Abstract
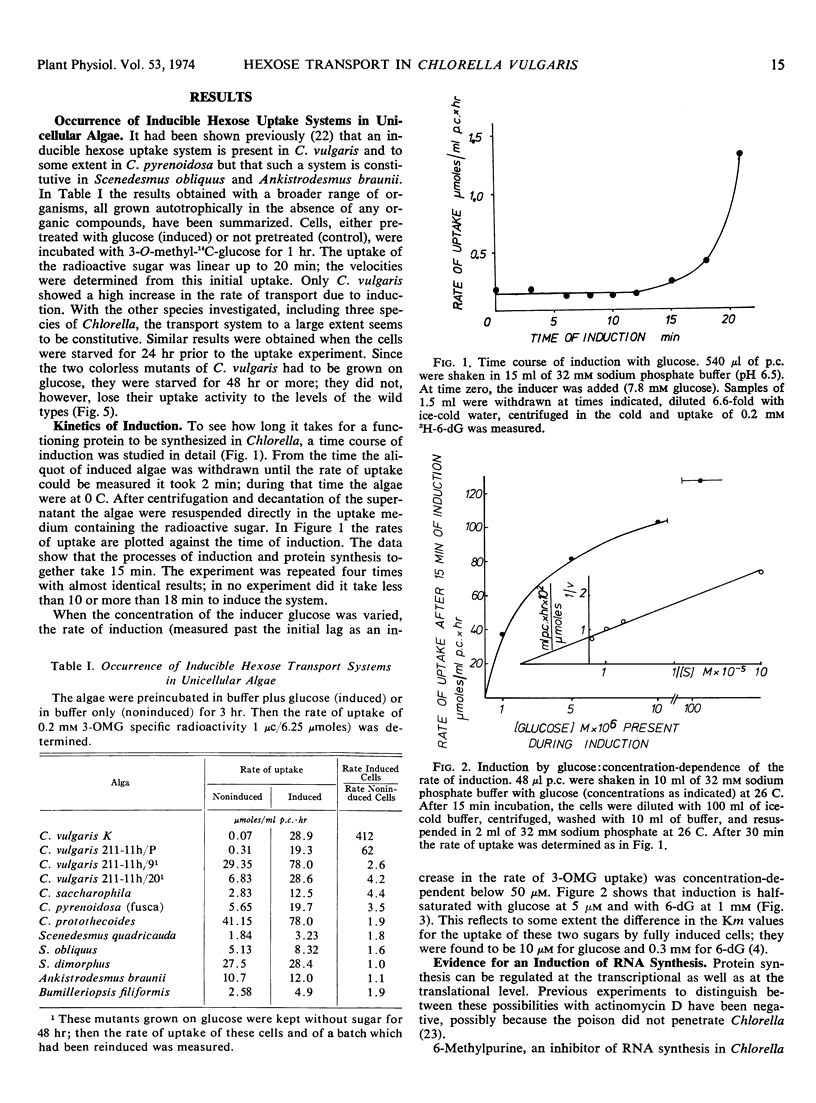
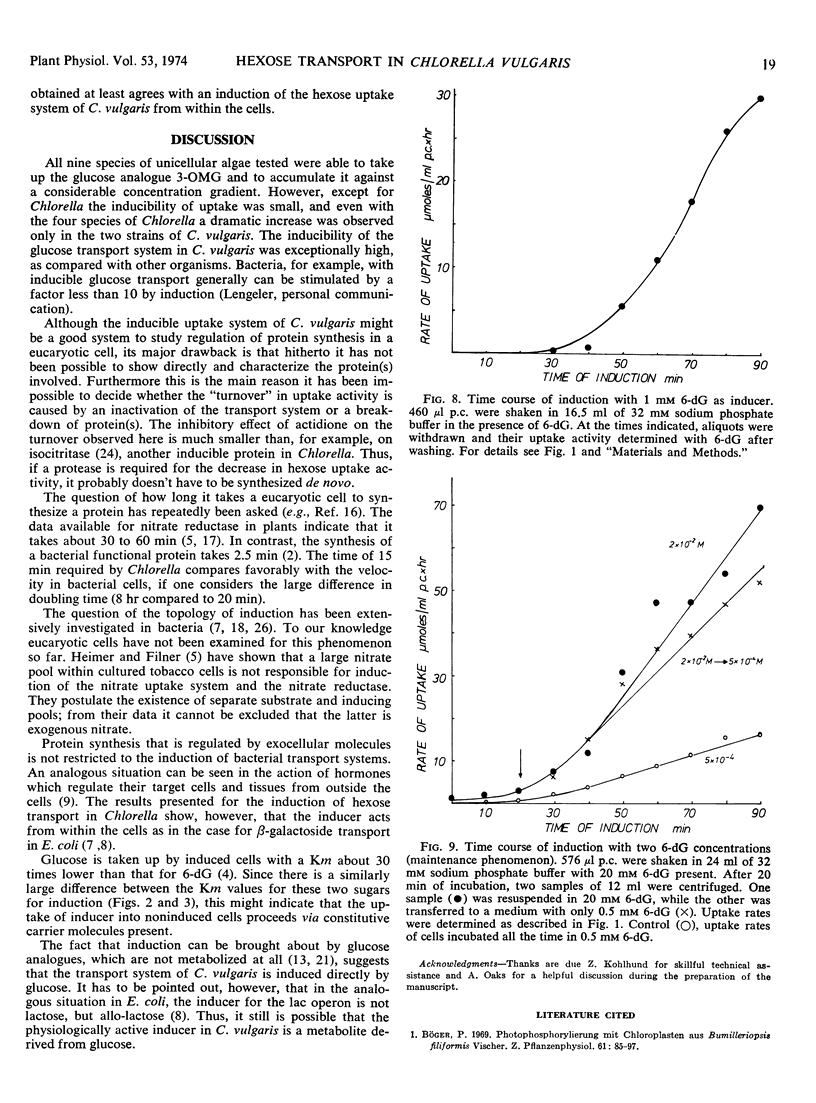
Of nine species of unicellular algae tested, Chlorella vulgaris showed the highest inducibility for an active hexose transport system. Whereas the rate of uptake in all other species was increased by induction less than 5-fold, it was increased more than 400-fold in one strain of C. vulgaris. With glucose as inducer, the minimum time necessary to synthesize inducible proteins of the transport system was 15 minutes. The Km for induction with glucose is 5 μm and with 6-deoxyglucose 1 mm. The inducing sugars have to penetrate the cells to be effective.
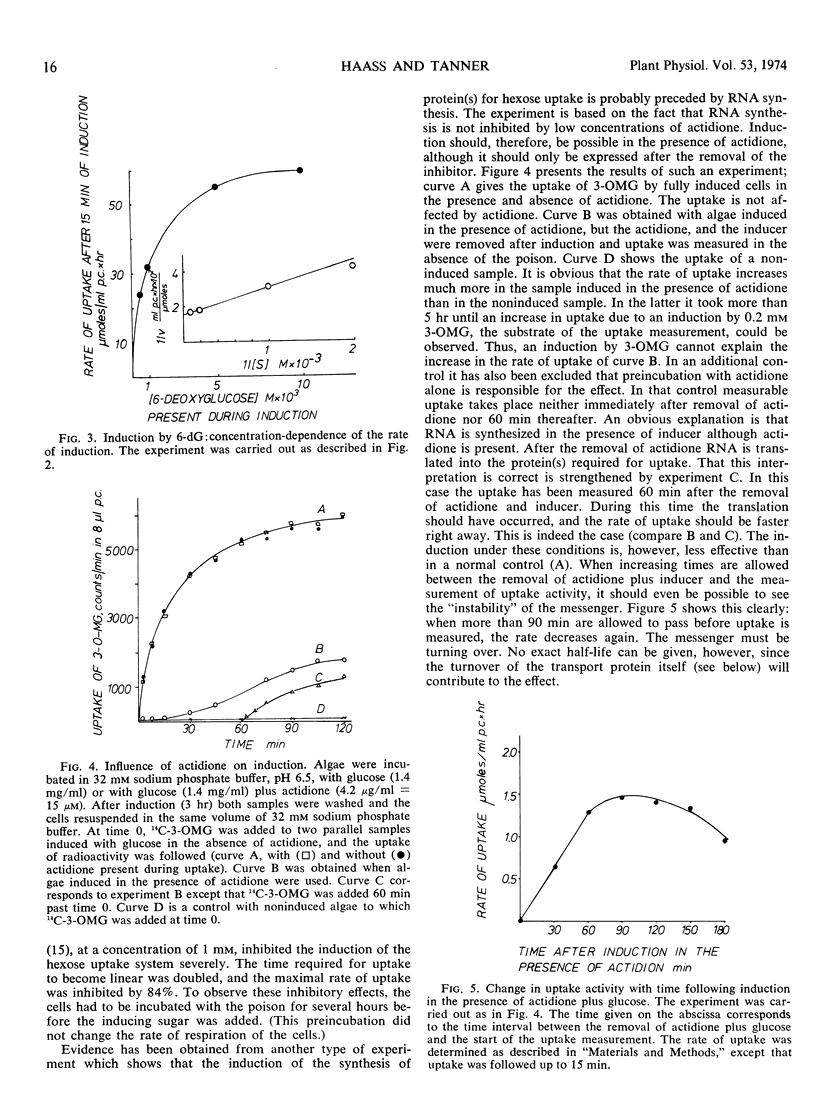
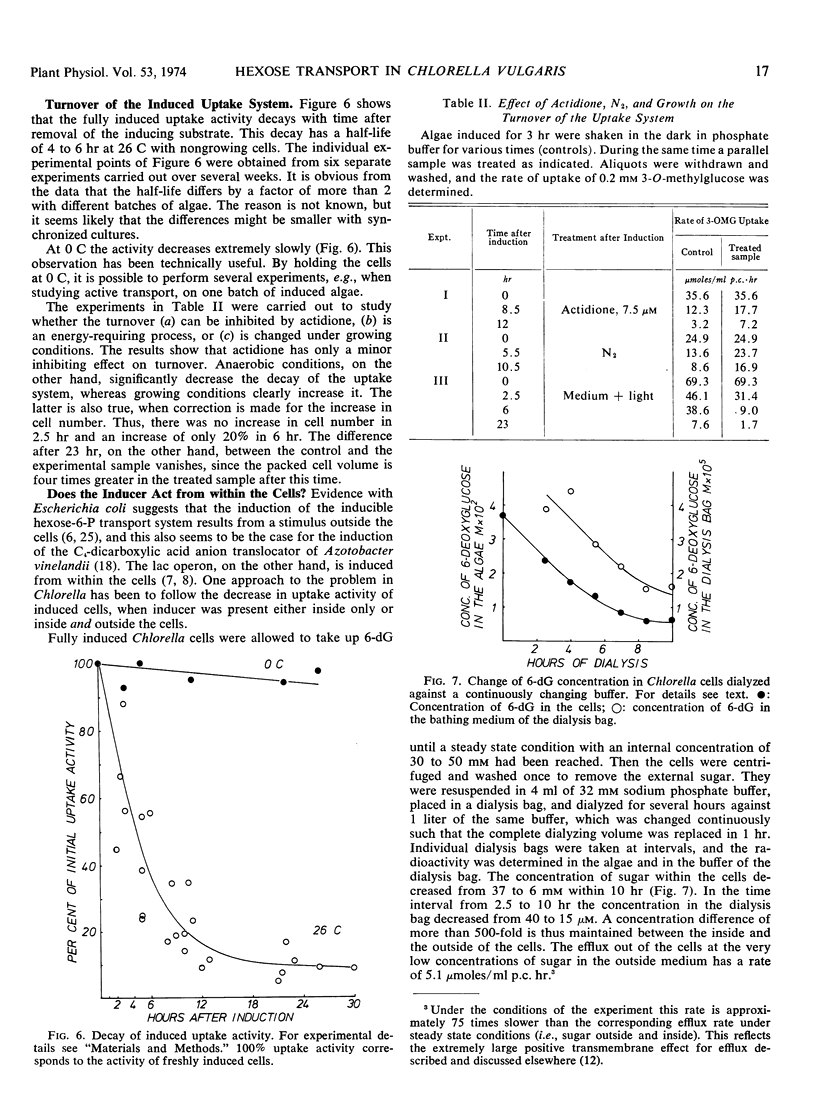
Evidence indicating that regulation of induction occurs at the transcriptional level was obtained. The induction was inhibited by 6-methylpurine. When cells were exposed to induce in the presence of actidione no increase in transport activity could be measured. After removal of actidione as well as the inducer, an increased uptake activity was observed after 30 to 60 minutes. The induced uptake system showed a turnover with a half-life of 4 to 6 hours at 26 C under nongrowing conditions; at 0 C turnover was negligible. Turnover was partly inhibited by anaerobic condition and by actidione; it was accelerated under growing conditions.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Branscomb E. W., Stuart R. N. Induction lag as a function of induction level. Biochem Biophys Res Commun. 1968 Aug 21;32(4):731–738. doi: 10.1016/0006-291x(68)90300-8. [DOI] [PubMed] [Google Scholar]
- COHN M. Contributions of studies on the beta-galactosidase of Escherichia coli to our understanding of enzyme synthesis. Bacteriol Rev. 1957 Sep;21(3):140–168. doi: 10.1128/br.21.3.140-168.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decker M., Tanner W. Respiratory increase and active hexose uptake of Chlorella vulgaris. Biochim Biophys Acta. 1972 Jun 20;266(3):661–669. doi: 10.1016/0006-3002(72)90009-1. [DOI] [PubMed] [Google Scholar]
- HERZENBERG L. A. Studies on the induction of beta-galactosidase in a cryptic strain of Escherichia coli. Biochim Biophys Acta. 1959 Feb;31(2):525–538. doi: 10.1016/0006-3002(59)90029-0. [DOI] [PubMed] [Google Scholar]
- Heimer Y. M., Filner P. Regulation of the nitrate assimilation pathway in cultured tobacco cells. 3. The nitrate uptake system. Biochim Biophys Acta. 1971 Feb 23;230(2):362–372. doi: 10.1016/0304-4165(71)90223-6. [DOI] [PubMed] [Google Scholar]
- Jobe A., Bourgeois S. lac Repressor-operator interaction. VI. The natural inducer of the lac operon. J Mol Biol. 1972 Aug 28;69(3):397–408. doi: 10.1016/0022-2836(72)90253-7. [DOI] [PubMed] [Google Scholar]
- Komor E., Haass D., Tanner W. Unusual features of the active hexose uptake system of Chlorella vulgaris. Biochim Biophys Acta. 1972 Jun 20;266(3):649–660. doi: 10.1016/0006-3002(72)90008-x. [DOI] [PubMed] [Google Scholar]
- Komor E., Tanner W. Characterization of the active hexose transport system of Chlorella vulgaris. Biochim Biophys Acta. 1971 Jul 6;241(1):170–179. doi: 10.1016/0005-2736(71)90314-2. [DOI] [PubMed] [Google Scholar]
- Oaks A., Wallace W., Stevens D. Synthesis and turnover of nitrate reductase in corn roots. Plant Physiol. 1972 Dec;50(6):649–654. doi: 10.1104/pp.50.6.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuser A. J., Postma P. W. The induction of translocators for di- and tricarboxylic-acid anions in Azotobacter vinelandii. Eur J Biochem. 1973 Mar 15;33(3):584–592. doi: 10.1111/j.1432-1033.1973.tb02719.x. [DOI] [PubMed] [Google Scholar]
- Robbie J. P., Wilson T. H. Transmembrane effects of beta-galactosides on thiomethyl-beta-galactoside transport in Escherichia coli. Biochim Biophys Acta. 1969 Mar 11;173(2):234–244. doi: 10.1016/0005-2736(69)90107-2. [DOI] [PubMed] [Google Scholar]
- Tanner W. Light-driven active uptake of 3-O-methylglucose via an inducible hexose uptake system of Chlorella. Biochem Biophys Res Commun. 1969 Jul 23;36(2):278–283. doi: 10.1016/0006-291x(69)90326-x. [DOI] [PubMed] [Google Scholar]
- Thurston C. F., John P. C., Syrett P. J. The effect of metabolic inhibitors on the loss of isocitrate lyase activity from Chlorella. Arch Mikrobiol. 1973;88(2):135–145. doi: 10.1007/BF00424767. [DOI] [PubMed] [Google Scholar]
- Winkler H. H. Compartmentation in the induction of the hexose-6-phosphate transport system of Escherichia coli. J Bacteriol. 1970 Feb;101(2):470–475. doi: 10.1128/jb.101.2.470-475.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler H. H. Kinetics of exogenous induction of the hexose-6-phosphate transport system of Escherichia coli. J Bacteriol. 1971 Jul;107(1):74–78. doi: 10.1128/jb.107.1.74-78.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]


