Abstract
Intelligence is defined for wild plants and its role in fitness identified. Intelligent behaviour exhibited by single cells and systems similarity between the interactome and connectome indicates neural systems are not necessary for intelligent capabilities. Plants sense and respond to many environmental signals that are assessed to competitively optimize acquisition of patchily distributed resources. Situations of choice engender motivational states in goal-directed plant behaviour; consequent intelligent decisions enable efficient gain of energy over expenditure. Comparison of swarm intelligence and plant behaviour indicates the origins of plant intelligence lie in complex communication and is exemplified by cambial control of branch function. Error correction in behaviours indicates both awareness and intention as does the ability to count to five. Volatile organic compounds are used as signals in numerous plant interactions. Being complex in composition and often species and individual specific, they may represent the plant language and account for self and alien recognition between individual plants. Game theory has been used to understand competitive and cooperative interactions between plants and microbes. Some unexpected cooperative behaviour between individuals and potential aliens has emerged. Behaviour profiting from experience, another simple definition of intelligence, requires both learning and memory and is indicated in the priming of herbivory, disease and abiotic stresses.
Keywords: behaviour, intelligence, plant, signals, assessment, fitness
1. Problems of perception
The Earth is a planet dominated by green plants. They account for over 99% of eukaryotic life as the ratio of atmospheric oxygen/carbon dioxide (estimated at 660) indicates. But in the areas of behaviour and intelligence, investigation is almost entirely limited to those of animals. To most animal scientists, plants seem to do nothing; good examples of still life. Being animals ourselves, behaviour and intelligence are expected to involve movement within our time frame. If it is not easily visible it is assumed to be absent. A further assumption is that behaviour and intelligence requires a nervous system, something that has been called brain chauvinism [1]. Brains or nervous systems are not, however, needed for intelligent behaviour as is indicated later; they happen to be the route evolution charted for rapid movement and equally rapid assessment of circumstance by animal organisms. Assessment of circumstance is, however, equally crucial for plants.
When the first plant acquired a blue green alga (that with evolutionary time morphed into a chloroplast), it also required a relatively rigid wall to constrain the generated osmotically active products which can produce turgor pressure. But the wall inhibits easy flexibility and movement. Since light energy was relatively ubiquitous, rapid movement never became an evolutionary imperative. Once on land, multicellular plants used the wall as a skeleton and growth was limited to small regions, when wall strength was relaxed to permit division and cell expansion. In the present day plants, these are tip meristems, embryonic areas in root and shoot that generate new cells and tissues. The root meristem is about 5 mm long and the shoot about double that.
Like all organisms, plants must acquire the resources they need to grow, they need to deal with predators and disease and find mates. Instead of movement, the competitive fight for the essentials, light, minerals and water, led instead to fights over space. A branching structure with tip growth was the obvious solution. It provided the potential for maximum space occupation, resource acquisition and in turn helped deny resources to nearby competitors. Fierce competition for light drove plant evolution upwards in height and a new meristem, the cambium, to increase girth.
Resources for both plants and animals are rarely uniformly distributed. Just as the roving animal locates potential food and moves towards it, growing plants have to identify the locations of richest sources of resources in their surrounding space and grow towards and capture them. In this situation, growth acts like very slow movement. However, growth is very slow in all organisms and does not result in obvious and visible change, which is why plant behaviour is often discounted. But the skill required to efficiently and even maximize capture of resources is no different between animals and plants. The plant phenotype is plastic and reflects in part its environmental history. But it is not always growth. Motor cells in very limited areas of the plant do use turgor pressure to change the phenotype, often reversibly. In a very few species, these turgor changes do lead to visible movement and behaviour. But for most plants, turgor movements are again too slow and below our ability to easily see.
2. What is intelligence?
2.1. Agreeing a uniform definition of intelligence
I consider that intelligence in most animals and plants is concerned with improved survival in the wild and thus in turn fitness. A compendium of different descriptions and attributes of intelligence has been published [2]. These descriptions hinge around the ability of organisms to solve problems experienced during the life cycle. Behaviour that profits from experience, through forms of learning and memory, and improves survival and thus potentially fitness are considered intelligent.
Perhaps, the most useful summary is that of Legg & Hunter [3]. They collected some 70 different definitions of intelligence and summarized them as follows:
Intelligence:
(1) is a property that an individual has as it interacts with its environment or environments;
(2) is related to the agent's ability to succeed or profit with respect to some goal or objective; and
(3) depends on how able the agent is to adapt to different objectives or environments.
Category 1. This is simply behaviour. In plants, behaviour is concerned with the phenotypic and molecular response to changes in a multitude of environmental and internal signals.
Category 2. The goal for any wild organism is ultimately fitness and is equated to numbers of surviving siblings. The ability to profit from learning and memory and thus improve subsequent behaviour increases the chances of survival of the individual. Darwin [4] considered selection to take place at the level of the individual. The whole life cycle is subject to overall selection and fitness and intelligent behaviour becomes a critical part of subsequent fitness [5].
Category 3. The linking with environmental variation is crucial here. What is intelligent in one environment may not be so in another. For plants it is the ability to improve behaviour through experience and thus be adaptively variable through a multiplicity of different environments while continuing development throughout the life cycle.
2.2. There are numerous short descriptions of intelligence in the literature [2]
Some will be mentioned later in this article. ‘Adaptively variable behaviour within the lifetime of the individual’ [6], is a simplification that agrees with the definition above and used previously by me [7]. The emphasis here is adaptively variable. Adaptation represents improvement in subsequent behaviour as a result of life cycle experience. Adaptive behaviour that is expressed with greater rapidity, higher probability or lower cost or, in summary, improved efficiency during the life cycle, is more intelligent and should help place the individual at one or more fitness peaks in an adaptive landscape [8].
2.3. Clarifying the distinction between plant development and behaviour
Behaviour is not to be confused with acts of development which are essential to the individual's survival and reproduction. A good example here (many others follow later) is seed germination. Without it the individual does not develop at all. But when the seed germinates is an act of adaptive variability or plasticity and thus potentially intelligent in characteristics.
In the soil many wild seeds are fully imbibed. Germination in some seeds only advances (dormancy is broken) when the seed is in receipt of a plethora of signals which are then assessed and judged to be beneficial for the seedling and later developing plant. The skill in environmental interpretation, that is learning, determines which seeds will most accurately assess the time of germination and environmental conditions for the young plant. These are clearly the most intelligent.
Signals that are assessed are a limited range of local temperatures to indicate a suitable season of either summer, autumn or winter (which is usually counted as number of days below 4°C), water availability, various soil volatile organic compounds, perception of light, age of seed (a phenomenon called after-ripening and not understood), soil minerals and probably others not yet determined. Then in addition, maternal environmental conditions influence the decision of daughter seeds on the timing of germination too; that is, to grow immediately or to remain dormant. These conditions include the maternal experience of carbon dioxide levels, competition with other plant species, day length, fungal infection, growing season length, light quality, mineral nutrition, position in the ovary, defoliation and time of seed maturation [9]. The maternal learning experience is passed onto the next generation through obvious memory. Maternal-condition influences on germination are predictions of likely future environments in which the sibling grows. Sibling phenotypic characteristics are in turn influenced by implanted maternal memory potentially improving intelligent behaviour and thus fitness [10,11]. Individual seeds germinate when the plethora of direct signals and maternal information reinforce each other. Germination is adaptively plastic. ‘Intelligence is commonly held to consist of the modification of behaviour in accordance with experience. Intelligence is the correlation of experiences and actions’ [12]. The complexity of germination behaviour can be extraordinary and is exemplified by detailed studies on the wild oat [2].
3. Brains are not needed for organisms to act intelligently
The commonest problem in recognizing plant intelligence is the assumption that only organisms with brains can express the behaviour. Plants obviously lack a defined nervous system and the conglomerate of nerve cells that construct brains. However, they do use electrical signals for communication. If, however, single cells are capable of intelligent behaviour, then the lack of a nervous system is no longer a problem since plants are constructed from many millions of cells which already possess that capability. It then becomes a matter of how cells interact to generate intelligent behaviour.
This section is divided into two parts. Firstly, I compare the systems structure of a cell with a simple organism with a recognizable simple brain, Caenorhabditis elegans. Current systems investigations of the cell describe the complexity and distribution of interactions between all cellular proteins. Just as the word genome is a suitable term that summarizes and describes the haploid complement of chromosomes in a single nucleus, the word interactome references the complexity and details of protein–protein interactions in defined cells. The word connectome describes the interaction complexity between neurones in a simple nervous system and like genome and interactome use of the word simplifies discussion and description. The word ‘degree’ is in common use in systems descriptions and refers to the number of interactions or connections either between one protein or one neurone and its interacting partners. There is overall systems similarity between the connectome and the interactome as indicated below.
Secondly, I describe the behaviour of a known single cell, Physarum polycephalum. Detailed investigations indicate the presence of intelligent behaviour. Those observations suggest the systems structure of cell interactome and Caenorhabditis connectome represent the basics necessary for intelligent behaviour and the ability to assess signals within the context of specific environments. Behaviour is then directed accordingly.
3.1. How cells and nematodes process information
A system is a network of mutually dependent and thus, interconnected components, comprising a unified whole [13].
Eucaryotic cells contain about 100 000 protein species in both plant and animal cells, once post-translational modifications are considered. These proteins interact with each other in complexes of varying size to form the dynamic network, the interactome. Information flow through this network can be manipulated by constructing new connections and disposing of old ones or by modifying their strength. Protein phosphorylation is a common means of manipulating connection strength. Plants and animals have about 1000 protein kinases and hundreds of protein phosphatases with differing degrees of specificity and control constructing a phosphorylome. The system is controlled through hundreds of feedback and feedforward processes.
Caenorhabditis elegans, a simple nematode, processes information through the use of some 300 neurones that construct the connectome another dynamic network. Environmental information is processed through this neural network and can be manipulated by changing the interactions between such nerve cells through increasing, or decreasing synapse number or changing synaptic strength.
These two systems like others also possess the important property of distributed control. That is, the recognizable parts can vary and behave with a degree of apparent independence of the main system while remaining attached to it. Their behaviour is still constrained by the connections with the remaining system. Such network structures engender resilience [14].
3.2. Cells and nematodes respond to external signals and assessment follows a simple sequence
Nematodes process information from volatile and water soluble chemical signals, from touch signals, osmolarity, etc., using sensory cells connected to sensory neurons, amplification via interneurons where assessment is made and thence to motor neurons which excite different kinds of muscle [15,16]. Behaviour is modified by experience via non-associative and associative learning through adaptation, habituation and decision and choice capabilities when response has to be prioritized between two contrasting signals [15,17].
Cells commonly process information (when responding to many and numerous external signals) through specific receptor activation, amplification (through cytosolic Ca2+, G proteins and numerous protein kinases) ending on a motor output involving secretion, ion flux changes, gene expression and movement in those cells capable of responding. Cell behaviour is modified by adaptation, habituation and decision capabilities, as indicated later, and the whole behaviour is integrated through feedback and feedforward.
In both cell and nematode, a similar sequence is found; signal, assessment and response.
3.3. Interactome and connectome degree structures are similar
Connection patterns in many real networks, including cells and nematodes, converge to a similar architecture exhibiting a heterogeneous degree number between the components with the distribution of degree number characterized by a power law. A minority of components are densely connected (sometimes called nodes or hubs), whereas most have weaker numbers of interacting partners (sometimes called connectors).
Numerous high-quality interaction maps are required to detail the interactome that avoid the obvious problems of false positives [18]. The interactome of Homo sapiens presently covers about a third of potential proteins, and has an average degree of about 7; yeast with more than three-quarters of all proteins examined has an average degree of 10 [19]. High degree proteins in the cytoplasm are in combination with large numbers of others. Actin is thought to combine with upwards of 100 different protein species for example. Low degree proteins have far fewer interactions.
Mutation analyses indicate that high degree hubs are usually found to be essential for growth and division; effectively they obey a lethality–centrality principle; elimination of the core hub kills the cell [20]. A minimum dominating set (MD Set) has been defined as optimized subsets of proteins from where each protein in the subset may be immediately reached. These MD Set modules control network behaviour [19,21]. Not all MD Set proteins are high degree. Wuchty provides a toy model of the degree number in an MD Set [19]. In both yeast and humans, these MD Set proteins are about one-sixth of the total protein number; their average degree increased from 7 to 17 in Homo sapiens and in yeast, from 10 to 24. They do contain proteins implicated in the development of cancer and in viral infection. High degree proteins are thus involved in control of the whole network and its aberrant states. The normal behaviour of the cell is overcome in these situations.
In C. elegans, single neuron ablation studies and anatomical studies has provided potential functions of each neurone. An optimized wiring network has been deduced [15].
In the nematode connectome, the average degree is again about 7. The distribution of degrees is a power law with a minority of neurons with very high degrees of connection and a long tail down to 3–4, most notably in the posterior of the animal [17,22,23]. The connectome contains a ‘rich club’ of some 11–12 neurons with degree 44 and above [23,24]. Ablation of most of these, affects locomotion and it is considered that they represent core command and assessment neurones required to integrate the behaviour of the organism.
3.4. Core constituents and learning in the connectome and interactome
The interactome and connectome have both a core and a periphery distinguished on their degree number. This arrangement provides for the interpretation and assessment of numerous signals by the core for signals arriving at the periphery. Current information present in this core modifies the particular flow of information as it passes onwards to a response or motor system.
When the connectome learns, information flow is altered by changing connection strength; increasing or decreasing synaptic number or by plasticity in synaptic strength. In addition, new synapses open up new pathways for information to be directed. Memory is retained as long as the synapse remains. A kind of synaptic control may also operate to control hormone secretion [25].
The equivalent in an interactome to the connectome rich club neurons, might be enzymes, such as protein kinase C with an estimated degree of 50. The phosphorylome covers both the constituent protein kinases and the interactions between all phosphorylated proteins. Cross-phosphorylation between numerous protein kinases is common including MAP kinases [26]. The MD set itself does contain numerous protein kinases suggesting a core position. The signal-transduction pathway represents a learning process via alterations of the subsequent cellular cascade [27]. In plant cells information flow is rerouted often ending in specific changes in chromatin structure or ion flux. This now novel pathway will usually last as long as the new protein phosphorylation state remains. Entirely new pathways can also be constructed through novel tertiary structure changes in transduction proteins involving different phosphorylation sites and control [2].
Networks that control their own information flow are described as intelligent and this certainly applies to the cell [1]. Both the connectome and the interactome exhibit intelligent capabilities; these derive from the evident similarity in their network structure. Even in more complex brains differential degrees of interaction are recognized; some groups of neurones in columns are densely connected with each other; others less so [2,28].
4. Intelligent behaviour in the single cell, Physarum polycephalum
Physarum polycephalum is a large slime mould, a coenocyte but a single cell. It survives by ingesting detritus found in its environment which it surrounds, engulfs and digests. Investigations over the last 15 years have uncovered some surprising behaviour which a number of authors have identified as intelligent [29,30]; that is, adaptively variable behaviour during the lifetime of the individual. To follow the movement of Physarum requires the use of time lapse, the organism's behaviour is largely expressed as different patterns of growth and again growth is slow.
(1) A simple maze was constructed with four possible routes differing in length and food placed at two ends. The plasmodium eventually forms a single thick tube that connects both food sources and which represents the shortest route out of four. In other words, the cell optimizes the ratio of energy output to energy gain [29,30].
(2) Physarum is very sensitive to strong light which damages it. By illuminating one part of the shortest route with strong light, it was reported that Physarum constructs the next shortest but safest route to connect the food sources [31]. This situation is clearly one of choice and decision; the organism balances the risk of damage to itself and the need to efficiently find food to survive. The decision in this case is beneficial.
(3) The optimum nutritionally balanced food for Physarum was estimated based on a chemical analysis of the plasmodium. Physarum was then offered 11 different kinds of food and sampled all of them but finally selected and exploited the one that was optimal for its nutritional needs [32]. Physarum was also presented with tasks that required easy or difficult discrimination between separate food sources. When conditions of stress were imposed, individuals tended to make inaccurate or costly decisions [33]. This is an indication of a primary path of information flow which can be interfered with through cross reaction with others.
(4) Temporary cessations of growth of the plasmodium were observed after the administration of small electrical shocks. Three shocks separated by the same time interval were administered but the fourth was omitted. Temporary cessation of growth occurred to all three shocks and to the un-provided fourth shock indicating it had learnt to anticipate the administration of a potential shock and the time interval over the previous mild electrical shocks were administered [34,35].
(5) Habituation is a form of learning in which the organism decreases or ceases to respond to a signal after repeated presentations, if the signal is no longer biologically damaging. Apparatus was constructed which ensured that food was available only if the organism crossed an agar bridge containing either caffeine or quinine at toxic (but not killing) concentrations. Although initially the time taken to cross the bridge was slow, with successive attempts it eventually achieved that of ordinary agar. The organism was not fatigued, however, because replacing caffeine with quinine returned the organism to the very slow progress initially experienced with caffeine [36].
Descriptions of intelligent behaviour in Stentor, paramecium, protists that construct ‘houses’ from tiny pieces of gravel and cooperative hunting in amoeba can be found in [2,12,37,38].
4.1. Habituation in Mimosa pudica
Habituation in plant systems is rarely examined. One example, that of habituation to mechanical stimulation, has recently been reported in the sensitive Mimosa and it seems pertinent to mention it here with the references above to habituation [12]. Although a form of habituation was reported by Bose over a century ago, he failed to apply an alternative method of mechanical stimulation to show it was not simple fatigue [39]. That has now been properly investigated and proper habituation demonstrated by using two different methods of mechanical stimulation [40].
4.2. Conclusion on interactome, connectome and single cell intelligence
The interactome and connectome have systems similarity in their distribution of degrees (connections) among the constituents. Just as the systems structure of the connectome provides for intelligent behaviour so does that of the cell interactome and this intelligence is confirmed with investigations on the single cell, Physarum. Since plants are usually composed of millions of cells the potential for intelligence is clearly present and is now described further.
5. The intelligent behaviour of green plants
The notion of plant intelligence is not new. Darwin [41] after much experimentation concluded, ‘The tip of the root having the power of redirecting the movements of the adjoining parts acts like the brain of one of the lower animals receiving the impressions of sense organs and directing the several movements'. Von Hartmann an early psychologist was also surprised by leaf behaviour as he reported in 1875 [42]. ‘If one sees how many means are here to attain the same end, one will be almost tempted to believe that here dwells a secret intelligence which chooses the most appropriate means for the attainment of the end’. Frits Went the discoverer of auxin a major plant hormone in the early 1930s, concluded [43] ‘In tropistic movements, plants appear to exhibit a sort of intelligence; their movement is of subsequent advantage to them’. The nineteenth century German scientist, von Liebig (discoverer of the mineral requirements of plant growth) was quoted by Weaver in 1926 as saying, ‘Plants search for food as if they had eyes’ [44].
6. The variety of signals to which green plants respond
Fitness dictates that the individual should be highly sensitive to the resource parameters of its environment, minimizing energy expenditure while maximizing energy gain to acquire them. Stored resources are then used to provision seeds. There is a relationship between stored resource and seed number in the wild; seed number acts as a proxy for fitness. However, environmental conditions other than resources can interfere in resource acquisition and be damaging. Intelligent behaviour requires the plant to be as sensitive to as many signals issuing from its environment and take necessary action to optimize its fitness chances. Some signals can be all or none, others are graded in size. They can be divided into abiotic and biotic signals.
(1) Abiotic signals. Green plants on the whole have been shown to be sensitive to and respond to specific environmental changes in light quality, intensity and exposure length [2]. Temperature (higher or lower than ambient) institute radical shifts in behaviour, often not perceived but easily detected experimentally [2]. There are numerous kinds of mechanical signals such as touch, bending, wind [45] or even weight itself and proprioreception [46]; stress institutes different phenotypic effects to strain [47]. Soil obstacles initiate marked root phenotypic change [48]. Even vibrations from caterpillar munching initiate herbivore defence reactions [49] and sound itself has effect on organ growth direction [50]. Patchy mineral distributions and soil impedance variation change the root phenotype [51,52]. Loss of turgor due to low water availability and low light can institute radical shifts in the resources directed to either root or shoot [7,53]. The adaptive effects of gravity on shoot and root phenotype are well known, but most lead to branches placed at angles to the gravity vector rather than the commonly examined vertical growth found in seedlings [47]. Atmospheric variations in gas concentration such as carbon dioxide or even oxygen depletion by reducing photorespiration can greatly increase growth [54]. Anaerobic conditions resulting from flooding change both the root and shoot phenotype often lead to oxygen piping through aerenchyma development [55]. Numerous volatile chemicals and non-volatile chemicals adaptively influence phenotype and even the salivary composition of different predatory caterpillars can be distinguished [56]. Many of these are goal-directed and controlled in extent through feedback.
(2) Biotic signals. These include competition and cooperation, trampling, herbivory, disease, symbiosis and mutualism [2].
The range of plant sensitive signals is similar to those of the five familiar human senses of taste, vision, touch, sound and smell. The changes in phenotype take place in the lifetime of the individual and are adaptively variable and thus by definition intelligent in the right environment. Any one of these signals is modulated by the strength of stimulus and a host of other signals that are experienced at the same time and interact. It was observations like these that led Darwin to draw the analogy between the root tip and a brain [41].
6.1. The signal-induced extent of phenotypic plasticity
Phenotypic plasticity during the life cycle encompasses variations: in stem height or root length; numbers of root and shoot branches; angles of branches; thickness of stems, roots and branches; leaf numbers, thickness, degree of leaf overlap, shape, position to the light vector, leaf hair numbers, leaf density and chemical nature of surface cuticle; root hairs; shoot to root ratio; shape of root structure. Plasticity enables the individual to master its local environment and help the individual succeed in the battle to optimize fitness. Plasticity is one detectable facet of intelligence.
6.2. Communication is essential to these changes in signal-induced plasticity and forms the basis of intelligent behaviour
The internal signals that result in plasticity communicate information at different speeds [57] and move mainly through the vascular system. They include mRNA, numerous small inhibitory RNAs [58], hundreds of proteins and peptides [59], 10 hormones (there may be more), hydraulic and mineral signals. Information is also transmitted through action potentials that result in cytosolic Ca2+ waves underpinned by glutamate-dependent calcium channels and release of glutamate from vesicular sources; a mechanism also used for short-term memory in nerve cells [57]. Action potentials are generated by rapid changes in temperature, mechanical damage from herbivores or salt stress. The calcium wave is attenuated by waves of secreted reactive oxygen species [57]. In these respects, the communication behaviour of a single plant is analogous to that of a single cell.
Probably the fastest communicating signal moves through plant tissues at about 1 m s−1 and the slowest at about 1 cm h−1 for some hormones. But most average 1–8 mm s−1 through the vascular system. The action potential was first reported over a century ago in the sensitive Mimosa and even earlier in the Venus fly trap [39]. Mechanical signals may operate through directly through mechano-sensitive calcium channels [45,57].
6.3. Self-organization underpins signal-induced behaviour and intelligence
Plants are typically self-organizing individuals. There is no cell or tissue/organ that has an overall view that guides the eventual phenotype. Instead organization develops from the bottom up in a kind of Markovian series. Each stage of development, a complex molecular state, undergoes conversation with its changing environment to which it both contributes and responds. Each stage, acts as a platform for the next step [2,60]. Development is thus a learning process! Coordination of developmental change is accomplished by communication throughout. Initially, this is over short distances in the seedling. As the system increases in size, the characteristics and identity of the communicating signals and feedback control, also change [28]. The individual plant and its environment form both a corporate and a feedback system.
Experimentally a single bud on a shrub can be induced to break dormancy by a local chemical stimulus or by micro-beams of light, while the remainder of the plant remains dormant or fails to respond [61,62]. These are exceptional examples of distributed control. Normally there is a strong degree of coordination and even though only single tissues may respond, other parts of the plant provide essential assessment of the signal and indicate its acceptance. Selective root responses still reflect a contribution and assessment of root-sensed signals from the shoot itself. A good example is that of phosphate deficiency which is sensed by the root cap and a specific phosphate binding protein or receptor. Information is transmitted to the shoot which synthesizes novel sRNAs. When these arrive in the root they initiate branch root proliferation [63]. Associated accumulations of sucrose caused by the deficiency act as additional proliferation signals improving reliability.
7. Intelligent responses to mineral and light resources
7.1. The plant root cap
In Arabidopsis, the extreme tip of the root is covered by a cap, constructed of some 200 cells. The cap is dynamic. It is constructed from a layer of dividing cells, a cap meristem, that abut the root meristem proper. With each successive division of the cap meristem, the cap cells are gradually pushed outwards and on reaching the outer root surface they are eventually sloughed off. However, during their lifetime slowly moving to the front of the cap, they act as both sensing and assessment of a variety of different signals. Apart from the cap epidermal cells, the internal cells of the cap are referred to as the columella.
The cap both senses and assesses numerous signals indicated below. The response motor for many of these signals is located shoot-wards in the elongating and branching regions as a result of information transmission from the cap and from the shoot in certain cases. It is thought that auxin a growth hormone or growth substance enters the cap from the shoot via the vascular tissue and this is dispersed towards the root epidermis shoot-wards. The root epidermal cells constrain the growth of the root and increased auxin content inhibits growth, the opposite of the shoot. There are, however, some problems with this view that have not been resolved; electrical changes precede any change in auxin contents for example and there are several different elongation mechanisms [2,64,65].
Signals sensed by the cap are described below:
(1) Gravity using a statolith-sensing mechanism. Statoliths are not essential to gravity sensing [63–65] but their presence results in a much faster bending response.
(2) Touch. When the cap touches an obstacle (stone) in the soil, the shootward region of the root assumes an unusual dog-leg kind of structure placing the tip at an almost horizontal angle enabling it to slide over an obstacle surface with continued division and growth [41,48]. Touch inhibits gravity sensing and lengthy touch dismembers statoliths.
(3) Phosphate deficiency, cap signals are transmitted to the shoot via it is thought a phosphate binding protein which acts as receptor. The shoot in turn synthesizes novel sRNAs. When these reach the root, the phenotype changes [66].
(4) Soil patches rich in nitrate construct surrounding gradients. When one is sensed the root grows along them with an acceleration of growth along the gradient and virtual cessation when the rich source is encountered [67].
(5) When the cap enters a nitrate or ammonium ion-poor soil zone, this is sensed by a cap-based nitrate sensor. Similarly to phosphate deficiency, information is sent to the shoot which replies with signals (possibly other-or-equivalent sRNAs) that modify root branching and increase overall root growth [68].
(6) Humidity gradients are sensed by the root cap [69]. When water is in short supply, roots will follow the humidity gradient. Statoliths are dismembered preventing interference by gravity signals.
(7) Salt stress initiates long distance Ca2+ waves to the shoot [70,71].
(8) Roots are sensitive to light. Sometimes they are positively phototropic but in other species negatively phototropic. The cap contains phytochrome, a light sensor that may mediate these phenotypic changes [72].
The cap is able to respond to numerous signals, assess them and some of which directly control the motor tissue further back in the growing region. Others cause the transmission of longer-range signals that require intervention by the shoot via several or more signals [63]. Several signals make for better reliability and thus resilience in behaviour. A single root will experience many of these signals at the same time and assessment is needed to determine the priorities.
To characterize the contribution each cap cell makes to sensing and assessment, root cap cell ablation is one approach. Unfortunately, this has only been examined in detail with gravity signals [73]. Different cell groups were found to have differential inputs to five different gravi-response parameters. Effect of loss of particular cells causes a range from zero loss of sensitivity to nearly 90% of response indicating that approximately only 10% of the columella cells are the most critical for the gravitational signal. In the C. elegans connectome about 4% of neurons are critical.
A central controlling core of root cap cells (with high degree and high connectivity) surrounded by a less significant periphery (with low degree and lower connectivity) is indicated and probably the necessary controlling structure for intelligent behaviour. Mechanical signals in obstacle avoidance are initiated via cytosolic Ca2+ transients in the peripheral cells [48]. Although the root cap acts holistically, different sensory and assessment functions seem to be distributed among different cell types.
8. Adaptively plastic and intelligent responses to light
Plant shoots bending towards light sources are familiar observations. Etiolated seedlings are common experimental material to investigate these phenomena. Darwin [41] covered the tip of an etiolated grass seedling (the coleoptile) and observed a large reduction in sensitivity to a unilateral light stimulus; the bending occurred further down indicating signal transmission. Intermittent illumination a few minutes per hour was all that is needed for bending, the signal is learnt and remembered. Crucially bending occurs towards any unilateral light stimulus no matter where it is placed and the response is thus adaptively variable.
The coleoptile tip is probably again about 200 cells in size like the cap. The light gradient across an individual cell of the coleoptile tip must be extremely small. The implication is that the tip behaves holistically and communication must occur across it. Cell ablation studies would probably prove very revealing. When sensitive tissues are exposed to two beams of light on either side whose difference in intensity is not perceivable by the naked eye, the tissue does bend towards the slightly stronger source [74]. ‘Phototropism must be viewed as a complex biological response involving interactions of multiple photoreceptors, multiple hormones and multiple signalling pathways that together orchestrate the establishment of coordinated differential growth gradients’ [75]. Sunflower seedlings kept in very dim light can exhibit some remarkable behaviour detected by time lapse (http://plantsinmotion.bio.indiana.edu/plantmotion/movements/tropism/tropisms.html).
A fair assessment from the time lapse would be that some of these appeared to search their environment through the whole hemisphere above ground.
8.1. Shoots in weak light; light tunnels
Plants that grow beneath a mixed, sometimes high, canopy have to make decisions about the best directions to grow and maximize energy capture. Light is at a premium so identifying the strongest light source is imperative. Time lapse has brought plant behaviour to speeds that can now be perceived easily and is initiating a slow revolution in understanding. An excellent example is to be found on https://www.youtube.com/watch?v=aNjR4rVA8to. The reader should view it first before reading further.
The time lapse shows the behaviour of a bramble (Rubus, species unspecified) growing under a woodland canopy. The time lapse lasts only about a minute and is greatly accelerated since the shoot really grows about 8 cm d−1.
The first part of the time lapse of the shoot tip shows what Attenborough calls ‘shaking its head’ as though deciding which direction to take. Once performed and shaking completed, the shoot grows in a specific direction. When slowed to real time, the shaking is a familiar circumnutation used by many plants to identify accurate directions of signals particularly light, gravity or in certain cases volatile attractants such as by the parasitic plant, dodder [76].
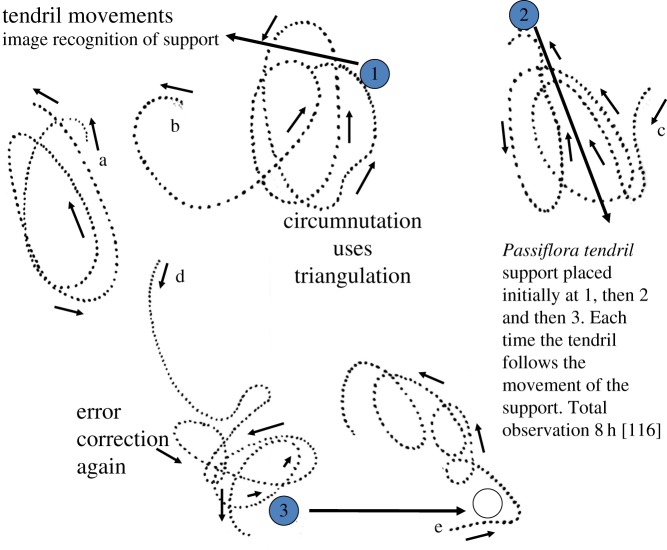
Circumnutation enables the sensitive shoot to accurately construct a picture of the light distribution and maximum direction ahead and grow towards it (figure 1). Although plants are very sensitive to light, they lack specific organs to detect its direction such as the complex eye in animals. Instead they move the light-sensitive tissue in a circle and remember how the intensity varies throughout, being very sensitive to even slight light differences [74]. Growth is then changed in direction to ensure that the intensity is equal throughout the circumnutation. This is a form of spherical or circular triangulation enabling efficient adaptation. Similarly, snakes use a split tongue or a tongue that moves from side to side and up and down. The presence and direction of the prey is assessed by the preponderance of volatile chemical particles on the various sides of its moving tongue or on the two sides of a split tongue. It has been described as having three-dimensional glasses for the snake and a similar conclusion applies to the circumnutating plant.
Figure 1.
Circumnutation uses a form of triangulation to locate the optimal direction of light. Large open arrow indicates growth direction. Smaller filled arrows indicate light direction and intensity. (a) The diagram illustrates the potential circular movement of a circumnutating seedling (dotted line). By movement in the form of a circle the shoot is able to compare continuously the light intensities at each stage of the rotation. The circumnutating shoot is using a form of spatial triangulation much as a snake uses its moving tongue to compare the density of prey chemicals on all sides and alter movement accordingly. In figure 1a, the assessed light intensity on all parts of the circle and on both sides would be about equal thus indicating a forward direction of growth. (b) In this case light (or in the case of dodder volatile chemicals) is to one side and thus the light intensity diminishes and then increases as the circle is moved through. This would signal a change in direction as indicated until again equal intensities throughout the circle are experienced. The requirements are memory of the variations in light intensity as the tissue moves around the circumnutatory circle and causing changes in movement to equalize out the light intensity. (Online version in colour.)
After this initial assessment, the Rubus shoot decides on its direction and moves swiftly without further circumnutation being necessary, except for minor adjustments later. Without time lapse, these observations could not have been made. Circumnutation is very common in seedlings and Darwin compiled large numbers of observations that he made [41].
8.2. Intelligent leaf movement in low light
Trying to optimize the energy gain/outlay ratio (costs versus benefit) in light-limiting circumstances, needs an assessment of the best distribution of the limited energy resource can be used for stem, root and leaf construction and how much energy will be gained back from the proposed leaves [77,78]. If the goal is also to reproduce, the energy drain for flower and seed construction has also to be factored in, as well as access by pollinators. If local competitors are few, then more of the limited resources can go to leaves; if many, height becomes more critical. Under a forest canopy, annuals of varying height are frequently observed so that the benefits of gaining more light are balanced by the commitment of resources to height and sufficient stabilizing root.
Under canopies or in forest gaps with low light, leaves tend to form monolayers, thus maximizing light gain for expenditure on leaf construction [79]. ‘Leaves are not that stupid. Leaves are positively phototactic at low light levels and tend to arrange themselves so as to avoid overlap. Since mutual shading may often be the most important way in which plants interact trophically, it may be profitable to study leaf height as an expression of altruism’ [75]. Which buds break dormancy to form branches is likewise assessed by light distributions.
8.3. Adaptive variability to light in response to crowds
In a crowded competitive environment of plants of similar height, access to light by the individual is diminished. To counteract this reduction, a set of phenotypic changes are initiated that are commonly called ‘shade avoidance’. Greater levels of resource are actively provided to the shoot at the expense of the root. Shoot growth is accelerated but on average the stem is thinner, internode distance is increased with fewer leaves, root growth is diminished, petioles (leaf stalks) are longer, leaves are angled to the horizontal [58,59]. The ultimate goal is to overgrow the opposition but if that is not possible, to place the reproductive organs in a position in which pollinators can easily locate and use them.
The altered spectral qualities, the ratio of far red/red light which is increased in light reflected from other leaves is one initiating signal of shade avoidance. However, alteration of the far red/red ratio also changes the composition of emitted volatile organic chemicals (VOCs) and these influence the biomass composition of adjacent plants of other varieties or species [80]. In crowds, however, leaves that touch each other assume a more vertical direction preceding the assessment of the far/red ratio and improving its subsequent assessment. The touch signal is initiated in the leaf blade and information transferred to the pulvinus which responds increasing the angle of elevation [81].
Since the shade avoidance syndrome can be instituted by reflected light before any loss of photosynthetic light, this decision clearly estimates a future circumstance rather than the present. For seedlings that perceive the shade signals, growth is totally directed away from the area of competition [82]. The shade avoidance response has been shown to be adaptively plastic and a prominent determinant of fitness [83–87]. Those individuals that assess by learning the environmental circumstance more accurately and adjust the plant body to best fit the prevailing condition are obviously more intelligent than others. They are more likely to survive and reproduce. In the appropriate circumstances this is a good example of intelligent behaviour.
9. Intelligent adaptive behaviour in mineral acquisition
When more major changes are required between overall root and shoot structure, then long range communication is essential. Local phosphate deficiency is sensed by the growing root tip and results in root proliferation. The root enormously increases the secretion of organic acids and phosphatases to release phosphate from the insoluble complexes with calcium, aluminium or iron and to breakdown organic phosphate derivatives [63,66,88]. The shoot is involved in this decision, it is not a simple diversion of resources to the root to increase growth rates as used to be thought [89].
When roots grow through a patch of soil rich in N, lateral roots proliferate [5]. Again long range communication from the shoot is likely to control the extent of root proliferation. But individual roots growing towards an N-rich patch may not require direct shoot permission, growth is accelerated as the patch is approached and then dramatically slows when the patch is reached, a phenomenon that needs more detailed investigation but analogous to movement of grazing animals [67]. Some seedling roots exhibit forms of circumnutation when grown in the absence of soil. If this continues in soil situations then this can be regarded as increasing the search for minerals or avoidance of toxins, analogous to reptiles that move their heads from side to side to improve prey detection.
10. Situations of choice and decision
The word intelligence is derived from the Latin inter-legere meaning to choose between and a reasonable description of intelligent behaviour. Choice and decision requires information on past behaviour, the presence of alternatives and critically an assessment of immediate futures enabling the beneficial choice to be made and furthering fitness. Making the less beneficial choice may have little immediate impact but will waste essential energy thus moderating fitness. The Charnov model [90] provides a simple way of assessing efficiency of excess energy or food gained during feeding. Observations of feeding behaviour in a number of animal organisms have indicated its general agreement with observation.
10.1. Motivational states in goal-directed behaviour, choice and decision
A two neuron system has been identified in the water snail, Lymnea that underpins decision-making during food search and consumption [91]. ‘Lymnea performs a sophisticated form of decision-making during feed searching behaviour using a core system of just two neuron types. The first reports the presence of food and the second the motivational state acting as a gain controller for adaptive behaviour in the absence of food.’ ‘Once it makes a perceptual decision about the presence of the stimulus, it often needs to perform further adaptive decisions to maximize the chances of achieving the goal; for example by changing search strategy if insufficient resource is localized. In the face of limited resources an important additional demand is that the goal is achieved with minimal energy expenditure’ [91].
Since Lymnea is making goal-directed choices, these comments from Rangel & Todd [92] are also relevant. ‘The problem can be solved by two different approaches. Animals can learn the value of each action through trial and error using reinforcement learning and then take the action with the highest value but this is only able to pick the optimal action on average. In another approach animals estimate the value associated with each action in every trial using knowledge about their costs and benefits. With sufficient knowledge this approach, often called goal-directed decision-making, can do much better since it is able to pick the optimal action at every trial.’ Goal-directed behaviour is common in plant behaviour [53].
10.2. Dodder search and feeding; suitable food located through both smell and taste
Observations on the parasitic behaviour of Cuscuta (dodder), indicates it performs analogous behaviour to Lymnea but without the need for control by a simple nervous system. Dodder is a typical parasitic plant in that it searches and locates host plants which in due course it exploits. Some 4500 angiosperm species exhibit varying degrees of parasitism.
The dodder seedling lacks a root. Consequently, it must find water quickly on germination. In this condition, it is clearly in a motivational state of search. The shoot circumnavigates and locates nearby hosts from the volatile organic compounds the host emits, as shown by time lapse [76]. From an initial, vertical circumnavigation of rotation, the circumnutation vector progressively changes to a horizontal one as the direction of host volatiles is detected. Receptors for these volatiles clearly must be present in dodder.
10.3. Motivational states of assessment in dodder of food suitability by taste
Once a suitable host is captured, dodder coils around its host and eventually develops haustoria; pegs that are driven into the host's circulatory system and provide essential sustenance for the parasite. Continued parasitic success leading to flowering, requires additional hosts and the search is helped by subsequent growth and branching. If there are several hosts, and thus choices in the vicinity, a decision must be made by any one branch to parasitize one host rather than the other. In the seedling stage if the two are equidistant say between a young tomato or a cereal plant the seedling plumps 90% of the time for the tomato even though the cereal does produce several chemical attractants [76]. In the field, dodder is known to prefer certain species more than others, a choice sometimes thought to relate to how much sodium is to be found in the host.
Kelly [93,94] offered numerous suitable hosts to dodder by tying them together and found a rejection rate of 50% within a few hours, indicated by the parasite branch growing away. The assessment period is thus completed in this short time and since contact is only surface in character, assessment is probably made of the volatile chemical signature of the host. Dodder is using taste like Lymnea and changing search strategy when the source is not satisfactory. The future assessment of host potential is made in these few earliest of hours. By feeding potential hosts with N, rejection can be reduced to 20–30%, suggesting additional N modifies host chemistry in terms of volatile organic compound synthesis and food suitability. The parasite can make a quantitative assessment of the future resource return.
A detailed time course of parasitism showed that typically the parasite coils around its host; a process continuing for about 4 days and which then ceases presumably controlled by feedback [93,94]. Once finished, haustorial formation commences and the numbers are determined by the number of coils. The energy outlay here is the extent of coiling while the subsequent parasite growth over 28 days indicates the energy gain. By making measurements on six different hosts and plotting energy gain/energy outlay a linear relation was observed indicating agreement with the Charnov [90] model of animal foraging. Thus dodder optimizes this crucial ratio. Estimates are made of the energy outlay in terms of number of coils to be produced in the first few hours of contact and before any commitment to exploit. It is not known how dodder makes these future intelligent assessments but they match many animal foraging assessments but, of course, without a neural system.
10.4. Dodder accomplishes behaviour which in Lymnea requires neural circuitry!
There is little doubt that a primary problem some biologists have with the concept of plant intelligence is the assumption that intelligence is limited to organisms with some sort of neural network. The observations above show that dodder is capable of equivalent behaviour in the absence of a neural network. Alternative methods of assessment exist in plants. Intelligent behaviour is not dependent on neural activity.
What is now required is understanding of the assessment processes involved in particular costs and benefits estimates, not only in parasitism but in shade avoidance, the various phototropic scenarios under canopies and in competitive circumstances for minerals too.
11. Self-organization: lessons from swarm intelligence
In complex multicellular animals, the immune system evolved to become a major assessment system, using both learning and memory. However, it is strongly diffuse compared with the centrality of the brain. Although trial and error immune learning is used together with memories of differing length, it is rarely referred to as intelligent. Some do, however, refer to it as the consciousness of the body [95]. Distinct analogies to swarm intelligence have been noted [96]. The diffuse behaviour and localized capability of the immune system always take place in conversation with the whole animal organism. Plants learn and remember both in a similar diffuse and localized fashion.
11.1. Swarm intelligence: a dynamic network of interrelations
Swarm intelligence deals with natural systems whose behaviour is coordinated via self-organization, decentralized and distributed control; all behavioural similarities to plants. The swarm system is composed of many individuals who are largely homogeneous and interact with each other to form complex dynamic networks and with the environment (e.g. stigmergy) via potentially simple rules. Feedback processes dictate function and control change. Parallel action is possible because individuals composing the swarm can perform different functions, making the overall behaviour more flexible. The system is largely fault tolerant; that is, some loss makes little difference to the overall behaviour. The coordinate behaviour requires no overall controller and without any individual being aware of the overall behaviour. As Maeterlinck commented with regard to white ant colonies ‘What is it that governs here? What is it that issues orders? Foresees the future, elaborates plans and preserves equilibrium’ [97].
Those rules allow thousands of relatively simple animals to form a collective brain able to make decisions and behave like a single organism. The behaviour of each individual is best described in probabilistic terms thus allowing a degree of variation among the individuals rather than exact responses. The behaviour is effectively scale free, small numbers of nest individuals exhibit similar behaviour to much larger numbers.
Present swarm intelligence analysis is largely confined to relatively simple arrangements but its origin derives from social insect colonies which are complex networks too. No insect member has an oversight of the behaviour of the whole just as no individual molecule has an oversight of the complex network that constructs a cell, or any tissue that constructs a whole plant. Most colonies grow with time and store resources against seasonal variation but growth is slow and not readily visible without measurement. It is the whole nest that is subject to selection and fitness; a product of the diverse interactions, feedbacks and communications between the individuals.
11.2. Analogous organization and behaviour between social insect colonies and angiosperm plants
The growing plant is self-organizing, there is no overall controller of the phenotype and development occurs from the bottom up than the top down. There are numerous leaves (equivalent to insect workers) and branch roots that do have qualitatively different but parallel functions in acquiring essential resources. Furthermore, leaves do communicate with other leaves [98,99]; roots communicate with other roots [100]. Self-evidently there is communication between root and shoot. Fault tolerance is shown by root pruning experiments in which substantial amounts can be removed without loss of shoot growth and shoot pruning which subsequently increases yield [89,101,102]. Feedback and feed forward processes control the growing plant and phenotypic plasticity. Plant individuals can be considered scale free; loss of some leaves or roots is tolerated but the individual remains. Leaves and roots are fixed on the same organism whereas in social insect colonies, the individuals move separately around but interacting with each other. Branch and leaf behaviour is best considered in probabilistic terms accounting for a degree of variation in numbers and position observed. Both plants and swarms later produce male and female for reproduction. Other analogous behaviour has been published which indicates quorum sensing as a basis for whole plant decisions [2]. The individual plant itself is subject to selection and fitness.
The beehive collects nectar (carbohydrate), pollen for N and water when required [103]. These are exactly the same constituents needed by plants. It is thought that low levels of protein in workers is the stimulus that instructs more workers to concentrate on collecting pollen needed for growing grubs. The plant equivalent, instigated by low protein levels in cells, is to increase root production and search for new sources of N. Nectar is used for honey, an energy resource to cover the winter. The numbers of honeycomb empty cells for storing honey are thought to be the stimulus, diverting more workers to collect nectar. Low levels of circulating carbohydrate are probably the stimulus for more leaf production, the equivalent of workers again. Water is only collected to cool the hive by evaporation. Part of the function of roots is to collect water and deficits institute increased search activity. Evaporation of water in the internal spaces of the leaf enables cooling.
Hives also maintain a relatively constant temperature. Recent research has demonstrated that leaves themselves on trees ranging from arboreal to the sub-tropical maintain a relatively common internal temperature of 21.4 ± 2.2°C [104]. This is an approximate optimum for photosynthesis; the external temperature of the boreal to sub-tropical averages ranged from 12° to 26°C. There are six ways known that help to maintain this apparent homeostasis to either cool or warm the leaf. The internal leaf temperature is regulated by manipulation of leaf blade orientation, control of stomatal aperture, chloroplast movement inside cells, changes in leaf hairiness and wax reflectance and numbers of leaves on branches [2,105–111]. The first three may take only some 15 min to change, the next two several days, and the final one maybe a week or two. Communication and feedback must be at the basis of these coordinate changes but what is communicated remains unknown at present. The coordinated events represent adaptive and thus intelligent plasticity. Those who more accurately assess the particular circumstances and deploy the optimal set of controls provide a greater probability of ultimate fitness.
11.3. Assessment and coherence in plants
What has always been problematic in plant intelligence is assessment. In animals, it becomes easy to identify the brain as an assessment organ that controls movement. The social insect colony in contrast acts holistically while allowing distributed control (e.g. individual food search). The search and collect behaviour of groups is analogous to phenotypic plasticity. The whole colony survives on complex messaging and negative and positive feedback between individuals.
On this analogy, the assessment of environmental signals and the necessary decisions to be taken by plants arise simply from the complex communication between cells, tissues and organs involving numerous feedback and feedforward processes. Assessment in plants does not necessarily require the equivalent of a brain although a functional equivalent may be present. But if swarm intelligence is the term to describe this behaviour in social insects, then the analogous behaviour in plants certainly deserves the description of intelligence too.
The intelligent behaviour of swarms and plants is indicated by a quote [111]. ‘Indeed it is not too much to say that a bee colony (individual plant) is capable of cognition in much the same sense that a human being is. The colony (plant) gathers and continually updates diverse information about its surroundings, combines this with information about its internal state (assessment) and makes decisions that reconcile its well-being with the environment’. That statement provides a challenge for future plant research.
12. The plant dance floor
One of the intelligent characteristics of the beehive is the way workers are redirected towards different food sources as they are discovered. The information exchange takes place on the ‘dance floor’, a meeting place where several different dances are used to manage and control information flow.
Commonly, there is internal competition among different shoot branches for root resources that are gained from the vascular system. Likewise there is internal competition among different roots for shoot resources [112,113]. Uncontrolled competition, however, cannot work. In the shoot, those branches nearest the root would gain most root resources and prevent much further shoot branch growth; similarly for the root system.
The cambium is a meristem producing new vascular tissue by cell division. Topologically it is like an inner skin continuously connected throughout the plant stem and root system and in direct contact with the vascular system. The cambium is responsible for much girth increase but it has a second function. It also generates the vascular tissue that connects newly growing buds and branches to the main vascular strands that connect shoot and root together [112].
As part of a continuous monitoring and arbitration programme, the cambium comparatively assesses the productivity of each branch. In simple terms, those branches that are very productive are provisioned with more vascular tissue and more root resource, those that are moderate remain unchanged and those doing poorly have some vascular tissue blocked. If totally unproductive, all connection is blocked and the branch dies. Branch formation well illustrates intelligent adaptive variability. This is also the case for leaves which are due to be abscised; the vascular tissue connection is blocked as an abscission zone secreting wall hydrolases is constructed enabling a clean break. A similar situation is probably found below ground.
Cambial cells must be in continuous communication with each other in order that comparative assessments can be made. It is a tissue that acts holistically in girth increase, gravitropic responses, cambial regeneration and wind sway responses indicating communication occurs throughout itself and thus capable of this critical role [2,47]. What information is used as comparative assessment is not known but clearly carbohydrate flux rates through the phloem adjacent to the cambium are one possibility; hormones are another.
The manipulation of the vascular system here also accounts for the controlled manipulation of all sorts of activity which come under the title of ‘source and sink’. Areas of carbohydrate availability like leaves, are regarded as sources and sinks areas that use carbohydrate. It is assumed that carbohydrate flows variably through the vascular system like water through a pipe. This may not be the case, movement will be controlled by the vascular system and increased movement will probably require more vascular tissue.
13. Correcting errors in behaviour and how plants count to five
The ability to correct errors in behaviour that is unproductive and launch it on a correct course, suggests an ability to compare what is actually happening with what should be happening; the current unproductive situation is directly compared to a potential and productive future. Error correction reflects intelligent adaptive variability and equates to intention; it should improve fitness by minimizing energy waste on fruitless enterprise. Although there are few examples of error correction in plants, I suspect many more occur during development itself and intrinsic correcting mechanisms are likely to be present. Something like error correction should operate during herbivory, disease or abiotic stresses using feedback to correct the current course to its desirable state.
13.1. Climbing plants
A number of plants climb on others and use various sensitive tissues, tendrils, leaf petioles, stems, for this purpose. When the sensitive tissue detects a support through a mechanical signal they usually attempt to encompass or wind around it. The energy consumption of climbers is reduced, compared with plants that provide their own independently supporting stem.
Climbing plants use circumnutatory movement to search for suitable supports. Some detect suitable support by mechanical stimulation from impact; others use reflected far red light or detection of volatile chemicals [76]. But provision of an unsuitable smooth support for climbers such as a glass rod leads to some initial winding followed by unwinding and continuation of the search for a suitable support elsewhere [2,114]. The same tendril can try four times or more with such a support before habituating and failing to further respond [114]. The initial curling then leads to an adaptive straightening before moving on elsewhere. Tendrils that contact each other also fail to wind around the partner; the recognition of unsuitable smoothness for grip may be the answer.
From my own observations of a vine in my greenhouse, tendrils normally attempt to assume the shape of whatever surface they come into contact with; that is, they learn progressively the shape of potential support characteristics. Coiling is common because most potential support structures are round or oval. Darwin [114] also recorded this behaviour too in climbing plants and observed that they often recoil from such situations. He offered a blackened zinc plate to a tendril-containing plant and noted that they ‘bent themselves around the edges of the zinc plate but they soon recoiled from these objects with what I can only call disgust and straightened themselves’. While disgust is clear in Darwin's anthropomorphic assessment, there is clear plant assessment made of the unsuitability of the support. I have observed similar recoil from an initial unsuitable support. Tendrils do require two signals, blue light and touch, to initiate curling. If touched in darkness and then the blue light signal given later, they exhibit curling for a period of up to 2 h before the excitation state from the touch stimulus disappears [115].
Figure 2 describes the movement of a Passiflora tendril when presented with a support that is periodically moved. The whole observation took 8 h and was monitored by the laborious method of placing a glass plate over the tendril and marking the position of the tip with a dot every 2–3 min [116]. The tendril clearly recognizes the support and moves towards it each time when it was moved.
Figure 2.
Circumnutating Passiflora tendril changing direction as an offered support made of green plant materials is moved successively from position 1 to eventually 3 before removal altogether. At first no support was presented (situation a). Then the support was placed at position 1 (situation b). Clearly although continuing circumnutation, the tendril approached the support using triangulation as explained in figure 1 legend. When the tendril was near the support, it was moved to position 2 (situation c). As the tendril approached the support again, the support was moved to position 3 (situation d). Finally, in situation e, the support was removed altogether. Small arrows indicate direction of movement of tendril. The position of the support was identified and the tendril changed behaviour to pursue it rather as an animal predator pursues prey. The behaviour is similar to that of a dodder seedling identifying a potential prey from volatile chemicals and moving the vector of circumnutation towards it [93]. (Online version in colour.)
13.2. Insectivorous plants
Insectivorous plants often live on N poor soils and need to supplement their diet with insects that they capture and digest. Darwin [117] authored a book on them which actually sold better initially than his Opus Magnus [3].
The Venus fly trap (Dionea) contains two sensitive hairs that must be touched within 20 s of each other for the trap to close. Each touch of the hair initiates an action potential that is conveyed to motor cells at the base of the trap that controls closure and engenders a decaying short-term memory. Two hairs are used to avoid erroneous closure induced by, for example, rain drops. However, closure on its own does not initiate the secretion of digestive enzymes and the formation of sodium channels to abstract a necessary mineral. Small insects can enter the trap and initiate just a two hair dependent closure. The top of the trap is grill-like and these can then escape and often do so. Under these error conditions, the trap opens in a day.
Larger insects that cannot escape continue to stimulate these hairs and a minimum of five action potentials (five touches of the hairs) are necessary to initiate the synthesis and secretion of digestive enzymes and sodium channel formation to uptake this important mineral. Under these conditions the trap remains closed for many weeks [118]. Thus this plant can count to five and by default 1, 3 and 4, a completely unexpected capability. In allowing small insects to escape, the plant is assessing the potential energy gain as unrewarding against a potential future of much larger and energy-rich prey.
Similarly for Drosera, the sundew. When insects land on the sticky tentacles, other tentacles bend to touch and enmesh the insect further with sticky solution. The whole leaf then folds in on itself, envelops the prey and digests it over many weeks. The signal indicating the response may be protein of any kind since milk and meat will initiate leaf envelopment but water or small stones or pieces of chalk only initiate slight tentacle movement which rapidly resets itself by tentacle straightening, correcting the erroneous response and resetting the trap [117].
14. Game theory and competition
Competition is the essence of Darwinian selection and thus individual behavioural interaction; the interaction that gives rise to a hierarchy of fitness. Populations are dependent on a mixture of demes and demes are composed of a mixture of individuals [119]. Individual competition is how Darwin assumed selection to operate. Game theory was originally constructed to analyse competition in economics among companies rather than individuals in biology. Maynard-Smith & Price [120] first used game theory, to describe animal conflict. Some plant competition studies have received attention and are described at greater length [2].
When plants share soil in close proximity, they proliferate their root tissue reducing shoot growth and potentially seed number [121]. The known aim is to sequester soil resources before they are removed by adjacent competitors and is another example of adaptive variability. How these plants recognize that they are growing next to aliens, compared with self, is not clear but evidence indicates a recognition process at work. Seedlings constructed to contain two shoots and two roots are can be split down the middle and the two daughters each containing a shoot and root separately cultivated. After a number of months apart, each daughter now responds to the other genetically identical partner (assayed as root proliferation) as alien [122]. Clearly a form of self-recognition is present. Observations made from of a number of excavated root systems in both the wild and agricultural settings indicates that roots of different species do avoid each other and leave space in between suggesting the potential for alien recognition may be common [123]. The recognition mechanisms are unknown but suggest intention. There are also indications that soil space itself is a signal and with greater space (but not greater resources) plant development and the phenotype is changed. If space is a signal, a mechanism involving recognition of the amount of soil space available, is currently unknown.
14.1. A third partner modifies competitive circumstances and leads to some cooperative behaviour among individuals
Some 80% or more of angiosperm plants exist in symbiotic combination with mycorrhizal fungi. Secretion of chemicals from roots like strigolactone attracts endo-mycorrhizal fungi whose hyphae penetrate the plant root and then the root cells, to form an arbuscular membrane. Carbohydrate is passed across this transit membrane to the symbiont and phosphate and iron is passed back to the host from the symbiont. In conifers and some dicot trees, ectomycorrhizae form a sheath around the root rather than penetration. Symbiosis requires a complex conversation between host and symbiont, otherwise defence reactions are initiated. Once the symbiotic state is established, disease resistance by the host against other pathogenic fungi is increased. The hyphae are much smaller than lateral roots and can penetrate soil particles and structures not open to them to mine iron and phosphate. The mycorrhizae form a very large complex network of hyphae in the soil. It has been claimed the network can extend to cover whole woodlands but whether as a single individual or anastomoses between different individuals is not clear [2].
However, some symbionts can cheat on the relationship. In the case of mycorrhizae, the individual can accumulate phosphate for itself and not pass it on. In that situation the plant has two known strategies [2]. The host can insist on a one-to-one exchange of phosphate for carbohydrate. Alternatively the host initiates defence reactions against the cheater.
Similar problems occur in the well-known legume rhizobial bacterial symbiosis. There are a very large number of rhizobial species only some of which are symbiotic in respect of exchanging N for carbohydrate. Some of these non-providers can enter the root through root cracks usually caused by lateral root breakthrough. The non-providers employ recognition conversations acquired from the symbiotic species. They live in spaces between cells but can be subject to defence reactions if they fail to provide fixed N. Others enter the anaerobic nodule cavity but they do not fix N either. Again the plant becomes aware of the cheating situation and reduces the anaerobic status of the nodule that damages cheater fitness [2]. Dealing with cheaters is obviously intelligent behaviour.
However, a single mycorrhizal individual can connect together several individual plants of the same or different species to form a common mycorrhizal network. Evidence suggests that various kinds of information can pass through this network. Partners subject to herbivore or disease attack do transfer some information from donor to adjacent receiver in the network enabling them to prepare suitable defence reactions via increased defence enzyme changes [124,125]. Within 24 h, the receiver plant had increased defence enzyme levels up to threefold [125]. A potential form of communication is through action potentials and cytosolic calcium waves induced by herbivore damage in the attacked plant [57]. Receipt of a substantive electrical signal by the root may pass easily into the penetrating mycorrhizae. Fungal hyphae have been reported to exhibit action potentials [126,127]. Such electrical information could continue through to receiver plants initiating action potentials in them, and inducing glutamate-sensitive calcium waves and de novo enzyme synthesis. The extent of cooperation between plants in the wild is still uncertain but clearly the potential for root fungal networks is present. Competition between plants is thus moderated and much earlier ecological studies on competition may need some reassessment. With two different plant species as partners in a common mycorrhizal network, a more intelligent partner can sequester more of the mycorrhizal material which has been observed.
The mycorrhizal network can also transfer water, some allelo-chemicals and some sugars to partners in the mycelial network [124,128,129]. How far these elements of communication can inform and affect other network partners is not known but limits may be strict unless there is information amplification by each partner.
14.2. Shoots and games
Shoot behaviour analysed by game theory has indicated the necessary trade-offs (decisions and choice) between plant height and foliage density of forest herbs growing competitively under a canopy or in full sunlight. Height is gained at the expense of leaf number and game theory explains how these herbs grow to different heights despite all being subject to similar reductions in light exposure [77]. Choice and decisions enter into the games as indicated earlier with obvious decisions about optimizing energy expenditure against energy gain using probabilistic assessments. Further analysis has investigated leaf competition on the same plant because older leaves get shaded by younger ones. In this model, the leaf area index (LAI, leaf area/unit ground area) was predicted to increase with an increasing degree of inhibition and light interception between genetically distinct neighbours. This implies that clonal plant stands with genetically identical daughters have a different LAI structure [2].
15. Volatile organic chemicals: the plant language?
The VOCs are emitted by leaves, shoots, roots, bark, fruits and flowers of probably all plants. About 1% of fixed carbon is used and emitted. The spectrum of emitted volatiles is changed by herbivore attack and disease and that can attract parasitoids of the herbivorous pests (so-called burglar alarm) [130,131]. Methyl jasmonate, ethylene, and methyl salicylate, all VOCs are involved in defence induction mechanisms [132,133]. These chemicals can diffuse to adjacent plants if they are sufficiently close together and initiate defence mechanisms in un-attacked plants. The limit of useful spread is about half a metre; beyond that insufficient stimulus is received. But many wild plants will be closer than half a metre. On that basis some communication will take place from an attacked plant. There is also evidence that kin respond better than aliens to adjacent signals indicating potential shoot recognition mechanisms [134]. If adjacent plants are partners in a mycorrhizal network then information is transferred between both roots and shoots as indicated in the previous section. A second function of herbivore-induced volatile production is to overcome some limitations of the vascular system of the attacked individual. Some parts of the plant, are not equally exposed to the circulating defence chemical messages and in this case, the VOC will help make up this deficiency [132,133].
The VOC spectrum is qualitatively different between individual species and even individuals [132,133]. Obvious fitness benefits arise from those emitted by flowers and fruits; variations in fragrance can mark out intelligent behaviour. Holopainen & Blande [133] have creatively suggested that the complexity and species individuality of VOC act as a plant vocabulary or language; individual volatiles are words and the VOC signature represents sentences. A sentence is an emergent property of the words used to construct it [5]. If the analogy is useful, it suggests that the whole VOC signature due to synergy between the words is essential; omission of one or two words (that is one or two VOCs) will fail, something now reported in one case [135].The VOCs emitted by damaged shoots elicit greater response in genetically identical relatives than aliens even from the same species suggesting the potential for self-recognition and perhaps altruism [132]. The concept of spontaneity suggests each individual will likely emit its own signature [2].
That plants can sense alien volatiles is known. The young seedlings of Dodder, a parasitic plant, home in on their prey by sensing the direction of emitted volatiles and potentially use emitted volatiles to assess the suitability of prey for exploitation [76,94]. Numerous VOCs are emitted by rhizosphere bacteria and mycorrhizae and alter root architecture in different ways ([136] and references therein). To enable these changes in form there have to be root sensing mechanisms via receptor proteins and a concurrent signal-transduction sequence. If alien species of plant root emit these volatiles they will induce root proliferation too.
15.1. Leaf mimicry through volatile organic chemical recognition?
Boquilia trifoliolata, a climbing vine in temperate rainforests, mimics the leaves of its supporting hosts in terms of size, shape, colour, orientation, petiole length and/or tip spineiness. The consequence of mimicry is to reduce herbivory. Mimicry on eight different hosts has been reported [137]. A vine, extending across different hosts, responds to each specifically in turn. The sensing mechanism is clearly unique to each host species and the most likely individualistic signal is released host bark VOCs. Again the sensing requires receptors able to discriminate the particular group of VOCs. However, Mancuso & Baluska [138] have creatively suggested that plants do possess primitive eyes, that is ocelli, based on a very early suggestion by Haberlandt. These might provide the necessary discrimination [139]. Anything that does discriminate between the fine details in its environment using light, justifiably has a tissue akin to an eye although obviously much simpler. A combination of smell and sight is more likely to lead to accurate recognition.
Some vines simply avoid trees on which the trunk is too smooth to enable climbing [2]. Again, recognition of a particular group of VOCs seems at present the most likely explanation.
15.2. Volatile organic chemical receptors?
The range of volatile chemicals produced below ground is quite extraordinary (e.g. [140–144]) and sufficient to account for the complexity of self and alien recognition which is known to occur. How are VOCs sensed? Since plants synthesize many VOCs, they do have enzymes with active sites that produce the chemical in the first place and thus have the potential with slight modification of producing a similar protein for sensing them. To simplify the detection of the VOC signature a single protein receptor detecting only partial structures of all the individual VOC signature complex is indicated and is characterized in odotope theory. These are forms of sensory capability akin to smell in animals.
16. Learning and priming from experience
Learning about a stimulus requires a transduction sequence process whereby the signal is processed either by cells or neural systems. For intelligent behaviour the learning process should institute a memory which can be accessed to modify subsequent behaviour. ‘Intelligence is commonly held to consist essentially of the modification (improvement) of behaviour in accord with experience. It is the correlations of experiences and actions that constitute intelligence’ [12]. Even in bacterial swimming, a few-second memory is required of the previous experience so that a new direction of swimming can be accessed and used. But memories have to be learnt first.
Learnt memories in animals usually result in obvious changes in behaviour detected by altered movements; but with the obvious exception of the immune system. There are many similar learning and memory events in plants that are simply discounted because the plant fails to visibly respond a reflection of the different plant lifestyle. But they can be experimentally detected. As with the immune system, the memory lengths can be diverse. In plants they vary from a few seconds to hours, weeks, months or even years dependent on the tissue and particular signal [2,7,53]. This variability is inevitable in the nature of the self-organizing structure of the plant phenotype.
16.1. Herbivory, disease and abiotic stress induce memories lasting months to years
Those plants that experience herbivory or disease become primed to further insults so that they now respond more quickly, to a greater extent and thus more robustly, than unchallenged plants [145,146]. This is clearly by definition intelligent behaviour. Priming can last for years and in certain cases survives meiosis. Chromatin structural modification, through epigenetic changes (specific histone acetylation or phosphorylation, DNA methylation), are one probable mechanism [147–149]. Perhaps unsurprisingly a similar chromatin modification or remodelling is involved in long-term memory in neural circuits [150]. The most recent addition to the potential for plant memory is that of prions recently discovered in plants and which provide a novel method of memory over long periods of time. Priming is quite simply learning and the memory is long-lived modifying future behaviour; priming could well be dependent on such protein memory [148].
Priming is now recognized to occur after repetitive heat, drought, cold and salt stresses which train the plant to respond more quickly and more robustly to repetition of these conditions [2,149,151]. The experience is learnt and remembered; the memory then participates in modifying subsequent behaviour to these abiotic insults and in ways that will impact and hopefully improve fitness. Repetitive treatments with the hormone, abscisic acid (ABA), primes ABA dependent genes in the same way [152]; their expression now responds more quickly and to a greater extent to subsequent hormone treatments.
Perhaps more intriguing is the obvious cross talk between many of these abiotic stressful conditions in which some of the same events are induced by separate stresses. Thus the response to one like heat, increases resistance to cold stress. Similarly, herbivore attack increases resistance to disease [150]. These observations represent kinds of conditioned behaviour in which one signal influences response to another and increases fitness.
The life history of individually cloned plants from the same parent determines their capability for stress response and priming. It illustrates how sensitive plants are to slight environmental variation and indicates the potential for fitness variation and intelligent behaviour [153].
Stress-induced signal-transduction pathways commonly involve information flow through cytosolic Ca2+-dependent processes and numerous interlinked protein kinase pathways. Concomitantly, the synthesis of the protein constituents of these pathways are increased, deepening the metabolic channel and increasing the direction through which information flows [2]. During learning by the brain, synaptic connections are strengthened and/or new connections made thereby deepening the channel of information flow through particular neural pathways. The analogous aspect of learning in both organisms is complete.
17. Conclusion
Learning, memory and intelligence are not common terms in plant science because of a belief that these behaviours are properties only of organisms with neural systems. Plant scientists, being of course animals themselves, have expectations that intelligence involves obvious visible movement. The animal immune system, single cells, the entire social insect colony, and the extensive evidence provided here on plants indicates the necessity for better appreciation of the intelligence that underpins them. Plant intelligence does not require an obvious brain; complex communication, still poorly understood in plants, may be sufficient. It is in the area of signal assessment that future investigation needs to concentrate.
The tenor of much plant research has concentrated on identifying signals, the positive feedbacks that initiate change. Perhaps the more crucial are the negative feedback interactions of which virtually nothing is known that indicate receipt of a signal and control its further expression. Nervous systems were an almost inevitable consequence of the need to move to find food, a particular lifestyle but just one of several, that we of course share. Intelligence, however, is an inevitable consequence for all organisms that consistently deal with a variable environment, both plant and animal. Without it, competition and fitness would never have energized evolutionary change in the way they have. From an initial and controversial beginning in 2003 [7], plant intelligence investigations are now spreading into different areas of study that find the concepts productive and even beginning the complex consideration of the meanings of plant cognition [154,155]. The frontier continues to expand.
Competing interests
I declare I have no competing interests.
Funding
I received no funding for this study.
References
- 1.Vertosick FT. 2002. The genius within. Discovering the intelligence of every living thing. New York, NY: Harcourt Inc. [Google Scholar]
- 2.Trewavas AJ. 2014. Plant behaviour and intelligence. Oxford, UK: Oxford University Press. [Google Scholar]
- 3.Legg S, Hutter M. 2007. A collection of definitions of intelligence. Front. Artif. Intell. Appl. 157, 17–24. [Google Scholar]
- 4.Darwin C. 1859. The origin of species by means of natural selection. London, UK: John Murray. [Google Scholar]
- 5.McNamara JM, Houston AI. 1996. State dependent life histories. Nature 380, 215–221. ( 10.1038/380215a0) [DOI] [PubMed] [Google Scholar]
- 6.Stenhouse D. 1974. The evolution of intelligence. London, UK: George Allen and Unwin. [Google Scholar]
- 7.Trewavas AJ. 2003. Aspects of plant intelligence. Ann. Bot. 92, 1–20. ( 10.1093/aob/mcg101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wright S. 1982. Character change, speciation and the higher taxa. Evolution 36, 427–443. ( 10.2307/2408092) [DOI] [PubMed] [Google Scholar]
- 9.Baskin CC, Baskin M. 2000. Seeds: ecology, biogeography and evolution of dormancy and germination. New York, NY: Academic Press. [Google Scholar]
- 10.Galloway LF. 2005. Maternal effects provide phenotypic adaptation to local environmental conditions. New Phytol. 166, 93–100. ( 10.1111/j.1469-8137.2004.01314.x) [DOI] [PubMed] [Google Scholar]
- 11.Galloway LF, Etterson JR. 2007. Transgenerational plasticity is adaptive in the wild. Science 318, 1134–1136. ( 10.1126/science.1148766) [DOI] [PubMed] [Google Scholar]
- 12.Jennings HS. 1923. Behaviour of the lower organisms. New York, NY: Columbia University Press. [Google Scholar]
- 13.Trewavas AJ. 2007. A brief history of systems biology. Plant Cell 18, 2420–2430. ( 10.1105/tpc.106.042267) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gao J, Barzel B, Barabasi A-L. 2016. Universal resilience patterns in complex networks. Nature 530, 307–312. ( 10.1038/nature16948) [DOI] [PubMed] [Google Scholar]
- 15.Giles AC, Rankin CH. 2009. Behavioural and genetic characterisation of habituation using Caenorhabditis elegans. Neurobiol. Learn. Mem. 92, 139–146. ( 10.1016/j.nlm.2008.08.004) [DOI] [PubMed] [Google Scholar]
- 16.Gushchin A, Tang A. 2015. Total wiring length minimisation of C. elegans neural network: a constrained optimization approach. PLoS ONE 10, e145029 ( 10.1371/journal.pone.0145029) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hobert O. 2003. Behavioural plasticity in C. elegans: paradigms, circuits, genes. J. Neurobiol. 54, 203–223. ( 10.1002/neu.10168) [DOI] [PubMed] [Google Scholar]
- 18.Yu H, et al. 2008. High quality binary protein interaction map of the yeast interactome network. Science 322, 104–110. ( 10.1126/science.1158684) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wuchty S. 2014. Controllability in protein interaction networks. Proc. Natl Acad. Sci. USA 111, 7156–7160. ( 10.1073/pnas.1311231111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zotenko E, Mestre J, O'Leary DP, Przytycka TM. 2008. Why do hubs in the yeast protein interaction network tend to be essential: re-examining the connection between the network topology and essentiality. PLoS Comput. Biol. 4, e1000140 ( 10.1371/journal.pcbi.1000140) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nacher JC, Akutsu T. 2012. Dominating scale-free networks with variable scaling exponent: heterogenous networks are not difficult to control. New J. Phys. 14, 073005 ( 10.1088/1367-2630/14/7/073005) [DOI] [Google Scholar]
- 22.Hall DH, Russell RL. 1991. The posterior nervous system of the nematode Caenorhabditis elegans: serial reconstruction of identified neurons and complete pattern of synaptic interactions. J. Neurosci. 11, 1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Varshney LR, Chen BL, Paniagua E, Hall DH, Chklovskii DB. 2011. Structural properties of the Caenorhabditis elegans neuronal network. PLoS Comput. Biol. 7, e1001066 ( 10.1371/journal.pcbi.1001066) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Towlson EK, Vertes PE, Ahnert SE, Schafer WR, Bullmore ET. 2013. The rich club of the C. elegans neuronal connectome. J. Neurosci. 33, 6380–6387. ( 10.1523/JNEUROSCI.3784-12.2013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baluska F, Schlicht M, Volkmann D, Mancuso S. 2008. Vesicular secretion of auxin; evidences and implications. Plant Signall. Behav. 3, 254–256. ( 10.4161/psb.3.4.5183) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Beitkrutz A, et al. 2010. A global protein kinase and phosphatase interaction network in yeast. Science 328, 1043–1046. ( 10.1126/science.1176495) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gee CE, Oertner TG. 2016. Pull out the stops for plasticity. Nature 529, 164 ( 10.1038/529164a) [DOI] [PubMed] [Google Scholar]
- 28.Trewavas AJ. 2008. Response to Alpi et al.: plant neurobiology—all metaphors have value. Trends Plant Sci. 12, 231–233. ( 10.1016/j.tplants.2007.04.006) [DOI] [PubMed] [Google Scholar]
- 29.Nakagaki T, Yamada H, Toth A. 2000. Maze solving by an amoeboid organism. Nature 407, 470 ( 10.1038/35035159) [DOI] [PubMed] [Google Scholar]
- 30.Nakagaki T, Kobayashi R, Nishiura Y, Ueda T. 2004. Obtaining multiple separate food sources: behavioural intelligence in the Physarum plasmodium. Proc. R. Soc. B 271, 2305–2310. ( 10.1098/rspb.2004.2856) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nakagaki T, Iima M, Ueda T, Nishiura Y, Saigusa T, Tero A, Kobayashi R, Showalter K. 2007. Minimum risk path finding by an adaptive amoebal network. Phys. Rev. Lett. 99, 068104 ( 10.1103/PhysRevLett.99.068104) [DOI] [PubMed] [Google Scholar]
- 32.Dussutour A, Latty T, Beekman M, Simpson SJ. 2010. Amoeboid organism solves complex nutritional challenges. Proc. Natl Acad. Sci. USA 107, 4607–4611. ( 10.1073/pnas.0912198107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Beekman M, Latty T. 2015. Brainless but multi-headed: decision making by the acellular slime mould Physarum polycephalum. J. Mol. Biol. 427, 3734–3743. ( 10.1016/j.jmb.2015.07.007) [DOI] [PubMed] [Google Scholar]
- 34.Saigusa T, Tero A, Nakagaki T, Kuramoto Y. 2008. Amoebae anticipate periodic events. Phys. Rev. Lett. 100, part. 018101 ( 10.1103/PhysRevLett.100.018101) [DOI] [PubMed] [Google Scholar]
- 35.Ball P. 2008. Cellular memory hints at the origin of intelligence. Nature 451, 385 ( 10.1038/451385a) [DOI] [PubMed] [Google Scholar]
- 36.Boisseau RP, Vogel D, Dussutour A. 2016. Habituation in non-neural organisms. Proc. R. Soc. B 283, 20160446 ( 10.1098/rspb.2016.0446) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wood DC. 1973. Stimulus specific habituation in a protozoan. Physiol. Behav. 11, 349–354. ( 10.1016/0031-9384(73)90011-5) [DOI] [PubMed] [Google Scholar]
- 38.Hansell M. 2011. Houses made by protists. Curr. Biol. 21, R485–R487. ( 10.1016/j.cub.2011.05.050) [DOI] [PubMed] [Google Scholar]
- 39.Bose JC. 1906. Plant response as a means of physiological investigation. New York, NY: Longmans Green & Co. [Google Scholar]
- 40.Gagliano M, Renton M, Depczynski M, Mancuso S. 2014. Experience teaches plants to learn faster and forget slower in environments where it matters. Oecologia 175, 63–72. ( 10.1007/s00442-013-2873-7) [DOI] [PubMed] [Google Scholar]
- 41.Darwin C. 1880. The power of movement in plants. London, UK: John Murray. [Google Scholar]
- 42.Von Hartmann E. 1875. (English translation, 1931) The unconscious and consciousness in the vegetable kingdom. In International library of psychology, philosophy and scientific method (ed. Ogden CK.), pp. 119–153. London, UK: Kegan Paul Trench & Trubner. [Google Scholar]
- 43.Went FW, Thimann KV. 1937. Phytohormones. New York, NY: MacMillan. [Google Scholar]
- 44.Weaver JE. 1926. Root development of field crops. New York, NY: McGraw-Hill. [Google Scholar]
- 45.Knight MR, Campbell AK, Smith SM, Trewavas AJ. 1991. Transgenic plant aequorin reports the effects of touch and cold-shock and elicitors on cytoplasmic calcium. Nature 352, 524–526. ( 10.1038/352524a0) [DOI] [PubMed] [Google Scholar]
- 46.Bastien R, Bohr T, Moulia B, Douady S. 2013. Unifying model of shoot gravitropism reveals proprioception as a central feature of posture control in plants. Proc. Natl Acad. Sci. USA 110, 755–760. ( 10.1073/pnas.1214301109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Niklas KJ. 1992. Plant biomechanics. Chicago, IL: Chicago University Press. [Google Scholar]
- 48.Massa GD, Gilroy S. 2003. Touch modulates gravity sensing to regulate the growth of primary roots of Arabidopsis thaliana. Plant J. 33, 435–445. ( 10.1046/j.1365-313X.2003.01637.x) [DOI] [PubMed] [Google Scholar]
- 49.Appel HM, Cocroft RB. 2014. Plants respond to leaf vibrations caused by insect herbivore chewing. Oecologia 175, 1257–1266. ( 10.1007/s00442-014-2995-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gagliano M, Mancuso S, Robert D. 2012. Towards understanding plant bioacoustics. Trends Plant Sci. 17, 323–325. ( 10.1016/j.tplants.2012.03.002) [DOI] [PubMed] [Google Scholar]
- 51.Bengough AG, Mullins CE. 1990. Mechanical impedance to root growth: a review of experimental techniques and root growth responses. J. Soil Sci. 41, 341–358. ( 10.1111/j.1365-2389.1990.tb00070.x) [DOI] [Google Scholar]
- 52.Bingham IJ, Bengough AG. 2003. Morphological plasticity of wheat and barley roots in response to spatial variation in soil strength. Plant Soil 250, 273–282. ( 10.1023/A:1022891519039) [DOI] [Google Scholar]
- 53.Trewavas AJ. 2009. What is plant behaviour? Plant Cell Environ. 32, 606–616. ( 10.1111/j.1365-3040.2009.01929) [DOI] [PubMed] [Google Scholar]
- 54.Tolbert NE, Benker C, Beck E. 1995. The oxygen and carbon dioxide compensation points of C3 plants. Proc. Natl Acad. Sci. USA 92, 11 230–11 233. ( 10.1073/pnas.92.24.11230) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Visser EJW, Voesink LACJ, Vartapetain BB, Jackson MB. 2003. Flooding and plant growth. Ann. Bot. 91 107–109. ( 10.1093/aob/mcg014) [DOI] [Google Scholar]
- 56.Acevedo FE, Rivera-Vega LJ, Chung SH, Ray S, Felton GW. 2015. Cues from chewing insects—the intersection of DAMPs, HAMPs, MAMPs and effectors. Curr. Opin. Plant Biol. 26, 80–86. ( 10.1016/j.pbi.2015.05.029) [DOI] [PubMed] [Google Scholar]
- 57.Choi W-G, Hilleary R, Swanson SJ, Kim S-U, Gilroy S. 2016. Rapid long-distance electrical and calcium signalling in plants. Annu. Rev. Plant Biol. 67, 287–307. ( 10.1146/annurev-arplant-043015-112130) [DOI] [PubMed] [Google Scholar]
- 58.Pierik R, deWit M. 2013. Shade avoidance: phytochrome signalling and other above ground neighbour detection cues. J. Exp. Bot. 65, 2815–2824. ( 10.1093/jxb/ert389) [DOI] [PubMed] [Google Scholar]
- 59.Aphalo PJ, Ballare CL, Scopel AL. 1999. Plant–plant signalling, the shade avoidance response and competition. J. Exp. Bot. 50, 1629–1634. ( 10.1093/jxb/50.340.1629) [DOI] [Google Scholar]
- 60.Sultan SE. 2015. Organism and environment. Oxford, UK: Oxford University Press. [Google Scholar]
- 61.De Kroon H, Huber H, Suefer JF, van Groenendael JM. 2005. A modular concept of phenotypic plasticity in plants. New Phytol. 166, 73–82. ( 10.1111/j.1469-8137.2004.01310.x) [DOI] [PubMed] [Google Scholar]
- 62.Denny FE, Stanton EN. 1928. Chemical treatments for shortening the rest period of pot grown woody plants. Am. J. Bot. 15, 327–336. ( 10.2307/2435734) [DOI] [Google Scholar]
- 63.Hammond JP, White PJ. 2011. Sugar signalling in root responses to low phosphate availability. Plant Physiol. 156, 1033–1040. ( 10.1104/pp.111.175380) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wolverton C, Ishikawa H, Evans ML. 2002. The kinetics of root gravitropism: dual motors and sensors. J. Plant Growth Regul. 21, 102–112. ( 10.1007/s003440010053) [DOI] [PubMed] [Google Scholar]
- 65.Wolverton C, Mullen JL, Ishikawa H, Evans ML. 2002. Root gravitropism in response to a signal originating outside of the cap. Planta 215, 153–157. ( 10.1007/s00425-001-0726-9) [DOI] [PubMed] [Google Scholar]
- 66.Svistoonoff S, Creff A, Reymond M, Sigoillot-Claude C, Ricaud L, Blanchet A, Nussaume L, Desnos T. 2007. Root tip contact with low-phosphate media reprograms plant root architecture. Nat. Gen. 39, 792–796. ( 10.1038/ng2041) [DOI] [PubMed] [Google Scholar]
- 67.McNickle GG, Cahill JF. 2009. Plant root growth and the marginal value theorem. Proc. Natl Acad. Sci. USA 106, 4747–4751. ( 10.1073/pnas.0807971106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Medici A, et al. 2014. AtNIGT1/HRS1 integrates nitrate and phosphate signals at the Arabidopsis root tip. Nat. Commun. 6, 6274 ( 10.1038/ncomms7274) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Eapen D, Barroso ML, Ponce G, Campos ME, Cassab GI. 2005. Hydrotropism: root growth responses to water. Trends Plant Sci. 10, 44–50. ( 10.1016/j.tplants.2004.11.004) [DOI] [PubMed] [Google Scholar]
- 70.Choi W-G, Toyota M, Kim S-H, Hilleary R, Gilroy S. 2014. Salt stress-induced Ca2+ waves are associated with rapid, long-distance root-to-shoot signaling in plants. Proc. Natl Acad. Sci USA 111, 6497–6502. ( 10.1073/pnas.1319955111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Evans MJ, Choi W-G, Gilroy S, Morris RJ. 2016. A ROS assisted calcium wave dependent on the At BOHD NADPH oxidase and TPC1 cation channel propagates the systemic response to salt stress. Plant Physiol. 171, 1771–1784. ( 10.1104/pp.16.00215) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kiss JZ, Mullen JL, Correll MJ, Hangartner RP. 2003. Phytochromes A and B mediate red-light induced positive phototropism in roots. Plant Physiol. 131, 1411–1417. ( 10.1104/pp.013847) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Blancaflor EB, Fasano JM, Gilroy S. 1998. Mapping the functional roles of cap cells in the response of Arabidopsis primary root to gravity. Plant Physiol. 116, 213–222. ( 10.1104/pp.116.1.213) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Palladin VI. 1923. Plant physiology, translated by BE. Livinston. Philadelphia, PA: P. Blakiston's Son and Co. [Google Scholar]
- 75.Whippo CS, Hangartner RP. 2006. Phototropism; bending towards enlightenment. Plant Cell 18, 1110–1119. ( 10.1105/tpc.105.039669) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Runyon JB, Mescher MC, de Moraes CM. 2006. Volatile chemical cues guide host location and host selection by parasitic plants. Science 313, 1964–1967. ( 10.1126/science.1131371) [DOI] [PubMed] [Google Scholar]
- 77.Givnish TJ. 1982. On the adaptive significance of leaf height in forest herbs. Am. Nat. 120, 353–381. ( 10.1086/283995) [DOI] [Google Scholar]
- 78.Givnish TJ. 1988. Adaptation to sun and shade: a whole plant perspective. Aust. J. Plant Physiol. 15, 63–92. ( 10.1071/PP9880063) [DOI] [Google Scholar]
- 79.Ackerley DD, Bazzaz FA. 1995. Seedling crown orientation and interception of diffuse radiation in tropical forest gaps. Ecology 76, 1134–1146. ( 10.2307/1940921) [DOI] [Google Scholar]
- 80.Kegge W, Ninkovic V, Glinwood R, Welschen RAM, Voesenek LACJ, Pierik R. 2015. Red: far-red light conditions affect the emission of volatile organic compounds from barley (Hordeum vulgare) leading to altered biomass allocation in neighbouring plants. Ann. Bot. 115, 961–970. ( 10.1093/aob/mcv036) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.De Wit M, Evers JB, Vergeer-van Eijk M, Gankema P, Voesenek LACJ, Pierek R. 2012. Plant neighbour detection through touching leaf tips precedes phytochrome signals. Proc. Natl Acad. Sci. USA 109, 14 705–14 710. ( 10.1073/pnas.1205437109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Novoplansky A. 1991. Developmental responses of Portulaca seedlings to conflicting spectral signals. Oecologia 88, 138–140. ( 10.1007/BF00328414) [DOI] [PubMed] [Google Scholar]
- 83.Donohue K, Pyle EH, Messiqua D, Heschel MS, Schmitt J. 2001. Adaptive divergence in plasticity in natural populations of Impatiens capensis and its consequence for performance in novel habitats. Evolution 55, 692–702. ( 10.1554/0014-3820(2001)055%5B0692:ADIPIN%5D2.0.CO;2) [DOI] [PubMed] [Google Scholar]
- 84.Dudley SA, Schmitt J. 1996. Testing the adaptive plasticity hypothesis: density-dependent selection of manipulated stem length in Impatiens capensis. Am. Nat. 147, 445–465. ( 10.1086/285860) [DOI] [Google Scholar]
- 85.Schmitt J, Dudley SA, Pigliucci M. 1999. Manipulative approaches to testing adaptive plasticity: phytochrome-mediated shade avoidance responses in plants. Am. Nat. 154, S43–S54. [DOI] [PubMed] [Google Scholar]
- 86.Botto JF, Smith H. 2002. Differential genetic variation in adaptive strategies to a common environmental signal in Arabidopsis accessions: phytochrome-mediated shade avoidance. Plant Cell Environ. 25, 53–63. ( 10.1046/j.0016-8025.2001.00812.x) [DOI] [Google Scholar]
- 87.Filiault DL, Maloof JN. 2012. A genome wide association study identifies variants underlying the Arabidopsis thaliana shade avoidance response. PLoS Genet. 8, e1002589 ( 10.1371/journal.pgen.1002589) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Thibaud M-C, Arrighi JF, Bayle V, Chiarenza S, Creff A, Bustos R, Paz-Ares J, Poirier Y, Nussaume L. 2010. Dissection of local and systemic transcriptional responses to phosphate starvation in Arabidopsis. Plant J. 64, 775–789. ( 10.1111/j.1365-313X.2010.04375.x) [DOI] [PubMed] [Google Scholar]
- 89.Wilson JB. 1988. A review of evidence on the control of shoot:root ratio in relation to models. Ann. Bot. 61, 433–449. ( 10.1093/oxfordjournals.aob.a087575) [DOI] [Google Scholar]
- 90.Charnov EL. 1976. Optimal foraging, the marginal value theorem. Theor. Popul. Biol. 9, 129–136. ( 10.1016/0040-5809(76)90040-X) [DOI] [PubMed] [Google Scholar]
- 91.Crossley M, Staras K, Kemenes G. 2015. A two neuron system for adaptive goal-directed decision-making in Lymnaea. Nat. Comm. 7, 11793 (doi.10.1038/ncomms11793) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rangel A, Todd H. 2010. Neural computations associated with goal-directed choice. Curr. Opin. Neurobiol. 20, 262–270. ( 10.1016/j.conb.2010.03.001) [DOI] [PubMed] [Google Scholar]
- 93.Kelly CK. 1990. Plant foraging: a marginal value model and coiling response in Cuscuta subinclusa. Ecology 71, 1916–1925. ( 10.2307/1937599) [DOI] [Google Scholar]
- 94.Kelly CK. 1992. Resource choice in Cuscuta europaea. Proc. Natl Acad. Sci. USA 89, 12 194–12 197. ( 10.1073/pnas.89.24.12194) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ihan A. 1997. Short note: distinguishing immune intelligence and immune response. Med. Hypotheses 49, 443–444. ( 10.1016/S0306-9877(97)90094-3) [DOI] [PubMed] [Google Scholar]
- 96.Timmis J, Andrews P, Hart E. 2010. On artificial immune systems and swarm intelligence. Swarm Intell. 4, 247–273. ( 10.1007/s11721-010-0045-5) [DOI] [Google Scholar]
- 97.Maeterlinck M. 1927. The life of the white ant. London, UK: George Allen and Unwin. [Google Scholar]
- 98.Lake JA, Quick WP, Beerling DJ, Woodward FI. 2001. Signals from mature to new leaves. Nature 411, 154 ( 10.1038/35075660) [DOI] [PubMed] [Google Scholar]
- 99.Lake JA, Woodward FI, Quick WP. 2002. Long distance CO2 signalling in plant. J. Exp. Bot. 53, 183–193. ( 10.1093/jexbot/53.367.183) [DOI] [PubMed] [Google Scholar]
- 100.Robinson D. 1994. The responses of plants to non-uniform supplies of nutrients. New Phytol. 127, 635–674. ( 10.1111/j.1469-8137.1994.tb02969.x) [DOI] [PubMed] [Google Scholar]
- 101.Andrews RE, Newman EI. 1968. The influence of root pruning on the growth and transpiration of wheat under different soil moisture conditions. New Phytol. 67, 617–630. ( 10.1111/j.1469-8137.1968.tb05488.x) [DOI] [Google Scholar]
- 102.Fanwoua J, Bairam E, Delaire M, Buck-Sorlin G. 2014. The role of branch architecture in assimilate production and partitioning: the example of apple. Front. Plant Sci. 5, 338 ( 10.3389/fpls.2014.00338) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Seeley TD. 1995. The wisdom of the hive. Cambridge, MA: Harvard University Press. [Google Scholar]
- 104.Helliker BR, Richter SL. 2008. Subtropical to boreal convergence of tree leaf temperatures. Nature 454, 511–514. ( 10.1038/nature07031) [DOI] [PubMed] [Google Scholar]
- 105.Murchie EH, Chen Y-Z, Hubbart S, Peng S, Horton P. 1999. Interactions between senescence and leaf orientation determine in situ patterns of photosynthesis and photo-inhibition in field grown rice. Plant Physiol. 119, 553–563. ( 10.1104/pp.119.2.553) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.He J, Chee CW, Goh CJ. 1996. Photoinhibition of Heliconia under natural tropical conditions: the importance of leaf orientation for light interception and leaf temperature. Plant Cell Environ. 19, 1238–1248. ( 10.1111/j.1365-3040.1996.tb00002.x) [DOI] [Google Scholar]
- 107.Medina E, Sobrado M, Herrera R. 1978. Significance of leaf orientation for leaf temperature in an Amazonian sclerophyll vegetation. Radiat. Environ. Biophys. 15, 131–140. ( 10.1007/BF01323262) [DOI] [PubMed] [Google Scholar]
- 108.Taylor E. 1969. The Redbud; adaptation for survival. Missouri Bot. Garden Bull. 57, 8–10. [Google Scholar]
- 109.Polko JK, Vosenek LACJ, Peeters AJM, Pierek R. 2011. Petiole hyponasty: an ethylene driven, adaptive response to changes in the environment. AoB Plants 2011, pplr031 ( 10.1093/aobpla/plr031) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Peak D, West JD, Messinger SM, Mott KA. 2004. Evidence for complex, collective dynamics and emergent distributed computation in plants. Proc. Natl Acad. Sci. USA 101, 918–922. ( 10.1073/pnas.0307811100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Seeley TD, Levien RA. 1987. A colony of mind: the beehive as thinking machine. Sciences 27, 38–43. ( 10.1002/j.2326-1951.1987.tb02955.x) [DOI] [Google Scholar]
- 112.Sachs T. 2006. How can plants choose the most promising organs? In Communication in plants (eds Baluska F, Mancuso S, Volkmann D), pp. 53–63. Berlin, Germany: Springer. [Google Scholar]
- 113.Sachs T, Novoplansky A, Cohen D. 1993. Plants as competing populations of redundant organs. Plant Cell Environ. 16, 765–770. ( 10.1111/j.1365-3040.1993.tb00498.x) [DOI] [Google Scholar]
- 114.Darwin C. 1891. The movements and habits of climbing plants. London, UK: John Murray. [Google Scholar]
- 115.Jaffe MJ, Shotwell M. 1980. Physiological studies on pea tendrils. XI. Storage of tactile sensory information prior to the light activation effect. Physiol. Plantarum 50, 78–82. ( 10.1111/j.1399-3054.1980.tb02688.x) [DOI] [Google Scholar]
- 116.Baillaud L. 1962. Mouvements autonomes des tiges, vrilles et autre organs. In Encyclopedia plant physiology: XVII. Physiology of movements, part 2 (ed. Ruhland W.), pp. 562–635. Berlin, Germany: Springer. [Google Scholar]
- 117.Darwin C. 1875. Insectivorous plants. London, UK: John Murray. [Google Scholar]
- 118.Bohm J, et al. 2016. The venus flytrap Dionaea muscipula counts prey-induced action potentials to induce sodium uptake. Curr. Biol. 26, 1–10. ( 10.1016/j.cub.2015.11.057) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Gould SJ. 2002. The structure of evolutionary theory. Cambridge, MA: Harvard University Press. [Google Scholar]
- 120.Maynard-Smith J, Price GR. 1973. The logic of animal conflict. Nature 246, 15–19. ( 10.1038/246015a0) [DOI] [Google Scholar]
- 121.Gersani M, Brown JS, O'Brien EE, Maina GM, Abramsky Z. 2001. Tragedy of the commons as a result of root competition. J. Ecol. 89, 660–669. ( 10.1046/j.0022-0477.2001.00609.x) [DOI] [Google Scholar]
- 122.Gruntmann M, Novoplansky A. 2004. Physiologically mediated self/non-self discrimination in roots. Proc. Natl Acad. Sci. USA 101, 3863–3867. ( 10.1073/pnas.0306604101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Schenk HJ, Callaway RA, Mahall BE. 1999. Spatial root segregation. Are roots territorial? Adv. Ecol. Res. 28, 145–180. ( 10.1016/S0065-2504(08)60032-X) [DOI] [Google Scholar]
- 124.Gorzelak MA, Asay AK, Pickles BJ, Simard SW. 2015. Inter-plant communication through mycorrhizal networks mediates complex adaptive behaviour in plant communities. AoB Plants 7, plv 050 ( 10.1093/aobpla/plv050) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Song YY, Simard SW, Carroll A, Mohn WW, Zeng RS. 2015. Defoliation of interior douglas fir elicits carbon transfer and stress signalling to ponderosa pine neighbours through ectomycorrhizal networks. Sci. Rep. 5, 8495 ( 10.1038/srep08495) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ollson S. 2009. Nutrient translocation and electrical signalling in mycelia. In The fungal colony (eds Gow NAR, Robson GD, Gadd GM), pp. 25–48. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 127.Ollson S, Hansson BS. 1995. Action potential-like activity found in fungal mycelia is sensitive to stimulation. Naturwissenschaften 82, 30–31. ( 10.1007/BF01167867) [DOI] [Google Scholar]
- 128.Barto EK, Weidenhamer JD, Cipollini D, Rillig MC. 2012. Fungal superhighways: do common mycorrhizal networks enhance below ground communication. Trends Plant Sci. 7, 633–637. ( 10.1016/j.tplants.2012.06.007) [DOI] [PubMed] [Google Scholar]
- 129.Song YY, Zeng RS, Xu JF, Li J, Shen X, Yihdego WG. 2010. Interplant communication of tomato plants through underground common mycorrhizal networks. PLoS ONE 5, e13324 ( 10.1371/journal.pone.0013324) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.De Moraes CM, Lewis WJ, Pare PW, Alborn HT, Tumlinson JH. 1998. Herbivore-infested plants selectively attract parasitoids. Nature 393, 570–573. ( 10.1038/31219) [DOI] [Google Scholar]
- 131.De Moraes CM, Schultz JC, Mescher MC, Tumlinson JH. 2004. Induced plant signalling and its implications for environmental sensing. J. Toxicol. Environ. Health A 67, 819–834. ( 10.1080/15287390490428288) [DOI] [PubMed] [Google Scholar]
- 132.Dudareva N, Negre F, Nagegowda DA, Orlova I. 2006. Plant volatiles: recent advances and future perspectives. Crit. Rev Plant Sci. 25, 417–440. ( 10.1080/07352680600899973) [DOI] [Google Scholar]
- 133.Holopainen JK, Blande JD. 2012. Molecular plant volatile communication. In Sensing in nature (ed. Lopez-Larrea C.), pp. 17–31. Berlin, Germany: Landes Bioscience and Springer Science +Business Media. [DOI] [PubMed] [Google Scholar]
- 134.Karban R, Shiojiri K, Ishizaki S, Wetzel WC, Evans RY. 2013. Kin recognition affects plant communication and defence. Proc R. Soc. B 280, 20123062 ( 10.1098/rspb.2012.3062) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kikuta Y, Ueda H, Nakayama K, Katsuda Y, Ozawa R, Takabayashi J, Hatanaka A, Matsuda K. 2011. Specific regulation of pyrethrin biosynthesis in Chrysanthemum cinerariaefolium by a blend of volatiles emitted from artificially damaged conspecific plants. Plant Cell Physiol. 52, 588–596. ( 10.1093/pcp/pcr017) [DOI] [PubMed] [Google Scholar]
- 136.Castulo-Rubio DY, Alejandre-Ramirez NA, Orozco-Mosqueda MC, Santoyo G, Macias-Rodriguez LI, Valencia-Cantero E. 2015. Volatile organic compounds produced by the Rhizobacterium Arthrobacter agilis UMCV2 modulate Sorghum bicolor (strategy II plant) morphogenesis and SbFR01 transcription in vitro. J. Plant Growth Regul. 34, 611–623. ( 10.1007/s00344-015-9495-8) [DOI] [Google Scholar]
- 137.Gianoli E, Carassco-Urr F. 2014. Leaf mimicry in a climbing plant protects against herbivory. Curr. Biol. 24, 984–987. ( 10.1016/j.cub.2014.03.010) [DOI] [PubMed] [Google Scholar]
- 138.Mancuso S, Baluska F. 2016. Vision in plants via plant specific ocelli. Trends Plant Sci. 21, 727–730. ( 10.1016/j.tplants.2016.07.008) [DOI] [PubMed] [Google Scholar]
- 139.Nilsson DE. 2013. Eye evolution and its functional basis. Vis. Neurosci. 30, 5–20. ( 10.1017/S0952523813000035) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Fiers M, Lognay G, Fauconnier M-L, Jijakli MH. 2013. Volatile compounds-mediated interactions between barley and pathogenic fungi in the soil. PLoS ONE 8, e66805 ( 10.1371/journal.pone.0066805) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Musah RA, Lesiak AD, Maron MJ, Cody RB, Edwards D, Fowbie KL, John Dane A, Long MC. 2016. Mechano-sensitivity below ground: touch sensitive smell-producing roots in the shy plant Mimosa pudica. Plant Physiol. 170, 1075–1089. ( 10.1104/pp.15.01705) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Palma R, Mutis A, Manosalva L, Ceballos R, Quiroz A. 2012. Behavioral and electrophysiological responses of Hylastinus obscurus to volatiles released from the roots of Trifolium pratense. J. Soil Sci. Plant Nutr. 12, 183–193. ( 10.4067/S0718-95162012000100015) [DOI] [Google Scholar]
- 143.Conrath U. 2011. Molecular aspects of defence priming. Trends Plant Sci. 16, 524–531. ( 10.1016/j.tplants.2011.06.004) [DOI] [PubMed] [Google Scholar]
- 144.Pieterse CMJ. 2012. Prime time for transgenerational defense. Plant Physiol. 158, 545 ( 10.1104/pp.112.900430) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Singh P, Yekondi S, Chen P-W, Tsai C-H, Yu C-W, Wu K, Zimmerli L. 2014. Environmental history modulates Arabidopsis pattern-triggered immunity in a histone acetyltransferase-dependent manner. Plant Cell 26, 2676–2688. ( 10.1105/tpc.114.123356) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Sweatt JD. 2016. Chromatin controls behaviour; dynamic regulation of chromatin remodelling controls learning and memory. Science 353, 218–219. ( 10.1126/science.aah4055) [DOI] [PubMed] [Google Scholar]
- 147.Latzel V, Rendina-Gonzalez AP, Rosenthal J. 2016. Epigenetic memory as a basis for intelligent behaviour in clonal plants. Front. Plant Sci. 7, 1354 ( 10.3389/fpls.2016.01354) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Chakrabortee S, Kayatekin C, Newby GA, Mendillo ML, Lancaster A, Lundquist S. 2016. Luminidependens (LD) is an Arabidopsis protein with prion behaviour. Proc. Natl Acad. Sci. USA 113, 6065–6070. ( 10.1073/pnas.1604478113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Ackerson RC. 1980. Stomatal response of cotton to water stress and abscisic acid as affected by water stress history. Plant Physiol. 65, 455–459. ( 10.1104/pp.65.3.455) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Koorneef A, Pieterse CMJ. 2008. Cross talk in defense signalling. Plant Physiol. 148, 839–844. ( 10.1104/pp.107.112029) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Ding Y, Fromm M, Avramova Z. 2012. Multiple exposures to drought ‘train’ transcriptional responses in Arabidopsis. Nat. Comm. 3, 740 ( 10.1038/ncomms1732) [DOI] [PubMed] [Google Scholar]
- 152.Goh CH, Nam HG, Park YS. 2003. Stress memory in plants: a negative regulation of stomatal response and transient induction of rd22 gene to light in abscisic acid-entrained Arabidopsis plants. Plant J. 36, 240–255. ( 10.1046/j.1365-313X.2003.01872.x) [DOI] [PubMed] [Google Scholar]
- 153.Raj S, Brautigam K, Hamanish ET, Wilkins O, Thomas BR, Schroeder W, Mansfield SD, Plant AL, Campbell MM. 2011. Clone history shapes Populus drought responses. Proc. Natl Acad. Sci. USA 108, 12 521–12 526. ( 10.1073/pnas.1103341108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Gagliano M. 2015. In a green frame of mind: perspectives on the behavioural ecology and cognitive nature of plants. AoB Plants 7, plu075 ( 10.1093/aobpla/plu075) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Van Loon LC. 2015. The intelligent behaviour of plants. Trends Plant Sci. 21, 286–294. ( 10.1016/j.tplants.2015.11.009) [DOI] [PubMed] [Google Scholar]