Abstract
Gene electrotransfer is a powerful method of DNA delivery offering several medical applications, among the most promising of which are DNA vaccination and gene therapy for cancer treatment. Electroporation entails the application of electric fields to cells which then experience a local and transient change of membrane permeability. Although gene electrotransfer has been extensively studied in in vitro and in vivo environments, the mechanisms by which DNA enters and navigates through cells are not fully understood. Here we present a comprehensive review of the body of knowledge concerning gene electrotransfer that has been accumulated over the last three decades. For that purpose, after briefly reviewing the medical applications that gene electrotransfer can provide, we outline membrane electropermeabilization, a key process for the delivery of DNA and smaller molecules. Since gene electrotransfer is a multipart process, we proceed our review in describing step by step our current understanding, with particular emphasis on DNA internalization and intracellular trafficking. Finally, we turn our attention to in vivo testing and methodology for gene electrotransfer.
Keywords: Electric field, Electroporation, Gene electrotransfer, Plasmid DNA, Gene therapy, DNA vaccination
1. Introduction
Gene therapy is based on the delivery of genetic material, mainly DNA, to the nucleus of cells in order to generate a therapeutic effect [1]. This method may be used to different ends, including the correction of a defective gene by silencing it or by providing a functional replacement, the introduction of a gene that encodes a foreign therapeutic protein or the introduction of a gene whose expression provokes cell death. On its journey to target cell nuclei, DNA will encounter several biological barriers, such as the extracellular matrix, the cell membrane, the cytoplasm, and the nuclear envelope [2]. The main drawback of gene therapy is its current limited efficiency and sometimes safety, which are both related to our insufficient knowledge about the underlying mechanisms. A massive effort has been allocated into developing techniques for the vectorization of nucleic acids, which are generally divided into viral and non-viral methods [3]. The advantage of using viral vectors is mainly their innate ability to transfer genetic information into the target cell, achieving high specificity and efficiency [4]. Nevertheless, insertional mutagenesis or excessive immune response of the host can render this method unsafe [5-8]. Therefore, establishing alternative non-viral methods for gene delivery is central for the broadening of medical care. Among gene-based vaccines, 43% of the clinical trials use the administration of naked/plasmid DNA, which shows that non-viral methods of gene delivery have already become prominent in clinical medicine [9]. Due to its safety, efficacy, flexibility, ease of application and low cost, gene electrotransfer is among the most promising substitutes to viral gene delivery methods.
Electroporation consists of exposing a cell or a tissue to an external electric field, which modifies cell membrane permeability to molecules that otherwise would cross the plasma membrane with low efficiency, or not at all [10, 11]. Electroporation has rapidly developed into different biotechnological [12] and biomedical applications [13]. The first of these involved delivery of anticancer drugs into cutaneous and subcutaneous tumor nodules [14-19]. This procedure, termed electrochemotherapy, is now used regularly in clinical practice for cancer treatment [20-23] and planning for treatment of deep-seated tumors is being developed [24, 25]. In addition to chemotherapeutic drugs, larger molecules such as DNA can be introduced into cells using electric pulses, which is referred to as electrogenetherapy in the context of medical care [26-28]. In the past 30 years, researchers have made considerable progress, and gene electrotransfer has been successfully performed on many tissues [29-31]. The first phase I clinical trial of gene electrotransfer has been conducted in patients with metastatic melanoma [32, 33] and several clinical studies for DNA vaccination have been monitored or are currently ongoing (http://clinicaltrials.gov) [34, 35]. In spite of the widespread use of gene electrotransfer and its noticeable applications in medical care, the fundamental molecular mechanisms that govern electropermeabilization of the plasma membrane and DNA delivery into cells remain to be untangled. Only on the basis of that knowledge, gene electrotransfer can be optimized and safely employed for disease treatment.
After revising the medical applications that gene electrotransfer can provide, we outline membrane electropermeabilization, a key process for the delivery of DNA and smaller molecules. Since gene electrotransfer is a multipart process, we proceed our review in describing step by step our current understanding, with particular emphasis on DNA internalization and intracellular trafficking. Finally, we turn our attention to in vivo testing and methodology for gene electrotransfer.
2. Medical applications in humans
DNA electrotransfer in vivo is in many cases more efficient than other non-viral methods of gene delivery, such as gene gun in the liver [36], liposomes in the brain or the cornea [37, 38], sonoporation in the muscle [39], or cationic lipids in the synovial tissue [40]. Gene expression is transient with durations between some weeks [38, 41, 42] and several months [29, 43, 44], and it is possible to repeat the electrotransfer procedure and reach identical levels of transfection as obtained following the first treatment [45, 46]. Electrotransfer of multiple genes in parallel is easily achieved [47] and by adapting the procedure to the target tissue, electrotransfer has been successfully applied in various species into various tissues including skeletal muscle, skin, tumors, liver, lungs, kidneys, brain, retina, cornea, and heart with minimal tissue damage [30, 48, 49].
The most widely used tissue for gene electrotransfer is skeletal muscle [49] because it is large, easy to access and its organization in long parallel fibers offers an optimal orientation relative to the direction of the electric field, promoting maximum delivery across the entire length of the fibers. Since skeletal muscle cells do not divide, gene expression following electrotransfer is stable for a long period. Most importantly, skeletal muscle produces biologically active proteins and releases them into the bloodstream. Therefore, muscle can be used as protein delivery system for distant targets [50]. The skin is the second most broadly used tissue for gene electrotransfer [51, 52]. It is accessible for treatment over large areas, and some epidermal cells (keratinocytes) can also produce and release proteins into the bloodstream. Other notable targets are antigen-presenting cells, which are major actors for immunotherapies such as vaccination. The first clinical trial on humans was for the treatment of skin cancer [32, 33]. However, therapeutic applications concern not only cancers [53] but also cardiovascular diseases [54], autoimmune diseases [55], monogenic diseases [56], organ specific disorders [57] and vaccination [58-60]. In the following sections, we focus on two of the applications of gene electrotransfer, DNA vaccination and cancer treatment.
2.1. DNA Vaccination
The idea behind genetic immunization simply consists of injecting a naked plasmid encoding a relevant antigen into muscle or skin that will produce antigens in sufficient amounts to initiate targeted immune response [61, 62]. This approach offers several advantages. The target tissue takes in charge the entire synthesis of the protein and its subsequent processing and presentation as an antigen to the lymphocytes. DNA is easy to produce compared to proteins or antigens (i.e. conventional vaccine material) and it is a stable molecule that can be stored for relatively long periods in normal conditions [63]. In addition, naked DNA is the only vector that does not generate anti-vector immune response, meaning that this approach is safer than the others in term of infection. Finally, because they are produced directly by the tissue, antigens are synthesized in their native form and in a stable manner. However, efficiencies in immunization are not as high as in classical vaccination techniques and the potential risk of DNA integration into the cell genome remains to be evaluated before larger scale use. This type of immunization is often developed for vaccination (virus, bacteria), for anticancer immunotherapy, and to induce the production of antibodies in high yields.
Comparison between DNA injection alone and injection followed by electroporation has demonstrated an increase in both cellular and humoral response after electric fields were applied. The addition of electroporation provides a 10-100 fold augmentation of immune response and defense against pathogens in humans and numerous animal models of diseases such as HIV/SIV, malaria, hepatitis B and C, human papilloma virus (HPV), anthrax and influenza [61, 64]. A recently completed human clinical trial of DNA vaccination against HIV infection showed that DNA injection followed by electroporation, compared to intramuscular DNA injection alone, considerably increases the rate, magnitude and duration of the immune response [65]. The vaccine contained two plasmids, each carrying two antigens whose expression was under the control of different promoters. The average magnitude of response was 70-fold higher for one antigen and about 20-fold higher for the others. Electroporation also permits a reduction in the quantity of injected vaccine, since a 1 mg dose combined with electroporation gave higher immune response rates than a 4 mg dose without electroporation. In parallel, delivery by electroporation enhances the quality of the T cell response through the production of several cytokines. Human clinical trials for the treatment of hepatitis B and C viruses have been conducted using gene electrotransfer alone or in combination with other treatments [66, 67]. Electroporation induces a robust T cell immune response, a production of antibodies and helped in reducing the viral replication. Another interesting clinical trial concerned the treatment of HPV16/18 delivering VGX-3100 vaccine using electroporation [68]. The production of antibody was significantly increased for both HPV16 and HPV 18 and persisted over several months. Additionally, specific T cells were detected in most of the patients which had from low to high dose. Other trials on humans using electroporation for the delivery of HIV, influenza, human papilloma virus and malaria vaccines have been performed or are ongoing [61, 62, 69]. The results confirm that human DNA vaccination using electroporation is safe and able to significantly elicit the immune response.
2.2. Cancer Treatment
Cancer treatment is currently the main application domain of gene therapies [70]. The four main approaches are the activation of immune response [71], the utilization of suicide genes [47, 72], the prevention of tumor angiogenesis [73] and the compensation of defective functions due to the loss of tumor suppressor genes or the creation of oncogenes [74]. These methods can be combined to obtain collaborative effects, for example the delivery by electroporation of HSV-TK suicide gene and IL-21 immune gene [75]. Electrogenetherapy can be used in combination with electrochemotherapy, because these two approaches employ different process to treat tumor cells. Anticancer agents, injected into the tumor eradicate the diseased cells, while DNA injected at the tumor periphery transfects healthy cells such that they can, for instance, stimulate the immune system [76, 77].
Treatments based on the electroporation-mediated expression of cytokines into tumors are widely studied. IL-12, IL-18, IL-2, IFN-α reduce the growth of different tumors and improve animal survival [53, 78-80]. The first clinical trial on humans was for the treatment of skin cancer (melanoma) via an electrotransferred IL-12-encoding plasmid [32, 33, 64]. The results showed the safety, reproducibility and clinical efficacy of the strategy, including cases of remission. Significant necrosis of most of the treated tumors was observed. Interestingly, this electroimmunotherapy results in the treatment of tumors unexposed to electric fields, which means that a systemic response is induced as well [53]. Phase II clinical trials using IL-12 plasmid are conducted on melanoma, carcinoma and lymphoma [64]. Since DNA electrotransfer in tumor cells is at the moment not very efficient, intramuscular or intradermal gene transfer can be efficiently performed for the treatment of tumors at distances. As described in the previous section, the produced protein can be released into the vascular system and exert therapeutic effects. This DNA vaccination strategy can be very useful for surgically inaccessible tumors and metastatic tumors.
Some cancer cells express tumor-specific or tumor-associated antigens [81] and vaccination strategies targeting these antigens can help to fight - for example skin, testicular and prostate cancer. Human clinical trials on prostate cancer through intramuscular electrotransfer of a plasmid encoding the prostate membrane specific antigen (PMSA) have been performed [82, 83]. There is also significant evidence of improved humoral immune response to DNA vaccination when delivered by electroporation. Although DNA, simply intramuscularly injected, induces some antibody responses (10-fold increase above basal state), DNA injection followed by electroporation is considerably more effective, inducing up to a 400-fold increase of antibody level. It also appears that multiple dosing with DNA combined with electroporation induces higher antibody levels and the response can persist for up to 18 months, whereas multiple injections alone result only in very slight changes of the antibody production [83]. Several other human clinical trials are currently active and attempt to treat prostate cancer (prostate specific antigen), melanoma (xenogenic tyrosinase), or leukemia (DOM-Wilm’s tumor epitope fusion antigen) [64].
Another strategy to treat cancer, termed antiangiogenesis, relies on depriving cancer cells from nutrients and oxygen by isolating tumors from the circulatory system. Currently, Phase I clinical trials are being performed to assess electroporation as a mean of delivering a plasmid encoding antiangiogenic metargidin peptide (AMEP) to treat melanoma [64, 84]. First results show a minimal toxicity of the method, the presence of AMEP mRNA only in the treated tumors, which means that the transfection was successful and due to gene electrotransfer. Additionally treated tumors had lower size increase by contrast to control non-treated tumors.
3. Cell membrane electropermeabilization
Electric fields are generated by the application of an electric current at the terminal of electrodes, which creates an electric potential difference U, or voltage, between the electrodes. The uniformity of this electric field is determined by electrode geometry. Two parallel plate electrodes produce a uniform electric field whose strength E is defined by the ratio between the voltage U and the gap g between the electrodes. Typical pulse wave shapes delivered by high voltage pulse generators are square-wave or exponentially decaying. Square-wave pulses generate an electric field having constant strength E and duration T. Exponentially decaying pulses create electric fields that decrease in strength over time following an exponential law. For electroporation of cells or tissues, parallel electrodes connected to a high voltage pulse generator delivering square-wave pulses are frequently used. Electroporation conditions are thus characterized by the strength E (equal to U/g for plate electrodes) of the electric field, the pulse duration T, the number of pulses N and the delay between two pulses, or period P, commonly given in frequency (f=1/P). The independent control of each parameter allows many combinations suited for different applications and the induction of specific effects on cells [85]. Efficient transfer of molecules in mammalian cells is obtained for electric field strengths between 100 V/cm and 1000 V/cm, with pulse durations ranging from µs to ms, repetition on the order of 10, and a frequency of approximately 1 Hz.
3.1. Induced Membrane Potential Difference
The cell membrane is an electric insulator that separates two ionic conductive media (the extra- and the intracellular media). The concentration gradient of ions between each side of the membrane generates a resting potential difference ΔΨ0 that is homogeneous all along the cell membrane. The value of ΔΨ0 depends on the cell type, but its value is near -70 mV. A cell subjected to an electric field will disturb the field lines [86]. The current is forced to flow around the cell and the ionic layers at the membrane interface are reorganized (Fig. 1). The larger line distortions are located at the sides of the membrane facing the field lines. With increasing electric field strength, the cell membrane progressively resists less until it reaches a critical state where the conductive intracellular medium contributes to the total conductance. At high electric fields, the membrane becomes conductive.
Fig. (1).
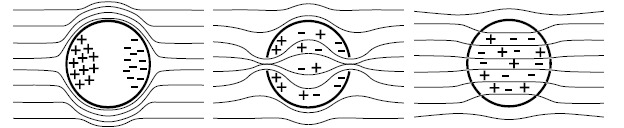
Schematic representation of the electric field lines around or through a spherical cell. The cell membrane conductivity is extremely low. The current flows and the field lines around the spherical cell are distorted. At a certain field value, the membrane allows for the field lines to cross towards the cell interior. Increasing the electric field increases the number of field lines crossing the cell and the cell membrane becomes conductive. Inspired from [86].
The redistribution of the ions at each side of the cell surface and the redistribution of the charges within the cell membrane create an induced potential difference ΔΨi through the cell membrane. ΔΨi can be modeled for a uniform electric field E by solving the Laplace equation and considering several assumptions (Fig. 2): the cell in suspension is approximated to a dielectric shell, the thickness d of the shell (membrane) is at any point negligible compared to the smallest semi-axis of the cell (radius r when the cell is spherical), and the intra- and extracellular media are pure conductors.
Fig. (2).
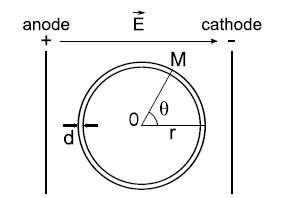
Schematic representation of a cell in a uniform electric field. The cell in suspension is represented as a spherical shell. The thickness d of the membrane is very small compared to the cell radius r. The uniform electric field is obtained by the application of an electric pulse at the extremity of two parallel conductive plates (of infinite length). Inspired from [87].
The induced potential difference ΔΨi at a point M of the cell membrane and at a time t after the rise of the electric field pulse is given by [88-92]:
where θ is the angle formed between the direction of the electric field and the normal of the point M on the membrane. f is a factor related to the shape of the cell which equals 1.5 if the cell is spherical. For other shapes such as ellipsoids, r is the semi-axis aligned along the electric field and f can be modeled [93]. g(λ) is a factor linked to the conductivity of the membrane (λm), of the intra (λi) and extracellular (λe) media. To take into account that the membrane is not a pure dielectric because some conducting leaks are present, the factor g is introduced, for a spherical cell:
When the membrane conductivity is extremely low compared to the conductivity of the intra and extracellular media, g(λ) = 1. τc is the charging time of the cell membrane given by, with Cm being the specific capacitance of the membrane:
When the membrane is a pure dielectric sphere (λm = 0), the charging time is maximal:
τc (~µs) is very small compared to conventional electric field pulse durations (longer than 100 µs) [85]. At the steady state, considering the cell as a spherical insulator shell, ΔΨi can be written in a simplified expression:
The induced potential difference at the cell membrane ΔΨi is, therefore, directly proportional to the size of the cell and the strength of the electric field. In addition, since it is correlated with the vectorial property of the electric field, it is not uniform along the cell membrane. ΔΨi is maximum at the side of the membrane facing the electrodes (θ = 0° or 180°) and decreases progressively along the cell surface up to the poles where ΔΨi = 0 (θ = 90° or 270°).
3.2. Threshold
During the application of the electric field, the induced potential difference towards the cell membrane ΔΨi is added to the resting potential difference of the cell ΔΨ0 [91, 94]. Considering the assumptions about the system described above (Fig. 2), ΔΨ is given by:
When the absolute value of the resulting ΔΨ reaches or is larger than a threshold value ΔΨc, corresponding to a critical electric field value Ep, a permeabilization state of the cell membrane is initiated. The critical potential difference ΔΨc varies according to the cell type and lies between 200 mV and 500 mV for animal, plant or bacteria cells [91, 95-98]. The existence of a threshold value ΔΨc for the membrane potential difference is the major characteristic of the membrane electropermeabilization.
The value ΔΨc is generally the same for most biological membranes (approx. 300 mV), which means that the electric field critical value Ep is simply related to the size (and the shape) of the cell. The smaller the cell, the higher the applied electric field has to be to reach the permeabilization state of the cell membrane. Thus, the conditions of the electric field have to be adjusted according to the target cells. For eukaryotic cells (diameter between 10 µm and 30 µm), Ep is close to 1000 V/cm and for bacteria (diameter between 1 µm and 3 µm) Ep is in the order of 6000 V/cm. Likewise, CHO cells which have a larger diameter than human erythrocytes need about half the electric field strength to be electropermeabilized [99]. The cell size dependence of the electric field threshold explains why adherent cells require lower electric field (300 V/cm for CHO cells) than cells in suspension (700 V/cm) to be permeabilized [100-102]. One can take advantage of the size dependence property for selective molecule delivery into the largest cells present in fluids or tissues. When pulsing the blood, leucocytes (immune cells) can be permeabilized whereas erythrocytes (red cells) are unaffected [103]. Mature adipocytes can be electroporated in adipose tissue allowing for their specific labeling in vivo [104].
With respect to the size of the different organelles existing in the cell cytoplasm, it appears that conditions necessary for the permeabilization of cell membranes should keep intracellular components unresponsive. For instance, electropermeabilization in vitro of isolated mitochondria requires 10 to 100 times higher electric field strength than the usual conditions for the permeabilization of the cell membranes [105]. By contrast, intense but very short pulses can induce permeabilization of small sized membranes (organelles) without affecting larger sized membranes (cell membranes). There, the critical value Ep can be achieved before the large sized membrane reaches its steady state. Recent technology based on the use of nanosecond pulsed electric fields (nsPEFs) consists in using large external fields (10-300 kV/cm) applied in pulses of nanosecond duration (10-300 ns) [106]. nsPEFs applied to cell suspensions generate cytosolic electric currents which exponentially decrease due to the charging time of the cell membrane [107-109]. This electric field present in the cytoplasm can induce a potential difference across intracellular membranes such as those of vesicles and other organelles, if higher than their critical value [106, 110-112].
3.3. Asymmetry
The position dependence, at the membrane, of the induced potential difference ΔΨi engenders a position dependence of the resulting potential difference ΔΨ as well. At the side of the membrane facing the anode, where the resting and induced potential difference are both negative, the membrane is hyperpolarized (Fig. 3a). By contrast, the side of the membrane facing the cathode is depolarized. The polarization of the cell surface has been experimentally demonstrated for instance on sea urchin eggs (Fig. 3b) [113-116].
Fig. (3).
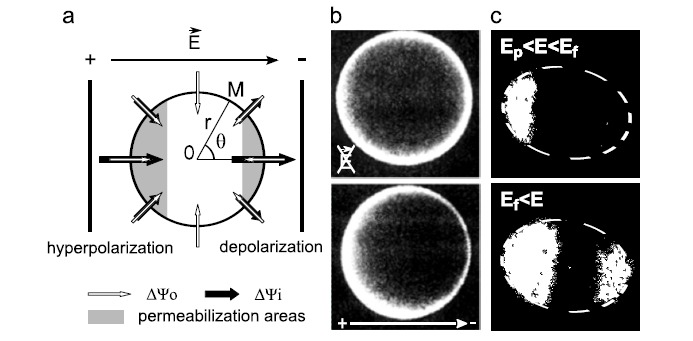
Asymmetry of the cell membrane potential and cell permeabilization due to electric fields. (a) Schematic representation of the resting and induced potential difference (resp. ΔΨ0, and ΔΨi) across the cell membrane. Inspired from [91] (b) Membrane potential difference of sea urchin eggs labeled with RH292 and observed using fluorescence microscopy before and during the application of an electric pulse [115] (c) Electropermeabilized areas of NIH3T3 cells labeled with ethidium bromide and observed using fluorescence microscopy after the application of electric fields of different strength [94]. E stands for electric field, Ep for electropermeabilization threshold and Ef for electrofusion threshold. The white dash lines in (c) represent the cell surface.
This asymmetry of the cell membrane potential can be transposed to the cell membrane electropermeabilization. The first area of the cell membrane reaching the permeabilization threshold is at the anode, for θ = 180°. Increasing the electric field above the corresponding value Ep expands the cell membrane area achieving ΔΨc. At a second threshold value of the electric field Ef (for electrofusion), ΔΨc is also reached at the side of the cell facing the negative electrode. Increasing the electric field above the Ef value increases the surface area being permeabilized on both sides of the cell membrane. Considering the cell as a sphere subjected to a uniform electric field, the permeabilized area Aperm of the cell surface can be predicted as follows [117, 118]:
where Atot is the cell surface, and E is the applied electric field strength.
Therefore, the electropermeabilization of the cell membrane is local and asymmetric (Fig. 16, step 1). The surface of the cell membrane being electropermeabilized only depends on the electric field strength (Fig. 4a). These predicted properties have been confirmed in different cell types via observations of the entry of external probes such as ethidium bromide (Fig. 3c) [94, 98, 119-122]. The area affected by the electric field also depends on the shape and the orientation of the cell within the electric field lines [123]. For a non-spherical cell at a given electric field strength, if the longest axis of the cell is parallel to the electric field lines, the permeabilization area is large whereas if the longest axis of the cell is perpendicular to the electric field, the permeabilization area is smaller.
Fig. (4).
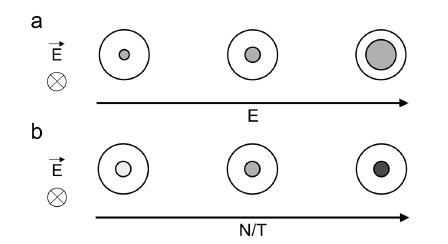
Influence of the parameters of the electric field on the surface permeabilization (electric field strength E, pulse duration T and number N). (a) At fixed N and T, the permeabilized area of the cell surface (gray) increases with increasing E. (b) At fixed E, the density of the transient permeable structures (light to dark gray) increases when increasing N and/or T. The electric field direction is perpendicular to the represented cell section. Inspired from [127].
3.4. Reversibility
The electropermeabilization of the cell is only transient [10]. For lipid bilayers, the lifetime duration of the permeated state is very short (~µs). Measurements of the membrane conductance showed that the reversibility process begins as soon as the electric field is turned off [124, 125]. For animal cells, the uptake of molecules after the application of the electric field demonstrates that the permeabilization state remains for some minutes after the last pulse, after which cells return to their initial impermeable state [117, 121, 126-129]. This process is called membrane resealing, and its duration is strongly dependent on the temperature [130, 131]. At room temperature, in isoosmolar buffer, half of the permeabilized CHO cells return to the impermeable state after 6 min [100]. At 37 °C, the resealing is faster, and at 4 °C, cells preserve the permeabilized state for more than 30 min [131]. Quantitative analysis of the resealing shows that it is a first order process with a rate constant under the control of the cumulated pulse duration NT [117]. The higher the pulsation duration and number, the longer is the resealing. A more recent analysis shows that several resealing processes coexist, while some have very short lifetimes (approx. 1ms), one persists for several minutes at room temperature [118, 132]. The application of electric fields significantly higher than the critical value Ep can induce an irreversible permeabilization of the cell membrane [133]. Subsequent cell death can be very fast (15 min) or delayed (24-48 h). Increasing the number or the duration of the electric pulses over the optimal conditions, determined for a given cell type, engenders cell death.
3.5. Associated Molecule Exchange
Electropermeabilization enables a cellular entry of small molecules (up to 4 kDa) of any chemical nature [134, 135]. This is observed during the seconds and minutes following the pulse. Most of the exchange takes place after the pulse [119, 136] and is naturally larger when the density of the membrane defects is high. Experimental results collected from measurements of conductance in cell suspension [88, 137] or from observations of single cells using fluorescence microscopy [119, 136] show that the level of permeabilization is strongly under the control of the pulse duration [117, 119]. If the delay between the pulses is sufficiently short (approx. 1s) such that the membrane resealing becomes negligible, successive pulses have additive effect [117]. The electropermeabilized surface of the cell is controlled by the electric field strength, and the density of the membrane defects is controlled by the pulse duration and number (Fig. 4) [127]. However, the effects of pulse duration and number do eventually saturate. At fixed electric field strength, for pulse durations larger than 100 µs and pulse number larger than 5, the permeabilization rate reaches a plateau [127, 138].
Small molecule delivery towards the electropermeabilized membrane is mostly driven by the concentration gradients existing across the membrane. Using Fick’s law, the flow Φ at a time t after the application of the electric field (strength E, duration T, number N) for a given solute S [117, 127, 139] is given by:
where, Ps is the permeability coefficient of S across the membrane.
where α is the partition coefficient of S between the medium and the membrane, D is the diffusion coefficient across the membrane and d is the thickness of the membrane [140]. ΔS is the concentration gradient of S across the membrane. f(N,T) is the fraction of the membrane brought into a permeable state. It is related to the density of the defects at the membrane, which depends on the pulse number and duration (N, T). k(N,T) is the resealing process constant. The lifetime duration of the defects follows a first order process with a rate constant under the control of the pulse number and duration (N, T). The entry of small molecules depends, therefore, on the surface of the membrane brought into the permeable state (under the control of E) and the level of permeability of that surface (under the control of N and T).
3.6. Transient Permeabilization Structures
The first observations of the effects of electric fields on membranes were conducted on lipid films [141]. Under the application of an electric field, free charges accumulate at each side of the films. Having opposite signs, they attract each other and create an electric compression force that stretches and shrinks the lipid film. Over a critical value of the electric field, this compression triggers an irreversible rupture. This model explains the cell death at high field conditions but does not explain the persistence and reversibility of the permeabilization. Other models involve the formation of reversible pores, which originally yielded the term electroporation [142-148]. These models are based on the fact that membranes are not perfect assemblies of lipids and proteins, but rather have structural defects that allow for the movement of lipids in the membrane matrix. The application of the electric field delivers the necessary energy to tip over the lipids and create hydrophilic pores [144, 146]. The pores open a passage for hydrophilic molecules, which in normal conditions do not cross the membrane. The pores are reversible as long as they do not exceed a critical size that would provoke membrane rupture. Many molecular simulations on lipid bilayers have supported a general model of pore formation that can be initiated with high surface tension and/or electric fields [109, 149-154]. According to this theoretical work, pore creation is in fact initiated by the formation of a water column driven by the electric field (Fig. 5). The electropore model is further supported by the observation of pores in giant vesicles [155-158] and by the observed diffusion of small molecules [94, 119-122].
Fig. (5).

Life cycle of an electropore. Only water (red) and phospholipid head groups (yellow) are shown. The creation of an electropore starts with the introduction of a water defect inside the lipid bilayer (pore initiation). This engenders a reorganization of the lipids around the defect (pore construction). As long as the electric field is present, this phenomenon expands until the formation of a mature pore (pore maturation). Once the electric field is turned off, pore annihilation begins. At this moment, the pore is quasi-stable (pore destabilization). The size of the pore decreases since water and phospholipid head groups move out of the bilayer interior (pore degradation). The head groups separate again into two distinct layers (pore deconstruction) and water is rapidly removed (pore dissolution) such that the initial structure of the membrane is restored. From [154].
As the lifetime of pores or any field-induced lipid structure is very short (approx. 1ms) [118, 159, 160], membrane resealing is expected to occur immediately after the application of the electric field, which is not the case. Moreover, this model does not fully explain the passage of macromolecules with sizes exceeding the pore sizes (1-20 nm) [142, 143, 145, 154, 159, 160]. Finally it has been shown that the lipids are not the only molecular structure involved in the permeabilization process. In fact, the membrane proteins and cytoskeleton are shown to be involved in the electropermeabilization [95, 99, 131, 161].
To conclude, membrane lipids are clearly involved in electropermeabilization. NMR analysis shows modification of the orientation of the polar head of the phospholipids in the permeabilized area of CHO cells [162]. Partial loss of the asymmetrical distribution of the phospholipids is shown in membrane of erythrocytes [163, 164] and fast phospholipid flip/flop occurs in the electropermeabilized membrane areas of CHO cells [165]. The electropore model, even if it does not elucidate the whole permeabilization process, explains the creation and expansion steps of the electropermeabilization and remains the best explanation for the diffusion of small molecules. The involvement of proteins is a key feature for the long-lived permeated state of the membrane following the electric field. Because the molecular structures responsible for the electropermeabilization of the biological membrane are not clearly defined, they are often referred to as transient permeable structures (TPS). Electropermeabilization can be thus described in five steps [166]:
creation (ns): the applied electric field induces a membrane potential difference and reaches the critical value Ep. Some membrane defects are generated and permeabilization begins.
expansion (µs): these defects propagate on the cell surface when the electric field strength is larger than Ep and the density of the defects increases as long as the field is present (cumulated duration).
stabilization (ms): points of cell permeabilization remains when the electric field is lower than Ep.
resealing (min): once the electric field is turned off, the cell membrane slowly loses its permeability returning to its initial impermeable state.
memory (h): the cell viability is preserved but some structural changes and physiological properties recover on a much longer time scale.
4. Gene electrotransfer
Gene electrotransfer appears to be a complex and multistep process which requires: (i) electropermeabilization of the plasma membrane, (ii) electrophoretic migration of the DNA towards membrane, (iii) DNA/membrane interaction, (iv) DNA translocation across the membrane, (v) intracellular migration of DNA through dense cell cytoplasm and finally (vi) DNA passage through the nuclear envelope and (vii) gene expression [167-170].
4.1. Threshold
For DNA electrotransfer to occur, the electric field strength has to be larger than a minimum value corresponding to the electropermeabilization threshold [171, 172]. In vitro, the threshold corresponds to the value Ef giving permeabilization of the side of the cell facing the cathode [171]. Regardless of cell viability, increasing the electric field strength improves DNA transfer up to a certain level where transfection efficiency decreases again [172-174]. This corresponds to cell viabilities between 30% and 80%. Moreover, the transfection efficiency has been shown to be directly proportional to (1-Ep/E), which corresponds to the definition of the electropermeabilized area [172]. Therefore, the larger the cell surface brought to the permeable state, the higher the transfection level. Nevertheless, cell survival is directly affected by electropermeabilization (Fig. 6) [133]. Thus successful gene electrotransfer occurs when both electropermeabilization and viability are optimal. These depend on the electrical field strength, the pulse number, and the pulse duration [121, 127, 172]. Furthermore, cell physiological condition plays an important role in cell survival. Even under mild conditions, cell death can occur after exposure to electric fields due to a prior poor physiological state such as loss of homoeostasis or membrane damage. For in vitro applications long pulses (from 1 ms to 5 ms) and combination of high and low voltage pulses have been suggested, whereas for in vivo applications, cell viability is better preserved with shorter duration pulses (from 100 µs to 500 µs) [175]. The importance of cell permeabilization has also been demonstrated in vivo using MRI [176]. Areas where permeabilization was detected, using a contrast agent, corresponded to areas where the plasmid coding for the β-galactosidase was expressed. Optimal DNA electrotransfer conditions depend on the cell type and the physiological state such as the phase in the cell-division cycle [168, 177-180].
Fig. (6).
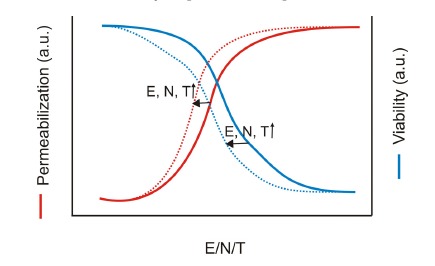
Schematic graph of cell membrane electropermeabilization and cell viability. Both strongly depend on the electric field strength (E), the number of pulses (N), and the pulse duration (T). The higher values these parameters have, the more cells are permeabilized but the less cells survive. Inspired from [127, 172].
4.2. Electrophoretic Component
DNA electrotransfer is possible only if DNA is present before the application of the electric field. When DNA is added as little as 2 s after electropermeabilization, transfection efficiency is insignificant in mammalian cells and tissues [29, 102, 172, 177, 181-184], yeast [184, 185] and bacteria [184, 186, 187]. It has been hypothesized that the permeable membrane structures allowing passage of DNA are short-lived and only present concurrently with the administration of an electric field [172]. Actually, DNA requires electrophoresis in order to interact with the cell membrane (Fig. 16, step 2). Since both DNA and the cell membrane are highly negatively charged, electrophoresis could be a means to overcome the electrostatic repulsion between DNA and membrane. The importance of the electrophoretic component has been highlighted first by growing cells in a monolayer such that the polarity of the field would bring molecules towards or away from the cells. Polarity inducing the migration of negatively charged compounds towards the cells engenders 10 times higher transfection efficiencies than inverted polarity [182]. Moreover, the addition of agents reducing the electrophoretic mobility, such as cations to reduce the net charge of the DNA or Ficoll to increase the viscosity of the medium, shows a concentration dependent decrease of the transfection efficiency. In addition, when a short and high voltage (HV) inducing electropermeabilization but little DNA electrotransfer is followed by a long low voltage (LV), DNA transfection becomes efficient [183]. The LV alone does not induce electropermeabilization and DNA electrotransfer. A recent study shows that, after using Ficoll to increase medium viscosity and so reduce DNA mobility and electrotransfer, the application of an LV pulse in addition to the HV one can recover to some extend the transfection efficiency. This reinforces the hypothesis of DNA electrophoresis being crucial for DNA electrotransfer [188]. This observation was confirmed both in vitro and in vivo for numerous tissues [29, 138, 189-195].
4.3. Asymmetry
The involvement of the electrophoretic component leads to an asymmetrical interaction of the DNA with the membrane (Fig. 16, step 3). Visualization at the single-cell level of fluorescently labeled DNA using microscopy confirmed that DNA interacts with the cell only on the side facing the cathode (Fig. 7a) [167, 171, 196-200]. During the application of an electric field, DNA molecules move along the field lines towards the anode. If a cell is in the path of migrating DNA and the electric field lower than the threshold value, DNA flows along the cell membrane as the field lines do [86, 201]. If a permeabilized cell is on its course, field lines traverse the membrane and DNA is brought to the cell surface. The asymmetrical interaction of the DNA with the cell membrane is directly related to the direction and the polarity of the electric fields [167, 171, 196-200]. When applying bipolar electric field (alternating current), DNA interaction is visible on both sides of the cell facing the electrodes (Fig. 7b). When applying bipolar and crossed electric fields, DNA interaction with the membrane is visible all along the cell membrane. In accordance with the idea that maximizing DNA-membrane interaction maximizes gene electrotransfer efficiency, changing electric field polarity and orientation has been shown to improve gene expression both in vitro and in vivo [121, 167, 193, 197].
Fig. (16).
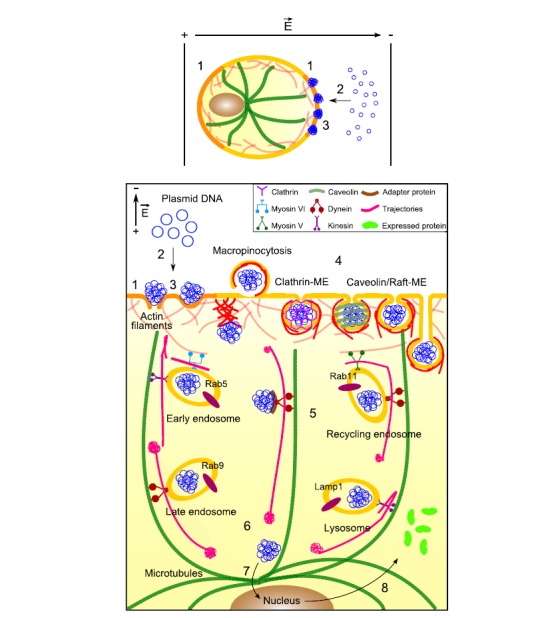
Schematic representation of the mechanism of DNA electrotransfer. During the application of the electric field, (1) the plasma membrane is permeabilized (orange), (2) DNA is electrophoretically pushed onto the cell membrane side facing the cathode, which results in (3) DNA-membrane interactions. DNA aggregates are inserted into the membrane (sites where membrane defects are present or not) and remain there for about ten minutes. After the application of the electric field and resealing of the membrane (yellow), (4) DNA is mainly internalized by endocytosis (macropinocytosis, clathrin-mediated endocytosis (Clathrin-ME), and caveolin/raft-mediated endocytosis (Caveolin/raft-ME). If DNA is internalized by other means than endocytosis, actin participation may take shape of bursts of polymerization. (5) While being actively transported in the cytoplasm (actin and tubulin networks, respectively in red and green), DNA aggregates pass through the different endosomal compartments (early endosomes, recycling endosomes, late endosomes, and lysosomes). Free DNA must interact with some adapter protein in order to be transported by motor proteins. For gene expression to occur, (6) DNA must escape from endosomal compartments. Once in the perinuclear region, (7) DNA must cross the nuclear envelope to be finally expressed and (8) yield proteins released into the cytoplasm. Inspired from [230, 251].
Fig. (7).
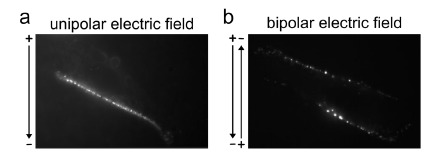
Asymmetry of the DNA interaction with the membrane on adherent cells. (a) Formation of DNA-membrane complexes only on one side of the cell surface (facing the cathode) under unipolar conditions. (b) Formation of DNA-membrane complexes on both sides of the cell surface in bipolar conditions. From [199].
4.4. DNA-membrane Interaction
DNA-membrane complexes are formed only when the electric field is present, and grow as local aggregates as a function of the electric field strength E and the cumulated duration NT (Fig. 8) [171, 196, 200, 202]. The distance L travelled by DNA by means of electrophoresis is given by:
Fig. (8).
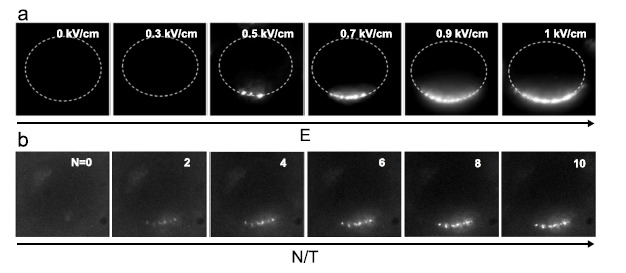
Influence of the parameters (E, T, N) of the electric field on the DNA accumulation at the cell surface. (a) At fixed N and T, the area of DNA accumulation or the number of DNA aggregates at the cell surface increases with increasing E. Each experiment was performed separately on different cells. (b) At fixed E, the amount of DNA accumulating in the aggregates increase when increasing N and/or T. The same cell is observed through the experiment. From [196, 200].
where µ is the electrophoretic mobility of the DNA molecules, which equals 1.5 10-4 cm2/Vs [201]. For an electric field applied as 10 pulses of 5 ms and 0.4 kV/cm, L = 30 µm, which means that the DNA being present over a length of 30 µm has been accumulated at the membrane, thus defining an accumulation factor of 30.
Once the field is turned off, the growth of the plasmid aggregates is stopped. The plasmid-membrane interaction is not evenly distributed on the permeabilized areas but is detected in association with membrane at some sites called competent sites with sizes ranging from 0.1 µm to 0.5 µm (Figs. 7, 8) [167, 171]. Already after the first pulse, the sites are defined and the following pulses induce only the linear accumulation of the DNA at these sites (Fig. 8b) [196, 200]. The electric field is necessary for the creation of these sites which are competent within a millisecond time range (Fig. 8a). The strength E controls the number of competent sites and the cumulated duration of the pulses controls the amount of DNA added in [171, 196, 200].
The amount of DNA interacting with the membrane seems to depend also on the presence of divalent cations. As shown on mammalian and yeast cells, increasing the concentration of magnesium or calcium in the medium during the application of the electric field leads to an enhancement of DNA-membrane interaction [202-204]. Since both DNA and the cell membrane are negatively charged, divalent cations could act a charge bridge and thus reduce electrostatic repulsion. The combination of electroporation and calcium loading has yielded excellent result in inducing tumor cell necrosis in vivo [205]. Nevertheless, concentrations higher than 1 mM hinder efficient gene electrotransfer [182, 202, 204], possibly by reducing the mobility of DNA across the cell membrane, and reducing cell viability.
DNA-membrane interaction is not only an accumulation of plasmids at the membrane surface level, but also an insertion of plasmids into the permeabilized membrane (Fig. 16, step 3). If electric field pulses with inverted polarities are applied, DNA complexes formed at one side of the cell remain present and thus resistant to electrophoresis [171, 197, 199]. The plasmid are then inserted or anchored in the plasma membrane. However, this anchorage strength depends on the delay between the pulses [167]. When this delay is short (100 µs or lower), the overall DNA-cell surface interaction (i.e. both sides of the cell) is higher under unipolar conditions compared to bipolar ones. If the delay is long enough (set to 10 s), the overall interaction under bipolar conditions is about twice that of the unipolar ones. In fact, plasmid DNA, first accumulated at the membrane, can leave the complexes through opposite electrophoretic force if applied within 10 s. Quantification of the DNA amount present at the membrane after each pulse, measured only at one side of the cell, shows indeed a removal of the DNA due to the inverted polarity [198]. This loss was partial and represented only about 20% with a delay of 2 s between inverted fields. Over the pulse application, the resulting DNA amount increases for the first 9 pulses after which neither accumulation nor removal is measured. These results reveal the existence of two classes of plasmid DNA-membrane complexes: i) complexes of low stability from which plasmid DNA can leave and return to the pulsation buffer, and ii) complexes of high stability, where plasmid DNA cannot be removed even by applying electric pulses of reversed polarity [167]. A time span between 2 s to 10 s appears to be needed to achieve stable plasmid-membrane complexes, after a 5 ms pulse. Complexes of high stability represent DNA irreversibly inserted into the membrane. An additional support for the hypothesis of DNA being inserted into the plasma membrane comes from the observation that a sequence consisting in applying first an LV pulse and then a HV pulse generates transfection efficiency drastically lower than the sequence HV pulse then LV pulse (and equivalent to a HV pulse alone) [190]. This means that for equivalent DNA electrophoresis, gene electrotransfer is more efficient when DNA encounters an already electropermeabilized membrane which can be interpreted as DNA being pushed, thus inserted, into the membrane.
The plasmid aggregates, which are inserted in the membrane after the electric field application, can nevertheless remain sensitive to the degrading action of nucleases added post-pulse, even if these are known not to cross the membrane [184]. For CHO cells, up to 1 min after the application of the electric field, the addition of DNase in the cell solution disturbs gene expression. This time varies according to the cell type or the tissue and can be much shorter [172, 182, 184-186]. The presence of DNase only 2-3 s before the application of the electric field was sufficient to suppress gene expression [182, 185]. Thus, DNA is inserted into and protected by some undefined structures that need about 1 min to be formed. DNA translocation through the membrane is relatively slow and achieved after the end of the electropulsation. Several minutes after the electropulsation, plasmids are still present at the cell surface [171]. The biophysical structure of the membrane-plasmid complex remains to be characterized.
4.5. DNA Internalization
The mechanism by which DNA is internalized is not yet well understood. Several models are proposed in the literature, but none can explain all experimental observations, and some remain speculative (Fig. 16, step 4).
4.5.1. Electropores
Krassowska’s model corroborates the first proposed mechanism, in which single DNA plasmid crosses the membrane via stable macropores [159, 206, 207]. These field-generated defects are due to the modulation of the cell membrane potential. The model relies on tension-coupled pores that do not bring to membrane rupture (a dramatic aspect of the classical electropore model [26, 208]). It predicts the creation of several hundreds of thousands of pores in which a large population (98%) contains small pores (1 nm radius) and a small population (2%) contains large pores (20 nm radius on average but up to 400 nm). The distribution of the pore populations at the poles of the cell facing the electrodes as well as the creation and expansion time scales are fairly consistent with experimental evidence. This model predicts pores large enough to allow for the plasmid uptake, even in its circular conformation, given that the effective diameter of a 6 kbp plasmid DNA varies between 8 nm and 22 nm, depending on salt concentration [209-211] (Fig. 9a). These pores stay opened for the entire duration of the electropulsation giving the necessary time for the plasmid to access the cytoplasm [212].
Fig. (9).
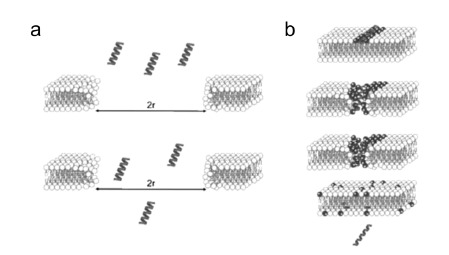
Models of DNA internalization in cells through electropores. (a) Electric pulses induce macropores large enough to let DNA diffusing through them (r represents the radius of the electropore). (b) DNA interacts with cationic lipids (darker grey) at the pore edges and via coalescence of the pores DNA passes through the membrane. From [213].
However, this model is confronted with experimental contradictions, the first of which is the membrane resealing time. The resealing time of such pores lies within a millisecond, as predicted by the model, which is far faster than the observed cell permeabilization of several minutes [121, 128, 129, 214]. In addition, for CHO cells, plasmid accessibility to DNase up to one minute following the end of electropulsation shows that the internalization of the plasmid takes place after electroporation [184]. Theoretical models calculate that stable pores have radiuses of only a few nanometers and that larger pores are unstable [215, 216]. These models have been partially confirmed experimentally [145, 161, 217]. Krassowska’s model can nonetheless be reconciled with the finding that plasmid entry into the cell occurs post-pulse. It has been proposed that plasmid translocation depends on the plasmid-membrane (cationic lipids like sphingosines) interactions and may occur by a coalescence of many small 1 nm pores (Fig. 9b) [127, 204, 218, 219]. The slow transfer of DNA through the electropermeabilized membrane (1 min) reflects the time needed for the pores occluded by DNA to coalesce into large enough pores to allow for its passage [220].
4.5.2. Electrophoresis
Another model proposes that, although electropores or defects are involved, DNA entry inside the cell takes place under the control of DNA electrophoresis. The latter concentrates the plasmid molecules near the membrane surface and pushes them through the putative electropores [177, 182, 183, 221, 222]. The external electric field imposed onto the cell does not penetrate through the initially intact membrane. As soon as electropores or defects are formed, the electric field crosses the membrane through these conducting structures (Fig. 1) [86]. The lines of the electric field are concentrated in the pores, so the strength of the electric field E in the pore and in its vicinity is higher than that in the bulk [182, 183]. At appropriate field polarity, the polyanionic DNA experiences a strong attraction to the pore. Even if the defect size (approx. 1 nm) is smaller than the effective diameter of DNA, DNA can enter the cell as the electrophoretic pressure of DNA onto the electropermeabilized membrane is strong enough to create a path [223]. The mechanical interaction between the pores and the plasmid induces an adjustment of the pore sizes and/or lifetimes that allow plasmid entry into the cell. This model assumes that the DNA molecule may prevent resealing of the membrane if it is still partially through the pore when the field is turned off. The plasmid may interact with the electropermeabilized membrane in three possible ways (Fig. 10) [183]:
Fig. (10).
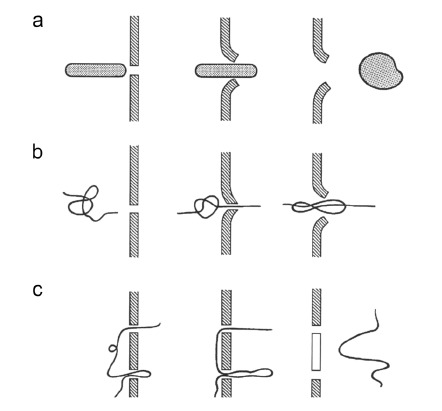
Models of DNA internalization in cells via electrophoresis. (a) Non-linear DNA is aligned with the electric field line and is electrophoretically pushed through one pore that becomes wider upon its passage. (b) Linear DNA has one end inserted in the pore and the electrophoretic force drives the DNA through it. (c) Linear DNA has two (or more) insertions in the membrane where the pores are and its electrophoresis cut the membrane between these pores (white part of the membrane). From [183].
DNA aligns according to the electric field direction and moves toward the permeabilized membrane. The plasmid may interact with a single membrane defect (pore) which becomes wider upon plasmid interaction by the action of electrophoretic forces (Fig. 10a).
The linear plasmid passage can be initiated by penetration of one end of the thread, which then leads the whole molecule through one pore under the electrophoretic force (Fig. 10b).
The DNA molecule can be involved in two (or more) pores and moved by electrophoretic forces. It cuts the membrane between these pores as a sharp thread can do (Fig. 10c).
This model, especially (i), can explain very well the observed accumulation and insertion of the non-linear DNA at the membrane [171, 196-199], including the minute range resealing and the slow translocation of the DNA through the cell membrane. However, if electrophoresis is the only driving force for plasmid translocation, comparable transfection efficiencies should be observed for equivalent ENT values (field strength E and cumulated pulse duration NT). In the case of HeLa cells, the number of transfected cells as a function of ET values is different according to whether short or long electric pulses are used [174]. Then, for constant ENT values, transfection level depends on T, which could mean that longer durations are favorable to the creation of the membrane defects leading to insertion sites, or to the DNA aggregation itself [127]. Therefore, electrophoretic movement cannot be the only driving force for plasmid internalization into cells, but it clearly supports the formation of DNA aggregates inserted in the membrane such that it is always included as a contribution in the mechanism of gene electrotransfer.
4.5.3. Endocytosis
A mechanism of DNA endocytosis-like internalization following electroporation was first suggested after the trapping of DNA inside a giant unilamellar vesicle (GUV) under the application of electric fields [224]. DNA entered the GUV filled with ethidium bromide, which is a DNA fluorescent probe, but no fluorescence was detected until sonification of the GUV, which is known to break membranes. This was interpreted by DNA entering the GUV via the formation of a vesicle. Recently, the ability to generate lipid vesicles and tubules inside GUV during electroporation has been observed using fluorescence microscopy [156]. The endocytic process for DNA internalization was first mentioned as a theoretical possibility by Klenchin et al. [182] and Tsong et al. [225]. Since then, endocytosis has received little consideration as a possible mechanism by which DNA could cross the plasma membrane. This was due to the absence of known cellular receptors for DNA, and because investigations in the field were more focused on the electropore model, since it explains very well the passage of small molecules across the cell membrane. Electropermeabilization of the plasma membrane remains a crucial step for gene delivery, but internalization of DNA via electropores is not easy to envision as, unlike small molecules, DNA forms distinct, stable and large clusters at the cell membrane prior to its passage into the cytoplasm. Recently, endocytosis has emerged as a valuable alternative model for DNA translocation given the growth of experimental evidences for its implication.
4.5.3.1. The Models
Within the endocytosis model, several mechanisms are proposed:
The term electroendocytosis often refers to endocytic-like vesicles formation under low electric fields (LEF) conditions that do not bring the membrane into the permeable state. Endocytosis would be stimulated by the redistribution of the charges (i.e. reorganization of charged lipids and proteins) in the cell membrane due to the electric field [226, 227]. The local lateral electrophoresis of proteins and lipids, in other words, the segregation of charged membrane components, only in the outer leaflet of the membrane would induce an asymmetric charge density between the outer and inner leaflet of the membrane thus responsible for spontaneous membrane curvature towards the cytoplasm (Fig. 11b). The difference of charge density would also be responsible for the membrane fission into vesicles [227].
It is believed that electrophoretically driven DNA can provide the necessary force to initiate a membrane invagination where membrane defects are present (Fig. 11a) [182, 183, 225]. This membrane invagination could then bud off inside the cell and the DNA would be trapped in endosome-like vesicles. This implies that the cellular endocytic machinery would take over at the stage of the membrane-scission, which is known to require dynamin (-like) proteins and cytoskeleton regulators [228].
Endocytosis (macropinocytosis, clathrin- and caveolin/raft-mediated) has now been shown to contribute to gene electrotransfer [229-233]. The insertion of the DNA into the membrane and/or the comparably enormous size of the DNA aggregates could exert on the membrane a curvature large enough to be recognized by the endocytic machinery which then would generate the membrane invagination and ultimately the vesicle. Another possibility is that the negatively charged DNA aggregates would mimic the clustering of the negatively charged molecules PIP2, which has been established as a crucial endocytosis and cytoskeleton regulator [234]. The engulfment of DNA via several pathways would be putatively based on the size of the aggregates. The involvement of macropinocytosis could be due to the collapse of electroinduced formation of ruffles, microvilli, or blebs at the surface of the membrane [235-237].
Tsong et al. [225] suppose that the denaturation of membrane proteins (e.g. protein channels) or any mechanical injury due to the electric field itself or the associated joule heating, leads to some cell repair mechanisms consisting in the internalization of the damaged proteins/membrane into vesicles for recycling. DNA being present nearby the surface (via electrophoresis or not) may then be engulfed in these vesicles.
Fig. (11).
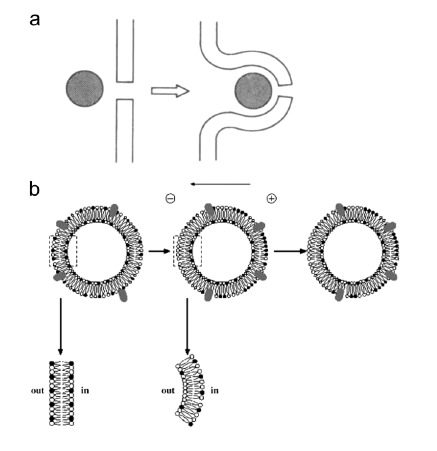
Models of DNA internalization in cells via endocytosis-like mechanism. (a) DNA electrophoresis brings the DNA molecule(s) at the membrane defect (pore) and provides the force to generate membrane invagination that buds off in vesicle containing DNA. From (148) (b) Low electric field conditions induce local segregation of charged membrane components (lipids, black and white circles, and proteins, grey ovals) in the outer leaflet of the cell membrane. This electrophoretic-induced segregation of charged membrane components induces an asymmetrical charge density across the membrane, which promotes spontaneous inward membrane curvature and fission. From (205).
4.5.3.2. Electroendocytosis
The involvement of electroendocytosis was shown to take place for LEF conditions (model (i) in section 3.5.3.1). LEF conditions mean series of direct current electric pulses with electric field strengths from 2 V/cm to 100 V/cm, pulse durations from 90 µs to 2 ms, frequencies between 100 Hz and 1000 Hz and exposure time range of 1-10 min [238]. A recent study compared the uptake of propidium iodide as an electropermeabilization probe and the uptake of FM4-64 as a lipid membrane probe. This work demonstrates that LEF conditions stimulate vesicle formation without bringing the cell into the permeable state [239]. The threshold of electroendocytosis is lower than that of electropermeabilization, and increasing the electric field strength increases the number of vesicles. Electric fields higher than the permeabilization threshold still stimulate endocytosis, therefore hinting at that electroendocytosis takes place in parallel to the electropermeabilization process.
However, electric field properties in LEF are substantially different from those necessary for successful DNA electrotransfer and only macromolecules such as BSA or dextran have been investigated. The absorption at the membrane and uptake across the membrane of FITC-BSA was highly increased under LEF exposure [226, 227, 238]. FITC-BSA observations using microscopy show vesicle patterns and colocalization with a membrane probe (labeled DHPE) confirming the presence of vesicles enclosing the macromolecules. The enhanced uptake of the small fluid-phase marker lucifer yellow confirms the presence of endocytic processes [226, 240]. Absorption of the macromolecule is temperature independent, but its uptake depends on temperature [226, 227]. The local lateral electrophoresis of charged lipids and proteins in the outer leaflet of the plasma membrane would induce a local depletion of negative charges at the membrane, which in turns would reduce repulsions between negatively charged macromolecules (BSA, or eventually DNA) and plasma membrane [227]. This mechanism can occur at low (4 °C) or physiological temperature (37 °C). Even if the local difference of charge density could generate spontaneous membrane curvature, the further invagination and the budding off of the vesicles, probably because of cell machinery involvement, is optimal at 37 °C. Indeed, the use of inhibitors showed that LEF stimulates vesiculation or uptake through microtubule- and clathrin-dependent pathways whereas caveolin-dependent endocytosis does not seem to be involved [226, 239]. Other LEF stimulated pathways should therefore concern macropinocytosis and pathways independent of clathrin and caveolin. Support for the contribution of macropinocytosis comes from measured enhancement of membrane ruffling at the cathode side under LEF [241].
It is interesting to note that when BSA is present during LEFs, it is significantly more absorbed to the membrane and internalized [227]. This is interpreted such that electrophoresis of the macromolecule increases the collision rate with the cell membrane. The contribution of the macromolecule electrophoresis represents 70% of the increased BSA absorption compared to non-field treated cells. The macromolecule electrophoretic component has a high importance as it is the case for DNA electrotransfer. Nevertheless, BSA added after the pulsation still can be internalized and LEF conditions do not bring the membrane into the permeable state, which is a minimum requirement for DNA to interact with the membrane and its further expression in cells. These fundamental differences suppose that in parallel to the mechanism involved in LEF conditions, must take place additional physical/chemical structures created only during electropermeabilization. It could be that this concerns the stable insertion of the DNA at sites where membrane defects (pores?) are present.
4.5.3.3. Cell-driven Endocytosis
The first evidence about the implication of endocytosis for the electrotransfer concerned the models (ii) and (iii) (see section 3.5.3.1) with the internalization of macromolecules such as albumin, and gold particles [235]. Observations with fluorescence and electron microscopy showed the macromolecules being trapped in vesicles. Electron microscopy showed also an increase of membrane ruffling and vesiculation for electroporated cells [126, 242-244]. Significant increases of FITC-BSA translocation into the cell after electrotransfer were measured [236, 245], and disruption of the actin cytoskeleton, using the cytochalasin B drug, inhibited its entry [236]. Additionally, Glogauer et al. measured an enhanced uptake of lucifer yellow, another fluid-phase endocytic marker, of membrane lipids and of membrane glycoproteins (Con A) due to electric fields. Observations using microscopy confirmed the presence of electroinduced vesicle patterns inside electroporated cells with FITC-BSA, FITC-Con A, β-galactosidase, 70 kDa FITC-dextran [236, 237, 245]. In addition, β-galactosidase entry was completely suppressed when microtubules were disrupted using colchicine [237]. All these investigations concerned proteins and not DNA. Fundamental differences between the entry of these two molecules reside in the fact that proteins, by contrast to DNA, can enter the cells when added up to 4 h after the application of the electric field, and their presence during the application of the electric field can reveal homogeneous labeling of the cytoplasm [237, 245]. The later observations of DNA in aggregates inserted into the membrane for ten minutes and then inside the cytoplasm, however, support an endocytic process [171, 197, 198, 200]. Moreover, post-pulse temperature has a strong effect on DNA expression [184, 246]. Cell placed at 4 °C only for 10 min after the application of the electric field showed almost no expression while increasing the temperature up to 37 °C increases the transfection efficiency. In addition, it was shown that the lifetime of the permeable state is dependent on the cytoskeleton [131]. The processes occurring in the 10 min following the pulsation are cell-dependent.
Actin Participation
A budding structure from the plasma membrane is a prerequisite for any endocytic pathway [247]. All types of endocytosis require the involvement of actin for both the budding step and the early stage of the endosomal transport. Recently, actin was shown to be recruited at the sites where DNA-membrane interactions occur [231]. Indeed, bursts of actin polymerization were detected as early as 3 min after the application of the electric field (Fig. 12). These actin patches were observed only at the side of the cell where DNA can interact with the membrane and, more importantly, only when DNA was present during the application of the electric field. The size, distribution, and persistence at or near the cell membrane coincided very well with those of DNA aggregates and additional experiments demonstrated a colocalization between these two structures. Moreover, disruption of the actin network, using the latrunculin B drug, led to a significant decrease in DNA accumulation at the plasma membrane, however without changing the appearance in aggregates. Actin disruption also caused a decrease in DNA expression, even when cells were treated 5 min after DNA electrotransfer [230, 231]. While actin patches started to disappear 15 min after the application of the electric field, some could remain visible for longer. Actin therefore does not appear to be implicated in the initial formation of the DNA-membrane interactions but it appears to contribute to the stabilization of DNA complexes at the membrane, to DNA internalization and to the early stages of intracellular transport. Membrane actin polymerization is known to occur when a high concentration of PIP2, which is highly negatively charged, is present in the membrane [248]. PIP2 recruits dynamin proteins that polymerize at areas of high membrane curvature [249]. Dynamin subsequently initiates actin polymerization. It is possible that the high local density of negative charge in the DNA aggregate could trigger a similar response from the actin network. As is the case for extracellular pathogens, membrane invaginations could also form due to the insertion of the comparably massive DNA aggregates into the membrane, without any assistance from the cell machinery [250]. Thus, binding to the membrane and a subsequent connection to the actin network could be a very general means for particle engulfment and transport which could be exploited by both pathogens (bacteria, viruses) and non-viral vectors.
Fig. (12).
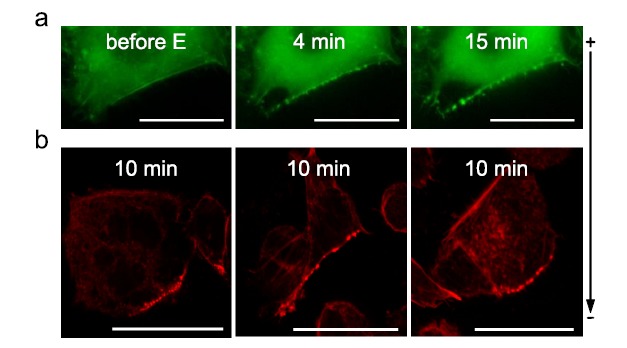
Actin patches formation after gene electrotransfer. DNA was electrotransferred into CHO cells via the application of 10 electric pulses of 5 ms at 1 Hz and 0.4 kV/cm. (a) Time lapse of EGFP-actin expressing cells electropermeabilized in the presence of DNA. Scale bar: 10 µm (b) Phalloidin-rhodamine labeled cells fixed 10 min after electropermeabilization in the presence of DNA. Scale bar: 20 µm. From [231].
Macropinocytosis of DNA
As noted above, macropinocytosis was the first among all endocytic pathways to be implicated in macromolecule internalization. The use of macropinocytosis inhibitors such as wortmannin and EIPA both significantly decrease DNA transfection efficiency [251]. EIPA has been used as the main diagnostic test to identify macropinocytosis, since it inhibits Na+/H+ exchangers that are very important for this endocytic process [252-254]. Wortmannin inhibits phosphoinositide 3-kinases (PI3Ks), which are responsible for the formation of lipid microdomains in membrane ruffles and the dynamics of macropinocytic cups [255]. Colocalization analysis yielded 25% of colocalization between DNA and 70 kDa dextran (Fig. 13) [251], which is predominantly labels macropinosomes in control cells, and wortmannin treatment eliminates endocytosis of 70 kDa dextran [255].
Fig. (13).
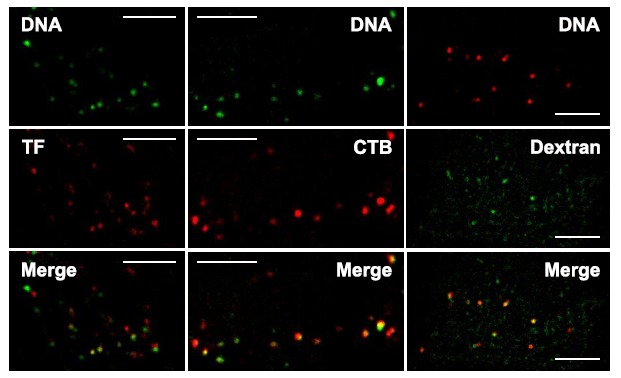
Colocalization of DNA with several endocytic markers. Transferrin (TF) highlights the involvement of clathrin-mediated endocytosis, cholera toxin B (CTB) the participation of caveolin/raft-mediated endocytosis, and 70 kDa dextran the contribution of fluid-phase endocytosis. DNA was electrotransferred into CHO cells via the application of 10 electric pulses of 5 ms at 1 Hz and 0.4 kV/cm. Images were taken sequentially using wide-field microscopy. Scale bar: 5 µm. From [251].
Therefore, several studies together show that DNA electrotransfer fulfills many of the conditions defining macropinocytosis [256]: electroporation induces membrane ruffles and blebbing [235-237], electrotransferred DNA colocalizes with the fluid-phase marker 70 kDa dextran, and gene expression is significantly reduced by inhibition of PI3K (wortmannin), Na+/H+ exchangers (EIPA), actin and microtubules dynamics (latrunculin B, jasplakinolide, nocodazole and taxol) [230, 231] and dynamin (dynasore and perhaps genistein) [233]. Macropinosomes would then represent about 25% of the DNA aggregates visible in cells.
Clathrin-mediated Endocytosis
Clathrin-mediated endocytosis is perhaps least suspected to participate in DNA electrotransfer, since DNA has no known receptors on cell membranes. Nevertheless, recent works show that it is partly involved in DNA internalization after electroporation [232]. The percentage of transfected CHO cells and their expression level are significantly decreased by treatment with both chlorpromazine and monodansylcadaverine [251], which are specific blockers of clathrin-mediated internalization [257]. Moreover, colocalization analysis between DNA and the clathrin-endocytosis marker transferrin establishes 25% colocalization between these two compounds (Fig. 13) [232]. These results are further supported by complementary evidence in B16 cells in which treatment with chlorpromazine or concanavalin A also affects gene expression [229, 233]. Additionally, Wu et al. report a drastic reduction of DNA expression after incubation with dynasore, an inhibitor of dynamin [233]. Dynamin, like actin, is crucial for numerous endocytic pathways including clathrin-mediated endocytosis [258].
Caveolin/raft-mediated Endocytosis of DNA
Caveolin- and raft-mediated endocytosis represent a large subset of pathways that share common features, among which is a strict requirement for cholesterol [259]. Cholesterol-sensitive internalization of lipid rafts can be classified into three major pathways including dynamin-dependent endocytosis of caveolae or non-caveolar vesicular carriers (IL-2), dynamin-independent endocytosis via non-caveolar tubular intermediates (GEECs), and dynamin-independent endocytosis of non-tubular carriers (flotillin, Arf6) [247, 259]. The depletion of cholesterol by MβCD and filipin have been shown to disrupt the composition of lipid rafts, which are therefore unable to segregate proteins and thus to perform endocytosis [260]. All of these pathways are also genistein- and actin dynamic-sensitive [261].
MβCD affects DNA expression drastically without altering either the cell membrane electropermeabilization or the DNA-membrane interaction [232]. Genistein, filipin and EIPA treatment also strongly impair gene expression [233, 251]. Genistein has also been shown to inhibit recruitment of dynamin at the surface of vesicles containing SV40 [262], which could indicate that non-clathrin, but dynamin-dependent endocytosis would be more affected by treatment with genistein (caveolae and IL-2 pathways). Recent work has demonstrated significant and long-lasting inhibition of DNA electrotransfer in mouse muscle after MβCD treatment [229]. This in vivo experiment very strongly supports the involvement of endocytosis for successful DNA electrotransfer. An earlier study demonstrated that although the internalization of adeno-associated virus is clearly mediated by the GEEC pathway, it remains sensitive to EIPA [263]. Therefore, EIPA appears to be also a GEEC endocytosis inhibitor. Consequently, a part of the diminution in the transfection efficiency after EIPA treatment [251] could be attributed to the involvement of the GEEC pathway in the internalization of electrotransferred DNA. The predominant raft marker CTB has been implicated in caveolar [264-268], non-caveolar [269, 270], flotillin-dependent [271] and the dynamin-independent GEEC pathway [269]. Colocalization study between DNA and CTB revealed about 50% of shared subcellular structures (Fig. 13), which probably reflects the contributions of several of the raft-mediated pathways (caveolin, flotillin, GEEC, IL-2 and Arf6) to DNA internalization [232].
Conclusion on Cell-driven Endocytosis
It appears therefore that cell-driven endocytosis is largely involved in DNA electrotransfer. Inserted DNA would be recognized by the cell, due to its size or charge, as cargo for endocytosis. DNA sensitivity to nuclease action up to one minute after pulse administration would reflect the time needed for the closure of membrane invaginations prior to endocytic transport. The persistence of DNA at the membrane for several minutes could then correspond to the time required for vesicles to bud off from the membrane. The DNA aggregates interacting with the plasma membrane range in size from 100 nm to 500 nm [171, 197]. This wide range of sizes may explain why DNA appears to be internalized by several endocytic pathways. A study on the uptake of microspheres showed that particles up to 200 nm were internalized mainly by the clathrin-mediated pathway. With increasing diameters, a shift to caveolin/raft-mediated endocytosis was observed and for 500 nm microspheres the latter was the predominant endocytic pathway [272]. Thus, particle size in itself can determine which pathway is followed.
4.5.3.4. Membrane Repair
It seems currently that model (iv) (see section 3.5.3.1) has no direct evidence for its involvement in the transfer of DNA or other molecules via electroporation. Cell membrane repair can lead to endocytosis for extracting damaged parts of the membrane or to compensate for exocytosis, which brings new functional components to the membrane [273, 274]. Pore forming proteins, such as bacterial toxins or the membrane attack complex (MAC) of the blood complement, leave some transmembrane pores that are eliminated by endocytosis. Any membrane repair mechanism involving exocytosis and endocytosis is initiated by calcium influx through the damaged membrane. With the use of microelectrodes, local intracellular calcium was shown to be recruited to the locus of the electroporated membrane [275]. Electroporation also activates exocytosis, since lysosome content is released at the cell surface. It has even been proposed to use electroporation to quantify the lysosomal exocytosis competence for the purpose of cell characterization [276]. Since exocytosis is activated after electroporation, one can expect endocytosis to occur to maintain cell membrane length. Electroinduced DNA binding to the cell surface and DNA expression due to electroporation are both improved in a concentration dependent way, up to a maximum threshold, if calcium is present in the pulsation buffer [187, 204]. It may be that calcium here activates cell repair and uptake of DNA together with the cell membrane. Whether this model of the endocytosis process occurs and whether DNA is present in these vesicles remain to be proved.
4.5.4. Conclusion on Endocytosis
Several studies point towards the contribution of endocytosis in the electrotransfer of DNA, but more investigations have to be performed in order to understand what type(s) of endocytosis would be involved. It is necessary to understand as well how electric fields could stimulate such processes. Also notably, any endocytosis model would only explain the internalization of large molecules as it does not support the free membrane crossing of small molecules. It has therefore to be considered to occur in parallel to another model valid for small molecule transmembrane exchange. One model that could reconcile all the DNA internalization models would be that DNA accumulates where pores are formed and that its electrophoretically driven insertion in the membrane pulls the pore and the plasma membrane around. This would generate membrane curvature that could be recognized as an emerging endocytic vesicle and induce a similar response from the cell as for an endocytic process, with the recruitment of actin, clathrin, caveolin, dynamin and other endocytic regulators.
4.6. Intracellular DNA Motion
The cytoplasm contains a dense cytoskeleton network (microfilaments, microtubules, and intermediate filaments), along with a variety of cellular organelles and abundant amounts of proteins and other molecules. This mesh-like structure hinders the diffusion of plasmid DNA. The mobility of small molecules (i.e. less than 500-750 Da) in the cytosol is reduced to only 3-4 fold less than in water, but the mobility declines rapidly with the larger sized molecules [277, 278]. The mobility of microinjected plasmid DNA is extremely small or even negligible in the cytoplasm or cell nucleus [277, 279-281]. Lukacs et al. have shown that DNA fragments longer than 2000 bp showed no translational diffusion through the cytoplasm [279]. Under the conditions induced during electropermeabilization, the time a plasmid DNA takes to reach the nucleus is significantly longer (several hours) than the time needed for a small molecule (few seconds) [171]. It is observed to cross the cytoplasm in the aggregated form. DNA expression is detected 3 h after the pulsation, reaches is maximum at around 12 h and stays at this level of expression for 16 additional hours before decreasing [219]. When 20 min depletion of ATP content in the cell is performed up to 2.5 h after electropulsation, the efficiency of transfection is affected but not the cell viability. Depletion performed 3 h or later after the electrotransfer does not affect DNA expression. These results show that the intracellular route of DNA is dependent on cellular energy levels and therefore is based on cell-driven processes.
4.6.1. Active Transport of the DNA Aggregates
DNA motion inside CHO cells has been recently studied using single-particle tracking (SPT) [230]. Electrotransferred DNA trajectories possess portions of active transport interrupted by phases of nearly immobility (Fig. 14). During the phases of active transport, DNA aggregates featured a motion on average having a velocity of 250 nm/s, persisting for 6 s and leading to a displacement of 1.3 µm. However, the distributions were rather broad with velocities from 50 nm/s to 3400 nm/s, displacements from 0.1 µm to 12 µm and active transport durations from 2 s to 30 s. These ranges are in agreements with other types of intracellular particle dynamics as observed for viruses [282, 283], polyplexes [284, 285], lipoplexes [286, 287], receptors [288, 289], endosomes [290, 291], and mitochondria [292]. Lower velocities were shown to correspond to actin-associated transport [230]. Indeed, after disruption of the microtubules using the nocodazole drug, active transport of the DNA still occurred and the measured velocities were in the range expected for myosin motors operating on actin – between 50 nm/s and 300 nm/s for myosin VI and between 250 nm/s and 500 nm/s for myosin V [293-295]. In addition to motor driven transport, actin-related movement could be also due to bursts of actin polymerization which was reported to drive viruses, bacteria or endosomes from the plasma membrane to the cytosol with mean velocities ranging from 50 nm/s to 600 nm/s [296, 297].
Fig. (14).
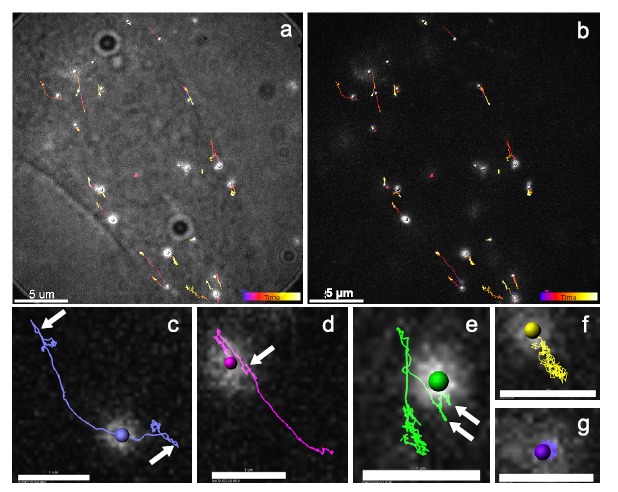
Single particle tracking of DNA aggregates in CHO cells after electrotransfer. (a, b) DNA aggregate trajectories in the cytoplasm after gene electrotransfer. (a) overlay between the transmission light image and the fluorescence depicted in (b). The color of the trajectories codes time with blue corresponding to 0 s and white to 35 s. (c-g) Some trajectories of the time series (b), scale bar: 1 µm. Trajectories show long (c, d) or short (e, f) distance excursions or almost immobile aggregates (g). White arrows point at the part of the trajectories exhibiting bidirectional motion. From [230].
SPT experiments have demonstrated that microtubule-driven transport is also responsible for electrotransferred DNA motion in the cell (Fig. 16, step 5). The quantified fast velocities corresponded well to the known mean velocities of dyneins, 220-1200 nm/s, and kinesins, 200 nm/s to 1600 nm/s [298-300]. Colocalization between DNA trajectories and microtubules and between DNA aggregates and the dynein motor supported these findings [230]. Moreover, nocodazole treatment effectively suppressed long-range transport of the DNA aggregates, unambiguously proving that fast active transport of DNA aggregates is microtubule-related. Interaction between plasmid DNA bearing specific sequences and microtubule-related motor proteins has been recently reported [301]. Disruption of microtubule and actin networks leads to a decrease of transfection level and efficiency [230]. While this confirms the importance of the microtubule and actin-related active transport of DNA for successful electrotransfer, it is interesting to note that Vaughan and Dean reported that the application of nocodazole or latrunculin B does not have any effect on gene expression following electroporation of human adenocarcinoma cells [302]. However, microinjected DNA expression in TC7 cells was decreased when microtubules were disrupted or dynein inhibited. This indicates that the relation between gene expression efficiency following electroporation and the active intracellular transport of DNA aggregates is not direct. A further hint in this direction comes from experiments in which the cellular microtubule network is stabilized. While application of taxol leads to a reduction of gene expression and of active transport in CHO cells [230], as expressed by significantly shorter overall displacements, slower velocities and decrease of the active transport events, Vaughan and Dean report a pronounced increase of gene expression in A549 cells [302]. For the same cell line, another study reports the suppression of high velocities after taxol treatment in the case of endocytic trafficking of the epidermal growth factor receptor [289]. The observation that stabilization of both the microtubules and the actin network does not enhance active transport dynamics [230] could thus be explained by the higher density of the respective cytoskeleton, which can act as a barrier for transport of the DNA aggregates, or can result from changed motor dynamics due to the drug application.
Additionally, SPT experiments indicated that several motor proteins are acting on one DNA aggregate [230]. This conclusion is suggested by two facts, namely the duration and displacement of active transport phases and the observed bidirectionality of the aggregate motion (Fig. 14). Single dynein, kinesin and myosin motors are described to run over distances from a few hundreds of nanometers to 2 µm with transport durations of less than 3 s [293-295, 298, 300, 303]. The longer travelled distances and the larger persistence of travelling measured suggest that DNA aggregates are driven by multiple motors of the same type, as is normally the case for cellular cargo transport [304, 305]. A similar conclusion can be drawn from the frequent observation of a bidirectional motion of the DNA aggregates, i.e. the active transport suddenly changes direction and the aggregate takes a nearly identical trajectory in the opposite direction. While this can be caused by (+) ends and (-) ends of microtubule/actin lying close to each other, it is more likely that the bidirectionality is caused by the presence of several oppositely directed motors on the same aggregate. If not one, but several motors act simultaneously on one aggregate, bidirectionality can then result from a tug of war of these motor proteins [304, 305].
Finally, measurements of the diffusion coefficient of the DNA aggregates in CHO cells revealed a distribution spread over several orders of magnitude (10-1 and 10-5 µm2/s) [230]. Similar profiles can be found for the diffusion of viruses [306], receptors [307], polyplexes [284] and lipoplexes [286]. Giving the Stokes-Einstein relation, Brownian motion depends on the viscosity of the medium and the size (radius) of the particles. A DNA aggregate of 100 nm, freely diffusing at 10-3 µm2/s, see an apparent viscosity of about 230 cP. Although DNA aggregates are expected to have a size distribution ranging from 100 nm to 500 nm, this cannot explain the observed distribution width [167, 171]. Moreover, the displacement of diffusing DNA particles did not depend on time, meaning that confined diffusion was the actual mode of diffusion of the DNA aggregates [230]. It is more likely that molecular crowding, exclusion, and obstruction in the cytoplasm control the mobility of particles [277]. In order to benefit from active transport, DNA must be in vesicles or interact with some adapter proteins that allow for its binding to the motor proteins. Therefore, the broad range of diffusion coefficient of the DNA could also reflect different sizes and maturation stages of the vesicles on their pathway from the plasma membrane to the nucleus [284].
4.6.2. Endosomal Trafficking of the DNA
Given the different models of internalization, DNA could enter the cell in a free form (electropores or electrophoresis) or could be trapped into some endosomal vesicles. In the first case, DNA would not remain free for a long time. It is known that DNA released in the cytoplasm, after a direct entry (microinjection) or after endosomal escape, will rapidly form complexes with DNA-binding proteins, polyamines and other polycations [308]. These complexes could serve as charge neutralization, and the induced condensation of the DNA, reducing therefore its size, may allow enhanced motion. It could as well protect DNA from its otherwise rapid degradation [309]. It is possible that some of the proteins that bind the DNA serve as adapters to interact with cytoskeleton motors. DNA does not bind directly to motor proteins, so the only way free DNA could use intracellular transport would be through intermediary proteins. If DNA enters via an endocytic process, the membrane of the endosomes already contains the proteins that interact with the motor proteins (Rabs) [297]. Endosomes, as cell organelles, are efficiently transported through the cell cytoplasm, and DNA could take advantage of this machinery to reach the nucleus. Coonrod et al. found that all exogenous DNA, including electrotransferred ones, colocalized to some extent with cathepsin B, a lysosomal protease, 3 h after the application of the electric field [310]. After electroporation, DNA can be routed to the lysosomal compartment and this could be due to a beforehand endosomal trafficking.
Quantitative colocalization study has shown that DNA is located in significant amounts in Rab5-, Rab11-, Rab9- and Lamp1-vesicles, which respectively correspond to early endosomes, recycling endosomes, late endosomes and lysosomes (Fig. 15) [251]. The analysis consisted in tracking simultaneously labeled DNA aggregates and EGFP-endosomal markers expressed transiently in CHO cells. In the hour following DNA electrotransfer, 70% of the DNA aggregates were in Rab5-structures, 50% in Rab11-compartments and 30% in Rab9-vesicles. Just 1-2 h after DNA electrotransfer, 60% of the DNA was located in Lamp1-containing structures. These results indicate that DNA is mostly engulfed into vesicles during its transport through the cytoplasm and that it follows the classical intracellular trafficking routes (Fig. 16, steps 5-6) [311, 312]. One should note that endosomal trafficking comprises a continuum of vesicles shuttling between compartments. Although the utilized molecular markers undoubtedly distinguish these compartments, they can also partially and transiently overlap [313]. This explains why the above percentages do not sum up to 100%. The earlier findings that DNA is present in aggregates along its way to the nucleus and remains as such even after its expression is achieved are consistent with an endosomal trafficking of the DNA after electrotransfer [171]. These results imply that a large part of the DNA aggregates is likely to be lost into recycling and degradation pathways. In addition to the established vesicles transport between the early and late endosomes to the Golgi [314], several publications mention the possibility of vesicles shuttling between lysosomes and the Golgi or the ER, which would give the endocytosed DNA a last chance to escape degradation [252, 313]. However, the engulfment of DNA into the trafficking pathways, although it sends some fraction of the internalized DNA into dead ends, may increase the overall stability of the DNA aggregates in the cytoplasm. Supporting this idea is a comparison between the half-life of microinjected DNA on its own and microinjected DNA encapsulated into lipid particles [309]. The latter had its degradation delayed for about 3-fold the time needed for naked DNA, for which the half-life was measured to be 1-2 h in HeLa and COS cells and 4 h in myotubes.
Fig. (15).
Dual-color SPT of DNA aggregates and endosomal proteins in CHO cells after electroporation. CHO cells separately expressing EGFP-Rab5, Rab11, Rab9 and Lamp1 plasmid constructs were electroporated in the presence of Cy5-labeled DNA. Using quantitative colocalization analysis, the respective movements of the objects are investigated. Correlated trajectories are highlighted in orange (DNA) and light purple (EGFP-markers), colocalized trajectories are drawn in blue (DNA) and pink (EGFP-markers) and the non-correlated and non-colocalized trajectories are in red (DNA) and green (EGFP-markers). (a) DNA in early endosomes (Rab5), (b) DNA in recycling endosomes (Rab11), (c) DNA in late endosomes (Rab9), and (d) DNA in lysosomes (Lamp1). Scale bar: 5 µm. From [251].
Understanding the routes taken by DNA into the cell interior gives potential insight into optimization of its delivery to the nucleus. One option would be the inhibition of lysosomal degradation activity by chloroquine, which induces higher gene expression in many transfection methods [315-318]. Another interesting possibility would consist in the use of nanosecond electric fields which were theoretically shown to achieve electropermeabilization of internal membranes without affecting the plasma membrane or the nuclear envelope [319]. In such case, DNA release from the endosomal trafficking pathway may be optimal at the late endosome level (about 1 h after DNA electrotransfer), meaning before DNA transfer into the degrading lysosomes but after DNA transport near the perinuclear region. The development of strategies fostering the efficient escape of DNA from late endosomes and lysosomes therefore appears to be an important step in the improvement of gene transfer methods based on electroporation.
4.7. Crossing the Nuclear Envelope
The nuclear envelope represents the last, but significant, obstacle to the expression of electrotransferred plasmid DNA (Fig. 16, step 7). Direct observations 24 h after the electrotransfer show that even in DNA-expressing cells, most of the DNA visible in aggregates remains at the perinuclear region [171]. Only a small fraction crossed the nuclear envelope, which was nevertheless sufficient to induce robust expression. The lack of efficient nuclear import of plasmid DNA from the cytoplasm was first identified more than 20 years ago. While molecules smaller than 40 kDa can diffuse through the nuclear pore complexes, larger molecules must carry a specific sequence so called the nuclear localization sequence, in order to cross [320]. The relatively large size of plasmid DNA (2-10 MDa, 1 kbp = 0.66 MDa) makes it unlikely that the nuclear entry occurs by passive diffusion.
With regard to quiescent cells, transfection levels in dividing cells are greatly higher, which means that DNA takes advantage of destabilization of the nuclear envelope during mitosis to enter into the nucleus. The synchronization of the electrotransfer with the mitotic phase has been proved to increase DNA delivery [168, 321-323], supporting the hypothesis that the denaturation of the nuclear membrane greatly facilitates the direct access of DNA to the nucleus. However, at least some of the plasmids used for electroporation contain the DTS sequence (DNA nuclear targeting sequence). The presence of this sequence is shown to significantly increase interactions with importins, proteins involved in the nuclear import, further nuclear localization of the DNA and expression of DNA [301, 324]. It is an interesting observation requiring further investigations in order to conclude about the possible importance of DNA transport via nuclear pores.
4.8. Gene Expression
The involvement of DNA electrophoresis as an important step for successful DNA electrotransfer implies, in turn, that the duration and number of pulses also control the transfection efficiency (Fig. 16, step 8). At fixed electric field strength, pulses of millisecond range achieve better transfection than pulses of microsecond range [29, 43, 127, 171-174, 184, 198, 325, 326]. Likewise, larger pulse numbers increase the transfection level. As a confirmation, when applying non-permeant LV pulse after permeant HV pulse, increasing the number and the duration of the LV pulses increases gene expression [138, 191, 327, 328]. The frequency of the pulse is also important. Increasing the delay between the pulses over 1 s for typical series of electric fields, or over 100 s for HV/LV combined electric fields, strongly decreases gene expression [172, 191]. In CHO cells, DNA transfection has been shown to linearly depend on the pulse number N and on the electropermeabilized surface [172]. The logarithm of the transfection depends linearly on the logarithm of the pulse duration T [172]. Therefore, the gene expression can be written, for field conditions not affecting to large extend the cell viability, [118, 172, 213]:
where K is a constant and f(DNA) a function depending on DNA. It is a complex dependence on, for instance, DNA concentration, which should take into account that above a certain threshold, high levels of DNA are toxic [172, 177, 183, 329, 330]. Like for small molecule exchange, gene expression depends on the surface of the membrane brought into the permeable state (under the control of E), and the level of permeability of that surface (under the control of N and T). However, for the transfer of small molecules, pulse durations are on the order of hundred microseconds, and for DNA transfer, pulse durations are in the millisecond range to ensure DNA electrophoresis necessary for DNA to interact with the membrane.
As early as in the pioneer article on cell electroporation, the importance of the DNA topology in gene electrotransfer has been established [26]. In mouse lyoma cells, electroporation in the presence of linear DNA reached higher level of transfection in comparison to cells electroporated with circular DNA. Following investigations in bacterial and mammalian cells, using two or three DNA isomers (linearized, circular supercoiled or circular relaxed DNA), showed that circular DNA, rather than linear DNA, is optimal for successful gene electrotransfer [169, 331, 332]. These studies revealed that DNA interaction with the membrane is not dependent on its topological isometry but DNA expression is significantly lower for linearized DNA. In bacterial cells, Xie et al. observed a lower stability of the linear DNA, which is interpreted as a rapid degradation by intracellular enzymes [332]. Based on DNA geometry and stiffness, one can suggest other hypotheses [331]. Since circular DNA has a more congealed form and a bigger diameter than linear DNA (20-30 nm vs. 2-3 nm), its electrophoretic migration could be more efficient and/or increase the size/lifetime of membrane defects (pores) or cause larger membrane invaginations near the absorbed DNA. The membrane permeabilization could thus be favored. Linear DNA, although having a small diameter, has a longer contour length (1.6 µm vs. 0.5 µm). When being absorbed to the cell surface, this large but thinner occupancy may decrease the percolation and coalescence of membrane defects engendering lower permeabilization. All these hypotheses imply that DNA internalization is the step being affected by the DNA topology, nevertheless DNA uptake does not seem to be dependant of the DNA isometry [169, 332].
5. Gene electrotransfer – in vivo aspects
A decade after the first in vitro gene electrotransfer was reported [26], the first in vivo transfer of DNA into tissue was published [333]. A mixture of two supercoiled plasmids was injected subcutaneously, and following a 10-60 minutes delay to allow for the dispersion of DNA, pulses were applied to the skin by a prototype pulse generator. From the treated skin, primary fibroblasts were obtained and stable gene electrotransfer was observed. The authors observed lower efficiency compared to cells treated in vitro and suggested that the difference could be due to the different cell environment. Since then, the use of in vivo gene electrotransfer has expanded, and many studies were presented in which different reporter genes and therapeutic genes were tested. It was shown that gene electrotransfer is a safe and efficient method in numerous tissues, including muscle, tumor, skin, liver, heart, cornea, brain, lung, kidney, and bladder [30, 48, 49].
Although abundant in vitro studies have attempted to explain gene electrotransfer mechanisms, we cannot entirely transfer our knowledge from the in vitro to the in vivo environment. Some of the gene electrotransfer mechanisms discovered using in vitro studies are still under debate for gene electrotransfer in tissues. Therefore in this section we will focus on gene electrotransfer mechanisms in tissues.
5.1. Electropermeabilization and Electrophoretic Component
As for in vitro experiments, two major steps are required for tissue-dwelling cells to be successfully transfected in vivo: electropermeabilization of the cell membrane and electrophoretically driven DNA migration through the tissue. The first in vivo study which discriminated between the effect of electropermeabilization and that of DNA electrophoresis was presented by Bureau et al. [190]. They studied both steps by applying combinations of two types of pulses in mouse tibial cranial muscle: (i) HV pulse, which permeabilizes cell membranes (short pulse - 100 µs; with high strength - 800 V/cm) and (ii) LV pulses, which electrophoretically drag DNA to the vicinity of the cell (long pulses – 83 ms; with low strength - 80 V/cm). It was shown that the electrophoretic role of the LV pulse is especially crucial in transfection efficacy. The author’s hypothesis is that the major role of DNA electrophoresis consists in driving the insertion of the DNA into the plasma membrane, rather than driving the translocation of the DNA across the plasma membrane. Satkauskas et al. also demonstrated that the electrophoretic component of LV pulses was sufficiently important that even if the cell membrane permeabilization caused by the HV pulse is not optimal, gene electrotransfer efficiency is still preserved if the LV pulse is present [191]. When DNA is injected after the LV pulse, no increase in gene electrotransfer efficiency is observed as compared to cells transfected with HV pulse only. On the other hand, when DNA is injected either before or after the HV pulse, but before LV pulse, gene electrotransfer efficiency is drastically increased. These results confirm the importance of the electrophoretic component of the LV pulse. An important consideration for in vivo applications, however, is that long pulses can nevertheless be detrimental for successful gene electrotransfer due to local increases of temperature [334, 335] which affects both DNA stability and target cell viability.
Some electric pulse generators can also deliver exponentially decaying pulses, which mimics the combination of HV and LV pulses. Such pulses have a peak voltage (i.e. HV component), which enables membrane permeabilization, followed by a low voltage tail (i.e. LV component), which enables electrophoretic DNA drag through the tissue matrix [336]. Since low voltage tails significantly decrease cell viability, exponentially decaying pulses are more frequently used for bacterial transformation than for in vivo gene delivery.
5.2. Electric Field Distribution in Tissues
The distribution of electric fields in tissues, which is not necessarily spatially homogeneous, is an additional important factor in the effective of in vivo DNA electrotransfer and it depends, for example, on tissue conductivity (Table 1). Since it is the local electric field that affects cell membranes and causes DNA migration, one must know the local electric field distribution that is generated by application of electric pulses in order to optimize DNA electrotransfer in intact tissue. Three-dimensional finite element models and corresponding in vivo studies were used to illustrate that local electric field distribution and tissue conductivity changes are highly heterogeneous [337], which is further affected by electrodes’ shape and placement [338].
Table 1.
Different tissue conductivities. From [339].
| Conductivity (S/m) | ||
|---|---|---|
| TUMOR | 0.22-0.4 | |
| FAT | 0.02-0.04 | |
| MUSCLE | transversal | 0.04-0.14 |
| longitudinal | 0.3-0.8 | |
| SKIN |
stratum corneum |
0.0000125 |
| lower skin layers |
0.227 | |
| HEART | 0.06-0.4 | |
| BONE | 0.01-0.006 | |
| KIDNEY | 0.6 | |
| LIVER | 0.023-0.2 | |
| LUNG (inflated) | 0.024-0.09 |
In order to electroporate tissue and retain cell viability, which is crucial for successful gene electrotransfer, one must know the electroporation threshold of tissue in addition to the electric field distribution to predict the optimal window for gene electrotransfer. The local electric field applied must induce reversible rather than irreversible electroporation. An experimental and theoretical study was performed on mouse skeletal muscle in which the electroporation threshold was determined with respect to muscle anisotropy [340]. When electric fields were applied parallel to the orientation of muscle fibers, the electroporation threshold was found to be 80 V/cm. When electric fields were applied perpendicular to muscle fibers, the electroporation threshold was found to be 200 V/cm (both for 8 pulses of 100 µs applied at 1 Hz). The exact determination of the local electroporation threshold of tissue is crucial not only for achieving efficient gene electrotransfer in clinics but also to avoid entering the irreversible electroporation domain. Furthermore, if excessively strong electric fields are used, or if they are applied with too many repetitions, sparking and high currents can occur, which, in addition to being harmful for tissue, can damage pulse generators. This phenomenon was studied on gel phantom and measured using ultrasound, magnetic resonance imaging, microphone and optical recording. The authors showed that electrical breakdown occurs across ionized electrolysis near the electrodes (mostly near the cathode), causing loud sounds, sparking and high currents [341].
5.3. Electrodes used In Vivo
Different electrodes can be used for gene electrotransfer in vivo, depending on whether the tissue to be electroporated is readily accessible (e.g. skin, muscle) or more embedded (e.g. deep-seated tumors). Several types of electrodes are currently used that differ in construction and material composition [336]. The chosen metal can be e.g. stainless steel, titanium, copper, aluminum, or platinum; which differ in their electrical conductivity, price, and resistance to corrosion. The release of metal particles into ions in the electroporated medium can be monitored by the electric field parameters [342]. For medical treatment, mostly electrodes made from stainless steel or titanium are used, but recent development proposes electrodes having a gallium core in order to absorb the heat, inherently generated, instead of the tissue [343]. Choosing the appropriate instrument is a matter of the desired electric field distribution and total area of gene electrotransfer, which are both highly dependent on electrode geometry (Fig. 17).
Fig. (17).
Effect of the diameter of needle electrodes on the electric field intensity and distribution, using the same pulse amplitude and distance between electrodes. (a) Needle diameter 0.3 mm, pulse amplitude 960 V. (b) Needle diameter 0.7 mm, pulse amplitude 960 V. (c) Needle diameter 1.1 mm, pulse amplitude 952 V. From [344].
The choice of electrodes is also important for avoiding the release of metallic ions during tissue treatment [345]. For most applications in which relatively low voltage electric fields are applied, stainless steel electrodes are the most appropriate [346, 347]. When higher voltage electric fields are required, it is important to use electrodes whose surfaces are least susceptible to electrochemical reactions (e.g., platinum coated electrodes) [348]. Most electrodes used for in vivo applications are plate or needle array electrodes, but others have recently been developed as well.
5.3.1. Plate Electrodes
Plate electrodes are composed of two parallel plates (Fig. 18a), which are shaped with round edges in order to minimize electric field heterogeneity and arcing along the plates' edges. These can be used transcutaneously on a variety of tissues and offer relatively good homogeneity of the administered electric field. However, it is sometimes difficult to pinch tissue into the narrow space between the electrode plates, which are therefore not useful for larger animals or humans. Furthermore if the target for gene electrotransfer is under the skin, stronger electric fields must be applied in order to permeabilize the tissue, which can cause skin burns.
Fig. (18).
Electrodes for in vivo applications. (a) plate electrodes used for tissue on surface (e.g. skin, muscle), (b) needle electrodes for deep-seated target, (c) multiple needle electrodes used for larger targets. Yellow areas represent the epidermis, red areas the dermis, and the grey round mass represent the targeted tissue. From [336].
5.3.2. Needle Array Electrodes
Needle array electrodes consist of two or more parallel needles between which an electric field is generated (Fig. 18b, c). As needle electrodes can penetrate the skin, they can be used to treat more deeply embedded and/or larger volume tissue. They also allow the application of electric fields in different directions without the need to move the electrodes, which improves the efficiency of gene electrotransfer [193, 349]. The primary disadvantages of needle electrodes are that the electric field between the needles is highly heterogeneous, and that the electric field near the tip of the needle is particularly strong, which can cause tissue burns [334, 350].
5.3.3. Surface Electrode Pins
Surface electrode pins, also termed multi-electrode arrays, consist of groups of tightly spaced pins around which an electric field can be generated. They are very useful for DNA delivery to skin, they are minimally invasive, cause no muscle stimulations or pain during use, and offer better control over the direction of the applied electric field.
5.3.4. Other Types of Electrodes
Specialty electrodes have also been developed for the treatment of tissues with idiosyncratic shapes. including spatula-shaped electrodes for gene electrotransfer of mouse muscles [351]; gutter-shaped electrodes for gene electrotransfer of arteries [352]; and long, single-needle electrodes with insulating tubing for treating deep-seated tissue. These electrodes are less invasive than multiple-array electrodes and since the electric field is applied only at the distal site of the needles, they leave the skin unexposed [353]. Other electrodes are non-invasive needle-free patch electrodes for treating the skin [354]; electrodes designed for endoscopic and/or laparoscopic applications [355]; grid electrodes with which pulses are applied more quickly and homogeneously [356]; flexible micro-needle array electrodes that adapt to the tissue surface profile while still achieving good electrotransfer efficiency with minimum tissue damage [357] and electrodes joined with a syringe, such that DNA can be injected into tissue and the tissue subjected to electrical pulses using the same device. The latter allow for a lower electric field to be applied in order to obtain the same transfection level as plate electrodes [358].
5.4. DNA Migration Through the Tissue
The main problem of in vivo gene electrotransfer is the poor DNA distribution and low DNA mobility through dense extracellular matrix. Beyond this physical impediment to gene delivery, extracellular DNases are ubiquitous and can degrade plasmid DNA before it reaches its target [359]; this problems can, however, be mitigated by adding DNase inhibitor to the carrier solution and injecting more DNA [360]. As a consequence, only a small amount of DNA is usually in contact with target cell membranes during pulse treatment [361]. In in vitro cell culture, DNA easily surrounds cell membrane, since there is no physical obstacle. A few studies on 3-D cell models, which more closely mimic in vivo conditions, have attempted to explain the important role of extracellular matrix material on gene electrotransfer efficiency [203, 361]. Extracellular matrix represents a major obstacle for DNA electrophoretic migration to the cell, and its rigidity is age dependent. Namely, it was shown, that in young rodents, gene transfer efficiency in muscle is quite high [362, 363]. But, in older or larger animals gene transfer efficiency drops due to the larger level of connective tissue in muscles [364]. However, when a hyaluronidase enzyme, that degrades hyaluronan, was added before pulse application, a significant increase of gene electrotransfer efficiency of muscle fibers was achieved [365].
The efficiency with which tumors can be treated by gene electrotransfer is also influenced by the local extracellular matrix, which varies between different tumors types. For tumors embedded high density extra-cellular matrix area was present, gene electrotransfer efficiency can be substantially increased by injection of hyaluronidase and collagenase prior to treatment with electric fields [170]. In contrast, pre-treating tumors with these enzymes did not rescue gene electrotransfer efficiency in tumors surrounded by low density extra-cellular matrix. It has also been suggested that gene transfer could be improved by decreasing cell volume or increasing the interstitial space in tumors. Henshaw et al. assessed this possibility by treating tumors with hyperosmotic mannitol solution to draw water from and thus shrink tumor-dwelling cells prior to treatment [366]. Using this treatment the authors observed an increase in the amount of DNA in proximity to treated cells and an increase in gene expression, both in vitro and in vivo.
5.5. Timing of Electric Pulses after DNA Administration
Another key parameter governing successful gene electrotransfer is the delay between the DNA administration into the tissue and the pulse application. The literature to date is not conclusive on this problem. In quadriceps mouse muscle, it was suggested that the optimal time window for applying pulses after DNA delivery is 30 min [367], while another study on tibial cranial mouse muscle showed no difference in gene electrotransfer efficiency when the time interval between DNA injection and application of electric pulses was between 20 s and 6 h [359]. Findings on murine melanoma showed that the optimal delay for pulse delivery can approximately be 1 min after DNA administration [102], or in soft tumors can also be between 5 and 30 min [368, 369]. The differences could be due to the age of animal, properties of tissue or carrier solution [370]. DNA can be administrated after tissue is given a permeabilizing pulse (HV pulse), but must be injected before the electrophoretic long duration pulse (LV pulse) [191].
5.6. Methods of DNA Injection
There are two ways in which DNA is delivered by means of injection: local injection into the targeted tissue or intravenous systemic injection, each of which possesses advantages and disadvantages. Direct injection offers speed and high local hydrostatic pressure, which increases gene electrotransfer efficiency up to 500 fold compared to low-pressure injection techniques [371]. This pressure effect is not as significant in tissues in which there is no capsulation (e.g., tumors) or in large animals, and it can also be detrimental to tissue if swelling occurs. Direct DNA injection can be suboptimal when the DNA carrier solution is not evenly distributed through the target tissue [359] and, more broadly, because not all tissues are readily accessible for DNA injection.
Systemically injected DNA has the obvious advantage of systemic circulation and thus broader potential access to internal tissues, to which DNA can be directly delivered in some instances (e.g., liver tissue). However, along with this advantage, systemically administered DNA is diluted upon reaching target tissue [372], does not have equal accessibility to all tissues due, for example to vascular lock [129] and has to cross the barrier between blood and tissue which is particularly difficult at the brain level. Furthermore, DNases in the blood stream can degrade DNA in circulation.
5.7. Other Contributing Factors
It was showed in vitro, that electroporation can prompt the formation of reactive oxygen species (ROS) near cell membranes, which can be detrimental to cell viability [133]. Antioxidants can retain the effects of ROS activity [373], and adding antioxidants after applying electric pulses could therefore increase cell viability and thereby increase gene electrotransfer efficiency. The primary study examining this phenomenon was performed on skeletal muscle in mice, in which the antioxidant tempol was injected together with plasmid DNA, and electric pulses were applied immediately after. The authors observed a 40% increase in transfected area compared to control treatments [374].
In in vivo environments, one must also consider local temperature increases that occur when using specific pulse parameters, which in turn can strongly affect gene electrotransfer efficiency due to DNA damage/denaturation or decreased cell viability [334]. Lackovic et al. analyzed standard pulse protocols used for in vivo gene electrotransfer, where also tissue electrical conductivity was taken into account in order to determine temperature distribution within the tissue. The authors showed that numerous factors affect tissue temperature increase due to Joule heating, including electrode geometry, tissue electrical conductivity, and electric pulse parameters [334]. By decreasing pulse amplitude, length or number, lower temperature increase in tissue could be achieved.
Extreme pH changes can also occur in tissues following electric pulse application, which damages tissue fibers [375]. Furthermore, alkaline pH values (especially above 12) permanently damage DNA. Therefore a study was conducted in order to determine the role of pH changes in tissue after gene electrotransfer. The authors observed a strong pH related tissue damage being higher when hyaluronidase was added before pulsing. Namely, although hyaluronidase enzyme increases DNA uptake [170], it also increases tissue conductivity and by that electric current, which has a strong effect on pH changes. Thus, greater tissue damage was observed especially near the electrodes [376].
Another important effect of electroporation on tissues (which could not be observed in vitro) is a blood flow modifying effect in different tissues (i.e. vascular lock) [129]. After application of electric pulses a blood volume reduction was observed in tumors, while systemic vasculature was not affected [377]. In muscles a short-term reduction of perfusion was also observed [129]. Direct observation with intravital fluorescence digitized microscopy imaging was done in order to determine the effects of electric pulses on the subcutaneous blood vessel dynamics and gene electrotransfer efficiency [378]. The study showed that plasmid DNA is sensitive to vascular lock. Namely, constriction of vasculature delays movement of large sized molecules through the vasculature wall. Therefore caution is needed when DNA is administered intravenously.
Conclusion
Gene electrotransfer represents a promising delivery system for introducing foreign genes into cells for a range of medical applications. This method was presented over 30 years ago using mammalian cells in vitro, and, since then, many experimental studies have aimed to clarify the mechanism of DNA delivery into cells via the application of electric pulses. Though numerous strides forward have been made in defining the mechanisms of DNA electrotransfer in vitro, several challenges remain for researchers in this field of study, such as a clear overview about the direct and indirect effects of the electric field on cells and membranes (DNA internalization and intracellular trafficking until the cell nuclei, transient alteration of the cytoskeleton). Understanding the relationship between findings in vitro and in vivo applications remains a high priority. In vivo gene electrotransfer faces further challenges since DNA delivery to the target cells is an additional step to overcome. For that purpose, development of plasmid design is mandatory in order to control gene expression both in space (target cells within tissues) and in time (duration of gene expression).
Numerous in vivo studies have examined different tissues and animals in order to provide guidelines for successful DNA delivery. However, a lack of consensus about the pulse parameters and the electroporators to use hinders the progress of the field. The major drawback of in vivo gene electrotransfer is its low efficiency, and overcoming this limitation remains an imperative problem for researchers aiming to translating the basic science of gene electrotransfer into clinically relevant applications. Additionally, upscaling from small animals to humans in terms of larger tissue volume is challenging as well. Fortunately we can be optimistic that working to improve gene electrotransfer methods will yield effective treatments. Today the most promising use of gene electrotransfer is DNA vaccination for prophylaxis and for administering immunotherapy in cancer patients. Phase II trials are already in progress.
ACKNOWLEDGEMENTS
We acknowledge Dr. Carey Nadell for his contribution to the correction of this review article. We acknowledge financial support from the Deutscher Akademischer Austauschdienst (DAAD), the Ministère des Affaires Etrangères (MAE) and the Ministère de l'Enseignement Supérieur et de la Recherche (MESR) (program PHC PROCOPE 2010-2011), the Agence Nationale pour la Recherche. Research was conducted in the scope of the EBAM European Associated Laboratory (LEA). This article is based upon work from COST TD1104 Action (www.electroporation.net), supported by COST (European Cooperation in Science and Technology).
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
References
- 1.Rogers S. Gene therapy: a potentially invaluable aid to medicine and mankind. Res. Commun. Chem. Pathol. Pharmacol. 1971;2(4):587–600. [PubMed] [Google Scholar]
- 2.Cian M.M., Helen O.M. Cancer Gene Therapy – Key Biological Concepts in the Design of Multifunctional Non-Viral Delivery Systems. INTECH Open Access Publisher; 2013. [Google Scholar]
- 3.Greco O, Scott SD, Marples B, et al. Cancer gene therapy: 'delivery, delivery, delivery'. . Front Biosci . 2002. [DOI] [PubMed]
- 4.Xiao P.J., Lentz T.B., Samulski R.J. Recombinant adeno-associated virus: clinical application and development as a gene-therapy vector. Ther. Deliv. 2012;3(7):835–856. doi: 10.4155/tde.12.63. [DOI] [PubMed] [Google Scholar]
- 5.Bester A.C., Schwartz M., Schmidt M., et al. Fragile sites are preferential targets for integrations of MLV vectors in gene therapy. Gene Ther. 2006;13(13):1057–1059. doi: 10.1038/sj.gt.3302752. [DOI] [PubMed] [Google Scholar]
- 6.Hacein-Bey-Abina S., von Kalle C., Schmidt M., et al. A serious adverse event after successful gene therapy for X-linked severe combined immunodeficiency. N. Engl. J. Med. 2003;348(3):255–256. doi: 10.1056/NEJM200301163480314. [DOI] [PubMed] [Google Scholar]
- 7.Hacein-Bey-Abina S., Von Kalle C., Schmidt M., et al. LMO2-associated clonal T cell proliferation in two patients after gene therapy for SCID-X1. Science. 2003;302(5644):415–419. doi: 10.1126/science.1088547. [DOI] [PubMed] [Google Scholar]
- 8.Marshall E. Gene therapy death prompts review of adenovirus vector. Science. 1999;286(5448):2244–2245. doi: 10.1126/science.286.5448.2244. [DOI] [PubMed] [Google Scholar]
- 9.Nakayama Y., Aruga A. Comparison of current regulatory status for gene-based vaccines in the U.S., europe and japan. Vaccines (Basel) 2015;3(1):186–202. doi: 10.3390/vaccines3010186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Neumann E., Rosenheck K. Permeability changes induced by electric impulses in vesicular membranes. J. Membr. Biol. 1972;10(3):279–290. doi: 10.1007/BF01867861. [DOI] [PubMed] [Google Scholar]
- 11.Stampfli R. Reversible electrical breakdown of the excitable membrane of a Ranvier node. An. Acad. Bras. Cienc. 1958;30:57–63. [Google Scholar]
- 12.Kotnik T., Frey W., Sack M., et al. Electroporation-based applications in biotechnology. Trends Biotechnol. 2015;33(8):480–488. doi: 10.1016/j.tibtech.2015.06.002. [DOI] [PubMed] [Google Scholar]
- 13.Yarmush M.L., Golberg A., Sersa G., et al. Electroporation-based technologies for medicine: principles, applications, and challenges. Annu. Rev. Biomed. Eng. 2014;16:295–320. doi: 10.1146/annurev-bioeng-071813-104622. [DOI] [PubMed] [Google Scholar]
- 14.Belehradek M., Domenge C., Luboinski B., et al. Electrochemotherapy, a new antitumor treatment. First clinical phase I-II trial. Cancer. 1993;72(12):3694–3700. doi: 10.1002/1097-0142(19931215)72:12<3694::aid-cncr2820721222>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 15.Okino M., Mohri H. Effects of a high-voltage electrical impulse and an anticancer drug on in vivo growing tumors. Jpn. J. Cancer Res. 1987;78(12):1319–1321. [PubMed] [Google Scholar]
- 16.Heller R., Jaroszeski M.J., Glass L.F., et al. Phase I/II trial for the treatment of cutaneous and subcutaneous tumors using electrochemotherapy. Cancer. 1996;77(5):964–971. doi: 10.1002/(sici)1097-0142(19960301)77:5<964::aid-cncr24>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 17.Miklavcic D., Sersa G., Brecelj E., et al. Electrochemotherapy: technological advancements for efficient electroporation-based treatment of internal tumors. Med. Biol. Eng. Comput. 2012;50(12):1213–1225. doi: 10.1007/s11517-012-0991-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Orlowski S., Belehradek J., Jr, Paoletti C., et al. Transient electropermeabilization of cells in culture. Increase of the cytotoxicity of anticancer drugs. Biochem. Pharmacol. 1988;37(24):4727–4733. doi: 10.1016/0006-2952(88)90344-9. [DOI] [PubMed] [Google Scholar]
- 19.Domenge C., Orlowski S., Luboinski B., et al. Antitumor electrochemotherapy: new advances in the clinical protocol. Cancer. 1996;77(5):956–963. doi: 10.1002/(sici)1097-0142(19960301)77:5<956::aid-cncr23>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 20.Spratt D.E., Spratt E.A., Wu S.H., et al. Efficacy of skin-directed therapy for cutaneous metastases from advanced cancer: A meta-analysis. J. Clin. Oncol. 2014;32(28):3144–3155. doi: 10.1200/JCO.2014.55.4634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Testori A., Rossi C.R., Tosti G. Utility of electrochemotherapy in melanoma treatment. Curr. Opin. Oncol. 2012;24(2):155–161. doi: 10.1097/CCO.0b013e32834fcaa8. [DOI] [PubMed] [Google Scholar]
- 22.Cadossi R., Ronchetti M., Cadossi M. Locally enhanced chemotherapy by electroporation: clinical experiences and perspective of use of electrochemotherapy. Future Oncol. 2014;10(5):877–890. doi: 10.2217/fon.13.235. [DOI] [PubMed] [Google Scholar]
- 23.Marty M., Sersa G., Garbay J.R., et al. Electrochemotherapy - An easy, highly effective and safe treatment of cutaneous and subcutaneous metastases: Results of ESOPE (European Standard Operating Procedures of Electrochemotherapy) study. Eur. J. Cancer, Suppl. 2006;4(11):3–13. [Google Scholar]
- 24.Edhemovic I., Brecelj E., Gasljevic G., et al. Intraoperative electrochemotherapy of colorectal liver metastases. J. Surg. Oncol. 2014;110(3):320–327. doi: 10.1002/jso.23625. [DOI] [PubMed] [Google Scholar]
- 25.Zupanic A., Kos B., Miklavcic D. Treatment planning of electroporation-based medical interventions: electrochemotherapy, gene electrotransfer and irreversible electroporation. Phys. Med. Biol. 2012;57(17):5425–5440. doi: 10.1088/0031-9155/57/17/5425. [DOI] [PubMed] [Google Scholar]
- 26.Neumann E., Schaefer-Ridder M., Wang Y., et al. Gene transfer into mouse lyoma cells by electroporation in high electric fields. EMBO J. 1982;1(7):841–845. doi: 10.1002/j.1460-2075.1982.tb01257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wong T.K., Neumann E. Electric field mediated gene transfer. Biochem. Biophys. Res. Commun. 1982;107(2):584–587. doi: 10.1016/0006-291x(82)91531-5. [DOI] [PubMed] [Google Scholar]
- 28.Young J.L., Dean D.A. Electroporation-mediated gene delivery. Adv. Genet. 2015;89:49–88. doi: 10.1016/bs.adgen.2014.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mir L.M., Bureau M.F., Gehl J., et al. High-efficiency gene transfer into skeletal muscle mediated by electric pulses. Proc. Natl. Acad. Sci. USA. 1999;96(8):4262–4267. doi: 10.1073/pnas.96.8.4262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mir L.M., Moller P.H., Andre F., et al. Electric pulse-mediated gene delivery to various animal tissues. Adv. Genet. 2005;54:83–114. doi: 10.1016/S0065-2660(05)54005-7. [DOI] [PubMed] [Google Scholar]
- 31.Gehl J. In: Somatic Genome Manipulation. Manipulation S.G., Li X-Q, Donnelly DJ, Jensen TG, editors. New York: Springer; 2015. pp. 3–15. [Google Scholar]
- 32.Daud A, Algazi AP, Ashworth MT, et al. Systemic antitumor effect and clinical response in a phase 2 trial of intratumoral electroporation of plasmid interleukin-12 in patients with advanced melanoma. . J Clin Oncol . 2014.
- 33.Daud A.I., DeConti R.C., Andrews S., et al. Phase I trial of interleukin-12 plasmid electroporation in patients with metastatic melanoma. J. Clin. Oncol. 2008;26(36):5896–5903. doi: 10.1200/JCO.2007.15.6794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hirao L.A., Wu L., Khan A.S., et al. Combined effects of IL-12 and electroporation enhances the potency of DNA vaccination in macaques. Vaccine. 2008;26(25):3112–3120. doi: 10.1016/j.vaccine.2008.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gothelf A., Gehl J. What you always needed to know about electroporation based DNA vaccines. Hum. Vaccin. Immunother. 2012;8(11):1694–1702. doi: 10.4161/hv.22062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Muramatsu T., Shibata O., Ryoki S., et al. Foreign gene expression in the mouse testis by localized in vivo gene transfer. Biochem. Biophys. Res. Commun. 1997;233(1):45–49. doi: 10.1006/bbrc.1997.6361. [DOI] [PubMed] [Google Scholar]
- 37.Tsujie M., Isaka Y., Nakamura H., et al. Electroporation-mediated gene transfer that targets glomeruli. J. Am. Soc. Nephrol. 2001;12(5):949–954. doi: 10.1681/ASN.V125949. [DOI] [PubMed] [Google Scholar]
- 38.Blair-Parks K., Weston B.C., Dean D.A. High-level gene transfer to the cornea using electroporation. J. Gene Med. 2002;4(1):92–100. doi: 10.1002/jgm.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sheyn D., Kimelman-Bleich N., Pelled G., et al. Ultrasound-based nonviral gene delivery induces bone formation in vivo. Gene Ther. 2008;15(4):257–266. doi: 10.1038/sj.gt.3303070. [DOI] [PubMed] [Google Scholar]
- 40.Ohashi S., Kubo T., Kishida T., et al. Successful genetic transduction in vivo into synovium by means of electroporation. Biochem. Biophys. Res. Commun. 2002;293(5):1530–1535. doi: 10.1016/S0006-291X(02)00386-8. [DOI] [PubMed] [Google Scholar]
- 41.Aihara H., Miyazaki J. Gene transfer into muscle by electroporation in vivo. Nat. Biotechnol. 1998;16(9):867–870. doi: 10.1038/nbt0998-867. [DOI] [PubMed] [Google Scholar]
- 42.Dean D.A., Machado-Aranda D., Blair-Parks K., et al. Electroporation as a method for high-level nonviral gene transfer to the lung. Gene Ther. 2003;10(18):1608–1615. doi: 10.1038/sj.gt.3302053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mir L.M., Bureau M.F., Rangara R., et al. Long-term, high level in vivo gene expression after electric pulse-mediated gene transfer into skeletal muscle. C. R. Acad. Sci. III. 1998;321(11):893–899. doi: 10.1016/s0764-4469(99)80003-1. [DOI] [PubMed] [Google Scholar]
- 44.Vicat J.M., Boisseau S., Jourdes P., et al. Muscle transfection by electroporation with high-voltage and short-pulse currents provides high-level and long-lasting gene expression. Hum. Gene Ther. 2000;11(6):909–916. doi: 10.1089/10430340050015518. [DOI] [PubMed] [Google Scholar]
- 45.Hoover F., Magne Kalhovde J. A double-injection DNA electroporation protocol to enhance in vivo gene delivery in skeletal muscle. Anal. Biochem. 2000;285(1):175–178. doi: 10.1006/abio.2000.4730. [DOI] [PubMed] [Google Scholar]
- 46.Payen E., Bettan M., Rouyer-Fessard P., et al. Improvement of mouse beta-thalassemia by electrotransfer of erythropoietin cDNA. Exp. Hematol. 2001;29(3):295–300. doi: 10.1016/s0301-472x(00)00679-2. [DOI] [PubMed] [Google Scholar]
- 47.Goto T., Nishi T., Kobayashi O., et al. Combination electro-gene therapy using herpes virus thymidine kinase and interleukin-12 expression plasmids is highly efficient against murine carcinomas in vivo. Mol. Ther. 2004;10(5):929–937. doi: 10.1016/j.ymthe.2004.07.028. [DOI] [PubMed] [Google Scholar]
- 48.Trezise A.E. In vivo DNA electrotransfer. DNA Cell Biol. 2002;21(12):869–877. doi: 10.1089/104454902762053837. [DOI] [PubMed] [Google Scholar]
- 49.Prud'homme G.J., Glinka Y., Khan A.S., et al. Electroporation-enhanced nonviral gene transfer for the prevention or treatment of immunological, endocrine and neoplastic diseases. Curr. Gene Ther. 2006;6(2):243–273. doi: 10.2174/156652306776359504. [DOI] [PubMed] [Google Scholar]
- 50.Goldspink G. Skeletal muscle as an artificial endocrine tissue. Best Pract. Res. Clin. Endocrinol. Metab. 2003;17(2):211–222. doi: 10.1016/s1521-690x(03)00015-0. [DOI] [PubMed] [Google Scholar]
- 51.Gothelf A., Gehl J. Gene electrotransfer to skin; review of existing literature and clinical perspectives. Curr. Gene Ther. 2010;10(4):287–299. doi: 10.2174/156652310791823443. [DOI] [PubMed] [Google Scholar]
- 52.Denet A.R., Vanbever R., Preat V. Skin electroporation for transdermal and topical delivery. Adv. Drug Deliv. Rev. 2004;56(5):659–674. doi: 10.1016/j.addr.2003.10.027. [DOI] [PubMed] [Google Scholar]
- 53.Heller L.C., Heller R. Electroporation gene therapy preclinical and clinical trials for melanoma. Curr. Gene Ther. 2010;10(4):312–317. doi: 10.2174/156652310791823489. [DOI] [PubMed] [Google Scholar]
- 54.Adachi O., Nakano A., Sato O., et al. Gene transfer of Fc-fusion cytokine by in vivo electroporation: application to gene therapy for viral myocarditis. Gene Ther. 2002;9(9):577–583. doi: 10.1038/sj.gt.3301691. [DOI] [PubMed] [Google Scholar]
- 55.Bloquel C., Bessis N., Boissier M.C., et al. Gene therapy of collagen-induced arthritis by electrotransfer of human tumor necrosis factor-alpha soluble receptor I variants. Hum. Gene Ther. 2004;15(2):189–201. doi: 10.1089/104303404772679995. [DOI] [PubMed] [Google Scholar]
- 56.Gollins H., McMahon J., Wells K.E., et al. High-efficiency plasmid gene transfer into dystrophic muscle. Gene Ther. 2003;10(6):504–512. doi: 10.1038/sj.gt.3301927. [DOI] [PubMed] [Google Scholar]
- 57.Tanaka T., Ichimaru N., Takahara S., et al. In vivo gene transfer of hepatocyte growth factor to skeletal muscle prevents changes in rat kidneys after 5/6 nephrectomy. Am. J. Transplant. 2002;2(9):828–836. doi: 10.1034/j.1600-6143.2002.20904.x. [DOI] [PubMed] [Google Scholar]
- 58.Bakker J.M., Bleeker W.K., Parren P.W. Therapeutic antibody gene transfer: An active approach to passive immunity. Mol. Ther. 2004;10(3):411–416. doi: 10.1016/j.ymthe.2004.06.865. [DOI] [PubMed] [Google Scholar]
- 59.Perez N., Bigey P., Scherman D., et al. Regulatable systemic production of monoclonal antibodies by in vivo muscle electroporation. Genet. Vaccines Ther. 2004;2(1):2. doi: 10.1186/1479-0556-2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vasan S. Electroporation-mediated administration of candidate DNA vaccines against HIV-1. Methods Mol. Biol. 2014;1121:291–307. doi: 10.1007/978-1-4614-9632-8_26. [DOI] [PubMed] [Google Scholar]
- 61.Sardesai N.Y., Weiner D.B. Electroporation delivery of DNA vaccines: prospects for success. Curr. Opin. Immunol. 2011;23(3):421–429. doi: 10.1016/j.coi.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rochard A., Scherman D., Bigey P. Genetic immunization with plasmid DNA mediated by electrotransfer. Hum. Gene Ther. 2011;22(7):789–798. doi: 10.1089/hum.2011.092. [DOI] [PubMed] [Google Scholar]
- 63.Schleef M., Blaesen M., Schmeer M., et al. Production of non viral DNA vectors. Curr. Gene Ther. 2010;10(6):487–507. doi: 10.2174/156652310793797711. [DOI] [PubMed] [Google Scholar]
- 64.Heller R., Heller L.C. Gene electrotransfer clinical trials. Adv. Genet. 2015;89:235–262. doi: 10.1016/bs.adgen.2014.10.006. [DOI] [PubMed] [Google Scholar]
- 65.Vasan S., Hurley A., Schlesinger S.J., et al. In vivo electroporation enhances the immunogenicity of an HIV-1 DNA vaccine candidate in healthy volunteers. PLoS One. 2011;6(5):e19252. doi: 10.1371/journal.pone.0019252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Weiland O., Ahlen G., Diepolder H., et al. Therapeutic DNA vaccination using in vivo electroporation followed by standard of care therapy in patients with genotype 1 chronic hepatitis C. Mol. Ther. 2013;21(9):1796–1805. doi: 10.1038/mt.2013.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yang F.Q., Yu Y.Y., Wang G.Q., et al. A pilot randomized controlled trial of dual-plasmid HBV DNA vaccine mediated by in vivo electroporation in chronic hepatitis B patients under lamivudine chemotherapy. J. Viral Hepat. 2012;19(8):581–593. doi: 10.1111/j.1365-2893.2012.01589.x. [DOI] [PubMed] [Google Scholar]
- 68.Bagarazzi M.L., Yan J., Morrow M.P., et al. Immunotherapy against HPV16/18 generates potent TH1 and cytotoxic cellular immune responses. Sci. Transl. Med. 2012;4(155):155ra38. doi: 10.1126/scitranslmed.3004414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bodles-Brakhop A.M., Heller R., Draghia-Akli R. Electroporation for the delivery of DNA-based vaccines and immunotherapeutics: current clinical developments. Mol. Ther. 2009;17(4):585–592. doi: 10.1038/mt.2009.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Burgain-Chain A., Scherman D. DNA Electrotransfer: An Effective Tool for Gene Therapy. In: Martin F., editor. Gene Therapy - Tools and Potential Applications. In Tech; 2013. [Google Scholar]
- 71.Bernal S.D., Ona E.T., Riego-Javier A., et al. Anticancer immune reactivity and long-term survival after treatment of metastatic ovarian cancer with dendritic cells. Oncol. Lett. 2012;3(1):66–74. doi: 10.3892/ol.2011.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shibata M.A., Morimoto J., Otsuki Y. Suppression of murine mammary carcinoma growth and metastasis by HSVtk/GCV gene therapy using in vivo electroporation. Cancer Gene Ther. 2002;9(1):16–27. doi: 10.1038/sj.cgt.7700415. [DOI] [PubMed] [Google Scholar]
- 73.Finetti F., Terzuoli E., Bocci E., et al. Pharmacological inhibition of microsomal prostaglandin E synthase-1 suppresses epidermal growth factor receptor-mediated tumor growth and angiogenesis. PLoS One. 2012;7(7):e40576. doi: 10.1371/journal.pone.0040576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Prabha S., Sharma B., Labhasetwar V. Inhibition of tumor angiogenesis and growth by nanoparticle-mediated p53 gene therapy in mice. Cancer Gene Ther. 2012;19(8):530–537. doi: 10.1038/cgt.2012.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hanari N., Matsubara H., Hoshino I., et al. Combinatory gene therapy with electrotransfer of midkine promoter-HSV-TK and interleukin-21. Anticancer Res. 2007;27(4B):2305–2310. [PubMed] [Google Scholar]
- 76.Heller L., Pottinger C., Jaroszeski M.J., et al. In vivo electroporation of plasmids encoding GM-CSF or interleukin-2 into existing B16 melanomas combined with electrochemotherapy induces long-term antitumour immunity. Melanoma Res. 2000;10(6):577–583. doi: 10.1097/00008390-200012000-00010. [DOI] [PubMed] [Google Scholar]
- 77.Sersa G., Teissie J., Cemazar M., et al. Electrochemotherapy of tumors as in situ vaccination boosted by immunogene electrotransfer. Cancer Immunol. Immunother. 2015;64(10):1315–1327. doi: 10.1007/s00262-015-1724-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cemazar M., Jarm T., Sersa G. Cancer electrogene therapy with interleukin-12. Curr. Gene Ther. 2010;10(4):300–311. doi: 10.2174/156652310791823425. [DOI] [PubMed] [Google Scholar]
- 79.Tamura T., Nishi T., Goto T., et al. Combination of IL-12 and IL-18 of electro-gene therapy synergistically inhibits tumor growth. Anticancer Res. 2003;23(2B):1173–1179. [PubMed] [Google Scholar]
- 80.Craig R., Cutrera J., Zhu S., et al. Administering plasmid DNA encoding tumor vessel-anchored IFN-alpha for localizing gene product within or into tumors. Mol. Ther. 2008;16(5):901–906. doi: 10.1038/mt.2008.40. [DOI] [PubMed] [Google Scholar]
- 81.Bei R, Scardino A. TAA polyepitope DNA-based vaccines: a potential tool for cancer therapy. . J Biomed Biotechnol . 2010. [DOI] [PMC free article] [PubMed]
- 82.Chudley L., McCann K., Mander A., et al. DNA fusion-gene vaccination in patients with prostate cancer induces high-frequency CD8(+) T-cell responses and increases PSA doubling time. Cancer Immunol. Immunother. 2012;61(11):2161–2170. doi: 10.1007/s00262-012-1270-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Low L., Mander A., McCann K., et al. DNA vaccination with electroporation induces increased antibody responses in patients with prostate cancer. Hum. Gene Ther. 2009;20(11):1269–1278. doi: 10.1089/hum.2009.067. [DOI] [PubMed] [Google Scholar]
- 84.Spanggaard I., Snoj M., Cavalcanti A., et al. Gene electrotransfer of plasmid antiangiogenic metargidin peptide (AMEP) in disseminated melanoma: safety and efficacy results of a phase I first-in-man study. Hum. Gene Ther. Clin. Dev. 2013;24(3):99–107. doi: 10.1089/humc.2012.240. [DOI] [PubMed] [Google Scholar]
- 85.Weaver J.C., Smith K.C., Esser A.T., et al. A brief overview of electroporation pulse strength-duration space: a region where additional intracellular effects are expected. Bioelectrochemistry. 2012;87:236–243. doi: 10.1016/j.bioelechem.2012.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zimmermann U. Electric field-mediated fusion and related electrical phenomena. Biochim. Biophys. Acta. 1982;694(3):227–277. doi: 10.1016/0304-4157(82)90007-7. [DOI] [PubMed] [Google Scholar]
- 87.Andre F., Mir L.M. DNA electrotransfer: its principles and an updated review of its therapeutic applications. Gene Ther. 2004;11(Suppl. 1):S33–S42. doi: 10.1038/sj.gt.3302367. [DOI] [PubMed] [Google Scholar]
- 88.Kinosita K., Jr, Tsong T.Y. Voltage-induced pore formation and hemolysis of human erythrocytes. Biochim. Biophys. Acta. 1977;471(2):227–242. doi: 10.1016/0005-2736(77)90252-8. [DOI] [PubMed] [Google Scholar]
- 89.Schwan H.P. Electrical properties of tissue and cell suspensions. Adv. Biol. Med. Phys. 1957;5:147–209. doi: 10.1016/b978-1-4832-3111-2.50008-0. [DOI] [PubMed] [Google Scholar]
- 90.Escoffre J.M., Dean D.S., Hubert M., et al. Membrane perturbation by an external electric field: a mechanism to permit molecular uptake. Eur. Biophys. J. 2007;36(8):973–983. doi: 10.1007/s00249-007-0194-7. [DOI] [PubMed] [Google Scholar]
- 91.Teissie J., Rols M.P. An experimental evaluation of the critical potential difference inducing cell membrane electropermeabilization. Biophys. J. 1993;65(1):409–413. doi: 10.1016/S0006-3495(93)81052-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zimmermann U., Pilwat G., Riemann F. Dielectric breakdown of cell membranes. Biophys. J. 1974;14(11):881–899. doi: 10.1016/S0006-3495(74)85956-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bernhardt J., Pauly H. On the generation of potential differences across the membranes of ellipsoidal cells in an alternating electrical field. Biophysik. 1973;10(3):89–98. doi: 10.1007/BF01189915. [DOI] [PubMed] [Google Scholar]
- 94.Tekle E., Astumian R.D., Chock P.B. Electro-permeabilization of cell membranes: effect of the resting membrane potential. Biochem. Biophys. Res. Commun. 1990;172(1):282–287. doi: 10.1016/s0006-291x(05)80206-2. [DOI] [PubMed] [Google Scholar]
- 95.Teissie J., Yow Tsong T. Voltage modulation of Na+/K+ transport in human erythrocytes. J. Physiol. (Paris) 1981;77(9):1043–1053. [PubMed] [Google Scholar]
- 96.Robello M., Gliozzi A. Conductance transition induced by an electric field in lipid bilayers. Biochim. Biophys. Acta. 1989;982(1):173–176. doi: 10.1016/0005-2736(89)90189-2. [DOI] [PubMed] [Google Scholar]
- 97.Tomov T.C., Tsoneva I.C. Changes in the surface-charge of cells induced by electrical pulses. Bioelectrochem. Bioenerg. 1989;22(2):127–133. [Google Scholar]
- 98.Marszalek P., Liu D.S., Tsong T.Y. Schwan equation and transmembrane potential induced by alternating electric-field. Biophys. J. 1990;58(4):1053–1058. doi: 10.1016/S0006-3495(90)82447-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Rols M.P., Teissie J. Experimental evidence for the involvement of the cytoskeleton in mammalian cell electropermeabilization. Biochim. Biophys. Acta. 1992;1111(1):45–50. doi: 10.1016/0005-2736(92)90272-n. [DOI] [PubMed] [Google Scholar]
- 100.Rols M.P., Teissie J. Modulation of electrically induced permeabilization and fusion of Chinese hamster ovary cells by osmotic pressure. Biochemistry. 1990;29(19):4561–4567. doi: 10.1021/bi00471a009. [DOI] [PubMed] [Google Scholar]
- 101.Golzio M., Rols M.P., Teissie J. In vitro and in vivo electric field-mediated permeabilization, gene transfer, and expression. Methods. 2004;33(2):126–135. doi: 10.1016/j.ymeth.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 102.Rols M.P., Delteil C., Golzio M., et al. In vivo electrically mediated protein and gene transfer in murine melanoma. Nat. Biotechnol. 1998;16(2):168–171. doi: 10.1038/nbt0298-168. [DOI] [PubMed] [Google Scholar]
- 103.Sixou S., Teissie J. Specific electropermeabilization of leucocytes in a blood sample and application to large volumes of cells. Biochim. Biophys. Acta. 1990;1028(2):154–160. doi: 10.1016/0005-2736(90)90149-i. [DOI] [PubMed] [Google Scholar]
- 104.Granneman J.G., Li P., Lu Y., et al. Seeing the trees in the forest: selective electroporation of adipocytes within adipose tissue. Am. J. Physiol. Endocrinol. Metab. 2004;287(3):E574–E582. doi: 10.1152/ajpendo.00567.2003. [DOI] [PubMed] [Google Scholar]
- 105.Collombet J.M., Wheeler V.C., Vogel F., et al. Introduction of plasmid DNA into isolated mitochondria by electroporation. A novel approach toward gene correction for mitochondrial disorders. J. Biol. Chem. 1997;272(8):5342–5347. doi: 10.1074/jbc.272.8.5342. [DOI] [PubMed] [Google Scholar]
- 106.Beebe S.J., Schoenbach K.H. Nanosecond pulsed electric fields: A new stimulus to activate intracellular signaling. J. Biomed. Biotechnol. 2005;(4):297–300. doi: 10.1155/JBB.2005.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Gowrishankar T.R., Esser A.T., Vasilkoski Z., et al. Microdosimetry for conventional and supra-electroporation in cells with organelles. Biochem. Biophys. Res. Commun. 2006;341(4):1266–1276. doi: 10.1016/j.bbrc.2006.01.094. [DOI] [PubMed] [Google Scholar]
- 108.Vernier P.T., Sun Y., Gundersen M.A. Nanoelectropulse-driven membrane perturbation and small molecule permeabilization. BMC Cell Biol. 2006;7:37. doi: 10.1186/1471-2121-7-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Vernier P.T., Sun Y.H., Marcu L., et al. Nanoelectropulse-induced phosphatidylserine translocation. Biophys. J. 2004;86(6):4040–4048. doi: 10.1529/biophysj.103.037945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Tekle E., Oubrahim H., Dzekunov S.M., et al. Selective field effects on intracellular vacuoles and vesicle membranes with nanosecond electric pulses. Biophys. J. 2005;89(1):274–284. doi: 10.1529/biophysj.104.054494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kolb J.F., Kono S., Schoenbach K.H. Nanosecond pulsed electric field generators for the study of subcellular effects. Bioelectromagnetics. 2006;27(3):172–187. doi: 10.1002/bem.20185. [DOI] [PubMed] [Google Scholar]
- 112.Napotnik T.B., Wu Y.H., Gundersen M.A., et al. Nanosecond electric pulses cause mitochondrial membrane permeabilization in Jurkat cells. Bioelectromagnetics. 2012;33(3):257–264. doi: 10.1002/bem.20707. [DOI] [PubMed] [Google Scholar]
- 113.Gross D., Loew L.M., Webb W.W. Optical imaging of cell membrane potential changes induced by applied electric fields. Biophys. J. 1986;50(2):339–348. doi: 10.1016/S0006-3495(86)83467-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kinosita K., Jr, Itoh H., Ishiwata S., et al. Dual-view microscopy with a single camera: real-time imaging of molecular orientations and calcium. J. Cell Biol. 1991;115(1):67–73. doi: 10.1083/jcb.115.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hibino M., Itoh H., Kinosita K. Time courses of cell electroporation as revealed by submicrosecond imaging of transmembrane potential. Biophys. J. 1993;64(6):1789–1800. doi: 10.1016/S0006-3495(93)81550-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hibino M., Shigemori M., Itoh H., et al. Membrane conductance of an electroporated cell analyzed by submicrosecond imaging of transmembrane potential. Biophys. J. 1991;59(1):209–220. doi: 10.1016/S0006-3495(91)82212-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Rols M.P., Teissie J. Electropermeabilization of mammalian cells. Quantitative analysis of the phenomenon. Biophys. J. 1990;58(5):1089–1098. doi: 10.1016/S0006-3495(90)82451-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Teissie J., Eynard N., Gabriel B., et al. Electropermeabilization of cell membranes. Adv. Drug Deliv. Rev. 1999;35(1):3–19. doi: 10.1016/s0169-409x(98)00060-x. [DOI] [PubMed] [Google Scholar]
- 119.Gabriel B., Teissie J. Direct observation in the millisecond time range of fluorescent molecule asymmetrical interaction with the electropermeabilized cell membrane. Biophys. J. 1997;73(5):2630–2637. doi: 10.1016/S0006-3495(97)78292-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Mehrle W., Zimmermann U., Hampp R. Evidence for asymmetrical uptake of fluorescent dyes through electro-permeabilized membranes of avena mesophyll protoplasts. FEBS Lett. 1985;185(1):89–94. [Google Scholar]
- 121.Tekle E., Astumian R.D., Chock P.B. Electroporation by using bipolar oscillating electric field: an improved method for DNA transfection of NIH 3T3 cells. Proc. Natl. Acad. Sci. USA. 1991;88(10):4230–4234. doi: 10.1073/pnas.88.10.4230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Djuzenova C.S., Zimmermann U., Frank H., et al. Effect of medium conductivity and composition on the uptake of propidium iodide into electropermeabilized myeloma cells. Biochim. Biophys. Acta. 1996;1284(2):143–152. doi: 10.1016/s0005-2736(96)00119-8. [DOI] [PubMed] [Google Scholar]
- 123.Valic B., Golzio M., Pavlin M., et al. Effect of electric field induced transmembrane potential on spheroidal cells: theory and experiment. Eur Biophys J Biophy. 2003;32(6):519–528. doi: 10.1007/s00249-003-0296-9. [DOI] [PubMed] [Google Scholar]
- 124.Benz R., Zimmermann U. The resealing process of lipid bilayers after reversible electrical breakdown. Biochim. Biophys. Acta. 1981;640(1):169–178. doi: 10.1016/0005-2736(81)90542-3. [DOI] [PubMed] [Google Scholar]
- 125.Benz R., Zimmermann U. Evidence for the presence of mobile charges in the cell-membrane of valonia-utricularis. Biophys. J. 1983;43(1):13–26. doi: 10.1016/S0006-3495(83)84318-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Escande-Geraud M.L., Rols M.P., Dupont M.A., et al. Reversible plasma membrane ultrastructural changes correlated with electropermeabilization in Chinese hamster ovary cells. Biochim. Biophys. Acta. 1988;939(2):247–259. doi: 10.1016/0005-2736(88)90068-5. [DOI] [PubMed] [Google Scholar]
- 127.Rols M.P., Teissie J. Electropermeabilization of mammalian cells to macromolecules: control by pulse duration. Biophys. J. 1998;75(3):1415–1423. doi: 10.1016/S0006-3495(98)74060-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Bier M., Hammer S.M., Canaday D.J., et al. Kinetics of sealing for transient electropores in isolated mammalian skeletal muscle cells. Bioelectromagnetics. 1999;20(3):194–201. doi: 10.1002/(sici)1521-186x(1999)20:3<194::aid-bem6>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 129.Gehl J., Skovsgaard T., Mir L.M. Vascular reactions to in vivo electroporation: characterization and consequences for drug and gene delivery. Biochim. Biophys. Acta. 2002;1569(1-3):51–58. doi: 10.1016/s0304-4165(01)00233-1. [DOI] [PubMed] [Google Scholar]
- 130.Kinosita K., Jr, Tsong T.Y. Formation and resealing of pores of controlled sizes in human erythrocyte membrane. Nature. 1977;268(5619):438–441. doi: 10.1038/268438a0. [DOI] [PubMed] [Google Scholar]
- 131.Teissie J., Rols M.P. Manipulation of cell cytoskeleton affects the lifetime of cell membrane electropermeabilization. Ann. N. Y. Acad. Sci. 1994;720:98–110. doi: 10.1111/j.1749-6632.1994.tb30438.x. [DOI] [PubMed] [Google Scholar]
- 132.Pucihar G., Kotnik T., Miklavcic D., et al. Kinetics of transmembrane transport of small molecules into electropermeabilized cells. Biophys. J. 2008;95(6):2837–2848. doi: 10.1529/biophysj.108.135541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Gabriel B., Teissie J. Control by electrical parameters of short- and long-term cell death resulting from electropermeabilization of Chinese hamster ovary cells. Biochim. Biophys. Acta. 1995;1266(2):171–178. doi: 10.1016/0167-4889(95)00021-j. [DOI] [PubMed] [Google Scholar]
- 134.Lombry C., Dujardin N., Preat V. Transdermal delivery of macromolecules using skin electroporation. Pharm. Res. 2000;17(1):32–37. doi: 10.1023/a:1007510323344. [DOI] [PubMed] [Google Scholar]
- 135.Sixou S., Teissie J. Exogenous uptake and release of molecules by electroloaded cells - a digitized videomicroscopy study. Bioelectrochem. Bioenerg. 1993;31(3):237–257. [Google Scholar]
- 136.Gabriel B., Teissie J. Time courses of mammalian cell electropermeabilization observed by millisecond imaging of membrane property changes during the pulse. Biophys. J. 1999;76(4):2158–2165. doi: 10.1016/S0006-3495(99)77370-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kinosita K., Jr, Tsong T.Y. Voltage-induced conductance in human erythrocyte membranes. Biochim. Biophys. Acta. 1979;554(2):479–497. doi: 10.1016/0005-2736(79)90386-9. [DOI] [PubMed] [Google Scholar]
- 138.Satkauskas S., Andre F., Bureau M.F., et al. Electrophoretic component of electric pulses determines the efficacy of in vivo DNA electrotransfer. Hum. Gene Ther. 2005;16(10):1194–1201. doi: 10.1089/hum.2005.16.1194. [DOI] [PubMed] [Google Scholar]
- 139.Montane M.H., Dupille E., Alibert G., et al. Induction of a long-lived fusogenic state in viable plant protoplasts permeabilized by electric fields. Biochim. Biophys. Acta. 1990;1024(1):203–207. doi: 10.1016/0005-2736(90)90227-f. [DOI] [PubMed] [Google Scholar]
- 140.Neumann E., Kakorin S., Toensing K. Fundamentals of electroporative delivery of drugs and genes. Bioelectrochem. Bioenerg. 1999;48(1):3–16. doi: 10.1016/s0302-4598(99)00008-2. [DOI] [PubMed] [Google Scholar]
- 141.Crowley J.M. Electrical breakdown of biomolecular lipid-membranes as an electromechanical instability. Biophys. J. 1973;13(7):711–724. doi: 10.1016/S0006-3495(73)86017-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Abidor I.G., Arakelyan V.B., Chernomordik L.V., et al. Electric breakdown of bilayer lipid-membranes. 1. main experimental facts and their qualitative discussion. Bioelectrochem. Bioenerg. 1979;6(1):37–52. [Google Scholar]
- 143.Chernomordik L.V., Sukharev S.I., Abidor I.G., et al. Breakdown of lipid bilayer-membranes in an electric-field. Biochim. Biophys. Acta. 1983;736(2):203–213. [Google Scholar]
- 144.Weaver J.C. Electroporation - a general phenomenon for manipulating cells and tissues. J. Cell. Biochem. 1993;51(4):426–435. doi: 10.1002/jcb.2400510407. [DOI] [PubMed] [Google Scholar]
- 145.Glaser R.W., Leikin S.L., Chernomordik L.V., et al. Reversible electrical breakdown of lipid bilayers: formation and evolution of pores. Biochim. Biophys. Acta. 1988;940(2):275–287. doi: 10.1016/0005-2736(88)90202-7. [DOI] [PubMed] [Google Scholar]
- 146.Sugar I.P., Neumann E. Stochastic model for electric field-induced membrane pores. Electroporation. Biophys. Chem. 1984;19(3):211–225. doi: 10.1016/0301-4622(84)87003-9. [DOI] [PubMed] [Google Scholar]
- 147.Sugar I.P., Forster W., Neumann E. Model of cell electrofusion. Membrane electroporation, pore coalescence and percolation. Biophys. Chem. 1987;26(2-3):321–335. doi: 10.1016/0301-4622(87)80033-9. [DOI] [PubMed] [Google Scholar]
- 148.Pavlin M., Kotnik T., Miklavčič D., et al. 2008. Electroporation of Planar Lipid Bilayers and Membranes. [Google Scholar]
- 149.Leontiadou H., Mark A.E., Marrink S.J. Molecular dynamics simulations of hydrophilic pores in lipid bilayers. Biophys. J. 2004;86(4):2156–2164. doi: 10.1016/S0006-3495(04)74275-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Tarek M. Membrane electroporation: A molecular dynamics simulation. Biophys. J. 2005;88(6):4045–4053. doi: 10.1529/biophysj.104.050617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Tieleman D.P. The molecular basis of electroporation. BMC Biochem. 2004;5:10. doi: 10.1186/1471-2091-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Vernier P.T., Ziegler M.J., Sun Y.H., et al. Nanopore-facilitated, voltage-driven phosphatidylserine translocation in lipid bilayers - in cells and in silico. Phys. Biol. 2006;3(4):233–247. doi: 10.1088/1478-3975/3/4/001. [DOI] [PubMed] [Google Scholar]
- 153.Wohlert J., den Otter W.K., Edholm O., et al. Free energy of a trans-membrane pore calculated from atomistic molecular dynamics simulations. J. Chem. Phys. 2006;124(15):154905. doi: 10.1063/1.2171965. [DOI] [PubMed] [Google Scholar]
- 154.Levine Z.A., Vernier P.T. Life cycle of an electropore: field-dependent and field-independent steps in pore creation and annihilation. J. Membr. Biol. 2010;236(1):27–36. doi: 10.1007/s00232-010-9277-y. [DOI] [PubMed] [Google Scholar]
- 155.Zhelev D.V., Needham D. Tension-stabilized pores in giant vesicles: determination of pore size and pore line tension. Biochim. Biophys. Acta. 1993;1147(1):89–104. doi: 10.1016/0005-2736(93)90319-u. [DOI] [PubMed] [Google Scholar]
- 156.Portet T., Camps i Febrer F., Escoffre J.M., et al. Visualization of membrane loss during the shrinkage of giant vesicles under electropulsation. Biophys. J. 2009;96(10):4109–4121. doi: 10.1016/j.bpj.2009.02.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Tekle E., Astumian R.D., Friauf W.A., et al. Asymmetric pore distribution and loss of membrane lipid in electroporated DOPC vesicles. Biophys. J. 2001;81(2):960–968. doi: 10.1016/S0006-3495(01)75754-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Riske K.A., Dimova R. Electro-deformation and poration of giant vesicles viewed with high temporal resolution. Biophys. J. 2005;88(2):1143–1155. doi: 10.1529/biophysj.104.050310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Krassowska W., Filev P.D. Modeling electroporation in a single cell. Biophys. J. 2007;92(2):404–417. doi: 10.1529/biophysj.106.094235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Shil P., Bidaye S., Vidyasagar P.B. Analysing the effects of surface distribution of pores in cell electroporation for a cell membrane containing cholesterol. J. Phys. D Appl. Phys. 2008;41(5) [Google Scholar]
- 161.Schwister K., Deuticke B. Formation and properties of aqueous leaks induced in human erythrocytes by electrical breakdown. Biochim. Biophys. Acta. 1985;816(2):332–348. doi: 10.1016/0005-2736(85)90501-2. [DOI] [PubMed] [Google Scholar]
- 162.Lopez A., Rols M.P., Teissie J. P-31 Nmr analysis of membrane phospholipid organization in viable, reversibly electropermeabilized chinese-hamster ovary cells. Biochemistry. 1988;27(4):1222–1228. doi: 10.1021/bi00404a023. [DOI] [PubMed] [Google Scholar]
- 163.Dressler V., Schwister K., Haest C.W., et al. Dielectric-breakdown of the erythrocyte-membrane enhances transbilayer mobility of phospholipids. Biochim. Biophys. Acta. 1983;732(1):304–307. doi: 10.1016/0005-2736(83)90216-x. [DOI] [PubMed] [Google Scholar]
- 164.Haest C.W., Kamp D., Deuticke B. Transbilayer reorientation of phospholipid probes in the human erythrocyte membrane. Lessons from studies on electroporated and resealed cells. Bba-Biomembranes. 1997;1325(1):17–33. doi: 10.1016/s0005-2736(96)00239-8. [DOI] [PubMed] [Google Scholar]
- 165.Escoffre J.M., Bellard E., Faurie C., et al. Membrane disorder and phospholipid scrambling in electropermeabilized and viable cells. Biochim. Biophys. Acta. 2014;1838(7):1701–1709. doi: 10.1016/j.bbamem.2014.02.013. [DOI] [PubMed] [Google Scholar]
- 166.Teissie J., Golzio M., Rols M.P. Mechanisms of cell membrane electropermeabilization: a minireview of our present (lack of? ) knowledge. Biochim. Biophys. Acta. 2005;1724(3):270–280. doi: 10.1016/j.bbagen.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 167.Faurie C., Rebersek M., Golzio M., et al. Electro-mediated gene transfer and expression are controlled by the life-time of DNA/ membrane complex formation. J. Gene Med. 2010;12(1):117–125. doi: 10.1002/jgm.1414. [DOI] [PubMed] [Google Scholar]
- 168.Golzio M., Teissie J., Rols M.P. Cell synchronization effect on mammalian cell permeabilization and gene delivery by electric field. Biochim. Biophys. Acta. 2002;1563(1-2):23–28. doi: 10.1016/s0005-2736(02)00369-3. [DOI] [PubMed] [Google Scholar]
- 169.Xie T.D., Tsong T.Y. Study of mechanisms of electric field-induced DNA transfection. V. Effects of DNA topology on surface binding, cell uptake, expression, and integration into host chromosomes of DNA in the mammalian cell. Biophys. J. 1993;65(4):1684–1689. doi: 10.1016/S0006-3495(93)81208-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Cemazar M., Golzio M., Sersa G., et al. Hyaluronidase and collagenase increase the transfection efficiency of gene electrotransfer in various murine tumors. Hum. Gene Ther. 2012;23(1):128–137. doi: 10.1089/hum.2011.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Golzio M., Teissie J., Rols M.P. Direct visualization at the single-cell level of electrically mediated gene delivery. Proc. Natl. Acad. Sci. USA. 2002;99(3):1292–1297. doi: 10.1073/pnas.022646499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Wolf H., Rols M.P., Boldt E., et al. Control by pulse parameters of electric field-mediated gene transfer in mammalian cells. Biophys. J. 1994;66(2 Pt 1):524–531. doi: 10.1016/s0006-3495(94)80805-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Chu G., Hayakawa H., Berg P. Electroporation for the efficient transfection of mammalian cells with DNA. Nucleic Acids Res. 1987;15(3):1311–1326. doi: 10.1093/nar/15.3.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Hui S.W. Effects of pulse length and strength on electroporation efficiency. Methods Mol. Biol. 1995;55:29–40. doi: 10.1385/0-89603-328-7:29. [DOI] [PubMed] [Google Scholar]
- 175.Pavlin M., Kanduser M. New insights into the mechanisms of gene electrotransfer - experimental and theoretical analysis. Sci. Rep. 2015;5:9132. doi: 10.1038/srep09132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Paturneau-Jouas M., Parzy E., Vidal G., et al. Electrotransfer at MR imaging: tool for optimization of gene transfer protocols--feasibility study in mice. Radiology. 2003;228(3):768–775. doi: 10.1148/radiol.2283020482. [DOI] [PubMed] [Google Scholar]
- 177.Winterbourne D.J., Thomas S., Hermon-Taylor J., et al. Electric shock-mediated transfection of cells. Characterization and optimization of electrical parameters. Biochem. J. 1988;251(2):427–434. doi: 10.1042/bj2510427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Lurquin P.F. Gene transfer by electroporation. Mol. Biotechnol. 1997;7(1):5–35. doi: 10.1007/BF02821542. [DOI] [PubMed] [Google Scholar]
- 179.Kalinski T., Jaquet K., Langen R., et al. An optimized electroporation protocol for transfection of sensitive cell lines using basic laboratory equipment. Biotechnol. Tech. 1997;11(10):717–772. [Google Scholar]
- 180.Delgado-Canedo A., dos Santos D.G., Chies J.A., et al. Optimization of an electroporation protocol using the K562 cell line as a model: role of cell cycle phase and cytoplasmic DNAses. Cytotechnology. 2006;51(3):141–148. doi: 10.1007/s10616-006-9028-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Bahnson A.B., Boggs S.S. Addition of serum to electroporated cells enhances survival and transfection efficiency. Biochem. Biophys. Res. Commun. 1990;171(2):752–757. doi: 10.1016/0006-291x(90)91210-j. [DOI] [PubMed] [Google Scholar]
- 182.Klenchin V.A., Sukharev S.I., Serov S.M., et al. Electrically induced DNA uptake by cells is a fast process involving DNA electrophoresis. Biophys. J. 1991;60(4):804–811. doi: 10.1016/S0006-3495(91)82115-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Sukharev S.I., Klenchin V.A., Serov S.M., et al. Electroporation and electrophoretic DNA transfer into cells. The effect of DNA interaction with electropores. Biophys. J. 1992;63(5):1320–1327. doi: 10.1016/S0006-3495(92)81709-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Eynard N., Rols M.P., Ganeva V., et al. Electrotransformation pathways of prokaryotic and eukaryotic cells: recent developments. Bioelectrochem. Bioenerg. 1997;44(1):103–110. [Google Scholar]
- 185.Ganeva V., Galutzov B., Teissie J. Fast kinetic studies of plasmid DNA transfer in intact yeast cells mediated by electropulsation. Biochem. Biophys. Res. Commun. 1995;214(3):825–832. doi: 10.1006/bbrc.1995.2361. [DOI] [PubMed] [Google Scholar]
- 186.Taketo A. DNA transfection of Escherichia coli by electroporation. Biochim. Biophys. Acta. 1988;949(3):318–324. doi: 10.1016/0167-4781(88)90158-3. [DOI] [PubMed] [Google Scholar]
- 187.Xie T.D., Sun L., Tsong T.Y. Study of mechanisms of electric field-induced DNA transfection. I. DNA entry by surface binding and diffusion through membrane pores. Biophys. J. 1990;58(1):13–19. doi: 10.1016/S0006-3495(90)82349-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Sungailaite S., Ruzgys P., Satkauskiene I., et al. The dependence of efficiency of transmembrane molecular transfer using electroporation on medium viscosity. J. Gene Med. 2015;17(3-5):80–86. doi: 10.1002/jgm.2825. [DOI] [PubMed] [Google Scholar]
- 189.Andre F.M., Gehl J., Sersa G., et al. Efficiency of high and low-voltage pulse combinations for gene electrotransfer in muscle, liver, tumor, and skin. Hum. Gene Ther. 2008;19(11):1261–1271. doi: 10.1089/hum.2008.060. [DOI] [PubMed] [Google Scholar]
- 190.Bureau M.F., Gehl J., Deleuze V., et al. Importance of association between permeabilization and electrophoretic forces for intramuscular DNA electrotransfer. Biochim. Biophys. Acta. 2000;1474(3):353–359. doi: 10.1016/s0304-4165(00)00028-3. [DOI] [PubMed] [Google Scholar]
- 191.Satkauskas S., Bureau M.F., Puc M., et al. Mechanisms of in vivo DNA electrotransfer: respective contributions of cell electropermeabilization and DNA electrophoresis. Mol. Ther. 2002;5(2):133–140. doi: 10.1006/mthe.2002.0526. [DOI] [PubMed] [Google Scholar]
- 192.Pavselj N., Preat V. DNA electrotransfer into the skin using a combination of one high and one low-voltage pulse. J. Control. Release. 2005;106(3):407–415. doi: 10.1016/j.jconrel.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 193.Saito K., Lehar M., Li Z.B., et al. High efficiency gene delivery into laryngeal muscle with bidirectional electroporation. Otolaryng Head Neck. 2006;135(2):209–214. doi: 10.1016/j.otohns.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 194.Kanduser M., Miklavcic D., Pavlin M. Mechanisms involved in gene electrotransfer using high and low-voltage pulses-an in vitro study. Bioelectrochemistry. 2009;74(2):265–271. doi: 10.1016/j.bioelechem.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 195.Pavlin M., Flisar K., Kanduser M. The role of electrophoresis in gene electrotransfer. J. Membr. Biol. 2010;236(1):75–79. doi: 10.1007/s00232-010-9276-z. [DOI] [PubMed] [Google Scholar]
- 196.Escoffre J.M., Portet T., Favard C., et al. Electromediated formation of DNA complexes with cell membranes and its consequences for gene delivery. Biochim. Biophys. Acta. 2011;1808(6):1538–1543. doi: 10.1016/j.bbamem.2010.10.009. [DOI] [PubMed] [Google Scholar]
- 197.Faurie C., Phez E., Golzio M., et al. Effect of electric field vectoriality on electrically mediated gene delivery in mammalian cells. Biochim. Biophys. Acta. 2004;1665(1-2):92–100. doi: 10.1016/j.bbamem.2004.06.018. [DOI] [PubMed] [Google Scholar]
- 198.Phez E., Faurie C., Golzio M., et al. New insights in the visualization of membrane permeabilization and DNA/membrane interaction of cells submitted to electric pulses. Biochim. Biophys. Acta. 2005;1724(3):248–254. doi: 10.1016/j.bbagen.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 199.Rebersek M., Faurie C., Kanduser M., et al. Electroporator with automatic change of electric field direction improves gene electrotransfer in vitro. Biomed. Eng. Online. 2007;6:25. doi: 10.1186/1475-925X-6-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Escoffre J.M., Bellard E., Phez E., et al. Effect of electric field intensity on plasmid DNA/membrane interaction during in vitro gene electrotransfer. Drug Deliv. Lett. 2012;2(1):22–25. [Google Scholar]
- 201.Neumann E. Membrane electroporation and direct gene-transfer. Bioelectrochem. Bioenerg. 1992;28(1-2):247–267. [Google Scholar]
- 202.Haberl S., Kanduser M., Flisar K., et al. Effect of different parameters used for in vitro gene electrotransfer on gene expression efficiency, cell viability and visualization of plasmid DNA at the membrane level. J. Gene Med. 2013;15(5):169–181. doi: 10.1002/jgm.2706. [DOI] [PubMed] [Google Scholar]
- 203.Haberl S., Pavlin M. Use of collagen gel as a three-dimensional in vitro model to study electropermeabilization and gene electrotransfer. J. Membr. Biol. 2010;236(1):87–95. doi: 10.1007/s00232-010-9280-3. [DOI] [PubMed] [Google Scholar]
- 204.Neumann E., Kakorin S., Tsoneva I., et al. Calcium-mediated DNA adsorption to yeast cells and kinetics of cell transformation by electroporation. Biophys. J. 1996;71(2):868–877. doi: 10.1016/S0006-3495(96)79288-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Frandsen S.K., Gissel H., Hojman P., et al. Direct therapeutic applications of calcium electroporation to effectively induce tumor necrosis. Cancer Res. 2012;72(6):1336–1341. doi: 10.1158/0008-5472.CAN-11-3782. [DOI] [PubMed] [Google Scholar]
- 206.Smith K.C., Neu J.C., Krassowska W. Model of creation and evolution of stable electropores for DNA delivery. Biophys. J. 2004;86(5):2813–2826. doi: 10.1016/S0006-3495(04)74334-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Kennedy S.M., Ji Z., Rockweiler N.B., et al. The role of plasmalemmal-cortical anchoring on the stability of transmembrane electropores. IEEE Trans. Dielectr. Electr. Insul. 2009;16(5):1251–1258. doi: 10.1109/TDEI.2009.5293935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Weaver J.C., Chizmadzhev Y.A. Theory of electroporation: A review. Bioelectrochem. Bioenerg. 1996;41(2):135–160. [Google Scholar]
- 209.Rybenkov V.V., Vologodskii A.V., Cozzarelli N.R. The effect of ionic conditions on DNA helical repeat, effective diameter and free energy of supercoiling. Nucleic Acids Res. 1997;25(7):1412–1418. doi: 10.1093/nar/25.7.1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Rybenkov V.V., Vologodskii A.V., Cozzarelli N.R. The effect of ionic conditions on the conformations of supercoiled DNA. I. Sedimentation analysis. J. Mol. Biol. 1997;267(2):299–311. doi: 10.1006/jmbi.1996.0876. [DOI] [PubMed] [Google Scholar]
- 211.Yarmola E.G., Zarudnaya M.I., Lazurkin Yu S. Osmotic pressure of DNA solutions and effective diameter of the double helix. J. Biomol. Struct. Dyn. 1985;2(5):981–993. doi: 10.1080/07391102.1985.10507614. [DOI] [PubMed] [Google Scholar]
- 212.Sukharev S.I., Blount P., Martinac B., et al. A large-conductance mechanosensitive channel in E. coli encoded by mscL alone. Nature. 1994;368(6468):265–268. doi: 10.1038/368265a0. [DOI] [PubMed] [Google Scholar]
- 213.Escoffre J-M., Mauroy C., Portet T., et al. Gene electrotransfer: from biophysical mechanisms to in vivo applications. Biophys. Rev. 2009;1(4):177–184. doi: 10.1007/s12551-009-0022-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Rols M.P., Dahhou F., Mishra K.P., et al. Control of electric field induced cell membrane permeabilization by membrane order. Biochemistry. 1990;29(12):2960–2966. doi: 10.1021/bi00464a011. [DOI] [PubMed] [Google Scholar]
- 215.Joshi R.P., Schoenbach K.H. Electroporation dynamics in biological cells subjected to ultrafast electrical pulses: a numerical simulation study. Phys. Rev. E Stat. Phys. Plasmas Fluids Relat. Interdiscip. Topics. 2000;62(1 Pt B):1025–1033. doi: 10.1103/physreve.62.1025. [DOI] [PubMed] [Google Scholar]
- 216.Freeman S.A., Wang M.A., Weaver J.C. Theory of electroporation of planar bilayer-membranes - predictions of the aqueous area, change in capacitance, and pore-pore separation. Biophys. J. 1994;67(1):42–56. doi: 10.1016/S0006-3495(94)80453-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Kakorin S., Neumann E. Ionic conductivity of electroporated lipid bilayer membranes. Bioelectrochemistry. 2002;56(1-2):163–166. doi: 10.1016/s1567-5394(02)00040-3. [DOI] [PubMed] [Google Scholar]
- 218.Hristova N.I., Tsoneva I., Neumann E. Sphingosine-mediated electroporative DNA transfer through lipid bilayers. FEBS Lett. 1997;415(1):81–86. doi: 10.1016/s0014-5793(97)01097-1. [DOI] [PubMed] [Google Scholar]
- 219.Rols M.P., Delteil C., Golzio M., et al. Control by ATP and ADP of voltage-induced mammalian-cell-membrane permeabilization, gene transfer and resulting expression. Eur. J. Biochem. 1998;254(2):382–388. doi: 10.1046/j.1432-1327.1998.2540382.x. [DOI] [PubMed] [Google Scholar]
- 220.Neamtu S., Morariu V.V., Turcu I., et al. Pore resealing inactivation in electroporated erythrocyte membrane irradiated with electrons. Bioelectrochem. Bioenerg. 1999;48(2):441–445. doi: 10.1016/s0302-4598(99)00044-6. [DOI] [PubMed] [Google Scholar]
- 221.Tekle E., Astumian R.D., Chock P.B. Selective and asymmetric molecular transport across electroporated cell membranes. Proc. Natl. Acad. Sci. USA. 1994;91(24):11512–11516. doi: 10.1073/pnas.91.24.11512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Spassova M., Tsoneva I., Petrov A.G., et al. Dip patch clamp currents suggest electrodiffusive transport of the polyelectrolyte DNA through lipid bilayers. Biophys. Chem. 1994;52(3):267–274. doi: 10.1016/0301-4622(94)00097-4. [DOI] [PubMed] [Google Scholar]
- 223.Bananis E., Murray J.W., Stockert R.J., et al. Microtubule and motor-dependent endocytic vesicle sorting in vitro. J. Cell Biol. 2000;151(1):179–186. doi: 10.1083/jcb.151.1.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Chernomordik L.V., Sokolov A.V., Budker V.G. Electrostimulated uptake of DNA by liposomes. Biochim. Biophys. Acta. 1990;1024(1):179–183. doi: 10.1016/0005-2736(90)90222-a. [DOI] [PubMed] [Google Scholar]
- 225.Tsong T.Y. Electroporation of cell membranes. Biophys. J. 1991;60(2):297–306. doi: 10.1016/S0006-3495(91)82054-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Antov Y., Barbul A., Korenstein R. Electroendocytosis: stimulation of adsorptive and fluid-phase uptake by pulsed low electric fields. Exp. Cell Res. 2004;297(2):348–362. doi: 10.1016/j.yexcr.2004.03.027. [DOI] [PubMed] [Google Scholar]
- 227.Antov Y., Barbul A., Mantsur H., et al. Electroendocytosis: exposure of cells to pulsed low electric fields enhances adsorption and uptake of macromolecules. Biophys. J. 2005;88(3):2206–2223. doi: 10.1529/biophysj.104.051268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Ferguson S.M., De Camilli P. Dynamin, a membrane-remodelling GTPase. Nat. Rev. Mol. Cell Biol. 2012;13(2):75–88. doi: 10.1038/nrm3266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Markelc B., Skvarca E., Dolinsek T., et al. Inhibitor of endocytosis impairs gene electrotransfer to mouse muscle in vivo. Bioelectrochemistry. 2015;103:111–119. doi: 10.1016/j.bioelechem.2014.08.020. [DOI] [PubMed] [Google Scholar]
- 230.Rosazza C., Buntz A., Riess T., et al. Intracellular tracking of single-plasmid DNA particles after delivery by electroporation. Mol. Ther. 2013;21(12):2217–2226. doi: 10.1038/mt.2013.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Rosazza C., Escoffre J-M., Zumbusch A., et al. The actin cytoskeleton has an active role in the electrotransfer of plasmid DNA in mammalian cells. Mol. Ther. 2011;19(5):913–921. doi: 10.1038/mt.2010.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Rosazza C., Phez E., Escoffre J.M., et al. Cholesterol implications in plasmid DNA electrotransfer: Evidence for the involvement of endocytotic pathways. Int. J. Pharm. 2012;423(1):134–143. doi: 10.1016/j.ijpharm.2011.05.024. [DOI] [PubMed] [Google Scholar]
- 233.Wu M., Yuan F. Membrane binding of plasmid DNA and endocytic pathways are involved in electrotransfection of mammalian cells. PLoS One. 2011;6(6):e20923. doi: 10.1371/journal.pone.0020923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Di Paolo G., De Camilli P. Phosphoinositides in cell regulation and membrane dynamics. Nature. 2006;443(7112):651–657. doi: 10.1038/nature05185. [DOI] [PubMed] [Google Scholar]
- 235.Lambert H., Pankov R., Gauthier J., et al. Electroporation-mediated uptake of proteins into mammalian-cells. Biochem. Cell Biol. 1990;68(4):729–734. doi: 10.1139/o90-105. [DOI] [PubMed] [Google Scholar]
- 236.Glogauer M., Lee W., McCulloch C.A. Induced endocytosis in human fibroblasts by electrical fields. Exp. Cell Res. 1993;208(1):232–240. doi: 10.1006/excr.1993.1242. [DOI] [PubMed] [Google Scholar]
- 237.Rols M.P., Femenia P., Teissie J. Long-lived macropinocytosis takes place in electropermeabilized mammalian cells. Biochem. Biophys. Res. Commun. 1995;208(1):26–35. doi: 10.1006/bbrc.1995.1300. [DOI] [PubMed] [Google Scholar]
- 238.Rosemberg Y., Korenstein R. Incorporation of macromolecules into cells and vesicles by low electric fields: induction of endocytotic-like processes. Bioelectrochem. Bioenerg. 1997;42(2):275–281. [Google Scholar]
- 239.Lin R., Chang D.C., Lee Y.K. Single-cell electroendocytosis on a micro chip using in situ fluorescence microscopy. Biomed. Microdevices. 2011;13(6):1063–1073. doi: 10.1007/s10544-011-9576-9. [DOI] [PubMed] [Google Scholar]
- 240.Mahrour N., Pologea-Moraru R., Moisescu M.G., et al. In vitro increase of the fluid-phase endocytosis induced by pulsed radiofrequency electromagnetic fields: importance of the electric field component. Biochim. Biophys. Acta. 2005;1668(1):126–137. doi: 10.1016/j.bbamem.2004.11.015. [DOI] [PubMed] [Google Scholar]
- 241.Li X.F., Kolega J. Effects of direct current electric fields on cell migration and actin filament distribution in bovine vascular endothelial cells. J. Vasc. Res. 2002;39(5):391–404. doi: 10.1159/000064517. [DOI] [PubMed] [Google Scholar]
- 242.Gass G.V., Chernomordik L.V. Reversible large-scale deformations in the membranes of electrically-treated cells - electroinduced bleb formation. Biochim. Biophys. Acta. 1990;1023(1):1–11. doi: 10.1016/0005-2736(90)90002-6. [DOI] [PubMed] [Google Scholar]
- 243.Popov S.V., Margolis L.B. Formation of cell outgrowths by external force: a model study. J. Cell Sci. 1988;90(Pt 3):379–389. doi: 10.1242/jcs.90.3.379. [DOI] [PubMed] [Google Scholar]
- 244.Pehlivanova V.N., Tsoneva I.H., Tzoneva R.D. Multiple effects of electroporation on the adhesive behaviour of breast cancer cells and fibroblasts. Cancer Cell Int. 2012;12(1):9. doi: 10.1186/1475-2867-12-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Zimmermann U., Schnettler R., Klock G., et al. Mechanisms of electrostimulated uptake of macromolecules into living cells. Naturwissenschaften. 1990;77(11):543–545. doi: 10.1007/BF01139269. [DOI] [PubMed] [Google Scholar]
- 246.Rols M.P., Delteil C., Serin G., et al. Temperature effects on electrotransfection of mammalian-cells. Nucleic Acids Res. 1994;22(3):540. doi: 10.1093/nar/22.3.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Doherty G.J., McMahon H.T. Mechanisms of endocytosis. Annu. Rev. Biochem. 2009;78:857–902. doi: 10.1146/annurev.biochem.78.081307.110540. [DOI] [PubMed] [Google Scholar]
- 248.Schafer D.A. Regulating actin dynamics at membranes: a focus on dynamin. Traffic. 2004;5(7):463–469. doi: 10.1111/j.1600-0854.2004.00199.x. [DOI] [PubMed] [Google Scholar]
- 249.Roux A., Koster G., Lenz M., et al. Membrane curvature controls dynamin polymerization. Proc. Natl. Acad. Sci. USA. 2010;107(9):4141–4146. doi: 10.1073/pnas.0913734107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Romer W., Berland L., Chambon V., et al. Shiga toxin induces tubular membrane invaginations for its uptake into cells. Nature. 2007;450(7170):670–675. doi: 10.1038/nature05996. [DOI] [PubMed] [Google Scholar]
- 251.Rosazza C., Deschout H., Buntz A., et al. Endocytosis and endosomal trafficking of DNA after gene electrotransfer in vitro. Mol. Ther. Nucleic Acids. 2016;5:e286. doi: 10.1038/mtna.2015.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Mercer J., Schelhaas M., Helenius A. Virus entry by endocytosis. Annu. Rev. Biochem. 2010;79:803–833. doi: 10.1146/annurev-biochem-060208-104626. [DOI] [PubMed] [Google Scholar]
- 253.West M.A., Bretscher M.S., Watts C. Distinct endocytotic pathways in epidermal growth factor-stimulated human carcinoma A431 cells. J. Cell Biol. 1989;109(6 Pt 1):2731–2739. doi: 10.1083/jcb.109.6.2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Dowrick P., Kenworthy P., McCann B., et al. Circular ruffle formation and closure lead to macropinocytosis in hepatocyte growth factor/scatter factor-treated cells. Eur. J. Cell Biol. 1993;61(1):44–53. [PubMed] [Google Scholar]
- 255.Araki N., Johnson M.T., Swanson J.A. A role for phosphoinositide 3-kinase in the completion of macropinocytosis and phagocytosis by macrophages. J. Cell Biol. 1996;135(5):1249–1260. doi: 10.1083/jcb.135.5.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Mercer J., Helenius A. Virus entry by macropinocytosis. Nat. Cell Biol. 2009;11(5):510–520. doi: 10.1038/ncb0509-510. [DOI] [PubMed] [Google Scholar]
- 257.Ivanov A.I. Pharmacological inhibition of endocytic pathways: is it specific enough to be useful? Methods Mol. Biol. 2008;440:15–33. doi: 10.1007/978-1-59745-178-9_2. [DOI] [PubMed] [Google Scholar]
- 258.Qualmann B., Kessels M.M. Endocytosis and the cytoskeleton. Int. Rev. Cytol. 2002;220:93–144. doi: 10.1016/s0074-7696(02)20004-2. [DOI] [PubMed] [Google Scholar]
- 259.Lajoie P., Nabi I.R. Lipid rafts, caveolae, and their endocytosis. Int. Rev. Cell Mol. Biol. 2010;282:135–163. doi: 10.1016/S1937-6448(10)82003-9. [DOI] [PubMed] [Google Scholar]
- 260.Kabouridis P.S., Janzen J., Magee A.L., et al. Cholesterol depletion disrupts lipid rafts and modulates the activity of multiple signaling pathways in T lymphocytes. Eur. J. Immunol. 2000;30(3):954–963. doi: 10.1002/1521-4141(200003)30:3<954::AID-IMMU954>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 261.Sahay G., Alakhova D.Y., Kabanov A.V. Endocytosis of nanomedicines. J. Control. Release. 2010;145(3):182–195. doi: 10.1016/j.jconrel.2010.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Pelkmans L., Puntener D., Helenius A. Local actin polymerization and dynamin recruitment in SV40-induced internalization of caveolae. Science. 2002;296(5567):535–539. doi: 10.1126/science.1069784. [DOI] [PubMed] [Google Scholar]
- 263.Nonnenmacher M., Weber T. Adeno-associated virus 2 infection requires endocytosis through the CLIC/GEEC pathway. Cell Host Microbe. 2011;10(6):563–576. doi: 10.1016/j.chom.2011.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Henley J.R., Krueger E.W., Oswald B.J., et al. Dynamin-mediated internalization of caveolae. J. Cell Biol. 1998;141(1):85–99. doi: 10.1083/jcb.141.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Oh P., McIntosh D.P., Schnitzer J.E. Dynamin at the neck of caveolae mediates their budding to form transport vesicles by GTP-driven fission from the plasma membrane of endothelium. J. Cell Biol. 1998;141(1):101–114. doi: 10.1083/jcb.141.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Parton R.G., Joggerst B., Simons K. Regulated internalization of caveolae. J. Cell Biol. 1994;127(5):1199–1215. doi: 10.1083/jcb.127.5.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Lajoie P., Goetz J.G., Dennis J.W., et al. Lattices, rafts, and scaffolds: domain regulation of receptor signaling at the plasma membrane. J. Cell Biol. 2009;185(3):381–385. doi: 10.1083/jcb.200811059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Lajoie P., Nabi I.R. Regulation of raft-dependent endocytosis. J. Cell. Mol. Med. 2007;11(4):644–653. doi: 10.1111/j.1582-4934.2007.00083.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Kirkham M., Parton R.G. Clathrin-independent endocytosis: new insights into caveolae and non-caveolar lipid raft carriers. Biochim. Biophys. Acta. 2005;1745(3):273–286. doi: 10.1016/j.bbamcr.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 270.Lajoie P., Kojic L.D., Nim S., et al. Caveolin-1 regulation of dynamin-dependent, raft-mediated endocytosis of cholera toxin-B sub-unit occurs independently of caveolae. J. Cell. Mol. Med. 2009;13(9B):3218–3225. doi: 10.1111/j.1582-4934.2009.00732.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Glebov O.O., Bright N.A., Nichols B.J. Flotillin-1 defines a clathrin-independent endocytic pathway in mammalian cells. Nat. Cell Biol. 2006;8(1):46–54. doi: 10.1038/ncb1342. [DOI] [PubMed] [Google Scholar]
- 272.Rejman J., Oberle V., Zuhorn I.S., et al. Size-dependent internalization of particles via the pathways of clathrin and caveolae-mediated endocytosis. Biochem. J. 2004;377(Pt 1):159–169. doi: 10.1042/BJ20031253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Draeger A., Monastyrskaya K., Babiychuk E.B. Plasma membrane repair and cellular damage control. Chem. Phys. Lipids. 2011;164:S15–S6. doi: 10.1016/j.bcp.2010.12.027. [DOI] [PubMed] [Google Scholar]
- 274.Idone V., Tam C., Andrews N.W. Two-way traffic on the road to plasma membrane repair. Trends Cell Biol. 2008;18(11):552–559. doi: 10.1016/j.tcb.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Hai A., Spira M.E. On-chip electroporation, membrane repair dynamics and transient in-cell recordings by arrays of gold mushroom-shaped microelectrodes. Lab Chip. 2012;12(16):2865–2873. doi: 10.1039/c2lc40091j. [DOI] [PubMed] [Google Scholar]
- 276.Huynh C., Roth D., Ward D.M., et al. Defective lysosomal exocytosis and plasma membrane repair in Chediak-Higashi/beige cells. Proc. Natl. Acad. Sci. USA. 2004;101(48):16795–16800. doi: 10.1073/pnas.0405905101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Luby-Phelps K. Cytoarchitecture and physical properties of cytoplasm: volume, viscosity, diffusion, intracellular surface area. Int. Rev. Cytol. 2000;192:189–221. doi: 10.1016/s0074-7696(08)60527-6. [DOI] [PubMed] [Google Scholar]
- 278.Seksek O., Biwersi J., Verkman A.S. Translational diffusion of macromolecule-sized solutes in cytoplasm and nucleus. J. Cell Biol. 1997;138(1):131–142. doi: 10.1083/jcb.138.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Lukacs G.L., Haggie P., Seksek O., et al. Size-dependent DNA mobility in cytoplasm and nucleus. J. Biol. Chem. 2000;275(3):1625–1629. doi: 10.1074/jbc.275.3.1625. [DOI] [PubMed] [Google Scholar]
- 280.Dauty E., Verkman A.S. Actin cytoskeleton as the principal determinant of size-dependent DNA mobility in cytoplasm: a new barrier for non-viral gene delivery. J. Biol. Chem. 2005;280(9):7823–7828. doi: 10.1074/jbc.M412374200. [DOI] [PubMed] [Google Scholar]
- 281.Dowty M.E., Williams P., Zhang G., et al. Plasmid DNA entry into postmitotic nuclei of primary rat myotubes. Proc. Natl. Acad. Sci. USA. 1995;92(10):4572–4576. doi: 10.1073/pnas.92.10.4572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Arhel N., Genovesio A., Kim K.A., et al. Quantitative four-dimensional tracking of cytoplasmic and nuclear HIV-1 complexes. Nat. Methods. 2006;3(10):817–824. doi: 10.1038/nmeth928. [DOI] [PubMed] [Google Scholar]
- 283.Ruthardt N., Lamb D.C., Brauchle C. Single-particle tracking as a quantitative microscopy-based approach to unravel cell entry mechanisms of viruses and pharmaceutical nanoparticles. Mol. Ther. 2011;19(7):1199–1211. doi: 10.1038/mt.2011.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Suh J., Wirtz D., Hanes J. Real-time intracellular transport of gene nanocarriers studied by multiple particle tracking. Biotechnol. Prog. 2004;20(2):598–602. doi: 10.1021/bp034251y. [DOI] [PubMed] [Google Scholar]
- 285.de Bruin K., Ruthardt N., von Gersdorff K., et al. Cellular dynamics of EGF receptor-targeted synthetic viruses. Mol. Ther. 2007;15(7):1297–1305. doi: 10.1038/sj.mt.6300176. [DOI] [PubMed] [Google Scholar]
- 286.Akita H., Enoto K., Masuda T., et al. Particle tracking of intracellular trafficking of octaarginine-modified liposomes: a comparative study with adenovirus. Mol. Ther. 2010;18(5):955–964. doi: 10.1038/mt.2010.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Sauer A.M., de Bruin K.G., Ruthardt N., et al. Dynamics of magnetic lipoplexes studied by single particle tracking in living cells. J. Control. Release. 2009;137(2):136–145. doi: 10.1016/j.jconrel.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 288.Osborne K.D., Lee W., Malarkey E.B., et al. Dynamic imaging of cannabinoid receptor 1 vesicular trafficking in cultured astrocytes. ASN Neuro. 2009;1(5):e00022. doi: 10.1042/AN20090040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Li H., Duan Z.W., Xie P., et al. Effects of paclitaxel on EGFR endocytic trafficking revealed using quantum dot tracking in single cells. PLoS One. 2012;7(9):e45465. doi: 10.1371/journal.pone.0045465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Ichikawa T., Yamada M., Homma D., et al. Digital fluorescence imaging of trafficking of endosomes containing low-density lipoprotein in brain astroglial cells. Biochem. Biophys. Res. Commun. 2000;269(1):25–30. doi: 10.1006/bbrc.2000.2261. [DOI] [PubMed] [Google Scholar]
- 291.Toshima J.Y., Toshima J., Kaksonen M., et al. Spatial dynamics of receptor-mediated endocytic trafficking in budding yeast revealed by using fluorescent alpha-factor derivatives. Proc. Natl. Acad. Sci. USA. 2006;103(15):5793–5798. doi: 10.1073/pnas.0601042103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Pilling A.D., Horiuchi D., Lively C.M., et al. Kinesin-1 and Dynein are the primary motors for fast transport of mitochondria in Drosophila motor axons. Mol. Biol. Cell. 2006;17(4):2057–2068. doi: 10.1091/mbc.E05-06-0526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Sweeney H.L., Park H., Zong A.B., et al. How myosin VI coordinates its heads during processive movement. EMBO J. 2007;26(11):2682–2692. doi: 10.1038/sj.emboj.7601720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Pierobon P., Achouri S., Courty S., et al. Velocity, processivity, and individual steps of single myosin V molecules in live cells. Biophys. J. 2009;96(10):4268–4275. doi: 10.1016/j.bpj.2009.02.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Ali M.Y., Kennedy G.G., Safer D., et al. Myosin Va and myosin VI coordinate their steps while engaged in an in vitro tug of war during cargo transport. Proc. Natl. Acad. Sci. USA. 2011;108(34):535–541. doi: 10.1073/pnas.1104298108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Merrifield C.J., Moss S.E., Ballestrem C., et al. Endocytic vesicles move at the tips of actin tails in cultured mast cells. Nat. Cell Biol. 1999;1(1):72–74. doi: 10.1038/9048. [DOI] [PubMed] [Google Scholar]
- 297.Murray J.W., Wolkoff A.W. Roles of the cytoskeleton and motor proteins in endocytic sorting. Adv. Drug Deliv. Rev. 2003;55(11):1385–1403. doi: 10.1016/j.addr.2003.07.008. [DOI] [PubMed] [Google Scholar]
- 298.Mallik R., Petrov D., Lex S.A., et al. Building complexity: an in vitro study of cytoplasmic dynein with in vivo implications. Curr. Biol. 2005;15(23):2075–2085. doi: 10.1016/j.cub.2005.10.039. [DOI] [PubMed] [Google Scholar]
- 299.Jamison D.K., Driver J.W., Rogers A.R., et al. Two kinesins transport cargo primarily via the action of one motor: implications for intracellular transport. Biophys. J. 2010;99(9):2967–2977. doi: 10.1016/j.bpj.2010.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Bhat D., Gopalakrishnan M. Effectiveness of a dynein team in a tug of war helped by reduced load sensitivity of detachment: evidence from the study of bidirectional endosome transport in D. discoideum. Phys. Biol. 2012;9(4):046003. doi: 10.1088/1478-3975/9/4/046003. [DOI] [PubMed] [Google Scholar]
- 301.Badding M.A., Lapek J.D., Friedman A.E., et al. Proteomic and functional analyses of protein-DNA complexes during gene transfer. Mol. Ther. 2013;21(4):775–785. doi: 10.1038/mt.2012.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Vaughan E.E., Dean D.A. Intracellular trafficking of plasmids during transfection is mediated by microtubules. Mol. Ther. 2006;13(2):422–428. doi: 10.1016/j.ymthe.2005.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Wang Z., Khan S., Sheetz M.P. Single cytoplasmic dynein molecule movements: characterization and comparison with kinesin. Biophys. J. 1995;69(5):2011–2023. doi: 10.1016/S0006-3495(95)80071-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Soppina V., Rai A.K., Ramaiya A.J., et al. Tug-of-war between dissimilar teams of microtubule motors regulates transport and fission of endosomes. Proc. Natl. Acad. Sci. USA. 2009;106(46):19381–19386. doi: 10.1073/pnas.0906524106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Provance D.W., Jr, Addison E.J., Wood P.R., et al. Myosin-Vb functions as a dynamic tether for peripheral endocytic compartments during transferrin trafficking. BMC Cell Biol. 2008;9:44. doi: 10.1186/1471-2121-9-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Ewers H., Smith A.E., Sbalzarini I.F., et al. Single-particle tracking of murine polyoma virus-like particles on live cells and artificial membranes. Proc. Natl. Acad. Sci. USA. 2005;102(42):15110–15115. doi: 10.1073/pnas.0504407102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Spendier K., Lidke K.A., Lidke D.S., et al. Single-particle tracking of immunoglobulin E receptors (FcepsilonRI) in micron-sized clusters and receptor patches. FEBS Lett. 2012;586(4):416–421. doi: 10.1016/j.febslet.2012.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Fath K.R., Trimbur G.M., Burgess D.R. Molecular motors and a spectrin matrix associate with Golgi membranes in vitro. J. Cell Biol. 1997;139(5):1169–1181. doi: 10.1083/jcb.139.5.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Lechardeur D., Sohn K.J., Haardt M., et al. Metabolic instability of plasmid DNA in the cytosol: a potential barrier to gene transfer. Gene Ther. 1999;6(4):482–497. doi: 10.1038/sj.gt.3300867. [DOI] [PubMed] [Google Scholar]
- 310.Coonrod A., Li F.Q., Horwitz M. On the mechanism of DNA transfection: efficient gene transfer without viruses. Gene Ther. 1997;4(12):1313–1321. doi: 10.1038/sj.gt.3300536. [DOI] [PubMed] [Google Scholar]
- 311.Rajendran L., Knolker H.J., Simons K. Subcellular targeting strategies for drug design and delivery. Nat. Rev. Drug Discov. 2010;9(1):29–42. doi: 10.1038/nrd2897. [DOI] [PubMed] [Google Scholar]
- 312.Boisvert M., Tijssen P. Molecular Regulation of Endocytosis. InTech; 2012. Endocytosis of Non-Enveloped DNA Viruses. [Google Scholar]
- 313.Huotari J., Helenius A. Endosome maturation. EMBO J. 2011;30(17):3481–3500. doi: 10.1038/emboj.2011.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Perret E., Lakkaraju A., Deborde S., et al. Evolving endosomes: how many varieties and why? Curr. Opin. Cell Biol. 2005;17(4):423–434. doi: 10.1016/j.ceb.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 315.Fattakhova Gn., Masilamani M., Borrego F., et al. The high-affinity immunoglobulin-E receptor (FcepsilonRI) is endocytosed by an AP-2/clathrin-independent, dynamin-dependent mechanism. Traffic. 2006;7(6):673–685. doi: 10.1111/j.1600-0854.2006.00423.x. [DOI] [PubMed] [Google Scholar]
- 316.Frick M., Bright N.A., Riento K., et al. Coassembly of flotillins induces formation of membrane microdomains, membrane curvature, and vesicle budding. Curr. Biol. 2007;17(13):1151–1156. doi: 10.1016/j.cub.2007.05.078. [DOI] [PubMed] [Google Scholar]
- 317.Frischknecht F., Moreau V., Rottger S., et al. Actin-based motility of vaccinia virus mimics receptor tyrosine kinase signalling. Nature. 1999;401(6756):926–929. doi: 10.1038/44860. [DOI] [PubMed] [Google Scholar]
- 318.Fujimoto L.M., Roth R., Heuser J.E., et al. Actin assembly plays a variable, but not obligatory role in receptor-mediated endocytosis in mammalian cells. Traffic. 2000;1(2):161–171. doi: 10.1034/j.1600-0854.2000.010208.x. [DOI] [PubMed] [Google Scholar]
- 319.Retelj L., Pucihar G., Miklavcic D. Electroporation of intracellular liposomes using nanosecond electric pulses-A theoretical study. IEEE Trans. Biomed. Eng. 2013;60(9):2624–2635. doi: 10.1109/TBME.2013.2262177. [DOI] [PubMed] [Google Scholar]
- 320.Dean D.A., Dean B.S., Muller S., et al. Sequence requirements for plasmid nuclear import. Exp. Cell Res. 1999;253(2):713–722. doi: 10.1006/excr.1999.4716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Schwachtgen J.L., Ferreira V., Meyer D., et al. Optimization of the transfection of human endothelial-cells by electroporation. Biotechniques. 1994;17(5):882–887. [PubMed] [Google Scholar]
- 322.Takahashi M., Furukawa T., Nikkuni K., et al. Efficient introduction of a gene into hematopoietic-cells in S-phase by electroporation. Exp. Hematol. 1991;19(5):343–346. [PubMed] [Google Scholar]
- 323.Brunner S., Furtbauer E., Sauer T., et al. Overcoming the nuclear barrier: cell cycle independent nonviral gene transfer with linear polyethylenimine or electroporation. Mol. Ther. 2002;5(1):80–86. doi: 10.1006/mthe.2001.0509. [DOI] [PubMed] [Google Scholar]
- 324.Young J.L., Benoit J.N., Dean D.A. Effect of a DNA nuclear targeting sequence on gene transfer and expression of plasmids in the intact vasculature. Gene Ther. 2003;10(17):1465–1470. doi: 10.1038/sj.gt.3302021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Kubiniec R.T., Liang H., Hui S.W. Effects of pulse length and pulse strength on transfection by electroporation. Biotechniques. 1990;8(1):16–20. [PubMed] [Google Scholar]
- 326.Knutson J.C., Yee D. Electroporation: parameters affecting transfer of DNA into mammalian cells. Anal. Biochem. 1987;164(1):44–52. doi: 10.1016/0003-2697(87)90365-4. [DOI] [PubMed] [Google Scholar]
- 327.Cepurniene K., Ruzgys P., Treinys R., et al. Influence of plasmid concentration on DNA electrotransfer in vitro using high-voltage and low-voltage pulses. J. Membr. Biol. 2010;236(1):81–85. doi: 10.1007/s00232-010-9270-5. [DOI] [PubMed] [Google Scholar]
- 328.Cemazar M., Golzio M., Sersa G., et al. Control by pulse parameters of DNA electrotransfer into solid tumors in mice. Gene Ther. 2009;16(5):635–644. doi: 10.1038/gt.2009.10. [DOI] [PubMed] [Google Scholar]
- 329.Rols M.P., Coulet D., Teissie J. Highly efficient transfection of mammalian cells by electric field pulses. Application to large volumes of cell culture by using a flow system. Eur. J. Biochem. 1992;206(1):115–121. doi: 10.1111/j.1432-1033.1992.tb16908.x. [DOI] [PubMed] [Google Scholar]
- 330.Vandermeulen G., Staes E., Vanderhaeghen M.L., et al. Optimisation of intradermal DNA electrotransfer for immunisation. J. Control. Release. 2007;124(1-2):81–87. doi: 10.1016/j.jconrel.2007.08.010. [DOI] [PubMed] [Google Scholar]
- 331.Escoffre J.M., Nikolova B., Mallet L., et al. New insights in the gene electrotransfer process: Evidence for the involvement of the plasmid DNA topology. Curr. Gene Ther. 2012;12(5):417–422. doi: 10.2174/156652312802762554. [DOI] [PubMed] [Google Scholar]
- 332.Xie T.D., Sun L., Zhao H.G., et al. Study of mechanisms of electric field-induced DNA transfection. IV. Effects of DNA topology on cell uptake and transfection efficiency. Biophys. J. 1992;63(4):1026–1031. doi: 10.1016/S0006-3495(92)81675-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Titomirov A.V., Sukharev S., Kistanova E. In vivo electroporation and stable transformation of skin cells of newborn mice by plasmid DNA. Biochim. Biophys. Acta. 1991;1088(1):131–134. doi: 10.1016/0167-4781(91)90162-f. [DOI] [PubMed] [Google Scholar]
- 334.Lackovic I., Magjarevic R., Miklavcic D. Three-dimensional finite-element analysis of joule heating in electrochemotherapy and in vivo gene electrotransfer. IEEE Trans. Dielectr. Electr. Insul. 2009;16(5):1338–1347. [Google Scholar]
- 335.Pliquett U. Joule heating during solid tissue electroporation. Med. Biol. Eng. Comput. 2003;41(2):215–219. doi: 10.1007/BF02344892. [DOI] [PubMed] [Google Scholar]
- 336.Rebersek M., Miklavcic D., Bertacchini C., et al. Cell Membrane electroporation-Part 3: The equipment. IEEE Electr Insul Mag. 2014;30(3):8–18. [Google Scholar]
- 337.Corovic S., Mir L.M., Miklavcic D. In vivo muscle electroporation threshold determination: Realistic numerical models and in vivo experiments. J. Membr. Biol. 2012;245(9):509–520. doi: 10.1007/s00232-012-9432-8. [DOI] [PubMed] [Google Scholar]
- 338.Miklavcic D., Corovic S., Pucihar G., et al. Importance of tumour coverage by sufficiently high local electric field for effective electrochemotherapy. Ejc Suppl. 2006;4(11):45–51. [Google Scholar]
- 339.Miklavčič D., Pavšelj N., Hart F.X. Electric Properties of Tissues. Wiley Encyclopedia of Biomedical Engineering. NJ, USA: John Wiley & Sons, Inc.; 2006. [Google Scholar]
- 340.Corovic S., Zupanic A., Kranjc S., et al. The influence of skeletal muscle anisotropy on electroporation: in vivo study and numerical modeling. Med. Biol. Eng. Comput. 2010;48(7):637–648. doi: 10.1007/s11517-010-0614-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Guenther E., Klein N., Mikus P., et al. Electrical breakdown in tissue electroporation. Biochem. Biophys. Res. Commun. 2015;467(4):736–741. doi: 10.1016/j.bbrc.2015.10.072. [DOI] [PubMed] [Google Scholar]
- 342.Kotnik T., Miklavcic D., Mir L.M. Cell membrane electropermeabilization by symmetrical bipolar rectangular pulses. Part II. Reduced electrolytic contamination. Bioelectrochemistry. 2001;54(1):91–95. doi: 10.1016/s1567-5394(01)00115-3. [DOI] [PubMed] [Google Scholar]
- 343.Arena C.B., Mahajan R.L., Nichole Rylander M., et al. An experimental and numerical investigation of phase change electrodes for therapeutic irreversible electroporation. J. Biomech. Eng. 2013;135(11):111009. doi: 10.1115/1.4025334. [DOI] [PubMed] [Google Scholar]
- 344.Sel D., Cukjati D., Batiuskaite D., et al. Sequential finite element model of tissue electropermeabilization. IEEE Trans. Biomed. Eng. 2005;52(5):816–827. doi: 10.1109/TBME.2005.845212. [DOI] [PubMed] [Google Scholar]
- 345.Chafai D.E., Mehle A., Tilmatine A., et al. Assessment of the electrochemical effects of pulsed electric fields in a biological cell suspension. Bioelectrochemistry. 2015;106:249–257. doi: 10.1016/j.bioelechem.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 346.Tomov T., Tsoneva I. Are the stainless steel electrodes inert? Bioelectrochemistry. 2000;51(2):207–209. doi: 10.1016/s0302-4598(00)00069-6. [DOI] [PubMed] [Google Scholar]
- 347.Hannaman D. Electroporation Based TriGrid™ Delivery System (TDS) for DNA Vaccine Administration. In: Thalhamer J., Weiss R., Scheiblhofer S., editors. Gene Vaccines: Springer Vienna. 2012. pp. 163–181. [Google Scholar]
- 348.Kim H.B., Ahn S., Jang H.J., et al. Evaluation of corrosion behaviors and surface profiles of platinum-coated electrodes by electrochemistry and complementary microscopy: Biomedical implications for anticancer therapy. Micron. 2007;38(7):747–753. doi: 10.1016/j.micron.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 349.Todorovic V., Kamensek U., Sersa G., et al. Changing electrode orientation, but not pulse polarity, increases the efficacy of gene electrotransfer to tumors in vivo. Bioelectrochemistry. 2014;100:119–127. doi: 10.1016/j.bioelechem.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 350.Garcia P.A., Davalos R.V., Miklavcic D. A numerical investigation of the electric and thermal cell kill distributions in electroporation-based therapies in tissue. PLoS One. 2014;9(8):e103083. doi: 10.1371/journal.pone.0103083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Dona M., Sandri M., Rossini K., et al. Functional in vivo gene transfer into the myofibers of adult skeletal muscle. Mol. Cell Biol. Res. Commun. 2003;312(4):1132–1138. doi: 10.1016/j.bbrc.2003.11.032. [DOI] [PubMed] [Google Scholar]
- 352.Martin J.B., Young J.L., Benoit J.N., et al. Gene transfer to intact mesenteric arteries by electroporation. J. Vasc. Res. 2000;37(5):372–380. doi: 10.1159/000025753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Rebersek M., Corovic S., Sersa G., et al. Electrode commutation sequence for honeycomb arrangement of electrodes in electrochemotherapy and corresponding electric field distribution. Bioelectrochemistry. 2008;74(1):26–31. doi: 10.1016/j.bioelechem.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 354.Babiuk S., Baca-Estrada M.E., Foldvari M., et al. Needle-free topical electroporation improves gene expression from plasmids administered in porcine skin. Mol. Ther. 2003;8(6):992–998. doi: 10.1016/j.ymthe.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 355.Soden D.M., Larkin J.O., Collins C.G., et al. Successful application of targeted electrochemotherapy using novel flexible electrodes and low dose bleomycin to solid tumours. Cancer Lett. 2006;232(2):300–310. doi: 10.1016/j.canlet.2005.03.057. [DOI] [PubMed] [Google Scholar]
- 356.Castiello M., Dughiero F., Scandola F., et al. A new grid electrode for electrochemotherapy treatment of large skin tumors. IEEE Trans. Dielectr. Electr. Insul. 2014;21(3):1424–1432. [Google Scholar]
- 357.Wei Z.W., Zheng S.Q., Wang R.X., et al. A flexible microneedle array as low-voltage electroporation electrodes for in vivo DNA and siRNA delivery. Lab Chip. 2014;14(20):4093–4102. doi: 10.1039/c4lc00800f. [DOI] [PubMed] [Google Scholar]
- 358.Liu F., Huang L. A syringe electrode device for simultaneous injection of DNA and electrotransfer. Mol. Ther. 2002;5(3):323–328. doi: 10.1006/mthe.2002.0540. [DOI] [PubMed] [Google Scholar]
- 359.Bureau M.F., Naimi S., Torero Ibad R., et al. Intramuscular plasmid DNA electrotransfer: biodistribution and degradation. Biochim. Biophys. Acta. 2004;1676(2):138–148. doi: 10.1016/j.bbaexp.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 360.Blomberg P., Eskandarpour M., Xia S., et al. Electroporation in combination with a plasmid vector containing SV40 enhancer elements results in increased and persistent gene expression in mouse muscle. Biochem. Biophys. Res. Commun. 2002;298(4):505–510. doi: 10.1016/s0006-291x(02)02486-5. [DOI] [PubMed] [Google Scholar]
- 361.Wasungu L., Escoffre J.M., Valette A., et al. A 3D in vitro spheroid model as a way to study the mechanisms of electroporation. Int. J. Pharm. 2009;379(2):278–284. doi: 10.1016/j.ijpharm.2009.03.035. [DOI] [PubMed] [Google Scholar]
- 362.Danko I., Williams P., Herweijer H., et al. High expression of naked plasmid DNA in muscles of young rodents. Hum. Mol. Genet. 1997;6(9):1435–1443. doi: 10.1093/hmg/6.9.1435. [DOI] [PubMed] [Google Scholar]
- 363.Wells D.J., Goldspink G. Age and sex influence expression of plasmid DNA directly injected into mouse skeletal-muscle. FEBS Lett. 1992;306(2-3):203–205. doi: 10.1016/0014-5793(92)81000-c. [DOI] [PubMed] [Google Scholar]
- 364.Jiao S.S., Williams P., Berg R.K., et al. Direct gene-transfer into nonhuman primate myofibers in vivo. Hum. Gene Ther. 1992;3(1):21–33. doi: 10.1089/hum.1992.3.1-21. [DOI] [PubMed] [Google Scholar]
- 365.McMahon J.M., Signori E., Wells K.E., et al. Optimisation of electrotransfer of plasmid into skeletal muscle by pretreatment with hyaluronidase - increased expression with reduced muscle damage. Gene Ther. 2001;8(16):1264–1270. doi: 10.1038/sj.gt.3301522. [DOI] [PubMed] [Google Scholar]
- 366.Henshaw J., Mossop B., Yuan F. Enhancement of electric field-mediated gene delivery through pretreatment of tumors with a hyperosmotic mannitol solution. Cancer Gene Ther. 2011;18(1):26–33. doi: 10.1038/cgt.2010.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Cappelletti M., Zampaglione I., Rizzuto G., et al. Gene electro-transfer improves transduction by modifying the fate of intramuscular DNA. J. Gene Med. 2003;5(4):324–332. doi: 10.1002/jgm.352. [DOI] [PubMed] [Google Scholar]
- 368.Cemazar M., Pavlin D., Kranjc S., et al. Sequence and time dependence of transfection efficiency of electrically-assisted gene delivery to tumors in mice. Curr. Drug Deliv. 2006;3(1):77–81. doi: 10.2174/156720106775197556. [DOI] [PubMed] [Google Scholar]
- 369.Mesojednik S., Pavlin D., Sersa G., et al. The effect of the histological properties of tumors on transfection efficiency of electrically assisted gene delivery to solid tumors in mice. Gene Ther. 2007;14(17):1261–1269. doi: 10.1038/sj.gt.3302989. [DOI] [PubMed] [Google Scholar]
- 370.Cemazar M., Golzio M., Sersa G., et al. Electrically-assisted nucleic acids delivery to tissues in vivo: Where do we stand? Curr. Pharm. Des. 2006;12(29):3817–3825. doi: 10.2174/138161206778559740. [DOI] [PubMed] [Google Scholar]
- 371.Andre F.M., Cournil-Henrionnet C., Vernerey D., et al. Variability of naked DNA expression after direct local injection: the influence of the injection speed. Gene Ther. 2006;13(23):1619–1627. doi: 10.1038/sj.gt.3302827. [DOI] [PubMed] [Google Scholar]
- 372.Philipp A., Meyer M., Wagner E. Extracellular targeting of synthetic therapeutic nucleic acid formulations. Curr. Gene Ther. 2008;8(5):324–334. doi: 10.2174/156652308786071023. [DOI] [PubMed] [Google Scholar]
- 373.Pisoschi A.M., Pop A. The role of antioxidants in the chemistry of oxidative stress: A review. Eur. J. Med. Chem. 2015;96:55–74. doi: 10.1016/j.ejmech.2015.04.040. [DOI] [PubMed] [Google Scholar]
- 374.Markelc B., Tevz G., Cemazar M., et al. Muscle gene electrotransfer is increased by the antioxidant tempol in mice. Gene Ther. 2012;19(3):312–320. doi: 10.1038/gt.2011.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Maglietti F., Michinski S., Olaiz N., et al. The role of PH fronts in tissue electroporation based treatments. PLoS One. 2013;8(11):e80167. doi: 10.1371/journal.pone.0080167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Olaiz N., Signori E., Maglietti F., et al. Tissue damage modeling in gene electrotransfer: The role of pH. Bioelectrochemistry. 2014;100:105–111. doi: 10.1016/j.bioelechem.2014.05.001. [DOI] [PubMed] [Google Scholar]
- 377.Sersa G., Cemazar M., Parkins C.S., et al. Tumour blood flow changes induced by application of electric pulses. Eur. J. Cancer. 1999;35(4):672–677. doi: 10.1016/s0959-8049(98)00426-2. [DOI] [PubMed] [Google Scholar]
- 378.Bellard E., Markelc B., Pelofy S., et al. Intravital microscopy at the single vessel level brings new insights of vascular modification mechanisms induced by electropermeabilization. J. Control. Release. 2012;163(3):396–403. doi: 10.1016/j.jconrel.2012.09.010. [DOI] [PubMed] [Google Scholar]


