Abstract
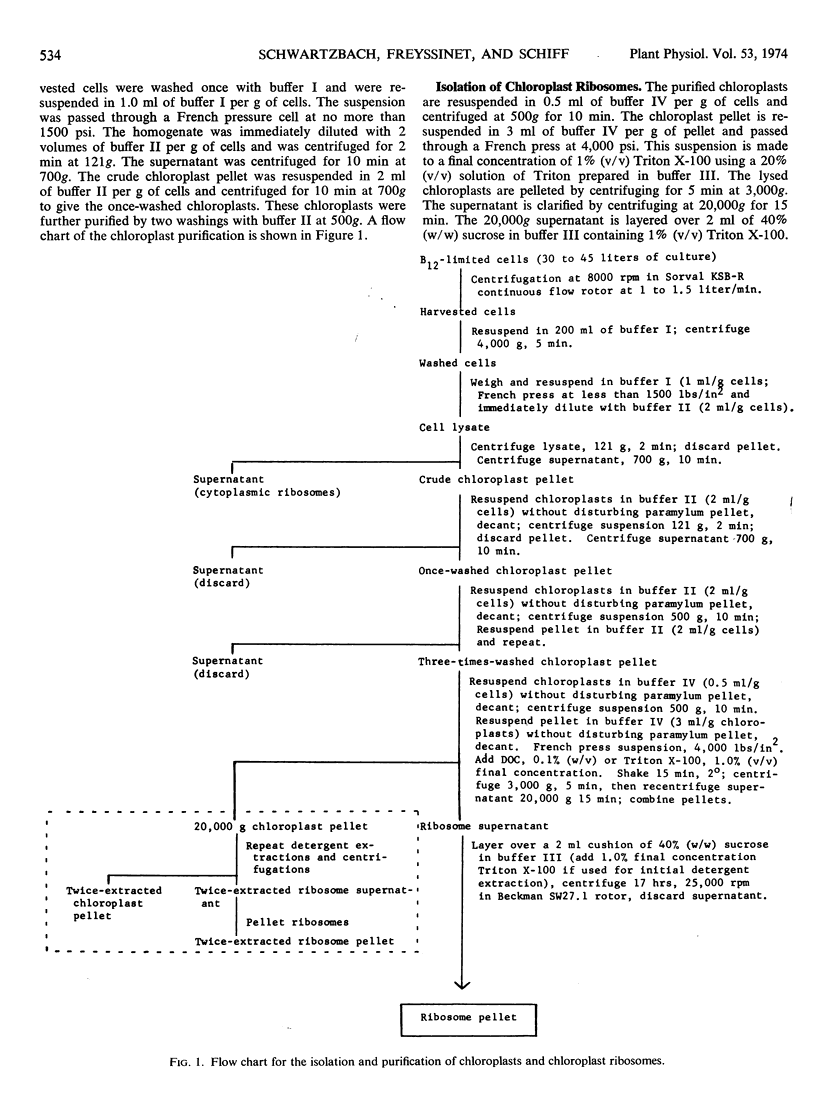
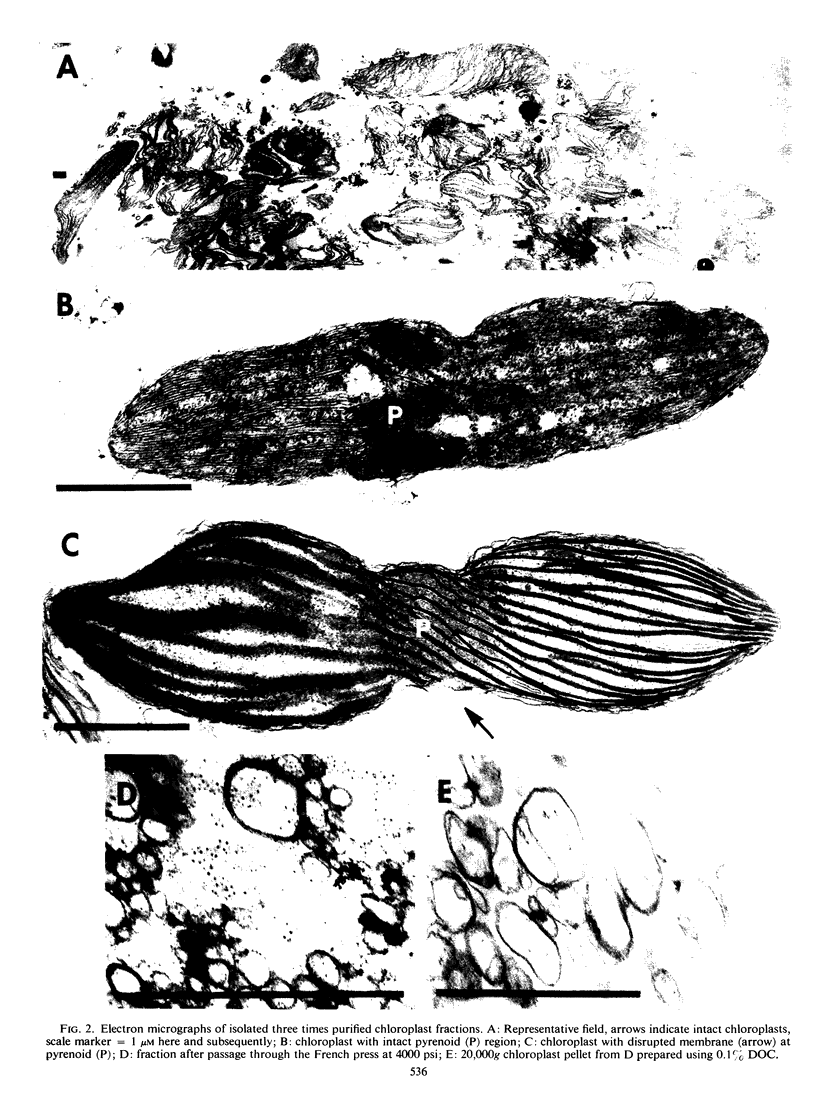
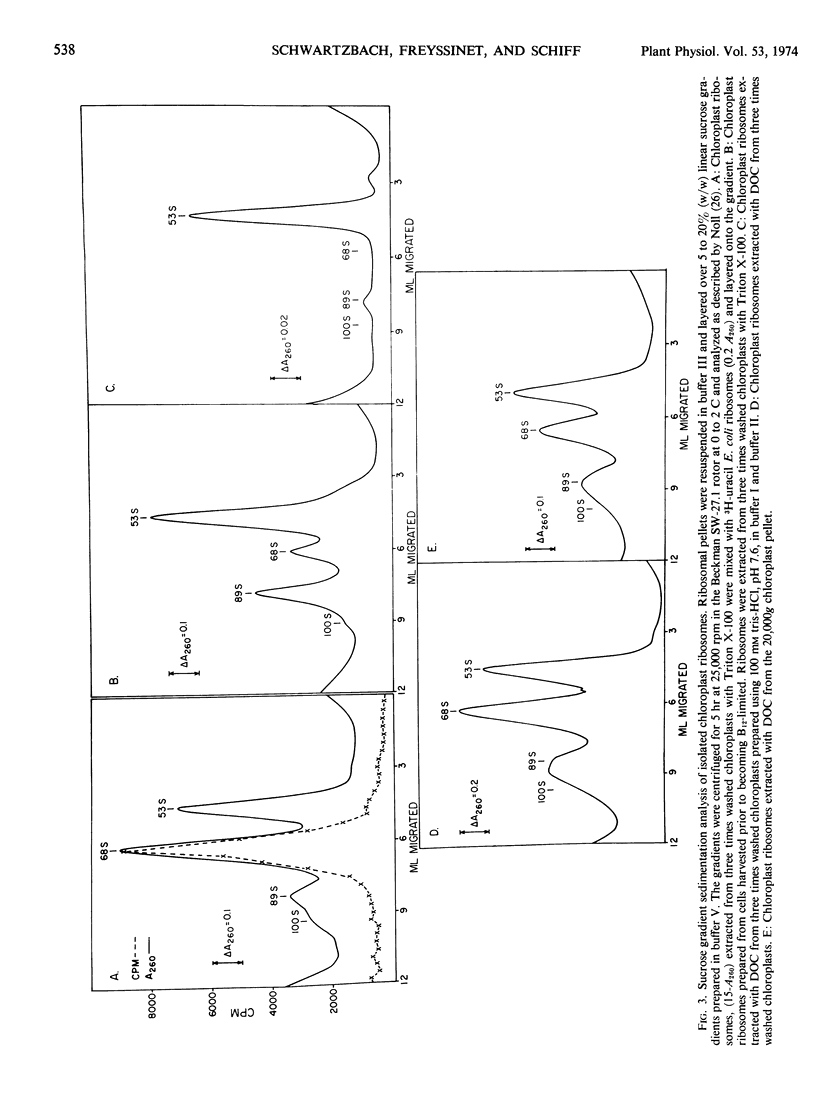
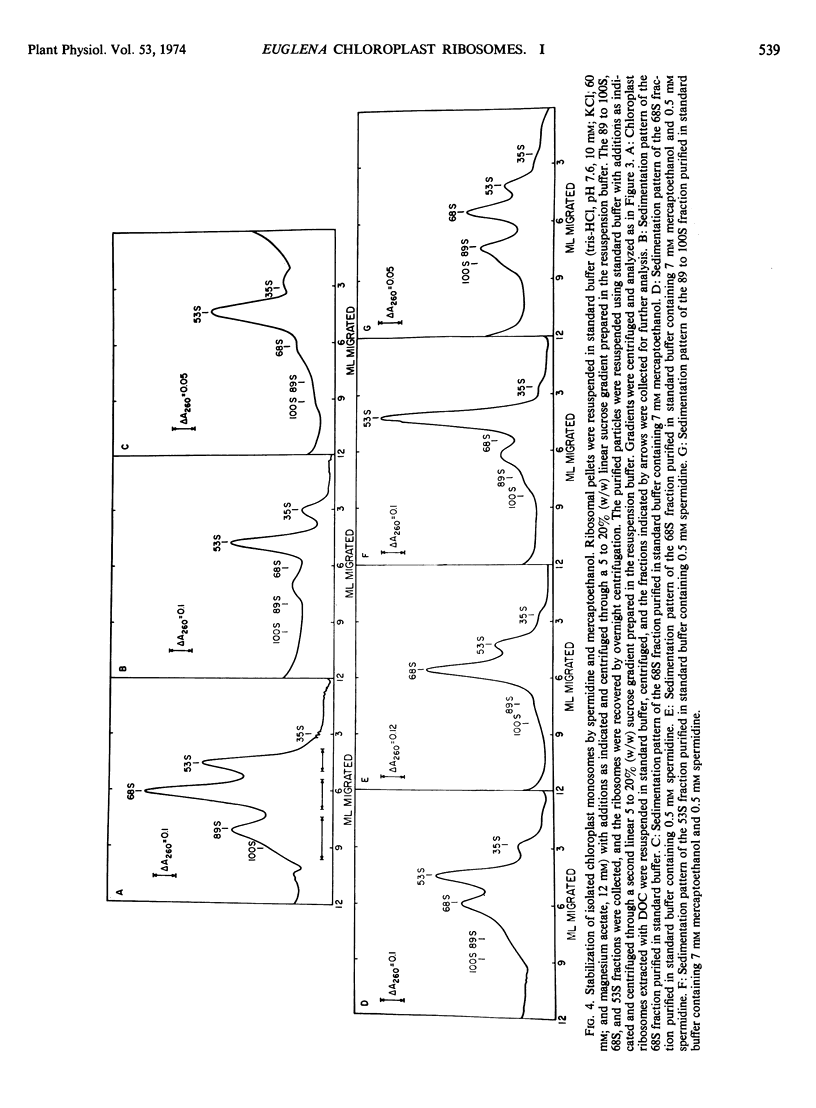
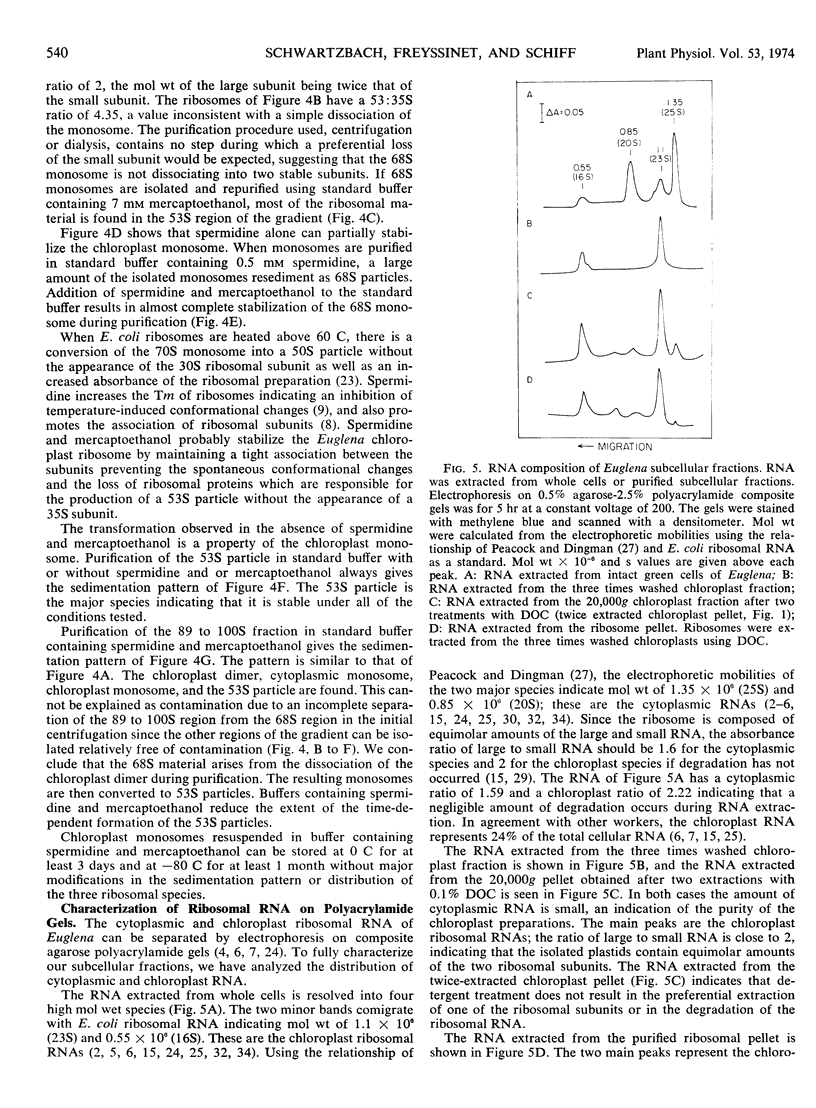
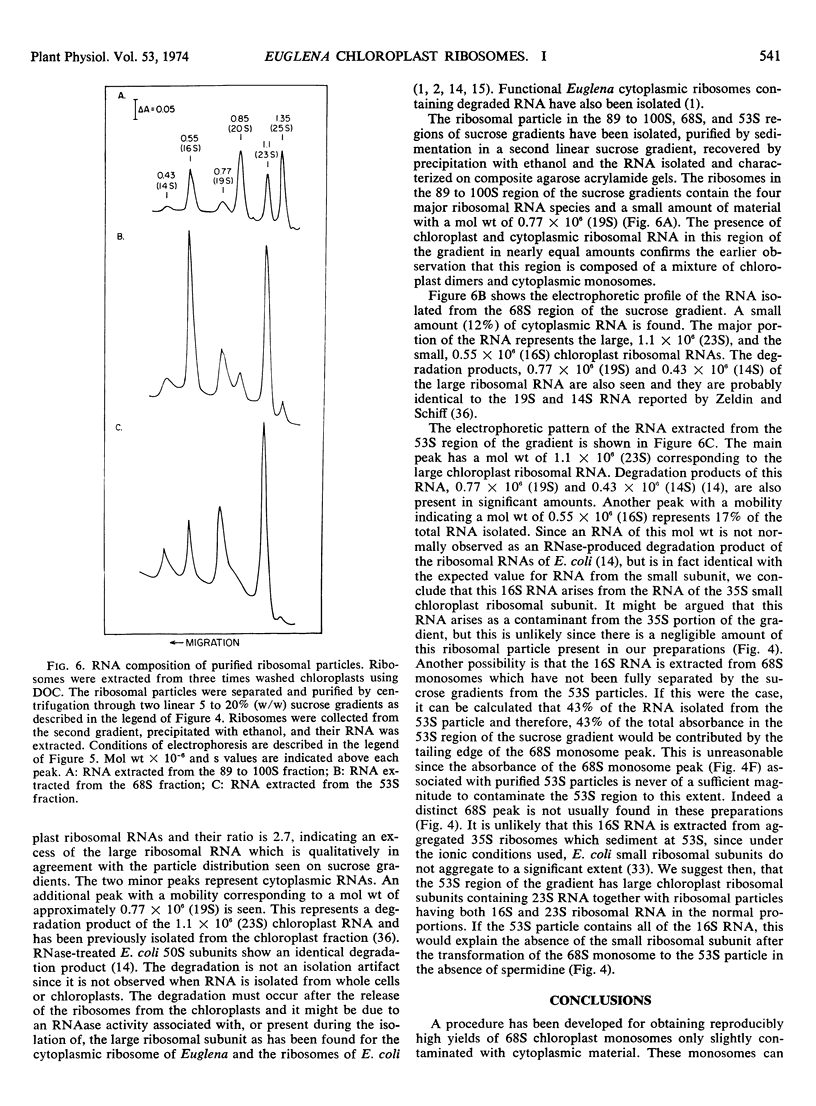
A new isolation procedure has resulted in an improved yield of stable 68S chloroplast ribosomes from Euglena gracilis var. bacillaris. Chloroplasts are isolated by suspending the cells in buffer I (sorbitol, 250 mm; sucrose, 250 mm; Ficoll, 2.5% [w/v]; magnesium acetate, 1 mm; bovine serum albumin, 0.01% [w/v]; mercaptoethanol, 14 mm; N-2-hydroxyethyl-piperazine-N′-2-ethanesulfonic acid, pH 7.6, 5 mm) and passing through a French press at less than 1500 pounds per square inch. The crude chloroplasts are purified by three washings with buffer II (sorbitol, 150 mm; sucrose, 150 mm; Ficoll, 2.5% [w/v]; magnesium acetate, 1 mm; bovine serum albumin, 0.01% [w/v]; mercaptoethanol, 14 mm; N-2-hydroxyethyl-piperazine-N′-2-ethanesulfonic acid, pH 7.6, 5 mm). Stable 68S chloroplast ribosomes are obtained when the isolated chloroplasts are resuspended in ribosome buffer (tris-HCI, pH 7.6, 10 mm; magnesium acetate, 12 mm; KCI, 60 mm) containing spermidine, 0.5 mm; mercaptoethanol, 14 mm; sucrose, 8% (w/w), passed through a French press at 4000 pounds per square inch and extracted with either 0.1% (w/v) sodium deoxycholate or 1.0% (v/v) Triton X-100. At 0 to 4 C in ribosome buffer, the purified 68S chloroplast monosome forms a 53S particle while the 35S particle, an expected product of monosome dissociation, cannot be detected. Spermidine and mercaptoethanol prevent the formation of 53S particles from 68S monosomes. The purified 53S particles derived from 68S monosomes contain 23S RNA as well as a significant amount of 16S RNA, suggesting that this particle may not be a true ribosomal subunit.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avadhani N. G., Buetow D. E. A proposed role for 5S ribosomal RNA. Biochem Biophys Res Commun. 1973 Jan 23;50(2):443–451. doi: 10.1016/0006-291x(73)90860-7. [DOI] [PubMed] [Google Scholar]
- Avadhani N. G., Buetow D. E. Isolation of active polyribosomes from the cytoplasm, mitochondria and chloroplasts of Euglena gracilis. Biochem J. 1972 Jun;128(2):353–365. doi: 10.1042/bj1280353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boynton J. E., Gillham N. W., Chabot J. F. Chloroplast ribosome deficient mutants in the green alga Chlamydomonas reinhardi and the question of chloroplast ribosome function. J Cell Sci. 1972 Mar;10(2):267–305. doi: 10.1242/jcs.10.2.267. [DOI] [PubMed] [Google Scholar]
- Brown R. D., Haselkorn R. Synthesis and maturation of cytoplasmic ribosomal RNA in Euglena gracilis. J Mol Biol. 1971 Aug 14;59(3):491–503. doi: 10.1016/0022-2836(71)90312-3. [DOI] [PubMed] [Google Scholar]
- COHEN S. S., LICHTENSTEIN J. Polyamines and ribosome structure. J Biol Chem. 1960 Jul;235:2112–2116. [PubMed] [Google Scholar]
- Carell E. F. Studies on chloroplast development and replication in Euglena. I. Vitamin B12 and chloroplast replication. J Cell Biol. 1969 May;41(2):431–440. doi: 10.1083/jcb.41.2.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlberg A. E., Dingman C. W., Peacock A. C. Electrophoretic characterization of bacterial polyribosomes in agarose-acrylamide composite gels. J Mol Biol. 1969 Apr 14;41(1):139–147. doi: 10.1016/0022-2836(69)90131-4. [DOI] [PubMed] [Google Scholar]
- Datta R. K., Sen S., Ghosh J. J. Effect of polyamines on the stability of brain-cortex ribosomes. Biochem J. 1969 Oct;114(4):847–854. doi: 10.1042/bj1140847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donachie W. D. Control of cell division in Escherichia coli: experiments with thymine starvation. J Bacteriol. 1969 Oct;100(1):260–268. doi: 10.1128/jb.100.1.260-268.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freyssinet G., Schiff J. A. The Chloroplast and Cytoplasmic Ribosomes of Euglena: II. Characterization of Ribosomal Proteins. Plant Physiol. 1974 Apr;53(4):543–554. doi: 10.1104/pp.53.4.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman K. A., Amaya J., Schachter E. M. Structure of RNA in ribosomes. Science. 1970 Oct 9;170(3954):171–173. doi: 10.1126/science.170.3954.171. [DOI] [PubMed] [Google Scholar]
- Holdsworth R. H. The isolation and partial characterization of the pyrenoid protein of Eremosphaera viridis. J Cell Biol. 1971 Nov;51(21):499–513. doi: 10.1083/jcb.51.2.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Infante A. A., Krauss M. Dissociation of ribosomes induced by centrifugation: evidence for doubting conformational changes in ribosomes. Biochim Biophys Acta. 1971 Aug 12;246(1):81–99. [PubMed] [Google Scholar]
- Kellems R. E., Butow R. A. Cytoplasmic-type 80 S ribosomes associated with yeast mitochondria. I. Evidence for ribosome binding sites on yeast mitochondria. J Biol Chem. 1972 Dec 25;247(24):8043–8050. [PubMed] [Google Scholar]
- Klein S., Schiff J. A., Holowinsky A. W. Events surrounding the early development of Euglena chloroplasts. II. Normal development of fine structure and the consequences of preillumination. Dev Biol. 1972 May;28(1):253–273. doi: 10.1016/0012-1606(72)90142-x. [DOI] [PubMed] [Google Scholar]
- Lee S. G., Evans W. R. Hybrid ribosome formation from Escherichia coli and chloroplast ribosome subunits. Science. 1971 Jul 16;173(3993):241–242. doi: 10.1126/science.173.3993.241. [DOI] [PubMed] [Google Scholar]
- Noll H. An automatic high-resolution gradient analyzing system. Anal Biochem. 1969 Jan;27(1):130–149. doi: 10.1016/0003-2697(69)90225-5. [DOI] [PubMed] [Google Scholar]
- Peacock A. C., Dingman C. W. Molecular weight estimation and separation of ribonucleic acid by electrophoresis in agarose-acrylamide composite gels. Biochemistry. 1968 Feb;7(2):668–674. doi: 10.1021/bi00842a023. [DOI] [PubMed] [Google Scholar]
- Peacock A. C., Dingman C. W. Resolution of multiple ribonucleic acid species by polyacrylamide gel electrophoresis. Biochemistry. 1967 Jun;6(6):1818–1827. doi: 10.1021/bi00858a033. [DOI] [PubMed] [Google Scholar]
- Rawson J. R., Crouse E. J., Stutz E. The integrity of the 25-S ribosomal RNA from Euglena gracilis 87-S ribosomes. Biochim Biophys Acta. 1971 Sep 24;246(3):507–516. doi: 10.1016/0005-2787(71)90788-x. [DOI] [PubMed] [Google Scholar]
- Rawson J. R., Stutz E. Isolation and characterization of Euglena gracilis cytoplasmic and chloroplast ribosomes and their ribosomal RNA components. Biochim Biophys Acta. 1969 Oct 22;190(2):368–380. doi: 10.1016/0005-2787(69)90087-2. [DOI] [PubMed] [Google Scholar]
- Scott N. S., Munns R., Smillie R. M. Chloroplast and cytoplasmic ribosomes in Euglena gracilis. FEBS Lett. 1970 Oct 5;10(3):149–152. doi: 10.1016/0014-5793(70)80439-2. [DOI] [PubMed] [Google Scholar]
- Spirin A. S., Belitsina N. V., Lishnevskaya E. B. On some artifacts of sucrose gradient sedimentation of ribosomes. FEBS Lett. 1972 Aug 1;24(2):219–224. doi: 10.1016/0014-5793(72)80771-3. [DOI] [PubMed] [Google Scholar]
- Vasconcelos A., Pollack M., Mendiola L. R., Hoffmann H. P., Brown D. H., Price C. A. Isolation of Intact Chloroplasts from Euglena gracilis by Zonal Centrifugation. Plant Physiol. 1971 Feb;47(2):217–221. doi: 10.1104/pp.47.2.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeldin M. H., Schiff J. A. RNA metabolism during light-induced chloroplast development in euglena. Plant Physiol. 1967 Jul;42(7):922–932. doi: 10.1104/pp.42.7.922. [DOI] [PMC free article] [PubMed] [Google Scholar]