Abstract
Bisphenol A (BPA) is an endocrine-disrupting compound used to manufacture plastics; it is present in linings of food cans, bottles, thermal receipts, and many other everyday items and is detectable in human urine and blood. Exposure to BPA during development can disrupt sexual differentiation of some brain regions. Moreover, BPA can have transgenerational effects on gene expression and behaviors. Here, we used a diet and breeding regimen that produces transgenerational effects on behaviors. C57BL/6J mice consumed control or BPA-containing diets during pregnancy. We examined vasopressin (AVP) and estrogen receptor α (ERα) immunoreactivity (ir) in sexually dimorphic brain regions from first-generation (F1) offspring and transgenerational effects of BPA in third-generation offspring. In all but one brain region examined, the expected sex differences were noted in both generations of control mice. In F1 mice, a diet by sex interaction was present for AVP-ir in the lateral septum and posterodorsal medial amygdala. In both regions, BPA exposure reduced immunoreactivity in male brains. An interaction between diet and sex for ERα-ir in the ventromedial hypothalamus was caused by reduced immunoreactivity in BPA-exposed females. Of interest, BPA had transgenerational effects on ERα-ir in the anteroventral periventricular nucleus and bed nucleus of the stria terminalis. Our data show that BPA produces immunoreactive differences in ERα-ir generations after exposure to BPA. We speculate that actions of BPA in utero on ERα-ir in brain have long-term consequences for reproduction and social behavior.
Bisphenol A (BPA) is a man-made endocrine-disrupting compound present in a variety of plastic products, including the linings of canned foods, thermal receipts, and some plastic bottles (1, 2). The National Health and Nutrition Examination Survey conducted in 2008 found that over 92% of the participants had detectable levels of BPA or its metabolites in their urine (3). BPA has complex actions on multiple target tissues, including the brain (4, 5), where it can bind to a variety of steroid hormone receptors (6, 7) and also influence expression of some of the enzymes in the steroidogenesis pathway (8). In this manner, BPA can disrupt sexual differentiation of neural circuits and behaviors (9).
Several studies have examined gestational and/or neonatal exposures to BPA and, in general, have found that exposure nullifies neural sex differences, as both sexes experiencing BPA tend to be less dimorphic than controls (10). One neurotransmitter that is disturbed by exposure to BPA is vasopressin (AVP) (11, 12). Estrogen receptor α (Esr1) messenger RNA (mRNA) in the rat hypothalamus is likewise changed by neonatal exposure to BPA (13). In a previous study from our laboratory, we noted that BPA exposure reduced the amount of both Avp and Esr1 mRNA in whole embryo brains (14). Moreover when F1 mice were bred to produce F3 lineages, without additional exposure to BPA, we found that the reduction in Avp mRNA persisted.
Here, we quantified AVP and ERα with immunocytochemistry (ICC) in several sexually dimorphic regions, which are part of the “social behavior network.” For AVP, we examined the posterodorsal medial amygdala (MePD), which contains AVP-synthesizing cells, the bed nucleus of the stria terminalis (BNST) that contains primarily AVP-containing fibers and the lateral septum (LS), which contains AVP projections from the aforementioned area (15, 16). As a control, we also examined AVP-ir in the paraventricular nucleus (PVN), an area that is not sexually dimorphic. Estrogen receptor α immunoreactive cells were measured in sexually dimorphic regions where BPA effects have been reported (17, 18); the anteroventral periventricular nucleus (AVPV), the BNST, MePD, and the ventromedial hypothalamus (VMH). Our main hypothesis was that sex differences would be lost in BPA-exposed F1 brains. We anticipated that some of these effects might be retained in F3 brains.
Materials and Methods
Animals and tissue collection
C57BL/6J mice were purchased from Jackson Laboratories (Bar Harbor, ME) and housed at the University of Virginia. All procedures used were in compliance with and approved by the University of Virginia Animal Use and Care Committees. Mice were maintained on a 12:12 light:dark cycle. Females were randomly assigned to a control phytoestrogen-free diet (Harlan Teklad TD95092, Madison, WI) or the diet made to order with 5 mg BPA added to each kilogram of diet (Harlan Teklad TD09386). We have used this dose in diet previously and have not noted any health effects in dams or pups (unpublished data). The concentrations of BPA produced in dam serum is equivalent to those found in humans (19). Female mice were placed on their assigned diets 7 to 10 days before pairing with a male. After 1 week, males were removed and females continued consuming their assigned diets ad libitum. Within 12 hours after birth, the pups from control and BPA fed dams were fostered to dams on control diet that had given birth within the previous 24 hours. At weaning, the F1 mice we used for breeding were switched to a phytoestrogen-containing chow (Harlan Teklad diet number 7912). This was done purely for financial reasons, as maintaining the mice for 3 generations on custom diet was prohibitively costly. Adult F1 offspring were paired, and their offspring (F2) were also bred, to produce third-generation (F3) lineages. In the exposure paradigm we used (during gestation), the first truly transgenerational offspring are the F3 because the germ cells that produce the F2 generation were exposed to BPA during the F1 gestation. For this reason, we only assessed F1 and F3 adults.
Adult F1 and F3 mice (about 90 days) were anesthetized (with Euthasol), and trunk blood was collected, centrifuged, and serum frozen. Brains were removed, fixed by immersion in 2% acrolein, stored in 30% sucrose for 1 to 2 days at 4°C, and then frozen and stored at –80°C until sectioning. The total number of brains used for each group was as follows: F1 generation: control male, n = 5; control female, n = 5; BPA male, n = 5; BPA female, n = 5, and F3 generation: control male, n = 11; control female, n = 12; BPA male, n = 11; BPA female, n = 12. No more than 2 pups were used from the same litter.
Immunocytochemistry
Fixed frozen brains were sectioned into a series of 3 30-μm coronal sections on a cryostat. Sections were stored in antifreeze [tris(hydroxymethyl)aminomethane-buffered saline, sucrose, polyvinylpyrrolidone-40, and ethylene glycol] at –20°C until they were processed for ICC. One vial was used for AVP, another vial for ERα, and the third for practice.
Both primary antibodies were rabbit polyclonals (anti-AVP, 1:5,000, DiaSorin; anti-ERα; 1:5,000, C1355, Upstate; Table 1). We have previously published our procedures for ICC (20). In brief, tissues were rinsed, made permeable, and incubated overnight in a primary antibody with blockers for nonspecific proteins. The tissue was rinsed and incubated in biotinylated secondary goat anti-rabbit serum (1:500, Vector Laboratories, Burlington, CA) for 75 minutes. Antigens were detected using the avidin-biotin complex ABC detection system (1:1,000, Vector Elite Kit, Vector Laboratories) coupled with nickel-intensified 3,3′-diaminobenzidine activated by 0.1% hydrogen peroxide. All tissues were developed in 3,3′-diaminobenzidine for the same length of time.
Table 1.
Antibodies Used for ICC
| Peptide/Protein Target | Antigen Sequence (If Known) | Name of Antibody | Manufacturer, Catalog No., and/or Name of Individual Providing the Antibody | Species Raised in; Monoclonal or Polyclonal | Dilution Used |
|---|---|---|---|---|---|
| AVP | DiaSorin, #22941 | polyclonal: rabbit | 1–5000 | ||
| ERα | C1355 | Upstate, #06-935 | polyclonal: rabbit | 1–5000 |
Image analysis
Tissue was mounted on gel-coated glass slides and coded to ensure the operator was blind to the diet and sex of the subject. Microscopic images were visualized with a BX60 microscope and captured using a charge-coupled device video camera (Photometric, Tucson, AZ) linked to a computer. For regions other than the PVN, the single section exhibiting the best match to the area of interest was quantified. We identified regions according to the Paxinos and Franklin mouse brain atlas (21) and the Allen Mouse Brain Atlas (http://mouse.brain-map.org). For each region, a standardized area of the same size and shape was used for measurements in all brains. Cell counts and grayscale images were quantified using MetaMorph software (version 4.5; Molecular Devices, West Chester, PA). We report cell counts for ERα-immunoreactivity (ir) as the antigen is contained in the cell nuclei that are largely nonoverlapping. On the other hand, AVP-ir fills cell bodies and fibers. Because the immunoreactivity is largely overlapping and contiguous, and we cannot use confocal microscopy with nonfluorescent sections, we are reporting area covered here and not distinguishing between cells and fibers.
The total area containing AVP immunoreactive staining was quantified in four regions. The regions examined were the LS, a region that only contains immunoreactive fibers and the BNST and MePD, both of which contain primarily fibers, with a few AVP immunoreactive cells. We also quantified the PVN, an area that has a dense population of cells and fibers. Assessment of AVP-ir in the PVN was limited to brains for which we had all PVN sections. Because we had small groups of F1 brains, the analysis was restricted to F3 samples. We quantified the size of the immunoreactive region on each section and expressed the data as an average (adjusted by numbers of sections). Areas in which ERα immunoreactive cells were counted were the BNST, the MePD, the AVPV, and the VMH.
Hormone assays
Serum from a subset of the original F3 mice used for ICC plus additional mice raised, treated, and euthanized in an identical manner were assayed. Only females were used for estradiol determination (enzyme-linked immunosorbent assay from Calbiotech; controls, n = 14; BPA, n = 12). The sensitivity of the estradiol assay was 3.0 pg/mL, and the intraassay coefficient of variation was 11.4% ± 3.1% [mean ± standard error of the mean (SEM)]. The interassay coefficient of variation was 9.68% ± 11.14%. Samples from F3 males (controls, n = 13; BPA, n = 10) were assayed for testosterone (enzyme-linked immunosorbent assay from IBL). For the testosterone assay, the level of sensitivity was 0.1 ng/mL and the intraassay coefficient of variation was 9.3% ± 1.6% (mean ± SEM). All samples were assayed in duplicate by the University of Virginia Ligand Assay Core (grant number P50-HD28934).
Statistical analysis
All data were analyzed using 2-way analysis of variances with sex and diet as the independent factors. All post hoc tests were performed with Bonferroni planned comparisons, which correct for multiple comparisons. ICC for F1 versus F3 mice was conducted in 2 separate runs. We did not perform a direct comparison between the F1 and F3 brains because the mice were consuming different diets throughout their lives, and the tissue sections were developed at different times. Previous work has shown that diet (particularly those with different amounts of phytoestrogens) has an impact on estradiol-induced progestin receptor- cell numbers in mouse brain and thus may have influenced these results (22). The hormone data were analyzed with Bonferroni multiple t tests.
Results
AVP-ir in F1 brains
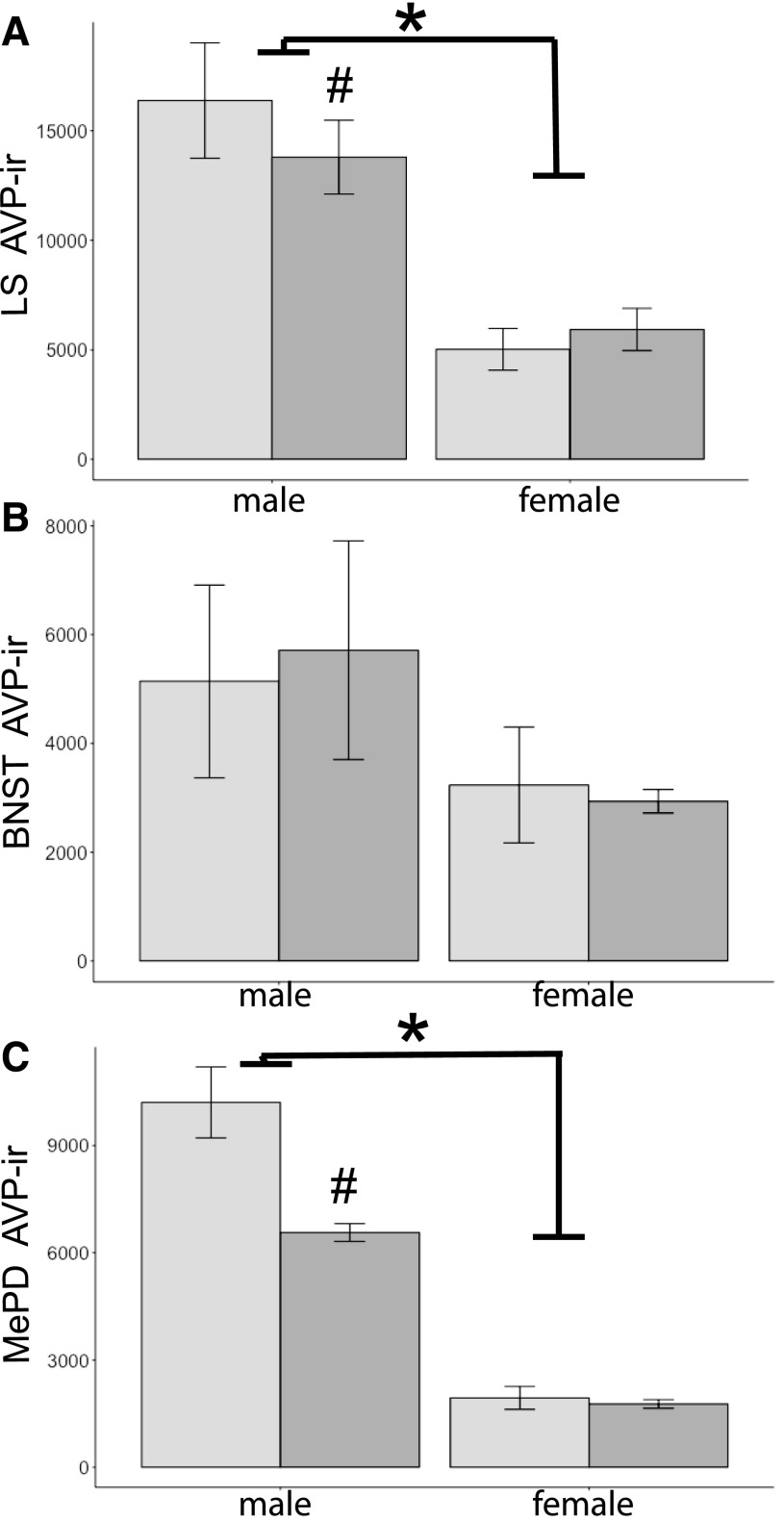
In F1 brains, significant main effects of sex on AVP-ir were noted. The area covered by AVP-ir in the LS and MePD was greater in male than in female brains [LS, F(1,19) = 64.1; MePD, F(1,18) = 195.3, P < 0.001; Figs. 1 and 2]. Gestational exposure to BPA affected AVP-ir in the MePD [F(1,18) = 16.6, P < 0.001], and BPA-exposed brains were less densely immunoreactive than the control brains. In both areas, an interaction between sex and diet was noted [MePD, F(1,18) = 13.8; LS, F(1,19) = 6.8, P < 0.02 at least; Figs. 1 and 2]. The interaction was the result of decreased immunoreactive areas in BPA-exposed males as compared with control males (P < 0.05; Fig. 2). Diet did not influence immunoreactivity in the LS [F(1,19) = 0.2]. In the BNST, there were no effects of diet [F(1,19) = 0.01] or sex [F(1,19) = 2.6, P = 0.125], nor was there an interaction between these two factors [F(1,19) = 0.1].
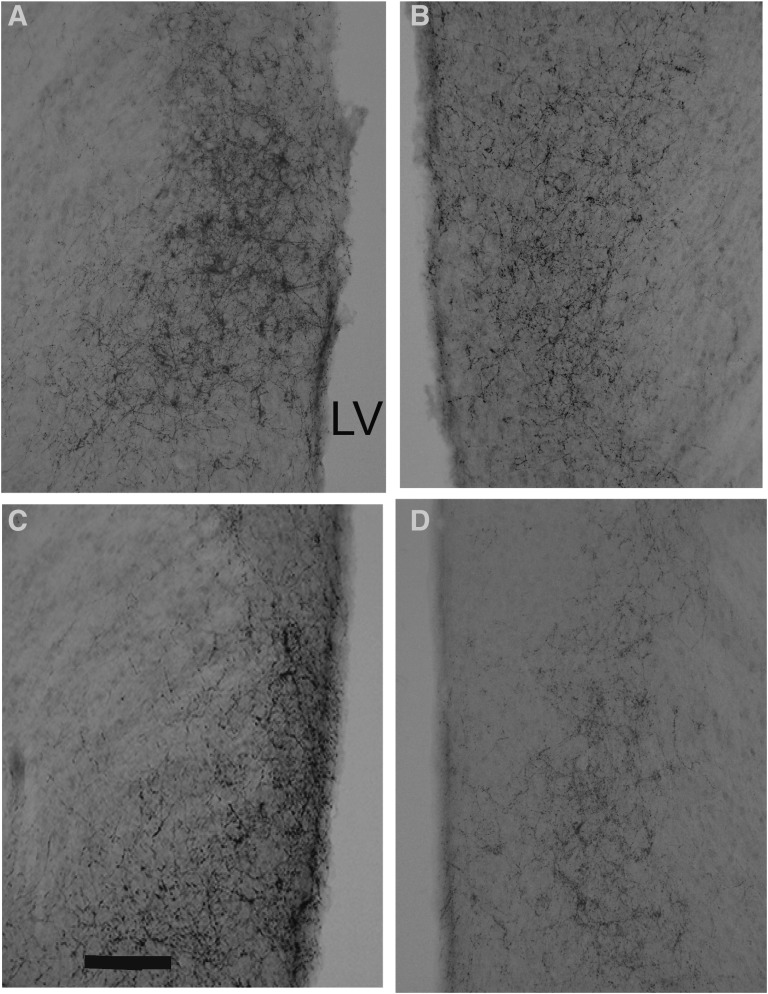
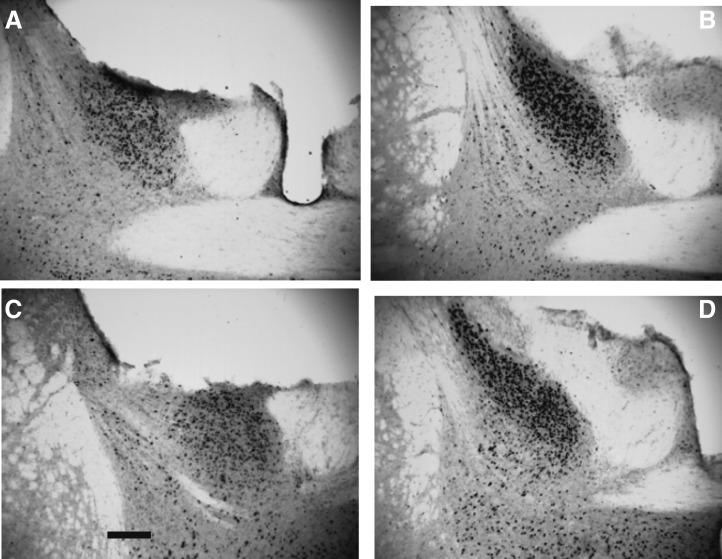
Figure 1.
Photomicrographs of immunoreactive AVP containing fibers in the LS of F1 mice. (A) Control male. (B) Control female. (C) BPA male. (D) BPA female. Scale bar in panel C = 50 μm. LV, lateral ventricle.
Figure 2.
Mean + SEM for AVP-ir–stained area (μm2) quantified in (A) the LS, (B) the BNST, and (C) the MePD. Mice were exposed to BPA (dark-gray histograms) or not (controls, light-gray histograms) during gestation. An asterisk signifies significant sex differences; males had more immunoreactivity than females (P < 0.001). A pound sign signifies significant interaction between diet and sex; BPA-exposed males had less AVP-ir than control males (P < 0.02). n = 5 per group, except for the MePD, in which n = 4 for control males.
ERα-ir in F1 brains
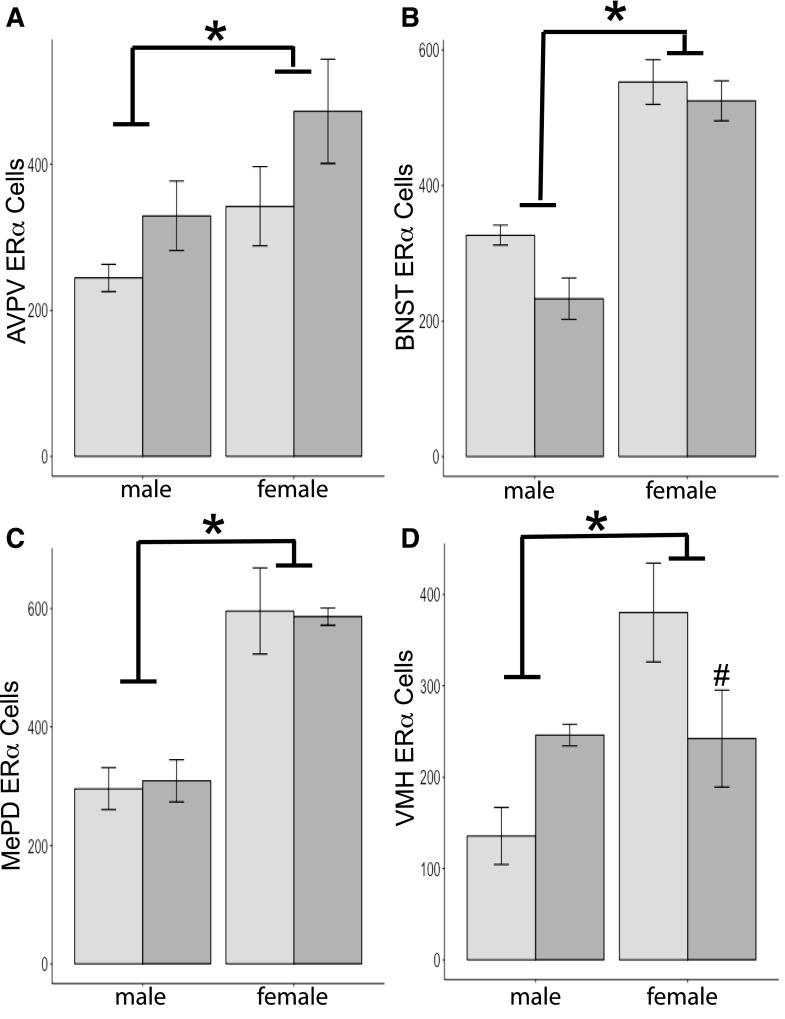
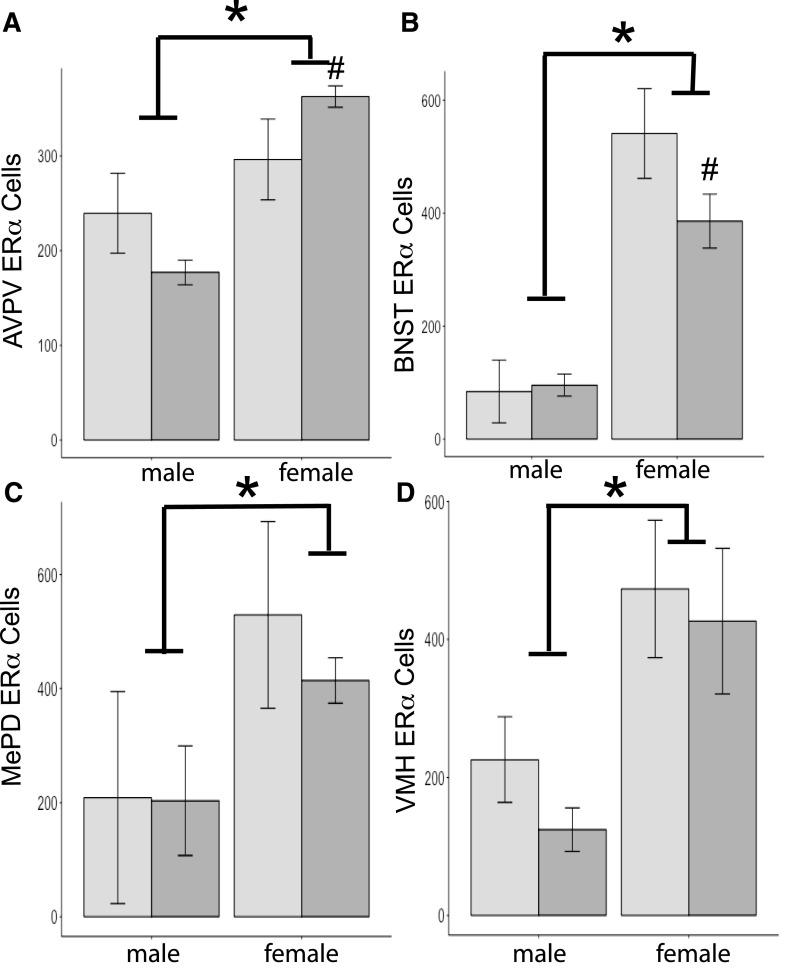
As expected, immunoreactive ERα cell numbers in all regions quantified were greater in female than male brains [AVPV, F(1,14) = 5.3; BNST, F(1,18) = 78.1; MePD, F(1,13) = 36.0; and VMH, F(1,12) = 10.0, P < 0.05 or less; Fig. 3]. Diet had no independent effect in any of the regions we examined [AVPV, F(1,14) = 4.2; BNST, F(1,18) = 4.3; MePD, F(1,13) = 0.001; and VMH, F(1,12) = 0.1; Fig. 3]. We found an interaction between sex and diet in ERα-ir cell numbers in one area, the VMH [F(1,12) = 10.6, P < 0.01]. The interaction was caused by differences between the groups of females. Females exposed to BPA had fewer ERα cells than control females (P < 0.05). No interactions between diet and sex were noted in the other regions we examined [AVPV, F(1,14) = 0.2; BNST, F(1,18) = 1.2; and MePD, F(1,13) = 0.05].
Figure 3.
Mean + SEM ERα cells quantified in F1 brains of mice either exposed to BPA during gestation (dark-gray histograms) or controls (light-gray histograms). (A) For the AVPV, n = 4 per group, except n = 3 for control females. (B) For the BNST, n = 5 per group, except n = 4 for control males. (C) For the MePD, n = 4 for control males, n = 5 for BPA males, n = 3 for control females, and n = 2 for BPA females. (D) For the VMH, n = 3 for all groups, except n = 4 for BPA males. An asterisk signifies significant effect of sex in all regions; females had more immunoreactive cells than males (P < 0.05). A pound sign signifies significant interaction produced by differences in ERα cell numbers in BPA-exposed versus control females (P < 0.05).
AVP-ir in F3
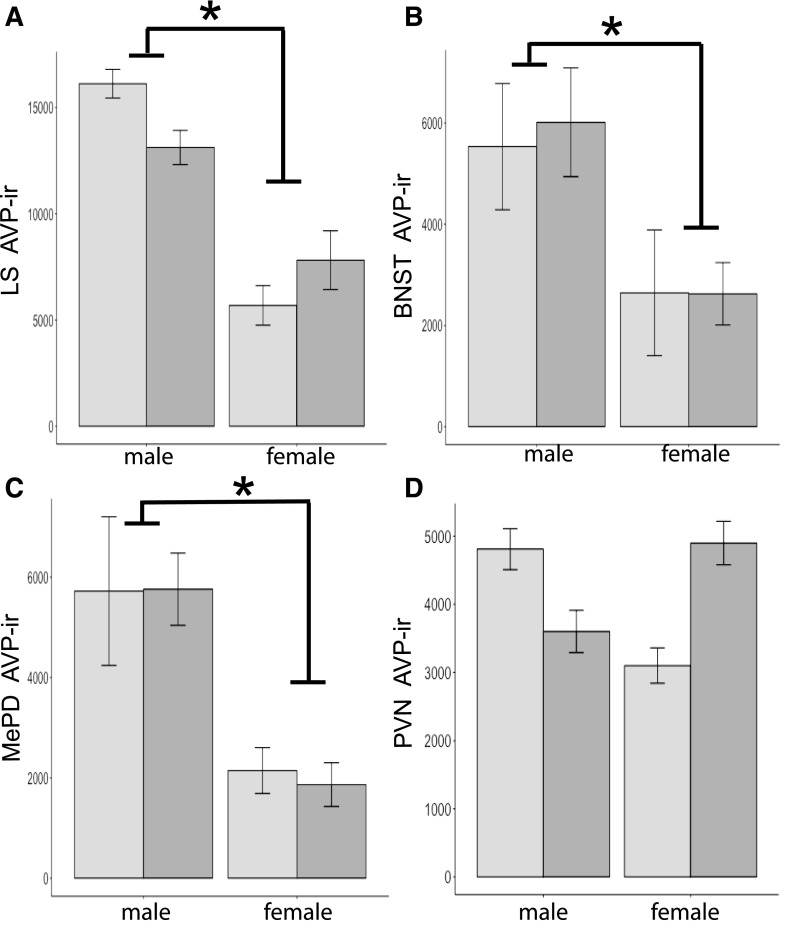
The predicted sex differences were present in F3 brains. Males had more immunoreactivity than females [LS, F(1,45) = 37.3; BNST, F(1,46) = 87.4; and MePD, F(1,43) = 17.6, P < 0.001 at least; Fig. 4]. We also quantified AVP-ir in the PVN in F3 brains. As expected, no sex differences were present in PVN [F(1,20) = 0.01]. No effects of diet were noted for any of the areas examined in F3 brains [LS, F(1,45) = 0.30; BNST, F(1,46) = 3.2; MePD, F(1,43) = 0.02; and PVN, F(1,20) = 0.5, P > 0.05]. In the PVN, a sex-by-diet interaction was present [F(1,20) = 5.4, P < 0.05], but none of the planned comparisons were significant, making the interaction impossible to interpret. Interactions were not found in any other regions, including the LS [F(1,45) = 1.2], the BNST [F(1,46) = 4.3], or the MePD [F(1,43) = 0.03].
Figure 4.
Mean + SEM AVP-ir–stained area (μm2) quantified in (A) the LS (n = 10 for control males, n = 11 for BPA males and control females, and n = 13 for BPA females), (B) the BNST (n = 11 for control and males, n = 12 for control females, and n = 13 for BPA females), (C) the MePD (n = 11 for control and males, n = 9 for control females, and n = 13 for BPA females), and (D) the PVN (n = 5 in all groups, except control females, where n = 6). Mice were the F3 generation from BPA (dark-gray histograms) or control (light-gray histograms) lineages. An asterisk signifies significant sex differences; males had more immunoreactivity than females (P < 0.001).
ERα-ir in F3 brains
Main effects of sex were noted in the F3 ERα cell counts for all regions examined. As expected, female brains had more ERα cells than male brains [AVPV, F(1,18) = 16.4; BNST, F(1,20) = 87.4; MePD, F(1,14) = 6.9; and VMH, F(1,16) = 11.8, P < 0.025 at least; Figs. 5 and 6]. No main effects of diet were found in any of the regions [AVPV, F(1,18) = 0.005; BNST, F(1,20) = 3.2; MePD, F(1,14) = 0.4; and VMH, F(1,16) = 0.8]. Interactions between diet and sex were present in the BNST [F(1,20) = 4.4] and the AVPV [F(1,18) = 4.6, P < 0.05; Fig. 5]. In the BNST, females from the BPA lineage had fewer ERα cells than the control lineage females (P < 0.05). In the AVPV, the reverse was noted, as more ERα cells were counted in BPA compared with control lineage female brains (P < 0.05). Interactions were not present in the MePD [F(1,14) = 0.3] or the VMH [F(1,16) = 0.1].
Figure 5.
Photomicrographs of ERα immunoreactivity in the BNST of F3 mice. (A) Control male. (B) Control female. (C) BPA male. (D) BPA female. Scale bar in panel C = 100 μm.
Figure 6.
Mean + SEM ERα cells quantified in F3 mice. Mice were from BPA (dark-gray histograms) or control (light-gray histograms) lineages. n = 5 per group except as noted below. (A) For the AVPV, n = 4 for control female group. (B) For the BNST, n = 6 for control males and n = 4 for BPA males and control females. (C) For the MePD, n = 3 for control males and female groups and n = 4 for BPA males. (D) For the VMH, n = 2 for control males and n = 4 for BPA males and control females. An asterisk signifies significant effect of sex in all regions; females had more immunoreactive cells than males (P < 0.05). A pound sign signifies significant interaction produced by differences in ERα cell numbers in BPA-exposed versus control females (P < 0.05).
No effects of BPA lineage on steroid hormones in F3 mice
In F3 mice, diet lineages had no significant effects on levels of estradiol in females (control = 6.73 ± 4.9 pg/mL; BPA = 6.92 ± 4.4 pg/mL; mean ± SEM) or testosterone in males (control = 1.22 ± 0.4 ng/mL; BPA = 2.00 ± 0.9 ng/mL; mean ± SEM).
Discussion
The most interesting finding from this study is that gestational BPA exposure produced transgenerational (F3) changes in ERα immunoreactive cell numbers in two brain regions. In all areas and both generations, we found the predicted sex difference in control mice; males had fewer ERα immunoreactive cells than did females. Diet effects were present in the VMH in F1 females, and BPA exposure in utero reduced ERα cell counts. Two other regions showed diet effects in F3 female brains. In the BNST, ERα cell numbers were reduced in females from the BPA lineage compared with control females. In the AVPV, the difference was in the opposite direction; BPA lineage females had more ERα cells than controls. The fact that the diet effects were in the opposite directions in these two areas suggests cell-specific interactions whereby other factors (coactivators or corepressors, for example) change the responses of ERα. Moreover, as estradiol levels were equivalent in F3 females, ERα-ir differences are not a reflection of peripheral hormone levels.
One potential mechanism suggested for these transgenerational effects is via BPA actions on germ cell DNA methylation (23). Our findings may be due to germ cell hypo- or hypermethylation of Esr1 in combination with the microenvironment in AVPV and BNST. Differences in DNA methylation of Ers1 have been reported in F1 mouse brain (24) but not yet in F3 brain. Our findings might be related to decreased fecundity, including a higher failure to maintain pregnancies, reported in F3 BPA lineage female mice (25). In addition, Esr1 mRNA was decreased in ovaries of F3 females from a BPA exposure lineage compared with controls, although there was no diet effect in F1 material (26).
Our data replicate sex differences found by others in adult rat, vole, and mouse brain for the expression of two different proteins, AVP and ERα (27–29). In adult F1 mice directly exposed to BPA during gestation, we observed disruptions in normal sexual differentiation of AVP expression in the LS and the MePD. F1 male mice exposed to BPA in utero had reduced AVP-ir area compared with control males and had similar immunoreactivity to female mice. Because this cell/fiber population is masculinized by early exposure to estrogens and androgens (20, 30), BPA had a demasculinizing action when given during gestation. In voles exposed post partum to a higher dose of BPA than the one used here, an increase in numbers of AVP immunoreactive neurons was noted in the anterior part of the PVN (12). The PVN did not prove to be sexually dimorphic in our study. However, we did find a diet by sex interaction in F3 mouse brains. Although no significance was noted in planned comparisons, the data suggest a sex difference in the controls (male > female) and a reversal in the BPA lineage. Further work needs to be done to shed light on this interesting trend.
In an earlier report from our laboratory, we noted a decrease in BPA-exposed F1 and F3 embryo (day 18.5) whole brains for AVP mRNA compared with controls (14). The fact that we did not find this in F3 brains using ICC is probably due to differences in the age of the animals and/or use of whole brains for mRNA. Because Avp is only produced in a small subset of neurons, use of whole brain dilutes the effects of BPA in small cell populations. To our knowledge, ours is the first examination of actions of BPA in F1 and F3 brains in these classically sexually differentiated regions in the AVP circuit. This circuit plays an important part in mediating social behaviors, especially social recognition. Differences in AVP synthesis and release could cause deficits in these behaviors, and we have found differences in social recognition in F1 and F3 juvenile mice exposed to BPA (14, 31). In both generations of BPA mice, habituation to the same female over repeated presentations takes longer than for control mice. This may be attributed to enhanced activity in the BPA-exposed mice; movement in the open field is elevated in male F3 BPA lineage mice compared with controls (31). The other aspect of social recognition we measured is dishabituation, in which a unique female replaces the familiar female after a series of 8 trials. F3 BPA lineage mice fail to display dishabituation. It is possible that this effect in females is produced by the drop in ERα cells we found in BNST. Of note, olfactory discrimination is normal in the BPA lineage F3 mice of both sexes (31).
Interactions between sex and diet were noted in ERα cell numbers in the VMH of F1 mice. BPA exposure in utero increased ERα-ir cell number in males, although it caused a decrease in females, and this eliminated the sex difference in the BPA-exposed mice. In our previous study, embryo brains of both sexes exposed to BPA had less Esr1 mRNA compared with controls (14). In rats, pre- and postnatal treatment (to dams and pups) with 17-ethinylestradiol or BPA (25 μg/kg body weight daily) increased Esr1 mRNA in the VMH in both sexes (17), whereas two other doses of BPA given for the first 3 days after birth had no impact on Esr1 mRNA in the VMH (32). When rats were treated around the time of puberty with 40μg/kg body weight of BPA, an increase in ERα cell numbers was noted in the female VMH. A high dose of BPA for 2 days after birth had no effect on ERα cells in the AVPV (33). The pattern we noted in the VMH, whereby a sex difference was present in ERα-ir in control mice but was eliminated in the BPA-treated animals, is caused by data from both sexes moving toward the mean. This same pattern has been noted by others (10, 17, 24) and for some sexually dimorphic behavioral effects of BPA exposure during development in mice and monkeys (34–36). The behavior most closely identified with estrogen receptors in the VMH is female sexual receptivity. Female C57BL/6J mice treated during the first 2 weeks of gestation with BPA (by gavage to dams) have elevated sexual receptivity compared with controls. Using a different antibody than the one used here, these authors did not find differences in ERα cell counts in the VMH of mice euthanized during diestrous (37).
In sum, our behavioral studies show robust and replicable transgenerational effects of BPA on social interactions (14, 31). Short-term changes in behavior may be mediated by BPA’s actions on AVP and ERα, transgenerational changesτρανσγενερατιοναλ χηανγεσ may be attributed to ERα. Our data further show that precise populations of neurons must be examined to pinpoint the specific actions of BPA. Use of whole brain, or gross dissections of large regions, may mask these effects. This is an important point, as some epigenetic methods require more tissue than small areas (like the BNST) provide and thus may miss some of the most important aspects of transgenerational actions of BPA. As new methods are becoming available, these may be used to shed light on the epigenetics responsible for the actions of endocrine-disrupting compounds on behavior.
Acknowledgments
We thank Ondrej Drobny for his work on some of the image analysis in this paper.
Current address: J.T.W. is with the Department of Pharmacology & Toxicology, Virginia Commonwealth University, Richmond, Virginia 23298.
Acknowledgments
This work was supported by National Institute for Health Grant RO1 ES022759 (to E.F.R.). J.T.W. was supported by grant F32 ES019404. The University of Virginia Center for Research in Reproduction Ligand Assay and Analysis Core is supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development /National Institute for Health (National Centers for Translational Research in Reproduction and Infertility) Grant P50-HD28934.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AVP
- vasopressin
- AVPV
- anteroventral periventricular nucleus
- BNST
- bed nucleus of the stria terminalis
- BPA
- bisphenol A
- ICC
- immunocytochemistry
- ir
- immunoreactivity
- LS
- lateral septum
- MePD
- posterodorsal medial amygdala
- mRNA
- messenger RNA
- PVN
- paraventricular nucleus
- SEM
- standard error of the mean
- VMH
- ventromedial hypothalamus
References
- 1.Vandenberg LN, Maffini MV, Wadia PR, Sonnenschein C, Rubin BS, Soto AM. Exposure to environmentally relevant doses of the xenoestrogen bisphenol-A alters development of the fetal mouse mammary gland. Endocrinology. 2007;148(1):116–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Biedermann S, Tschudin P, Grob K. Transfer of bisphenol A from thermal printer paper to the skin. Anal Bioanal Chem. 2010;398(1):571–576. [DOI] [PubMed] [Google Scholar]
- 3.Calafat AM, Ye X, Wong LY, Reidy JA, Needham LL. Exposure of the U.S. population to bisphenol A and 4-tertiary-octylphenol: 2003-2004. Environ Health Perspect. 2008;116(1):39–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yolton K, Xu Y, Strauss D, Altaye M, Calafat AM, Khoury J. Prenatal exposure to bisphenol A and phthalates and infant neurobehavior. Neurotoxicol Teratol. 2011;33(5):558–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Patisaul HB, Polston EK. Influence of endocrine active compounds on the developing rodent brain. Brain Res Brain Res Rev. 2008;57(2):352–362. [DOI] [PubMed] [Google Scholar]
- 6.Kuiper GG, Lemmen JG, Carlsson B, Corton JC, Safe SH, van der Saag PT, van der Burg B, Gustafsson JA. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor beta. Endocrinology. 1998;139(10):4252–4263. [DOI] [PubMed] [Google Scholar]
- 7.Gould JC, Leonard LS, Maness SC, Wagner BL, Conner K, Zacharewski T, Safe S, McDonnell DP, Gaido KW. Bisphenol A interacts with the estrogen receptor α in a distinct manner from estradiol. Mol Cell Endocrinol. 1998;142(1-2):203–214. [DOI] [PubMed] [Google Scholar]
- 8.Bonefeld-Jørgensen EC, Long M, Hofmeister MV, Vinggaard AM. Endocrine-disrupting potential of bisphenol A, bisphenol A dimethacrylate, 4-n-nonylphenol, and 4-n-octylphenol in vitro: new data and a brief review. Environ Health Perspect. 2007;115(Suppl 1):69–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arnold AP, Rissman EF, De Vries GJ. Two perspectives on the origin of sex differences in the brain. Ann N Y Acad Sci. 2003;1007:176–188. [DOI] [PubMed] [Google Scholar]
- 10.Rebuli ME, Patisaul HB. Assessment of sex specific endocrine disrupting effects in the prenatal and pre-pubertal rodent brain. J Steroid Biochem Mol Biol. 2016;160:148–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Adewale HB, Todd KL, Mickens JA, Patisaul HB. The impact of neonatal bisphenol-A exposure on sexually dimorphic hypothalamic nuclei in the female rat. Neurotoxicology. 2011;32(1):38–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sullivan AW, Beach EC, Stetzik LA, Perry A, D’Addezio AS, Cushing BS, Patisaul HB. A novel model for neuroendocrine toxicology: neurobehavioral effects of BPA exposure in a prosocial species, the prairie vole (Microtus ochrogaster). Endocrinology. 2014;155(10):3867–3881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ceccarelli I, Della Seta D, Fiorenzani P, Farabollini F, Aloisi AM. Estrogenic chemicals at puberty change ERα in the hypothalamus of male and female rats. Neurotoxicol Teratol. 2007;29(1):108–115. [DOI] [PubMed] [Google Scholar]
- 14.Wolstenholme JT, Edwards M, Shetty SR, Gatewood JD, Taylor JA, Rissman EF, Connelly JJ. Gestational exposure to bisphenol a produces transgenerational changes in behaviors and gene expression. Endocrinology. 2012;153(8):3828–3838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Leeuwen FW, Caffe AR, De Vries GJ. Vasopressin cells in the bed nucleus of the stria terminalis of the rat: sex differences and the influence of androgens. Brain Res. 1985;325(1-2):391–394. [DOI] [PubMed] [Google Scholar]
- 16.Koolhaas JM, Everts H, de Ruiter AJ, de Boer SF, Bohus B. Coping with stress in rats and mice: differential peptidergic modulation of the amygdala-lateral septum complex. Prog Brain Res. 1998;119:437–448. [DOI] [PubMed] [Google Scholar]
- 17.Cao J, Rebuli ME, Rogers J, Todd KL, Leyrer SM, Ferguson SA, Patisaul HB. Prenatal bisphenol A exposure alters sex-specific estrogen receptor expression in the neonatal rat hypothalamus and amygdala. Toxicol Sci. 2013;133(1):157–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yu CJ, Fang QQ, Tai FD. Pubertal BPA exposure changes central ERα levels in female mice. Environ Toxicol Pharmacol. 2015;40(2):606–614. [DOI] [PubMed] [Google Scholar]
- 19.Wolstenholme JT, Taylor JA, Shetty SR, Edwards M, Connelly JJ, Rissman EF. Gestational exposure to low dose bisphenol A alters social behavior in juvenile mice. PLoS One. 2011;6(9):e25448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scordalakes EM, Rissman EF. Aggression and arginine vasopressin immunoreactivity regulation by androgen receptor and estrogen receptor α. Genes Brain Behav. 2004;3(1):20–26. [DOI] [PubMed] [Google Scholar]
- 21.Franklin K, Paxinos G. The Mouse Brain in Stereotaxic Coordinates. 3rd ed. San Diego: Academic Press; 2008. [Google Scholar]
- 22.Kudwa AE, Harada N, Honda SI, Rissman EF. Regulation of progestin receptors in medial amygdala: estradiol, phytoestrogens and sex. Physiol Behav. 2009;97(2):146–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rissman EF, Adli M. Minireview: transgenerational epigenetic inheritance: focus on endocrine disrupting compounds. Endocrinology. 2014;155(8):2770–2780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kundakovic M, Gudsnuk K, Franks B, Madrid J, Miller RL, Perera FP, Champagne FA. Sex-specific epigenetic disruption and behavioral changes following low-dose in utero bisphenol A exposure. Proc Natl Acad Sci USA. 2013;110(24):9956–9961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ziv-Gal A, Wang W, Zhou C, Flaws JA. The effects of in utero bisphenol A exposure on reproductive capacity in several generations of mice. Toxicol Appl Pharmacol. 2015;284(3):354–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Berger A, Ziv-Gal A, Cudiamat J, Wang W, Zhou C, Flaws JA. The effects of in utero bisphenol A exposure on the ovaries in multiple generations of mice. Reprod Toxicol. 2016;60:39–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Simerly RB. Wired for reproduction: organization and development of sexually dimorphic circuits in the mammalian forebrain. Annu Rev Neurosci. 2002;25:507–536. [DOI] [PubMed] [Google Scholar]
- 28.DeVries GJ, Buijs RM, Van Leeuwen FW, Caffé AR, Swaab DF. The vasopressinergic innervation of the brain in normal and castrated rats. J Comp Neurol. 1985;233(2):236–254. [DOI] [PubMed] [Google Scholar]
- 29.Kelly DA, Varnum MM, Krentzel AA, Krug S, Forger NG. Differential control of sex differences in estrogen receptor α in the bed nucleus of the stria terminalis and anteroventral periventricular nucleus. Endocrinology. 2013;154(10):3836–3846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Han TM, De Vries GJ. Organizational effects of testosterone, estradiol, and dihydrotestosterone on vasopressin mRNA expression in the bed nucleus of the stria terminalis. J Neurobiol. 2003;54(3):502–510. [DOI] [PubMed] [Google Scholar]
- 31.Wolstenholme JT, Goldsby JA, Rissman EF. Transgenerational effects of prenatal bisphenol A on social recognition. Horm Behav. 2013;64(5):833–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cao J, Mickens JA, McCaffrey KA, Leyrer SM, Patisaul HB. Neonatal Bisphenol A exposure alters sexually dimorphic gene expression in the postnatal rat hypothalamus. Neurotoxicology. 2012;33(1):23–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Patisaul HB, Fortino AE, Polston EK. Neonatal genistein or bisphenol-A exposure alters sexual differentiation of the AVPV. Neurotoxicol Teratol. 2006;28(1):111–118. [DOI] [PubMed] [Google Scholar]
- 34.Gioiosa L, Parmigiani S, Vom Saal FS, Palanza P. The effects of bisphenol A on emotional behavior depend upon the timing of exposure, age and gender in mice. Horm Behav. 2013;63(4):598–605. [DOI] [PubMed] [Google Scholar]
- 35.Negishi T, Nakagami A, Kawasaki K, Nishida Y, Ihara T, Kuroda Y, Tashiro T, Koyama T, Yoshikawa Y. Altered social interactions in male juvenile cynomolgus monkeys prenatally exposed to bisphenol A. Neurotoxicol Teratol. 2014;44:46–52. [DOI] [PubMed] [Google Scholar]
- 36.Palanza P, Gioiosa L, vom Saal FS, Parmigiani S. Effects of developmental exposure to bisphenol A on brain and behavior in mice. Environ Res. 2008;108(2):150–157. [DOI] [PubMed] [Google Scholar]
- 37.Naulé L, Picot M, Martini M, Parmentier C, Hardin-Pouzet H, Keller M, Franceschini I, Mhaouty-Kodja S. Neuroendocrine and behavioral effects of maternal exposure to oral bisphenol A in female mice. J Endocrinol. 2014;220(3):375–388. [DOI] [PubMed] [Google Scholar]