Abstract
The ability of intact spinach (Spinacia oleracea) chloroplast preparations to catalyze CO2 fixation and photophosphorylation was examined. Under conditions optimal for CO2 fixation, only poor photophosphorylation was observed. Conditions optimal for photophosphorylation were found to be highly inhibitory to the CO2-fixing capacity of the intact chloroplast preparation.
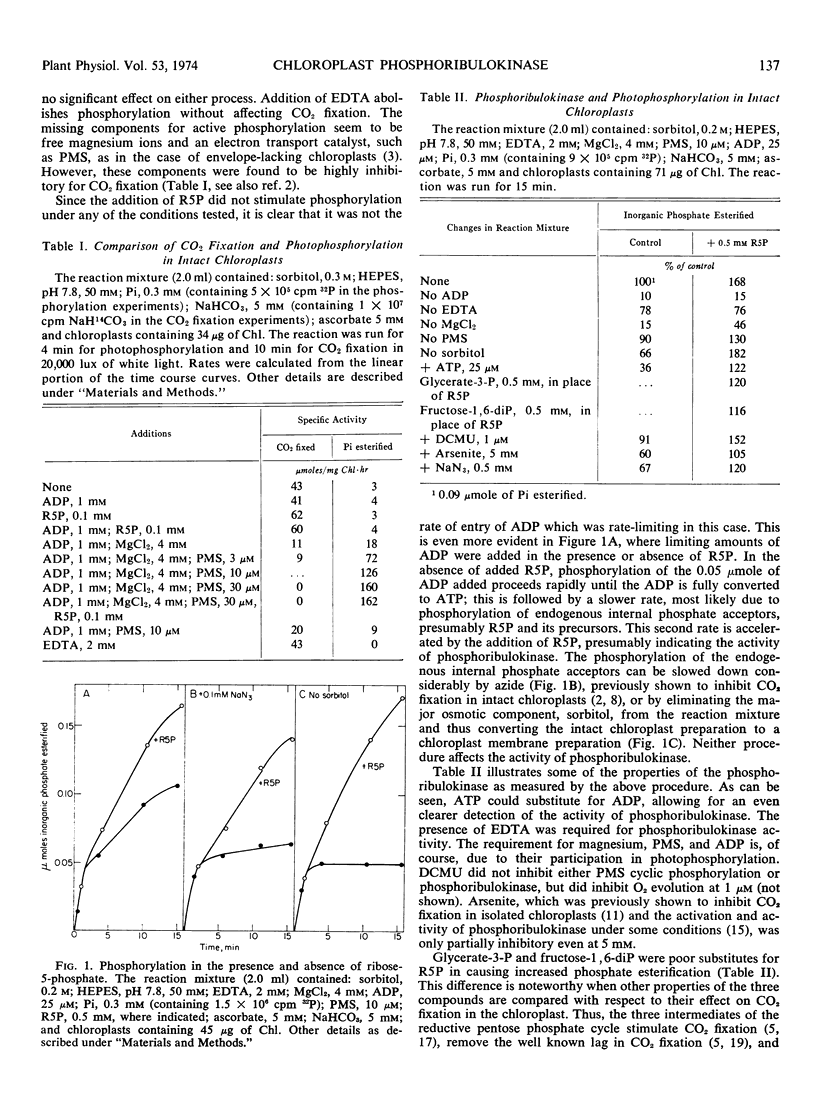
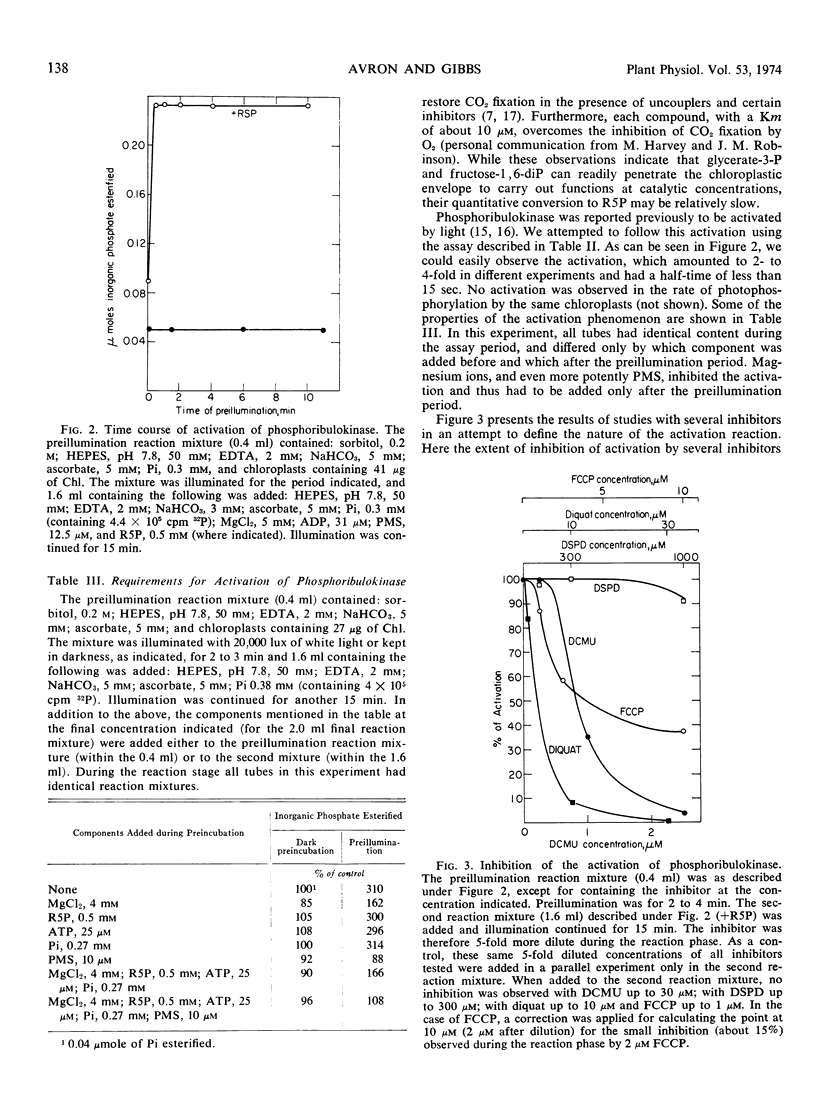
A method for following the activity of phosphoribulokinase in the intact chloroplast preparation was developed, and conditions for optimal activity were defined. The enzyme was found to be activated 2- to 4-fold by preillumination with a half-time of less than 15 seconds. Activation was inhibited by magnesium ions and selectively by inhibitors of photosynthetic electron transport. We concluded that activation was due to the effect of a photoproduced reductant in a site preceding ferredoxin in the electron transport chain. The photoactivated state of the enzyme decayed in the dark with a half-time of about 8 minutes.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avron M., Gibbs M. Carbon dioxide fixation in the light and in the dark by isolated spinach chloroplasts. Plant Physiol. 1974 Feb;53(2):140–143. doi: 10.1104/pp.53.2.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldry C. W., Walker D. A., Bucke C. Calvin-cycle intermediates in relation to induction phenomena in photosynthetic carbon dioxide fixation by isolated chloroplasts. Biochem J. 1966 Dec;101(3):642–646. doi: 10.1042/bj1010642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamberger E. S., Gibbs M. Effect of Phosphorylated Compounds and Inhibitors on CO(2) Fixation by Intact Spinach Chloroplasts. Plant Physiol. 1965 Sep;40(5):919–926. doi: 10.1104/pp.40.5.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Amotz A., Avron M. Is nicotinamide adenine dinucleotide phosphate an obligatory intermediate in photosynthesis? Plant Physiol. 1972 Feb;49(2):244–248. doi: 10.1104/pp.49.2.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockburn W., Walker D. A., Baldry C. W. Photosynthesis by isolated chloroplasts. Reversal of orthophosphate inhibition by Calvin-cycle intermediates. Biochem J. 1968 Mar;107(1):89–95. doi: 10.1042/bj1070089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIBBS M., CALO N. Studies on carbon dioxide fixation by chloroplasts, the effect of arsenite and other factors. Biochim Biophys Acta. 1960 Nov 4;44:341–347. doi: 10.1016/0006-3002(60)91570-5. [DOI] [PubMed] [Google Scholar]
- Heldt H. W. Adenine nucleotide translocation in spinach chloroplasts. FEBS Lett. 1969 Sep;5(1):11–14. doi: 10.1016/0014-5793(69)80280-2. [DOI] [PubMed] [Google Scholar]
- Latzko E., von Garnier R., Gibbs M. Effect of photosynthesis, photosynthetic inhibitors and oxygen on the activity of ribulose 5-phosphate kinase. Biochem Biophys Res Commun. 1970;39(6):1140–1144. doi: 10.1016/0006-291x(70)90678-9. [DOI] [PubMed] [Google Scholar]
- Schacter B., Eley J. H., Gibbs M. Involvement of Photosynthetic Carbon Reduction Cycle Intermediates in CO(2) Fixation and O(2) Evolution by Isolated Chloroplasts. Plant Physiol. 1971 Dec;48(6):707–711. doi: 10.1104/pp.48.6.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker D. A., Crofts A. R. Photosynthesis. Annu Rev Biochem. 1970;39:389–428. doi: 10.1146/annurev.bi.39.070170.002133. [DOI] [PubMed] [Google Scholar]
- Zweig G., Shavit N., Avron M. Diquat (I,I'-ethylene-2,2'-dipyridylium dibromide) in photo-reactions of isolated chloroplasts. Biochim Biophys Acta. 1965 Nov 29;109(2):332–346. [PubMed] [Google Scholar]


