Abstract
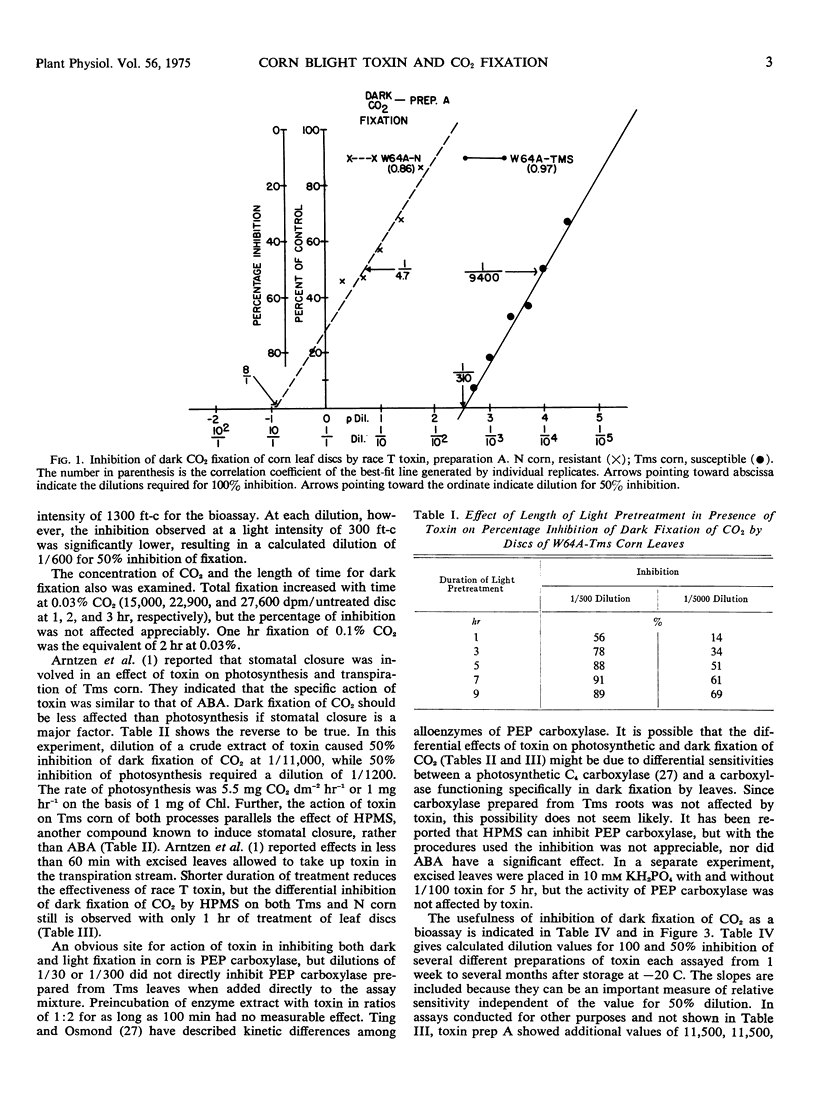
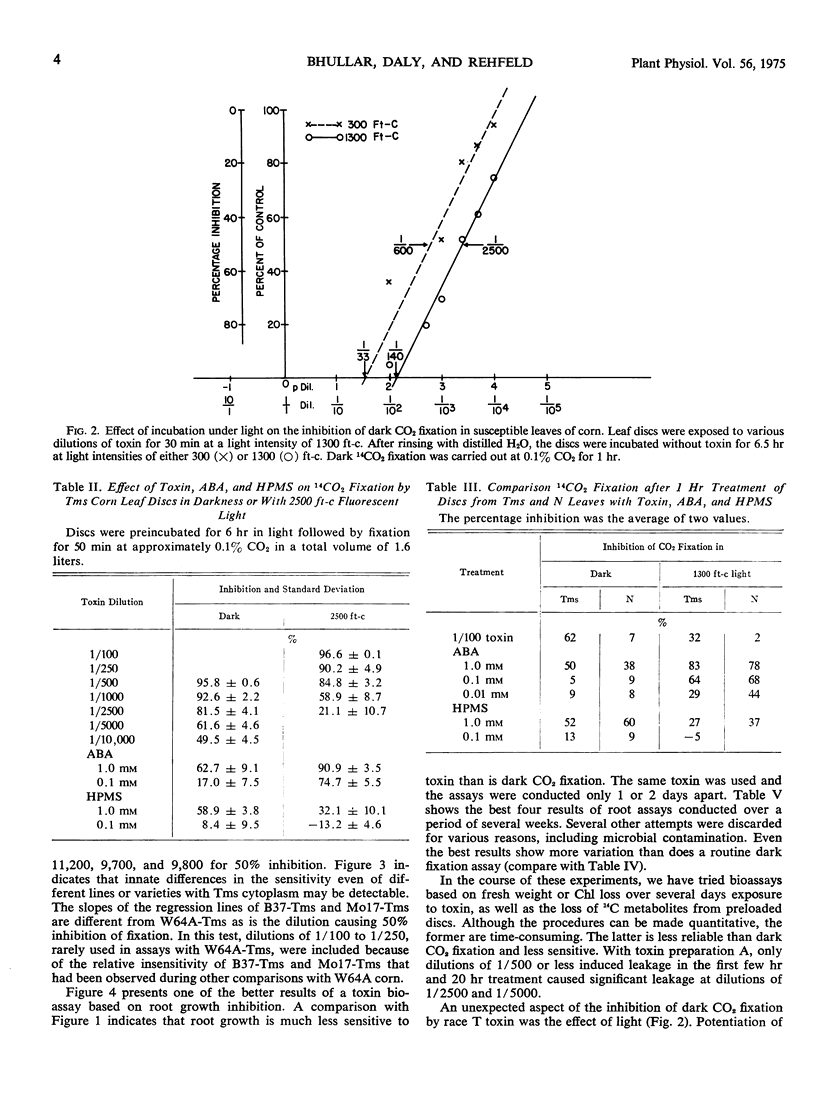
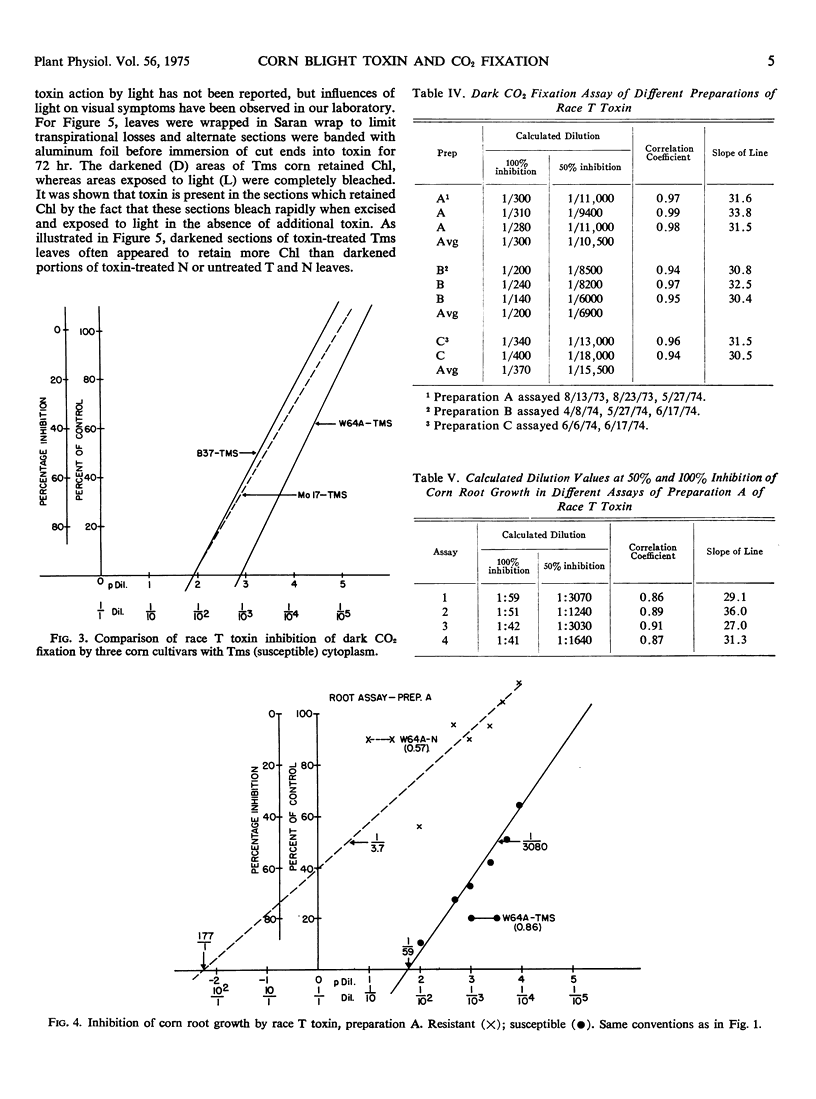
The host-specific toxin produced by Helminthosporium maydis, race T, causes 50% inhibition of dark fixation of 14CO2 by leaf discs of susceptible (Texas male sterile) corn when it is diluted to approximately 1/10,000 of the volume of the original fungus culture filtrate. Dilutions of 1/10 or less are required for equivalent inhibition of discs prepared from resistant (N) corn. Root growth and photosynthesis were considerably less sensitive (dilution values 1/3000 and 1/1200, respectively), as was leakage of 14C induced by toxin from preloaded discs. Based on literature values for dilutions causing ion leakage or inhibition of mitochondrial oxidation, toxin dilutions several orders of magnitude greater bring about inhibition of dark CO2 fixation. Preincubation of discs in light increased sensitivity of dark fixation to toxin and an effect of light on symptom development was shown. Phosphoenolypruvate carboxylase activity in extracts of roots or leaves was not affected by toxin nor was the enzyme level altered in excised leaves treated with toxin. Inhibition of dark fixation of CO2 provides a bioassaay for race T toxin which is both reliable and rapid.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
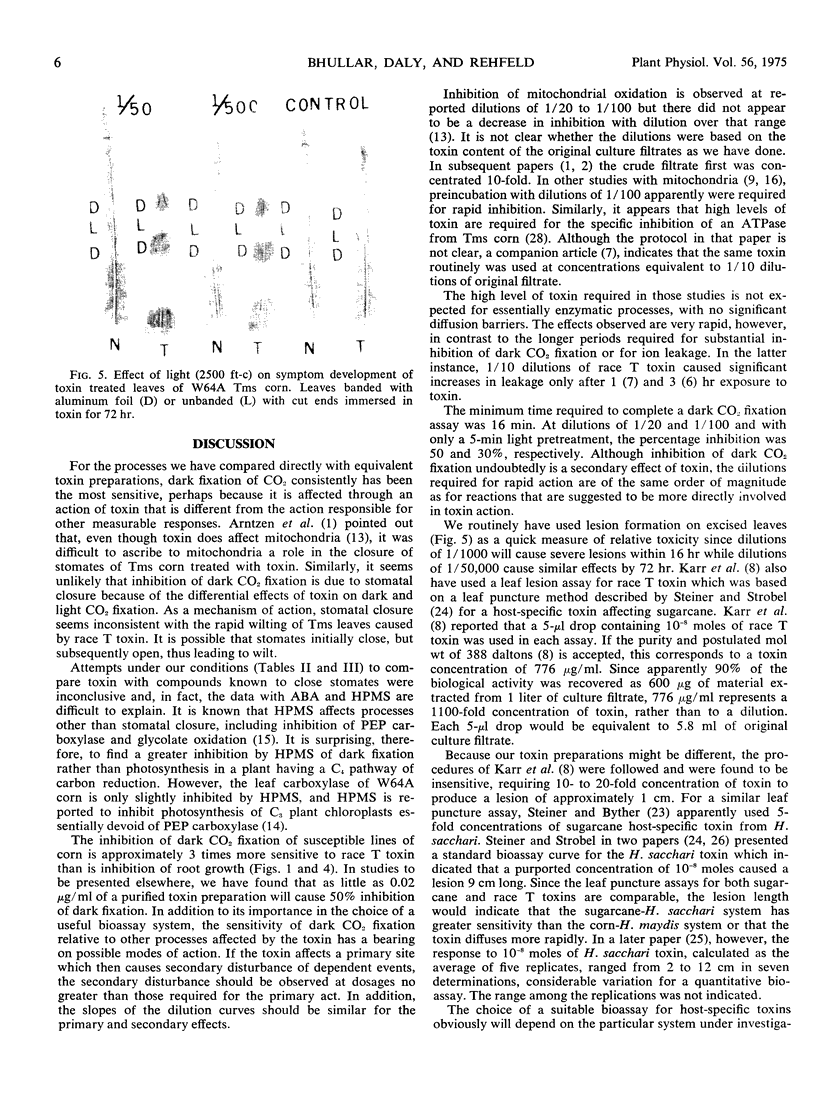
- Arntzen C. J., Haugh M. F., Bobick S. Induction of Stomatal Closure by Helminthosporium maydis Pathotoxin. Plant Physiol. 1973 Dec;52(6):569–574. doi: 10.1104/pp.52.6.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karr A. L., Karr D. B., Strobel G. A. Isolation and Partial Characterization of Four Host-specific Toxins of Helminthosporium maydis (Race T). Plant Physiol. 1974 Feb;53(2):250–257. doi: 10.1104/pp.53.2.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osmond C. B., Avadhani P. N. Inhibition of the beta-Carboxylation Pathway of CO(2) Fixation by Bisulfite Compounds. Plant Physiol. 1970 Feb;45(2):228–230. doi: 10.1104/pp.45.2.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHEFFER R. P., PRINGLE R. B. A selective toxin produced by Periconia circinata. Nature. 1961 Aug 26;191:912–913. doi: 10.1038/191912a0. [DOI] [PubMed] [Google Scholar]
- Samaddar K. R., Scheffer R. P. Effect of the Specific Toxin in Helminthosporium victoriae on Host Cell Membranes. Plant Physiol. 1968 Jan;43(1):21–28. doi: 10.1104/pp.43.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner G. W., Strobel G. A. Helminthosporoside, a host-specific toxin from Helminthosporium sacchari. J Biol Chem. 1971 Jul 10;246(13):4350–4357. [PubMed] [Google Scholar]
- Strobel G. A. The helminthosporoside-binding protein of sugarcane. Its properties and relationship to susceptibility to the eye spot disease. J Biol Chem. 1973 Feb 25;248(4):1321–1328. [PubMed] [Google Scholar]
- Ting I. P., Osmond C. B. Multiple forms of plant phosphoenolpyruvate carboxylase associated with different metabolic pathways. Plant Physiol. 1973 Mar;51(3):448–453. doi: 10.1104/pp.51.3.448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tipton C. L., Mondal M. H., Uhlig J. Inhibition of the K + -stimulated ATPase of maize root microsomes by Helminthosporium maydis race T pathotoxin. Biochem Biophys Res Commun. 1973 Apr 2;51(3):725–728. doi: 10.1016/0006-291x(73)91375-2. [DOI] [PubMed] [Google Scholar]