Abstract
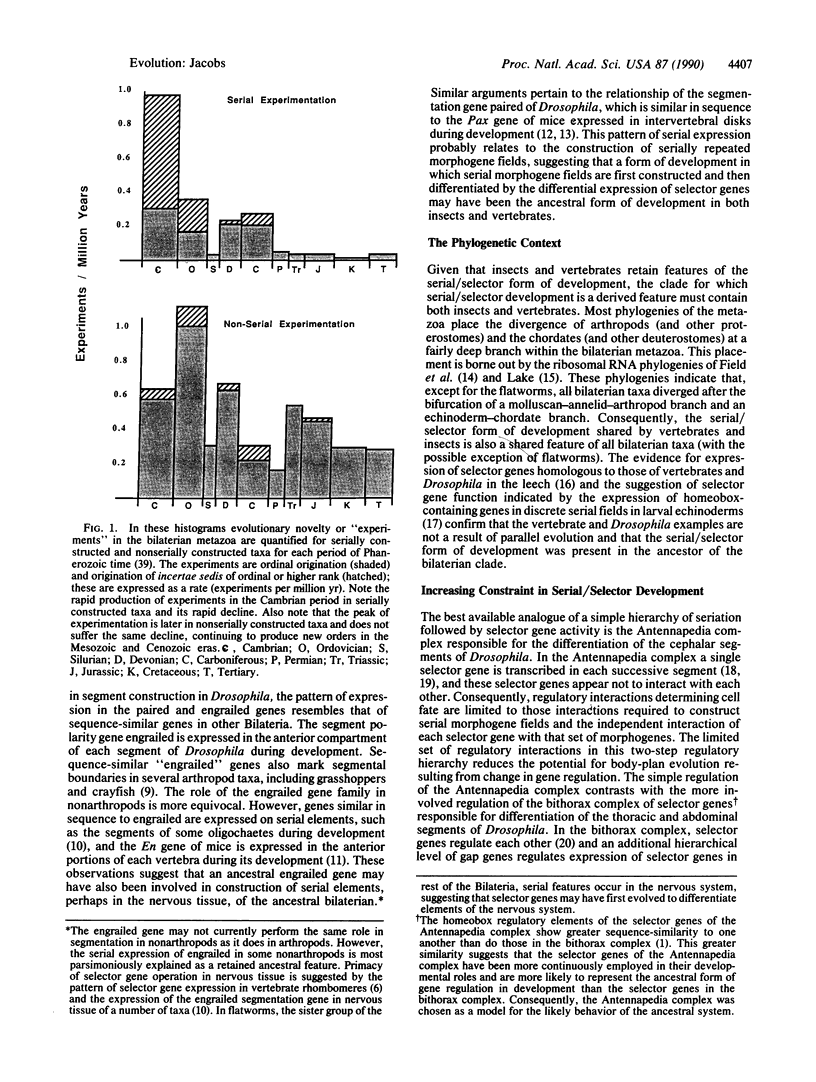
There is a significantly greater post-Cambrian decline in frequency of ordinal origination among serially constructed Bilateria, such as arthropods, than in nonserially constructed Bilateria. Greater decline in arthropod ordinal origination is not predicted by ecologic, diversity-dependent models of decline in the production of higher taxa. Reduction in ordinal origination indicates increased constraint on arthropod body-plan evolution. The dispersal of selector genes in the genomes of arthropods in conjunction with the retention of a simple regulatory hierarchy in development may have caused the increased constraint seen. Increased constraint would not be expected in those organisms that are not serially constructed and presumably have not retained the simple ancestral regulatory hierarchy in development of selector gene differentiation of serial elements. The hypothesis of differential constraint tested against the fossil record in this paper can be further tested by examination of the distribution of selector genes in the genomes of arthropods.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balling R., Deutsch U., Gruss P. undulated, a mutation affecting the development of the mouse skeleton, has a point mutation in the paired box of Pax 1. Cell. 1988 Nov 4;55(3):531–535. doi: 10.1016/0092-8674(88)90039-6. [DOI] [PubMed] [Google Scholar]
- Barad M., Jack T., Chadwick R., McGinnis W. A novel, tissue-specific, Drosophila homeobox gene. EMBO J. 1988 Jul;7(7):2151–2161. doi: 10.1002/j.1460-2075.1988.tb03054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casanova J., Sánchez-Herrero E., Morata G. Developmental analysis of a hybrid gene composed of parts of the Ubx and abd-A genes of Drosophila. EMBO J. 1988 Apr;7(4):1097–1105. doi: 10.1002/j.1460-2075.1988.tb02918.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casanova J., Sánchez-Herrero E., Morata G. Identification and characterization of a parasegment specific regulatory element of the abdominal-B gene of Drosophila. Cell. 1986 Nov 21;47(4):627–636. doi: 10.1016/0092-8674(86)90627-6. [DOI] [PubMed] [Google Scholar]
- Colberg-Poley A. M., Voss S. D., Chowdhury K., Stewart C. L., Wagner E. F., Gruss P. Clustered homeo boxes are differentially expressed during murine development. Cell. 1985 Nov;43(1):39–45. doi: 10.1016/0092-8674(85)90010-8. [DOI] [PubMed] [Google Scholar]
- Davidson D., Graham E., Sime C., Hill R. A gene with sequence similarity to Drosophila engrailed is expressed during the development of the neural tube and vertebrae in the mouse. Development. 1988 Oct;104(2):305–316. doi: 10.1242/dev.104.2.305. [DOI] [PubMed] [Google Scholar]
- Deutsch U., Dressler G. R., Gruss P. Pax 1, a member of a paired box homologous murine gene family, is expressed in segmented structures during development. Cell. 1988 May 20;53(4):617–625. doi: 10.1016/0092-8674(88)90577-6. [DOI] [PubMed] [Google Scholar]
- Duboule D., Dollé P. The structural and functional organization of the murine HOX gene family resembles that of Drosophila homeotic genes. EMBO J. 1989 May;8(5):1497–1505. doi: 10.1002/j.1460-2075.1989.tb03534.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar B. A., O'Farrell P. H. Genetic control of cell division patterns in the Drosophila embryo. Cell. 1989 Apr 7;57(1):177–187. doi: 10.1016/0092-8674(89)90183-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erwin D. H., Valentine J. W., Sepkoski J. J., Jr A comparative study of diversification events: the early Paleozoic versus the Mesozoic. Evolution. 1987;41(6):1177–1186. [PubMed] [Google Scholar]
- Field K. G., Olsen G. J., Lane D. J., Giovannoni S. J., Ghiselin M. T., Raff E. C., Pace N. R., Raff R. A. Molecular phylogeny of the animal kingdom. Science. 1988 Feb 12;239(4841 Pt 1):748–753. doi: 10.1126/science.3277277. [DOI] [PubMed] [Google Scholar]
- Foe V. E. Mitotic domains reveal early commitment of cells in Drosophila embryos. Development. 1989 Sep;107(1):1–22. [PubMed] [Google Scholar]
- Gaunt S. J., Krumlauf R., Duboule D. Mouse homeo-genes within a subfamily, Hox-1.4, -2.6 and -5.1, display similar anteroposterior domains of expression in the embryo, but show stage- and tissue-dependent differences in their regulation. Development. 1989 Sep;107(1):131–141. doi: 10.1242/dev.107.1.131. [DOI] [PubMed] [Google Scholar]
- Gaunt S. J. Mouse homeobox gene transcripts occupy different but overlapping domains in embryonic germ layers and organs: a comparison of Hox-3.1 and Hox-1.5. Development. 1988 May;103(1):135–144. doi: 10.1242/dev.103.1.135. [DOI] [PubMed] [Google Scholar]
- Gehring W. J. Homeo boxes in the study of development. Science. 1987 Jun 5;236(4806):1245–1252. doi: 10.1126/science.2884726. [DOI] [PubMed] [Google Scholar]
- Graham A., Papalopulu N., Krumlauf R. The murine and Drosophila homeobox gene complexes have common features of organization and expression. Cell. 1989 May 5;57(3):367–378. doi: 10.1016/0092-8674(89)90912-4. [DOI] [PubMed] [Google Scholar]
- Holland P. W. Homeobox genes and the vertebrate head. Development. 1988;103 (Suppl):17–24. [PubMed] [Google Scholar]
- Kuziora M. A., McGinnis W. Autoregulation of a Drosophila homeotic selector gene. Cell. 1988 Nov 4;55(3):477–485. doi: 10.1016/0092-8674(88)90034-7. [DOI] [PubMed] [Google Scholar]
- Lake J. A. Origin of the Metazoa. Proc Natl Acad Sci U S A. 1990 Jan;87(2):763–766. doi: 10.1073/pnas.87.2.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahaffey J. W., Diederich R. J., Kaufman T. C. Novel patterns of homeotic protein accumulation in the head of the Drosophila embryo. Development. 1989 Jan;105(1):167–174. doi: 10.1242/dev.105.1.167. [DOI] [PubMed] [Google Scholar]
- Patel N. H., Kornberg T. B., Goodman C. S. Expression of engrailed during segmentation in grasshopper and crayfish. Development. 1989 Oct;107(2):201–212. doi: 10.1242/dev.107.2.201. [DOI] [PubMed] [Google Scholar]
- Patel N. H., Martin-Blanco E., Coleman K. G., Poole S. J., Ellis M. C., Kornberg T. B., Goodman C. S. Expression of engrailed proteins in arthropods, annelids, and chordates. Cell. 1989 Sep 8;58(5):955–968. doi: 10.1016/0092-8674(89)90947-1. [DOI] [PubMed] [Google Scholar]
- Rowe A., Akam M. The structure and expression of a hybrid homeotic gene. EMBO J. 1988 Apr;7(4):1107–1114. doi: 10.1002/j.1460-2075.1988.tb02919.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneuwly S., Kuroiwa A., Gehring W. J. Molecular analysis of the dominant homeotic Antennapedia phenotype. EMBO J. 1987 Jan;6(1):201–206. doi: 10.1002/j.1460-2075.1987.tb04739.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott M. P., Weiner A. J. Structural relationships among genes that control development: sequence homology between the Antennapedia, Ultrabithorax, and fushi tarazu loci of Drosophila. Proc Natl Acad Sci U S A. 1984 Jul;81(13):4115–4119. doi: 10.1073/pnas.81.13.4115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struhl G. Differing strategies for organizing anterior and posterior body pattern in Drosophila embryos. Nature. 1989 Apr 27;338(6218):741–744. doi: 10.1038/338741a0. [DOI] [PubMed] [Google Scholar]
- Wilkinson D. G., Bhatt S., Cook M., Boncinelli E., Krumlauf R. Segmental expression of Hox-2 homoeobox-containing genes in the developing mouse hindbrain. Nature. 1989 Oct 5;341(6241):405–409. doi: 10.1038/341405a0. [DOI] [PubMed] [Google Scholar]
- Wysocka-Diller J. W., Aisemberg G. O., Baumgarten M., Levine M., Macagno E. R. Characterization of a homologue of bithorax-complex genes in the leech Hirudo medicinalis. Nature. 1989 Oct 26;341(6244):760–763. doi: 10.1038/341760a0. [DOI] [PubMed] [Google Scholar]