Abstract
Niacin, as an antidyslipidemic drug, elicits a strong flushing response by release of prostaglandin (PG) D2. However, whether niacin is beneficial for inflammatory bowel disease (IBD) remains unclear. Here, we observed niacin administration‐enhanced PGD2 production in colon tissues in dextran sulfate sodium (DSS)‐challenged mice, and protected mice against DSS or 2,4,6‐trinitrobenzene sulfonic acid (TNBS)‐induced colitis in D prostanoid receptor 1 (DP1)‐dependent manner. Specific ablation of DP1 receptor in vascular endothelial cells, colonic epithelium, and myeloid cells augmented DSS/TNBS‐induced colitis in mice through increasing vascular permeability, promoting apoptosis of epithelial cells, and stimulating pro‐inflammatory cytokine secretion of macrophages, respectively. Niacin treatment improved vascular permeability, reduced apoptotic epithelial cells, promoted epithelial cell update, and suppressed pro‐inflammatory gene expression of macrophages. Moreover, treatment with niacin‐containing retention enema effectively promoted UC clinical remission and mucosal healing in patients with moderately active disease. Therefore, niacin displayed multiple beneficial effects on DSS/TNBS‐induced colitis in mice by activation of PGD2/DP1 axis. The potential efficacy of niacin in management of IBD warrants further investigation.
Keywords: DP1 receptor, niacin, prostaglandin, retention enema, ulcerative colitis
Subject Categories: Digestive System, Immunology
Introduction
Ulcerative colitis (UC) is a chronic inflammatory bowel disease (IBD) characterized by recurrent episodes of active disease, which commonly affects the colon, the rectum, or both simultaneously. Histologically, it displays chronic inflammatory alterations limited to the mucosa and submucosa with cryptitis and crypt abscesses (Danese & Fiocchi, 2011). Despite UC‐related mortality being low, its morbidity remains high and 10–20% of affected individuals undergo colectomy. Although the UC etiology is largely unknown, accumulated evidence supports an interaction between genetic predisposition and microbial/environmental factors that trigger pro‐colitogenic perturbations of the host–commensal relationship and an aberrant mucosal immune response (Khor et al, 2011). Genome‐wide association studies (GWAS) have identified 47 genetic susceptibility loci for UC, 28 of which are shared between Crohn's disease (CD) and UC (Franke et al, 2010; Anderson et al, 2011). Indeed, these risk loci implicated in IBD are involved in different key signal pathways which are essential for intestinal homeostasis, such as epithelial restitution, barrier function, innate and adaptive immune regulation, microbial defense, cellular stress, and metabolism (Khor et al, 2011). Moreover, vascular injury including dilated vessels and increased vascular permeability also contributes to the inflammatory disorder of colonic mucosa in UC patients (Deng et al, 2013).
Niacin (nicotinic acid) is also known as vitamin B3 and serves as a precursor for coenzymes such as nicotinamide adenine dinucleotide (NAD) and nicotinamide adenine dinucleotide phosphate (NADP), which are essential for living cells. Niacin has been used for more than five decades to treat dyslipidemia, because it reduces low‐density lipoprotein cholesterol (LDLc), very low‐density lipoprotein cholesterol (VLDLc), and triglycerides (TGs), and elevates high‐density lipoprotein cholesterol (HDLc) (Song & FitzGerald, 2013). The orphan G‐protein‐coupled receptor GPR109A, also known as hydroxycarboxylic acid 2 (HCA2) in mice, and as HM74A in humans, can be activated by niacin (Wise et al, 2003). The beneficial effect of niacin on free fatty acid and TGs is mediated by GPR109A suppression of lipolysis; however, the effects on HDLc and LDLc are not mediated by the GPR109A receptor (Bodor & Offermanns, 2008). GPR109A expression is markedly upregulated in macrophages upon inflammatory stimulation (Feingold et al, 2014). Moreover, emerging evidence demonstrated that niacin displays multiple anti‐inflammatory properties through GPR109A receptor activation (Holzhauser et al, 2011; Digby et al, 2012; Godin et al, 2012; Zandi‐Nejad et al, 2013; Zhou et al, 2014). Thus, the potential therapeutic efficacy of niacin on patients with UC warrants further clinical investigation.
One unpleasant side effect caused by niacin is cutaneous flushing. Niacin stimulates prostaglandin D2 (PGD2) release in both mice and humans (Hanson et al, 2010; Song & FitzGerald, 2013), which plays a central role in the niacin‐induced flushing. Low‐dose aspirin could depress niacin‐evoked PGD2 release and reduce the associated flushing (Cefali et al, 2007; Song & FitzGerald, 2013). PGD2 promotes the niacin‐evoked flushing through its specific D prostanoid receptor 1 (DP1). Blockade of DP1 receptor completely inhibits niacin‐induced vasodilation in mice and humans without affecting its effects on lipid metabolism (Cheng et al, 2006; Paolini et al, 2008; Maccubbin et al, 2009). In addition, PGD2 mediates active resolution of inflammation through DP1 receptor (Rajakariar et al, 2007; Kong et al, 2016). Interestingly, marked elevation of PGD2 production was observed in inflamed colon tissues from both UC patients and experimental colitis murine models (Ajuebor et al, 2000; Vong et al, 2010), which is associated with long‐term remission in humans (Vong et al, 2010). Yet, it remains to be determined whether niacin‐mediated protection against UC depends on PGD2 production.
In this study, we investigated the therapeutic effect of niacin on colitis both in mice and in patients with moderately active UC. We found that niacin shows anti‐inflammatory and anti‐apoptotic properties through downregulation of colonic inflammatory cytokine levels, suppression of vascular permeability, and inhibition of colonic epithelium apoptosis by activation of DP1 receptor in macrophages, endothelial cells, and colonic epithelium. Furthermore, treatment with retention enema containing niacin effectively promoted clinical remission and mucosal healing in patients with moderately active UC.
Results
Niacin boosts PGD2 generation in mice
To explore whether niacin protects against inflammatory bowel diseases (IBDs) through releasing PGD2, we first examined niacin‐induced PGD2 production in colon tissues and urinary secretion of PGD2 metabolites‐ 11,15‐Dioxo‐9α‐hydroxy‐2,3,4,5‐tetranorprostan‐1,20‐dioic acid (tetranor PGDM) from DSS‐induced colitis mouse model by using mass spectrometry analysis. Indeed, PGD2 production in homogenized colons and urinary tetranor PGDM was markedly elevated by niacin administration in DSS‐challenged mice in a dose‐dependent manner (Fig 1A and B). In addition, niacin treatment induced PGF2α product in colon tissues (Fig EV1A) and increased urinary metabolites of PGE2, PGI2, and PGF2α (Fig EV1B) in DSS‐challenged mice, indicating niacin may upregulate PG biosynthesis pathway. Accordingly, we observed niacin treatment upregulated cytosolic phospholipase A2 (cPLA2), COX‐2, and hematopoietic PGD synthase (hPGDS) in peritoneal macrophages (Fig 1C–E). However, niacin had no markedly influence on specialized pro‐resolving mediators (SPMs) in colon tissues from DSS‐challenged mice, such as lipoxin (LX) A4, resolvin (Rv) E1 (Fig EV1C).
Figure 1. Niacin induces PGD2 secretion in DSS‐challenged mice.
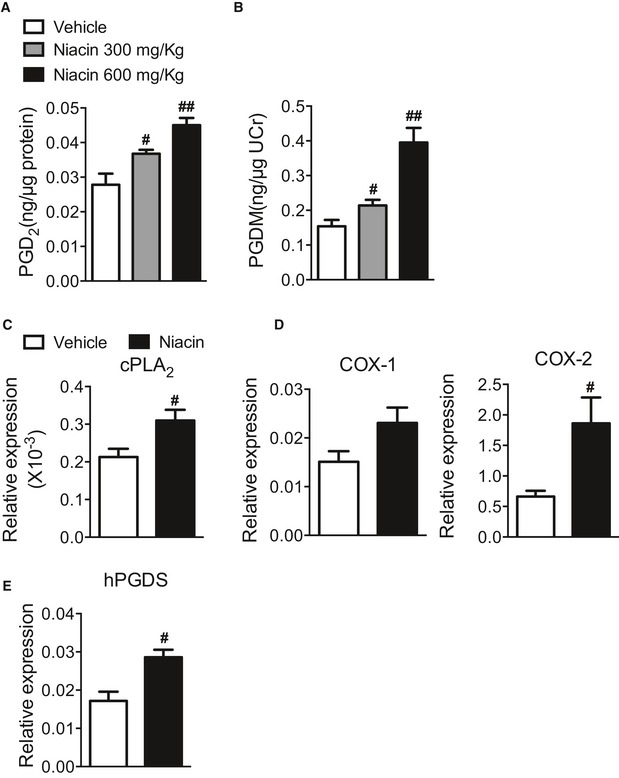
-
AMass spectrometry analysis of PGD2 production in colons from niacin‐treated mice after DSS challenge.
-
BMass spectrometry analysis of urinary PGD2 metabolites (PGDM) from niacin‐treated mice after DSS challenge. PGDM, 11,15‐dioxo‐9 alpha‐hydroxy‐2,3,4,5‐tetranorprostan‐1,20‐dioic acid.
-
C–EReal‐time PCR analysis of cPLA2, COX‐1, COX‐2, and hPGDS expression in peritoneal macrophage treated with niacin.
Figure EV1. Effect of niacin on PG, RvE1, and LXA4 production in mice after DSS challenge.
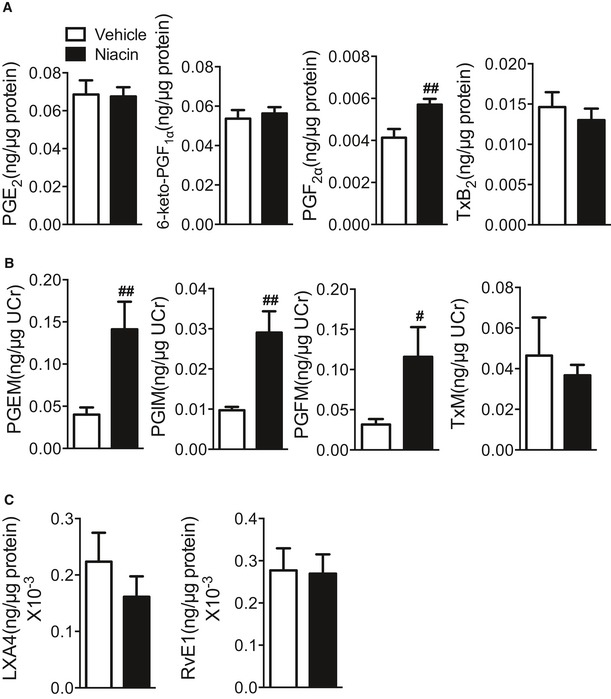
- Mass spectrometry analysis of PG production in colons from niacin (600 mg/kg)‐treated mice after DSS challenge. Vehicle, n = 6; niacin 600 mg/kg, n = 7.
- Measurement of urinary metabolite of PGs in niacin (600 mg/kg)‐treated mice. PGEM, 11α‐hydroxy‐9,15‐dioxo‐2,3,4,5‐tetranor‐prostane‐1,20‐dioic acid; PGIM, 2,3‐dinor‐6‐keto‐PGF1α; TxM, 2,3‐dinor‐TxB2; PGFM, 13,14‐dihydro‐15‐keto‐PGF2α. n = 6.
- Mass spectrometry analysis of RvE1 and LXA4 production in colons from niacin (600 mg/kg)‐treated mice after DSS challenge. n = 6.
Disruption of DP1 receptor deteriorates both DSS‐ and TNBS‐induced colitis in mice
PGD2 specifically binds and activates two distinct D prostanoid receptors DP1 and DP2. Next, we investigated the effects of PGD2 receptor deficiency on development of DSS‐ or TNBS‐induced colitis in mice. Interestingly, mice with global DP1 disruption (Fig 2A) lost over 12% more weight than wild‐type (WT) controls (Fig 2B), and had significantly higher DAI than WT after DSS challenge (2.33 ± 0.33 vs. 0.42 ± 0.22, P < 0.01, Fig 2C). Accordingly, DP1 deletion augmented the severity of DSS‐induced colitis in mice including reduction of colon length (Fig 2D), increase of epithelial cell lost, thickening of intestinal wall, enhanced infiltration of inflammatory cells in colon tissues (Fig 2E), and increase of overall mortality (Fig 2F). Likewise, DP1−/− mice were also more vulnerable to TNBS‐induced colitis (Fig EV2). However, DP2 deficiency (Satoh et al, 2006) did not influence DSS‐induced colitis in mice (Fig 2A–F). Thus, activation of DP1 receptor, not DP2, protects mice against DSS/TNBS‐induced colitis.
Figure 2. DP1 knockout augments DSS‐induced colitis in mice.
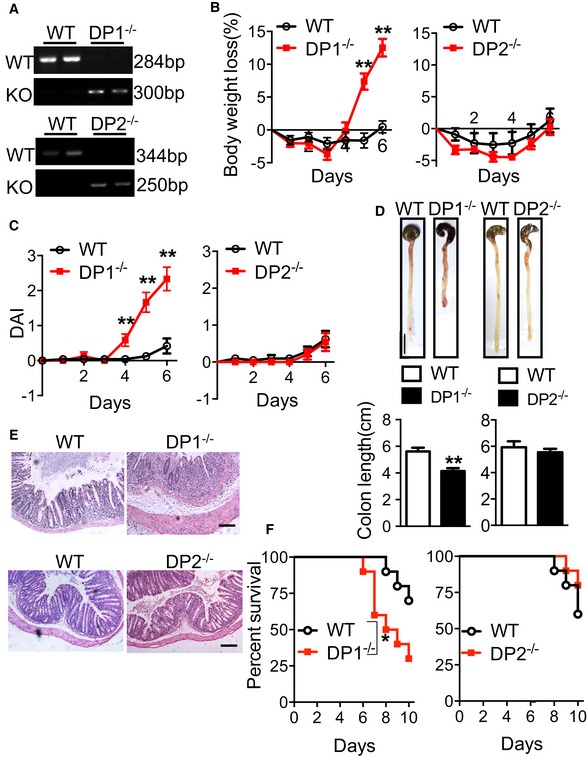
-
APCR analysis of tail genomic DNA from DP1−/−, DP2−/−, and WT mice.
-
B–DBody weight loss (B) and disease activity index (C), and colon length (D) of DP1−/−, DP2−/−, and WT mice in response to DSS challenge. Scale bar: 1 cm.
-
EH&E staining of histological sections in the distal colon from the mice administered with DSS for 6 days. Scale bars: 100 μm. Graphs represent overall histology score.
-
FSurvival rates of DSS‐challenged DP1−/−, DP2−/− mice, and WT controls.
Figure EV2. DP1 deficiency augments TNBS‐induced colitis in mice.
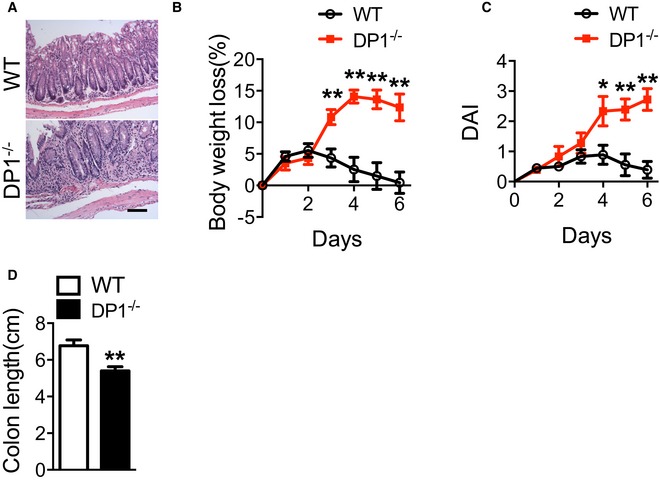
-
AH&E staining of histological sections in the distal colon from the mice after TNBS challenge. Scale bar: 100 μm. Graphs represent overall histology score.
-
B–DBody weight loss (B), disease activity index (DAI, C), and colon length (D) of DP1−/− and WT mice in response to 2.5% TNBS challenge.
Niacin ameliorates DSS/TNBS‐induced colitis in mice through DP1 receptor
Treatment with niacin promotes PGD2 release in colon tissues (Fig 1A), and disruption of DP1 receptor worsens DSS/TNBS‐induced colitis in mice (Figs 2 and EV2). We hypothesized niacin could improve clinical manifestation of colitis induced by DSS or TNBS. Indeed, administration of niacin (600 mg/kg by gavage, once a day, Fig 3A) markedly delayed body weight loss (7.94 ± 1.44% vs. 14.25 ± 1.03%, P < 0.01, Fig 3B), elevation of DAI (1.46 ± 0.14 vs. 2.58 ± 0.16, P < 0.01, Fig 3C), and shortening of colon length (6.26 ± 0.19 cm vs. 4.43 ± 0.27 cm, P < 0.01, Fig 3D and E) caused by DSS challenge in WT mice, and consequently reduced mortality (Fig 3F). In addition, niacin also ameliorated body weight loss and shortening of colon length caused by TNBS challenge in mice (Fig EV3). In contrast, these beneficial effects of niacin were not observed in DP1‐deficient mice (Figs 3B–F and EV3).
Figure 3. Niacin protects mice from DSS‐induced colitis.
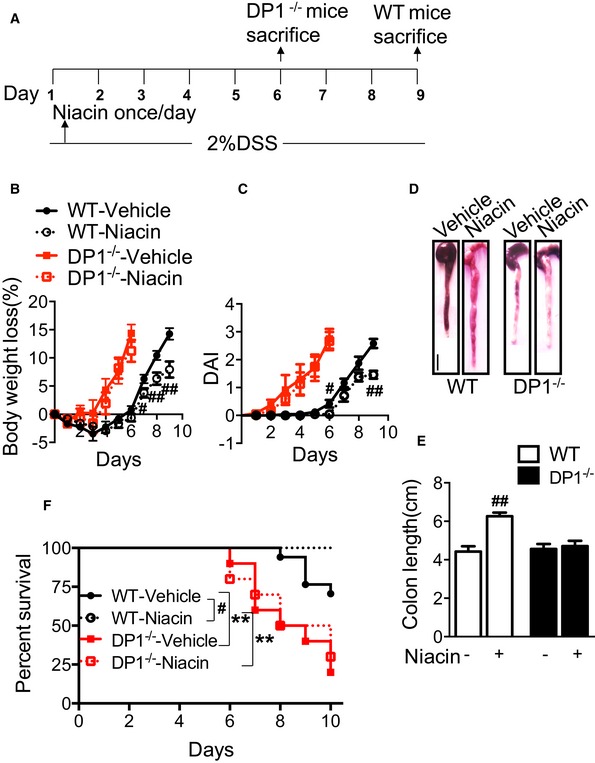
-
AProtocol for niacin treatment on DSS‐induced colitis in mice.
-
B, CEffect of niacin treatment on body weight loss (B) and disease activity index (C) of DP1−/− and WT mice in response to DSS challenge.
-
DMacroscopic appearance of colons from DSS‐challenged mice after niacin treatment. Scale bar: 1 cm.
-
EEffect of niacin treatment on colon length (centimeter) of DP1−/− and WT mice in response to DSS challenge.
-
FEffect of niacin treatment on survival rates of DSS‐challenged DP1−/− mice and WT controls.
Figure EV3. Niacin protects mice against from TNBS‐induced colitis.
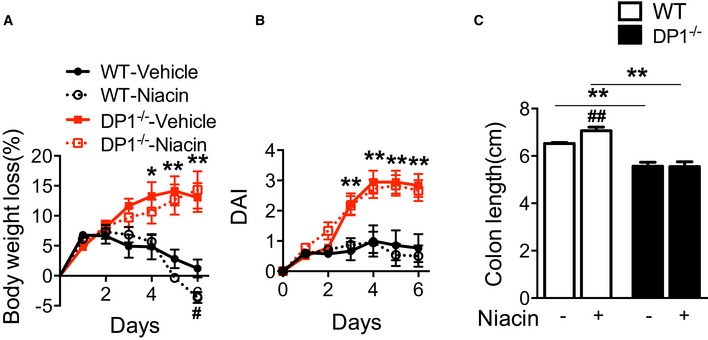
-
A–CEffect of niacin treatment on body weight loss (A), disease activity index (DAI, B), and colon length (centimeter) (C) of DP1−/− and WT mice in response to 2.5% TNBS challenge.
We also examined the effect of niacin on oxidative stress and plasma lipids in mice. As shown in Appendix Fig S1, the levels of 8‐isoprostane PGF2α in colon tissues and urine were not altered (Appendix Fig S1A) by niacin treatment in mice, while plasma cholesterol and triglyceride were markedly decreased after niacin treatment (Appendix Fig S1B).
DP1 deletion in vascular endothelial cells, epithelial cells, and myeloid cells, but not in smooth muscle cells, exacerbates the development of DSS/TNBS‐induced colitis in mice
DP1 receptor is expressed in colon tissues from healthy subjects, but its downregulation was detected in patients with active colitis (Vong et al, 2010). To determine which cell types express DP1 receptor, we performed double immunofluorescence staining of DP1 and different cell type‐specific markers in inflamed colon tissues from both UC patients and DSS‐challenged mice. Sections were stained for DP1 along with a α‐actin, a smooth muscle cell (SMA)‐specific marker, CD31, an endothelial cell marker, pan‐keratin, an epithelial cell marker, or CD68, a macrophage marker. Doubly stained cells were observed among all these marker positive cells (Fig 4A and B), indicating DP1 was expressed variably in multiple cell types including smooth muscle cells, vascular endothelial cells, colonic epithelial cells, and macrophages.
Figure 4. DSS‐induced colitis in endothelial cell, epithelial cell, macrophage, or smooth muscle cell‐specific DP1‐deficient mice.
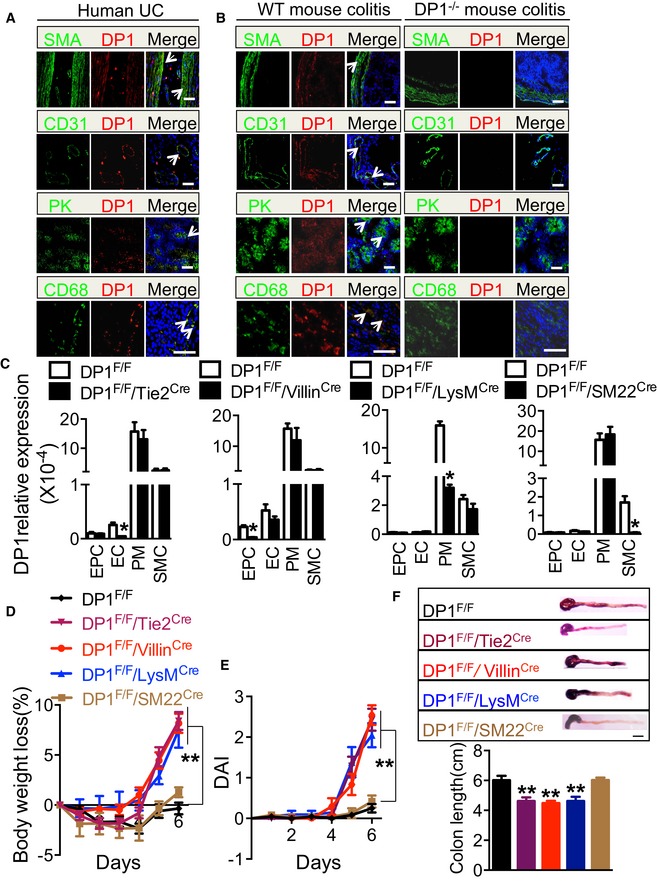
-
A, BDP1 receptor expression in post‐operative colon tissues from UC patients (A) and inflamed colons from DSS‐challenged wild‐type mice (B). The arrows indicate DP1+ cells. Scale bars: 100 μm.
-
CDP1 expression in endothelial cells (EC), colonic epithelial cell (EPC), peritoneal macrophages (PM), and smooth muscle cells (SMC) in tissue‐specific DP1‐deficient mice.
-
D, EBody weight loss (D) and disease activity index (E) of DP1F/F/Tie2Cre mice, DP1F/F/VillinCre mice, DP1F/F/LysMCre mice, and DP1F/F/SM22Cre mice in response to DSS administration.
-
FMacroscopic appearance of representative colons from DSS‐challenged DP1F/F/Tie2Cre mice, DP1F/F/VillinCre mice, DP1F/F/LysMCre mice, and DP1F/F/SM22Cre mice (upper) and quantitation of colon length (bottom). Scale bar: 1 cm.
To identify the cell types in which loss of DP1 function exaggerated the murine colitis, DP1Flox/Flox (DP1F/F) mice were crossed with Tie2Cre, VillinCre, LysMCre, or SM22Cre transgenic mice to generate vascular endothelial cell, colonic epithelial cell, macrophage, or smooth muscle cell‐specific DP1‐deficient mice, respectively (Fig 4C). Since Tie2 kinase is also expressed in hematopoietic progenitors (Zhou et al, 2009), DP1 downregulation (~22%) in colonic macrophages was detected in DP1F/F/Tie2Cre mice (data not shown). As shown in Figs 4D–F and EV4A, DP1F/F/Tie2Cre, DP1F/F/VillinCre, and DP1F/F/LysMCre mice displayed considerably more body weight loss (Figs 4D and EV4A), higher DAI (Figs 4E and EV4A), and shorter colon (Figs 4F and EV4A) after DSS or TNBS challenge, as compared to DP1F/F mice. In contrast, DP1F/F/SM22Cre mice did not differ from control mice in response to DSS or TNBS challenge (Figs 4D–F and EV4A). Together, these results suggest that DP1 receptor in the vascular endothelial cells, epithelial cells, and myeloid cells may mediate protective effects against DSS/TNBS‐induced colitis in mice.
Figure EV4. TNBS‐induced colitis in cell‐specific DP1‐deficient mice.
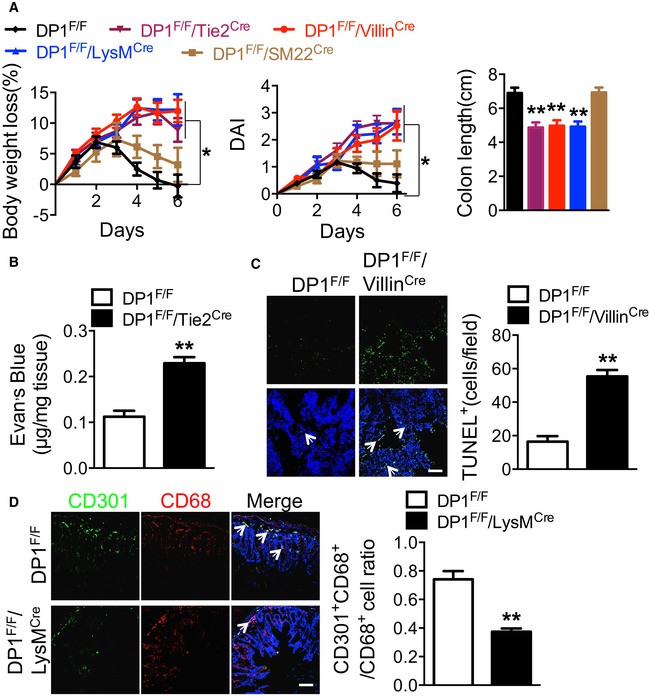
- Body weight loss, disease activity index (DAI), and colon length of DP1F/F/Tie2Cre mice, DP1F/F/VillinCre mice, DP1F/F/LysMCre mice, and DP1F/F/SM22Cre mice in response to TNBS treatment.
- Quantitative measurement of vascular permeability by dye leakage in the colonic mucosa from TNBS‐challenged DP1F/F/Tie2Cre and DP1F/F mice. The mice were sacrificed at day 6.
- TUNEL assay (left) and quantitation (right) in colonic tissues from TNBS‐challenged DP1F/F/VillinCre and DP1F/F mice. The arrows indicate the TUNEL+ cells (left). Scale bar: 100 μm. DP1F/F, n = 7; DP1F/F/VillinCre, n = 8.
- Representative immunofluorescent staining (left) and quantitation (right) of CD301+CD68+ cells in colonic tissues from TNBS‐challenged DP1F/F/LysMCre and DP1F/F mice. The arrows indicate the CD301+CD68+ cells (left). Scale bar: 100 μm. DP1F/F, n = 8; DP1F/F/LysMCre, n = 6.
Niacin suppresses vascular permeability in experimental colitis
Endothelial damage, increased colonic vascular permeability, perivascular edema, and subsequent epithelial hypoxia are essential for the development of ulcerative colitis (Deng et al, 2013). Deletion of DP1 receptor in vascular endothelial cells exacerbated DSS‐induced colitis in mice including aggravated perivascular edema and enhanced infiltration of inflammation cells (Fig 5A). In the ear model of LPS‐evoked vascular permeability, endothelial cell deficiency of DP1 significantly augmented leakage as measured by Evan's blue dye extravasation (A610: 0.27 ± 0.01 vs. 0.21 ± 0.01, P < 0.01, Fig 5B and C). Administration of DP1 agonist BW245C (3 mg/kg by subcutaneous injection) markedly inhibited LPS‐triggered vascular permeability in DP1F/F mice (A610: 0.10 ± 0.01 vs. 0.21 ± 0.01, P < 0.01, Fig 5B and C) but not in DP1F/F/Tie2Cre mice (Fig 5B and C). Consistent with this observation, more extravasated Evan's blue in colonic tissues was observed in DSS‐ or TNBS‐challenged DP1F/F/Tie2Cre mice than DP1F/F controls (0.26 ± 0.02 μg/mg vs. 0.11 ± 0.01 μg/mg, P < 0.01, Fig 5D; 0.23 ± 0.01 μg/mg vs. 0.11 ± 0.01 μg/mg, P < 0.01 Fig EV4B). The administration of niacin reduced Evan's blue extravasation in the inflamed intestines at both day 6 (0.10 ± 0.01 μg/mg vs. 0.13 ± 0.01 μg/mg, P = 0.06) and day 9 (0.14 ± 0.02 μg/mg vs. 0.26 ± 0.03 μg/mg, P < 0.01, Fig 5E). This reduction of vascular permeability was entirely blocked by DP1 deletion in vascular endothelial cells (Fig 5E), indicating niacin inhibits vascular permeability in intestinal tissues through activation of PGD2/DP1 signal in endothelial cells.
Figure 5. Niacin inhibits DSS‐induced vascular permeability.
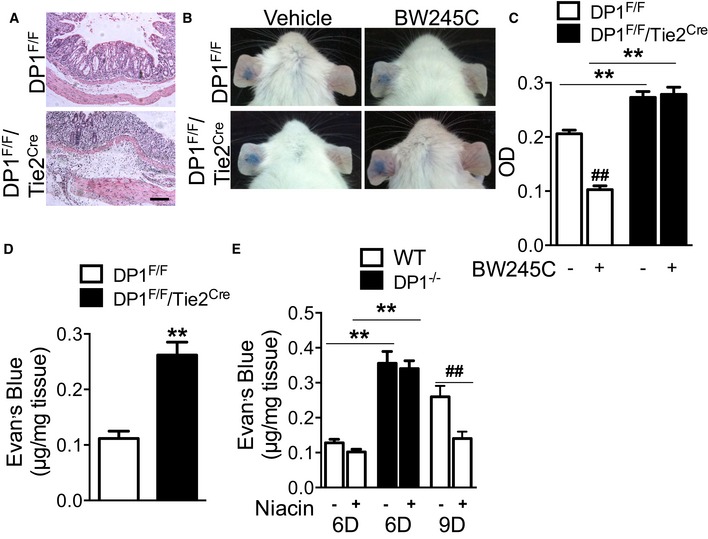
- H&E staining of distal colons from DSS‐challenged DP1F/F/Tie2Cre and DP1F/F mice. Scale bar: 100 μm.
- Representative images of Evan's blue extravasation in ears from DP1F/F/Tie2Cre and DP1F/F mice. Dye leakage is induced by LPS after 30 min in BW245C or vehicle pre‐treated mice.
- Quantitation of vascular permeability by measurement of dye absorbance at 610 nm in ear biopsies from DP1F/F/Tie2Cre and DP1F/F mice. DP1F/F‐vehicle, n = 6; DP1F/F‐niacin, n = 7; DP1F/F/Tie2Cre‐vehicle, n = 7; DP1F/F/Tie2Cre‐niacin, n = 6.
- Quantitative measurement of vascular permeability by dye leakage in the colonic mucosa from DSS‐challenged DP1F/F/Tie2Cre and DP1F/F mice. The mice were sacrificed at day 6. n = 6.
- Effect of niacin on Evan's blue extravasation in the colonic mucosa from DP1−/− and WT mice. Mice were sacrificed at day 6 or day 9 as indicated. n = 6.
Niacin suppresses apoptosis of intestinal epithelial cells in experimental colitis in mice
Intestinal epithelium barrier breakdown is a hallmark of colitis. Increased apoptosis and decreased proliferation contribute to a breakdown of the epithelial barrier function in DSS‐induced colitis (Araki et al, 2010). Indeed, DP1 deletion in intestinal epithelium (DP1F/F/VillinCre) resulted in greater crypt and epithelial cell loss in mice with DSS‐induced colitis as compared with DP1F/F mice (Fig 6A). TUNEL staining clearly showed higher frequency of apoptotic epithelial cells in DSS‐ or TNBS‐challenged DP1F/F/VillinCre mice than in control mice (54.75 ± 4.99 cells/field vs. 24.80 ± 1.66 cells/field, P < 0.01, Fig 6B; 55.38 ± 3.80 cells/field vs. 16.43 ± 3.34 cells/field, P < 0.01, Fig EV4C), while DP1 deletion had inhibited proliferation of epithelial cells as measured by the Ki‐67 immunoreactivity (P < 0.05, Fig 6C). Similarly, in primary cultured epithelial cells, DP1 deficiency augmented IL‐13‐induced epithelial apoptosis (39.65 ± 0.52% vs. 27.46 ± 0.55%, P < 0.01, Fig 6D). Interestingly, niacin protected colonic epithelial cells against DSS‐induced apoptosis and promoted cell proliferation in WT mice, but not in DP1‐deficient mice (Fig 6E–F). Thus, niacin helps maintain the intestinal epithelium barrier by activation of the DP1 receptor.
Figure 6. DP1 receptor contributes to the protection of niacin against DSS‐induced epithelial cell apoptosis.
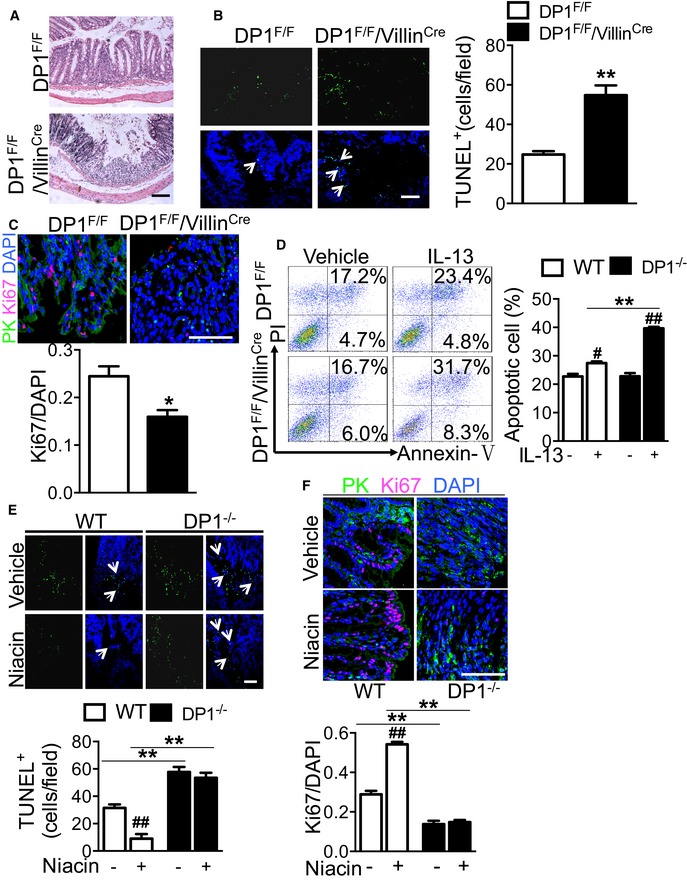
-
AH&E staining on distal colons from DSS‐challenged DP1F/F/VillinCre and DP1F/F mice. Scale bars: 100 μm.
-
BTUNEL assay (left) and quantitation (right) in colonic tissues from DP1F/F/VillinCre and DP1F/F mice. The arrows indicate the TUNEL+ cells (left). The arrows indicate the TUNEL+ cells. Scale bars: 100 μm. DP1F/F, n = 5; DP1F/F/VillinCre, n = 4.
-
CRepresentative Ki67 staining (upper) and quantitation (bottom) of Ki67 in colonic tissues from DP1F/F/VillinCre and DP1F/F mice. Anti‐pan‐keratin (PK) antibody and anti‐Ki67 antibody were used. Scale bars: 100 μm. n = 4.
-
DRepresentative flowcharts (left) and quantitation (right) of Annexin V‐positive IL‐13‐treated colonic epithelial cells from DP1F/F/VillinCre and DP1F/F mice. n = 3.
-
E, FEffect of niacin on DSS‐induced epithelial cell apoptosis and proliferation inhibition in DP1−/− and WT mice. The arrows indicate the TUNEL+ cells. Scale bars: 100 μm. n = 4.
Niacin depresses intestinal inflammatory reaction by promoting M2 polarization in experimental colitis in mice
Recently, we found DP1 deficiency in macrophages led to M1 polarization and delayed resolution in zymosan‐induced peritonitis in mice (Kong et al, 2016, 2017), and deletion of the DP1 receptor in macrophages (DP1F/F/LysMCre) aggravated DSS‐induced colitis (Fig 4D–F). We hypothesize that activation of the DP1 receptor in macrophages (i.e., niacin intake) may reduce intestinal inflammation by directing macrophage polarization toward anti‐inflammatory M2‐like cells. As shown in Figs 7A and B and EV4D, disruption of the DP1 receptor in macrophages decreased the proportion of M2‐like macrophages (CD301+CD68+ or CD206+F4/80+) infiltrated in inflamed intestines in both DSS‐ and TNBS‐challenged mice. Consistent with this finding, in intestinal macrophages separated by flow cytometry (Fig 7C), the expression of pro‐inflammatory genes [tumor necrosis factor‐α (TNF‐α), MCP‐1] is markedly induced and expression of anti‐inflammatory genes (IL‐4, IL‐5, and IL‐10) is suppressed by DP1 deficiency (Fig 7D). Interestingly, some inflammatory genes, such as IL‐1β and TGF‐β, displayed extremely reduced expression in intestinal macrophages (data not shown). We also examined whether niacin may delay DSS‐induced colitis partially through modulation of macrophage polarization. Indeed, niacin facilitated intestinal macrophage polarization toward M2 status by increasing CD301+CD68+ cell ratio in DSS‐challenged intestines (Fig 7E), downregulating pro‐inflammatory genes, and provoking anti‐inflammatory gene expression (Fig 7F). However, the phenotypic turnover of macrophage by niacin was not observed in myeloid DP1‐deficient mice, suggesting DP1 receptor mediates the M2 polarization elicited by niacin (Fig 7G).
Figure 7. Niacin suppresses pro‐inflammatory cytokine expression in macrophages in DSS‐induced colitis in mice.
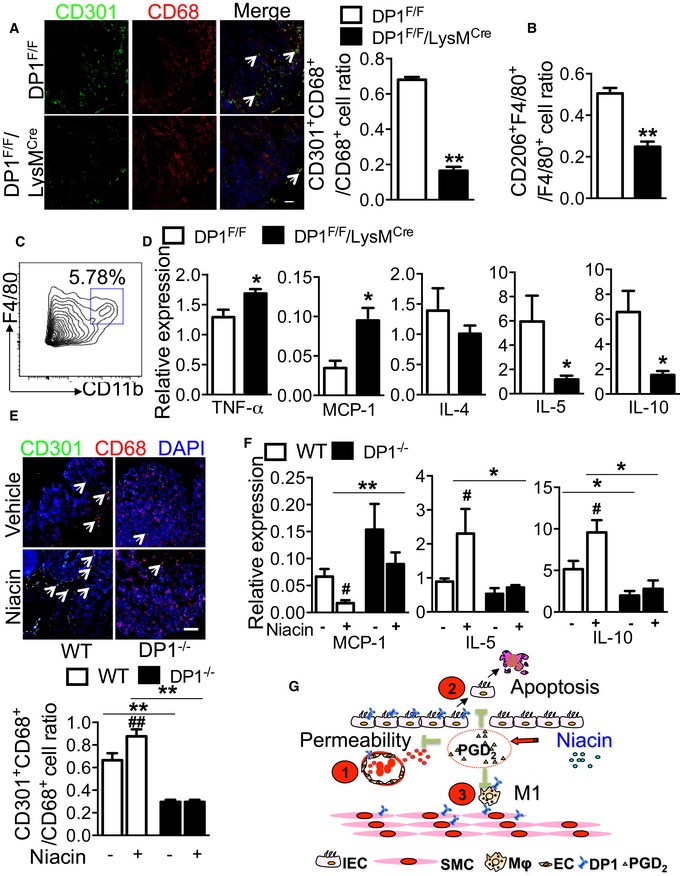
- Representative immunofluorescent staining (left) and quantitation (right) of CD301+CD68+ cells in colonic tissues from DSS‐challenged DP1F/F/LysMCre and DP1F/F mice. The arrows indicate the CD301+CD68+ cells (left panel). Scale bar: 100 μm. n = 4.
- Flow cytometry analysis of CD206+F4/80+ cells in colons DSS‐challenged DP1F/F/LysMCre and DP1F/F mice. n = 5.
- Flow cytometry analysis of CD11b+F4/80+ cells in colons from DSS‐challenged mice.
- Real‐time PCR analysis of TNF‐α, MCP‐1, IL‐4, IL‐5, and IL‐10 expression in colonic F4/80+CD11b+ cells in DP1F/F/LysMCre mice and DP1F/F mice. n = 6.
- Effect of niacin on colonic macrophage infiltration in DSS‐challenged DP1−/− and WT mice. The arrows indicate the CD301+CD68+ cells. Scale bar: 100 μm. WT‐vehicle, DP1−/−‐vehicle, DP1−/−‐niacin: n = 4; WT‐niacin, n = 7.
- Effect of niacin on MCP‐1, IL‐5, and IL‐10 expression in colonic F4/80+CD11b+ cells in DP1−/− mice and WT mice. n = 6.
- Schematic illustration of the protective mechanisms of PGD2/DP1 axis in UC. Niacin stimulates PGD2 release in the inflamed colon tissues, which
 inhibits vascular permeability,
inhibits vascular permeability,  suppresses DSS‐induced apoptosis, and
suppresses DSS‐induced apoptosis, and  downregulates pro‐inflammatory cytokine secretion in macrophages through activation of DP1 receptor. IECs, intestinal epithelial cells; SMCs, smooth muscle cells; MΦ: macrophage.
downregulates pro‐inflammatory cytokine secretion in macrophages through activation of DP1 receptor. IECs, intestinal epithelial cells; SMCs, smooth muscle cells; MΦ: macrophage.
PGD2 infusion ameliorates DSS‐induced colitis in mice
To further confirm that niacin relieves UC through releasing PGD2 in mice, we directly infused PGD2 to DSS‐challenged mice. As shown in Fig EV5A–C, PGD2 ameliorates DSS‐induced colitis in mice as evidenced by decreased body weight loss, DAI, and shortening of the colon after DSS challenge. As expected, PGD2 administration significantly inhibited Evan's blue extravasation in the inflamed intestines at day 9 (0.13 ± 0.01 μg/mg vs. 0.25 ± 0.02 μg/mg, P < 0.01, Fig EV5D), markedly decreased DSS‐induced apoptosis of epithelial cells (Fig EV5E), and increased the proportion of M2‐like macrophages (Fig EV5F).
Figure EV5. PGD2 infusion ameliorates DSS‐induced colitis in mice.
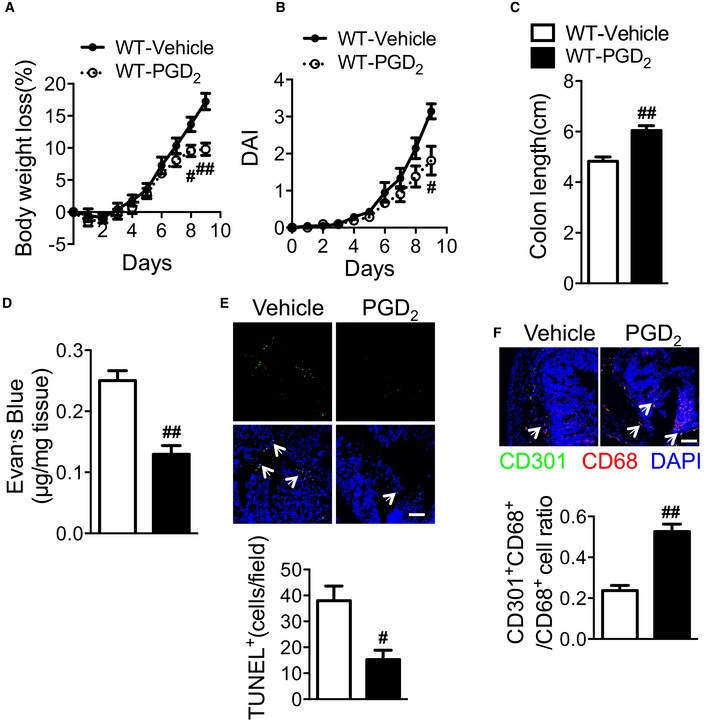
-
A–CEffect of PGD2 treatment on body weight loss (A), disease activity index (B), and colon length (C, centimeter) of WT mice in response to DSS challenge.
-
DEffect of PGD2 on Evan's blue extravasation in the colonic mucosa from WT mice.
-
EEffect of PGD2 on DSS‐induced epithelial cell apoptosis in WT mice. Scale bars: 100 μm. The arrows indicate TUNEL+ cells.
-
FEffect of PGD2 on colonic macrophage infiltration in DSS‐challenged WT mice. Scale bars: 100 μm. The arrows indicate CD301+CD68+ cells.
Niacin induces clinical remission in patients with moderately active UC
We investigated whether niacin induced clinical remission in patients with moderately active UC. Twenty‐six UC patients (Dubinsky et al, 2003; Annese et al, 2005), who did not respond to conventional therapies, were recruited. Demographics and baseline characteristics of patients are summarized in Appendix Table S1. Patients were assigned to receive retention enema treatment (including 300 mg niacin/100 ml) daily for 6 weeks (Fig 8A). As we expected, PGD2 production as measured by urinary tetranor PGDM was elevated in patients after niacin administration (Fig 8B). PGF2α production was also increased by niacin treatment without any influence on urinary 8‐isoprostane PGF2α (Appendix Fig S2A and B). Surprisingly, the proportion of patients with clinical response was 92.3% (24/26), and the proportion of clinical remissions was 88.5% (23/26). Compared to baseline, 23 out of 26 patients achieved mucosal healing (Fig 8C). Patients receiving niacin treatment had significant improvement in the Mayo score (Fig 8D). Each subscore, such as stool frequency, rectal bleeding, endoscopic findings, or physician's global assessment, is reduced significantly after niacin treatment (Appendix Table S2). Moreover, 24 out of 26 patients that received niacin treatment underwent overall histological improvement with normal epithelium, mucosal architecture, and lamina propia cellularity and few inflammatory cell infiltration (Fig 8E). C‐reactive protein (CRP) and erythrocyte sedimentation rate (ESR) levels in plasma and platelet activities of UC patients were not markedly altered after niacin treatment (data not shown). No serious adverse effect of niacin, including flushing and urticaria, was observed (Appendix Table S3). And niacin retention enema did not influence lipid profile of patients (Appendix Table S4). Overall, enema treatment in combination with niacin is well tolerated and effective in inducing clinical remission in UC patients.
Figure 8. The therapeutic effect of niacin retention enema on patients with moderately active UC.
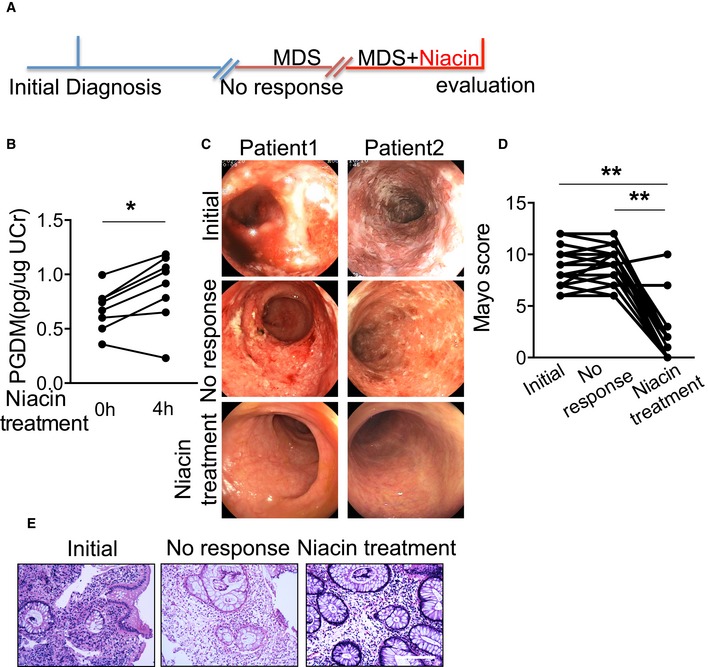
- Clinical study design of niacin retention enema therapy. MDS: metronidazole, dexamethasone, starch.
- Effect of niacin retention enema on urinary tetranor PGDM production in patients.
- Representative endoscopic images of colon mucosa from patients involved in this study. Superficial ulceration, granulocyte infiltration, and distorted/branching crypts are apparent in biopsies from patients with active disease, whereas those from the healthy subjects or those in remission appear normal.
- Changes of Mayo score of UC patients before and after niacin retention enema therapy.
- Representative H&E staining colon mucosa from UC patients. Scale bar: 100 μm.
Discussion
Ulcerative colitis is a chronic, relapsing inflammatory bowel disease, and the pathological changes of the colonic tissues affected include crypt branching, irregularity of size and shape of crypt, inflammatory cell infiltration in the lamina propia, and even erosion (Danese & Fiocchi, 2011). In this study, we demonstrated that niacin displayed multifaceted protective effects against DSS/TNBS‐induced colitis in mice through activation of the DP1 receptor, including inhibition of vascular leakage, suppression of colonic epithelium apoptosis, and reduction of pro‐inflammatory cytokine secretion. These disorders interactively promote pathological progression of UC (Su et al, 2009; Khor et al, 2011). Retention enema with niacin facilitated mucosal healing in patients with moderately active UC.
Prostaglandins are synthesized from arachidonic acid through the action of phospholipases and cyclooxygenases and are involved in many inflammatory processes (Zhang et al, 2010; Fattahi & Mirshafiey, 2012; Dennis & Norris, 2015; Cheng et al, 2016). Non‐steroidal anti‐inflammatory drugs (NSAID), which are widely utilized as analgesic and anti‐inflammatory agents for the treatment of arthritis and other inflammatory disorders, have been reported to exacerbate IBD (Felder et al, 2000; Bonner, 2001; Matuk et al, 2004). Mice lacking either COX‐1 or COX‐2 show increased sensitivity to DSS, and inducible COX‐2‐deficient mice are more susceptible than COX‐1‐deficient mice (Morteau et al, 2000). COX‐2 expression in myeloid cells and endothelial cells confers protection against DSS‐induced colitis (Ishikawa et al, 2011). Indeed, PGD2 is important for induction and maintenance of UC remission in both rodents and humans (Ajuebor et al, 2000; Vong et al, 2010). Consistent with this function, blockade of PGD2 downstream receptor DP1 worsens DSS‐induced colitis in mice (Cheng et al, 2006; Sturm et al, 2014). We found that niacin induces remission of UC in mice via DP1. Moreover, DP1 receptor expression in vascular endothelial cells, colonic epithelium, and myeloid cells is critical to protect the colonic mucosa from injury in DSS/TNBS‐induced colitis mouse models. These observations suggest that PGD2 derived from COX enzymes (mostly COX‐2) and perhaps PGE2 (Tessner et al, 1998; Tanaka et al, 2009) are responsible for NSAID‐exacerbated colitis. However, we failed to detect a significant effect of DP2 knockout on DSS‐induced colitis in mice, which does not seem to match the observations obtained from pharmacological inhibition of the DP2 receptor (Sturm et al, 2014).
Niacin reduces plasma levels of free fatty acids and cholesterol through activation of GPR109A receptor on adipocytes while causing an unwanted facial flushing side effect due to the GPR109A‐mediated PGD2 release on Langerhans cells and keratinocytes (Benyo et al, 2006; Maciejewski‐Lenoir et al, 2006; Hanson et al, 2010). Besides adipose tissue and immune cells (Feingold et al, 2014), GPR109A receptor is also expressed in colonic epithelium (Ganapathy et al, 2013). Indeed, PGD2 production was induced by niacin treatment in colon tissues. The disruption of DP1 receptor augmented inflammatory response by increasing pro‐inflammatory cytokine expression, enhanced apoptosis in colonic epithelium, and vascular permeability in DSS‐ or TNBS‐challenged mice. Thus, niacin ameliorated DSS/TNBS‐induced colitis by inhibition of colonic epithelium apoptosis and of inflammatory reactions in the lamina propia. In agreement with our results, niacin has been found to display anti‐inflammatory properties in other inflammatory diseases such as coronary artery disease (Kuvin et al, 2006) and atherosclerosis (Yu & Zhao, 2007). In addition, the activation of GPR109A in myeloid cells induces IL‐10 expression and subsequent differentiation of regulatory T cells (Treg cells) and IL‐10‐producing T cells (Singh et al, 2014). IL‐10 secretion was dramatically reduced in DP1‐deficient myeloid cells in DSS‐induced colitis mice, strongly indicating that the niacin/GPR109A axis‐mediated anti‐inflammatory IL‐10 secretion may depend on PGD2/DP1 signaling in myeloid cells.
Retention enema in combination with niacin induces clinical remission and endoscopic mucosal healing of the eligible patients with moderately active UC (limited to the left half of the colon and rectum). It is an explorative and self‐controlled follow‐up study. However, there are several limitations of this clinical study, such as small sample size, lack of placebo control, relatively short period of medication, short follow‐up period, and the fact that all the recruited patients are unresponsive to conventional treatment. Moreover, it remains to be investigated whether oral intake of niacin would have an equivalent effect on UC as the retention enema.
In summary, we demonstrated that niacin treatment ameliorates UC by boosting PGD2 release in both mice and patients, indicating niacin may serve as an effective therapeutic option for UC patients, especially those with an inadequate or no response to conventional treatment.
Materials and Methods
Mice
VillinCre mice were purchased from Model Animal Research Center of Nanjing University, and EllaCre, Tie2Cre, LysMCre, and SM22Cre mice were obtained from Jackson Laboratory (Bar Harbor, Maine, USA). All the colonies including DP1 (Kong et al, 2016) and DP2 (Satoh et al, 2006) mutants were maintained on a C57BL/6 genetic background. DP1Flox/Flox (DP1F/F) mice were crossed with Tie2Cre, VillinCre, LysMCre, or SM22Cre transgenic mice to generate cell‐specific DP1‐deficient mice, respectively. Animals were maintained and experiments were carried out with the approval of the Institutional Animal Care and Use Committee of the Institute for Nutritional Sciences, Chinese Academy of Sciences.
Reagents
Dextran sulfate sodium (DSS) was purchased from MP Biomedicals (LLC, Santa Ana, California, USA). TNBS, LPS, Evan's blue, and niacin were purchased from Sigma Chemical Company (Sigma‐Aldrich, St. Louis, MO, USA). PGD2 and BW245C were obtained from Cayman Chemical Company (Cayman Chemical, Ann Arbor, MI, USA). Percoll solution was from Biosharp (Biosharp, Hefei, China). IL‐13 was purchased from Peprotech (Peprotech, Rocky Hill, USA). TdT fluorescence in situ Apoptosis Detection kit was from Yeasen Biological Technology (Yeasen, Shanghai, China). Annexin V‐FITC Apoptosis Detection Assay kit was obtained from Dojindo Laboratories (Dojindo, Shanghai, China).
Induction of mouse colitis
For DSS‐induced colitis, 6‐ to 8‐week‐old male mice were subjected DSS administration (molecular weight: 36,000–50,000 D) through drinking water (2%) for 6–9 days as indicated. As for TNBS‐induced colitis, 6‐ to 8‐week‐old male mice were pre‐sensitized with 1% TNBS at day 1 and then challenged with 2.5 % TNBS (100 μl) intrarectally at day 8 (Wirtz et al, 2007). Niacin was used to treat mice by gavage at a dose of 600 mg/kg/day, which is comparable to the therapeutic dose used in dyslipidemic patients if the 10 times faster metabolism of mice as compared to humans is taken into account (van der Hoorn et al, 2008). Body weight was measured every day from the beginning of the DSS and TNBS administration. The body weight loss was calculated as the percentage change compared to the original body weight. The stool consistency and gross bleeding were monitored daily. The disease activity index (DAI) was calculated by the combined scores of the following parameters divided by 3: weight loss (0, normal; 1, 0–5%; 2, 5–10%; 3, 10–20%; 4, > 20%), stool consistency (0, normal; 2, loose stools; 4, watery/diarrhea), and gross bleeding in stool (0, negative; 2, positive in hemoculture; 4, macroscopic hematochezia) (Cooper et al, 1993).
Primary cell culture
For preparation of peritoneal macrophages, mice were injected intraperitoneally with 1 ml of 10% thioglycollate medium (Scharlau®, Barcelona, Spain). On the fourth day, the mice were euthanized and injected intraperitoneally with 7–8 ml of ice‐cold phosphate‐buffered saline (PBS), and then peritoneal macrophages were collected and cultured in Roswell Park Memorial Institute (RPMI) 1,640 medium with 10% fetal bovine serum at 37°C, 5% CO2 (Wang et al, 2014).
For preparation of colonic epithelial cells and lamina propria mononuclear cells (LPMCs), intestine biopsies were opened longitudinally and cut into 1‐cm pieces. They were incubated in 5 ml pre‐digestion solution Hanks' balanced salt solution (HBSS) containing 5 mM ethylenediaminetetraacetic acid (EDTA) and 1 mM dithiothreitol (DTT) for 1 h at 37°C, and the intestine pieces were subjected to 100‐μm cell strainer to get single primary cells. The cells were further centrifuged with a 50% Percoll solution at 500 g for 20 min, and the epithelial cells were then equilibrated at the interface (Evans et al, 1992).
For preparation of intestinal LPMCs, intestine biopsies were cut into 1‐mm pieces and incubated in a digestion solution containing 0.05 g of collagenase D (Roche, Basel, Switzerland), 0.05 g of DNase I (Sigma‐Aldrich, St. Louis, MO, USA), and 0.3 g of dispase II (Roche) for about 40 min until all the pieces were fully digested. The cell pellets were obtained after centrifugation at 500 g for 10 min, followed by resuspension and separation in 40%/80% Percoll solution by centrifugation at 1,000 g for 20 min, and LPMCs could be visible as a white ring at the interface (Weigmann et al, 2007).
Vascular smooth muscle cells and endothelial cells were prepared from aortas and lungs as previously reported (Zhang et al, 2013; Lu et al, 2015), respectively. Vascular endothelial cells at 2–3 passages were used for experiments.
Flow cytometry
The lamina propia mononuclear cells were stained with fluorescence‐tagged primary antibodies (Brilliant Violet 421‐F4/80, Biolegend, San Diego, CA, USA; FITC‐anti‐CD11b, MACS, Bergisch Gladbach, Germany, FITC‐anti‐CD206, Biolegend, San Diego, CA, USA) for 45 min at 4°C. Flow cytometry was performed using a BD LSRFortessa™ cell analyzer (BD Biosciences, San Jose, CA, USA).
Hematoxylin and eosin and Immunofluorescence staining
For hematoxylin and eosin (H&E) staining, mouse colon tissues were fixed in 4% paraformaldehyde and embedded in paraffin and then incised a 4‐μm section. The sections were stained with both hematoxylin and eosin, and then photographed using an electron microscope.
For immunofluorescence staining, colon biopsies from UC patients and mice were embedded in O.C.T. compound (Tissue Tek, Sakura, Torrance, CA, USA), and then incised 8‐μm sections and processed for immunostaining. The DP1 expression was examined with polyclonal anti‐DP1 primary antibody (Cayman Chemical, Ann Arbor, MI, USA). The endothelial cells were marked with anti‐CD31 (1:200, BD Biosciences, San Diego, CA, USA). To detect epithelial cell and macrophage, anti‐pan‐keratin‐FITC antibody (Cell Signaling Technology, Danvers, MA, USA) and anti‐CD68 (1:200, AbD Serotec, Kidlington, UK) primary antibody were used. To label the smooth muscle cells, the anti‐α‐actin‐FITC antibody (1:200, Sigma‐Aldrich, St. Louis, MO, USA) was used. Anti‐CD301 (1:100 Bio‐Rad, California, USA) primary antibody was used to mark M2‐like macrophage, and anti‐Ki67 (1:500, Epitomics, Burlingame, CA, USA) primary antibody was used to label proliferating cell.
Mouse genotyping
Genomic DNA from mouse tail biopsies was extracted as template. PCR products were resolved by electrophoresis in a 2% agarose gel (Biowest, Verkiu, Lithuania), and stained with ethidium bromide. The images were digitally captured with a SynGene gel image system (Tanon 2500, Shanghai, China). The primers used in this study are shown in Appendix Table S5.
RNA preparation and RT–PCR analysis
Total RNA was isolated with TRIzol reagent (Invitrogen, San Diego, CA, USA). The reverse transcription reactions were performed by use of Reverse Transcription Reagent kits (Takara, Otsu, Shiga, Japan). Real‐time PCR was conducted with SYBR Green mix (Applied Biosystems®, CA, USA). The primers used in this study are summarized in Appendix Table S5.
Vascular permeability assay
After anesthesia, both ears of one group of mouse were injected intradermally with 3 mg/kg (in 10 μl) BW245C and the ears of the other group were injected with vehicle. After 30 min, 10 μl (1 mg/ml) LPS was injected in the left ear of all mice, whereas only PBS was injected on the right ear. 30 min later, mice received 100 μl of 1% Evan's blue in PBS through tail vein injection. All animals were euthanized after 15 min by CO2 inhalation. Ear biopsies were collected with a 6‐mm Acu‐Punch, (Acuderm Inc., Ft. Lauderdale, FL, USA) and immersed in 1 ml of formamide overnight in a water bath at 55°C. Evan's blue dye was extracted from ear biopsies and measured by absorbance at 610 nm using a spectrophotometer.
For vascular permeability in DSS‐induced UC, mice with DSS‐induced colitis received intravenously 100 μl of 1% Evan's blue in PBS 15 min before being sacrificed. After autopsy, the colon tissues were dried and weighed. Evan's blue was extracted and quantitated.
Apoptosis analysis
For terminal deoxynucleotidyl transferase (TdT)‐mediated biotin‐16‐dUTP nick‐end labeling (TUNEL) assay, the colon tissues from mice with DSS administration were imbedded in O.C.T. compound and made into 8‐μm frozen sections. The frozen sections were stained with a TUNEL fluorescence in situ Apoptosis Detection kit (Yeasen, Shanghai, China) according to the manufacturer's manual.
For Annexin V‐FITC Apoptosis Detection, after treatment with IL‐13 or vehicle, the adherent primary epithelial cells were prepared and stained with Annexin V‐FITC Apoptosis Detection kit according to the manufacturer's instructions. Annexin V binding was analyzed by flow cytometry within 1 h.
Mass spectral analysis
Urinary prostanoid metabolites, 8‐isoprostane prostaglandin F2α, were extracted and quantitated as previously reported (Zhang et al, 2013). In brief, mouse urine was collected for 12 h in metabolic cages after niacin treatment. Samples (100 μl) were spiked with internal standards [tetranor PGDM‐d6, tetranor PGEM‐d6, 13,14‐dihydro‐15‐keto‐PGF2α (PGFM)‐d4, 2,3‐dinor‐6‐keto PGF1α (PGIM)‐d4, 11‐dehydro‐thromboxane B2 (TxM)‐d4, and 8‐iso‐PGF2α‐d4, (5 μl)] contained in acetonitrile (ACN). 200 μl methoxy‐amine HCl (1 g/ml), an aqueous solution, was added. After standing for 30 min at room temperature, make capacity to 1 ml by water. The samples were applied to the cartridge conditioned with 1 ml of acetonitrile and equilibrated with 1 ml of water. After being washed with 1 ml of 5% acetonitrile in water, they were dried with vacuum for 15 min. After being subjected to elution using 1 ml of 5% acetonitrile in ethyl acetate, the samples were dissolved in 100 μl 10% ACN in water and passed through small centrifugal filters with a 0.2‐μm nylon membrane prior to analysis by mass spectrometry. The urinary creatinine was used to normalize the prostaglandin metabolites, 8‐isoprostane prostaglandin F2α.
Colon tissues from mice were homogenated and centrifuged and 500 μl of supernatant was collected for PG and SPM production analysis. In brief, internal standards (PGD2‐d4, 6‐keto‐PGF1α‐d4, PGF2α‐d4, PGE2‐d4, TxB2‐d4, RvD1‐d5, RvE1‐d5, 8‐iso‐PGF2α‐d4 and 5(S)6(R)‐LXA4‐d5 (5 μl)) were added to the samples in 40 μl of citric acid (1 M) and 5 μl of 10% butylated hydroxytoluene, and then the samples were vigorously vortexed with 1 ml solvent (normal hexane:ethyl acetate, 1:1). The organic phase supernatant was collected after centrifugation (6,000 g, 10 min). After being subjected to elution using 1 ml of 5% acetonitrile in ethyl acetate, the samples were dissolved in 100 μl 10% ACN in water and passed through small centrifugal filters with a 0.2‐μm nylon membrane prior to analysis by mass spectrometry. PG and SPM production was normalized to total protein of extracted tissues.
Plasma lipid measurements
Plasma triglyceride (TG), total cholesterol (TC), low‐density lipoprotein (LDL), and high‐density lipoprotein (HDL) levels were measured using commercial kits (Jiancheng, Nanjing, China).
Clinical study design and patients
This study was conducted to evaluate niacin as an effective alternative therapeutic agent for patients with UC unresponsive to conventional treatment. All patients were recruited from the Department of Gastroenterology of Ruijin Hospital in Shanghai Jiao Tong University School of Medicine from March 2015 to December 2016. The trial is registered with Chinese Clinical Trial Registry (ChiCTR; www.chictr.org.cn; ChiCTR‐IOR‐15006400). Eligible patients were 18 years old or above and had a diagnosis of moderately active left‐sided UC, including the descending colon, the sigmoid colon, and rectum with a Mayo Clinic score greater than or equal to 6, a rectal bleeding subscore of 1 or higher, and endoscopic subscore of 2 or higher. Additional inclusion criteria were documentation of inadequate or no response to conventional retention enema treatment (5‐aminosalicylate, metronidazole, dexamethasone, starch) and regular oral medicines in the past 1–2 years, such as 5‐aminosalicylate (5‐ASA) and/or corticosteroids. Briefly, eligible patients had no clinical response to following sequential therapies according to clinical practice guidelines for the medical management of ulcerative colitis within 6 months before the study (Mowat et al, 2011; Bressler et al, 2015): (i) oral 5‐aminosalicylate (5‐ASA, 4 g of Pentasa per day) induction therapy for 8 weeks; (ii) oral corticosteroids (30–40 mg of oral prednisolone or the equivalent per day) for 4 weeks; and (iii) oral 5‐aminosalicylate (5‐ASA, 4 g of Pentasa per day) with consecutive regular retention enema treatment (5 mg of dexamethasone, 0.5 g of metronidazole, and 5 g of starch in 100 ml saline per day) for 6 weeks. Patients were excluded if they had extremely severe UC, severe colonic stricture, infectious enteritis, a history of bowel surgery, major organ dysfunction, malignant neoplasm, pregnancy, hypertension, diabetes, and concomitant use of immunosuppressants such as azathioprine (AZA), mercaptopurine (MP), anti‐TNF therapy. The study was reviewed and approved by the Ruijin Hospital Ethics Committee of Shanghai Jiao Tong University School of Medicine, and conducted in accordance with the Good Clinical Practice, the Belmont report, the Declaration of Helsinki, and other relevant rules and regulations. All patients provided written informed consent.
Clinical study protocol
In the study, eligible patients received retention enema treatment with niacin (0.5 g metronidazole, 5 mg dexamethasone, 5 g starch, 300 mg niacin in 100 ml saline) daily for 6 weeks, who continued to take oral 5‐aminosalicylate (4 g of Pentasa per day) throughout the study. The patients were examined at the beginning and the 6th week. Mayo Clinic scores were calculated, colonoscopy was performed with biopsy, and blood samples were collected for hematologic testing. In addition, the urine samples were obtained at 0 and 4 h after retention enema treatment for PG metabolite measurement. The primary endpoint was a clinical response, defined by a decrease in the Mayo Clinic score ≥ 3 points and ≥ 30% from the baseline score, with a decrease ≥ 1 point from the baseline score on the rectal bleeding subscore or a rectal bleeding subscore of 0 or 1. Clinical remission was defined as a Mayo Clinic score of 2 or lower with no subscore > 1. Mucosal healing was defined as an endoscopic subscore of 0 or 1 as assessed by a professional endoscopist (Yoshimura et al, 2015).
Statistical analysis
All data are expressed as the mean ± standard error of the mean (SEM). Data were analyzed using GraphPad Prism software, version 5.0 (GraphPad Software, San Diego, CA, USA). The two‐tailed unpaired Student's t‐test was performed to compare the two datasets. Multiple comparisons were tested with two‐way ANOVA followed by Bonferroni's post‐test. A P‐value of less than 0.05 was considered statistically significant. For the clinical trial, paired Student's t‐test was used to compare the values before and after niacin treatment. The exact P‐values in each figure are listed in Appendix Table S6.
Author contributions
JL, LWa, and Ying Yu designed research; JL, DK, QW, WW, YT, TB, LG, LWe, QZha, Yu Yu, YQ, SZ, GL, QL, SW, YZ, YW, QZhu, DJ and WY performed research and analyzed data; YJ, HY, MN, ML, and RMB contributed experimental reagents; JL, LWa, and Ying Yu wrote the manuscript.
Conflict of interest
The authors declare that they have no conflict of interest.
The paper explained.
Problem
Niacin is an antidyslipidemic drug that elicits a strong flushing response through release of prostaglandin (PG) D2. Ulcerative colitis (UC) is a chronic inflammatory bowel disease; however, it remains unclear whether niacin is beneficial for UC.
Results
Niacin boosted PGD2 release in vivo and improved both DSS‐ and TNBS‐induced colitis in mice via the D prostanoid receptor 1 (DP1). DP1 expression varied between vascular wall, colonic epithelium, and infiltrated macrophages in the inflamed colons of both humans and mice. DP1 receptor deficiency in vascular endothelial cells, colonic epithelium, and myeloid cells intensified the DSS‐ or TNBS‐induced colitis in mice through increasing vascular permeability, promoting apoptosis of epithelial cells, and stimulating pro‐inflammatory cytokine secretion from macrophages, respectively. Niacin treatment improved vascular permeability, reduced apoptosis of epithelial cells, and suppressed pro‐inflammatory cytokine expression from macrophages. Moreover, treatment with niacin‐containing retention enema effectively promoted UC clinical remission and mucosal healing in patients with moderately active disease.
Impact
Niacin displays multiple beneficial effects on colitis in mice and humans by activation of the PGD2/DP1 axis. These results suggest niacin may become an effective therapeutic option for UC patients.
Supporting information
Appendix
Expanded View Figures PDF
Review Process File
Acknowledgements
This work was supported by the National Natural Science Foundation of China (81525004, 91439204, 81272263, 81672719) and the Science and Technology Commission of Shanghai Municipality (15140902000, 14JC1407400). Ying Yu is a fellow at the Jiangsu Collaborative Innovation Center for Cardiovascular Disease Translational Medicine.
EMBO Mol Med (2017) 9: 571–588
Contributor Information
Lifu Wang, Email: lifuwang@sjtu.edu.cn.
Ying Yu, Email: yuying@sibs.ac.cn, Email: yuying@tmu.edu.cn.
References
- Ajuebor MN, Singh A, Wallace JL (2000) Cyclooxygenase‐2‐derived prostaglandin D(2) is an early anti‐inflammatory signal in experimental colitis. Am J Physiol Gastrointest Liver Physiol 279: G238–G244 [DOI] [PubMed] [Google Scholar]
- Anderson CA, Boucher G, Lees CW, Franke A, D'Amato M, Taylor KD, Lee JC, Goyette P, Imielinski M, Latiano A et al (2011) Meta‐analysis identifies 29 additional ulcerative colitis risk loci, increasing the number of confirmed associations to 47. Nat Genet 43: 246–252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annese V, Latiano A, Rossi L, Lombardi G, Dallapiccola B, Serafini S, Damonte G, Andriulli A, Magnani M (2005) Erythrocytes‐mediated delivery of dexamethasone in steroid‐dependent IBD patients‐a pilot uncontrolled study. Am J Gastroenterol 100: 1370–1375 [DOI] [PubMed] [Google Scholar]
- Araki Y, Mukaisyo K, Sugihara H, Fujiyama Y, Hattori T (2010) Increased apoptosis and decreased proliferation of colonic epithelium in dextran sulfate sodium‐induced colitis in mice. Oncol Rep 24: 869–874 [DOI] [PubMed] [Google Scholar]
- Benyo Z, Gille A, Bennett CL, Clausen BE, Offermanns S (2006) Nicotinic acid‐induced flushing is mediated by activation of epidermal langerhans cells. Mol Pharmacol 70: 1844–1849 [DOI] [PubMed] [Google Scholar]
- Bodor ET, Offermanns S (2008) Nicotinic acid: an old drug with a promising future. Br J Pharmacol 153(Suppl 1): S68–S75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner GF (2001) Exacerbation of inflammatory bowel disease associated with use of celecoxib. Am J Gastroenterol 96: 1306–1308 [DOI] [PubMed] [Google Scholar]
- Bressler B, Marshall JK, Bernstein CN, Bitton A, Jones J, Leontiadis GI, Panaccione R, Steinhart AH, Tse F, Feagan B et al (2015) Clinical practice guidelines for the medical management of nonhospitalized ulcerative colitis: the Toronto consensus. Gastroenterology 148: 1035–1058.e3 [DOI] [PubMed] [Google Scholar]
- Cefali EA, Simmons PD, Stanek EJ, McGovern ME, Kissling CJ (2007) Aspirin reduces cutaneous flushing after administration of an optimized extended‐release niacin formulation. Int J Clin Pharmacol Ther 45: 78–88 [DOI] [PubMed] [Google Scholar]
- Cheng K, Wu TJ, Wu KK, Sturino C, Metters K, Gottesdiener K, Wright SD, Wang Z, O'Neill G, Lai E et al (2006) Antagonism of the prostaglandin D2 receptor 1 suppresses nicotinic acid‐induced vasodilation in mice and humans. Proc Natl Acad Sci USA 103: 6682–6687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng J, Dackor RT, Bradbury JA, Li H, DeGraff LM, Hong LK, King D, Lih FB, Gruzdev A, Edin ML et al (2016) Contribution of alveolar type II cell‐derived cyclooxygenase‐2 to basal airway function, lung inflammation, and lung fibrosis. FASEB J 30: 160–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper HS, Murthy SN, Shah RS, Sedergran DJ (1993) Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab Invest 69: 238–249 [PubMed] [Google Scholar]
- Danese S, Fiocchi C (2011) Ulcerative colitis. N Engl J Med 365: 1713–1725 [DOI] [PubMed] [Google Scholar]
- Deng X, Szabo S, Khomenko T, Tolstanova G, Paunovic B, French SW, Sandor Z (2013) Novel pharmacologic approaches to the prevention and treatment of ulcerative colitis. Curr Pharm Des 19: 17–28 [DOI] [PubMed] [Google Scholar]
- Dennis EA, Norris PC (2015) Eicosanoid storm in infection and inflammation. Nat Rev Immunol 15: 511–523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Digby JE, Martinez F, Jefferson A, Ruparelia N, Chai J, Wamil M, Greaves DR, Choudhury RP (2012) Anti‐inflammatory effects of nicotinic acid in human monocytes are mediated by GPR109A dependent mechanisms. Arterioscler Thromb Vasc Biol 32: 669–676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubinsky MC, Feldman EJ, Abreu MT, Targan SR, Vasiliauskas EA (2003) Thioguanine: a potential alternate thiopurine for IBD patients allergic to 6‐mercaptopurine or azathioprine. Am J Gastroenterol 98: 1058–1063 [DOI] [PubMed] [Google Scholar]
- Evans GS, Flint N, Somers AS, Eyden B, Potten CS (1992) The development of a method for the preparation of rat intestinal epithelial cell primary cultures. J Cell Sci 101(Pt 1): 219–231 [DOI] [PubMed] [Google Scholar]
- Fattahi MJ, Mirshafiey A (2012) Prostaglandins and rheumatoid arthritis. Arthritis 2012: 239310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feingold KR, Moser A, Shigenaga JK, Grunfeld C (2014) Inflammation stimulates niacin receptor (GPR109A/HCA2) expression in adipose tissue and macrophages. J Lipid Res 55: 2501–2508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felder JB, Korelitz BI, Rajapakse R, Schwarz S, Horatagis AP, Gleim G (2000) Effects of nonsteroidal antiinflammatory drugs on inflammatory bowel disease: a case‐control study. Am J Gastroenterol 95: 1949–1954 [DOI] [PubMed] [Google Scholar]
- Franke A, McGovern DP, Barrett JC, Wang K, Radford‐Smith GL, Ahmad T, Lees CW, Balschun T, Lee J, Roberts R et al (2010) Genome‐wide meta‐analysis increases to 71 the number of confirmed Crohn's disease susceptibility loci. Nat Genet 42: 1118–1125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganapathy V, Thangaraju M, Prasad PD, Martin PM, Singh N (2013) Transporters and receptors for short‐chain fatty acids as the molecular link between colonic bacteria and the host. Curr Opin Pharmacol 13: 869–874 [DOI] [PubMed] [Google Scholar]
- Godin AM, Ferreira WC, Rocha LT, Ferreira RG, Paiva AL, Merlo LA, Nascimento EB Jr, Bastos LF, Coelho MM (2012) Nicotinic acid induces antinociceptive and anti‐inflammatory effects in different experimental models. Pharmacol Biochem Behav 101: 493–498 [DOI] [PubMed] [Google Scholar]
- Hanson J, Gille A, Zwykiel S, Lukasova M, Clausen BE, Ahmed K, Tunaru S, Wirth A, Offermanns S (2010) Nicotinic acid‐ and monomethyl fumarate‐induced flushing involves GPR109A expressed by keratinocytes and COX‐2‐dependent prostanoid formation in mice. J Clin Investig 120: 2910–2919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzhauser E, Albrecht C, Zhou Q, Buttler A, Preusch MR, Blessing E, Katus HA, Bea F (2011) Nicotinic acid has anti‐atherogenic and anti‐inflammatory properties on advanced atherosclerotic lesions independent of its lipid‐modifying capabilities. J Cardiovasc Pharmacol 57: 447–454 [DOI] [PubMed] [Google Scholar]
- van der Hoorn JW, de Haan W, Berbee JF, Havekes LM, Jukema JW, Rensen PC, Princen HM (2008) Niacin increases HDL by reducing hepatic expression and plasma levels of cholesteryl ester transfer protein in APOE*3Leiden.CETP mice. Arterioscler Thromb Vasc Biol 28: 2016–2022 [DOI] [PubMed] [Google Scholar]
- Ishikawa TO, Oshima M, Herschman HR (2011) Cox‐2 deletion in myeloid and endothelial cells, but not in epithelial cells, exacerbates murine colitis. Carcinogenesis 32: 417–426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khor B, Gardet A, Xavier RJ (2011) Genetics and pathogenesis of inflammatory bowel disease. Nature 474: 307–317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong D, Shen Y, Liu G, Zuo S, Ji Y, Lu A, Nakamura M, Lazarus M, Stratakis CA, Breyer RM et al (2016) PKA regulatory IIalpha subunit is essential for PGD2‐mediated resolution of inflammation. J Exp Med 213: 2209–2226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong D, Li J, Shen Y, Liu G, Zuo S, Tao B, Ji Y, Lu A, Lazarus M, Breyer RM et al (2017) Niacin promotes cardiac healing after myocardial infarction through activation of the myeloid prostaglandin D2 receptor subtype 1. J Pharmacol Exp Ther 360: 435–444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuvin JT, Dave DM, Sliney KA, Mooney P, Patel AR, Kimmelstiel CD, Karas RH (2006) Effects of extended‐release niacin on lipoprotein particle size, distribution, and inflammatory markers in patients with coronary artery disease. Am J Cardiol 98: 743–745 [DOI] [PubMed] [Google Scholar]
- Lu A, Zuo C, He Y, Chen G, Piao L, Zhang J, Xiao B, Shen Y, Tang J, Kong D et al (2015) EP3 receptor deficiency attenuates pulmonary hypertension through suppression of Rho/TGF‐beta1 signaling. J Clin Investig 125: 1228–1242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maccubbin D, Koren MJ, Davidson M, Gavish D, Pasternak RC, Macdonell G, Mallick M, Sisk CM, Paolini JF, Mitchel Y (2009) Flushing profile of extended‐release niacin/laropiprant versus gradually titrated niacin extended‐release in patients with dyslipidemia with and without ischemic cardiovascular disease. Am J Cardiol 104: 74–81 [DOI] [PubMed] [Google Scholar]
- Maciejewski‐Lenoir D, Richman JG, Hakak Y, Gaidarov I, Behan DP, Connolly DT (2006) Langerhans cells release prostaglandin D2 in response to nicotinic acid. J Invest Dermatol 126: 2637–2646 [DOI] [PubMed] [Google Scholar]
- Matuk R, Crawford J, Abreu MT, Targan SR, Vasiliauskas EA, Papadakis KA (2004) The spectrum of gastrointestinal toxicity and effect on disease activity of selective cyclooxygenase‐2 inhibitors in patients with inflammatory bowel disease. Inflamm Bowel Dis 10: 352–356 [DOI] [PubMed] [Google Scholar]
- Morteau O, Morham SG, Sellon R, Dieleman LA, Langenbach R, Smithies O, Sartor RB (2000) Impaired mucosal defense to acute colonic injury in mice lacking cyclooxygenase‐1 or cyclooxygenase‐2. J Clin Investig 105: 469–478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mowat C, Cole A, Windsor A, Ahmad T, Arnott I, Driscoll R, Mitton S, Orchard T, Rutter M, Younge L et al (2011) Guidelines for the management of inflammatory bowel disease in adults. Gut 60: 571–607 [DOI] [PubMed] [Google Scholar]
- Paolini JF, Mitchel YB, Reyes R, Kher U, Lai E, Watson DJ, Norquist JM, Meehan AG, Bays HE, Davidson M et al (2008) Effects of laropiprant on nicotinic acid‐induced flushing in patients with dyslipidemia. Am J Cardiol 101: 625–630 [DOI] [PubMed] [Google Scholar]
- Rajakariar R, Hilliard M, Lawrence T, Trivedi S, Colville‐Nash P, Bellingan G, Fitzgerald D, Yaqoob MM, Gilroy DW (2007) Hematopoietic prostaglandin D2 synthase controls the onset and resolution of acute inflammation through PGD2 and 15‐deoxyDelta12 14 PGJ2. Proc Natl Acad Sci USA 104: 20979–20984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh T, Moroi R, Aritake K, Urade Y, Kanai Y, Sumi K, Yokozeki H, Hirai H, Nagata K, Hara T et al (2006) Prostaglandin D2 plays an essential role in chronic allergic inflammation of the skin via CRTH2 receptor. J Immunol 177: 2621–2629 [DOI] [PubMed] [Google Scholar]
- Singh N, Gurav A, Sivaprakasam S, Brady E, Padia R, Shi H, Thangaraju M, Prasad PD, Manicassamy S, Munn DH et al (2014) Activation of Gpr109a, receptor for niacin and the commensal metabolite butyrate, suppresses colonic inflammation and carcinogenesis. Immunity 40: 128–139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song WL, FitzGerald GA (2013) Niacin, an old drug with a new twist. J Lipid Res 54: 2586–2594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturm EM, Radnai B, Jandl K, Stancic A, Parzmair GP, Hogenauer C, Kump P, Wenzl H, Petritsch W, Pieber TR et al (2014) Opposing roles of prostaglandin D2 receptors in ulcerative colitis. J Immunol 193: 827–839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su L, Shen L, Clayburgh DR, Nalle SC, Sullivan EA, Meddings JB, Abraham C, Turner JR (2009) Targeted epithelial tight junction dysfunction causes immune activation and contributes to development of experimental colitis. Gastroenterology 136: 551–563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K, Suemasu S, Ishihara T, Tasaka Y, Arai Y, Mizushima T (2009) Inhibition of both COX‐1 and COX‐2 and resulting decrease in the level of prostaglandins E2 is responsible for non‐steroidal anti‐inflammatory drug (NSAID)‐dependent exacerbation of colitis. Eur J Pharmacol 603: 120–132 [DOI] [PubMed] [Google Scholar]
- Tessner TG, Cohn SM, Schloemann S, Stenson WF (1998) Prostaglandins prevent decreased epithelial cell proliferation associated with dextran sodium sulfate injury in mice. Gastroenterology 115: 874–882 [DOI] [PubMed] [Google Scholar]
- Vong L, Ferraz JG, Panaccione R, Beck PL, Wallace JL (2010) A pro‐resolution mediator, prostaglandin D(2), is specifically up‐regulated in individuals in long‐term remission from ulcerative colitis. Proc Natl Acad Sci USA 107: 12023–12027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, He Y, Shen Y, Zhang Q, Chen D, Zuo C, Qin J, Wang H, Wang J, Yu Y (2014) Vitamin D inhibits COX‐2 expression and inflammatory response by targeting thioesterase superfamily member 4. J Biol Chem 289: 11681–11694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigmann B, Tubbe I, Seidel D, Nicolaev A, Becker C, Neurath MF (2007) Isolation and subsequent analysis of murine lamina propria mononuclear cells from colonic tissue. Nat Protoc 2: 2307–2311 [DOI] [PubMed] [Google Scholar]
- Wirtz S, Neufert C, Weigmann B, Neurath MF (2007) Chemically induced mouse models of intestinal inflammation. Nat Protoc 2: 541–546 [DOI] [PubMed] [Google Scholar]
- Wise A, Foord SM, Fraser NJ, Barnes AA, Elshourbagy N, Eilert M, Ignar DM, Murdock PR, Steplewski K, Green A et al (2003) Molecular identification of high and low affinity receptors for nicotinic acid. J Biol Chem 278: 9869–9874 [DOI] [PubMed] [Google Scholar]
- Yoshimura N, Watanabe M, Motoya S, Tominaga K, Matsuoka K, Iwakiri R, Watanabe K, Hibi T, AJMS Group (2015) Safety and efficacy of AJM300, an oral antagonist of alpha4 integrin, in induction therapy for patients with active ulcerative colitis. Gastroenterology 149: 1775–1783.e2 [DOI] [PubMed] [Google Scholar]
- Yu BL, Zhao SP (2007) Anti‐inflammatory effect is an important property of niacin on atherosclerosis beyond its lipid‐altering effects. Med Hypotheses 69: 90–94 [DOI] [PubMed] [Google Scholar]
- Zandi‐Nejad K, Takakura A, Jurewicz M, Chandraker AK, Offermanns S, Mount D, Abdi R (2013) The role of HCA2 (GPR109A) in regulating macrophage function. FASEB J 27: 4366–4374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Gong Y, Yu Y (2010) PG F(2alpha) receptor: a promising therapeutic target for cardiovascular disease. Front Pharmacol 1: 116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Zou F, Tang J, Zhang Q, Gong Y, Wang Q, Shen Y, Xiong L, Breyer RM, Lazarus M et al (2013) Cyclooxygenase‐2‐derived prostaglandin E(2) promotes injury‐induced vascular neointimal hyperplasia through the E‐prostanoid 3 receptor. Circ Res 113: 104–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Seo KH, He HZ, Pacholczyk R, Meng DM, Li CG, Xu J, She JX, Dong Z, Mi QS (2009) Tie2cre‐induced inactivation of the miRNA‐processing enzyme Dicer disrupts invariant NKT cell development. Proc Natl Acad Sci USA 106: 10266–10271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou E, Li Y, Yao M, Wei Z, Fu Y, Yang Z (2014) Niacin attenuates the production of pro‐inflammatory cytokines in LPS‐induced mouse alveolar macrophages by HCA2 dependent mechanisms. Int Immunopharmacol 23: 121–126 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix
Expanded View Figures PDF
Review Process File


