Abstract
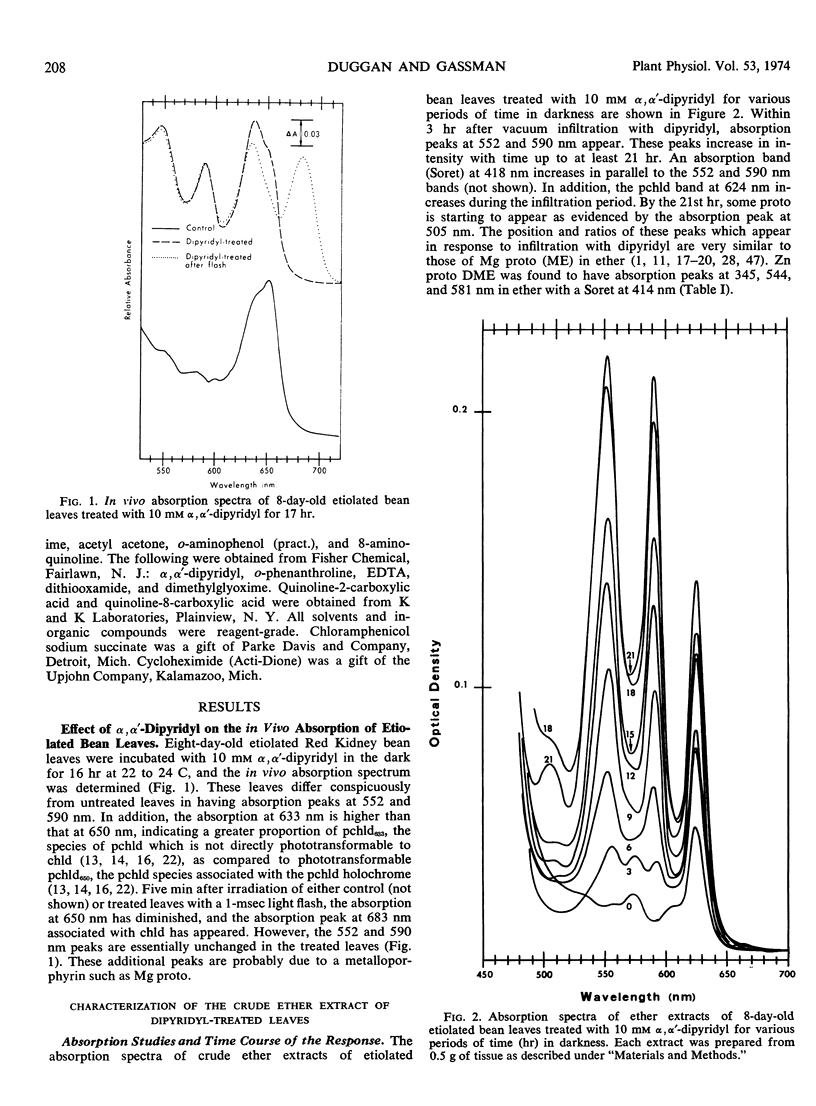
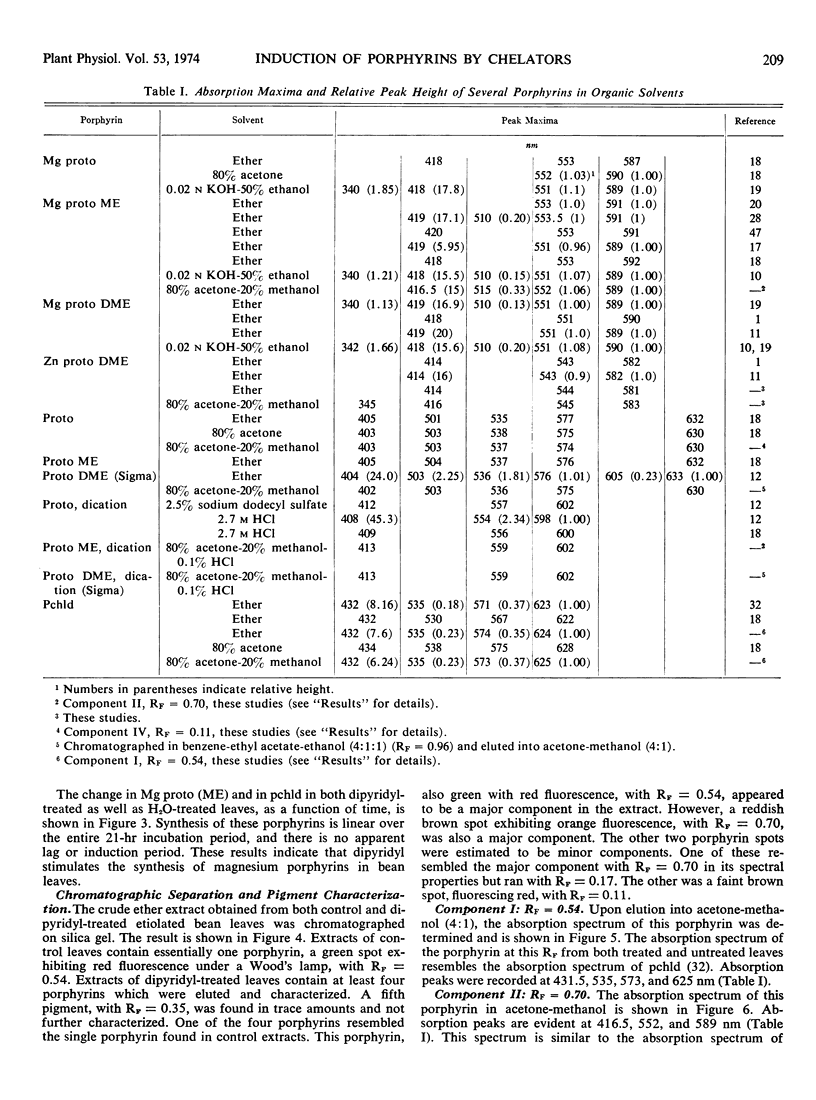
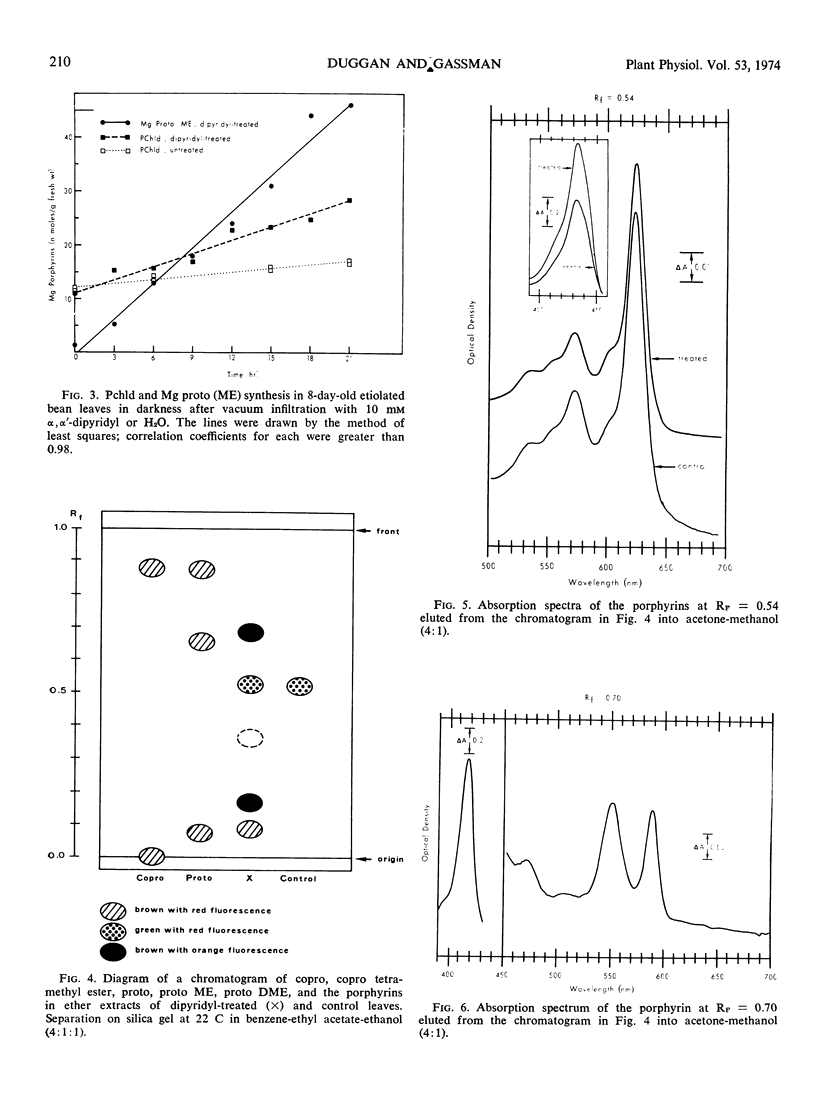
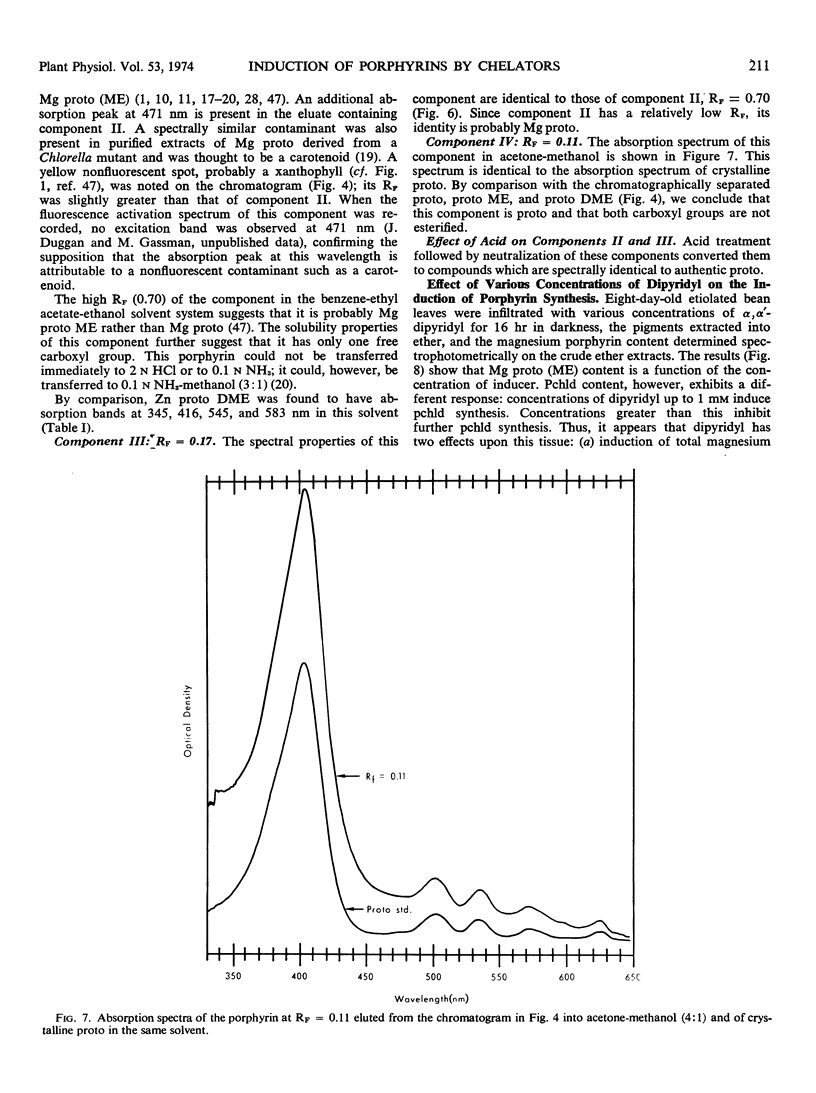
Primary leaves of 7- to 9-day-old etiolated seedlings of Phaseolus vulgaris L. var. Red Kidney infiltrated in darkness with aqueous solutions of α, α′-dipyridyl, o-phenanthroline, pyridine-2-aldoxime, pyridine-2-aldehyde, 8-hydroxyquinoline, or picolinic acid synthesize large amounts of magnesium protoporphyrin monomethyl ester and lesser amounts of magnesium protoporphyrin, protoporphyrin, and protochlorophyllide. Pigment formation proceeds in a linear manner for up to 21 hours after vacuum infiltration with 10 mm α, α′-dipyridyl. Etiolated tissues of Zea mays L., Cucumis sativus L., and Pisum sativum L. respond in the same way to dipyridyl treatment. Compounds active in eliciting this response are aromatic heterocyclic nitrogenous bases which also act as bidentate chelators and form extremely stable complexes with iron; other metal ion chelators, such as ethylenediaminetetraacetic acid, salicylaldoxime, and sodium diethyldithiocarbamate, do not elicit any pigment synthesis. The ferrous, ferric, cobaltous, and zinc chelates of α, α′-dipyridyl are similarly ineffective. If levulinic acid is supplied to etiolated bean leaves together with α, α′-dipyridyl, porphyrin production is inhibited and δ-aminolevulinic acid accumulates in the tissue. Synthesis of porphyrins proceeds in the presence of 450 micrograms per milliliter chloramphenicol or 50 micrograms per milliliter cycloheximide with only partial diminution. We propose that heme or an iron-protein complex blocks the action of the enzyme(s) governing the synthesis of δ-aminolevulinic acid in etiolated leaves in the dark and that iron chelators antagonize this inhibition, leading to the biosynthesis of δ-aminolevulinic acid and porphyrins.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURNHAM B. F., PIERCE W. S., WILLIAMS K. R., BOYER M. H., KIRBY C. K. delta-aminolaevulate dehydratase from Rhodopseudomonas spheroides. Biochem J. 1963 Jun;87:462–472. doi: 10.1042/bj0870462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum S. J., Ellsworth R. K. The chromatographic separation of magnesium protoporphyrin IX dimethyl esters from zinc protoporphyrin IX dimethyl esters. J Chromatogr. 1970 Mar 31;47(3):503–505. doi: 10.1016/0021-9673(70)80078-4. [DOI] [PubMed] [Google Scholar]
- Beale S. I., Castelfranco P. A. 14 C incorporation from exogenous compounds into -aminolevulinic acid by greening cucumber cotyledons. Biochem Biophys Res Commun. 1973 May 1;52(1):143–149. doi: 10.1016/0006-291x(73)90966-2. [DOI] [PubMed] [Google Scholar]
- Beale S. I. Studies on the Biosynthesis and Metabolism of delta-Aminolevulinic Acid in Chlorella. Plant Physiol. 1971 Sep;48(3):316–319. doi: 10.1104/pp.48.3.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beale S. I. The biosynthesis of delta-aminolevulinic acid in Chlorella. Plant Physiol. 1970 Apr;45(4):504–506. doi: 10.1104/pp.45.4.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COOPER R. THE BIOSYNTHESIS OF COPROPORPHYRINOGEN, MAGNESIUM PROTOPORPHYRIN MONOMETHYL ESTER AND BACTERIOCHLOROPHYLL BY RHODOPSEUDOMONAS CAPSULATA. Biochem J. 1963 Oct;89:100–108. doi: 10.1042/bj0890100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRANICK S. Magnesium protoporphyrin as a precursor of chlorophyll in Chlorella. J Biol Chem. 1948 Aug;175(1):333–342. [PubMed] [Google Scholar]
- GRANICK S. Magnesium protoporphyrin monoester and protoporphyrin monomethyl ester in chlorophyll biosynthesis. J Biol Chem. 1961 Apr;236:1168–1172. [PubMed] [Google Scholar]
- GRANICK S., URATA G. Increase in activity of alpha-aminolevulinic acid synthetase in liver mitochondria induced by feeding of 3,5-dicarbethoxy-1,4-dihydrocollidine. J Biol Chem. 1963 Feb;238:821–827. [PubMed] [Google Scholar]
- Gassman M. L. Absorbance and fluorescence properties of protochlorophyllide in etiolated bean leaves. Biochem Biophys Res Commun. 1973 Aug 6;53(3):693–702. doi: 10.1016/0006-291x(73)90149-6. [DOI] [PubMed] [Google Scholar]
- Gassman M., Bogorad L. Control of chlorophyll production in rapidly greening bean leaves. Plant Physiol. 1967 Jun;42(6):774–780. doi: 10.1104/pp.42.6.774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gassman M., Bogorad L. Studies on the regeneration of protochlorophyllide after brief illumination of etiolated bean leaves. Plant Physiol. 1967 Jun;42(6):781–784. doi: 10.1104/pp.42.6.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorchein A. Magnesium protoporphyrin chelatase activity in Rhodopseudomonas spheroides. Studies with whole cells. Biochem J. 1972 Mar;127(1):97–106. doi: 10.1042/bj1270097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gough S. Defective synthesis of porphyrins in barley plastids caused by mutation in nuclear genes. Biochim Biophys Acta. 1972 Nov 24;286(1):36–54. doi: 10.1016/0304-4165(72)90086-4. [DOI] [PubMed] [Google Scholar]
- Harel E., Klein S. Light dependent formation of -aminolevulinic acid in etiolated leaves of higher plants. Biochem Biophys Res Commun. 1972 Oct 17;49(2):364–370. doi: 10.1016/0006-291x(72)90419-6. [DOI] [PubMed] [Google Scholar]
- Ho Y. K., Lascelles J. -aminolevulinic acid dehydratase of Spirillum itersonii and the regulation of tetrapyrrole synthesis. Arch Biochem Biophys. 1971 Jun;144(2):734–740. doi: 10.1016/0003-9861(71)90381-x. [DOI] [PubMed] [Google Scholar]
- Hsu W. P., Miller G. W. Chlorophyll and porphyrin synthesis in relation to iron in Nicotiana tabacum, L. Biochim Biophys Acta. 1965 Dec 16;111(2):393–402. doi: 10.1016/0304-4165(65)90049-8. [DOI] [PubMed] [Google Scholar]
- JONES O. T. MAGNESIUM 2,4-DIVINYLPHAEOPORPHYRIN A5 MONOMETHYL ESTER, A PROTOCHLOROPHYLL-LIKE PIGMENT PRODUCED BY RHODOPSEUDOMONAS SPHEROIDES. Biochem J. 1963 Nov;89:182–189. doi: 10.1042/bj0890182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JONES O. T. THE INHIBITION OF BACTERIOCHLOROPHYLL BIOSYNTHESIS IN RHODOPSEUDOMONAS SPHEROIDES BY 8-HYDROXYQUINOLINE. Biochem J. 1963 Aug;88:335–343. doi: 10.1042/bj0880335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JONES O. T. The production of magnesium protoporphyrin monomethyl ester by Rhodopseudomonas spheroides. Biochem J. 1963 Mar;86:429–432. doi: 10.1042/bj0860429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOSKI V. M., SMITH J. H. C. The isolation and spectral absorption properties of protochlorophyll from etiolated barley seedlings. J Am Chem Soc. 1948 Nov;70(11):3558–3562. doi: 10.1021/ja01191a006. [DOI] [PubMed] [Google Scholar]
- LABBE R. F., HUBBARD N. Preparation and properties of the iron-protoporphyrin chelating enzyme. Biochim Biophys Acta. 1960 Jul 1;41:185–191. doi: 10.1016/0006-3002(60)90001-9. [DOI] [PubMed] [Google Scholar]
- LASCELLES J. Adaptation to form bacteriochlorophyll in Rhodopseudomonas spheroides: changes in activity of enzymes concerned in pyrrole synthesis. Biochem J. 1959 Jul;72:508–518. doi: 10.1042/bj0720508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LASCELLES J. Synthesis of tetrapyrroles by microorganisms. Physiol Rev. 1961 Apr;41:417–441. doi: 10.1152/physrev.1961.41.2.417. [DOI] [PubMed] [Google Scholar]
- LASCELLES J. The synthesis of porphyrins and bacteriochlorophyll by cell suspensions of Rhodopseudomonas spheroides. Biochem J. 1956 Jan;62(1):78–93. doi: 10.1042/bj0620078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levere R. D., Granick S. Control of hemoglobin synthesis in the cultured chick blastoderm by delta-aminolevulinic acid synthetase: increase in the rate of hemoglobin formation with delta-aminolevulinic acid. Proc Natl Acad Sci U S A. 1965 Jul;54(1):134–137. doi: 10.1073/pnas.54.1.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAUZERALL D., GRANICK S. The occurrence and determination of delta-amino-levulinic acid and porphobilinogen in urine. J Biol Chem. 1956 Mar;219(1):435–446. [PubMed] [Google Scholar]
- Marsh H. V., Evans H. J., Matrone G. Investigations of the Role of Iron in Chlorophyll Metabolism I. Effect of Iron Deficiency on Chlorophyll and Heme Content and on the Activities of Certain Enzymes in Leaves. Plant Physiol. 1963 Nov;38(6):632–638. doi: 10.1104/pp.38.6.632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh H. V., Evans H. J., Matrone G. Investigations of the Role of Iron in Chlorophyll Metabolism. II. Effect of Iron Deficiency on Chlorophyll Synthesis. Plant Physiol. 1963 Nov;38(6):638–642. doi: 10.1104/pp.38.6.638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadler K., Granick S. Controls on chlorophyll synthesis in barley. Plant Physiol. 1970 Aug;46(2):240–246. doi: 10.1104/pp.46.2.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nandi D. L., Shemin D. Delta-aminolevulinic acid dehydratase of Rhodopseudomonas spheroides. 3. Mechanism of porphobilinogen synthesis. J Biol Chem. 1968 Mar 25;243(6):1236–1242. [PubMed] [Google Scholar]
- Ramaswamy N. K., Nair P. M. -Aminolevulinic acid synthetase from cold-stored potatoes. Biochim Biophys Acta. 1973 Jan 12;293(1):269–277. doi: 10.1016/0005-2744(73)90399-9. [DOI] [PubMed] [Google Scholar]
- Rebeiz C. A., Crane J. C., Nishijima C. The biosynthesis of metal porphyrins by subchloroplastic fractions. Plant Physiol. 1972 Jul;50(1):185–186. doi: 10.1104/pp.50.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebeiz C. A., Haidar M. A., Yaghi M. Porphyrin Biosynthesis in Cell-free Homogenates from Higher Plants. Plant Physiol. 1970 Oct;46(4):543–549. doi: 10.1104/pp.46.4.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SANO S., GRANICK S. Mitochondrial coproporphyrinogen oxidase and protoporphyrin formation. J Biol Chem. 1961 Apr;236:1173–1180. [PubMed] [Google Scholar]
- Strand L. J., Manning J., Marver H. S. The induction of -aminolevulinic acid synthetase in cultured liver cells. The effects of end product and inhibitors of heme synthesis. J Biol Chem. 1972 May 10;247(9):2820–2827. [PubMed] [Google Scholar]
- Süzer S., Sauer K. The sites of photoconversion of protochlorophyllide to chlorophyllide in barley seedlings. Plant Physiol. 1971 Jul;48(1):60–63. doi: 10.1104/pp.48.1.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TOWNSLEY P. M., NEILANDS J. B. The iron and porphyrin metabolism of Micrococcus lysodeikticus. J Biol Chem. 1957 Feb;224(2):695–705. [PubMed] [Google Scholar]
- Tait G. H. Coproporphyrinogenase activity in extracts from Rhodopseudomonas spheroides. Biochem Biophys Res Commun. 1969 Sep 24;37(1):116–122. doi: 10.1016/0006-291x(69)90888-2. [DOI] [PubMed] [Google Scholar]
- URATA G., GRANICK S. Biosynthesis of alpha-aminoketones and the metabolism of aminoacetone. J Biol Chem. 1963 Feb;238:811–820. [PubMed] [Google Scholar]


