Abstract
Despite recently reported associations between air pollution and acute psychiatric outcomes, the association with depression onset has not, to our knowledge, been previously examined. We conducted a prospective cohort study among 41,844 women in the Nurses’ Health Study, in the United States. The women had an average age of 66.6 (standard deviation, 7.6) years, were depression-free in 1996, and were followed through 2008. May–September ozone exposures were predicted by interpolating concentrations from the 5 nearest monitors. One-, 2-, and 5-year average concentrations of particulate matter with an aerodynamic diameter less than or equal to 2.5 μm (PM2.5) were predicted at each participant's residence using a spatiotemporal model. We defined depression as report of doctor's diagnosis or use of antidepressant medication. We estimated adjusted hazard ratios with time-varying Cox models. Hazard ratios for both pollutants were elevated (per 10-parts-per-billion increase in ozone, hazard ratio (HR) = 1.06; 95% confidence interval (CI): 1.00, 1.12; per 10-μg/m3 increase in 1-year PM2.5, HR = 1.08; 95% CI: 0.97, 1.20). Associations were stronger when only antidepressant use was used to define cases (for ozone, HR = 1.08; 95% CI: 1.02, 1.14; for PM2.5, HR = 1.12; 95% CI: 1.00, 1.25). To our knowledge, these results represent the first identification of a possible association between both long-term ozone and PM2.5 exposure and depression onset. Although the stronger association specifically with antidepressant use may reflect that this endpoint better captures the onset time and milder cases, our findings should be interpreted with caution.
Keywords: air pollution, depression onset, Nurses’ Health Study, ozone, particulate matter
Major depressive disorder has consistently been identified as a key contributor to the global disease burden and has increased in prevalence (1). The disability-adjusted life years attributable to major depressive disorder increased by 37% between 1990 and 2010, and major depressive disorder was the 11th leading cause of disability-adjusted life years globally and the 5th in North America (1). Depression has also been linked to decreased work productivity and lost labor time (2, 3) and lower quality of life (4). In the United States, the age-standardized prevalence of depression was 9.1% in 2008, with higher estimates in the Southeast (5). The prevalence of depression and depressive symptoms of clinical significance is greater among women (5, 6).
Recent studies have reported adverse associations between air pollution exposures and the nervous system (7) and decreased cognitive function (8, 9). More recently, there has been increasing interest in whether air pollution affects mental health (10), and air pollution exposures have been found to cause depression-like behaviors in animal studies (11, 12). A few studies have reported associations between air pollution and suicide (13, 14) or elevated anxiety (15). Consistent with these findings, many time-series studies examining acute associations have reported increased depression-related hospital admissions with increasing pollution levels (16–18). The associations between short-term air pollution exposures and depressive symptoms specifically, and not depression-related admissions, however, have not been consistent (19, 20). On the other hand, there is reason to suspect that longer-term air pollution exposure would be of relevance for depression.
Extensive experimental and epidemiologic data over several decades indicate an association between longer-term exposure to air pollution and cardiovascular disease, stroke, and cardiovascular risk factors such as local and systemic inflammation, increased blood viscosity and pressure, atherosclerosis, and impaired vascular tone (21–24). Many of these cardiovascular risk factors, in turn, have been found to predict depression (25, 26). To our knowledge, the association between long-term air pollution exposure and onset of depression has not yet been examined. Identification of modifiable risk factors for depression onset, such as air pollution, would provide the basis for targeted regulations and interventions.
In the current study, we investigated the association between exposures to summer ozone and particulate matter with an aerodynamic diameter less than or equal to 2.5 μm (PM2.5) over the prior few years and onset of depression among a nationwide cohort of mid-life and older women, between 1996 and 2008.
METHODS
Data collection
Study population
The Nurses’ Health Study (NHS) is a prospective cohort of women; in 1976, 121,701 married registered nurses between the ages of 30 and 55, living in 11 states, were enrolled. As of the mid-1990s, at least 10 participants lived in each of the 48 continental states. Biennial questionnaires are mailed to all participants, with a response rate >90% for each cycle (27). Questions regarding antidepressant use were first included on the 1996 questionnaire (baseline for this analysis); the 5-item Mental Health Inventory was first included in 1992 and was repeated in 1996 and 2000. In 2000, participants were asked whether they had ever received a depression diagnosis by a physician and, if so, the year of the diagnosis (1996 or earlier, 1997–1998, 1999, or 2000 or after).
In order to exclude women with depression prior to 1996 (baseline), we excluded participants who had reported antidepressant use in 1996 or earlier or who had an abbreviated Mental Health Inventory score ≤52 (indicative of severe depression symptoms) on either the 1992 or 1996 questionnaire. We further excluded women who, when asked on the 2000 questionnaire, either reported physician-diagnosed depression prior to 1996 or were missing information on their depression history. We further excluded women with incomplete information regarding their depressive symptoms in 1992 or 1996. Our baseline population, therefore, consisted of women with complete depression and exposure information between 1996 and 2000 and who were free of depression at baseline. Information about diagnosis by a physician/clinician and use of antidepressant medication was updated every 2 years thereafter.
High prevalence of depression has been reported among patients with several conditions, such as cancer (28, 29), myocardial infarction (MI) (30, 31), and stroke (32, 33). Given the association between air pollution exposure and these outcomes (23, 34, 35) and to reduce potential outcome misclassification, we censored subjects that developed any of these conditions during follow-up in a time-varying manner, (i.e., at the cycle before the first report of these conditions).
Our analyses were approved by the Institutional Review Board of the Brigham and Women's Hospital and the Human Subjects Committee of the Harvard T.H. Chan School of Public Health. The nurses provided implied informed consent by completion and return of each questionnaire.
Outcome definition
We defined as depression onset the first report of either a physician diagnosis or use of antidepressant medication, as previously described for this cohort (36, 37). In secondary analyses to determine whether our results varied by case definition, we repeated analyses using only the first report of doctor diagnosis or the first report of use of antidepressant medication, separately, to define incident cases. For each of these separate analyses we allowed for overlap across definitions (e.g., in the analyses using antidepressant medication use as the outcome, we did not censor subjects that received a doctor's diagnosis prior to antidepressant use). We also ran sensitivity analyses in which we did censor subjects who reported a doctor's diagnosis before use of antidepressant medication when antidepressant use was the outcome (and vice versa).
Exposure assessment
All residential mailing addresses for NHS participants, updated every 2 years, have been geocoded to obtain latitude and longitude. To assess exposure to PM2.5, we predicted monthly PM2.5 concentrations at the residence of each participant, using a nationwide spatiotemporal model (38). Briefly, this model uses a generalized additive model to predict monthly PM2.5 concentrations based on data from governmental and research monitoring networks together with geographic information system–based covariates, such as population density, proximity to nearest roads, urban land use, elevation, weather variables, and point-source emissions. We calculated time-varying 1-, 2-, and 5-year average PM2.5 exposures.
To assess exposure to ozone, we used monitoring data from the US Environmental Protection Agency's Air Quality System database (39). Data from up to 5 monitors were used to estimate monthly averaged ozone concentrations at each participant's residential address. We included participants with at least 1 monitor within 50 km (30 mi) of the participant's house (22), and we allowed monitors within 200 km (120 mi) to contribute information. We then calculated a weighted monthly average from these monitors for each participant's home, using as weights the squares of the distances between the home and each of the monitors. We were only able to assess ozone concentrations averaged across the summer months (May–September) as time-varying exposures, because ozone concentrations are not available throughout the year at many locations.
Both ozone and PM2.5 time-varying exposures were assigned at the beginning of each 2-year cycle during which case status was determined.
Covariates
Information on potential confounders is available at each cycle (every 2 years), and information on diet is collected every 4 years. Covariate information was reassigned for all participants at the beginning of each 2-year cycle. Specifically, we adjusted for calendar year and month at questionnaire return (to control for long-term and seasonal trends), census region (Northeast, Midwest, West, and South), and living in a Metropolitan Statistical Area (yes/no). Further, we adjusted for other variables that could also act as potential confounders or surrogates for known confounders such as socioeconomic status, which we selected using directed acyclic graphs (DAGs) (40). Specifically, we controlled for race (white: yes/no), physical activity (metabolic equivalent hours/week), body mass index (calculated as weight (kg)/height (m)2), pack-years of smoking (as number of packs/day times the number of years of cigarette smoking, continuous), smoking status (current/former/never), dietary habits (using a continuous cumulative summary score based on the Alternate Healthy Eating Index, which incorporates alcohol consumption (41)), multivitamin intake (yes/no), participation in social groups (yes/no), and baseline abbreviated Mental Health Inventory score (continuous). To adjust for individual-level socioeconomic status, we included information on the educational level of the participants, the education of both their parents when the participant was 16 years old, marital status, and husband's education, if applicable. To adjust for community-level socioeconomic status, we used information from the US Census 2000 on tract-level median household income and house value as well as population density. We used missing indicators to handle missing covariate observations. Other than 14% missing observations for husband's education, which includes observations of nonmarried participants, all covariates had ≤1% missing values. All reported results refer to models adjusting for the above covariates unless otherwise specified.
Data analysis
We used time-varying Cox proportional hazards models, using proc phreg in SAS, version 9.3 (SAS Institute, Inc., Cary, North Carolina), to assess the association between ozone and PM2.5 exposures and onset of depression, using questionnaire cycles since baseline as the time metameter. Participants contributed person-time starting in June 1996 until depression onset, death, loss to follow-up, or end of follow-up (May 2008), whichever occurred earliest. We estimated hazard ratios and 95% confidence intervals, stratified by age in years.
We assessed potential deviations from linearity in the outcome-exposure association using penalized splines. To examine potentially varying associations by region (due to regional differences both in particle composition (42) and depression prevalence (5)), we also examined potential effect modification by census region. Finally, to account for any potential copollutant confounding, we repeated analyses including both ozone and 1-year PM2.5 exposures in the model simultaneously.
Cardiovascular-related disorders could be mediators in the causal pathway between air pollution and depression. In a sensitivity analysis, therefore, we included in our models self-reported hypertension, hypercholesterolemia, physician-diagnosed diabetes, and family history of MI to assess the impact of their inclusion in the estimated associations. We also repeated analyses without censoring subjects for cancer, MI, or stroke.
All hazard ratios are presented per 10-parts-per-billion (ppb) increase in ozone concentration or per 10-μg/m3 increase in PM2.5 concentration, for comparison with other air pollution studies. All statistical analyses were conducted using SAS, version 9.3 (SAS Institute, Inc.), and R, version 3.0.3 (R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
We identified 41,844 NHS participants that satisfied our criteria for inclusion in the baseline population in 1996. The average age of the participants was 66.6 (standard deviation (SD), 7.6) years. During follow-up, we identified 5,003 incident cases (12.0%) who reported either receiving a doctor's diagnosis or initiating use of antidepressant medication. The average summer ozone concentration was 31.9 (SD, 5.3) ppb and the average 1-year PM2.5 was 12.6 (SD, 2.9) μg/m3 (Table 1). We observed a low correlation between ozone and PM2.5 (r = 0.13). We observed a decrease in PM2.5 concentrations during follow-up (from 13.1 (SD, 3.0) μg/m3 in 1996 to 11.7 (SD, 2.5) μg/m3 in 2008). We did not observe a decreasing trend in ozone concentrations (30.9 (SD, 4.9) ppb in 1996 and 31.9 (SD, 4.4) ppb in 2008).
Table 1.
Selected Characteristics of Study Participants (n = 41,844), Nurses’ Health Study, United States, 1996–2008
| Characteristic | Mean (SD) | % |
|---|---|---|
| Age, years | 66.6 (7.6) | |
| BMIa | 26.2 (5.1) | |
| Smoking, pack-yearsb | 11.5 (18.2) | |
| AHEI score | 207.6 (64.1) | |
| Summerc ozone concentration, ppb | 31.9 (5.3) | |
| 1-year PM2.5 concentration, μg/m3 | 12.6 (2.9) | |
| White | 95 | |
| Smoking status | ||
| Never smoker | 47 | |
| Former smoker | 45 | |
| Current smoker | 8 | |
| Physical activity, MET-hours/week | ||
| <3.0 | 18 | |
| 3.0–8.9 | 21 | |
| 9.0–17.9 | 22 | |
| 18.0–26.9 | 14 | |
| ≥27.0 | 25 | |
| Registered nurse degree | 93 | |
| Married | 84 | |
| Husband's educationd | ||
| Less than high school | 5 | |
| High school graduate | 33 | |
| Beyond high school | 48 | |
| Hypertension | 35 | |
| Hypercholesterolemia | 38 | |
| Diabetes | 5 | |
| Use of antianxiety medication | 40 | |
| Living in MSA | 93 |
Abbreviations: AHEI, Alternative Healthy Eating Index; BMI, body mass index; MET, metabolic equivalent of task; MSA, Metropolitan Statistical Area; PM2.5, particulate matter with an aerodynamic diameter less than or equal to 2.5 μm; ppb, parts per billion; SD, standard deviation.
a BMI was calculated as weight (kg)/height (m)2.
b Among ever smokers.
c Summer was defined as May–September.
d For husband's education, 14% of observations were missing; ≤1% were missing for the other covariates.
We observed an increase in reported incident cases over the number of participants at risk in the first cycles (from 2.5% in 1998 to 3.2% in 2002) and a decrease after that (1.9% in 2008). We observed regional differences in the cumulative incidence across regions, ranging from 10.7% in the Northeast to 12.5% in the Midwest. The pollutant concentrations also varied across regions, with highest levels in the Midwest and lowest levels in the South for both ozone and PM2.5 (Table 2).
Table 2.
Incident Cases and Pollutant Concentrations According to Census Region, Nurses’ Health Study, United States, 1996–2008
| Outcome | Northeast | Midwest | West | South | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | % | Mean (SD) | No. | % | Mean (SD) | No. | % | Mean (SD) | No. | % | Mean (SD) | |
| Antidepressant use or depression diagnosis | 2,349 | 10.7 | 959 | 12.5 | 769 | 12.1 | 926 | 11.9 | ||||
| Depression diagnosis | 1,126 | 5.7 | 464 | 6.0 | 370 | 5.8 | 445 | 5.6 | ||||
| Use of antidepressant medication | 2,125 | 9.7 | 867 | 11.3 | 687 | 10.8 | 837 | 10.7 | ||||
| Ozone, ppb | 32.1 (4.2) | 32.6 (3.8) | 31.6 (8.4) | 30.6 (5.8) | ||||||||
| PM2.5, μg/m3 | 12.6 (2.4) | 13.9 (2.2) | 12.2 (4.7) | 11.6 (2.4) | ||||||||
Abbreviations: PM2.5, particulate matter with an aerodynamic diameter less than or equal to 2.5 μm; ppb, parts per billion; SD, standard deviation.
In our main analysis, defining depression onset as the first report of either doctor diagnosis or antidepressant use, we observed increased hazard ratios for exposures to both ozone and PM2.5 (Table 3). We observed similar hazard ratios for 1- and 2-year PM2.5 exposures, but the association with 5-year PM2.5 exposure was attenuated. We observed no deviations from linearity for any exposure in analyses using penalized splines.
Table 3.
Hazard Ratios for Depression-Related Outcomes According to Per-Increment Increase in Ozone or Fine Particulate Matter Exposure, Nurses’ Health Study, United States, 1996–2008
| Outcome | No. of Cases | Person-Years | Adjustment | Per 10-ppb Increase in Summera Ozone Concentration | Per 10-μg/m3 Increase in PM2.5 Concentration | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1-year | 2-year | 5-yearb | |||||||||
| HR | 95% CI | HR | 95% CI | HR | 95% CI | HR | 95% CI | ||||
| Antidepressant use or depression diagnosis | 5,003 | 376,306 | Cruded | 1.08 | 1.02, 1.14 | 1.02 | 0.92, 1.12 | 1.03 | 0.93, 1.13 | 1.02 | 0.92, 1.12 |
| Adjustede | 1.06 | 1.00, 1.12 | 1.08 | 0.97, 1.20 | 1.08 | 0.97, 1.20 | 1.07 | 0.95, 1.19 | |||
| Depression diagnosisc | 2,405 | 391,117 | Cruded | 1.02 | 0.95, 1.10 | 0.92 | 0.80, 1.06 | 0.94 | 0.82, 1.09 | 0.92 | 0.79, 1.06 |
| Adjustede | 1.00 | 0.92, 1.08 | 0.98 | 0.84, 1.15 | 1.00 | 0.85, 1.17 | 0.97 | 0.83, 1.14 | |||
| Use of antidepressant medicationc | 4,516 | 379,605 | Cruded | 1.10 | 1.04, 1.16 | 1.05 | 0.95, 1.17 | 1.05 | 0.95, 1.17 | 1.03 | 0.93, 1.15 |
| Adjustede | 1.08 | 1.02, 1.14 | 1.12 | 1.00, 1.25 | 1.11 | 0.99, 1.25 | 1.09 | 0.97, 1.23 | |||
Abbreviations: CI, confidence interval; HR; hazard ratio; PM2.5, particulate matter with an aerodynamic diameter less than or equal to 2.5 μm; ppb, parts per billion.
a Summer was defined as May–September.
b For 5-year PM2.5 concentration, the person-years were 376,254, 391,063, and 379,553 for antidepressant use or depression diagnosis, depression diagnosis only, and use of antidepressant medication only, respectively.
c Doctor's diagnosis and antidepressant use were not assumed to be mutually exclusive. When we repeated analyses looking at doctor diagnoses and censoring subjects who reported prior antidepressant use (and vice versa), the results remained the same.
d Crude effect estimates are adjusted for age and questionnaire cycle.
e Adjusted effect estimates were additionally adjusted for calendar year, month at questionnaire return, census region, living in a Metropolitan Statistical Area, race, physical activity, body mass index, pack-years of smoking, smoking status, dietary habits, participation in social groups, baseline abbreviated Mental Health Inventory score, educational level, parental education, marital status, husband's education (if applicable), tract-level median income, house value, and population density.
When we included ozone and 1-year PM2.5 in the same model, the results for ozone did not change, but the PM2.5 effect estimates were slightly attenuated. Specifically, we found a hazard ratio of 1.06 (95% confidence interval (CI): 1.00, 1.12) per 10-ppb increase in ozone and a hazard ratio of 1.06 (95% CI: 0.95, 1.18) per 10-μg/m3 increase in PM2.5.
Regional analyses
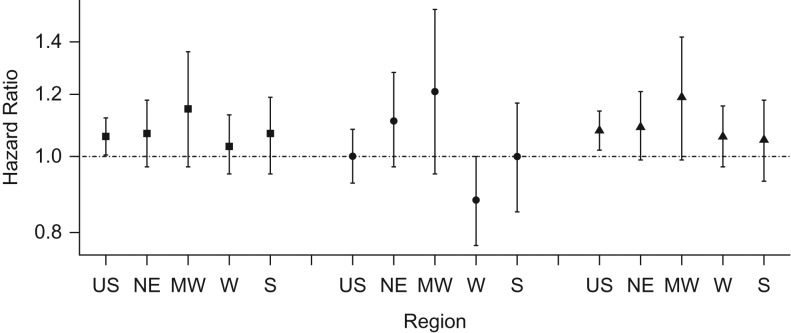
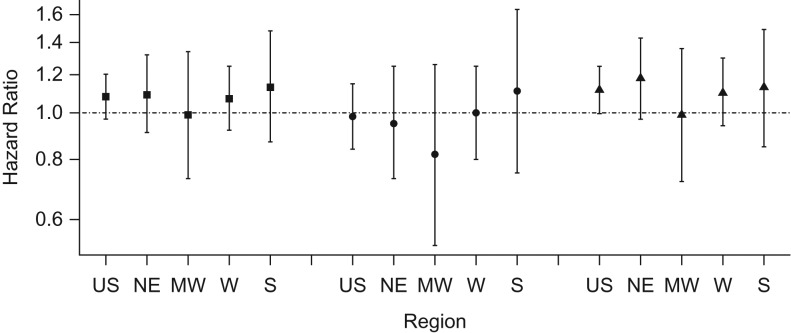
We observed no evidence of effect modification by census region for any exposure (Figures 1 and 2). For ozone exposures, the highest hazard ratio was observed in the Midwest (per 10-ppb increase, hazard ratio = 1.15; 95% CI: 0.97, 1.36), while for 1-year PM2.5 the highest was in the South (per 10-μg/m3 increase, hazard ratio = 1.13; 95% CI: 0.87, 1.48).
Figure 1.
Adjusted hazard ratios and 95% confidence intervals per 10-parts-per-billion increase in ozone exposures across the United States and by region, Nurses’ Health Study, 1996–2008. Square: antidepressant use or doctor diagnosis; circle: doctor diagnosis only; triangle: antidepressant use only. MW, Midwest; NE, Northeast; S, South; US, United States; W, West.
Figure 2.
Adjusted hazard ratios and 95% confidence intervals per 10-μg/m3 increase in 1-year exposures to particulate matter with an aerodynamic diameter less than or equal to 2.5 μm across the United States and by region, Nurses’ Health Study, 1996–2008. Square: antidepressant use or doctor diagnosis; circle: doctor diagnosis only; triangle: antidepressant use only. MW, Midwest; NE, Northeast; S, South; US, United States; W, West.
Doctor's diagnosis
We also repeated analyses, assessing whether air pollution exposure was associated with first report of a doctor diagnosis or antidepressant use separately. We identified 2,405 (5.7%) incident cases for doctor's diagnosis and 4,516 (10.8%) for antidepressant use. The highest incidence was observed in the Midwest and the lowest in the Northeast for both doctor's diagnosis and antidepressant use (Table 2).
We observed no association between air pollution and first reported doctor's diagnosis (Table 3). We observed an effect modification by region for the association between ozone and doctor's diagnosis (P = 0.03, Figure 1). Although we observed some variation in the associations across regions according to PM2.5 exposure, these differences were small (Figure 2).
Antidepressant use
We observed the highest effect estimates when depression was defined as incident antidepressant use (Table 3). Specifically, we found a hazard ratio of 1.08 (95% CI: 1.02, 1.14) per 10-ppb increase in ozone and a hazard ratio of 1.12 (95% CI: 1.00, 1.25) per-10 μg/m3 increase in 1-year PM2.5. We observed no effect modification by region for either pollutant (Figures 1 and 2).
Sensitivity analyses
Adjusting for cardiovascular-related variables (hypertension, hypercholesterolemia, diabetes and family history of MI) or antianxiety medication use did not change the estimated hazard ratios (Appendix Table 1). Not censoring participants who had had stroke, MI, or cancer resulted in somewhat attenuated effect estimates (Appendix Table 2).
DISCUSSION
We conducted a nationwide, prospective cohort study to assess the association between air pollution exposures and onset of depression among middle-aged and older women. Our findings suggest modest increased risks for depression onset for both ozone and PM2.5 exposures. Although modest, increases of similar magnitude are commonly observed for long-term air pollution exposures and other outcomes, such as mortality (43, 44). Given the widespread exposure to ozone and PM2.5, even modest increases are of great population relevance. Although both the cumulative incidence and pollutant concentrations varied by census region, the observed hazard ratios did not vary across regions. Mutually adjusting for ozone and PM2.5 did not materially change the observed hazard ratios.
The association between air pollution and mood disorders and mental health has received increasing interest in the scientific literature. Two recent European studies have reported that increases in air pollution are negatively associated with self-reported happiness (45), with high related costs (46). Time-series studies of acute events have linked higher air pollution levels with increases in suicides (13) and depression-related admissions (18). Lim et al. (19) found PM10 and ozone exposures to be strongly associated with depressive symptoms among the elderly, while Wang et al. (20) reported a protective association between PM2.5 and depressive symptoms.
Findings from toxicological studies support our findings of an association between air pollution and depression. Fonken et al. (11) found that exposure to PM2.5 is associated with increased depression-like responses in mice, while Mokoena et al. (12) reported anxiety and depression-like behavior in rats following chronic ozone inhalation. Jones and Thomsen (47), in a review article, reported that increases in proinflammatory cytokines result in behaviors that are related to psychiatric disorders in animals, such as social withdrawal. Air pollution has been consistently associated with increases in proinflammatory markers in the blood (48). Air pollution exposures have also been found to increase systemic oxidative stress (49, 50), which in turn is known to play an important role in psychiatric disorders such as depression (51, 52).
In our results, the association with air pollution was apparent for incident use of antidepressant medication rather than for doctor's diagnosis of depression. Antidepressant use has significantly increased in the last 2 decades (53, 54). Moreover, there has been a significant increase in prescription of antidepressants by primary care physicians without a psychiatric diagnosis, from less than 60% of the total prescriptions for antidepressants in 1996 to 73% in 2007 (55, 56). Mojtabai and Olfson (56) reported that a very large, and still growing, proportion of prescriptions for antidepressant medication occurs during medical visits without giving an accompanying clinical psychiatric diagnosis, which they suggest is attributed to milder types of depression. To the extent that these prescriptions are capturing the onset of depression (among persons who get a doctor's diagnosis either later or not at all), then this could, at least in part, explain the observed null association with incident doctor's diagnosis and the weaker effect estimates in the analysis using the combined indicator of diagnosis or antidepressant use as the outcome. We cannot, however, rule out the possibility that this finding is due to chance. It is also possible that antidepressants are being prescribed for other conditions, and our results could reflect an association between air pollution and those other conditions. In our main analyses, however, we censored NHS participants who developed cancer, MI, or stroke. In the sensitivity analyses including these subjects, the observed effect estimates were attenuated. Associations between air pollution and these conditions, therefore, likely do not account for our findings.
Strengths of our study include the very large nationwide cohort, well-validated spatiotemporal models for PM2.5 exposure, and extensive individual-level covariate data. Nonetheless, our findings should be interpreted in light of some limitations. Outcome assessment was based on self-report and therefore some misclassification is likely both for doctor's diagnosis and antidepressant use. Any such misclassification, however, is expected to bias our results towards the null. Exposure measurement error is also likely and is expected to be higher for ozone, for which interpolated monitor concentrations were assigned for NHS participants, than for PM2.5, for which a spatiotemporal model was used and concentrations were predicted at each participant's residence (57). Exposure measurement error, however, is also expected to bias our results towards the null (57). An additional limitation is lack of information on the precise timing of depression onset in a 2-year cycle, potentially contributing to exposure error. Although residual confounding cannot be excluded, NHS is a rather homogeneous population with detailed information on individual- and community-level factors as well as lifestyle factors for which we have controlled in our analyses. Retirement status, which is available only in 1996 and 2000, could also be a confounder. However, air pollution levels were lower among retired participants in both these years (results not shown), and some data suggest that retirement is a possible risk factor for depression (58). Any resulting bias, thus, would likely bias our results downwards. Finally, our findings might not be generalizable to the general population; our cohort is composed of middle-aged and older women, among whom, in general, depression is more prevalent (5, 6).
In conclusion, we conducted a nationwide, prospective cohort study to assess the association between exposure to PM2.5 and ozone and depression onset. Our findings suggest that both ozone and PM2.5 exposures are potential risk factors for depression and, specifically, incident antidepressant use. Our results could lend further support for the role of air pollution in neurobehavioral disorders.
ACKNOWLEDGMENTS
Author affiliations: Department of Environmental Health, Harvard T.H. Chan School of Public Health, Boston, Massachusetts (Marianthi-Anna Kioumourtzoglou, Jaime E. Hart, Brent A. Coull, Francine Laden, Marc G. Weisskopf); Department of Epidemiology and Biostatistics, Milken Institute School of Public Health, George Washington University, Washington, DC (Melinda C. Power); Channing Division of Network Medicine, Department of Medicine at Brigham and Women's Hospital and Harvard Medical School, Boston, Massachusetts (Jaime E. Hart, Olivia I. Okereke, Francine Laden); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, Massachusetts (Olivia I. Okereke, Francine Laden, Marc G. Weisskopf); Department of Psychiatry, Brigham and Women's Hospital and Harvard Medical School, Boston, Massachusetts (Olivia I. Okereke); and Department of Biostatistics, Harvard T.H. Chan School of Public Health, Boston, Massachusetts (Brent A. Coull). M.A.K. is currently at the Department of Environmental Health Sciences, Mailman School of Public Health, Columbia University, New York, New York.
This work was supported by the National Institutes of Health (grant UM1 CA186107) and National Institute of Environmental Health Sciences (grants R21 ES019982, R01 ES017017, P30 ES000002, and P30 ES009089). M.A.K. was supported by a training grant from the National Institutes of Health (grant T32 ES007069) and M.C.P. by a training grant from the National Institute on Aging (grant T32 AG027668).
The funding agencies had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication. The authors were independent of the study funders.
Conflict of interest: none declared.
APPENDIX
Appendix Table 1.
Hazard Ratios From Sensitivity Analyses for Ozone and Fine Particulate Matter Exposures, Adjusted for Cardiovascular-Related Variables or Antianxiety Medications, Nurses’ Health Study, United States, 1996–2008
| Outcome | No. of Cases | Person-Years | Per 10-ppb Increase in Summera Ozone Concentration | Per 10-μg/m3 Increase in 1-year PM2.5 Concentration | ||
|---|---|---|---|---|---|---|
| HRb | 95% CI | HRb | 95% CI | |||
| Adjusted for Hypertension, Hypercholesterolemia, Diabetes, and Family History of Myocardial Infarction | ||||||
| Antidepressant use or depression diagnosis | 5,003 | 376,306 | 1.06 | 1.00, 1.12 | 1.08 | 0.97, 1.20 |
| Depression diagnosis | 2,405 | 391,117 | 1.00 | 0.92, 1.08 | 0.98 | 0.84, 1.15 |
| Use of antidepressant medication | 4,516 | 379,605 | 1.08 | 1.02, 1.14 | 1.12 | 1.00, 1.25 |
| Adjusted for Use of Antianxiety Medication | ||||||
| Antidepressant use or depression diagnosis | 5,003 | 376,306 | 1.05 | 0.99, 1.11 | 1.07 | 0.96, 1.19 |
| Depression diagnosis | 2,405 | 391,117 | 1.00 | 0.92, 1.08 | 0.98 | 0.83, 1.14 |
| Use of antidepressant medication | 4,516 | 379,605 | 1.07 | 1.01, 1.13 | 1.11 | 0.99, 1.24 |
Abbreviations: CI, confidence interval; HR; hazard ratio; PM2.5, particulate matter with an aerodynamic diameter less than or equal to 2.5 μm; ppb, parts per billion.
a Summer was defined as May–September.
b All models adjusted for calendar year, month at questionnaire return, census region, living in a Metropolitan Statistical Area, race, physical activity, body mass index, pack-years of smoking, smoking status, dietary habits, alcohol consumption, multivitamin intake, participation in social groups, baseline abbreviated Mental Health Inventory score, educational level, parental education, marital status, husband's education (if applicable), tract-level median income, house value, and population density.
Appendix Table 2.
Adjusted Hazard Ratios From Sensitivity Analyses for Ozone and Fine Particulate Matter Exposures, Including Subjects With Cancer, Myocardial Infarction, or Stroke, Nurses’ Health Study, United States, 1996–2008
| Outcome | No. of Cases | Person Years | Per 10-ppb Increase in Summera Ozone Concentration | Per 10-μg/m3 Increase in 1-year PM2.5 Concentration | ||
|---|---|---|---|---|---|---|
| HRb | 95% CI | HRb | 95% CI | |||
| Antidepressant use or depression diagnosis | 6,627 | 465,165 | 1.03 | 0.98, 1.08 | 1.06 | 0.96, 1.16 |
| Depression diagnosis | 3,205 | 485,497 | 1.01 | 0.94, 1.08 | 0.96 | 0.84, 1.10 |
| Use of antidepressant medication | 5,989 | 469,752 | 1.04 | 0.99, 1.10 | 1.08 | 0.98, 1.19 |
Abbreviations: CI, confidence interval; HR; hazard ratio; PM2.5, particulate matter with an aerodynamic diameter less than or equal to 2.5 μm; ppb, parts per billion.
a Summer was defined as May–September.
b All models adjusted for calendar year, month at questionnaire return, census region, living in a Metropolitan Statistical Area, race, physical activity, body mass index, pack-years of smoking, smoking status, dietary habits, alcohol consumption, multivitamin intake, participation in social groups, baseline abbreviated Mental Health Inventory score, educational level, parental education, marital status, husband's education (if applicable), tract-level median income, house value, and population density.
REFERENCES
- 1. Murray CJ, Vos T, Lozano R, et al. . Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2197–2223. [DOI] [PubMed] [Google Scholar]
- 2. Stewart WF, Ricci JA, Chee E, et al. . Cost of lost productive work time among US workers with depression. JAMA. 2003;289(23):3135–3144. [DOI] [PubMed] [Google Scholar]
- 3. Ekman M, Granström O, Omérov S, et al. . The societal cost of depression: evidence from 10,000 Swedish patients in psychiatric care. J Affect Disord. 2013;150(3):790–797. [DOI] [PubMed] [Google Scholar]
- 4. Ruo B, Rumsfeld JS, Hlatky MA, et al. . Depressive symptoms and health-related quality of life: the Heart and Soul Study. JAMA. 2003;290(2):215–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Centers for Disease Control and Prevention Current depression among adults—United States, 2006 and 2008. MMWR Morb Mortal Wkly Rep. 2010;59(38):1229–1235. [PubMed] [Google Scholar]
- 6. Thielke SM, Diehr P, Unutzer J. Prevalence, incidence, and persistence of major depressive symptoms in the Cardiovascular Health Study. Aging Ment Health. 2010;14(2):168–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Block ML, Elder A, Auten RL, et al. . The outdoor air pollution and brain health workshop. Neurotoxicology. 2012;33(5):972–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Power MC, Weisskopf MG, Alexeeff SE, et al. . Traffic-related air pollution and cognitive function in a cohort of older men. Environ Health Perspect. 2011;119(5):682–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ranft U, Schikowski T, Sugiri D, et al. . Long-term exposure to traffic-related particulate matter impairs cognitive function in the elderly. Environ Res. 2009;109(8):1004–1011. [DOI] [PubMed] [Google Scholar]
- 10. Tzivian L, Winkler A, Dlugaj M, et al. . Effect of long-term outdoor air pollution and noise on cognitive and psychological functions in adults. Int J Hyg Environ Health. 2015;218(1):1–11. [DOI] [PubMed] [Google Scholar]
- 11. Fonken LK, Xu X, Weil ZM, et al. . Air pollution impairs cognition, provokes depressive-like behaviors and alters hippocampal cytokine expression and morphology. Mol Psychiatry. 2011;16(10):987–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mokoena ML, Harvey BH, Viljoen F, et al. . Ozone exposure of Flinders Sensitive Line rats is a rodent translational model of neurobiological oxidative stress with relevance for depression and antidepressant response. Psychopharmacology (Berl). 2015;232(16):2921–2938. [DOI] [PubMed] [Google Scholar]
- 13. Kim C, Jung SH, Kang DR, et al. . Ambient particulate matter as a risk factor for suicide. Am J Psychiatry. 2010;167(9):1100–1107. [DOI] [PubMed] [Google Scholar]
- 14. Szyszkowicz M, Willey JB, Grafstein E, et al. . Air pollution and emergency department visits for suicide attempts in Vancouver, Canada. Environ Health Insights. 2010;4:79–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Power MC, Kioumourtzoglou MA, Hart JE, et al. . The relation between past exposure to fine particulate air pollution and prevalent anxiety: observational cohort study. BMJ. 2015;350:h1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cho J, Choi YJ, Suh M, et al. . Air pollution as a risk factor for depressive episode in patients with cardiovascular disease, diabetes mellitus, or asthma. J Affect Disord. 2014;157:45–51. [DOI] [PubMed] [Google Scholar]
- 17. Szyszkowicz M. Air pollution and emergency department visits for depression in Edmonton, Canada. Int J Occup Med Environ Health. 2007;20(3):241–245. [DOI] [PubMed] [Google Scholar]
- 18. Szyszkowicz M, Rowe BH, Colman I. Air pollution and daily emergency department visits for depression. Int J Occup Med Environ Health. 2009;22(4):355–362. [DOI] [PubMed] [Google Scholar]
- 19. Lim YH, Kim H, Kim JH, et al. . Air pollution and symptoms of depression in elderly adults. Environ Health Perspect. 2012;120(7):1023–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wang Y, Eliot MN, Koutrakis P, et al. . Ambient air pollution and depressive symptoms in older adults: results from the MOBILIZE Boston study. Environ Health Perspect. 2014;122(6):553–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Brook RD, Rajagopalan S, Pope CA, et al. . Particulate matter air pollution and cardiovascular disease: an update to the scientific statement from the American Heart Association. Circulation. 2010;121(21):2331–2378. [DOI] [PubMed] [Google Scholar]
- 22. Miller KA, Siscovick DS, Sheppard L, et al. . Long-term exposure to air pollution and incidence of cardiovascular events in women. N Engl J Med. 2007;356(5):447–458. [DOI] [PubMed] [Google Scholar]
- 23. Puett RC, Hart JE, Yanosky JD, et al. . Chronic fine and coarse particulate exposure, mortality, and coronary heart disease in the Nurses’ Health Study. Environ Health Perspect. 2009;117(11):1697–1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pope CA, Dockery DW. Health effects of fine particulate air pollution: lines that connect. J Air Waste Manage Assoc. 2006;56(6):709–742. [DOI] [PubMed] [Google Scholar]
- 25. Alexopoulos GS, Meyers BS, Young RC, et al. . “Vascular depression” hypothesis. Arch Gen Psychiatry. 1997;54(10):915–922. [DOI] [PubMed] [Google Scholar]
- 26. Naarding P, Schoevers RA, Janzing JG, et al. . A study on symptom profiles of late-life depression: the influence of vascular, degenerative and inflammatory risk-indicators. J Affect Disord. 2005;88(2):155–162. [DOI] [PubMed] [Google Scholar]
- 27. Colditz G, Manson J, Hankinson S. The Nurses’ Health Study: 20-year contribution to the understanding of health among women. J Womens Health. 1997;6(1):49–62. [DOI] [PubMed] [Google Scholar]
- 28. Breitbart W, Rosenfeld B, Pessin H, et al. . Depression, hopelessness, and desire for hastened death in terminally ill patients with cancer. JAMA. 2000;284(22):2907–2911. [DOI] [PubMed] [Google Scholar]
- 29. Massie MJ. Prevalence of depression in patients with cancer. J Natl Cancer Inst Monogr. 2004;(32):57–71. [DOI] [PubMed] [Google Scholar]
- 30. Frasure-Smith N, Lespérance F, Talajic M. Depression following myocardial infarction. Impact on 6-month survival. JAMA. 1993;270(15):1819–1825. [PubMed] [Google Scholar]
- 31. Ladwig KH, Röll G, Breithardt G, et al. . Post-infarction depression and incomplete recovery 6 months after acute myocardial infarction. Lancet. 1994;343(8888):20–23. [DOI] [PubMed] [Google Scholar]
- 32. Carson AJ, MacHale S, Allen K, et al. . Depression after stroke and lesion location: a systematic review. Lancet. 2000;356(9224):122–126. [DOI] [PubMed] [Google Scholar]
- 33. Hackett ML, Yapa C, Parag V, et al. . Frequency of depression after stroke: a systematic review of observational studies. Stroke. 2005;36(6):1330–1340. [DOI] [PubMed] [Google Scholar]
- 34. Loomis D, Grosse Y, Lauby-Secretan B, et al. . The carcinogenicity of outdoor air pollution. Lancet Oncol. 2013;14(13):1262–1263. [DOI] [PubMed] [Google Scholar]
- 35. Wellenius GA, Boyle LD, Wilker EH, et al. . Ambient fine particulate matter alters cerebral hemodynamics in the elderly. Stroke. 2013;44(6):1532–1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Chocano-Bedoya PO, O'Reilly EJ, Lucas M, et al. . Prospective study on long-term dietary patterns and incident depression in middle-aged and older women. Am J Clin Nutr. 2013;98(3):813–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Pan A, Sun Q, Czernichow S, et al. . Bidirectional association between depression and obesity in middle-aged and older women. Int J Obes. 2012;36(4):595–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yanosky JD, Paciorek CJ, Laden F, et al. . Spatio-temporal modeling of particulate air pollution in the conterminous United States using geographic and meteorological predictors. Environ Health. 2014;13:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. US Environmental Protection Agency Air Quality System Database. http://www.epa.gov/ttn/airs/airsaqs/detaildata/downloadaqsdata.htm Washington, DC: US Environmental Protection Agency; 2013. Accessed July 12, 2013.
- 40. Hernán M, Robins JM Causal Inference Book. http://www.hsph.harvard.edu/miguel-hernan/causal-inference-book/. Updated August 27, 2015. Accessed November 16, 2015.
- 41. Chiuve SE, Fung TT, Rimm EB, et al. . Alternative dietary indices both strongly predict risk of chronic disease. J Nutr. 2012;142(6):1009–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bell ML, Dominici F, Ebisu K, et al. . Spatial and temporal variation in PM2.5 chemical composition in the United States for health effects studies. Environ Health Perspect. 2007;115(7):989–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Beelen R, Raaschou-Nielsen O, Stafoggia M, et al. . Effects of long-term exposure to air pollution on natural-cause mortality: an analysis of 22 European cohorts within the multicentre ESCAPE project. Lancet. 2014;383(9919):785–795. [DOI] [PubMed] [Google Scholar]
- 44. Jerrett M, Burnett RT, Pope CA 3rd, et al. . Long-term ozone exposure and mortality. N Engl J Med. 2009;360(11):1085–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mackerron G, Mourato S. Life satisfaction and air quality in London. Ecol Econ. 2009;68(5):1441–1453. [Google Scholar]
- 46. Welsch H. Environment and happiness: Valuation of air pollution using life satisfaction data. Ecol Econ. 2006;58(4):801–813. [Google Scholar]
- 47. Jones KA, Thomsen C. The role of the innate immune system in psychiatric disorders. Mol Cell Neurosci. 2013;53:52–62. [DOI] [PubMed] [Google Scholar]
- 48. Thompson AM, Zanobetti A, Silverman F, et al. . Baseline repeated measures from controlled human exposure studies: Associations between ambient air pollution exposure and the systemic inflammatory biomarkers IL-6 and fibrinogen. Environ Health Perspect. 2010;118(1):120–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kelly FJ. Oxidative stress: its role in air pollution and adverse health effects. Occup Environ Med. 2003;60(8):612–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Risom L, Møller P, Loft S. Oxidative stress-induced DNA damage by particulate air pollution. Mutat Res. 2005;592(1-2):119–137. [DOI] [PubMed] [Google Scholar]
- 51. Ng F, Berk M, Dean O, et al. . Oxidative stress in psychiatric disorders: evidence base and therapeutic implications. Int J Neuropsychopharmacol. 2008;11(6):851–876. [DOI] [PubMed] [Google Scholar]
- 52. Ozcan ME, Gulec M, Ozerol E, et al. . Antioxidant enzyme activities and oxidative stress in affective disorders. Int Clin Psychopharmacol. 2004;19(2):89–95. [DOI] [PubMed] [Google Scholar]
- 53. Mojtabai R. Increase in antidepressant medication in the US adult population between 1990 and 2003. Psychother Psychosom. 2008;77(2):83–92. [DOI] [PubMed] [Google Scholar]
- 54. Olfson M, Marcus SC. National patterns in antidepressant medication treatment. Arch Gen Psychiatry. 2009;66(8):848–856. [DOI] [PubMed] [Google Scholar]
- 55. Larson MJ, Miller K, Fleming KJ. Treatment with antidepressant medications in private health plans. Adm Policy Ment Health. 2007;34(2):116–126. [DOI] [PubMed] [Google Scholar]
- 56. Mojtabai R, Olfson M. Proportion of antidepressants prescribed without a psychiatric diagnosis is growing. Health Aff (Millwood). 2011;30(8):1434–1442. [DOI] [PubMed] [Google Scholar]
- 57. Kioumourtzoglou MA, Spiegelman D, Szpiro AA, et al. . Exposure measurement error in PM2.5 health effects studies: a pooled analysis of eight personal exposure validation studies. Environ Health. 2014;13(1):2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Reitzes DC, Mutran EJ, Fernandez ME. Does retirement hurt well-being? Factors influencing self-esteem and depression among retirees and workers. Gerontologist. 1996;36(5):649–656. [DOI] [PubMed] [Google Scholar]