomach, intestine, liver, pancreas, spleen, kidneys, adrenal gland, urinary bladder, and reproductive system (male: testes, seminal vesicles; female: ovaries, uterus). Any other tissue in which lesions were observed by gross inspection were removed for further examination. Fixed tissues were embedded in paraffin, sectioned at 5 μm, and stained with hematoxylin-eosin. Diagnosis of each histopathological lesion was made using the classifications for aging mouse pathology previously described (Bronson and Lipman 1993; Ikeno et al. 2005). See Supplemental Experimental Methods for further pathology description.
Probable cause of death
The probable cause of death for each mouse was determined by the severity of the diseases found at necropsy, which was assessed independently by two pathologists blinded to the treatment groups. In the case of neoplastic lesions, grade 3 and 4 lesions were categorized as death by neoplastic disease. For non-neoplastic diseases, lesions that were severe, e.g., grade 4, associated with other histopathological changes (pleural effusion, ascites, etc.) were categorized as death by non-neoplastic disease. In more than 90% of the cases, there was agreement by the two pathologists. In cases where disease was considered not severe enough, the cause of death was categorized as unknown.
Statistical analysis
Two independent biostatisticians assisted with statistical analyses in this study. Aside from the survival analysis (described below), data are presented as mean ± SEM. Analysis and figure preparation was performed using SigmaPlot version 11 (Systat Software, San Jose, CA), JMP 11 (SAS, Cary, NC), and SAS. Each animal was classified as a unit of analysis. No differences between the lifespan data for the individual control groups (WT10d, GFP5m, GFP15m) were observed; thus, the three control groups were pooled and collectively used as the control group. Pairwise multivariate analysis was performed on the entire dataset. When relevant, one-way ANOVA with post hoc Bonferonni testing was used to identify differences between the multiple treatment groups and aged or young controls. Chi-squared analysis was used to compare the percentages of animals in each group with the identified pathology. A p value less than 0.05 was considered statistically significant.
Cox proportional hazard model fitting, log rank testing for differences between Kaplan-Meier survival curves, and Boschloo testing for difference in extreme survival were all performed on the survival data with specific functions implemented in the R package survival. Using the distributions of ages at death, we tested among competing models for acceleration in the rate of increase in mortality based on the two-parameter Gompertz model as implemented in the R package eha. Quantile regression, as described in Koenker and implemented in the R package quantreg, was used to compute and compare mean, 30, 50, and 90% survival times for each group (Koenker 2008). Time until cancer-induced death was studied with similar survival techniques, treating deaths from other causes as censored observations.
Electronic supplementary material
(PDF 991 kb)
Acknowledgements
This study could not have been performed without the support of Dr. Shoshanna Yakar, who provided the mouse strain used to derive these cohorts. The authors would also like to thank the staff of the Rodent Barrier Facility at the University of Oklahoma Health Sciences Center, especially Bonita Johnson, for superb animal care throughout this study, as well as Jessica Landoll, Eileen Parks, Alex Yeganeh, and Dr. Arlan Richardson for their scientific input. This work was supported by grants from the National Institute on Aging (R01-AG038747 to W.E.S, R01-AG047879 to A.C., F32AG048728 to N.M.A), the American Federation for Aging Research (AFAR Breakthroughs in Gerontology Award to W.E.S.), the Ellison Medical Research Foundation Senior Scholar Award (to W.E.S.), the Arkansas Claude Pepper Older Americans Independence Center at University of Arkansas Medical Center (to Z.U.; P30 AG028718), the Oklahoma Center for the Advancement of Science and Technology (to A.C., Z.U., W.E.S.), the San Antonio Nathan Shock Center Pathology Core (P30 AG013319), the Oklahoma Nathan Shock Center of Excellence in the Biology of Aging (P30 AG050911), and the Donald W. Reynolds Foundation.
References
- Amato G, et al. Body composition, bone metabolism, and heart structure and function in growth hormone (GH)-deficient adults before and after GH replacement therapy at low doses. J Clin Endocrinol Metab. 1993;77:1671–1676. doi: 10.1210/jcem.77.6.8263158. [DOI] [PubMed] [Google Scholar]
- Arum O, Rickman DJ, Kopchick JJ, Bartke A. The slow-aging growth hormone receptor/binding protein gene-disrupted (GHR-KO) mouse is protected from aging-resultant neuromusculoskeletal frailty. Age. 2014;36:117–127. doi: 10.1007/s11357-013-9551-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashpole NM, et al. IGF-1 regulates vertebral bone aging through sex-specific and time-dependent mechanisms. J Bone Miner Res Off J Am Soc Bone Miner Res. 2015 doi: 10.1002/jbmr.2689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashpole NM, et al. Differential effects of IGF-1 deficiency during the life span on structural and biomechanical properties in the tibia of aged mice. Age. 2016;38:38. doi: 10.1007/s11357-016-9902-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashpole NM, et al. IGF-1 regulates vertebral Bone aging through sex-specific and time-dependent mechanisms. J Bone Miner Res Off J Am Soc Bone Miner Res. 2016;31:443–454. doi: 10.1002/jbmr.2689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austad SN, Fischer KE. Sex differences in lifespan. Cell Metab. 2016;23:1022–1033. doi: 10.1016/j.cmet.2016.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey-Downs LC, et al. Liver-specific knockdown of IGF-1 decreases vascular oxidative stress resistance by impairing the Nrf2-dependent antioxidant response: a novel model of vascular aging. J Gerontol A Biol Sci Med Sci. 2012;67:313–329. doi: 10.1093/gerona/glr164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey-Downs LC, et al. Growth hormone and IGF-1 deficiency exacerbate high-fat diet-induced endothelial impairment in obese Lewis dwarf rats: implications for vascular aging. J Gerontol A Biol Sci Med Sci. 2012;67:553–564. doi: 10.1093/gerona/glr197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bansal A, Zhu LJ, Yen K, Tissenbaum HA. Uncoupling lifespan and healthspan in Caenorhabditis elegans longevity mutants. Proc Natl Acad Sci U S A. 2015;112:E277–E286. doi: 10.1073/pnas.1412192112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartke A, Brown-Borg H. Life extension in the dwarf mouse. Curr Top Dev Biol. 2004;63:189–225. doi: 10.1016/S0070-2153(04)63006-7. [DOI] [PubMed] [Google Scholar]
- Bartke A, et al. Growth hormone and aging. Journal of the American Aging Association. 2000;23:219–225. doi: 10.1007/s11357-000-0021-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bokov AF et al. (2011) Does reduced IGF-1R signaling in Igf1r(+/−) mice alter aging? Plos ONE 6:e26891. doi:10.1371/journal.pone.0026891 [DOI] [PMC free article] [PubMed]
- Bronson RT, Lipman RD. The role of pathology in rodent experimental gerontology. Aging-Clin Exp Res. 1993;5:253–257. doi: 10.1007/BF03324169. [DOI] [PubMed] [Google Scholar]
- Brown-Borg HM. The somatotropic axis and longevity in mice. Am J Phys Endocrinol Metab. 2015;309:E503–E510. doi: 10.1152/ajpendo.00262.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown-Borg HM, Borg KE, Meliska CJ, Bartke A. Dwarf mice and the ageing process. Nature. 1996;384:33. doi: 10.1038/384033a0. [DOI] [PubMed] [Google Scholar]
- Coschigano KT, Clemmons D, Bellush LL, Kopchick JJ. Assessment of growth parameters and life span of GHR/BP gene-disrupted mice. Endocrinology. 2000;141:2608–2613. doi: 10.1210/endo.141.7.7586. [DOI] [PubMed] [Google Scholar]
- Coschigano KT, Holland AN, Riders ME, List EO, Flyvbjerg A, Kopchick JJ. Deletion, but not antagonism, of the mouse growth hormone receptor results in severely decreased body weights, insulin, and insulin-like growth factor I levels and increased life span. Endocrinology. 2003;144:3799–3810. doi: 10.1210/en.2003-0374. [DOI] [PubMed] [Google Scholar]
- De Boer H, Blok GJ, Voerman HJ, De Vries PM, van der Veen EA. Body composition in adult growth hormone-deficient men, assessed by anthropometry and bioimpedance analysis. J Clin Endocrinol Metab. 1992;75:833–837. doi: 10.1210/jcem.75.3.1517374. [DOI] [PubMed] [Google Scholar]
- Eriksson JG, Forsen TJ, Kajantie E, Osmond C, Barker DJ. Childhood growth and hypertension in later life. Hypertension. 2007;49:1415–1421. doi: 10.1161/HYPERTENSIONAHA.106.085597. [DOI] [PubMed] [Google Scholar]
- Eriksson JG, Forsen T, Tuomilehto J, Winter PD, Osmond C, Barker DJ. Catch-up growth in childhood and death from coronary heart disease: longitudinal study. BMJ. 1999;318:427–431. doi: 10.1136/bmj.318.7181.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong Z, et al. Reductions in serum IGF-1 during aging impair health span. Aging Cell. 2014;13:408–418. doi: 10.1111/acel.12188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison DE, et al. Acarbose, 17-alpha-estradiol, and nordihydroguaiaretic acid extend mouse lifespan preferentially in males. Aging Cell. 2014;13:273–282. doi: 10.1111/acel.12170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hascup KN, et al. Enhanced cognition and hypoglutamatergic signaling in a growth hormone receptor knockout mouse model of successful aging. J Gerontol A Biol Sci Med Sci. 2016 doi: 10.1093/gerona/glw088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herenu CB, Cristina C, Rimoldi OJ, Becu-Villalobos D, Cambiaggi V, Portiansky EL, Goya RG. Restorative effect of insulin-like growth factor-I gene therapy in the hypothalamus of senile rats with dopaminergic dysfunction. Gene Ther. 2007;14:237–245. doi: 10.1038/sj.gt.3302870. [DOI] [PubMed] [Google Scholar]
- Holzenberger M, et al. IGF-1 receptor regulates lifespan and resistance to oxidative stress in mice. Nature. 2003;421:182–187. doi: 10.1038/nature01298. [DOI] [PubMed] [Google Scholar]
- Ikeno Y, Bronson RT, Hubbard GB, Lee S, Bartke A. Delayed occurrence of fatal neoplastic diseases in Ames dwarf mice: correlation to extended longevity. J Gerontol a-Biol. 2003;58:291–296. doi: 10.1093/gerona/58.4.B291. [DOI] [PubMed] [Google Scholar]
- Ikeno Y, Hubbard GB, Lee S, Richardson A, Strong R, Diaz V, Nelson JF. Housing density does not influence the longevity effect of calorie restriction. J Gerontol a-Biol. 2005;60:1510–1517. doi: 10.1093/gerona/60.12.1510. [DOI] [PubMed] [Google Scholar]
- Ikeno Y, et al. Reduced incidence and delayed occurrence of fatal neoplastic diseases in growth hormone receptor/binding protein knockout mice. J Gerontol A Biol Sci Med Sci. 2009;64:522–529. doi: 10.1093/gerona/glp017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson JO, Fowelin J, Landin K, Lager I, Bengtsson BA. Growth hormone-deficient adults are insulin-resistant. Metab Clin Exp. 1995;44:1126–1129. doi: 10.1016/0026-0495(95)90004-7. [DOI] [PubMed] [Google Scholar]
- Kajantie E, Osmond C, Barker DJ, Forsen T, Phillips DI, Eriksson JG. Size at birth as a predictor of mortality in adulthood: a follow-up of 350 000 person-years. Int J Epidemiol. 2005;34:655–663. doi: 10.1093/ije/dyi048. [DOI] [PubMed] [Google Scholar]
- Koenker R. Censored quantile regression redux. J Stat Softw. 2008;27:1–25. doi: 10.18637/jss.v027.i06. [DOI] [Google Scholar]
- Ladiges W, Van Remmen H, Strong R, Ikeno Y, Treuting P, Rabinovitch P, Richardson A. Lifespan extension in genetically modified mice. Aging Cell. 2009;8:346–352. doi: 10.1111/j.1474-9726.2009.00491.x. [DOI] [PubMed] [Google Scholar]
- Markowska AL, Mooney M, Sonntag WE. Insulin-like growth factor-1 ameliorates age-related behavioral deficits. Neuroscience. 1998;87:559–569. doi: 10.1016/S0306-4522(98)00143-2. [DOI] [PubMed] [Google Scholar]
- Merola B, et al. Cardiac structural and functional abnormalities in adult patients with growth hormone deficiency. J Clin Endocrinol Metab. 1993;77:1658–1661. doi: 10.1210/jcem.77.6.8263155. [DOI] [PubMed] [Google Scholar]
- Miller RA, et al. An Aging Interventions Testing Program: study design and interim report. Aging Cell. 2007;6:565–575. doi: 10.1111/j.1474-9726.2007.00311.x. [DOI] [PubMed] [Google Scholar]
- Miller RA, et al. Rapamycin, but not resveratrol or simvastatin, extends life span of genetically heterogeneous mice. J Gerontol A Biol Sci Med Sci. 2011;66:191–201. doi: 10.1093/gerona/glq178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller RA, et al. Rapamycin-mediated lifespan increase in mice is dose and sex dependent and metabolically distinct from dietary restriction. Aging Cell. 2014;13:468–477. doi: 10.1111/acel.12194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milman S, Atzmon G, Huffman DM, Wan J, Crandall JP, Cohen P, Barzilai N. Low insulin-like growth factor-1 level predicts survival in humans with exceptional longevity. Aging Cell. 2014;13:769–771. doi: 10.1111/acel.12213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osmond C, Kajantie E, Forsen TJ, Eriksson JG, Barker DJ. Infant growth and stroke in adult life: the Helsinki birth cohort study. Stroke; a journal of cerebral circulation. 2007;38:264–270. doi: 10.1161/01.STR.0000254471.72186.03. [DOI] [PubMed] [Google Scholar]
- Podlutsky A, et al. The GH/IGF-1 axis in a critical period early in life determines cellular DNA repair capacity by altering transcriptional regulation of DNA repair-related genes: implications for the developmental origins of cancer. Geroscience. 2017 doi: 10.1007/s11357-017-9966-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radaelli E, Arnold A, Papanikolaou A, Garcia-Fernandez RA, Mattiello S, Scanziani E, Cardiff RD. Mammary tumor phenotypes in wild-type aging female FVB/N mice with pituitary prolactinomas. Vet Pathol. 2009;46:736–745. doi: 10.1354/vp.08-VP-0280-R-FL. [DOI] [PubMed] [Google Scholar]
- Ramsey MM, Weiner JL, Moore TP, Carter CS, Sonntag WE. Growth hormone treatment attenuates age-related changes in hippocampal short-term plasticity and spatial learning. Neuroscience. 2004;129:119–127. doi: 10.1016/j.neuroscience.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Richardson A, Liu F, Adamo ML, Van Remmen H, Nelson JF. The role of insulin and insulin-like growth factor-I in mammalian ageing. Best Pract Res Clin Endocrinol Metab. 2004;18:393–406. doi: 10.1016/j.beem.2004.02.002. [DOI] [PubMed] [Google Scholar]
- Rincon M, Rudin E, Barzilai N. The insulin/IGF-1 signaling in mammals and its relevance to human longevity. Exp Gerontol. 2005;40:873–877. doi: 10.1016/j.exger.2005.06.014. [DOI] [PubMed] [Google Scholar]
- Sonntag WE, Csiszar A, deCabo R, Ferrucci L, Ungvari Z. Diverse roles of growth hormone and insulin-like growth factor-1 in mammalian aging: progress and controversies. J Gerontol A Biol Sci Med Sci. 2012;67:587–598. doi: 10.1093/gerona/gls115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonntag WE, Lynch CD, Cefalu WT, Ingram RL, Bennett SA, Thornton PL, Khan AS. Pleiotropic effects of growth hormone and insulin-like growth factor (IGF)-1 on biological aging: inferences from moderate caloric-restricted animals. J Gerontol A Biol Sci Med Sci. 1999;54:B521–B538. doi: 10.1093/gerona/54.12.B521. [DOI] [PubMed] [Google Scholar]
- Sonntag WE, Lynch C, Thornton P, Khan A, Bennett S, Ingram R. The effects of growth hormone and IGF-1 deficiency on cerebrovascular and brain ageing. J Anat. 2000;197(Pt 4):575–585. doi: 10.1017/S002187829900713X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonntag WE, et al. Adult-onset growth hormone and insulin-like growth factor I deficiency reduces neoplastic disease, modifies age-related pathology, and increases life span. Endocrinology. 2005;146:2920–2932. doi: 10.1210/en.2005-0058. [DOI] [PubMed] [Google Scholar]
- Suh Y, et al. Functionally significant insulin-like growth factor I receptor mutations in centenarians. Proc Natl Acad Sci U S A. 2008;105:3438–3442. doi: 10.1073/pnas.0705467105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth P, et al. IGF-1 deficiency impairs cerebral myogenic autoregulation in hypertensive mice. Journal of cerebral blood flow and metabolism: official journal of the International Society of Cerebral Blood Flow and Metabolism. 2014;34:1887–1897. doi: 10.1038/jcbfm.2014.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth P, et al. IGF-1 deficiency impairs neurovascular coupling in mice: implications for cerebromicrovascular aging. Aging Cell. 2015 doi: 10.1111/acel.12372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dam PS, et al. Childhood-onset growth hormone deficiency, cognitive function and brain N-acetylaspartate. Psychoneuroendocrinology. 2005;30:357–363. doi: 10.1016/j.psyneuen.2004.10.002. [DOI] [PubMed] [Google Scholar]
- van Heemst D, et al. Reduced insulin/IGF-1 signalling and human longevity. Aging Cell. 2005;4:79–85. doi: 10.1111/j.1474-9728.2005.00148.x. [DOI] [PubMed] [Google Scholar]
- Xu J, Gontier G, Chaker Z, Lacube P, Dupont J, Holzenberger M. Longevity effect of IGF-1R(+/−) mutation depends on genetic background-specific receptor activation. Aging Cell. 2014;13:19–28. doi: 10.1111/acel.12145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakar S, Liu JL, Stannard B, Butler A, Accili D, Sauer B, LeRoith D. Normal growth and development in the absence of hepatic insulin-like growth factor I. Proc Natl Acad Sci U S A. 1999;96:7324–7329. doi: 10.1073/pnas.96.13.7324. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 991 kb)