Abstract
17β-estradiol (E2) regulates central and peripheral mechanisms that control energy and glucose homeostasis predominantly through estrogen receptor α (ERα) acting via receptor binding to estrogen response elements (EREs). ERα signaling is also involved in mediating the effects of E2 on diet-induced obesity (DIO), although the roles of ERE-dependent and -independent ERα signaling in reducing the effects of DIO remain largely unknown. We hypothesize that ERE-dependent ERα signaling is necessary to ameliorate the effects of DIO. We addressed this question using ERα knockout (KO) and ERα knockin/knockout (KIKO) female mice, the latter expressing an ERα that lacks a functional ERE binding domain. Female mice were ovariectomized, fed a low-fat diet (LFD) or a high-fat diet (HFD), and orally dosed with vehicle or estradiol benzoate (EB) (300 μg/kg). After 9 weeks, body composition, glucose and insulin tolerance, peptide hormone and inflammatory cytokine levels, and hypothalamic arcuate nucleus and liver gene expression were assessed. EB reduced body weight and body fat in wild-type (WT) female mice, regardless of diet, and in HFD-fed KIKO female mice, in part by reducing energy intake and feeding efficiency. EB reduced fasting glucose levels in KIKO mice fed both diets but augmented glucose tolerance only in HFD-fed KIKO female mice. Plasma insulin and interleukin 6 were elevated in KIKO and KO female mice compared with LFD-fed WT female mice. Expression of arcuate neuropeptide and receptor genes and liver fatty acid biosynthesis genes was altered by HFD and by EB through ERE-dependent and -independent mechanisms. Therefore, ERE-independent signaling mechanisms in both the brain and peripheral organs mediate, in part, the effects of E2 during DIO.
Obesity is a growing global health concern that is due, in part, to changes in diet and lifestyle (1). Postmenopausal women are more susceptible to obesity and its associated diseases (e.g., cardiovascular disease, type II diabetes, and metabolic syndrome) than premenopausal women due, in part, to a reduction in circulating 17β-estradiol (E2) (2–5). During the menstrual cycle in humans and primates, feeding behavior varies with a periovulatory nadir and a luteal phase peak in food consumption, illustrating E2’s effects (6–9). Consequently, the decrease in circulating estrogens due to menopause is associated with positive weight gain in humans and primates (10) leading to an increased risk for the associated diseases (2–5). Many of these effects of menopause can be ameliorated by hormone replacement therapy (11).
The primary receptor mediating E2’s effects on energy homeostasis is estrogen receptor α (ERα). ERα knockout (KO) female mice exhibit an obese phenotype with increased visceral adiposity, decreased energy expenditure, altered glucose homeostasis, and insulin resistance (12–14). ERα is also necessary for the attenuation of body weight gain after ovariectomy (OVX, surgical menopause) (13, 15). Interestingly, the restoration of estrogen response element (ERE)–independent ERα signaling normalizes energy balance in KO female mice (16). Female mice expressing an ERα that lacks the ability to bind to ERE due to mutations in the DNA-binding domain, called ERα knockin/knockout (KIKO), do not become obese like their KO counterparts (16). Using the KO and KIKO mouse models, we have previously shown that ERE-independent ERα signaling is not sufficient to suppress postovariectomy body weight gain and fat accumulation or augment oxygen consumption and anorexigenic neuropeptide signaling in chow-fed female mice (17).
Peripheral expression of ERα in the liver, adipose tissue, skeletal muscle, and the pancreas mediates E2’s actions in metabolism and glucose homeostasis (18, 19). E2 reduces insulin resistance induced by a high-fat diet (HFD) and improves insulin signaling in skeletal muscles through an ERα-mediated mechanism yet elevates inflammatory cytokines [interleukin 6 (IL-6), tumor necrosis factor (TNF)-α] in the plasma in HFD-fed OVX female mice (20). E2 replacement in HFD-fed OVX female mice also restores oxygen consumption and improves glucose homeostasis and insulin sensitivity (21). In HFD-fed OVX female mice, E2 suppresses expression of a number of hepatic lipogenic and gluconeogenic genes, including glucose 6-phosphatase (G6pase), stearoyl-CoA desaturase (Scd1), and peroxisome proliferator-activated receptor γ (Pparγ) (22). Although the ERα-mediated signaling mechanisms involved in these effects on liver function are not clearly understood, analysis of E2-induced regulation of the liver transcriptome suggests that the DNA binding domain of ERα is necessary to control liver gene expression (23). However, it is unknown if E2 requires a functional DNA-binding domain to reduce the effect of diet-induced obesity (DIO) in OVX female mice. Therefore, we hypothesize that ERE-dependent ERα signaling is necessary to ameliorate the effects of DIO on body weight, adiposity, glucose homeostasis, and gene expression in the arcuate nucleus (ARC) of the hypothalamus and the liver.
Materials and Methods
Animals
All animal treatments were in accordance with institutional guidelines based on National Institutes of Health standards and were performed with approval from the Rutgers University Institutional Animal Care and Use Committee. Female wild-type (WT C57BL/6J), ERαKO (KO), and ERαKIKO (KIKO) transgenic mice (provided by Dr. Ken Korach, National Institute of Environmental Health Sciences) (24, 25) were selectively bred in-house and maintained under controlled temperature (23°C) and photoperiod conditions (12/12-hour light/dark cycle) with food and water ad libitum. WT/KO heterozygous male and female mice were mated to produce ERαKO female mice. Nonclassical ERα knockin heterozygous male mice (WT/KI) and WT/KO heterozygous female mice were crossed to generate ERαKIKO female mice. WT female mice were generated from both colonies and used with their KIKO and KO littermates. At weaning, female mice were tagged and ear-clipped for genotyping. Genotype was determined by polymerase chain reaction (PCR) of extracted DNA using previously published protocols (24, 25).
Drugs and diets
Estradiol benzoate (EB) and 4-(2-aminoethyl)benzenesulfonyl fluoride hydrochloride were purchased from Sigma-Aldrich (St. Louis, MO). Ketamine, Marcaine, and Rimadyl were purchased from Henry Schein Animal Health (Dublin, OH). EB was dissolved in 100% ethanol (Sigma) prior to dissolving in sesame oil (Sigma). Diets were purchased from Research Diets (New Brunswick, NJ): low-fat diet (LFD) (10% kcal fat; D12450B) and HFD (45% kcal fat; D12451).
Experimental design
Our study used a postmenopausal mouse model with or without E2 replacement to mimic hormone replacement therapy in women. Adult female mice (8 to 10 weeks old) were OVX via a single ventral incision under isoflurane anesthesia [2% in O2/N2O (2:1)] delivered by face mask with a local injection of Marcaine (2 mg/kg) followed by 48 hours of pain management using an injection of Rimadyl (4 mg/kg, every 24 hours; Henry Schein). Female mice from each genotype were fed a LFD ad libitum (n = 20) or a HFD (n = 20) for 9 weeks. Half of each diet group was administered sesame oil or EB (300 μg/kg, in sesame oil) daily perorally via a peanut butter carrier [all groups (LFD-oil, LFD-EB, HFD-oil, HFD-EB): n = 10, except for KIKO LFD-oil and HFD-EB: n = 9] (26). Average age for each genotype was 9.9 ± 0.2 weeks (n = 40) for WT, 8.2 ± 0.1 weeks (n = 38) for KIKO, and 8.5 ± 0.1 weeks (n = 40) for KO female mice. We chose peroral EB replacement for the DIO studies to reduce the stress-inducing effects of repeated injections and to maintain a constant systemic level of E2 in the blood (27). Female mice were housed in genotype-matched pairs, and body weight and food intake were measured weekly. At the end of 9 weeks, body composition was measured using an EchoMRI 3-in-1 Body Composition Analyzer (Echo Medical Systems, Houston, TX). A glucose tolerance test (GTT) was performed on each female mouse. Female mice were fasted overnight (1700 hours to 0900 hours) in a new cage. At the start of the test and 30 minutes after application of a local anesthetic (Lidocaine; Henry Schein) to the tail, mice were placed in Plexiglass restrainers and tails were nicked to collect a baseline (time 0) glucose reading using a glucometer (AlphaTRAK2). Immediately after baseline reading, female mice were injected intraperitoneally (IP) with a bolus of glucose (2.0 g/kg body weight) and placed back individually into clean cages with no food or water. Tail blood samples were collected at 15, 30, 60, 90, 120, and 180 minutes postinjection. After sufficient recovery (∼3 to 4 days), an insulin tolerance test (ITT) was performed after a 5-hour fast (900 hours to 1400 hours) in a similar manner as the GTT with an IP injection of insulin (0.75 units/kg). Tail blood samples were collected in individual cages at 15, 30, 60, 90, and 120 minutes postinjection. After each test, each mouse was returned to its home cage with its original cage mate with ad libitum access to water and food. Experimenters were blind to the experimental treatment groups (28, 29).
Brain and body dissections
After sufficient recovery from the ITT (∼1 week), female mice were dosed with EB at 0900 hours and decapitated after sedation with ketamine (100 µL of 100 mg/mL, IP) at 1000 hours. Food was removed at the last dosing. Trunk blood was collected in a K+ EDTA collection tube. Plasma was prepared for analysis of peptide hormone and inflammatory cytokine by adding the protease inhibitor 4-(2-aminoethyl)benzenesulfonyl fluoride hydrochloride (1 mg/mL) to each collection tube. Samples were maintained on ice until centrifugation at 3000 rpm for 10 minutes at 4°C. Plasma was stored at –80°C until analysis. Ghrelin (active), insulin, leptin, monocyte chemoattractant protein 1 (MCP-1), and TNF-α were determined by multiplex assay (#MMHMAG-44K; EMD Millipore, Billerica, MA). Total plasma E2 levels were measured using Mouse/Rat Estradiol ELISA kit (ES180S-100; Calbiotech, El Cajon, CA).
Abdominal cavity was dissected for liver tissue (secondary lobe) and the uterus. Each uterus was weighed (wet weight). Liver tissue was fixed in RNAlater (Life Technologies, Carlsbad, CA) and stored at –80°C. Liver RNA was extracted using a standard TRIzol extraction (Life Technologies) coupled with NucleoSpin RNA extraction and DNase-I kit (Macherey-Nagel, Bethlehem, PA). The brain was immediately extracted from the skull and rinsed in ice-cold Sorensen’s buffer for 30 seconds. The brain was cut using a brain matrix (Ted Pella, Redding, CA) into 1-mm-thick coronal rostral and caudal blocks corresponding to plates 42 to 47 and plates 48 to 53, respectively, from The Mouse Brain in Stereotaxic Coordinates (30). Blocks of the basal hypothalamus (BH) were transferred to RNAlater (Life Technologies) and stored overnight at 4°C. The rostral and caudal parts of the ARC were dissected from slices using a dissecting microscope. Dissected tissue was stored at –80°C. Total RNA was extracted from the combined nucleus (rostral and caudal arcuate) using Ambion RNAqueous-Micro Kits (Life Technologies) according to the manufacturer’s protocol. Total RNA was also DNase I-treated, using the extraction kits, at 37°C for 30 minutes to minimize genomic DNA contamination. Liver and arcuate RNA quantity and quality were determined using a NanoDrop ND-2000 spectrophotometer (Thermo Fisher Scientific, Waltham, MA) and an Agilent 2100 Bioanalyzer with RNA 6000 Nano kits (Agilent Technologies, Santa Clara, CA). Only samples with RNA integrity number >8 were used in the analysis of gene expression.
Quantitative real-time PCR
Complementary DNA (cDNA) was synthesized from 500 ng of total liver RNA and 200 ng of total ARC RNA using Superscript III reverse transcription (Life Technologies), 4 μL 5× buffer, 25 mM MgCl2, 10 mM dNTP (Clontech Laboratories, Mountain View, CA), 100 ng random hexamer primers (Promega, Madison, WI), 40 U/μL Rnasin (Promega), and 100 mM dithiothreitol in diethylpyrocarbonate-treated water (Bioexpress, Kaysville, UT) in a total volume of 20 μl. Reverse transcription was conducted using the following protocol: 5 minutes at 25°C, 60 minutes at 50°C, and 15 minutes at 70°C. The cDNA was diluted to 1:20 with nuclease-free water (Bioexpress) for a final cDNA concentration of 1.25 ng/μl for liver and 0.5 ng/μl for ARC and stored at –20°C. Untreated liver and BH test tissue RNA was used for the calibrator and negative control (no reverse transcription) and processed simultaneously with the experimental samples.
All primers were designed to span exon-exon junctions and synthesized by Life Technologies using Clone Manager 5 software (Sci Ed Software, Denver, CO). Supplemental Table 1 (28.3KB, docx) provides a list of all the primer sequences used for quantitative real-time PCR. For quantitative PCR, 4 μl of cDNA template was amplified using either PowerSYBR Green (Life Technologies) or Sso Advanced SYBR Green (BioRad, Hercules, CA) on CFX-Connect Real-time PCR instrument (BioRad). Standard curves for each primer pair were prepared using serial dilutions of liver or BH cDNA in triplicate to determine the efficiency [E = 10(−1/m) – 1; m = slope] of each primer pair. All efficiencies, expressed as percent efficiency, were approximately equal at 1 doubling per cycle (90% to 110%). The relative mRNA expression was calculated using the ΔΔCQ method with diluted (1:20) cDNA from liver or BH of an untreated male mouse used as a calibrator. The amplification protocol for all the genes was as follows: initial denaturing at 95°C for 10 minutes (PowerSYBR) or 3 minutes (SsoAdvanced) followed by 40 cycles of amplification at 94°C for 10 seconds (denaturing), 60°C for 45 seconds (annealing), and completed with a dissociation step for melting point analysis with 60 cycles of 95°C for 10 seconds, 65°C to 95°C (in increments of 0.5°C) for 5 seconds, and 95°C for 5 seconds. The geometric mean of the reference genes Actb (β-actin), Hprt (hypoxanthine-guanine phosphoribosyltransferase), and Gapdh (glyceraldehyde-3-phosphate dehydrogenase) was used to calculate ΔCQ values. Calibrator, negative controls, and water blank were added to each plate. Quantification values were generated only from samples showing a single product at the expected melting point. All gene expression data are expressed as an n-fold difference relative to the calibrator (31, 32).
Statistical analysis
All data are expressed as mean ± SEM. All data were analyzed using a multifactorial (steroid, diet, genotype) analysis of variance (ANOVA) followed by a post hoc Newman-Keuls test using Statistica 7.1 software (StatSoft, Tulsa, OK). Cumulative weight gain, GTT, and ITT data were analyzed using repeated-measures, 2-way ANOVA with a post hoc Newman-Keuls test. All post hoc tests compared differences within genotype between treatments for diet and steroid effects and compared differences across genotypes within the same diet × steroid treatment. All gene expression data were analyzed using 2-way ANOVA with a post hoc Newman-Keuls test within each genotype because expression was normalized to the LFD-Oil samples of each genotype. All ANOVA statistics are reported in Supplemental Tables 2, 3, and 4 (28.3KB, docx) . In all experiments, effects were considered significant at α ≤ 0.05.
Results
Body weight and body composition
On the day of surgery, intact WT female mice weighed 19.8 ± 0.1 g (n = 40), intact KIKO female mice weighed 18.6 ± 0.2 g (n = 38), and intact KO female mice weighed 20.6 ± 0.2 g (n = 40). KO female mice weighed more than WT (P < 0.01) or KIKO female mice (P < 0.0001). For cumulative body weight, EB suppressed post-OVX weight gain in both LFD-fed and HFD-fed WT female mice [Fig. 1(A)]. The attenuation of body weight was significant by week 6 (P < 0.01) in the LFD-fed female mice and by week 4 (P < 0.01) in the HFD-fed female mice. In KIKO female mice, EB suppressed post-OVX weight gain only in HFD-fed female mice [Fig. 1(B)]. The attenuation of body weight was significant initially by week 4 (P < 0.05) and then continuously from week 6 to week 9 (P < 0.05) in the HFD-fed female mice. In KO female mice, EB did not suppress post-OVX weight gain in either LFD-fed or HFD-fed groups [Fig. 1(C)].
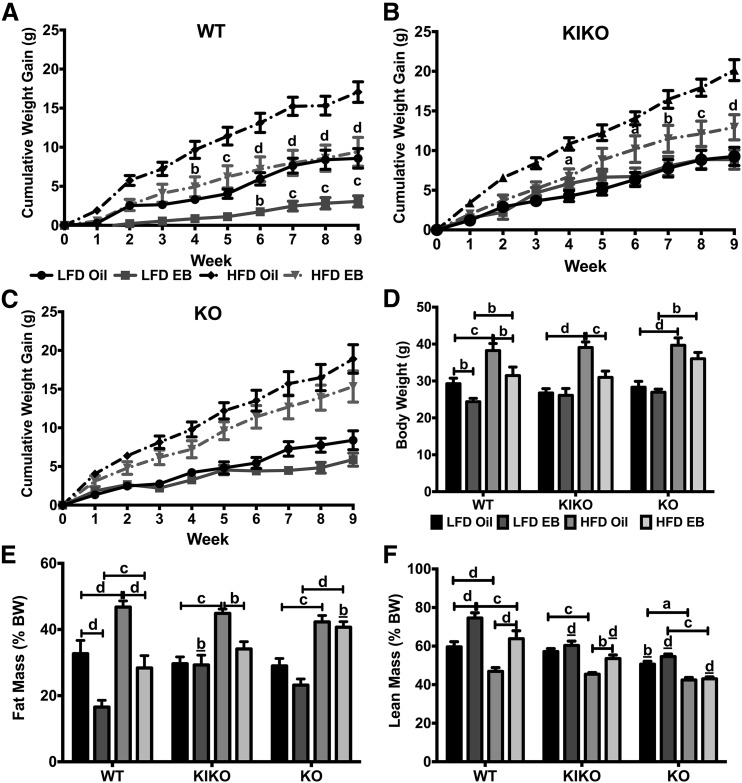
Figure 1.
Cumulative body weight gain and body fat accumulation were reduced in WT and HFD-fed KIKO female mice by EB. (A) WT. (B) KIKO. (C) KO. Cumulative body weight gain (g). (D) Week 9 body weights (g). (E) Percent body fat (fat mass/body weight). (F) Percent lean mass (lean mass/body weight). Data were analyzed by multifactorial ANOVA with post hoc Newman-Keuls test. Sample sizes were 9 to 10 per group. For A, B, and C, letters denote comparisons between oil and EB within the same diet. For D, E, and F, capped lines denote comparisons within genotype between steroid (oil vs EB) and diet (LFD vs HFD) combinations, and underlined letters denote comparisons between WT and either KIKO or KO within the same treatment combination (a, P < 0.05; b, P < 0.01; c, P < 0.001; d, P < 0.0001).
After 9 weeks, oil-treated HFD-fed female mice from all genotypes weighed more than their LFD-fed counterparts (WT: P < 0.001; KIKO: P < 0.0001; KO: P < 0.0001) [Fig. 1(D)]. EB suppressed body weight in WT and HFD-fed KIKO female mice compared with their oil-treated counterparts. LFD-oil WT female mice (n = 10) weighed 29.3 ± 1.5 g, and LFD-EB WT mice (n = 10) weighed 24.4 ± 0.9 g (P < 0.01). HFD-oil WT female mice (n = 10) weighed 38.2 ± 1.9 g, and HFD-EB WT female mice (n = 10) weighed 31.5 ± 2.3 g (P < 0.01). LFD-oil KIKO female mice (n = 9) weighed 26.8 ± 1.2 g, and LFD-EB KIKO female mice (n = 10) weighed 26.1 ± 1.8 g (not significant). HFD-oil KIKO (n = 10) weighed 39.1 ± 1.5 g and HFD-EB KIKO (n = 9) weighed 30.9 ± 1.7 g (P < 0.001). LFD-oil KO female mice (n = 10) weighed 28.3 ± 1.6 g, and LFD-EB KO mice (n = 10) weighed 26.9 ± 0.8 g (not significant). HFD-oil KO female mice (n = 10) weighed 39.7 ± 2.0 g, and HFD-EB KO female mice (n = 10) weighed 36.0 ± 1.7 g (not significant).
Body fat accumulation (% of body weight) was affected by both steroid treatment and diet. As previously reported (17), body fat accumulation after 9 weeks in the LFD-fed WT female mice was reduced by EB treatment (P < 0.0001) [Fig. 1(E)]. EB also reduced fat accumulation in HFD-fed WT female mice (P < 0.0001), although between EB-treated female mice, fat accumulation was higher in HFD-fed than LFD-fed WT female mice (P < 0.001). Among KIKO female mice, HFD increased body fat accumulation in oil-treated female mice (P < 0.001), which was abrogated by EB treatment (P < 0.01). Interestingly, percent body fat in LFD-fed KIKO female mice was higher than in LFD-fed WT mice (P < 0.01). There was no effect of EB on fat mass in KO female mice, regardless of diet, although HFD increased body fat accumulation in KO female mice (oil: P < 0.001; EB: P < 0.0001). HFD-EB KO female mice were also fatter than HFD-EB WT female mice (P < 0.01).
Lean mass (% of body weight) was also affected by steroid, diet, and genotype. As expected, HFD reduced lean mass in oil-treated (P < 0.0001) and EB-treated (P < 0.001) WT female mice [Fig. 1(F)]. Lean mass was elevated in EB-treated WT female mice compared with oil-treated WT female mice (LFD: P < 0.0001; HFD: P < 0.0001). Similar to WT female mice, EB treatment increased lean mass but only in HFD-fed KIKO female mice (P < 0.01). In KO female mice, HFD reduced lean mass, regardless of steroid treatment (oil: P < 0.05; EB: P < 0.001). KIKO and KO female mice had less lean mass compared with WT female mice when orally dosed with EB (P < 0.0001).
Food intake and efficacy of oral EB treatment
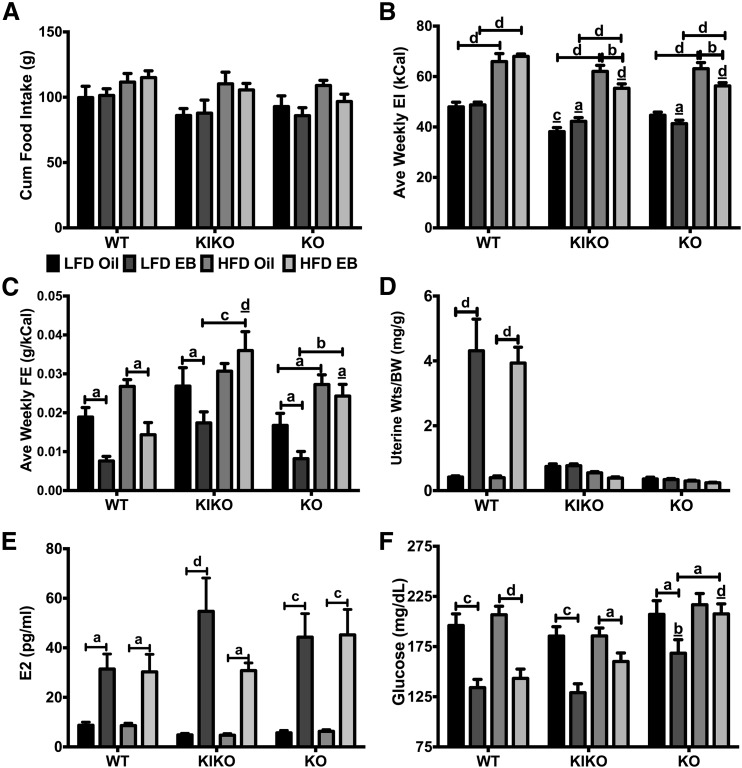
Cumulative amount of food consumed per pair (each cage, n = 5/group) from week 2 to week 9 [Fig. 2(A)], not including week 1 during recovery from surgery, was not altered by steroid or diet in any genotype. However, when analyzed as average weekly food intake (data not shown), HFD-fed WT, KIKO, and KO female mice consumed more food than their LFD-fed counterparts except for EB-treated KIKO female mice. As previously reported (17), KIKO and KO female mice consumed less food than WT female mice except for HFD-fed, oil-treated female mice. A similar pattern was found when analyzed as average weekly energy intake (kCal) [Fig. 2(B)]. HFD increased energy intake in all genotypes. EB reduced weekly energy intake in HFD-fed KIKO and KO female mice (both P < 0.01) but not in HFD-fed WT female mice. Feeding efficiency (g gained/kCal consumed) is an indirect measure of metabolism. In our study, EB reduced feeding efficiency in LFD- and HFD-fed WT female mice (both P < 0.05) and in LFD-fed KIKO (P < 0.05) and KO (P < 0.05) female mice [Fig. 2(C)]. HFD elevated feeding efficiency in EB-treated KIKO (P < 0.001) and KO female mice (P < 0.01) compared with LFD-fed counterparts.
Figure 2.
EB reduced feeding efficiency and fasting glucose in WT and LFD-fed KIKO and KO female mice. (A) Cumulative food intake (g). (B) Average weekly energy intake (EI; kCal). (C) Average weekly feeding efficiency (FE; g gained/kCal consumed). For A, B, and C, all food intake data were per cage (n = 5). (D) Uterine weights normalized to body weights (mg/g). (E) Plasma E2 levels (pg/mL). (F) Fasting glucose levels (mg/dL). Data were analyzed by multifactorial ANOVA with post hoc Newman-Keuls test. For D, E, and F, sample sizes were 9 to 10 per group. Capped lines denote comparisons within genotype between steroid (oil vs EB) and diet (LFD vs HFD) combinations, and underlined letters denote comparisons between WT and either KIKO or KO within the same treatment combination (a, P < 0.05; b, P < 0.01; c, P < 0.001; d, P < 0.0001). BW, body weight; Wts, weights.
Uterine wet weight was also measured to confirm the hypertrophic actions of EB, an ERα-mediated process (33). Past studies in our laboratory have found that subcutaneous injection of EB (250 ng/dose, every other day) for 4 weeks significantly increased the uterine weight in WT female mice (17). In the current study, EB increased the uterine weight in both LFD- and HFD-fed WT female mice (both P < 0.0001) [Fig. 2(D)] but did not increase uterine weight in KIKO and KO female mice. Plasma E2 levels were higher in all the EB-treated female mice regardless of genotype or diet [Fig. 2(E)].
Glucose and insulin tolerance
To determine the interactions of EB and HFD on glucose homeostasis, we conducted glucose and insulin tolerance tests on all female mice. For the GTT, all mice were fasted overnight (1700 hours to 0900 hours). Fasting glucose levels were suppressed by EB in all genotypes, regardless of diet, except for HFD-fed KO female mice [Fig. 2(F)]. EB-treated KO female mice fed a LFD (P < 0.01) or a HFD (P < 0.0001) had elevated glucose levels compared with their WT and KIKO counterparts.
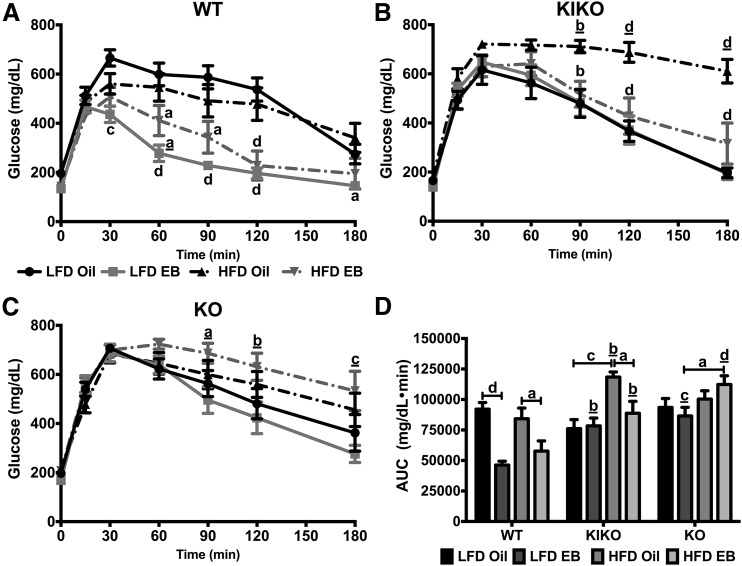
Glucose tolerance was determined over 180 minutes after an IP injection of glucose (2 g/kg). Among WT female mice, EB augmented glucose tolerance in both LFD- and HFD-fed female mice [Fig. 3(A)]. Among KIKO female mice, HFD reduced glucose clearance in oil-treated KIKO female mice, with EB augmenting glucose clearance in HFD-fed female mice [Fig. 3(B)]. In EB-treated KO female mice, HFD reduced glucose tolerance [Fig. 3(C)]. The effects of genotype, diet, and steroid on glucose tolerance are further illustrated in Fig. 3(D), including an effect of genotype where EB-treated KIKO and KO female mice exhibited slower glucose clearance compared with EB-treated WT female mice (P < 0.01, P < 0.01, P < 0.001, and P < 0.0001, respectively).
Figure 3.
Glucose tolerance after glucose injection was increased by EB in WT and HFD-fed KIKO female mice. GTT data from (A) WT, (B) KIKO, and (C) KO female mice. Data were analyzed by repeated-measures, 2-way ANOVA with post hoc Newman-Keuls test. (D) Area under the curve analysis (mg/dL/min). Data were analyzed by a multifactorial ANOVA with post hoc Newman-Keuls test. Sample sizes were 9 to 10 per group. For A, B, and C, letters denote an effect of steroid and underlined letters denote an effect of diet. For D, capped lines denote comparisons within genotype between steroid (oil vs EB) and diet (LFD vs HFD) combinations, and underlined letters denote comparisons between WT and either KIKO or KO within the same treatment combination (a, P < 0.05; b, P < 0.01; c, P < 0.001; d, P < 0.0001).
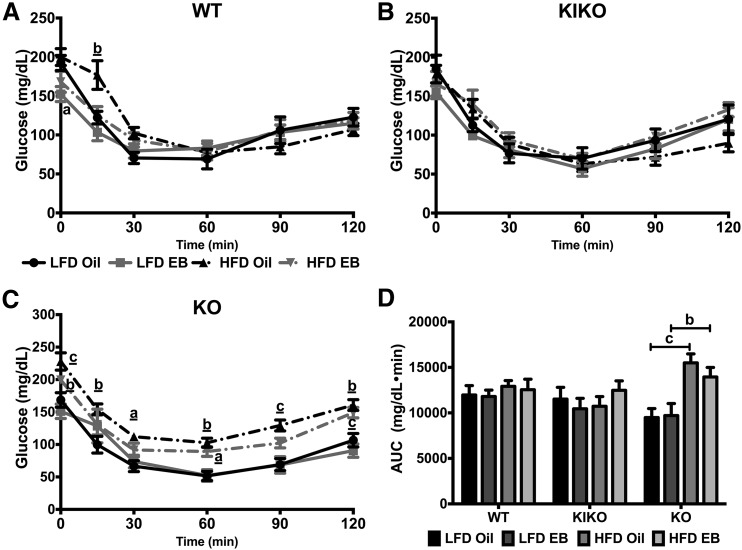
Insulin tolerance was measured over 120 minutes after an IP injection of insulin. There were no effects of diet or steroid in WT or KIKO female mice [Fig. 4(A) and 4(B)]. HFD decreased glucose clearance at 15 minutes in oil-treated WT female mice (P < 0.01) but did not affect insulin tolerance in KIKO female mice. HFD reduced the response to insulin in oil-treated KO female mice at all time points and in EB-treated KO female mice at 0, 60, and 120 minutes [Fig. 4(C)]. The effects of diet on insulin tolerance are further illustrated in Fig. 4(D) especially the effect of HFD in KO female mice.
Figure 4.
Glucose clearance after insulin injection was not affected by diet or EB in WT and KIKO female mice. ITT from (A) WT, (B) KIKO, and (C) KO female mice. Data were analyzed by repeated-measures, 2-way ANOVA with post hoc Newman-Keuls test. (D) Area under the curve (AUC) analysis (mg/dL/min). Data were analyzed by a multifactorial ANOVA with post hoc Newman-Keuls test. Sample sizes were 9 to 10 per group. For A, B, and C, letters denote an effect of steroid and underlined letters denote an effect of diet. For D, capped lines denote comparisons within genotype between steroid (oil vs EB) and diet (LFD vs HFD) combinations, and underlined letters denote comparisons between WT and either KIKO or KO within the same treatment combination (a, P < 0.05; b, P < 0.01; c, P < 0.001; d, P < 0.0001).
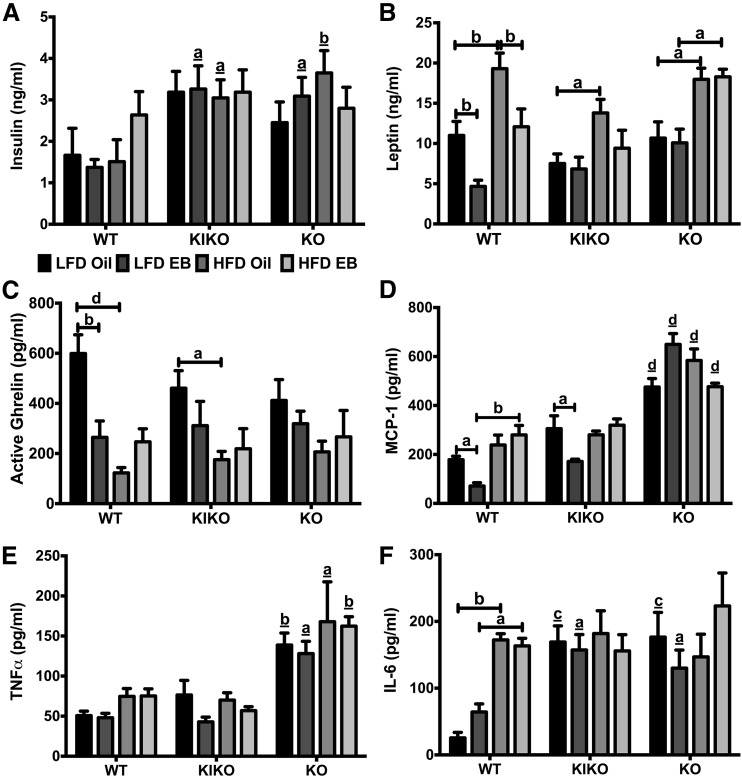
Peripheral peptide hormones and inflammatory cytokines
To determine the effects of diet and EB on peptide hormones and inflammatory cytokines, we analyzed plasma samples using multiplex assays. Plasma insulin levels were elevated in LFD-EB and HFD-Oil KIKO and KO female mice compared with WT [Fig. 5(A)], with those groups expressing two to three times as much insulin. HFD elevated plasma leptin levels in oil-treated WT (P < 0.01), KIKO (P < 0.05), and KO (P < 0.05) female mice and in EB-treated KO female mice (P < 0.05) [Fig. 5(B)]. EB suppressed leptin in both LFD-fed (P < 0.01) and HFD-fed (P < 0.01) WT female mice but had no effect in KIKO or KO female mice. Ghrelin (active), an orexigenic peptide hormone, was suppressed by both EB (P < 0.01) and HFD (P < 0.0001) in WT and by HFD (P < 0.05) in KIKO female mice [Fig. 5(C)].
Figure 5.
Regulation of peripheral peptide hormones and inflammatory cytokines by EB and HFD. (A) Plasma insulin levels (ng/mL). (B) Plasma leptin levels (ng/mL). (C) Plasma ghrelin levels (pg/mL). (D) Plasma MCP-1 levels (pg/mL). (E) Plasma TNF-α levels (pg/mL). (F) Plasma IL-6 levels (pg/mL). Data were analyzed by a multifactorial ANOVA with post hoc Newman-Keuls test. Sample sizes were 9 to 10 per group. Capped lines denote comparisons within genotype between steroid (oil vs EB) and diet (LFD vs HFD) combinations, and underlined letters denote comparisons between WT and either KIKO or KO within the same treatment combination (a, P < 0.05; b, P < 0.01; c, P < 0.001; d, P < 0.0001).
The selected inflammatory cytokines—MCP-1, TNF-α, and IL-6—are all implicated in obesity (34). MCP-1 levels were suppressed by EB in the LFD-fed WT and KIKO female mice (both P < 0.05). HFD nullified EB’s effect in WT and KIKO female mice [Fig. 5(D)]. MCP-1 levels in KO female mice were elevated compared with their counterparts in both WT and KIKO female mice (P < 0.0001 for all comparisons). There was no effect of steroid or diet on TNF-α levels in any genotype [Fig. 5(E)]. However, TNF-α levels in KO female mice were elevated compared with WT and KIKO female mice in all groups (P < 0.01, P < 0.05, P < 0.05, and P < 0.01, respectively). Plasma IL-6 levels were elevated by HFD only in WT female mice, regardless of steroid (oil: P < 0.01; EB: P < 0.05), and were elevated in LFD-fed KIKO (oil: P < 0.001; EB: P < 0.05) and KO (oil: P < 0.001; EB: P < 0.05) female mice compared with WT female mice [Fig. 5(F)].
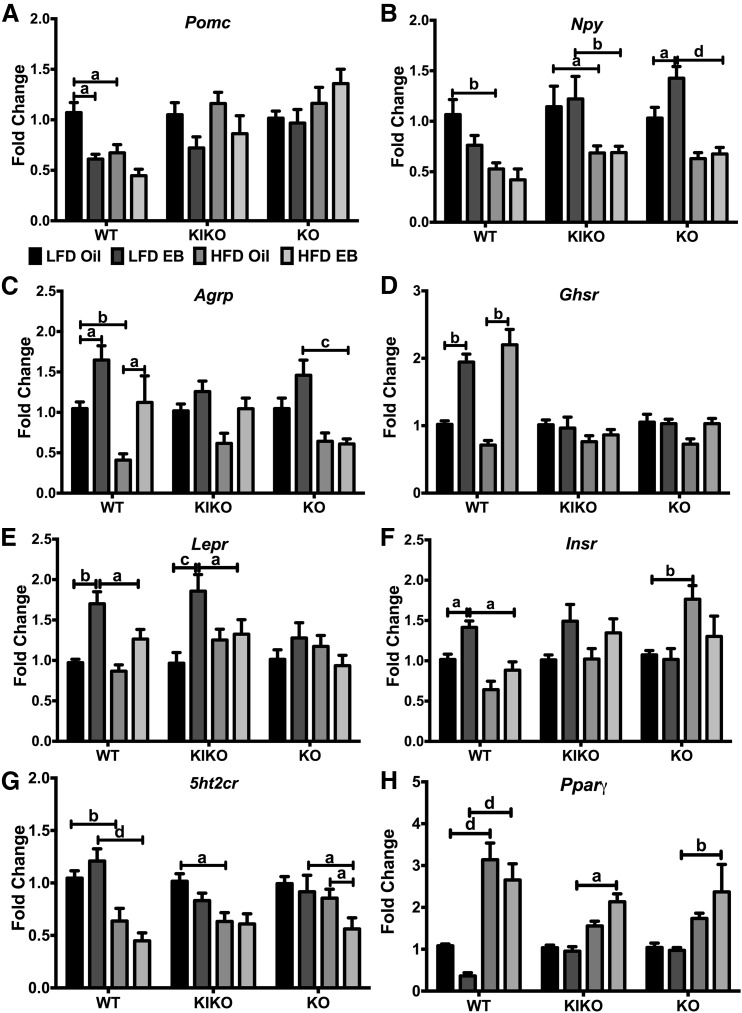
ARC and liver gene expression
To determine the interactions of EB and HFD within each genotype on ARC gene expression, we analyzed expression of neuropeptides and receptors for hormones, fatty acids, and neurotransmitters involved in energy balance (35, 36). For the anorectic neuropeptide Pomc (proopiomelanocortin), EB and HFD reduced expression only in WT female mice [Fig. 6(A)]. There was no effect of diet or steroid in any genotype on Cart (cocaine- and amphetamine-regulated transcript) expression (data not shown). For orexigenic Npy (neuropeptide Y), HFD suppressed expression in WT, KIKO, and KO female mice, and EB augmented Npy expression in LFD-fed KO female mice [Fig. 6(B)]. EB enhanced Agrp (agouti-related peptide) expression in WT, whereas HFD suppressed Agrp in WT and KO female mice [Fig. 6(C)].
Figure 6.
Regulation of arcuate neuropeptide and receptor genes by EB and HFD. (A) Pomc, (B) Npy, (C) Agrp, (D) Ghsr, (E) Lepr, (F) Insr, (G) 5ht2cr, and (H) Pparγ expression calculated within each genotype. Data were analyzed by a 2-way ANOVA with post hoc Newman-Keuls test within each genotype. Sample size was 8 per group. Capped lines denote comparisons within genotype between steroid (oil vs EB) and diet (LFD vs HFD) combinations (a, P < 0.05; b, P < 0.01; c, P < 0.001; d, P < 0.0001).
EB augmented the expression of Ghsr (ghrelin’s receptor) only in WT mice, regardless of diet [Fig. 6(D)]. In both LFD-fed WT and KIKO female mice, EB augmented Lepr (leptin receptor) expression, and HFD nullified this effect [Fig. 6(E)]. EB augmented expression of Insr (insulin receptor) in WT female mice, which was nullified by HFD [Fig. 6(F)], and HFD augmented Insr expression in oil-treated KO female mice. In all genotypes, HFD suppressed expression of 5ht2cr (serotonin 5HT2c receptor) [Fig. 6(G)] while augmenting the expression of the fatty acid receptor Pparγ (PPARγ) in all genotypes [Fig. 6(H)]. Expression of the ERα gene, Esr1, was suppressed by EB only in WT, with no effect of HFD, as has been previously reported (37) (data not shown).
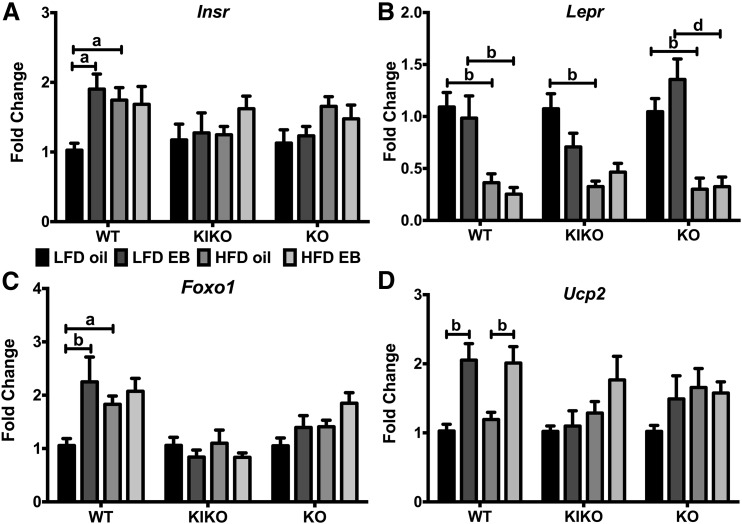
Previous studies have found that ERα DNA binding is required for E2 regulation of liver gene expression (23), including genes for receptors, signaling proteins, and enzymes that control glucose and triglyceride production, lipid biosynthesis, and fatty acid catabolism. In our study, Insr was augmented by EB in LFD-fed WT and by HFD in oil-treated WT [Fig. 7(A)]. Lepr expression was suppressed by HFD in WT, KIKO, and KO female mice [Fig. 7(B)]. Expression of Foxo1 (forkhead box O1), a transcription factor that mediates hepatic gluconeogenesis by glucagon and insulin (38), was augmented in LFD-fed WT female mice by EB and by HFD [Fig. 7(C)]. Mitochondrial Ucp2 (uncoupling protein 2) expression was augmented by EB in WT female mice, regardless of diet [Fig. 7(D)]. In KO female mice, HFD augmented Pepck (phosphoenolpyruvate carboxykinase) expression (data not shown). There were no effects of steroid or diet on G6pc (glucose 6-phosphatase), Dgat2c (diglyceride acyltransferase 2c), Pgc1α (peroxisome proliferator-activated receptor gamma coactivator 1α), Pparγ, or Esr1 expression in the liver in any genotype (data not shown).
Figure 7.
Regulation of receptor and signaling genes by EB and HFD in the liver. (A) Insr, (B) Lepr, (C) Foxo1, and (D) Ucp2 expression calculated within each genotype. Data were analyzed by a 2-way ANOVA with post hoc Newman-Keuls test within each genotype. Sample size was 8 per group. Capped lines denote comparisons within genotype between steroid (oil vs EB) and diet (LFD vs HFD) combinations (a, P < 0.05; b, P < 0.01; c, P < 0.001; d, P < 0.0001).
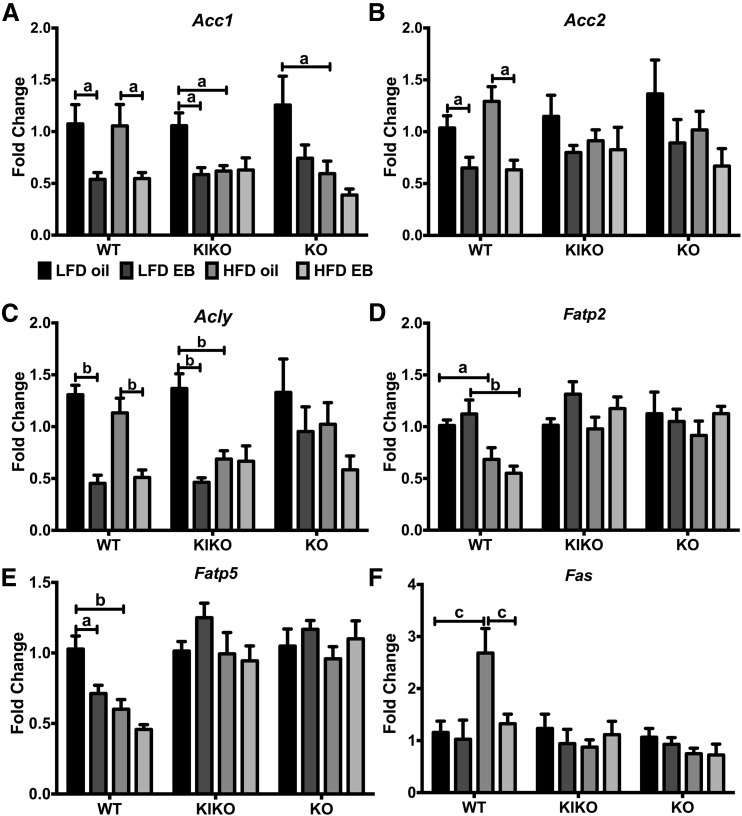
Genes involved in lipid biosynthesis and fatty acid catabolism, including Acc1, Acc2 (acetyl-CoA carboxylase1 and 2), Acly (ATP citrate lyase), Fatp2 (very long-chain acyl-CoA synthetase), Fatp5 (bile acyl-CoA synthetase), and Fas (fatty acid synthase), were analyzed. Expression of Acc1 was reduced by EB, regardless of diet, in WT and in LFD-fed KIKO female mice [Fig. 8(A)]. Acc1 was also reduced by HFD in oil-treated KIKO and KO female mice. In WT female mice, Acc2 expression was suppressed by EB, regardless of diet [Fig. 8(B)]. Likewise, Acly expression was reduced by EB, regardless of diet, in WT female mice and by EB and HFD in KIKO female mice [Fig. 8(C)]. Fatp2 expression was reduced by HFD [Fig. 8(D)], and Fatp5 was suppressed by EB and HFD only in WT female mice [Fig. 8(E)]. Fas was augmented by HFD in oil-treated WT and suppressed by EB in HFD-fed WT female mice [Fig. 8(F)].
Figure 8.
Regulation of liver genes involved fatty acid metabolism by EB and HFD. (A) Acc1, (B) Acc2, (C) Acly, (D) Fatp2, (E) Fatp5, and (F) Fas expression calculated within each genotype. Data were analyzed by a 2-way ANOVA with post hoc Newman-Keuls test within each genotype. Sample size was 8 per group. Capped lines denote comparisons within genotype between steroid (oil vs EB) and diet (LFD vs HFD) combinations (a, P < 0.05; b, P < 0.01; c, P < 0.001; d, P < 0.0001).
Discussion
E2 regulates central and peripheral mechanisms that control energy and glucose homeostasis and ameliorates the effects of DIO in OVX female mice predominantly through ERα binding to ERE. In the current study, we characterized the role of ERE-independent ERα signaling in controlling energy homeostasis and reducing the impact of DIO in a mouse postmenopausal model. We found that E2 reduced the effects of OVX and DIO on weight gain, adiposity, feeding efficiency, and glucose homeostasis (fasting glucose and tolerance) in WT female mice. As we previously reported (17), E2 did not reduce body weight and adiposity in LFD-fed KIKO but did reduce the effects of DIO in KIKO female mice. These diet-dependent effects in KIKO suggest that ERE-independent ERα signaling is activated by a constituent of a diet high in fatty acids and/or during states of positive energy balance.
Our study indicates that the DNA-binding domain, which recognizes the ERE promoter sequence, is not solely necessary to block many of the effects of DIO in female mice. Another transgenic ERα KO mouse model that lacks the AF-2 ligand-binding domain of ERα is similar to KO mice in adiposity and glucose tolerance and is more susceptible to DIO, which produces insulin resistance when compared with WT (39). Consequently, E2 replacement in HFD-fed OVX female mice requires the AF-2 domain to ameliorate the effects of DIO on energy and glucose homeostasis. That study and our current study, when considered together, demonstrate that ERE-independent, but not ligand-independent, ERα signaling is protective against the deleterious effects of DIO in female mice.
Another recent study characterized the activation of membrane-initiated E2 signaling in OVX female mice using an estrogen dendrimer conjugate (40). Unlike E2, the estrogen dendrimer conjugate did not abrogate the effects of HFD on glucose homeostasis or adiposity but did improve diet-induced hepatic steatosis, due to the suppression of fatty acid and triglyceride synthesis genes in the liver. When considered with our study, these data suggest that nuclear-initiated ERE-independent signaling is sufficient to control adiposity and glucose homeostasis, but membrane-initiated ERE-independent signaling is central to the control of liver lipogenesis by E2. Although the exact cellular pathways are unknown, these ERE-independent mechanisms, which include both nuclear- and membrane-initiated pathways, act in the hypothalamus, the liver, adipose tissue, and other peripheral organs (18, 19).
E2 signaling through ERα in the ARC is a central pathway for E2 to control feeding behavior (41), although activation of the Gq-mER expressed in POMC and NPY neurons also controls feeding behavior in rodents (42–44). In our study, weekly food and energy intake was reduced by EB in both HFD-fed KIKO and KO female mice, indicating that another ER (e.g., Gq-mER) (43) may compensate for ERα in controlling food intake in the KIKO and KO during DIO. Furthermore, our data suggest that a primary effect of ovariectomy in the WT and LFD-fed KIKO and KO female mice is a decrease in metabolism (as indicated by the decrease in feeding efficiency), which is improved by E2 replacement and corroborates our previous study (17).
The differential expression of ARC neuropeptides (Pomc, Npy, Agrp) across the genotypes did not directly correlate with the effects on food intake, which has been reported in mice (45), rats (46, 47), and guinea pigs (43, 48). However, another study found that differences in arcuate orexigenic gene expression between intact and OVX female mice is lost after long-term (∼5 weeks) ovariectomy despite observable effects on metabolism (e.g., increase in body weight) (49). Perhaps, the same compensatory mechanisms underlying arcuate gene expression are functional in our long-term study. Nevertheless, an increase in Agrp, which increases food intake by blocking α-melanocyte stimulating hormone at the melanocortin 3/4 receptors expressed in downstream hypothalamic neurons (50), is counterintuitive to E2’s well-characterized suppression of food intake in most rodent models (43, 51, 52). However, an increase in Agrp mRNA expression does not necessarily lead to an increase in AgRP peptide release, especially because E2 reduces the excitability of NPY/agouti-related protein neurons via an upregulation of the M-current (KNCQ channels) (53) and reduces NPY release in the paraventricular hypothalamus (PVH) (46).
The ARC nucleus is not the only brain region involved in E2’s control of feeding, energy expenditure, and activity. These brain regions include the ventromedial hypothalamus (VMH), the PVH, and discreet hindbrain nuclei, such as the nucleus tractus solitarius (54–57). For example, specific deletion of ERα in VMH SF-1 neurons in female mice decreases energy expenditure, activity, and heat production (41), and specific ERα knockdown by RNAi in the VMH of female rats induces a phenotype defined by obesity, hyperphagia, glucose intolerance, and reduced activity (energy expenditure) that is resistant to E2’s actions (55). In the nucleus tractus solitarius, E2 via ERα also augments the control of food intake (meal size) by cholecystokinin, a satiety hormone from the small intestine (13, 56). These key brain regions may compensate for the orexigenic profile of arcuate neuropeptide expression (EB suppressed Pomc and augmented Agrp) and may account for the lack of E2’s effect on feeding in WT female mice.
The lack of E2’s effects on food intake may also be due to the experimental design. In our study, female mice were housed in pairs to reduce the stress induced by single housing, to which KIKO and KO female mice are especially sensitive, as observed previously (17). Numerous other studies illustrating E2’s effect on food intake used either singly housed female mice and/or cages specifically designed to measure discreet feeding parameters (meal duration, frequency, size) (41, 52, 54, 55, 58). Although our paired-housing design reduced stress, it diminishes the sensitivity of our weekly food intake measurements. Furthermore, our study used daily doses of EB to maintain a continuous level of systemic E2 similar to hormone replacement therapy in postmenopausal women, whereas other studies have used cyclical (52), bidaily (17, 43, 48), or short-term (12 to 24 hours) E2 treatment (45, 59) to examine food intake and ARC neuropeptide expression.
In female rodents and premenopausal women, E2 controls the deposition of adipose tissue by increasing subcutaneous fat accumulation and decreasing visceral fat deposition, primarily through an ERα-mediated mechanism (60). In OVX rodents and postmenopausal women, the loss of E2 produces an increase in abdominal or visceral adiposity. In our study, fat mass was reduced by EB in WT and HFD-fed KIKO mice, suggesting that ERE-independent ERα signaling can rectify, in part, the effects of DIO on adiposity. However, recent evidence suggests that another membrane ER, GPER1, controls adiposity in female mice during DIO and may underlay some of the effects on adiposity found in the KIKO female mice (61).
E2 controls glucose metabolism primarily through ERα acting in the liver, adipose tissue, and skeletal muscle (18, 19). In our study, glucose clearance was reduced by HFD in both WT and KIKO female mice but was restored by EB. One of the potential mechanisms subject to E2’s actions is the regulation of glucose transporter type 4 (GLUT4) expression and activity in skeletal muscle. E2 augments GLUT4 expression and insulin-induced trafficking to the membrane (18, 62, 63), which may use both ERE-dependent and -independent signaling to regulate expression and activity. Although skeletal muscle GLUT4 expression is augmented by ERα activation in the extensor digitorum longus (63), the GLUT4 promoter lacks a consensus ERE, which suggests that this regulation is mediated through ERE-independent mechanisms. Our data reveal that these ERE-independent mechanisms are key to the restoration of normal KIKO glucose clearance (uptake) after DIO. Furthermore, the loss of ERE-dependent signaling diminishes E2’s control of glucose transport into skeletal muscle and/or adipose tissue and slows glucose clearance as illustrated by an elevated area under the curve in the LFD- and HFD-fed KIKO female mice compared with WT in the GTT.
HFD reduced insulin tolerance only in KO female mice, which, along with elevated plasma insulin in KO (and KIKO) female mice, indicates potential insulin resistance. The loss of ERE-dependent ERα signaling sensitizes female mice to the effects of DIO on insulin sensitivity. In fed animals, insulin signaling in the liver suppresses hepatic gluconeogenesis, in part, via the phosphorylation of FoxO1 and the subsequent inhibition of FoxO1-mediated transcription of Pepck and G6pase (38). In our study, Insr and Foxo1 expression in the WT liver was augmented by HFD and EB, the latter through ERE-dependent transcription. Because insulin receptor and FoxO1 oppose each other in hepatic gluconeogenesis, an enhancement of Insr would augment insulin actions in the liver and suppress gluconeogenesis, whereas a boost in Foxo1 would counteract insulin’s actions.
Elevated plasma IL-6 levels observed in HFD-fed WT female mice (and all KIKO and KO groups) may abrogate this apparent interaction of EB and HFD on insulin signaling in the liver because acute IL-6 administration blocks the suppression of hepatic gluconeogenesis by insulin (64). Alternatively, elevated IL-6 without other inflammatory signals promotes insulin production and glucose-stimulated insulin secretion from the pancreas (65–68). In our study, both WT and KIKO female mice expressed elevated IL-6 levels when fed a HFD without elevated TNF-α or MCP1. These female mice were also resistant to the effects of HFD on glucose and insulin tolerance, potentially by the actions of IL-6 on glucose-stimulated insulin secretion from the pancreas.
Low-grade inflammation is a hallmark of DIO, with most of the inflammatory cytokines being produced by the increasing deposition of adipose tissue. These cytokines are transported through cardiovascular circulation to other organs that control the metabolic process, including the liver, brain, and muscle (14, 69, 70). In a previous study, E2 reduced HFD-induced insulin resistance, improved insulin signaling in skeletal muscles, and increased plasma levels of inflammatory cytokines (IL-6, TNF-α) in OVX female mice through an ERα-mediated mechanism (20). Although EB had no effect on plasma IL-6 or TNF-α levels in our study, KO female mice exhibited elevated levels of these cytokines and MCP-1 compared with WT and KIKO female mice, indicating that the loss of ERα signaling sensitizes female mice to OVX- and DIO-induced inflammation. Presumably, the elevated levels of inflammatory cytokines in the KO female mice are an underlying mechanism behind the disruption in glucose homeostasis and insulin sensitivity in this genotype (14). ERE-independent ERα signaling is involved in the regulation of MCP-1 and IL-6 expression in MCF-7 cells through the response element for another transcription factor, nuclear factor kappa-light-chain-enhancer of activated B cells (34). Our data support this hypothesis, with EB reducing plasma MCP-1 in LFD-fed WT and KIKO female mice, although abrogated by HFD.
ERα is expressed in the liver and activates both nuclear-initiated and membrane-initiated mechanisms (18, 19). Although the ERα-mediated signaling mechanisms involved in liver function are not fully understood, it is suggested that the DNA binding domain of ERα is necessary for E2’s regulation of liver gene expression (23). For example, via ERα, E2 controls the expression of signal transducer and activator of transcription 3 in the liver to improve insulin sensitivity and regulate lipogenic genes (Pparγ, Fas, Acc1), which are augmented by OVX in mice (71–73). E2 protects against fatty liver and associated insulin resistance, in part, by reducing lipid synthesis concurrently with activation of lipid oxidation in HFD-fed OVX female mice (21, 72, 74). E2 also alters expression of a number of hepatic lipogenic and gluconeogenic genes, including G6pase and Pparγ in HFD-fed OVX female mice (22), and attenuates the increase in Acc1 and Fas due to OVX, reducing hepatic steatosis (75). Whereas E2 had no effect on G6pase and Pparγ in the liver in our study, EB did reduce the expression of lipid biosynthesis genes (Acc1, Acc2, Acly, Fatp2, and Fatp5) in WT female mice, thus controlling lipid production and deposition in the liver during DIO. One such ERE-dependent mechanism is the regulation of a microRNA, miR-125b, by E2 in the liver via ERα, which subsequently targets Fas expression to reduce lipid deposition (76).
Two lipogenic genes (Acc1, Acly) were suppressed by E2 in LFD-fed KIKO female mice, which indicates that ERE-independent ERα signaling affects liver lipogenesis. In KIKO but not in WT female mice, HFD reduced the expression of Acc1 and Acly, suggesting that HFD overwhelms ERE-independent ERα-mediated regulation of Acc1 and Acly expression. Another gene involved in liver metabolism and lipogenesis is Ucp2 (uncoupling protein 2), which was augmented by EB through ERE-dependent signaling in the WT liver, regardless of diet. An increase in Ucp2 expression in WT acts as a buffer against the production of reactive oxygen species by fatty acid beta-oxidation and subsequently promotes mitochondrial biogenesis (77, 78), thereby reducing the effects of DIO on liver metabolism and lipid deposition.
In conclusion, the current study demonstrates that ERα mediates E2’s control of energy homeostasis through ERE-dependent and -independent mechanisms contingent upon the diet consumed in a postmenopausal mouse model. These mechanisms include regulation of hypothalamic and liver gene expression, adiposity, energy intake, peptide hormone production, and glucose clearance. Thus, our study identifies several unique cellular and gene targets for mechanistic characterization of ERα signaling, both ERE-dependent and -independent, in these tissues. A prime example of ERα- and ERE-independent signaling is activation of the Gq-mER, which is expressed throughout the hypothalamus. Activation of the Gq-mER by its selective ligand, STX, reduces post-OVX body weight gain by modulating feeding behavior and controls thermoregulation and bone remodeling, all of which are disrupted during menopause (43). The identification of similar membrane-initiated E2 signaling in vivo is necessary to develop selective therapies to treat the dysregulation of energy and glucose homeostasis in menopausal women, which increases the risk for obesity, metabolic syndrome, and type 2 diabetes.
Acknowledgments
The authors thank Dr. Sara Campbell for the use of the EMD Millipore MAGPIX Multiplex System, Dr. Judy Storch for the use of the EchoMRI Body Composition Analyzer, Dr. Nicholas T. Bello for careful review and critique of the manuscript, and the many undergraduate students who assisted in genotyping and weighing the mice.
Acknowledgments
This work was supported by funds from the United States Department of Agriculture–National Institute of Food and Agriculture (Grant NJ06107) and the National Institutes of Health (Grants R00DK083457, R00DK083457-S1, and P30ES005022).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- ANOVA
- analysis of variance
- ARC
- arcuate nucleus
- BH
- basal hypothalamus
- cDNA
- complementary DNA
- DIO
- diet-induced obesity
- E2
- 17β-estradiol
- EB
- estradiol benzoate
- ERα
- estrogen receptor α
- ERE
- estrogen response element
- GLUT4
- glucose transporter type 4
- GTT
- glucose tolerance test
- HFD
- high-fat diet
- IL-6
- interleukin 6
- IP
- intraperitoneally
- ITT
- insulin tolerance test
- KI
- knock in
- KO
- knockout
- LFD
- low-fat diet
- MCP-1
- monocyte chemoattractant protein 1
- NPY
- neuropeptide Y
- OVX
- ovariectomy
- PCR
- polymerase chain reaction
- TNF-α
- tumor necrosis factor α
- VMH
- ventromedial hypothalamus
- WT
- wild-type
References
- 1.Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999-2010. JAMA. 2012;307(5):491–497. [DOI] [PubMed] [Google Scholar]
- 2.Moore TR. Adolescent and adult obesity in women: a tidal wave just beginning. Clin Obstet Gynecol. 2004;47:884–889. [DOI] [PubMed] [Google Scholar]
- 3.Hodson L, Banerjee R, Rial B, Arlt W, Adiels M, Boren J, Marinou K, Fisher C, Mostad IL, Stratton IM, Barrett PH, Chan DC, Watts GF, Harnden K, Karpe F, Fielding BA. Menopausal status and abdominal obesity are significant determinants of hepatic lipid metabolism in women. J Am Heart Assoc. 2015;4(10):e002258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Patil SR. Prevalence of carotid artery calcification in postmenopausal women and its correlation with atherogenic risk factors. J Nat Sci Biol Med. 2015;6(Suppl 1):S1–S6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Orgaz Gallego MP, Bermejo López P, Tricio Armero MA, Abellán Alemán J, Solera Albero J, Tárraga López PJ. Metabolic syndrome and its components in Spanish postmenopausal women. Nutr Hosp. 2015;32(2):656–666. [DOI] [PubMed] [Google Scholar]
- 6.Johnson ZP, Lowe J, Michopoulos V, Moore CJ, Wilson ME, Toufexis D. Oestradiol differentially influences feeding behaviour depending on diet composition in female rhesus monkeys. J Neuroendocrinol. 2013;25(8):729–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Paolisso G, Rizzo MR, Mazziotti G, Rotondi M, Tagliamonte MR, Varricchio G, Carella C, Varricchio M. Lack of association between changes in plasma leptin concentration and in food intake during the menstrual cycle. Eur J Clin Invest. 1999;29(6):490–495. [DOI] [PubMed] [Google Scholar]
- 8.Buffenstein R, Poppitt SD, McDevitt RM, Prentice AM. Food intake and the menstrual cycle: a retrospective analysis, with implications for appetite research. Physiol Behav. 1995;58(6):1067–1077. [DOI] [PubMed] [Google Scholar]
- 9.Dye L, Blundell JE. Menstrual cycle and appetite control: implications for weight regulation. Hum Reprod. 1997;12(6):1142–1151. [DOI] [PubMed] [Google Scholar]
- 10.Sullivan EL, Shearin J, Koegler FH, Cameron JL. Selective estrogen receptor modulator promotes weight loss in ovariectomized female rhesus monkeys (Macaca mulatta) by decreasing food intake and increasing activity. Am J Physiol Endocrinol Metab. 2012;302(7):E759–E767. [DOI] [PubMed] [Google Scholar]
- 11.Lobo RA, Davis SR, De Villiers TJ, Gompel A, Henderson VW, Hodis HN, Lumsden MA, Mack WJ, Shapiro S, Baber RJ. Prevention of diseases after menopause. Climacteric. 2014;17(5):540–556. [DOI] [PubMed] [Google Scholar]
- 12.Heine PA, Taylor JA, Iwamoto GA, Lubahn DB, Cooke PS. Increased adipose tissue in male and female estrogen receptor-alpha knockout mice. Proc Natl Acad Sci USA. 2000;97(23):12729–12734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Geary N, Asarian L, Korach KS, Pfaff DW, Ogawa S. Deficits in E2-dependent control of feeding, weight gain, and cholecystokinin satiation in ER-α null mice. Endocrinology. 2001;142(11):4751–4757. [DOI] [PubMed] [Google Scholar]
- 14.Ribas V, Nguyen MTA, Henstridge DC, Nguyen AK, Beaven SW, Watt MJ, Hevener AL. Impaired oxidative metabolism and inflammation are associated with insulin resistance in ERalpha-deficient mice. Am J Physiol Endocrinol Metab. 2010;298(2):E304–E319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thammacharoen S, Geary N, Lutz TA, Ogawa S, Asarian L. Divergent effects of estradiol and the estrogen receptor-α agonist PPT on eating and activation of PVN CRH neurons in ovariectomized rats and mice. Brain Res. 2009;1268:88–96. [DOI] [PubMed] [Google Scholar]
- 16.Park CJ, Zhao Z, Glidewell-Kenney C, Lazic M, Chambon P, Krust A, Weiss J, Clegg DJ, Dunaif A, Jameson JL, Levine JE. Genetic rescue of nonclassical ERα signaling normalizes energy balance in obese Erα-null mutant mice. J Clin Invest. 2011;121(2):604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mamounis KJ, Yang JA, Yasrebi A, Roepke TA. Estrogen response element-independent signaling partially restores post-ovariectomy body weight gain but is not sufficient for 17β-estradiol’s control of energy homeostasis. Steroids. 2014;81:88–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barros RPA, Gustafsson J-Å. Estrogen receptors and the metabolic network. Cell Metab. 2011;14(3):289–299. [DOI] [PubMed] [Google Scholar]
- 19.Mauvais-Jarvis F, Clegg DJ, Hevener AL. The role of estrogens in control of energy balance and glucose homeostasis. Endocr Rev. 2013;34(3):309–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Riant E, Waget A, Cogo H, Arnal JF, Burcelin R, Gourdy P. Estrogens protect against high-fat diet-induced insulin resistance and glucose intolerance in mice. Endocrinology. 2009;150(5):2109–2117. [DOI] [PubMed] [Google Scholar]
- 21.Camporez JPG, Jornayvaz FR, Lee HY, Kanda S, Guigni BA, Kahn M, Samuel VT, Carvalho CR, Petersen KF, Jurczak MJ, Shulman GI. Cellular mechanism by which estradiol protects female ovariectomized mice from high-fat diet-induced hepatic and muscle insulin resistance. Endocrinology. 2013;154(3):1021–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bryzgalova G, Lundholm L, Portwood N, Gustafsson JA, Khan A, Efendic S, Dahlman-Wright K. Mechanisms of antidiabetogenic and body weight-lowering effects of estrogen in high-fat diet-fed mice. Am J Physiol Endocrinol Metab. 2008;295(4):E904–E912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ahlbory-Dieker DL, Stride BD, Leder G, Schkoldow J, Trölenberg S, Seidel H, Otto C, Sommer A, Parker MG, Schütz G, Wintermantel TM. DNA binding by estrogen receptor-alpha is essential for the transcriptional response to estrogen in the liver and the uterus. Mol Endocrinol. 2009;23(10):1544–1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hewitt SC, Kissling GE, Fieselman KE, Jayes FL, Gerrish KE, Korach KS. Biological and biochemical consequences of global deletion of exon 3 from the ER alpha gene. FASEB J. 2010;24(12):4660–4667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hewitt SC, Korach KS. Estrogenic activity of bisphenol A and 2,2-bis(p-hydroxyphenyl)-1,1,1-trichloroethane (HPTE) demonstrated in mouse uterine gene profiles. Environ Health Perspect. 2011;119(1):63–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yasrebi A, Hsieh A, Mamounis KJ, Krumm EA, Yang JA, Magby J, Hu P, Roepke TA. Differential gene regulation of GHSR signaling pathway in the arcuate nucleus and NPY neurons by fasting, diet-induced obesity, and 17β-estradiol [published correction appears in Mol Cell Endocrinol. 2016;428:171–173]. Mol Cell Endocrinol. 2016;422:42–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ingberg E, Theodorsson A, Theodorsson E, Strom JO. Methods for long-term 17β-estradiol administration to mice. Gen Comp Endocrinol. 2012;175(1):188–193. [DOI] [PubMed] [Google Scholar]
- 28.McGuinness OP, Ayala JE, Laughlin MR, Wasserman DH. NIH experiment in centralized mouse phenotyping: the Vanderbilt experience and recommendations for evaluating glucose homeostasis in the mouse. Am J Physiol Endocrinol Metab. 2009;297(4):E849–E855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Andrikopoulos S, Blair AR, Deluca N, Fam BC, Proietto J. Evaluating the glucose tolerance test in mice. Am J Physiol Endocrinol Metab. 2008;295(6):E1323–E1332. [DOI] [PubMed] [Google Scholar]
- 30.Paxinos G, Franklin KBJ. The Mouse Brain in Stereotaxic Coordinates, Compact: The Coronal Plates and Diagrams. 3rd ed. London, UK: Academic Press; 2008. [Google Scholar]
- 31.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25(4):402–408. [DOI] [PubMed] [Google Scholar]
- 32.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3(6):1101–1108. [DOI] [PubMed] [Google Scholar]
- 33.Kurita T, Lee K, Saunders PT, Cooke PS, Taylor JA, Lubahn DB, Zhao C, Mäkelä S, Gustafsson JA, Dahiya R, Cunha GR. Regulation of progesterone receptors and decidualization in uterine stroma of the estrogen receptor-alpha knockout mouse. Biol Reprod. 2001;64(1):272–283. [DOI] [PubMed] [Google Scholar]
- 34.Ye J, McGuinness OP. Inflammation during obesity is not all bad: evidence from animal and human studies. Am J Physiol Endocrinol Metab. 2013;304(5):E466–E477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Coll AP, Farooqi IS, O’Rahilly S. The hormonal control of food intake. Cell. 2007;129(2):251–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Koch M, Horvath TL. Molecular and cellular regulation of hypothalamic melanocortin neurons controlling food intake and energy metabolism. Mol Psychiatry. 2014;19(7):752–761. [DOI] [PubMed] [Google Scholar]
- 37.Morselli E, Fuente-Martin E, Finan B, Kim M, Frank A, Garcia-Caceres C, Navas CR, Gordillo R, Neinast M, Kalainayakan SP, Li DL, Gao Y, Yi CX, Hahner L, Palmer BF, Tschöp MH, Clegg DJ. Hypothalamic PGC-1α protects against high-fat diet exposure by regulating ERα. Cell Reports. 2014;9(2):633–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Oh K-J, Han H-S, Kim M-J, Koo S-H. CREB and FoxO1: two transcription factors for the regulation of hepatic gluconeogenesis. BMB Rep. 2013;46(12):567–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Handgraaf S, Riant E, Fabre A, Waget A, Burcelin R, Lière P, Krust A, Chambon P, Arnal JF, Gourdy P. Prevention of obesity and insulin resistance by estrogens requires ERα activation function-2 (ERαAF-2), whereas ERαAF-1 is dispensable. Diabetes. 2013;62(12):4098–4108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chambliss KL, Barrera J, Umetani M, Umetani J, Kim SH, Madak-Erdogan Z, Huang L, Katzenellenbogen BS, Katzenellenbogen JA, Mineo C, Shaul PW. Non-nuclear estrogen receptor activation improves hepatic steatosis in female mice. Endocrinology. 2016;157(10):3731–3741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xu Y, Nedungadi TP, Zhu L, Sobhani N, Irani BG, Davis KE, Zhang X, Zou F, Gent LM, Hahner LD, Khan SA, Elias CF, Elmquist JK, Clegg DJ. Distinct hypothalamic neurons mediate estrogenic effects on energy homeostasis and reproduction. Cell Metab. 2011;14(4):453–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Qiu J, Bosch MA, Tobias SC, Krust A, Graham SM, Murphy SJ, Korach KS, Chambon P, Scanlan TS, Rønnekleiv OK, Kelly MJ. A G-protein-coupled estrogen receptor is involved in hypothalamic control of energy homeostasis. J Neurosci. 2006;26(21):5649–5655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Roepke TA, Bosch MA, Rick EA, Lee B, Wagner EJ, Seidlova-Wuttke D, Wuttke W, Scanlan TS, Rønnekleiv OK, Kelly MJ. Contribution of a membrane estrogen receptor to the estrogenic regulation of body temperature and energy homeostasis. Endocrinology. 2010;151(10):4926–4937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Smith AW, Bosch MA, Wagner EJ, Rønnekleiv OK, Kelly MJ. The membrane estrogen receptor ligand STX rapidly enhances GABAergic signaling in NPY/AgRP neurons: role in mediating the anorexigenic effects of 17β-estradiol. Am J Physiol Endocrinol Metab. 2013;305(5):E632–E640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pelletier G, Li S, Luu-The V, Labrie F. Oestrogenic regulation of pro-opiomelanocortin, neuropeptide Y and corticotrophin-releasing hormone mRNAs in mouse hypothalamus. J Neuroendocrinol. 2007;19(6):426–431. [DOI] [PubMed] [Google Scholar]
- 46.Bonavera JJ, Dube MG, Kalra PS, Kalra SP. Anorectic effects of estrogen may be mediated by decreased neuropeptide-Y release in the hypothalamic paraventricular nucleus. Endocrinology. 1994;134(6):2367–2370. [DOI] [PubMed] [Google Scholar]
- 47.Santollo J, Yao D, Neal-Perry G, Etgen AM. Middle-aged female rats retain sensitivity to the anorexigenic effect of exogenous estradiol. Behav Brain Res. 2012;232(1):159–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Roepke TA, Xue C, Bosch MA, Scanlan TS, Kelly MJ, Rønnekleiv OK. Genes associated with membrane-initiated signaling of estrogen and energy homeostasis. Endocrinology. 2008;149(12):6113–6124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Clegg DJ, Brown LM, Zigman JM, Kemp CJ, Strader AD, Benoit SC, Woods SC, Mangiaracina M, Geary N. Estradiol-dependent decrease in the orexigenic potency of ghrelin in female rats. Diabetes. 2007;56(4):1051–1058. [DOI] [PubMed] [Google Scholar]
- 50.Garfield AS, Li C, Madara JC, Shah BP, Webber E, Steger JS, Campbell JN, Gavrilova O, Lee CE, Olson DP, Elmquist JK, Tannous BA, Krashes MJ, Lowell BB. A neural basis for melanocortin-4 receptor-regulated appetite. Nat Neurosci. 2015;18(6):863–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Asarian L, Geary N. Modulation of appetite by gonadal steroid hormones. Philos Trans R Soc Lond B Biol Sci. 2006;361(1471):1251–1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Asarian L, Geary N. Cyclic estradiol treatment normalizes body weight and restores physiological patterns of spontaneous feeding and sexual receptivity in ovariectomized rats. Horm Behav. 2002;42(4):461–471. [DOI] [PubMed] [Google Scholar]
- 53.Roepke TA, Qiu J, Smith AW, Rønnekleiv OK, Kelly MJ. Fasting and 17β-estradiol differentially modulate the M-current in neuropeptide Y neurons. J Neurosci. 2011;31(33):11825–11835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Santollo J, Torregrossa AM, Eckel LA. Estradiol acts in the medial preoptic area, arcuate nucleus, and dorsal raphe nucleus to reduce food intake in ovariectomized rats. Horm Behav. 2011;60(1):86–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Musatov S, Chen W, Pfaff DW, Mobbs CV, Yang XJ, Clegg DJ, Kaplitt MG, Ogawa S. Silencing of estrogen receptor alpha in the ventromedial nucleus of hypothalamus leads to metabolic syndrome. Proc Natl Acad Sci USA. 2007;104(7):2501–2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Thammacharoen S, Lutz TA, Geary N, Asarian L. Hindbrain administration of estradiol inhibits feeding and activates estrogen receptor-α-expressing cells in the nucleus tractus solitarius of ovariectomized rats. Endocrinology. 2008;149(4):1609–1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Asarian L, Geary N. Estradiol enhances cholecystokinin-dependent lipid-induced satiation and activates estrogen receptor-alpha-expressing cells in the nucleus tractus solitarius of ovariectomized rats. Endocrinology. 2007;148(12):5656–5666. [DOI] [PubMed] [Google Scholar]
- 58.Santollo J, Wiley MD, Eckel LA. Acute activation of ER alpha decreases food intake, meal size, and body weight in ovariectomized rats. Am J Physiol Regul Integr Comp Physiol. 2007;293(6):R2194–R2201. [DOI] [PubMed] [Google Scholar]
- 59.Thornton JE, Loose MD, Kelly MJ, Rönnekleiv OK. Effects of estrogen on the number of neurons expressing beta-endorphin in the medial basal hypothalamus of the female guinea pig. J Comp Neurol. 1994;341(1):68–77. [DOI] [PubMed] [Google Scholar]
- 60.Shi H, Seeley RJ, Clegg DJ. Sexual differences in the control of energy homeostasis. Front Neuroendocrinol. 2009;30(3):396–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang A, Luo J, Moore W, Alkhalidy H, Wu L, Zhang J, Zhen W, Wang Y, Clegg DJ, Bin Xu, Cheng Z, McMillan RP, Hulver MW, Liu D. GPR30 regulates diet-induced adiposity in female mice and adipogenesis in vitro. Sci Rep. 2016;6:34302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jelenik T, Roden M. How estrogens prevent from lipid-induced insulin resistance. Endocrinology. 2013;154(3):989–992. [DOI] [PubMed] [Google Scholar]
- 63.Gorres BK, Bomhoff GL, Morris JK, Geiger PC. In vivo stimulation of oestrogen receptor α increases insulin-stimulated skeletal muscle glucose uptake. J Physiol. 2011;589(8):2041–2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kim HJ, Higashimori T, Park SY, Choi H, Dong J, Kim YJ, Noh HL, Cho YR, Cline G, Kim YB, Kim JK. Differential effects of interleukin-6 and -10 on skeletal muscle and liver insulin action in vivo. Diabetes. 2004;53(4):1060–1067. [DOI] [PubMed] [Google Scholar]
- 65.da Silva Krause M, Bittencourt A, Homem de Bittencourt PI Jr, McClenaghan NH, Flatt PR, Murphy C, Newsholme P. Physiological concentrations of interleukin-6 directly promote insulin secretion, signal transduction, nitric oxide release, and redox status in a clonal pancreatic β-cell line and mouse islets. J Endocrinol. 2012;214(3):301–311. [DOI] [PubMed] [Google Scholar]
- 66.Suzuki T, Imai J, Yamada T, Ishigaki Y, Kaneko K, Uno K, Hasegawa Y, Ishihara H, Oka Y, Katagiri H. Interleukin-6 enhances glucose-stimulated insulin secretion from pancreatic β-cells: potential involvement of the PLC-IP3-dependent pathway. Diabetes. 2011;60(2):537–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ellingsgaard H, Hauselmann I, Schuler B, Habib AM, Baggio LL, Meier DT, Eppler E, Bouzakri K, Wueest S, Muller YD, Hansen AM, Reinecke M, Konrad D, Gassmann M, Reimann F, Halban PA, Gromada J, Drucker DJ, Gribble FM, Ehses JA, Donath MY. Interleukin-6 enhances insulin secretion by increasing glucagon-like peptide-1 secretion from L cells and alpha cells. Nat Med. 2011;17(11):1481–1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Allen TL, Whitham M, Febbraio MA. IL-6 muscles in on the gut and pancreas to enhance insulin secretion. Cell Metab. 2012;15(1):8–9. [DOI] [PubMed] [Google Scholar]
- 69.Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444(7121):860–867. [DOI] [PubMed] [Google Scholar]
- 70.Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. J Clin Invest. 2006;116(7):1793–1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lundholm L, Bryzgalova G, Gao H, Portwood N, Fält S, Berndt KD, Dicker A, Galuska D, Zierath JR, Gustafsson JA, Efendic S, Dahlman-Wright K, Khan A. The estrogen receptor α-selective agonist propyl pyrazole triol improves glucose tolerance in ob/ob mice; potential molecular mechanisms. J Endocrinol. 2008;199(2):275–286. [DOI] [PubMed] [Google Scholar]
- 72.Gao H, Bryzgalova G, Hedman E, Khan A, Efendic S, Gustafsson JA, Dahlman-Wright K. Long-term administration of estradiol decreases expression of hepatic lipogenic genes and improves insulin sensitivity in ob/ob mice: a possible mechanism is through direct regulation of signal transducer and activator of transcription 3. Mol Endocrinol. 2006;20(6):1287–1299. [DOI] [PubMed] [Google Scholar]
- 73.Rogers NH, Perfield JW II, Strissel KJ, Obin MS, Greenberg AS. Reduced energy expenditure and increased inflammation are early events in the development of ovariectomy-induced obesity. Endocrinology. 2009;150(5):2161–2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhu L, Brown WC, Cai Q, Krust A, Chambon P, McGuinness OP, Stafford JM. Estrogen treatment after ovariectomy protects against fatty liver and may improve pathway-selective insulin resistance. Diabetes. 2013;62(2):424–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fu X, Xing L, Xu W, Shu J. Treatment with estrogen protects against ovariectomy-induced hepatic steatosis by increasing AQP7 expression. Mol Med Rep. 2016;14(1):425–431. [DOI] [PubMed] [Google Scholar]
- 76.Zhang ZC, Liu Y, Xiao LL, Li SF, Jiang JH, Zhao Y, Qian SW, Tang QQ, Li X. Upregulation of miR-125b by estrogen protects against non-alcoholic fatty liver in female mice. J Hepatol. 2015;63(6):1466–1475. [DOI] [PubMed] [Google Scholar]
- 77.Du X, Edelstein D, Obici S, Higham N, Zou M-H, Brownlee M. Insulin resistance reduces arterial prostacyclin synthase and eNOS activities by increasing endothelial fatty acid oxidation. J Clin Invest. 2006;116(4):1071–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Andrews ZB. Central mechanisms involved in the orexigenic actions of ghrelin. Peptides. 2011;32(11):2248–2255. [DOI] [PubMed] [Google Scholar]