Heart failure (HF) is associated with significant physical and emotional concerns1,2 as well as cognitive consequences.3,4 HF-related cognitive deficits predict hospitalization and mortality over and above the physical symptoms of the disease5 and are linked to high rates of disability.6 Despite the clinical significance of HF-related cognitive impairment, few studies have examined specific mechanisms for why cognitive deficits are associated with poorer HF outcomes. Riegel and Dickson (2015) in their Situation-Specific Theory of HF Self-Care state that effective self-care relies on maintenance, symptom perception, and symptom management.7 Cognitive deficits predict poorer self-reports of self-care such as maintenance of daily tasks8 and self-management of symptoms9 reflecting the complexity of heart failure management.
Daily weighing is recommended in the American College of Cardiology/AHA Guidelines for the Management of Heart Failure.10 The primary function of daily weighing is to detect rapid increases in weight, an indicator of fluid retention or decompensating HF and contributor to hospitalizations.11,12 Despite the observed benefits of daily weight monitoring, less than 40% of patients weigh daily and less than 33% know how to act appropriately in regard to information about their weight gain.13,14 Weight gain is a marker for ineffective self-care demonstrating an inability to adhere to the required components of HF management.
Despite the logical link between cognitive function, daily weighing, and weight gain, to our knowledge, no studies have examined whether cognitive function predicts adherence to daily weighing and actual weight gain as an indicator of the need to take action. This evidence could help our understanding of whether or not cognitive deficits result in poor HF outcomes because they promote poor adherence to daily weighing, greater weight gain, or failure to act appropriately in response to weight gain. Thus, the purpose of this study was (a) to examine whether cognition is associated with adherence to daily weighing and weight gain incidence, and (b) to describe the relationship between weight gain awareness and self-management behaviors. We hypothesized that greater cognitive deficits would be associated with reduced adherence to daily weighing and greater weight gain incidences.
Methods
Participants
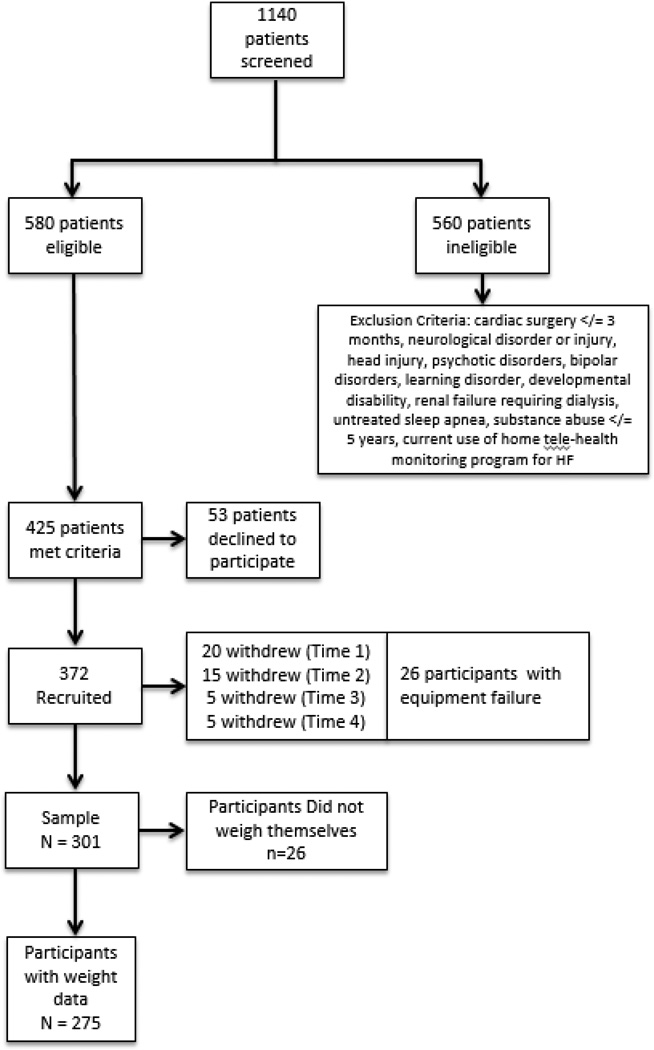
The parent study consisted of 372 patients with HF and age 55 or older enrolled in the Heart Failure Adherence, Behavior, and Cognition Study (Heart ABC). The majority of participants were recruited from the outpatient settings (n=350). See Figure 1 for a sample diagram explaining eligibility criteria and attrition. We have described the sample in detail in previous reports.15–17 The current study used data from 301 participants who provided data on weighing adherence and 275 participants who provided actual weight data to determine weight gain incidence. There were no differences between those who provided data and those who did not on baseline demographic or study variables.
Figure 1.
Study Sample
Procedures
All patients were recruited from inpatient and/or outpatient cardiology practices in Northeast Ohio and gave their consent to participate. Procedures were approved by the Institutional Review Boards of Kent State University, Summa Health Systems, Inc., and University Hospitals Case Medical Center. A research assistant conducted a series of assessments during visits to the patients’ homes.
Neuropsychological testing and self-report questionnaires were administered at the first home visit (Visit #1). Participants were given a HF Self-management knowledge test to identify baseline HF knowledge. If a deficit was noted, instruction was given related to taking medications, adhering to a 2 gram sodium diet, weighing themselves daily, and calling their physician for weight gain of 2–3 pounds in 24 hours or 5 pounds in one week at the beginning of the study. At the second home visit 1–2 weeks later, the research staff installed a scale in the participant’s home and instructed the participant on use of the scale (Visit #2). The research staff provided contact information and written instructions in case the participant encountered any difficulties. Participants were instructed to weigh themselves as they normally would and to record their weights on a paper calendar that was provided. The number of days between visits 2 and 3 served as a run-in period for the participant to familiarize themselves with the scale and to ensure proper use (run-in period = 1 week). The run-in period was important to identify that participants did not have device failure and understood the directions. The time period between home Visit # 3 and Visit # 4 (21 days) was used to calculate the main outcome, daily weighing adherence. There were no reports of difficulty using the paper calendar weight logs.
Measures
Cognitive Functioning
Cognitive functioning was measured across multiple domains using neuropsychological tests that have strong validity and reliability. The three cognitive domains were: (1) Attention -The capacity to attend to and process information; (2) Executive function - The capacity to problem-solve, plan, inhibit, and reason; and (3) Memory - The capacity to retain and recall verbal information. The individual tests used to assess each of these domains are presented in Table 1 and included the following: the Rey Auditory Verbal Learning Test,18 the Stroop Test,19 the Trail Making Test,20 Letter-Number Sequencing from the Wechsler Adult Intelligence Scale,21 and the Frontal Assessment Battery.22
Table 1.
Characteristics of Participants (N = 301)
| M ± SD or N(%) | |
|---|---|
| Demographic, Medical, and Psychosocial Factors (Possible Range) | |
| Age | 68.7 ± 9.7 |
| Female | 150 (40.3) |
| Non-whitea | 104 (28.0) |
| Education Level | |
| 8th Grade or Less | 10 (2.7) |
| 9–11th Grade | 37 (9.9) |
| High School | 100 (26.9) |
| Technical or Trade School | 38 (10.2) |
| Some College | 102 (27.4) |
| Bachelor’s Degree | 50 (13.4) |
| Master’s Degree | 35 (9.4) |
| SES z-score | −0.1 ± 4.4 |
| Charlson Comorbidity Indexb | 3.4 ± 1.8 |
| Medication Regimen Complexity Index | 22.3 ± 12.1 |
| Self-reported HF Severity at Baseline (NYHA) | |
| Class I | 37 (9.9) |
| Class II | 86 (23.1) |
| Class III | 230 (61.8) |
| Class IV | 19 (5.1) |
| Depressive Symptoms (PHQ-9) (0–27) | 4.7 ± 4.9 |
| Anxiety Symptoms (PROMIS-Anxiety) (5–35) | 13.0 ± 5.4 |
| Social Support (MSPSS) (12–84) | 68.7 ± 14.5 |
| Medical Term Recognition Test (METER) (0–40) | 35.3 ± 6.3 |
| Cognitive Function T-scores (20–80) | |
| Attention Composite | 44.4 ± 7.5 |
| Stroop Word | 43.1 ± 9.4 |
| Stroop Color | 45.2 ± 9.6 |
| Trails A | 42.2 ± 10.4 |
| Letter-Number Sequencing | 46.9 ± 10.5 |
| Executive Function Composite | 45.8 ± 8.1 |
| Stroop Color Word | 45.0 ± 10.2 |
| Trails B | 41.4 ± 12.0 |
| Frontal Assessment Battery | 50.9 ± 8.4 |
| Memory Composite | 47.7 ± 7.9 |
| Learning over Time | 49.3 ± 10.8 |
| True Hits | 48.9 ± 9.1 |
| Short Delay | 45.5 ± 10.9 |
| Long Delay | 47.1 ± 9.5 |
| Weight-related Variables | |
| Average 21-Day Adherence (0–100%) | 76.5 ± 33.9 |
| Adherent at least 80% of days | 221(71.8) |
| Experienced weight-gain incidence (3lb/day) | 110(36.6) |
| Baseline knowledge of when to call provider about weight gain | 131(35.3) |
Note. SES = socioeconomic status. HF = heart failure. NYHA = New York Heart Association. PHQ-9 = Patient Health Questionnaire-9. PROMIS-Anxiety = Patient Reported Outcomes Measurement Information System Anxiety Scale. MSPSS = Multidimensional Scale of Perceived Social Support. Means and standard deviations are presented for continuous variables. Sample size and percentages are presented for categorical variables.
Of the non-white participants, 96% identified as African American.
Most common comorbidities reported on the Charlson and corresponding % of participants: myocardial infarction (51.3%), diabetes (44.1%), and chronic obstructive pulmonary disease; COPD (27.4%).
Daily Weighing Adherence
All participants were provided an electronic scale and instructed to weigh themselves as they usually would and record their weight on a paper calendar provided by a research assistant. Daily weighing adherence was defined as the number of days that the participant weighed themselves divided by the total number of days monitored (maximum of 21 days), excluding days that the participant knowingly did not use the scale (e.g., out of town, hospitalized).
Weight Gain Incidences
Research staff calculated the number of times that a patient gained 3 or more pounds in 1 day considering three consecutive day intervals by examining the daily weights. The patient was classified as having a weight gain incidence if the research staff identified at least one of these incidences in their daily weighing calendar.
Weight Gain Symptom Awareness and Management Behavior
Self-care behaviors related to management of weight gain was measured using the Self-Care of Heart Failure Index (SCHFI).23 We used the SCHFI data from the Visit #4 just after weight monitoring ended. We modified the original SCHIFI item which asks about breathing or ankle swelling to include: “Have you had a weight gain of at least 2–3 pounds in a day or 5 pounds in a week?” Also, we added, “If you had an increase in weight, how likely are you to try a symptom remedy? (i.e., reduce salt or fluid, take water pill, or call your doctor).” At baseline, participants were also asked when they should call a doctor or nurse regarding weight gain. Participants were considered correct if they answered “2–3 pounds in a day or 5 pounds in a week.” Participants who answered with the incorrect answer were provided standard education on daily weight monitoring.
Covariate
Age, gender, race, and body mass index (BMI) were collected from patients’ medical charts. Comorbidity was measured using the Charlson Comorbidity Index (CCI), which is a summary score of comorbid medical conditions collected from the medical chart.24 Socioeconomic Status was estimated by calculating a z-score using indicators of income and education for each Zip Code, with higher scores indicating higher socioeconomic status.25 Participants self-reported their highest level of education completed. Participants also self-reported symptoms of HF used to classify their HF severity based on The NYHA functional classification (Class I: No limitation, Class II: Slight limitation, Class III: Marked limitation, and Class IV: Severe limitation). Depression was assessed with the Patient Health Questionnaire-9 (PHQ-9).26 The Multidimensional Scale of Perceived Social Support was used to measure social support (MSPSS).27 The Medical Term Recognition Test (METER) was used to assess health literacy.28
Data Analysis
Raw neuropsychological test scores were converted to age-adjusted scaled scores using normative data for each cognitive test. These scaled scores were converted to T-scores (M = 50, SD = 10) and were averaged to create a composite T-score in order to generate the most accurate measure of cognition for each domain: attention, executive function, and memory. Higher scores indicate more intact cognitive functioning, while lower scores reflect more impaired cognitive functioning.
The characteristics of participants were summarized using mean ± standard deviation for continuous variables and frequencies and percentages for categorical variables. For a variety of reasons there were missing values in multiple variables and the missing data were assumed to be missing at random. Multiple imputations for unbiased parameter estimates were used to impute 20 datasets using a multivariate normal regression model and standard errors of the estimates were corrected appropriately. The multivariate normal regression method used an iterative Markov chain Monte Carlo (MCMC) method to impute missing values. There were no differences in results comparing the imputed and non-imputed data.
The outcome of interest, daily weighing adherence, was negatively skewed; no transformation method could be applied to make the distribution normal. Therefore, median regression, a robust regression procedure, was used to analyze the association between the covariates (demographic, medical, and psychosocial factors) and daily weighing adherence. Then, the associations between cognitive function, daily weighing adherence, and weight gain were examined while adjusting for covariates associated with the outcomes using a multivariable median regression model. Next, logistic regression was used to analyze the associations between the covariates (demographic, medical, and psychosocial factors) and experience of a weight gain incidence. Then, the association between cognitive function and experience of a weight gain incidence was examined using logistic regression while adjusting for significant covariates. All the analyses were performed using Stata 13 software (StataCorp LP, College Station, TX). Descriptive and chi squared statistics were used to describe weight gain symptom awareness and management of weight gain.
Results
Demographic, Medical, and Psychosocial Characteristics of the Sample
Baseline descriptive data for the study sample (N=301) are listed in Table 1. Of the 8.6% (n=26) that did not provide weight data, there were no differences in baseline demographic and cognitive variables compared with those who provided data. The reasons these participants did not provide weight data included not writing down their weight in their log or not using the electronic scale. We recruited 22 patients from the in-patient setting and these participants were younger than the out-patient participants but were not different on any other baseline factors. The majority of participants were older (68.7 ± 9.7 years), White (72.0%), male (59.7%), with at least a high school education (87.3%). Most were categorized as NYHA Class II or III heart failure severity (84.9%) with an average comorbidity score (3.4 ± 1.8). Mean composite scores for each cognitive domain and individual test are presented (Table 1). On average, participants weighed themselves nearly 77% of the days. About 36% (n = 108) of the participants experienced a weight gain incidence during the study period and only 35% (n=105) had baseline knowledge of when to call the doctor regarding weight gain.
Predictors of Daily Weighing Adherence
None of the demographic, medical, psychosocial, or cognitive variables were significantly associated with daily weighing adherence (Table 2). None of the cognitive variables (attention, executive function, memory) were associated with daily weighing adherence in the unadjusted or adjusted median regression models (Table 3).
Table 2.
Unadjusted Median Regressions of Demographic, Medical, Psychosocial, and Cognitive Factors Predicting Daily Weighing Adherence (N=301)
| Factors | β (95% CI) |
SE | p | |
|---|---|---|---|---|
| Demographic/Medical | ||||
| Age | 0.00 (−.29, .29) |
0.15 | 1.000 | |
| Race-ethnicity | 0.00 (−6.22, 6.22) |
3.16 | 1.000 | |
| Sex | −.24 (−5.30, 4.83) |
2.57 | .926 | |
| SES | 0.00 (−0.66, 0.66) |
0.33 | .997 | |
| NYHA Class II | −4.76 (−14.45, 4.93) |
4.92 | .334 | |
| NYHA Class III | −5.00 (−13.81, 3.82) |
4.48 | .265 | |
| NYHA Class IV | 0.00 (−14.28, 14.28) |
7.25 | 1.00 | |
| Psychosocial | ||||
| Depression (PHQ-9) | −0.03 (−0.60, 0.54) |
0.29 | .919 | |
| Social Support (MSPSS) | 0.02 (−0.18, 0.22) |
0.10 | .861 | |
| Health Literacy (METER) | 0.00 (−0.47, 0.47) |
0.24 | 1.000 | |
| Cognitive | ||||
| Attention | 0.00 (−0.38, 0.38) |
0.19 | 1.00 | |
| Executive Function | 0.00 (−0.28, 0.28) |
0.14 | 1.00 | |
| Memory | 0.00 (−0.35, 0.35) |
0.18 | 1.00 | |
Note. CI= confidence interval. SE = standard error. SES = socioeconomic status. NYHA = New York Heart Association. PHQ-9 = Patient Health Questionnaire-9. MSPSS = Multidimensional Scale of Perceived Social Support. METER = Medical Term Recognition Test
Table 3.
Adjusted Median Regressions of Demographic, Medical, Psychosocial, and Cognitive Factors Predicting Daily Weighing Adherence
| Models for each of the Cognitive Variables | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Attention | Executive Attention | Memory | ||||||||
| Variables | β (95%CI) |
SE | p | β (95%CI) |
SE | p | β (95%CI) |
SE | p | |
| Demographic/Medical | ||||||||||
| Age | 0.59 (−0.260, 0.37) |
1.61 | .729 | 0.57 (−0.27, 0.38) |
0.16 | .728 | 0.01 (−0.31, 0.33) |
1.64 | .942 | |
| Race-ethnicity | −1.00 (−8.92, 6.91) |
4.03 | .740 | −1.09 (−9.09, 6.92) |
4.07 | .789 | −1.60 (−9.50, 6.29) |
4.02 | .690 | |
| Sex | −0.74 (−6.95, 5.48) |
3.16 | .795 | −0.89 (−7.28, 5.50) |
3.25 | .785 | 0.86 (−5.92, 7.63) |
3.44 | .803 | |
| SES | −0.22 (−1.01, 0.57) |
0.40 | .570 | −0.24 (−1.04, 0.57) |
0.41 | .561 | −0.25 (−1.06, 0.56) |
0.41 | .540 | |
| Self-reported NYHA | ||||||||||
| Class II | −2.52 (−13.19, 8.16) |
5.43 | .640 | −3.23 (−14.08, 7.62) |
5.51 | .558 | −3.82 (−14.64, 7.02) |
5.50 | .489 | |
| Class III | −5.70 (−15.65, 4.31) |
5.08 | .263 | −5.31 (−15.45, 4.84) |
5.15 | .304 | −6.02 (−16.19, 4.15) |
5.16 | .245 | |
| Class IV | 0.21 (−16.08, 16.50) |
8.26 | .966 | −0.07 (−16.71, 16.57) |
8.46 | .993 | −1.59 (−18.15, 14.96) |
8.40 | .850 | |
| Psychosocial | ||||||||||
| Depression (PHQ-9) |
0.07 (−0.60, 0.73) |
0.34 | .863 | 0.06 (−0.62, 0.74) |
0.35 | .871 | 0.04 (−0.63, 0.72) |
0.34 | .910 | |
| Social Support (MSPSS) |
0.15 (−0.07, 0.37) |
0.11 | .172 | 0.15 (−0.08, 0.39) |
0.12 | .194 | 0.11 (−0.11, 0.34) |
0.11 | .330 | |
| Health Literacy (METER) |
0.05 (−0.43, 0.54) |
0.25 | .833 | 0.05 (−0.43, 0.54) |
0.25 | .836 | −0.01 (−0.49, 0.46) |
0.24 | .950 | |
| Cognitive Domains | ||||||||||
| Attention | −0.19 (−0.65, 0.27) |
0.23 | .424 | -- | -- | -- | -- | -- | -- | |
| Executive Function |
-- | -- | -- | −0.12 (−0.47, 0.23) |
0.18 | .498 | -- | -- | -- | |
| Memory | -- | -- | -- | -- | -- | -- | −0.04 (−0.44, 0.36) |
0.20 | 0.85 | |
Predictors of Weight Gain Incidence
In the adjusted logistic model, all three cognitive variables remained significantly associated with the odds of experiencing a weight gain incidence (Table 4). For every unit increase in attention, participants were 6% less likely to experience a weight gain incidence. For every unit increase in executive function, participants were 3% less likely to experience a weight gain incidence. For every unit increase in memory, participants were 5% less likely to experience a weight gain incidence.
Table 4.
Adjusted Logistic Regressions of Demographic, Medical, Psychosocial, and Cognitive Factors Predicting Experience of a Weight Gain Incident (N=275)
| Models for each of the Cognitive Variables | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Unadjusted | Attention | Executive Attention | Memory | ||||||
| Variables |
OR (95% CI) |
p |
OR (95% CI) |
p |
OR (95% CI) |
p |
OR (95% CI) |
p | |
| Demographic/Medical | |||||||||
| Age | 0.95 (0.92–0.97) |
.001* | 0.95 (0.92–0.98) |
.001* | 0.95 (0.91–0.97) |
.001* | 0.95 (0.92–0.97) |
.001 | |
| Race-ethnicity | 1.65 (0.97–2.80) |
.064† | 1.14 (0.56–2.30) |
.721 | 1.14 (0.56–2.32) |
.712 | 1.24 (0.62–2.49) |
.535 | |
| Sex | 0.84 (0.51–1.37) |
.483 | 0.70 (0.40–1.24) |
.221 | 0.70 (0.40–1.23) |
.211 | 0.85 (0.47–1.56) |
.609 | |
| SES | 0.96 (0.91–1.02) |
.192 | 10.2 (0.95–1.09) |
.619 | 1.02 (0.95–1.09) |
.629 | 1.01 (0.94–1.08) |
.705 | |
| Self-reported NYHA | |||||||||
| Class II | 0.45 (0.18–1.10) |
.080† | 0.50 (0.19–1.27) |
.145 | 0.49 (0.19–1.26) |
.140 | 0.50 (0.19–1.28) |
.148 | |
| Class III | 0.72 (0.32–1.60) |
.420 | 0.65 (0.27–1.56) |
.335 | 0.69 (0.29–1.63) |
.400 | 0.74 (0.30–1.76) |
.502 | |
| Class IV | 0.55 (0.21–2.73) |
.663 | 0.67 (0.16–2.76) |
.580 | 0.70 (0.17–2.88) |
.616 | 0.62 (0.15–2.56) |
.513 | |
| Psychosocial | |||||||||
| Depression (PHQ-9) |
1.03 (0.98–1.08) |
.237 | 1.02 (0.96–1.08) |
.621 | 1.01 (0.95–1.08) |
.653 | 1.01 (0.96–1.07) |
.677 | |
| Social Support (MSPSS) |
1.00 (0.98–1.02) |
.898 | 1.00 (0.99–1.03) |
.346 | 1.01 (0.99–1.03) |
.331 | 1.00 (0.99–1.02) |
.679 | |
| Health Literacy (METER) |
1.00 (0.95–1.03) |
.624 | 1.01 (0.97–1.06) |
.646 | 1.01 (0.98–1.06) |
.611 | 1.00 (0.96–1.05) |
.802 | |
| Cognitive Domains | |||||||||
| Attention | 0.94 (0.91–0.98) |
.001* | 0.95 (0.91–0.99) |
.016* | -- | -- | -- | -- | |
| Executive Function |
0.97 (0.94–0.99) |
.008* | -- | -- | 0.96 (0.93–1.00) |
.026* | -- | -- | |
| Memory | 0.95 (0.92–0.98) |
.003* | -- | -- | -- | -- | 0.96 (0.92–0.99) |
.023* | |
Note. SE = standard error. SES = socioeconomic status. NYHA = New York Heart Association. PHQ-9 = Patient Health Questionnaire-9. MSPSS = Multidimensional Scale of Perceived Social Support. METER = Medical Term Recognition Test
Significant at p < .05
Significant at p < .10
Self-reported Weight Gain Symptom Awareness and Management Behaviors
At baseline, only 35% (n=105) of our sample knew when to call a provider regarding weight gain. Likewise, among those who experienced weight gain (n=102), only 36% knew this information at baseline. Of the 102 individuals who had a weight gain and filled out the SCHFI, 65% (n=66) did not self-report the weight increase on the SCHFI. Problematically, if patients did not identify this symptom, they were then unlikely to answer the question regarding which management strategy they pursued to address it (i.e., 12% of the patients with a weight gain who completed the SCHFI failed to answer the question regarding management strategies/remedies). Accordingly, it may not be surprising that the patients with a weight gain did not report a higher likelihood of using any of the following symptom management strategies compared to patients without a gain: reduce salt (χ2(3, 251) = 2.35, p = .502); reduce fluid intake (χ2(3, 251) = 6.37, p = .095); take a water pill (χ2(3, 252) = 1.32, p = .724); or call their provider (χ2(3, 251) = 0.987, p = .804).
Discussion
Our hypothesis that greater cognitive deficits would be associated with reduced adherence to daily weighing and greater weight gain incidences was partially supported. We found that cognitive function was not associated with daily weighing behavior in patients with HF, but diminishing cognitive function increased the likelihood of experiencing a clinically significant weight gain. This effect remained after adjustment for multiple demographic and psychosocial variables and HF severity.
One potential reason that we did not detect an association between cognitive function and daily weighing behavior may be that individuals in this sample on average had good adherence to daily weighing (76.5%). This high rate of adherence may have resulted from our study design, in which we provided all participants with a scale, a written log (calendar), and instructions to record daily weights. These instructions and tools could have unintentionally served as an intervention to increase daily weighing behavior among study participants. It is also possible that the sample was biased due to the attrition rate (i.e. those who dropped out had reduced adherence); however, no differences in baseline study variables were noted.
Despite the lack of association between weighing behavior and cognitive function, our finding that patients with cognitive impairment are at increased risk for clinically significant weight gain has important implications. Sudden increases in weight are associated with increased hospitalization11 and rates of mortality.29 Thus, our findings suggests that adults with cognitive impairment need to be monitored more closely for increased weight gain incidences. The clinical implications include identification of patients at risk and implementing a system for reporting daily weights. Telehealth strategies would be beneficial for patients with cognitive impairment to improve communication of weight status.
Poorer outcomes may partially result from a lack of awareness/knowledge of what constitutes a clinically significant increase in weight that would require intervention such as contacting a healthcare provider. Baseline testing indicated that over 60% of participants who experienced a clinically significant weight gain were unaware at baseline of when to call a doctor or nurse regarding weight gain (despite being given instruction at the beginning of the study). Such findings suggest that interventions to increase daily weighing may not be effective at improving health outcomes if adults do not recognize the weight gain or pursue symptom management such as calling the doctor/nurse regarding weight gain. In line with this possibility, White and colleagues demonstrated that daily weight monitoring was not associated with medical-seeking behavior.30 Although 75% of the participants experienced a clinically significant weight gain, only one sought medical attention.30 Future studies should examine potential ways to increase the cue to action by possibly visually graphing data so that increases can be seen, setting an alarm to alert adults with HF that a clinically significant weight gain has been detected, or simulation to practice the behavior to increase efficacy.
Delay in responding to heart failure symptoms has been reported. Contextual factors for delay in action include prior illness experiences, social and emotional factors.31 In addition, not reporting weight gain may be related to patients’ interpretation that the weight gain as unrelated to their HF condition. If these patterns are detected, family members can be encouraged to assist by monitoring weight and providing assistance for reporting.
Several limitations of this study are noted. First, generalizability of the findings to individuals of other race-ethnicities and geographical locations may be an issue given that the participants were mostly Whites (72%) who reside in the Midwest U.S. Second, the weighing behavior collected in this study may not represent the participants’ typical routine, given that the study design may have encouraged daily weighing (i.e., a pseudo- intervention effect). Although these limitations exist, there are also several strengths in this study. First, the study consisted of a large sample size. Further, the data collected included a rigorous array of gold standard neuropsychological tests to ensure the most accurate estimate of cognitive function.
Cognitive function was not associated with daily weighing adherence in this sample of older adults with HF. Given a weight scale, calendar, and instructions, most participants had high adherence to daily weighing recommendations regardless of cognitive status. Those with diminishing cognitive function, however, were at increased risk of weight gain. Unfortunately, the majority of those who experienced a clinically significant weight gain were unaware of when to seek medical attention regarding that bodily change. Findings suggests that HF patients may benefit most when health professionals not only promote daily weighing, but also emphasize the importance of recognizing clinically significant weight gain and seeking medical attention when this weight gain is detected.
What’s New?
Poorer cognitive function increased the likelihood of experiencing a clinically significant weight gain in adult patients with heart failure.
Over 60% of participants with a clinically significant weight gain were unaware at baseline of when to call a provider regarding weight gain (despite being given instruction at study initiation).
HF patients may benefit most when health providers not only promote daily weighing but also emphasize the importance of recognizing clinically significant weight gain and seeking medical attention when this weight gain is detected
Acknowledgments
Funding: This work was supported by the National Heart, Lung, and Blood Institute [R01 HL096710-01A1 to M.D. and J.W.H].
Footnotes
Compliance with Ethical Standards: All procedures were reviewed and approved by the appropriate Institutional Review Boards and all participants provided written informed consent.
The authors have no conflicts of interest.
References
- 1.Rutledge T, Reis VA, Linke SE, Greenberg BH, Mills PJ. Depression in Heart Failure: A Meta-Analytic Review of Prevalence, Intervention Effects, and Associations With Clinical Outcomes. J. Am. Coll. Cardiol. 2006;48(8):1527–1537. doi: 10.1016/j.jacc.2006.06.055. [DOI] [PubMed] [Google Scholar]
- 2.Watson R, Gibbs C, Lip G. ABC of heart failure: clinical features and complications. BMJ: British Medical Journal. 2000;320(7229):236. doi: 10.1136/bmj.320.7229.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harkness K, Demers C, Heckman GA, McKelvie RS. Screening for cognitive deficits using the montreal cognitive assessment tool in outpatients≥ 65 years of age with heart failure. The American journal of cardiology. 2011;107(8):1203–1207. doi: 10.1016/j.amjcard.2010.12.021. [DOI] [PubMed] [Google Scholar]
- 4.Pressler SJ, Subramanian U, Kareken D, et al. Cognitive deficits in chronic heart failure. Nursing Research. 2010;59(2):127. doi: 10.1097/NNR.0b013e3181d1a747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zuccalà G, Pedone C, Cesari M, et al. The effects of cognitive impairment on mortality among hospitalized patients with heart failure. The American Journal of Medicine. 2003;115(2):97–103. doi: 10.1016/s0002-9343(03)00264-x. [DOI] [PubMed] [Google Scholar]
- 6.Zuccala G, Onder G, Pedone C, et al. Cognitive dysfunction as a major determinant of disability in patients with heart failure: results from a multicentre survey. Journal of Neurology, Neurosurgery & Psychiatry. 2001;70(1):109–112. doi: 10.1136/jnnp.70.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Riegel B, Dickson VV, Faulkner K. The Situation-Specific Theory of Heart Failure Self-Care. Review of Literature. 2016;31(3):226–235. doi: 10.1097/JCN.0000000000000244. [DOI] [PubMed] [Google Scholar]
- 8.Currie K, Rideout A, Lindsay G, Harkness the association between mild cognitive impairment and self-care in adults with chronic heart failure: A systematic Review and narrative synthesis. Journal of Cardiovascular Nursing. 2015;30(5):382–391. doi: 10.1097/JCN.0000000000000173. [DOI] [PubMed] [Google Scholar]
- 9.Cameron J, Worrall-Carter L, Page K, Riegel B, Lo SK, Stewart S. Does cognitive impairment predict poor self-care in patients with heart failure? Eur. J. Heart Fail. 2010;12(5):508–515. doi: 10.1093/eurjhf/hfq042. [DOI] [PubMed] [Google Scholar]
- 10.Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA Guideline for the Management of Heart FailureA Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J. Am. Coll. Cardiol. 2013;62(16):e147–e239. doi: 10.1016/j.jacc.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 11.Chaudhry SI, Wang Y, Concato J, Gill TM, Krumholz HM. Patterns of weight change preceding hospitalization for heart failure. Circulation. 2007;116(14):1549–1554. doi: 10.1161/CIRCULATIONAHA.107.690768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang J, Goode KM, Cuddihy PE, Cleland JG. Predicting hospitalization due to worsening heart failure using daily weight measurement: analysis of the Trans-European Network-Home-Care Management System (TEN-HMS) study. Eur. J. Heart Fail. 2009;11(4):420–427. doi: 10.1093/eurjhf/hfp033. [DOI] [PubMed] [Google Scholar]
- 13.Sulzbach-Hoke L, Kagan S, Craig K. Weighing behavior and symptom distress of clinic patients with CHF. Medsurg nursing: official journal of the Academy of Medical-Surgical Nurses. 1997;6(5):288–293. 314. [PubMed] [Google Scholar]
- 14.Wal MH, Jaarsma T, Veldhuisen DJ. Non-compliance in patients with heart failure; how can we manage it? European journal of heart failure. 2005;7(1):5–17. doi: 10.1016/j.ejheart.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 15.Hawkins MA, Gunstad J, Dolansky MA, et al. Greater body mass index is associated with poorer cognitive functioning in male heart failure patients. Journal of cardiac failure. 2014;20(3):199–206. doi: 10.1016/j.cardfail.2013.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hawkins MA, Dolansky MA, Schaefer JT, et al. Cognitive function in heart failure is associated with nonsomatic symptoms of depression but not somatic symptoms. Journal of Cardiovascular Nursing. 2015;30(5):E9–E17. doi: 10.1097/JCN.0000000000000178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hawkins MA, Gathright EC, Gunstad J, et al. The MoCA and MMSE as screeners for cognitive impairment in a heart failure population: A study with comprehensive neuropsychological testing. Heart & Lung: The Journal of Acute and Critical Care. 2014;43(5):462–468. doi: 10.1016/j.hrtlng.2014.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meyers JE, Meyers KR. Rey Complex Figure test and Recognition Trial. Odessa, FL: Psychological Assessment Resources; 1995. [Google Scholar]
- 19.Golden JC. Stroop Color and Word Test. Chicago, IL: Stoelting; 1978. [Google Scholar]
- 20.Reitan RM. Validity of the Trail Making Test as an indicator of organic brain damage. Perceptual and motor skills. 1958;8(3):271–276. [Google Scholar]
- 21.Wechsler D. Wechsler Adult Intelligence Scale-Third Edition. San Antonio, TX: The Psychological Corporation; 1997. [Google Scholar]
- 22.Dubois B, Slachevsky A, Litvan I, Pillon B. The FAB A frontal assessment battery at bedside. Neurology. 2000;55(11):1621–1626. doi: 10.1212/wnl.55.11.1621. [DOI] [PubMed] [Google Scholar]
- 23.Vellone E, Riegel B, Cocchieri A, et al. Psychometric testing of the self-care of heart failure index version 6.2. Research in nursing & health. 2013;36(5):500–511. doi: 10.1002/nur.21554. [DOI] [PubMed] [Google Scholar]
- 24.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J. Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 25.Roux AVD, Merkin SS, Arnett D, et al. Neighborhood of residence and incidence of coronary heart disease. N. Engl. J. Med. 2001;345(2):99–106. doi: 10.1056/NEJM200107123450205. [DOI] [PubMed] [Google Scholar]
- 26.Kroenke K, Spitzer RL, Williams JB. The Phq-9. Journal of general internal medicine. 2001;16(9):606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zimet GD, Powell SS, Farley GK, Werkman S, Berkoff KA. Psychometric characteristics of the multidimensional scale of perceived social support. Journal of personality assessment. 1990;55(3–4):610–617. doi: 10.1080/00223891.1990.9674095. [DOI] [PubMed] [Google Scholar]
- 28.Rawson KA, Gunstad J, Hughes J, et al. The METER: a brief, self-administered measure of health literacy. Journal of general internal medicine. 2010;25(1):67–71. doi: 10.1007/s11606-009-1158-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goldberg LR, Piette JD, Walsh MN, et al. Randomized trial of a daily electronic home monitoring system in patients with advanced heart failure: the Weight Monitoring in Heart Failure (WHARF) trial. Am. Heart J. 2003;146(4):705–712. doi: 10.1016/S0002-8703(03)00393-4. [DOI] [PubMed] [Google Scholar]
- 30.White MM, Howie-Esquivel J, Caldwell MA. Improving heart failure symptom recognition: a diary analysis. J. Cardiovasc. Nurs. 2010;25(1):7–12. doi: 10.1097/JCN.0b013e3181b7af9e. [DOI] [PubMed] [Google Scholar]
- 31.Jurgens CY, Hoke L, Brynes J, Riegel B. Why do elders delay responding to heart failure symptoms. Nursing Research. 2009;58(4):274–282. doi: 10.1097/NNR.0b013e3181ac1581. [DOI] [PubMed] [Google Scholar]