Abstract
Obesity, diabetes, and cardiovascular disease (CVD) present important unmet prevention and treatment challenges. Dietary pulses are sustainable, affordable, and nutrient‐dense foods that have shown a wide range of health benefits in the prevention and management of these conditions. Despite these findings, recommendations for pulse intake continue to vary across chronic disease guidelines, and intake levels continue to remain low. Here, we summarize findings from recent systematic reviews and meta‐analyses assessing the relationship between dietary pulse consumption and cardiometabolic health and assess the overall strength of the evidence using the Grading of Recommendations, Assessment, Development, and Evaluation tool. We conclude that systematic reviews and meta‐analyses of prospective cohort studies assessing the relationship between legumes and the risk of coronary heart disease appear to provide moderate‐quality evidence of a benefit, and several systematic reviews and meta‐analyses of randomized controlled trials assessing the effect of pulses on cardiometabolic risk factors provide low‐ to moderate‐quality evidence of a benefit. There remains an urgent need, however, for more high‐quality prospective cohort studies and large, high‐quality, randomized trials to clarify the benefits of dietary pulses in the prevention and management of overweight/obesity, diabetes, and CVD.
Keywords: dietary pulses, cardiometabolic health, review, GRADE
Introduction
The dual epidemics of obesity and type 2 diabetes continue to increase and threaten the recent gains made in cardiovascular disease (CVD) prevention.1, 2, 3 Chronic disease guidelines recommend diet and lifestyle as the cornerstones of therapy for the prevention and management of obesity, type 2 diabetes, and CVD4, 5, 6, 7, 8 and important alternatives to those who do not tolerate many of the medications used to manage these conditions.6, 9, 10 Dietary pulses, the edible dried seeds of legumes (i.e., beans, lentils, chickpeas, and peas) that are high in fiber, plant protein, and various micronutrients and low in fat and glycemic index (GI),11, 12, 13 have shown a wide range of health benefits for the prevention and management of type 2 diabetes and CVD.14, 15, 16, 17, 18, 19 In addition, they have shown to be more environmentally sustainable,20 which is a growing global concern.
Dietary pulses are not well recognized for these advantages. Recommendations for dietary pulse intake vary across chronic disease guidelines. The Canadian Diabetes Association and the European Association for the Study of Diabetes recommend that individuals with diabetes consume dietary pulses to help manage glycemic control8 and legumes (which include pulses, soybeans, peanuts, fresh peas, and fresh beans) to help meet minimum requirements for fiber intake.21 The American Diabetes Association, however, has made no specific recommendations to consume pulses, recommending instead various dietary patterns that may be high in dietary pulses (i.e., Mediterranean, Dietary Approaches to Stop Hypertension (DASH), vegetarian, and vegan).7 Similarly, heart‐healthy guidelines from the American Heart Association encourage intake of legumes as part of a diet aimed at reducing CVD risk,5 whereas the Canadian Cardiovascular Society6 and the European guidelines for CVD prevention22 have not made any specific recommendations for the intake of dietary pulses. Current levels of dietary pulse consumption remain low.11, 12, 23 It has been reported that only 13% of Canadians and 7.9% of Americans consume pulses on any given day, where average intakes ranged from 13 to 294 g/day among Canadian consumers and 23 to 277 g/day among American consumers (approximately less than 0.25–1.75 cups/day or less than 0.5–2.5 servings/day of cooked pulses based on Health Canada's Food Guide serving size).11, 12 European data show a similar pattern of low consumption.23
The purpose of this review was to summarize the findings from recent systematic reviews and meta‐analyses assessing the relationship between dietary pulses and cardiometabolic health and to assess the overall strength of the evidence using the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) tool.
Study selection and grading of the evidence
Systematic reviews and meta‐analyses of randomized controlled trials and prospective cohort studies are the highest levels of evidence to inform public health policy and clinical practice guidelines. We sought to identify the most recent systematic reviews and meta‐analyses assessing the relationships of dietary pulses with incident cardiometabolic diseases (overweight/obesity, diabetes, hypertension, CVD, stroke, etc.) in prospective cohort studies and the effects of dietary pulses on cardiometabolic risk factors (glycemic control, blood lipids, adiposity, blood pressure, etc.) in randomized controlled trials. We included our own Canadian Institutes of Health Research (CIHR)‐funded systematic reviews and meta‐analyses (ClinicalTrials.gov identifier: NCT01594567) and searched PubMed (which includes the MEDLINE and NLM databases) through December 9, 2016 using the following search terms: “pulses” OR “legumes” AND “meta‐analysis.” Figure 1 shows the literature search and selection process.
Figure 1.
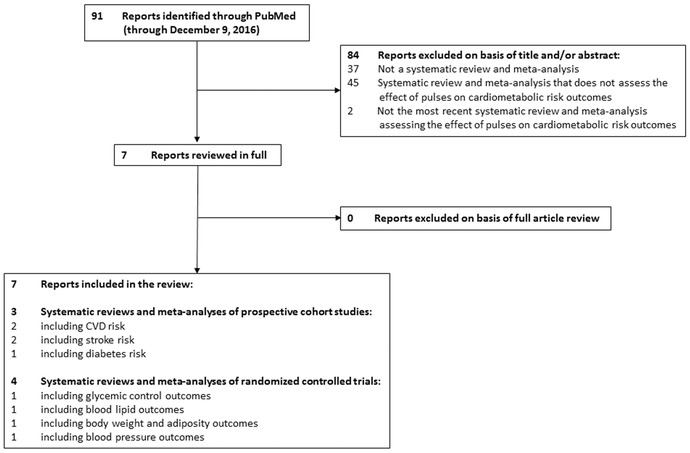
Literature search and selection process.
We summarized all of the available evidence and assessed the overall quality of the evidence using the GRADE tool.25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37 This tool allows evidence to be graded as high, moderate, low, or very low quality. Randomized controlled trials start as high‐quality evidence, and observational studies start as low‐quality evidence. Both can then be downgraded or upgraded on the basis of prespecified criteria. The criteria used to downgrade evidence from randomized controlled trials and prospective cohort studies include study limitations (weight of studies showing risk of bias as assessed by the Cochrane Risk of Bias Tool or the New Castle Ottawa Scale, unless otherwise specified), inconsistency (substantial unexplained interstudy heterogeneity, I 2 > 50% and P < 0.10), indirectness (presence of factors that limit the generalizability of the results), imprecision (the 95% confidence intervals (95% CIs) for mean differences and risk estimates are wide or cross a minimally important difference), and publication bias (significant evidence of small‐study effects). The criteria used to upgrade the quality of evidence are restricted to observational studies. These criteria include a large magnitude effect, a dose–response gradient, and attenuation by plausible confounding effects. (Plausible confounding would decrease the observed risk estimate or increase the observed risk estimate if no effect was observed.)
Systematic reviews and meta‐analyses of prospective cohort studies
No systematic reviews and meta‐analyses have assessed the relationship between dietary pulses and incident cardiometabolic diseases. A relationship, however, has been assessed between legumes, which include dietary pulses, and incidence of cardiometabolic diseases in prospective cohort studies.
Cardiovascular disease risk
One systematic review and meta‐analysis assessed the relationship between legumes in the context of a Mediterranean diet and composite CVD outcomes (including incidence and mortality of CVD, coronary heart disease (CHD), myocardial infarction, and stroke)38 (Fig. 2). It included eight prospective cohort studies (n = 222,021; 14,395 events) conducted in various countries, including the United States (two studies), Spain (two studies), Greece (two studies), Germany (one study), and several other European countries (one study), with follow‐up durations ranging from 4.9 to 20 years.38 Legume consumption in the context of a Mediterranean diet was found to significantly reduce CVD risk (relative risk (RR) = 0.91 (95% CI: 0.83–0.98)), which showed good consistency across the included studies (I 2 = 33%).38
Figure 2.
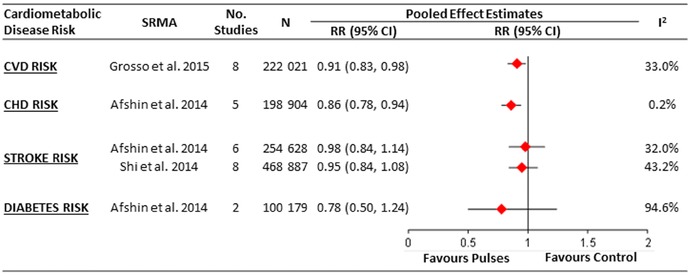
Summary of the pooled effect estimates from the most recent systematic reviews and meta‐analyses of prospective cohort studies assessing the relationship between legume consumption and cardiometabolic disease risk. CHD, chronic heart disease; CVD, cardiovascular disease; N, number of participants; RR, relative risk; SRMA, systematic review and meta‐analysis.
Suggested reasons for this observed CVD risk benefit may be related to specific nutrients and properties found in legumes (i.e., fiber, magnesium, potassium, folate, phytochemicals, low GI, etc.),39, 40, 41, 42, 43, 44 observed benefits of non‐soy legumes on various cardiometabolic risk factors14, 15, 16, 18, 45 (see below), and, potentially, the replacement of certain foods with legumes (i.e., red meat and high‐GI foods).46, 47, 48, 49
Table S1 shows our GRADE assessment of the overall strength of the evidence for the relationship between legumes and CVD risk. The evidence was rated as very low for the association of legumes in the context of a Mediterranean diet and composite CVD outcomes, owing to a downgrade for imprecision in the pooled risk estimates. This assessment suggests that legumes, which include dietary pulses, may have a meaningful cardiovascular benefit, but the estimate remains uncertain.
Coronary heart disease risk
One systematic review and meta‐analysis assessed the relationship between legumes and CHD risk19 (Fig. 2). It included five prospective cohort studies (n = 198,904; 6514 events) that were conducted in various countries, including the United States (two studies), Spain (one study), Greece (one study), and Japan (one study), with follow‐up durations ranging from 10 to 26 years.19 Legume consumption of at least four weekly 100‐g servings (please note that the serving size used in this systematic review and meta‐analysis of 100 g is less than Health Canada's Food Guide serving size of ¾ cup or ∼130 g/day of cooked legumes) was found to be inversely associated with CHD risk (RR = 0.86 (95% CI: 0.78–0.94)), which showed good consistency across the included studies (I 2 = 0.2%).19 Suggested reasons for this observed CHD risk benefit are similar to those described for CVD risk (see above).
Table S1 shows our GRADE assessment of the overall strength of the evidence for the relationship between legumes and CHD risk. The evidence was rated as moderate for the association of legumes with CHD owing to an upgrade for a significant inverse dose–response gradient with no downgrades. This assessment suggests that legumes, which include dietary pulses, may have advantages for CHD prevention, but more studies are required to clarify the association.
Stroke risk
Two systematic reviews and meta‐analyses assessed the relationship between legumes and stroke risk19, 50 (Fig. 2).The same systematic review and meta‐analysis that found an inverse association between legume consumption and CHD showed no association with stroke risk when intakes were ≥4 weekly 100‐g servings of legumes (six prospective cohort studies; n = 254,628; 6690 events; follow‐up range: 10.6–26 years; RR = 0.98 (95% CI: 0.84–1.14)). The association estimates again showed good consistency across the included studies (I 2 = 32.0%), but a meaningful benefit could not be ruled out given the wide confidence interval.19
These findings were consistent with those from another systematic review and meta‐analysis assessing stroke risk in six prospective cohort studies (n = 173,229; 4030 events) conducted in the United States (two studies), Finland (one study), Japan (two studies), and the Netherlands (one study) with follow‐up durations ranging from 6.3 to 26 years.50 Legume consumption was again not associated with risk of stroke when comparing the highest and the lowest level of intake (RR = 0.95 (95% CI: 0.84–1.08); I 2 = 43.2%).50
Table S1 shows our GRADE assessment of the overall strength of the evidence for the relationship between legumes and stroke. The evidence was rated as very low owing to a downgrade for imprecision in the pooled risk estimates. This assessment suggests that legumes, which include dietary pulses, may or may not affect stroke risk. The relationship remains uncertain, with future studies likely to have an important influence on risk estimates.
Diabetes risk
One systematic review and meta‐analysis assessed the relationship between legumes and diabetes risk.19 It included only two prospective cohort studies (n = 100,179; 2746 events). No association was found between diabetes risk and ≥4 weekly 100‐g servings of legumes (RR = 0.78 (95% CI: 0.50–1.24); Fig. 2).19 The two included prospective cohort studies produced very different results, as indicated by the substantial degree of inconsistency in their association estimates (I 2 = 94.6%). One of the cohort studies, which was conducted in over 60,000 middle‐aged Chinese women over an average follow‐up duration of approximately 5 years, showed that legume intake was inversely associated with type 2 diabetes risk when comparing the upper quintile of intake to the lower quintile (RR = 0.62 (95% CI: 0.51–0.74)).51 The other cohort study, which was conducted in over 35,000 older women participating in the Iowa Women's Health Study over a 6‐year follow‐up duration, showed no significant association between mature bean intake and type 2 diabetes risk when comparing the highest to lowest quintile of intake (RR = 0.96 (95% CI: 0.76–1.20)).52
Table S1 shows our GRADE assessment of the overall strength of the evidence for the relationship between legumes and diabetes. The evidence for this association was rated as very low, owing to downgrades for inconsistency, indirectness, and imprecision. This assessment suggests that legumes, which include dietary pulses, may or may not affect diabetes risk. The relationship remains uncertain, with future studies likely to have an important influence on risk estimates.
Systematic reviews and meta‐analyses of randomized controlled trials
We have successfully conducted a series of CIHR‐funded systematic reviews and meta‐analyses of randomized controlled trials of the effect of dietary pulse consumption on the following cardiometabolic risk factors: glycemic control,18 lipids,14 adiposity,16 and blood pressure15 (ClinicalTrials.gov Identifier, NCT01594567). To be included, trials had to be randomized, with isocaloric comparisons between the intervention and control arms and a follow‐up duration ≥3 weeks. We summarize each and provide its respective GRADE assessments below.
Glycemic control
Our first systematic review and meta‐analysis investigated the effect of dietary pulse consumption on the following glycemic control outcomes: fasting glucose, fasting insulin, homeostatic model assessment insulin resistance (HOMA‐IR), and glycosylated blood proteins (i.e., HbA1c or fructosamine).18 We included a total of 41 randomized controlled trials involving 1656 participants with and without diabetes. Separate meta‐analyses were conducted for three different types of dietary pulse interventions: dietary pulses alone (11 trials; n = 253), dietary pulses as part of low‐GI diets (19 trials; n = 762), and dietary pulses as part of high‐fiber diets (11 trials; n = 641). Table 1 shows a summary of the characteristics of included randomized controlled trials, and Figure 3 shows the overall pooled effect estimates of dietary pulses on glycemic control outcomes in each of the three separate meta‐analyses. Dietary pulses alone at a median dose of 120 g/day (approximately just over 0.5 cup/day or less than one serving/day of cooked dietary pulses) were found to lower fasting glucose (standardized mean difference (SMD) = –0.82 (95% CI: –1.36 to –0.27)) and insulin (SMD = –0.49 (95% CI: –0.93 to –0.04)), with no significant effects on HOMA‐IR or glycated blood proteins. Dietary pulses in low‐GI diets (median GI = 67.3, range: 54.3–85.7; median GI difference (low‐GI minus control) = –21.4, range: –1.7 to –46.2, with values based on the bread scale) were found to lower glycated blood proteins (SMD = –0.28 (95% CI: –0.42 to –0.14)), with no significant effects on fasting glucose and insulin or HOMA‐IR. Dietary pulses in high‐fiber diets (median fiber intake = 50 g/day, range: 36.7–96.6 g/day; median fiber intake difference (high‐fiber minus control) = 29.3 g/day, range: 13–79 g/day) were found to lower fasting glucose (SMD = –0.32 (95% CI: –0.49 to –0.15)) and glycated blood proteins (SMD = –0.27 (95% CI: –0.45 to −0.09)), with no significant effects on fasting insulin or HOMA‐IR. Given that these data are expressed as SMDs, values <0.4, between 0.4 and 0.7, and >0.7 were interpreted as having a small, moderate, and large effect size, respectively.53 Dietary pulses were determined to have a meaningful effect on the main clinical marker of glycemic control (HbA1c). When the SMDs of glycosylated blood proteins were used to calculate the mean absolute reduction in HbA1c for dietary pulses as part of low‐GI diets in individuals with type 2 diabetes, the results corresponded to an absolute reduction of approximately 0.5% in HbA1c. This reduction exceeds the clinically meaningful threshold proposed by the U.S. Food and Drug Administration for the development of new drugs for diabetes (≥0.3%)54 and may be beneficial for reducing the risk of developing micro‐ and macrovascular complications.55, 56 Interstudy heterogeneity, however, was high and unexplained across most glycemic control outcomes.
Table 1.
Summary of characteristics of included trials in the most recent systematic reviews and meta‐analyses of randomized controlled trials assessing the effect of dietary pulses on cardiometabolic risk factors
| Cardiometabolic risk factor | SRMA | Total no. of RCTs | Total n | Median sample size (range) | Metabolic phenotypes: no. of trials a | Median age (range) | Median follow‐up (range) | Trial design: no. of trials | Median pulse dose (range) | Pulse type: no. of trials | Pulse form: no. of trials | Comparator: no. of trials b |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Glycemic control | Sievenpiper et al.18 | |||||||||||
| Pulses alone | 11 | 253 | 20 (6–55) |
HC: 7 T2DM: 1 CAD: 1 OH: 4 |
NR | 5 weeks (1–16 weeks) |
C: 7 P: 4 |
120 g/day (15.5–465 g/day) |
Chickpeas: 2 Beans: 8 Various: 1 |
Whole: 6 Flour/powder: 3 Flakes: 1 Both: 1 |
CHO foods: 11 | |
| Pulses in low‐GI diets | 19 | 762 | 16 (6–162) |
T2DM: 13 T1DM: 3 CAD: 1 OH: 3 |
NR | 6 weeks (2–52 weeks) |
C: 12 P: 7 |
– |
Lentils: 2 Beans: 5 Various: 12 |
Whole: 19 |
High‐GI diet: 16 Low‐CHO diet: 1 Diabetes diet: 2 Food exchange diet: 2 |
|
| Pulses in high‐fiber diets | 11 | 641 | 14 (9–450) |
T2DM: 6 T1DM: 5 OH: 2 |
NR | 6 weeks (1.4–156 weeks) |
C: 9 P: 2 |
– |
Beans: 3 Lupins: 1 Various: 7 |
Whole: 10 Pulse fiber: 1 |
Low‐fiber diet: 11 | |
| Blood lipids | Ha et al.14 | 26 | 1037 | 29 (6–121) |
O/OW: 5 HC: 8 T2DM: 3 MetS features: 2 IR: 2 OH: 7 |
51.1 years (4–36 years) | 6 weeks (3–52 weeks) |
C: 13 P: 13 |
130 g/day (50–377 g/day) |
Lentils: 1 Chickpeas: 2 Peas: 2 Beans: 14 Various: 7 |
Whole: 19 Flour/powder: 3 Both: 3 NR: 1 |
CHO foods: 14 High‐GI diet: 1 AP: 2 Diet without pulses: 9 |
| Body weight and adiposity | Kim et al.16 | 21 | 940 | 27 (6–123) |
O/OW: 9 HC: 4 T2DM: 2 MetS features: 1 OH: 6 |
51.3 years (28.1–64 years) | 6 weeks (3–48 weeks) |
C: 9 P: 12 |
132 g/day (80–278 g/day) |
Chickpeas: 3 Peas: 1 Beans: 8 Various: 9 |
Whole: 14 Flour/powder: 4 Both: 3 |
CHO foods: 10 High‐GI diet: 1 Low‐CHO diet: 1 Diet without pulses: 9 |
| Blood pressure | Jayalath et al.15 | 8 | 554 | 78 (18–121) |
O/OW: 4 T2DM: 1 MetS features: 1 OH: 2 |
49 years (28–60 years) | 10 weeks (4–52 weeks) |
C: 2 P: 8 |
127.5 g/day (81–275 g/day) |
Lupins: 2 Various: 6 |
Whole: 5 Flour/powder: 3 |
CHO foods: 4 Diet without pulses: 4 |
AP, animal protein; Apo‐B, apolipoprotein B; BF%, body fat percentage; both, whole and flour form; BW, body weight; C, crossover; CAD, coronary heart disease; CHO, carbohydrate; CHO foods, wheat‐based foods (e.g., white bread and cereals), oat bran, pasta (e.g., spaghetti and chicken soup), potato and potato flakes, rice, carrots, high‐ and/or low‐fiber foods; DBP, diastolic blood pressure; FG, fasting glucose; FI, fasting insulin; GBP, glycosylated blood proteins (HbA1c or fructosamine); HC, hypercholesterolemia; HOMA‐IR, homeostatic model assessment insulin resistance; IR, insulin resistant; MAP, mean arterial pressure; MetS, metabolic syndrome; n, number of participants; no., number; NR, not reported; OH, otherwise healthy; OW/O, overweight or obese; P, parallel; SBP, systolic blood pressure; SRMA, systematic review and meta‐analysis; T1DM, type 1 diabetes mellitus; T2DM, type 2 diabetes mellitus; Various, ≥1 pulse type used (i.e., lentils, chickpeas, peas, and/or beans); WC, waist circumference.
Number of trials may not add up to total number of RCTs because one trial could have consisted of participants who had more than one metabolic phenotype (i.e., HC and T2DM). In this case, this trial would be placed in more than one category.
Number of trials may not add up to total number of RCTs because some RCTs had more than one control arm (i.e., a high‐GI diet arm and low‐CHO diet arm). Although our analyses usually combined these arms to create a pair‐wise comparison, in this table, a trial would be placed in more than one category.
Figure 3.
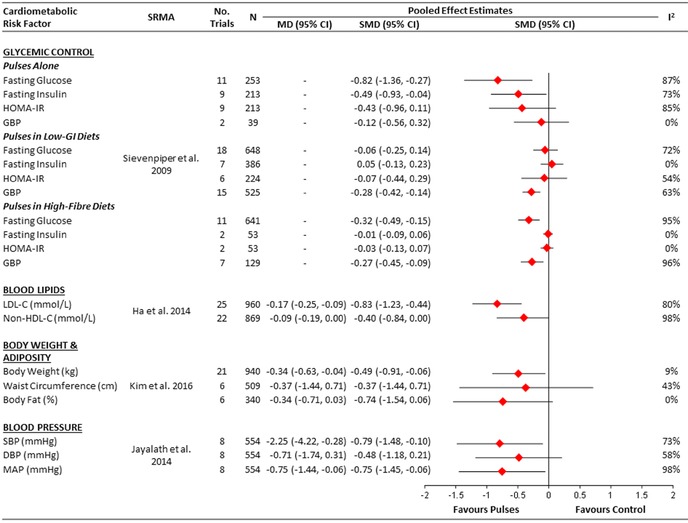
Summary of the pooled effect estimates from the most recent systematic reviews and meta‐analyses of randomized controlled trials assessing the effect of dietary pulses on cardiometabolic risk factors. To allow the summary estimates for each end point to be displayed on the same axis, mean differences (MDs) were transformed to standardized mean differences (SMDs) and pseudo‐95% CIs, which were derived directly from the original mean difference and 95% CI. DBP, diastolic blood pressure; GBPs, glycosylated blood proteins; MAP, mean arterial pressure; MD, mean difference; N, number of participants; SBP, systolic blood pressure; SMD, standardized mean difference; SRMA, systematic review and meta‐analysis.
The mechanisms by which dietary pulses improve glycemic control are thought to relate to a slow release mechanism. Data from acute and chronic studies showing reductions in postprandial glucose and insulin excursions after dietary pulse intake57, 58, 59, 60, 61, 62, 63, 64, 65, 66 suggest that dietary pulses slow absorption in the small intestine and thereby lower the GI of the diet. This slowed absorption may be attributed to various components within dietary pulses, such as their high viscous fiber content,67 the presence of certain compounds that may act as enzyme inhibitors,68, 69, 70 or their high amylose to amylopectin ratio.70, 71
Table S2 shows our GRADE assessment of the overall strength of the evidence for the effect of dietary pulses on glycemic control outcomes. The evidence ratings ranged from low‐to‐moderate, very‐low‐to‐high, and low‐to‐moderate for the effects of dietary pulses alone, dietary pulses in low‐GI diets, and dietary pulses in high‐fiber diets, respectively, owing to downgrades for inconsistency, indirectness, and imprecision. This assessment suggests that dietary pulses consumed alone or as part of low‐GI and high‐fiber diets may lead to meaningful improvements in medium‐ to longer‐term glycemic control. The effect estimates, however, remain uncertain for most glycemic outcomes, calling for more large, high‐quality, randomized trials to clarify the glycemic benefits of dietary pulses. As we are aware of several randomized controlled trials72, 73, 74, 75, 76, 77, 78, 79, 80, 81, 82 that have been published since the census for our systematic review and meta‐analysis, we are currently conducting an updated systematic review and meta‐analysis.
Blood lipids
We conducted a systematic review and meta‐analysis of randomized controlled trials of the effect of dietary pulse consumption on the following established therapeutic lipid targets for cardiovascular risk reduction: low‐density lipoprotein C (LDL‐C), apolipoprotein B (apo B), and non‐high‐density lipoprotein C (non‐HDL‐C).14 We included a total of 26 randomized controlled trials involving 1037 predominantly middle‐aged participants at moderate risk of coronary artery disease. Table 1 shows a summary of the characteristics of included randomized controlled trials and Figure 3 shows the overall pooled effect estimates of dietary pulses on lipids. Dietary pulses at a median dose of 130 g/day (∼0.75 cup/day or one serving/day of cooked dietary pulses) were found to lower the main lipid‐lowering targets used clinically: LDL‐C (mean difference (MD) = −0.17 mmol/L (95% CI: −0.25 to −0.09 mmol/L)) and non‐HDL‐C (MD = −0.09 mmol/L (95% CI: −0.19 to 0.00 mmol/L)). Only one trial was identified that measured apo B, which found no significant difference between the dietary pulse intervention and control arm (MD = 0.02 g/L (95% CI: −0.04 to 0.08 g/L)).75 There was substantial unexplained interstudy heterogeneity for both LDL‐C and non‐HDL‐C (I 2 = 80% and 98%, respectively). These reductions are consistent with the findings from two previous meta‐analyses45, 83 and are at a level that would be considered clinically meaningful. As there is a one‐to‐one relationship between LDL‐C lowering and cardiovascular risk reduction,84, 85 the 5% reduction from baseline in LDL‐C observed in our meta‐analysis would translate into a similar 5% risk reduction in major cardiovascular events.
Several mechanisms may explain the lipid‐lowering benefit of dietary pulses. First, dietary pulses are high in the viscous soluble fiber pectin.86, 87 Viscous fibers have been shown to bind bile acids in the intestine, preventing their enterohepatic recycling.78, 88, 89, 90 The resulting increase in the liver's production of bile acids decreases its pool of cholesterol, increasing the liver's uptake of LDL‐C from the blood. The decrease in cholesterol in turn depletes intracellular cholesterol, upregulating the expression of LDL receptors on cell surfaces, which exert further decreases in cholesterol levels.78, 88, 89, 91 Second, the fermentation products of viscous soluble fiber from dietary pulses may decrease cholesterol production. Soluble fiber in the colon is fermented to short‐chain fatty acids, such as propionate and butyrate,92 both of which have been shown to have cholesterol‐lowering activity in cell culture and animal models as well as randomized controlled trials in humans.93, 94, 95 Finally, the 7S globulins component of dietary pulses may decrease LDL‐C. The 7S globulins are a family of proteins commonly found in legumes that have been shown to be involved in the cholesterol‐lowering effects of various legumes, including soybeans96 and dietary pulses,97 in cell culture and animal studies. The exact mechanism by which this protein lowers cholesterol, however, is currently unclear.97
Table S3 shows the GRADE assessments for the effect of dietary pulses on lipids. The evidence was rated as moderate quality for an LDL‐C–lowering benefit owing to a downgrade for inconsistency and low quality for a non‐HDL‐C–lowering benefit owing to downgrades in inconsistency and imprecision. This assessment suggests that dietary pulses may result in meaningful reductions in established therapeutic lipid targets for cardiovascular risk reduction. Sources of uncertainty, however, remain. There is a need for further large, high‐quality, randomized controlled trials to clarify the lipid‐lowering benefits of dietary pulses.
Body weight and adiposity
We conducted a systematic review and meta‐analysis of randomized controlled trials looking at the effect of dietary pulse consumption on the following measures of adiposity: body weight, waist circumference, and body fat.16 We included a total of 21 randomized controlled trials consisting of 940 middle‐aged participants who were predominately overweight or obese. Table 1 shows a summary of the characteristics of included randomized controlled trials and Figure 3 shows the overall pooled effect estimates of dietary pulses on measures of adiposity. Dietary pulses at a median dose of 132 g/day (∼0.75 cup/day or one serving/day of cooked dietary pulses) were found to lower body weight (MD = –0.34 kg (95% CI: –0.63 to –0.04 kg)) and body fat nonsignificantly (MD = –0.34% (95% CI: –0.71 to 0.03)), with no effect on waist circumference. Dietary pulses lowered body weight significantly more than control diets under both neutral (weight maintaining) and negative (calorie‐restricted weight loss) energy‐balance conditions. The treatment effects for body weight, body fat, and waist circumference were robust to differences in the participants and trial conditions, with no evidence of interstudy heterogeneity (I 2 <50%). By adding data from 16 new randomized controlled trials, our systematic review and meta‐analysis builds on an earlier systematic review and meta‐analysis that showed a nonsignificant weight loss benefit.83
There are two main mechanisms that may explain the weight loss seen with dietary pulses. The first relates to the satiating properties of dietary pulses, which include high fiber and protein content and a low GI.70, 98, 99 High‐fiber foods require longer chewing time and promote gastric distention, which may reduce the rate of ingestion and trigger signals of fullness.100 Both high‐fiber and low‐GI foods can slow digestion and delay absorption of nutrients, which could delay hunger and subsequent energy intake.100, 101 As protein is generally more satiating than carbohydrate or fat of similar energy content,102 it has been suggested that protein from dietary pulses may contain bioactive components that stimulate secretion of gastrointestinal hormones involved in increasing satiety, such as cholecystokinin and glucagon‐like peptide‐1.70, 103 Overall, these satiating properties of pulses are supported by the results of a recent systematic review and meta‐analysis of acute feeding trials (nine trials; n = 126) conducted by our group, which showed that pulses increased acute satiety in generally healthy, younger participants.17 Specifically, dietary pulses were shown to produce a 31% greater satiety incremental area under the curve (ratio of means = 1.31 (95% CI: 1.09–1.58)) in comparison with controls,17 which is likely to be a clinically meaningful effect.104 The second main mechanism relates to reduced bioavailability of calories from dietary pulses. The reduced bioavailability is largely attributed to the presence of intact cell walls and the highly resistant starch and fiber content of pulses, which do not get broken down into absorbable units by the stomach and small intestine.100 Pulses contain protease and amylase inhibitors, as well as a number of phenolic compounds that may interfere with digestibility and energy availability.70 The Atwater factors used to assign calories to different foods may overestimate the amount of calories in dietary pulses.105 The implication is that the calories labeled may not be the calories metabolized, resulting in lower energy density.
Table S4 shows the GRADE assessments for the effect of dietary pulses on adiposity. The evidence for all three outcomes (body weight, waist circumference, and body fat) was rated as moderate owing to downgrades for imprecision in each case. This assessment suggests that, although dietary pulses may result in reductions in body weight under both weight‐maintaining and weight loss conditions, more large, high‐quality, randomized controlled trials are needed to clarify the size of the benefit.
Blood pressure
We conducted the only systematic review and meta‐analysis of randomized controlled trials assessing the effect of dietary pulse consumption on blood pressure outcomes, which included systolic blood pressure (SBP), diastolic blood pressure (DBP), and mean arterial pressure (MAP).15 A total of eight randomized controlled trials were included, involving 554 middle‐aged participants with and without hypertension. Table 1 shows a summary of the characteristics of the included randomized controlled trials and Figure 3 shows the overall pooled effect estimates of dietary pulses on blood pressure. Dietary pulses at a median dose of 127.5 g/day (∼0.75 cup/day or one serving/day of cooked dietary pulses) were found to lower SBP (MD = −2.25 mmHg (95% CI: −4.22 to −0.28 mmHg)) and MAP (MD = −0.75 mmHg (95% CI: −1.44 to −0.06 mmHg)), with no significant effects on DBP. There was substantial unexplained interstudy heterogeneity across all outcomes (I 2 > 50%). The observed 2.25‐mmHg lowering in SBP by dietary pulses is clinically relevant. This is supported by evidence from prospective studies showing that even a 2‐mmHg reduction in SBP may potentially lower mortality risk from stroke and CHD or other vascular causes in the average middle‐aged population by about 10% and 7%, respectively.106
Several potential mechanisms may explain the effect of dietary pulse consumption on lowering blood pressure. Dietary pulses are good sources of fiber, potassium, and magnesium,11, 12 all of which have shown to have blood pressure–lowering effects.107, 108, 109 Dietary pulses have also been shown to lower postprandial insulin levels,57, 58, 59, 60, 61, 62, 63, 64, 65, 66 which is associated with reduced salt retention and blood pressure.110, 111 Finally, the observed lowering in blood pressure may be mediated through weight loss,112, 113 which is supported by the results of our systematic review and meta‐analysis assessing the effect of dietary pulses on body weight.16
Table S5 shows the GRADE assessments for the effect of dietary pulses on blood pressure. The evidence for all three outcomes (SBP, DBP, and MAP) was rated as low owing to downgrades for inconsistency and imprecision in each case. This assessment suggests that dietary pulses may result in reductions in blood pressure. The relationship, however, remains uncertain, with future randomized controlled trials likely to have an important influence on risk estimates.
Conclusions
Dietary pulses are sustainable and affordable sources of fiber, plant protein, and various micronutrients that, as part of a healthy dietary pattern, appear to play important roles in modifying cardiometabolic risk. Systematic reviews and meta‐analyses of prospective cohort studies assessing the relationship between legumes, which include dietary pulses, and the risk of CHD at doses ≥4 weekly 100‐g servings provide moderate‐quality evidence of a benefit. Given the paucity of prospective cohort studies in this area, more prospective cohort studies that directly assess the relationship between dietary pulses and the incidence of various cardiometabolic diseases are needed to increase our understanding of the role of dietary pulses in the primary prevention of diabetes, CHD, and stroke. A direct benefit of dietary pulses, however, is supported by several systematic reviews and meta‐analyses of randomized controlled trials of the effect of dietary pulses on cardiometabolic risk factors. Dietary pulses at doses of 120–132 g/day (0.5–0.75 cups/day, the equivalent of about one serving/day) have shown meaningful reductions in established cardiometabolic risk factors, including HbA1c, LDL‐C, body weight, and blood pressure, with the evidence for these specific reductions ranging from low to moderate quality. There remains an urgent need for more large, high‐quality, randomized trials to clarify the benefits of dietary pulses in the prevention and management of overweight/obesity, diabetes, and CVD. In the meantime, the available evidence suggests that there is a potential opportunity for Western populations, which as a whole consume much less than one serving/day/person,11, 12 to increase their intake of dietary pulses to improve cardiometabolic health.
Conflicts of interest
Dr. Cyril C.W. Kendall has received research support from the Advanced Foods and Material Network, Agrifoods and Agriculture Canada, the Almond Board of California, the American Pistachio Growers, Barilla, the California Strawberry Commission, the Calorie Control Council, CIHR, the Canola Council of Canada, the Coca‐Cola Company (investigator initiated, unrestricted grant), Hain Celestial, the International Tree Nut Council Nutrition Research and Education Foundation, Kellogg, Kraft, Loblaw Companies Ltd., Orafti, Pulse Canada, Saskatchewan Pulse Growers, Solae, and Unilever. He has received travel funding, consultant fees, and/or honoraria from Abbott Laboratories, the Almond Board of California, the American Peanut Council, the American Pistachio Growers, Barilla, Bayer, the Canola Council of Canada, the Coca‐Cola Company, Danone, General Mills, the International Tree Nut Council Nutrition Research and Education Foundation, Kellogg, Loblaw Companies Ltd., the Nutrition Foundation of Italy, Oldways Preservation Trust, Orafti, Paramount Farms, the Peanut Institute, PepsiCo, Pulse Canada, Sabra Dipping Co., Saskatchewan Pulse Growers, Solae, Sun‐Maid, Tate and Lyle, and Unilever. He has served on the scientific advisory board for the Almond Board of California, the International Tree Nut Council, Oldways Preservation Trust, Paramount Farms, and Pulse Canada. He is a member of the International Carbohydrate Quality Consortium (ICQC), executive board member of the Diabetes and Nutrition Study Group (DNSG) of the European Association for the Study of Diabetes (EASD); is on the Clinical Practice Guidelines Expert Committee for Nutrition Therapy of the EASD; and is a director of the Toronto 3D Knowledge Synthesis and Clinical Trials Foundation. Dr. John L. Sievenpiper has received research support from the Canadian Institutes of Health Research (CIHR), the Canadian Diabetes Association (CDA), the PSI Foundation, the Calorie Control Council, the Banting and Best Diabetes Centre (BBDC), the American Society for Nutrition (ASN), the Dr. Pepper Snapple Group (investigator initiated, unrestricted donation), the INC International Nut and Dried Fruit Council, and the University of Toronto (through an unrestricted donation from Tate & Lyle). He has received speaker fees and/or honoraria from the Canadian Diabetes Association (CDA), the Canadian Nutrition Society (CNS), the University of Alabama at Birmingham, Abbott Laboratories, the Canadian Sugar Institute, the Dr. Pepper Snapple Group, the Coca‐Cola Company, the Dairy Farmers of Canada, C3 Collaborating for Health, White Wave Foods, Rippe Lifestyle, mdBriefcase, Alberta Milk, Food Minds, PepsiCo, and Pulse Canada. He has ad hoc consulting arrangements with Winston & Strawn LLP, Perkins Coie LLP, and Tate & Lyle. He is a member of the European Fruit Juice Association Scientific Expert Panel. He is on the Clinical Practice Guidelines Expert Committees of the Canadian Diabetes Association (CDA), the European Association for the study of Diabetes (EASD), and the Canadian Cardiovascular Society (CCS), as well as an expert writing panel of the American Society for Nutrition (ASN). He serves as an unpaid scientific advisor for the Food, Nutrition, and Safety Program (FNSP) and the Technical Committee on Carbohydrates of the International Life Science Institute (ILSI) North America. He is a member of the International Carbohydrate Quality Consortium (ICQC), executive board member of the Diabetes and Nutrition Study Group (DNSG) of the EASD, and director of the Toronto 3D Knowledge Synthesis and Clinical Trials foundation. His wife is an employee of Unilever Canada. No competing interests were declared by Effie Viguiliouk or Dr. Sonia Blanco Mejia.
Supporting information
Table S1. GRADE assessments of systematic reviews and meta‐analyses of prospective cohort studies assessing the relationship between legume consumption and cardiometabolic disease risk
Table S2. GRADE assessments of systematic reviews and meta‐analyses of randomized controlled trials assessing the effect of dietary pulses on glycemic control
Table S3. GRADE assessment of the systematic review and meta‐analysis of randomized controlled trials assessing the effect of dietary pulses on blood lipids
Table S4. GRADE assessment of the systematic review and meta‐analysis of randomized controlled trials assessing the effect of dietary pulses on body weight and adiposity
Table S5. GRADE assessment of the systematic review and meta‐analysis of randomized controlled trials assessing the effect of dietary pulses on blood pressure
Acknowledgments
This review was presented as part of the “Little Beans, Big Opportunities: Realizing the Potential of Pulses to Meet Today's Global Health Challenges” conference held at the New York Academy of Sciences, New York, New York on November 19, 2015. Aspects of this work were funded by the Canadian Institutes of Health Research (Funding Reference Number 119797). Effie Viguiliouk was supported by a Toronto 3D Knowledge Synthesis and Clinical Trials Foundation Internship Award. Dr. John L. Sievenpiper was supported by a PSI Foundation Graham Farquharson Knowledge Translation Fellowship, a Canadian Diabetes Association (CDA) Clinician Scientist Award, a Banting & Best Diabetes Centre Sun Life Financial New Investigator Award, and a CIHR INMD/CNS New Investigator Partnership Prize.
References
- 1. International Diabetes Federation . 2015. IDF Diabetes Atlas. 7th ed Accessed August 30, 2016. http://www.diabetesatlas.org/resources/2015-atlas.html. [Google Scholar]
- 2. O'Rourke, K. , VanderZanden A., Shepard D. & Leach‐Kemon K.. 2015. Cardiovascular disease worldwide, 1990–2013. JAMA 314: 1905. [Google Scholar]
- 3. World Health Organization (WHO) . Obesity. Accessed August 30, 2016. http://www.who.int/gho/ncd/risk_factors/obesity_text/en/.
- 4. Chobanian, A.V. , Bakris G.L., Black H.R., et al 2003. The seventh report of the Joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure: the JNC 7 report. JAMA 289: 2560–2572. [DOI] [PubMed] [Google Scholar]
- 5. American Heart Association Nutrition Committee , Lichtenstein A.H., Appel L.J., et al 2006. Diet and lifestyle recommendations revision 2006: a scientific statement from the American Heart Association Nutrition Committee. Circulation 114: 82–96. [DOI] [PubMed] [Google Scholar]
- 6. Anderson, T.J. , Gregoire J., Hegele R.A., et al 2013. 2012 update of the Canadian Cardiovascular Society guidelines for the diagnosis and treatment of dyslipidemia for the prevention of cardiovascular disease in the adult. Can. J. Cardiol. 29: 151–167. [DOI] [PubMed] [Google Scholar]
- 7. Evert, A.B. , Boucher J.L., Cypress M., et al 2014. Nutrition therapy recommendations for the management of adults with diabetes. Diabetes Care 37(Suppl. 1): S120–S143. [DOI] [PubMed] [Google Scholar]
- 8. Canadian Diabetes Association Clinical Practice Guidelines Expert Committee , Dworatzek P.D., Arcudi K., et al 2013. Nutrition therapy. Can. J. Diabetes 37(Suppl. 1): S45–S55. [DOI] [PubMed] [Google Scholar]
- 9. American Diabetes Association . 2016. Approaches to glycemic treatment. Sec. 7. In Standards of Medical Care in Diabetes—2016. Diabetes Care 39(Suppl. 1): S52–S59.26696682 [Google Scholar]
- 10. Canadian Diabetes Association Clinical Practice Guidelines Expert Committee , Harper W., Clement M., et al 2013. Pharmacologic management of type 2 diabetes. Can. J. Diabetes 37(Suppl. 1): S61–S68. [DOI] [PubMed] [Google Scholar]
- 11. Mitchell, D.C. , Lawrence F.R., Hartman T.J., et al 2009. Consumption of dry beans, peas, and lentils could improve diet quality in the US population. J. Am. Diet. Assoc. 109: 909–913. [DOI] [PubMed] [Google Scholar]
- 12. Mudryj, A.N. , Yu N., Hartman T.J., et al 2012. Pulse consumption in Canadian adults influences nutrient intakes. Br. J. Nutr. 108(Suppl. 1): S27–S36. [DOI] [PubMed] [Google Scholar]
- 13. Foster‐Powell, K. , Holt S.H. & Brand‐Miller J.C.. 2002. International table of glycemic index and glycemic load values: 2002. Am. J. Clin. Nutr. 76: 5–56. [DOI] [PubMed] [Google Scholar]
- 14. Ha, V. , Sievenpiper J.L., de Souza R.J., et al 2014. Effect of dietary pulse intake on established therapeutic lipid targets for cardiovascular risk reduction: a systematic review and meta‐analysis of randomized controlled trials. CMAJ 186: E252–E262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jayalath, V.H. , de Souza R.J., Sievenpiper J.L., et al 2014. Effect of dietary pulses on blood pressure: a systematic review and meta‐analysis of controlled feeding trials. Am. J. Hypertens. 27: 56–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kim, S.J. , de Souza R.J., Choo V.L., et al 2016. Effects of dietary pulse consumption on body weight: a systematic review and meta‐analysis of randomized controlled trials. Am. J. Clin. Nutr. 103: 1213–1223. [DOI] [PubMed] [Google Scholar]
- 17. Li, S.S. , Kendall C.W., de Souza R.J., et al 2014. Dietary pulses, satiety and food intake: a systematic review and meta‐analysis of acute feeding trials. Obesity 22: 1773–1780. [DOI] [PubMed] [Google Scholar]
- 18. Sievenpiper, J.L. , Kendall C.W.C., Esfahani A., et al 2009. Effect of non‐oil‐seed pulses on glycaemic control: a systematic review and meta‐analysis of randomised controlled experimental trials in people with and without diabetes. Diabetologia 52: 1479–1495. [DOI] [PubMed] [Google Scholar]
- 19. Afshin, A. , Micha R., Khatibzadeh S., et al 2014. Consumption of nuts and legumes and risk of incident ischemic heart disease, stroke, and diabetes: a systematic review and meta‐analysis. Am. J. Clin. Nutr. 100: 278–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pulse Canada . 2016. Pulses and sustainability. Accessed August 30, 2016. http://www.pulsecanada.com/environment/sustainability. [Google Scholar]
- 21. Mann, J.I. , De Leeuw I., Hermansen K., et al 2004. Evidence‐based nutritional approaches to the treatment and prevention of diabetes mellitus. Nutr. Metab. Cardiovasc. Dis. 14: 373–394. [DOI] [PubMed] [Google Scholar]
- 22. Piepoli, M.F. , Hoes A.W., Agewall S., et al 2016. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: the Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts) developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur. Heart J. 37: 2315–2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Halkjaer, J. , Olsen A., Bjerregaard L.J., et al 2009. Intake of total, animal and plant proteins, and their food sources in 10 countries in the European Prospective Investigation into Cancer and Nutrition. Eur. J. Clin. Nutr. 63(Suppl. 4): S16–S36. [DOI] [PubMed] [Google Scholar]
- 24. Health Canada . 2011. Eating well with Canada's food guide. Acessed December 13, 2016. http://www.hc-sc.gc.ca/fn-an/food-guide-aliment/index-eng.php. [Google Scholar]
- 25. Guyatt, G. , Oxman A.D., Akl E.A., et al 2011. GRADE guidelines: 1. Introduction—GRADE evidence profiles and summary of findings tables. J. Clin. Epidemiol. 64: 383–394. [DOI] [PubMed] [Google Scholar]
- 26. Guyatt, G.H. , Oxman A.D., Kunz R., et al 2011. GRADE guidelines: 2. Framing the question and deciding on important outcomes. J. Clin. Epidemiol. 64: 395–400. [DOI] [PubMed] [Google Scholar]
- 27. Balshem, H. , Helfand M., Schünemann H.J., et al 2011. GRADE guidelines: 3. Rating the quality of evidence. J. Clin. Epidemiol. 64: 401–406. [DOI] [PubMed] [Google Scholar]
- 28. Guyatt, G.H. , Oxman A.D., Vist G., et al 2011. GRADE guidelines: 4. Rating the quality of evidence—study limitations (risk of bias). J. Clin. Epidemiol. 64: 407–415. [DOI] [PubMed] [Google Scholar]
- 29. Guyatt, G.H. , Oxman A.D., Montori V., et al 2011. GRADE guidelines: 5. Rating the quality of evidence—publication bias. J. Clin. Epidemiol. 64: 1277–1282. [DOI] [PubMed] [Google Scholar]
- 30. Guyatt, G.H. , Oxman A.D, Kunz R., et al 2011. GRADE guidelines 6. Rating the quality of evidence—imprecision. J. Clin. Epidemiol. 64: 1283–1293. [DOI] [PubMed] [Google Scholar]
- 31. Guyatt, G.H. , Oxman A.D., Kunz R., et al 2011. GRADE guidelines: 7. Rating the quality of evidence—inconsistency. J. Clin. Epidemiol. 64: 1294–1302. [DOI] [PubMed] [Google Scholar]
- 32. Guyatt, G.H. , Oxman A.D., Kunz R., et al 2011. GRADE guidelines: 8. Rating the quality of evidence—indirectness. J. Clin. Epidemiol. 64: 1303–1310. [DOI] [PubMed] [Google Scholar]
- 33. Guyatt, G.H. , Oxman A.D., Sultan S., et al 2011. GRADE guidelines: 9. Rating up the quality of evidence. J. Clin. Epidemiol. 64: 1311–1316. [DOI] [PubMed] [Google Scholar]
- 34. Brunetti, M. , Shemilt I., Pregno S., et al 2013. GRADE guidelines: 10. Considering resource use and rating the quality of economic evidence. J. Clin. Epidemiol. 66: 140–150. [DOI] [PubMed] [Google Scholar]
- 35. Guyatt, G. , Oxman A.D., Sultan S., et al 2013. GRADE guidelines: 11. Making an overall rating of confidence in effect estimates for a single outcome and for all outcomes. J. Clin. Epidemiol. 66: 151–157. [DOI] [PubMed] [Google Scholar]
- 36. Guyatt, G.H. , Oxman A.D., Santesso N., et al 2013. GRADE guidelines: 12. Preparing summary of findings tables—binary outcomes. J. Clin. Epidemiol. 66: 158–172. [DOI] [PubMed] [Google Scholar]
- 37. Guyatt, G.H. , Thorlund K., Oxman A.D., et al 2013. GRADE guidelines: 13. Preparing summary of findings tables and evidence profiles—continuous outcomes. J. Clin. Epidemiol. 66: 173–183. [DOI] [PubMed] [Google Scholar]
- 38. Grosso, G. , Marventano S., Yang J., et al 2015. A comprehensive meta‐analysis on evidence of Mediterranean diet and cardiovascular disease: are individual components equal? Crit. Rev. Food Sci. Nutr. [Epub ahead of print.] [DOI] [PubMed] [Google Scholar]
- 39. D'Elia, L. , Barba G., Cappuccio F.P., et al 2011. Potassium intake, stroke, and cardiovascular disease a meta‐analysis of prospective studies. J. Am. Coll. Cardiol. 57: 1210–1219. [DOI] [PubMed] [Google Scholar]
- 40. Dong, J.Y. , Zhang Y.H., Wang P., et al 2012. Meta‐analysis of dietary glycemic load and glycemic index in relation to risk of coronary heart disease. Am. J. Cardiol. 109: 1608–1613. [DOI] [PubMed] [Google Scholar]
- 41. Fan, J. , Song Y., Wang Y., et al 2012. Dietary glycemic index, glycemic load, and risk of coronary heart disease, stroke, and stroke mortality: a systematic review with meta‐analysis. PLoS One 7: e52182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Fang, X. , Liang C., Li M., et al 2016. Dose–response relationship between dietary magnesium intake and cardiovascular mortality: a systematic review and dose‐based meta‐regression analysis of prospective studies. J. Trace Elem. Med. Biol. 38: 64–73. [DOI] [PubMed] [Google Scholar]
- 43. Kim, Y. & Je Y.. 2016. Dietary fibre intake and mortality from cardiovascular disease and all cancers: a meta‐analysis of prospective cohort studies. Arch. Cardiovasc. Dis. 109: 39–54. [DOI] [PubMed] [Google Scholar]
- 44. Mirrahimi, A. , de Souza R.J., Chiavaroli L., et al 2012. Associations of glycemic index and load with coronary heart disease events: a systematic review and meta‐analysis of prospective cohorts. J. Am. Heart Assoc. 1: e000752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Bazzano, L.A. , Thompson A.M., Tees M.T., et al 2011. Non‐soy legume consumption lowers cholesterol levels: a meta‐analysis of randomized controlled trials. Nutr. Metab. Cardiovasc. Dis. 21: 94–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Micha, R. , Wallace S.K. & Mozaffarian D.. 2010. Red and processed meat consumption and risk of incident coronary heart disease, stroke, and diabetes mellitus: a systematic review and meta‐analysis. Circulation 121: 2271–2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Venn, B.J. , Perry T., Green T.J., et al 2010. The effect of increasing consumption of pulses and wholegrains in obese people: a randomized controlled trial. J. Am. Coll. Nutr. 29: 365–372. [DOI] [PubMed] [Google Scholar]
- 48. Viguiliouk, E. , Stewart S.E., Jayalath V.H., et al 2015. Effect of replacing animal protein with plant protein on glycemic control in diabetes: a systematic review and meta‐analysis of randomized controlled trials. Nutrients 7: 9804–9824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Malik, V.S. , Li Y., Tobias D.K., et al 2016. Dietary protein intake and risk of type 2 diabetes in US men and women. Am. J. Epidemiol. 183: 715–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Shi, Z.Q. , Tang J.J., Wu H., et al 2014. Consumption of nuts and legumes and risk of stroke: a meta‐analysis of prospective cohort studies. Nutr. Metab. Cardiovasc. Dis. 24: 1262–1271. [DOI] [PubMed] [Google Scholar]
- 51. Villegas, R. , Gao Y.T., Yang G., et al 2008. Legume and soy food intake and the incidence of type 2 diabetes in the Shanghai Women's Health Study. Am. J. Clin. Nutr. 87: 162–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Meyer, K.A. , Kushi L.H., D.R. Jacobs, Jr. , et al 2000. Carbohydrates, dietary fiber, and incident type 2 diabetes in older women. Am. J. Clin. Nutr. 71: 921–930. [DOI] [PubMed] [Google Scholar]
- 53. Higgins, J.P.T. & Green S., Eds. 2011. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration; Accessed August 30, 2016. www.cochrane-handbook.org. [Google Scholar]
- 54. Center for Drug Evaluation and Research . 2008. Guidance for industry: diabetes mellitus: developing drugs and therapeutic biologics for treatment and prevention (DRAFT GUIDANCE). U.S. Department of Health and Human Services Food and Drug Administration, Silver Spring, Maryland: Accessed August 30, 2016. http://www.fda.gov/downloads/Drugs/.../Guidances/ucm071624.pdf. [Google Scholar]
- 55. Stratton, I.M. , Adler A.I., Neil H.A., et al 2000. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ 321: 405–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Group, A.C. , Patel A., MacMahon S., et al 2008. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N. Engl. J. Med. 358: 2560–2572. [DOI] [PubMed] [Google Scholar]
- 57. Nestel, P. , Cehun M. & Chronopoulos A.. 2004. Effects of long‐term consumption and single meals of chickpeas on plasma glucose, insulin, and triacylglycerol concentrations. Am. J. Clin. Nutr. 79: 390–395. [DOI] [PubMed] [Google Scholar]
- 58. Augustin, L.S. , Chiavaroli L., Campbell J., et al 2016. Post‐prandial glucose and insulin responses of hummus alone or combined with a carbohydrate food: a dose–response study. Nutr. J. 15: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Mollard, R.C. , Wong C.L., Luhovyy B.L., et al 2014. Second‐meal effects of pulses on blood glucose and subjective appetite following a standardized meal 2 h later. Appl. Physiol. Nutr. Metab. 39: 849–851. [DOI] [PubMed] [Google Scholar]
- 60. Anderson, G.H. , Liu Y., Smith C.E., et al 2014. The acute effect of commercially available pulse powders on postprandial glycaemic response in healthy young men. Br. J. Nutr. 112: 1966–1973. [DOI] [PubMed] [Google Scholar]
- 61. Mollard, R.C. , Zykus A., Luhovyy B.L., et al 2012. The acute effects of a pulse‐containing meal on glycaemic responses and measures of satiety and satiation within and at a later meal. Br. J. Nutr. 108: 509–517. [DOI] [PubMed] [Google Scholar]
- 62. Mollard, R.C. , Wong C.L., Luhovyy B.L., et al 2011. First and second meal effects of pulses on blood glucose, appetite, and food intake at a later meal. Appl. Physiol. Nutr. Metab. 36: 634–642. [DOI] [PubMed] [Google Scholar]
- 63. Mollard, R.C. , Luhovyy B.L., Smith C., et al 2014. Acute effects of pea protein and hull fibre alone and combined on blood glucose, appetite, and food intake in healthy young men—a randomized crossover trial. Appl. Physiol. Nutr. Metab. 39: 1360–1365. [DOI] [PubMed] [Google Scholar]
- 64. Wursch, P. , Acheson K., Koellreutter B., et al 1988. Metabolic effects of instant bean and potato over 6 hours. Am. J. Clin. Nutr. 48: 1418–1423. [DOI] [PubMed] [Google Scholar]
- 65. Tappy, L. , Wursch P., Randin J.P., et al 1986. Metabolic effect of pre‐cooked instant preparations of bean and potato in normal and in diabetic subjects. Am. J. Clin. Nutr. 43: 30–36. [DOI] [PubMed] [Google Scholar]
- 66. Torsdottir, I. , Alpsten M., Andersson H., et al 1989. Gastric emptying and glycemic response after ingestion of mashed bean or potato flakes in composite meals. Am. J. Clin. Nutr. 50: 1415–1419. [DOI] [PubMed] [Google Scholar]
- 67. Jenkins, D.J. , Wolever T.M., Leeds A.R., et al 1978. Dietary fibres, fibre analogues, and glucose tolerance: importance of viscosity. Br. Med. J. 1: 1392–1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Thompson, L.U. , Yoon J.H., Jenkins D.J., et al 1984. Relationship between polyphenol intake and blood glucose response of normal and diabetic individuals. Am. J. Clin. Nutr. 39: 745–751. [DOI] [PubMed] [Google Scholar]
- 69. Marshall, J.J. & Lauda C.M.. 1975. Purification and properties of phaseolamin, an inhibitor of alpha‐amylase, from the kidney bean, Phaseolus vulgaris. J. Biol. Chem. 250: 8030–8037. [PubMed] [Google Scholar]
- 70. McCrory, M.A. , Hamaker B.R., Lovejoy J.C., et al 2010. Pulse consumption, satiety, and weight management. Adv. Nutr. 1: 17–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Zhou, K. , Slavin M., Lutterodt H., et al 2012. “Cereals and legumes” (chapter 1). Biochemical changes in raw foods (part 1) In Biochemistry of Foods. 3rd ed Eskin N.A.M. & Shahidi F., Eds.: 3–48. Oxford: Academic Press. [Google Scholar]
- 72. Tonstad, S. , Malik N. & Haddad E.. 2014. A high‐fibre bean‐rich diet versus a low‐carbohydrate diet for obesity. J. Hum. Nutr. Diet. 27(Suppl. 2): 109–116. [DOI] [PubMed] [Google Scholar]
- 73. Saraf‐Bank, S. , Esmaillzadeh A., Faghihimani E., et al 2015. Effects of legume‐enriched diet on cardiometabolic risk factors among individuals at risk for diabetes: a crossover study. J. Am. Coll. Nutr. 35: 31–40. [DOI] [PubMed] [Google Scholar]
- 74. Abete, I. , Parra D. & Martinez J.A.. 2009. Legume‐, fish‐, or high‐protein‐based hypocaloric diets: effects on weight loss and mitochondrial oxidation in obese men. J. Med. Food 12: 100–108. [DOI] [PubMed] [Google Scholar]
- 75. Gravel, K. , Lemieux S., Asselin G., et al 2010. Effects of pulse consumption in women presenting components of the metabolic syndrome: a randomized controlled trial. Mediterr. J. Nutr. Metab. 3: 143–151. [Google Scholar]
- 76. Hodgson, J.M. , Lee Y.P., Puddey I.B., et al 2010. Effects of increasing dietary protein and fibre intake with lupin on body weight and composition and blood lipids in overweight men and women. Int. J. Obes. 34: 1086–1094. [DOI] [PubMed] [Google Scholar]
- 77. Belski, R. , Mori T.A., Puddey I.B., et al 2011. Effects of lupin‐enriched foods on body composition and cardiovascular disease risk factors: a 12‐month randomized controlled weight loss trial. Int. J. Obes. 35: 810–819. [DOI] [PubMed] [Google Scholar]
- 78. Abeysekara, S. , Chilibeck P.D., Vatanparast H., et al 2012. A pulse‐based diet is effective for reducing total and LDL‐cholesterol in older adults. Br. J. Nutr. 108(Suppl. 1): S103–S110. [DOI] [PubMed] [Google Scholar]
- 79. Alizadeh, M. , Gharaaghaji R. & Gargari B.P.. 2014. The effects of legumes on metabolic features, insulin resistance and hepatic function tests in women with central obesity: a randomized controlled trial. Int. J. Prev. Med. 5: 710–720. [PMC free article] [PubMed] [Google Scholar]
- 80. Hartman, T.J. , Albert P.S., Zhang Z., et al 2010. Consumption of a legume‐enriched, low‐glycemic index diet is associated with biomarkers of insulin resistance and inflammation among men at risk for colorectal cancer. J. Nutr. 140: 60–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Hermsdorff, H.H. , Zulet M.A., Abete I., et al 2011. A legume‐based hypocaloric diet reduces proinflammatory status and improves metabolic features in overweight/obese subjects. Eur. J. Nutr. 50: 61–69. [DOI] [PubMed] [Google Scholar]
- 82. Marinangeli, C.P. & Jones P.J.. 2011. Whole and fractionated yellow pea flours reduce fasting insulin and insulin resistance in hypercholesterolaemic and overweight human subjects. Br. J. Nutr. 105: 110–117. [DOI] [PubMed] [Google Scholar]
- 83. Anderson, J.W. & Major A.W.. 2002. Pulses and lipaemia, short‐ and long‐term effect: potential in the prevention of cardiovascular disease. Br. J. Nutr. 88(Suppl. 3): S263–S271. [DOI] [PubMed] [Google Scholar]
- 84. Robinson, J.G. , Smith B., Maheshwari N., et al 2005. Pleiotropic effects of statins: benefit beyond cholesterol reduction? A meta‐regression analysis. J. Am. Coll. Cardiol. 46: 1855–1862. [DOI] [PubMed] [Google Scholar]
- 85. Manson, J.E. , Tosteson H., Ridker P.M., et al 1992. The primary prevention of myocardial infarction. N. Engl. J. Med. 326: 1406–1416. [DOI] [PubMed] [Google Scholar]
- 86. Chibbar, R.N. , Ambigaipalan P. & Hoover R.. 2010. Molecular diversity in pulse seed starch and complex carbohydrates and its role in human nutrition and health. Cereal Chem. 87: 342–352. [Google Scholar]
- 87. Tosh, S.M. & Yada S.. 2010. Dietary fibres in pulse seeds and fractions: characterization, functional attributes, and applications. Food Res. Int. 43: 450–460. [Google Scholar]
- 88. Duane, W.C. 1997. Effects of legume consumption on serum cholesterol, biliary lipids, and sterol metabolism in humans. J. Lipid Res. 38: 1120–1128. [PubMed] [Google Scholar]
- 89. Galisteo, M. , Duarte J. & Zarzuelo A.. 2008. Effects of dietary fibers on disturbances clustered in the metabolic syndrome. J. Nutr. Biochem. 19: 71–84. [DOI] [PubMed] [Google Scholar]
- 90. van Bennekum, A.M. , Nguyen D.V., Schulthess G., et al 2005. Mechanisms of cholesterol‐lowering effects of dietary insoluble fibres: relationships with intestinal and hepatic cholesterol parameters. Br. J. Nutr. 94: 331–337. [DOI] [PubMed] [Google Scholar]
- 91. Fernandez, M.L. 2001. Soluble fiber and nondigestible carbohydrate effects on plasma lipids and cardiovascular risk. Curr. Opin. Lipidol. 12: 35–40. [DOI] [PubMed] [Google Scholar]
- 92. Cummings, J.H. 1981. Short chain fatty acids in the human colon. Gut 22: 763–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Alvaro, A. , Sola R., Rosales R., et al 2008. Gene expression analysis of a human enterocyte cell line reveals downregulation of cholesterol biosynthesis in response to short‐chain fatty acids. IUBMB Life 60: 757–764. [DOI] [PubMed] [Google Scholar]
- 94. Anderson, J.W. , Story L., Sieling B., et al 1984. Hypocholesterolemic effects of oat‐bran or bean intake for hypercholesterolemic men. Am. J. Clin. Nutr. 40: 1146–1155. [DOI] [PubMed] [Google Scholar]
- 95. Hara, H. , Haga S., Aoyama Y., et al 1999. Short‐chain fatty acids suppress cholesterol synthesis in rat liver and intestine. J. Nutr. 129: 942–948. [DOI] [PubMed] [Google Scholar]
- 96. Lovati, M.R. , Manzoni C., Corsini A., et al 1996. 7S globulin from soybean is metabolized in human cell cultures by a specific uptake and degradation system. J. Nutr. 126: 2831–2842. [DOI] [PubMed] [Google Scholar]
- 97. Ferreira, E.S. , Amaral A.L., Demonte A., et al 2015. Hypocholesterolaemic effect of rat‐administered oral doses of the isolated 7S globulins from cowpeas and adzuki beans. J. Nutr. Sci. 4: e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Leathwood, P. & Pollet P.. 1988. Effects of slow release carbohydrates in the form of bean flakes on the evolution of hunger and satiety in man. Appetite 10: 1–11. [DOI] [PubMed] [Google Scholar]
- 99. Murty, C.M. , Pittaway J.K. & Ball M.J.. 2010. Chickpea supplementation in an Australian diet affects food choice, satiety and bowel health. Appetite 54: 282–288. [DOI] [PubMed] [Google Scholar]
- 100. Howarth, N.C. , Saltzman E. & Roberts S.B.. 2001. Dietary fiber and weight regulation. Nutr. Rev. 59: 129–139. [DOI] [PubMed] [Google Scholar]
- 101. Wolever, T.M. , Jenkins D.J., Ocana A.M., et al 1988. Second‐meal effect: low‐glycemic‐index foods eaten at dinner improve subsequent breakfast glycemic response. Am. J. Clin. Nutr. 48: 1041–1047. [DOI] [PubMed] [Google Scholar]
- 102. Paddon‐Jones, D. , Westman E., Mattes R.D., et al 2008. Protein, weight management, and satiety. Am. J. Clin. Nutr. 87: 1558S–1561S. [DOI] [PubMed] [Google Scholar]
- 103. Sufian, M.K. , Hira T., Asano K., et al 2007. Peptides derived from dolicholin, a phaseolin‐like protein in country beans (Dolichos lablab), potently stimulate cholecystokinin secretion from enteroendocrine STC‐1 cells. J. Agric. Food Chem. 55: 8980–8986. [DOI] [PubMed] [Google Scholar]
- 104. Blundell, J. , de Graaf C., Hulshof T., et al 2010. Appetite control: methodological aspects of the evaluation of foods. Obes. Rev. 11: 251–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Zou, M.L. , Moughan P.J., Awati A., et al 2007. Accuracy of the Atwater factors and related food energy conversion factors with low‐fat, high‐fiber diets when energy intake is reduced spontaneously. Am. J. Clin. Nutr. 86: 1649–1656. [DOI] [PubMed] [Google Scholar]
- 106. Lewington, S. , Clarke R., Qizilbash N., et al 2002. Age‐specific relevance of usual blood pressure to vascular mortality: a meta‐analysis of individual data for one million adults in 61 prospective studies. Lancet 360: 1903–1913. [DOI] [PubMed] [Google Scholar]
- 107. Streppel, M.T. , Arends L.R., van't Veer P., et al 2005. Dietary fiber and blood pressure: a meta‐analysis of randomized placebo‐controlled trials. Arch. Intern. Med. 165: 150–156. [DOI] [PubMed] [Google Scholar]
- 108. Aburto, N.J. , Hanson S., Gutierrez H., et al 2013. Effect of increased potassium intake on cardiovascular risk factors and disease: systematic review and meta‐analyses. BMJ 346: f1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Zhang, X. , Li Y., Del Gobbo L.C., et al 2016. Effects of magnesium supplementation on blood pressure: a meta‐analysis of randomized double‐blind placebo‐controlled trials. Hypertension 68: 324–333. [DOI] [PubMed] [Google Scholar]
- 110. DeFronzo, R.A. , Cooke C.R., Andres R., et al 1975. The effect of insulin on renal handling of sodium, potassium, calcium, and phosphate in man. J. Clin. Invest. 55: 845–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Friedberg, C.E. , van Buren M., Bijlsma J.A. & Koomans H.A.. 1991. Insulin increases sodium reabsorption in diluting segment in humans: evidence for indirect mediation through hypokalemia. Kidney Int. 40: 251–256. [DOI] [PubMed] [Google Scholar]
- 112. Staessen, J. , Fagard R. & Amery A.. 1988. The relationship between body weight and blood pressure. J. Hum. Hypertens. 2: 207–217. [PubMed] [Google Scholar]
- 113. Gilardini, L. , Redaelli G., Croci M., et al 2016. Effect of a modest weight loss in normalizing blood pressure in obese subjects on antihypertensive drugs. Obes. Facts 9: 251–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. GRADE assessments of systematic reviews and meta‐analyses of prospective cohort studies assessing the relationship between legume consumption and cardiometabolic disease risk
Table S2. GRADE assessments of systematic reviews and meta‐analyses of randomized controlled trials assessing the effect of dietary pulses on glycemic control
Table S3. GRADE assessment of the systematic review and meta‐analysis of randomized controlled trials assessing the effect of dietary pulses on blood lipids
Table S4. GRADE assessment of the systematic review and meta‐analysis of randomized controlled trials assessing the effect of dietary pulses on body weight and adiposity
Table S5. GRADE assessment of the systematic review and meta‐analysis of randomized controlled trials assessing the effect of dietary pulses on blood pressure


