Abstract
Membrane proteins play vital roles in inside-out and outside-in signal transduction by responding to inputs that include mechanical stimuli. Mechanical gating may be mediated by the membrane or by protein(s) but evidence for the latter is scarce. Here we use force spectroscopy, protein engineering and bacterial growth assays to investigate the effects of force on complexes formed between TonB and TonB-dependent transporters (TBDT) from Gram-negative bacteria. We confirm the feasibility of protein-only mediated mechanical gating by demonstrating that the interaction between TonB and BtuB (a TBDT) is sufficiently strong under force to create a channel through the TBDT. In addition, by comparing the dimensions of the force-induced channel in BtuB and a second TBDT (FhuA), we show that the mechanical properties of the interaction are perfectly tuned to their function by inducing formation of a channel whose dimensions are tailored to the ligand.
Bacterial outer membrane TonB-dependent transporters (TBDTs) mediate the influx of several nutrients. Here the authors use single-molecule force spectroscopy to show that the interaction between TonB and Escherichia coli TBDT BtuB is mechanically resistant to the pulling that gates the BtuB channel.
Belying its name, the outer-membrane (OM) envelope of Gram-negative bacteria is relatively devoid of lipid and instead is packed with β-barrel OM proteins (OMPs), which function as enzymes, foldases, assembly platforms and both specific and non-specific transporters1. Many of these functions, such as transport against a concentration gradient, are energy dependent and, as the periplasmic space is devoid of adenosine triphosphate (ATP), it is necessary to couple OMP transporters to machinery from the energized inner-membrane (IM) to facilitate these processes. A well-studied example is the interaction of OM transporters with TonB—a periplasmic protein tethered to the IM. The TonB-dependent transporter family (TBDTs)2,3,4 bind and then transport scarce but vital nutrients such as maltodextrin (MalA), sucrose (SuxA) and metallo-organic compounds including vitamin B12 (BtuB), haem (HasR and HemR) and siderophores (FepA, FhuA, FecA and FhuE)3,5. Their importance to cell viability results in TBDTs being virulence factors in pathogenic bacteria6,7. Coincidentally, TBDTs are also hijacked by colicins and phage for cell entry8.
Structurally, TBDTs are characterized by 22-stranded β-barrels whose lumens are occluded by an N-terminal globular ‘plug' domain (Fig. 1a). The plug domain contains a conserved binding motif known as the Ton box9 on the periplasmic side, which upon binding of extracellular substrate becomes disordered10,11. This transition allows the formation of a non-covalent complex with the C-terminal globular periplasmic domain of TonB (TonBCTD) (Fig. 1b)12,13. TonB is anchored to the IM by a single N-terminal transmembrane helix and these domains are linked by a central proline-rich periplasmic spanning domain. This linker adopts an extended polyproline type II helical rod conformation14, allowing TonBCTD to reach the OM. In vivo, TonB forms a complex with the IM proteins ExbB and ExbD15. These proteins are thought to function as a scaffold (ExbB)16,17 and to harness the proton motive force (ExbD)18, a pre-requisite for TonB-dependent activity.
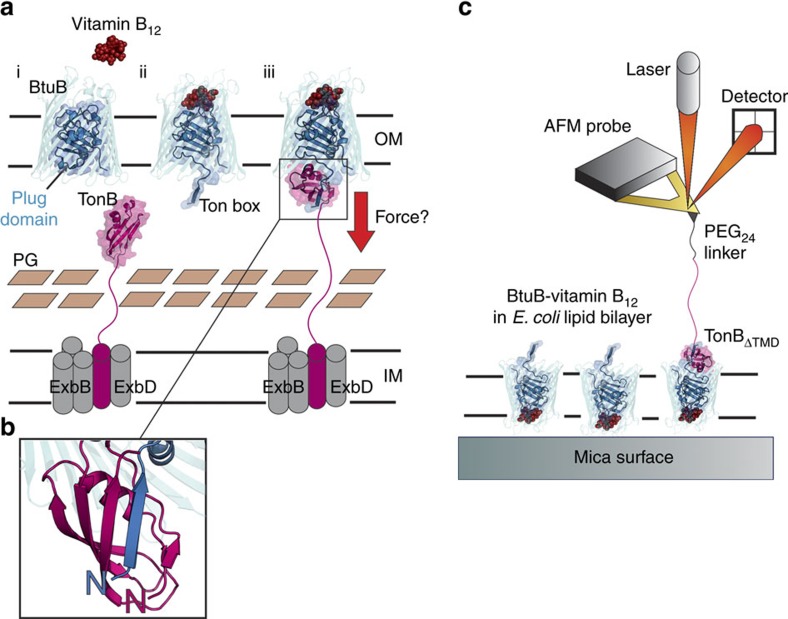
Figure 1. Investigating TonB-dependent transport by single-molecule force spectroscopy.
(a) Schematic of TonB-dependent vitamin B12 transport in E. coli. PG, peptidoglycan. (i) The lumen of BtuB, a 22-stranded β-barrel OM protein (PDB: 1NQE), is occluded by an N-terminal plug domain (arrow) preventing transit of vitamin B12 (red space filling model), (ii) the binding of vitamin B12 induces an allosteric rearrangement of the plug domain, releasing the Ton box into the periplasmic space, where it forms a 1:1 complex (PDB: 2GSK) with the C-terminal domain of TonB (TonBCTD, residues 153–233 (PDB: 1XX3). The transmembrane helix at the N-terminus of TonB forms a complex with ExbB and ExbD (grey cylinders), which is necessary, together with an energized IM, for TonB-dependent transport, (iii) linkage of the OM and IM via this non-covalent complex is thought to trigger the full or partial unfolding of the plug domain, allowing the passage of vitamin B12. While the precise mechanisms vary, most models for TonB-dependent transport suggest that remodelling is induced by application of force (red arrow). (b) Detail of the TonB:BtuB interaction showing the parallel orientation of the β-strand augmentation interaction of the Ton box of BtuB (blue) with TonBCTD (pink). Note: for TonBCTD, N designates the start of the C-terminal domain. (c) Simplified schematic to describe the single-molecule force spectroscopy approach used in this study. The interaction between soluble TonB (residues 33–239, TonBΔTMD, that is, deletion of the N-terminal transmembrane helix) attached to the AFM probe and BtuB inserted into E. coli polar lipid extract liposomes and adsorbed onto a mica surface, is measured using an AFM. Note: adsorption of liposomes onto mica generates a heterogeneous surface with regions of single- and double-bilayer thicknesses.
While it is known that TonB-mediated transport requires TonB to be tethered to an energized IM19, the mechanism by which TonB remodels the plug domains of TBDTs remains unclear20. Currently favoured models, such as the pulling hypothesis21,22 or the rotational surveillance and energy transfer23 model, suggest that TonB applies a mechanical remodelling force driven by its interaction with the ExbBD complex in the energized IM. This induces partial or full-plug domain unfolding via the non-covalent interaction of the Ton box with TonBCTD3,24,25,26. For these models to be viable, the Ton box–TonB inter-protein interaction must be sufficiently stable under tension to allow the unfolding of the plug domain before its dissociation. Intriguingly, the crystal structure of BtuB (the vitamin B12 TBDT receptor of E. coli) in complex with TonB shows that the Ton box binds to the β-sheet of TonBCTD in a parallel orientation and that this augmented β-sheet is rotated ∼90° with respect to the β-strands of the plug domain12. If the plug domain of BtuB is extended by TonB in vivo, the relative geometry of the β-strands involved in these inter- and intra-protein interactions are ideally oriented to engender these mechanical phenotypes27,28 as shown using molecular dynamics (MD)22.
To investigate the mechanism of TBDT function and more generally, the role of protein–protein interactions in membrane protein gating, we first employ single-molecule force spectroscopy using an atomic force microscope (AFM) to show that the non-covalent interaction between the Ton box from BtuB (TBBtuB) and TonB is surprisingly durable under extension. Repeating this experiment using BtuB reconstituted in E. coli polar liposomes adsorbed onto a mica surface and TonB immobilized onto an AFM tip (Fig. 1c), we find that the TBBtuB–TonB complex is sufficiently durable under force to allow the unfolding of half of the plug domain before its dissociation. The presence and extent of the mechanically labile subdomain is confirmed by repeating the experiments on disulfide crosslinked variants and the consequences of preventing plug remodelling is assessed using a vitamin B12 transport assay. Finally, we show using mutagenesis that destabilization of the mechanically strong plug subdomain results in complete plug unfolding under force before dissociation of TonB and use an antibiotic sensitivity assay to show that this also occurs in vivo. Together, these data provide evidence for the direct mechanical gating of a membrane protein via protein–protein interactions and show that TBDTs maintain the integrity of the OM during transport by formation of a channel whose dimensions matches that of their cargo.
Results
TBBtuB interaction with TonB is mechanically strong
The BtuB–TonB complex is thought to span the periplasm and, ignoring the membrane-embedded components, comprises three mechanical elements: the plug domain of BtuB, the TBBtuB–TonB linkage and the polyproline linker domain of TonB. As a first step towards understanding the effects of mechanical extension on this network, we focussed on the non-covalent TonB–TBBtuB interaction using a minimal system comprising TonBΔTMD (TonB without the transmembrane domain (residues 1–32, see ‘Methods' section and Fig. 2a) and a synthetic TBBtuB peptide (residues 5–14 of BtuB, see ‘Methods' section). Before AFM experiments, the affinity of TBBtuB–TonBΔTMD was measured by microscale thermophoresis yielding a KD of 9.4±0.3 μM (Fig. 2b), similar to that previously published (36 μM) for the interaction between the Ton box peptide of FhuA and TonBCTD29. TBBtuB and TonBΔTMD were then immobilized to an AFM probe and a silicon nitride surface, respectively, using flexible NHS-PEG24-maleimide linkers (see ‘Methods' section, Fig. 2a). The mechanical strength of this complex was then quantified by accumulating data from multiple approach-retract cycles whereby complex formation was allowed to occur in buffered solution by approaching the AFM probe to the surface at 1 μm s−1 and, after application of a 300 pN trigger force, retracting at a velocity of 1 μm s−1. Cycles that result in formation of the complex (7.5%) are identified by a distinctive single ‘saw-tooth' force–extension profile (red traces Fig. 2c, left) resulting from extension of the linkers and the complex and subsequent dissociation of TBBtuB–TonBΔTMD at a certain force. The rising edge of such profiles can be fitted with a worm-like chain model30 (WLC, black solid lines Fig. 2c left, ‘Methods' section) to obtain the maximum end-to-end length (or contour length Lc) of the force-resistant structure that comprises the protein complex and linkers at rupture. Data from ∼400 force–extension profiles (per experiment) are used to generate force- and contour length-frequency histograms (see Supplementary Fig. 1), allowing quantification of the most probable unbinding force (FU) and most probable Lc at rupture. Finally, as both the FU and Lc for the unbinding of a specific interaction would be expected to be both correlated and reproducible, combining these data in a scatterplot allows the specificity of the interaction to be assessed by the presence or absence of a ‘hot-spot'. The specificity of the data presented here is evidenced by comparing the scatterplots obtained for TonBΔTMD with wild-type TBBtuB (hotspot present, see Fig. 2c, right and Supplementary Fig. 2a) and a binding deficient variant of TBBtuB31 that substitutes a proline for leucine at position 8 (no hotspot, Supplementary Fig. 2a).
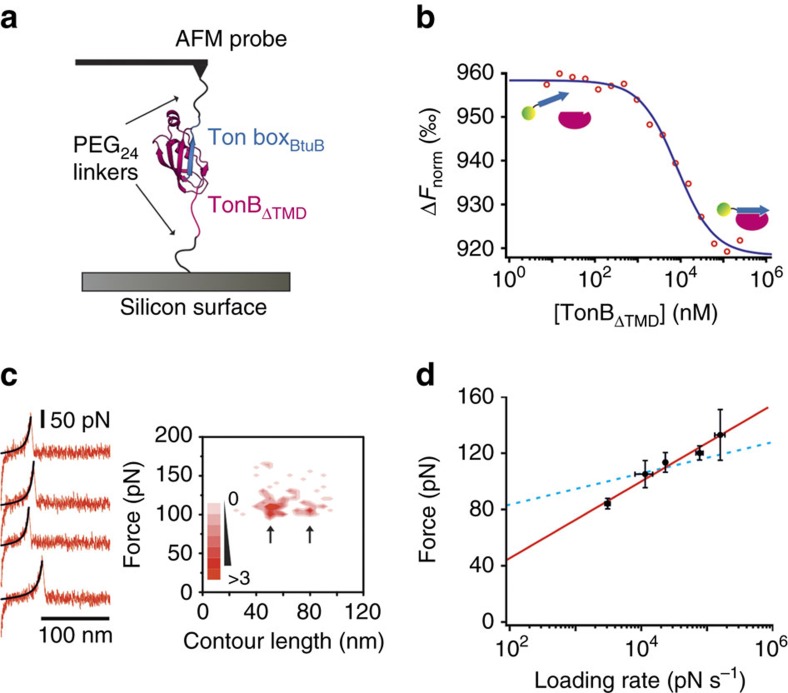
Figure 2. Characterization of the Ton boxBtuB peptide:TonBΔTMD interaction.
(a) Cartoon schematic of the AFM experiment used to characterize the mechanical strength of TBBtuB:TonBΔTMD. (b) MST of the Ton box of BtuB (TBBtuB) labelled with Alexa Fluor-488 C5 maleimide in the presence of 7 nM-244 μM TonBΔTMD. Data (open red circles) are fitted using the law of mass action with the Nanotemper software to give a KD of 9.4±0.3 μM. (c) Left: example retraction traces (red) showing the extension and rupture of TBBtuB:TonBΔTMD at a retraction velocity of 1 μm s−1 fitted with a worm-like chain model (WLC, black). Right: a contour length (Lc) versus rupture force (FU) scatterplot of 90 dissociation events reveals two distinct hot-spots (arrows). Scatterplot is coloured by density of data points (5 nm–10 pN). (d) The dynamic force spectrum (a plot of FU versus the natural logarithm of the force loading rate (pN s−1)) of the TBBtuB:TonBΔTMD interaction. The error bars show the range of measurements from the triplicate mean. Data are fitted to a linear regression (red line). The dynamic force spectrum obtained for the highly avid E9:Im9 (E9 crosslinked between residues 20–66) is shown for comparison (dashed blue line)33.
Closer examination of the individual force–extension profiles (Fig. 2c, left), distance histograms (Supplementary Fig. 1b) and scatterplots (Supplementary Fig. 2a) obtained over a range of retraction velocities (0.2–5 μm s−1) reveal that while dissociation occurs at a single force (FU=113±13 pN at 1 μm s−1 (average of the mode of the Gaussian fit of triplicate data sets±half of the range), two Lc distributions are evident with modal values of 47 and 74 nm. The latter value is consistent with the sum of the end-to-end length of the PEG24 linkers (19 nm) and the expected end-to-end length of the TonBΔTMD–TBBtuB complex (5.5 nm) with an unstructured or unfolded 118-residue linker domain (47.2 nm, see ‘Methods' section and Supplementary Fig. 2b).The shorter modal Lc value (coincidentally also 47 nm) suggests that the linker domain of TonBΔTMD must also populate a force-resistant conformation ∼22.5 nm in length (47–19–5.5 nm). To test this hypothesis, these experiments were repeated using TonBCTD (that is, the globular C-terminal domain without the linker). These experiments yielded a unimodal distance-frequency histogram with a Gaussian fit value (22.4 nm) close to the predicted value (24.5 nm, the sum of the PEG24 linkers (19 nm) and TonBCTD–TBBtuB complex (5.5 nm), Supplementary Fig. 2c).
As forced-dissociation experiments are typically performed far from equilibrium, FU depends on the rate at which force is loaded onto proteins and their complexes32. To quantify the loading rate dependence of this system, TonBΔTMD–TBBtuB was extended at retraction velocities of 200–5,000 nm s−1 (corresponding to force loading rates of 103–105 pN s−1 (see ‘Methods' section). The resultant dynamic force spectrum reveals that rupture occurs at 84–132 pN (Fig. 2d) at the relatively high-loading rates applied using AFM. This is a remarkably strong interaction for a complex with a relatively low affinity (μM) if the association rate is assumed to be diffusion limited. For example, at similar loading rates, the dissociation of E920–66-Im9 (Kd=10−14 M, koff=1.4 × 10−6 s−1)33 and an antibody and its epitope (Kd=10−9 M, koff=4.4 × 10−3 s−1)34 occurs at a force of ∼100 pN and ∼160 pN, respectively. These comparisons indicate that the TonB–Ton box complex is indeed mechanically robust and provide support for a slow association rate29. It is interesting to note that the β-strand augmentation mechanism which underlies complex formation is well known for being mechanically ‘strong'12 and is found in other complexes evolved to withstand mechanical stress28.
Force-induced partial unfolding of BtuB by TonBΔTMD
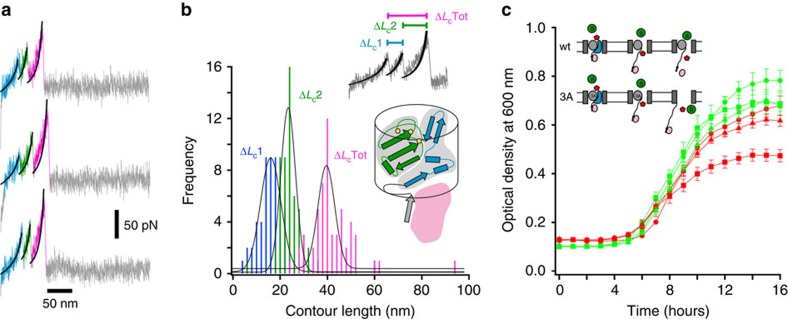
We have demonstrated above that TonBΔTMD–TBBtuB is able to resist levels of force that are sufficient to unfold protein domains with moderate mechanical strength35,36 at similar applied loading rates. This raised the possibility that the plug domain, which is contiguous with the Ton box, may fully or partially unfold to open the lumen before dissociation of TonB from BtuB. To test this theory, wild-type BtuB was overexpressed and purified from the OM of E. coli and reconstituted into liposomes composed of E. coli polar lipid extract in the presence of cyanocobalamin (a synthetic form of vitamin B12) (see ‘Methods' section). The lipid vesicles containing BtuB were then adsorbed onto a freshly cleaved mica surface and TonBΔTMD immobilized to the AFM probe (Fig. 1c). Pressing the probe against the surface with 60–90 pN of force and withdrawing at a velocity of 1 μm s−1 (force loading rate ∼10 nNs−1) after dwelling for 1 s led to two distinctive force–extension profiles. Approximately a third of the profiles (31%) displayed a single rupture event (FU=97±8 pN, Lc=51±3 nm) similar in force to that observed for the minimal TonB–TBBtuB interaction at the same loading rate (FU=113±7 pN). The majority, however, displayed two rupture events (Fig. 3a) at FU and Lc values of 61±4 and 91±23 pN and 58±3 and 77±7 nm, respectively (Supplementary Table 1). These more frequent events indicate that the complex must undergo some partial unfolding before dissociation and that unfolding is more likely than dissociation of the complex at this loading rate. As we have shown that TonB does not unfold at these forces, force would presumably be propagated into the plug domain via the contiguous Ton box. These data thus suggest that the strength of intra-polypeptide interactions in the plug domain are weaker than those of the inter-polypeptide interactions between TonBΔTMD and BtuB, leading to partial/full unfolding of the plug before unbinding. As the mechanical strength of proteins and their complexes is kinetically controlled, the loading rate may affect unfolding and unbinding differently. Interestingly, analysis of the speed dependence of the putative unfolding and unbinding events demonstrates that the relative strength of intra- and inter-protein interactions at the same loading rate remains constant across the dynamic range of experiment (Fig. 3b).
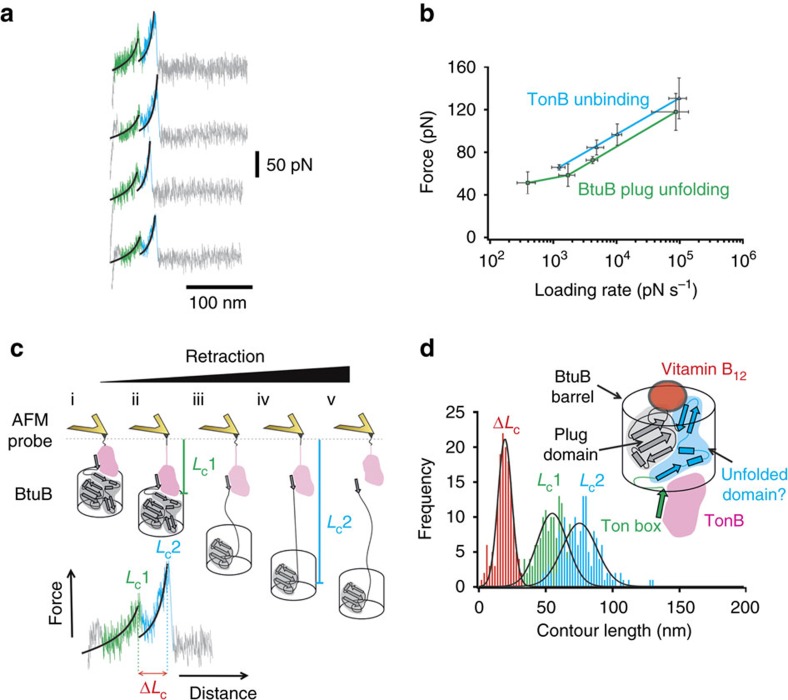
Figure 3. Pulling on the BtuB–TonB complex partially unfolds the plug domain.
(a) Representative force–extension profiles that show two rupture events (the leading edge of the first and second event is coloured green and blue, respectively). Each is fitted to the WLC model (black). (b) Dynamic force spectrum of unfolding (green) and unbinding (blue) events at 200, 500, 1,000 and 5,000 nm s−1, error bars show average and range of fitted mode of three triplicate data sets. (c) Schematic describing the origin of each rupture event (an example trace with WLC fits is shown below) and their relationship to Lc1, Lc2 and ΔLc. The barrel of BtuB is shown as an open cylinder with the secondary structure of the plug domain (grey shaded region) and TonB (pink), which is attached to the AFM probe. (i) The AFM probe is pressed against the surface bringing the TonB–BtuB complex together; (ii) TonB bound to BtuB is fully extended causing the force to increase; (iii) part of the plug domain is unfolded and the force sharply decreases; (iv) the unfolded region is extended, causing an increase in the entropic restoring force and (v) TBBtuB dissociates from TonB and the force sharply falls to zero. The difference in fitted contour lengths (ΔLc) between the two peaks can be used to calculate the extent of unfolding. (d) Contour length frequency distributions from WLC fits to double-peaked force–extension profiles from a single dataset (n=153) accumulated at a retraction velocity 1,000 nm s−1. Frequency distributions obtained for the remodelling/unfolding event (Lc1, green), unbinding (Lc2, blue) and ΔLc (red) are fitted to Gaussian functions (black lines). Inset: the presence of an unfolding event before dissociation reveals that the plug domain comprises a mechanically weak subdomain (the 50 amino acids downstream of the Ton box (residues 23–73, shaded blue)) and a mechanically recalcitrant subdomain (shaded grey). Residues that extend before the first event are coloured green. TBBtuB is shown as a green arrow bound to TonB (shaded in pink).
Identifying a force-induced channel through the plug domain
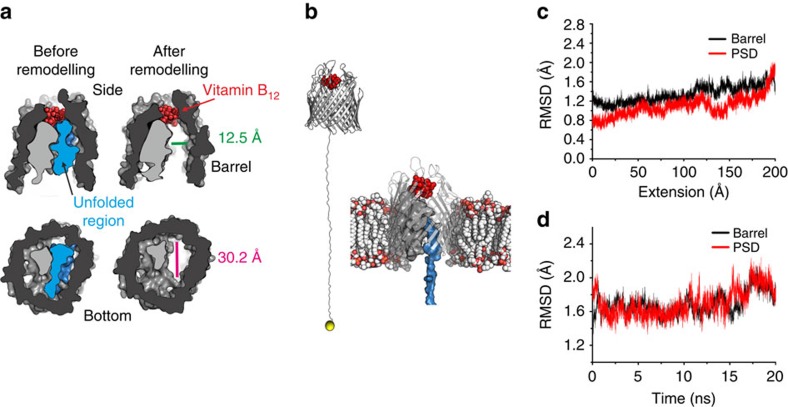
To quantify the extent of unfolding, force–extension profiles showing two events were analysed further by fitting worm-like chain models to the unfolding (Lc1) and unbinding (Lc2) events. The difference in these values (ΔLc) reveals how much of the protein complex becomes unfolded at the first rupture event (Fig. 3c). These data in the form of a histogram are shown in Fig. 3d and reveal a modal value of ΔLc=20±2 nm that is independent of the retraction velocity used (Supplementary Table 1), or importantly, the presence or absence of the TonB linker domain (Supplementary Fig. 3 and Supplementary Table 2). This extension corresponds to the unfolding of 50 amino acids—approximately half of the residues within the plug domain. While these data reveal the extent of unfolding it does not pinpoint the region involved within the plug. To do this, we reasoned that the 50 residues directly downstream of the Ton box motif (residues 23–73) would most likely unfold first (see Fig. 3c,d, inset), as these residues are initially exposed to mechanical deformation as force propagates through the plug. To test this assumption, BtuB variants that contained a disulfide bridge within the plug were generated. First, a variant designated XLbarrel (Fig. 4a schematic) was designed to prevent any unfolding by linking the N-terminus of the plug domain (residue 23 located between the Ton box and the first β-strand of the plug domain) and residue 374 in strand 12 of the barrel domain (BtuB L23C/S374C, yellow-filled circles joined by red bar, Fig. 4a, left). For this crosslinked variant, 74% of the force–extension profiles analysed contained single rupture events with an FU of 100±6 pN at a Lc of 47±2 nm (Fig. 4b, red traces and histogram). The Lc value is in accord with the distance expected for the rupture of the complex without remodelling, while the FU value is close to that observed for the dissociation of the minimal TBBtuB–TonB complex observed previously at the same retraction velocity (1 μm s−1, 113±13 pN). Addition of reductant (2 mM dithiothreitol, DTT) to the buffer solution during the AFM experiments resulted in the re-appearance of the double-peaked events observed for the wild-type receptor (Fig. 4b, green and blue traces and histogram) with a modal ΔLc value of 21±4 nm (Fig. 4b, grey histogram) and FU=64±1 and 99±7 pN for the unfolding and unbinding forces, respectively (Supplementary Table 3). To localize the unfolding event further, a V29C/V45C variant (XLloop) was designed to crosslink strand 1 (V29C) and helix 2 (V45C) of the plug domain (yellow-filled circles joined by red bar, Fig. 4a, right). Upon disulfide bond formation, this creates a covalent 15 amino-acid loop within the region predicted to unfold (blue-shaded area in Fig. 4a schematic) shortening ΔLc by ∼6 nm. Similar to the wild-type data, force–extension profiles for BtuB XLloop–TonB dissociation contained a high proportion (94%) of double-peaked events (Fig. 4c, inset). Most importantly, the ΔLc-frequency histogram (Fig. 4c, grey histogram) yielded a modal value 6 nm shorter relative to wild-type (14±3 and 20±1 nm), confirming unfolding in the N-terminal portion of the plug. Once again, the force–extension profiles for BtuB XLloop–TonB dissociation reverted to wild-type profiles upon addition of 2 mM DTT (ΔLc=20±1 nm) (Fig. 4c, bottom grey histogram). Together these data suggest that the first ∼50 residues of the plug domain are denatured before the non-covalent TonB–BtuB complex dissociates. The structural consequence of partially unfolding the plug domain was visualized by removing the 50 residues directly downstream of TBBtuB from the crystal structure of BtuB bound to vitamin B12 and TonBCTD12. The topology of the plug domain is such that this deletion results in the appearance of 12.5 × 30.2 Å-wide channel through the receptor (Fig. 5a). To assess the effect of unfolding half of the plug domain, we performed MD simulations of the system. These simulations were similar to that undertaken by Gumbart and colleagues in 2007 apart from the presence of bound vitamin B12 in this study (Supplementary Fig. 4). After the system was equilibrated (Supplementary Fig. 4), a force ramp was applied by retracting a harmonic spring at constant velocity (also sometimes called steered MD) until the Ton box was extended 20 nm away from its original position (Fig. 5b, left). Similar to the previous MD study on BtuB–TonB22 (and also observed for the Neisseria TbpA–TonB complex37), the plug domain unfolded directly downstream of the Ton box creating a continuous channel through the receptor (Fig. 5b, right). Furthermore, the mechanically strong subdomain was found to remain native-like during (Fig. 5c) and up to 20 ns after unfolding (Fig. 5d) of the mechanically weak subdomain.
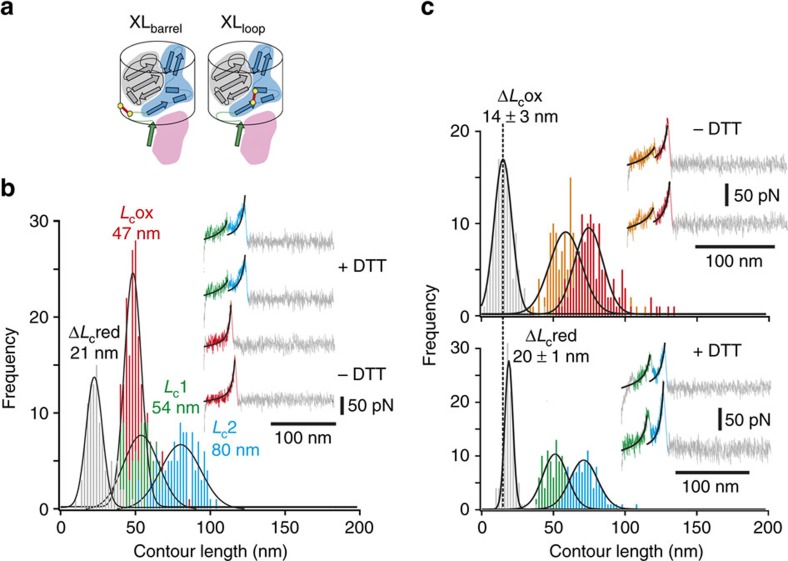
Figure 4. Using disulfide crosslinking to locate the mechanically weak plug subdomain.
(a) Secondary structure and topology of the plug domain showing location of the disulfide bridges (bold red line linked by yellow circles). The mechanically weak and strong subdomains are shaded blue and grey, respectively, and TBBtuB is shown as a green arrow bound to TonB (shaded in pink). (b) Lc distributions of single rupture events observed for BtuB XLbarrel–TonBΔTMD in the absence of reductant (n=196, red histogram, red force–extension traces (inset)) and, after addition of 2 mM DTT (n=109), double rupture events resulting from partial unfolding (Lc1, green histogram and green force–extension profiles, inset) and unbinding (Lc2, blue histogram and blue force–extension profiles, inset) of XLbarrel plug domain from TBBtuB. When fitted to a Gaussian distribution, the ΔLc-frequency histogram (grey bars) for these double events has a modal value of 21±4 nm. (c) Top: Lc distributions and force–extension profiles (inset) for BtuB XLloop–TonBΔTMD in the absence of DTT (n=154). Partial unfolding (Lc1) and unbinding (Lc2) are shown in orange and red, respectively, for both histograms and force–extension profiles. The ΔLc (grey histogram) modal value is shown by the dashed line. Bottom: Lc distributions and force–extension profiles (inset) for BtuB XLloop–TonBΔTMD in the presence of 2 mM DTT (n=122). Partial unfolding (Lc1) and unbinding (Lc2) are shown in green and blue, respectively, for both histograms and force–extension profiles. In the presence of DTT, the ΔLc (grey histogram) modal value (20±1) reverts to that of wild-type BtuB (20±2). All data collected at 1,000 nm s−1.
Figure 5. Creation of a channel through the receptor by partial unfolding of the plug domain.
(a) Surface representation of vertical and horizontal cross-sections of BtuB showing the effect of removing 50 amino acids (blue) downstream of the Ton box. The dimensions of the resultant channel are shown. (b) Left: snapshot after 20 nm SMD from the Ton box (yellow sphere), the membrane and water are hidden for clarity. BtuB is shown as a grey cartoon with vitamin B12 shown as red spheres. Right: snapshot of BtuB equilibrated in a POPC bilayer (red and white spheres) after 20 nm of plug unfolding induced by extension of the Ton box by SMD. The unfolded mechanically weak domain is shown in blue surface representation, the recalcitrant plug subdomain (PSD) is shown in grey surface representation and vitamin B12 as red spheres. (c) Root mean square deviation (a measure of the average deviation of the atomic positions from the experimental structure) of the recalcitrant PSD and barrel domain during 20 nm of SMD. The simulations show that the root mean square deviation of the recalcitrant PSD and the β-barrel are stable and of similar magnitude, demonstrating that the mechanically strong PSD is conformationally stable over this timescale. (d) Equilibration of the system after 20 nm of unfolding by SMD.
The effect of preventing or diverting the force propagation pathway through the plug domain in vivo was next assessed by examining the growth phenotypes of an E. coli metE strain expressing wild-type BtuB or its variants in the presence of different concentrations of vitamin B12. This strain requires vitamin B12 to synthesize methionine and requires functional BtuB for full growth on minimal medium in the presence of 0.1–1 nM vitamin B12. The colony size of E. coli RK5016 (btub metE38) cells expressing wild-type BtuB or the crosslinked variants under the control of a native promoter (pAG1 (ref. 39)) grown on minimal medium agar plates supplemented with vitamin B12 (0.1–100 nM) or methionine (5 μg ml−1) were compared after 48 h (Table 1). Full growth was observed for wild-type and XLloop BtuB (Table 1) at all concentrations of vitamin B12, while reduced growth was observed at all vitamin B12 concentrations for BtuB XLbarrel. Full growth was observed for all BtuB variants in the presence of methionine (Supplementary Fig. 5). The phenotypes of these variants support the hypothesis that application of force (via TonB) unravels the mechanically weak plug subdomain from the N-terminus to create a channel between the plug and wall of the β-barrel.
Table 1. Methionine assay to assess the growth phenotype of the BtuB variants.
| Strain | Growth on: | ||||
|---|---|---|---|---|---|
| [Vitamin B12] (nM) | |||||
| RK5016 | Met | 0.1 | 1 | 10 | 100 |
| pAG1 (wt BtuB) | ++ | ++ | ++ | ++ | ++ |
| L8P | ++ | − | − | + | + |
| XLbarrel | ++ | + | + | + | + |
| XLloop | ++ | ++ | ++ | ++ | ++ |
Growth of E. coli RK5016 metE btub cells transformed with BtuB variants after 48 h incubation on Davis minimal agar supplemented with 0.1–100 nM vitamin B12 or 5 μg ml−1 methionine. − indicates no observable colonies, + indicates partial growth (small colonies) and ++ indicates full growth (comparable to methionine plate controls (colony diameter=2 mm)). Two biological replicates were performed.
To determine whether channel formation by force-induced remodelling of the plug via TonB is a feature general to all TBDTs, we repeated the force spectroscopy experiments on the ferric hydroxamate uptake (FhuA) receptor from E. coli, which transports ferrichrome (740.52 Da)—a significantly smaller cargo than vitamin B12 (1355.38 Da). After purification and immobilization (see ‘Methods' section), two rupture events (74±11 and 105±24 pN and 62±4 and 85±9 nm; Supplementary Fig. 6a and Supplementary Table 4) were detected reminiscent of those observed for BtuB. The change in contour length for FhuA, however, was found to be greater than for BtuB (ΔLc=25±1 nm) (Supplementary Table 4 and Supplementary Fig. 6b), corresponding to the removal of 63 residues (residues 20–83). Importantly, while remodelling once again results in the formation of a channel across the receptor (Supplementary Fig. 6c), its dimensions are slightly smaller (14 × 26 Å) reflecting the smaller size of its cargo.
Destabilizing the mechanically strong plug subdomain
The presence of different sized mechanically strong plug subdomains in BtuB and FhuA suggests that these TBDTs have evolved to limit their channel dimensions to allow passage of their substrate. To investigate the consequences of enlarging the channel to the dimensions of the β-barrel lumen (∼35–40 Å diameter), the C-terminal plug subdomain of BtuB (residues 73–136) was destabilized by the introduction of three large hydrophobic deletion substitutions (I80A/L85A/L96A, designated as BtuB3A). By contrast to wild-type BtuB and the other variants studied, the force–extension profiles for BtuB3A–TonBΔTMD displayed three rupture events (Fig. 6a and Supplementary Table 5). The increase in contour length between the first and second rupture event (ΔLc1=19±4 nm) was similar to that found for wild-type BtuB–TonBΔTMD (ΔLc=20±1 nm) identifying this event as the unfolding of the mechanically weak subdomain. If the additional rupture event (FU=94±9 pN) observed for BtuB3A is due to the unfolding of the remaining 64 plug residues, an increase in contour length of ∼25 nm would be expected, in good agreement with the measured value (ΔLc2=26±3 nm). The difference in contour length between the first and last event (total ΔLc=45±6 nm) is equivalent to the unfolding of 112±13 residues, in good agreement with the number of residues in BtuB plug (114 (residues 22–136), Fig. 6b).
Figure 6. Destabilizing the mechanically strong subdomain leads to complete unfolding.
(a) Force–extension profiles for BtuB3A–TonBΔTMD display triple rupture events. Each event is fitted to a WLC model (black). Data were obtained at a retraction velocity of 1,000 nm s−1. (b) ΔLc frequency histograms (n=55) for the difference in Lc between the first and second event (ΔLc1, blue histogram), the second and third event (ΔLc2, green histogram) and the first and third event (ΔLcTot, pink histogram) (see top inset). As the length of an amino acid is known (0.4 nm), these data can be used to calculate the number of amino acids unfolded at each event, allowing the unfolding events to be mapped onto the secondary structure schematic (bottom inset: residues unfolded at the first and second rupture events coloured blue and green, respectively. Yellow dots represent the location of each alanine mutation). (c) Bacitracin sensitivity assay. RK5016 cells transformed with BtuB or variants (circles: wild-type, squares: BtuB3A and triangles: BtuB3A,L8P) under a native promoter were used to inoculate LB medium supplemented with selection antibiotics and 200 μg ml−1 bacitracin in the absence (red data) or presence of 200 μg ml−1 (green data) of Ton box pentapeptide in a 96-well format with shaking at 200 r.p.m. at 37 °C. The absorbance at 600 nm was monitored over 15 h. Data points are averages of three technical repeats with error bars showing s.d. Inset: the mechanically strong plug subdomain (grey ellipse) of wild-type BtuB does not unfold upon extension via TonB (pink ellipse). The channel formed by unfolding the mechanically weak domain (blue shaded region) thus allows passage of vitamin B12 (red pentagon) but not bacitracin (green circle). By contrast, upon destabilizing the mechanically strong subdomain (BtuB3A), extension via TonB results in the unfolding of both subdomains, leading to the formation of a channel that allows passage of both substrate and antibiotic.
In vitro, the drastic weakening of the mechanically robust plug subdomain allows full plug unfolding before dissociation of the TonB–BtuB complex. If this mechanical hierarchy is preserved in vivo, which would be necessary for any force-modulated gating mechanism, this would result in the formation of a transient ∼3.5 × 3.5 nm pore within the OM. Consequently, an antibiotic sensitivity screen was performed using bacitracin, a large (1.4 kDa) inhibitor of cell wall synthesis. E. coli are normally unaffected by bacitracin at concentrations less than 200 μg ml−1, as the OM provides a natural barrier to this large antibiotic40. If the integrity of the OM is compromised by addition of polymyxin B40 or by the presence of a TBDT without a folded plug domain41, sensitivity is increased. Accordingly, the growth of RK5016 cells expressing wild-type or variant BtuBs under the control of the native promoter was measured in the presence of 200 μg ml−1 bacitracin. Cells expressing BtuB3A exhibited reduced growth (typically reaching 50% of the maximal growth of cultures expressing wild-type BtuB, Fig. 6c) suggesting an increased accessibility of bacitracin to the periplasm. To rule out the possibility that the increase in bacitracin sensitivity was simply due to the inability of BtuB3A to form a stable natively folded plug domain, 200 μg ml−1 Ton box pentapeptide (ETVIV) was added to the growth medium to prevent active unfolding of the plug by TonB42. The addition of 200 μg ml−1 Ton box pentapeptide yielded a phenotype similar to wild-type BtuB (Fig. 6c). Introduction of the L8P substitution (known to disrupt formation of TonB–BtuB complex in vitro) within the Ton box of BtuB3A resulted in bacteria with significantly reduced sensitivity to bacitracin relative to BtuB3A. These results demonstrate that BtuB3A is active and able to form a native-like luminal plug in vivo. More importantly, the differential sensitivity to bacitracin of wild-type and BtuB3A in the presence and absence of Ton box peptide accords with the in vitro AFM data above, suggesting that in vivo, TonB drives partial (wild-type BtuB) or complete unfolding (BtuB3A) of the plug before complex dissociation.
Discussion
The work presented here demonstrates that the TonB–TBBtuB interaction is sufficiently strong under extension to allow the mechanical unfolding of a defined region of the plug domain of BtuB to create a substrate channel through the receptor before its dissociation. This mechanism is similar to that originally proposed a decade ago21, which was supported by an in silico study which showed that extension of TBBtuB by ∼20 nm leads to the creation of a channel that would allow substrate passage22. The mechanisms of both inside-out energy transduction and the resultant vertical displacement of TonB by a distance similar to the width of the periplasm43 are still unclear. Despite differences in their details, however, all the suggested models involve the formation of a force-transduction pathway, which apply mechanical force to the plug domain. In these models, the vertical displacement of TBBtuB that is required for channel opening may be a result of ‘passive' processes (for example, variations in the width of the periplasmic space44 and/or the differential diffusion of the IM and OM proteins45). Alternatively, active processes dependent on the proton motive force may play a role. This includes a conformational change of the TonB linker resulting in a shortening in the end-to-end length of some part of the linker region (this work), by a transition of residues 66–100 (ref. 46) from an extended polyproline type II to a shorter polyproline type I helix14, or by the ExbBD-dependent23,47 rotary motion of TonB itself.
The direct identification of two distinct subdomains in the luminal plugs of FhuA and BtuB rationalizes a wealth of previous observations from mutational and crosslinking studies. For example, the first 51 residues of the FepA plug domain were found to be more susceptible to labelling relative to residues 91–142 (ref. 48), while the introduction of crosslinks between the N- or C-terminal portions of the FhuA plug to the barrel were found to prevent or support ferrichrome transport, respectively49,50. A lack of plug domain cooperativity has also been observed in vitro: electron paramagnetic resonance experiments and trypsin cleavage have shown that the N-terminal regions of the BtuB and FhuA plug domains (respectively) unfold in the presence of 4 M urea51,52 and that, for BtuB, the unfolded subdomain rapidly refolds upon removal of the denaturant51. Finally, previous AFM studies, which investigated the mechanical unfolding pathway of the barrel of FhuA (by adsorbing FhuA to the AFM tip in a non-specific manner in the absence of TonB) showed that the plug domain unfolded in two steps (residues 1–91, FU=∼50 pN and residues 92–161, FU=163±50 pN at a retraction velocity of 2,200 nm s−1 (ref. 53)).
Here we demonstrate that complete unfolding allows the free diffusion of large antibiotics, such as bacitracin, into the cell. This observation, taken with others22,48,51,52, suggests that TBDTs have evolved to minimize the deleterious effect of compromising the outermost barrier of Gram-negative bacteria by allowing the remodelling of the fewest residues required for transit of their cargo. The mechanically recalcitrant C-terminal plug subdomain of BtuB identified here, together with subdomains identified in FecA and FhuA, have been shown by evolution-based statistical analysis to contain a network of conserved residues believed to be involved in the allosteric communication that drives the release of the Ton box into the periplasm upon ligand association54. We therefore propose that the plug domain is divided into two separate functional parts: a mechanically strong subdomain involved in this signalling and a mechanically weak subdomain that is unfolded to allow substrate transport. In this regard, it is interesting to note that the mechanically weak subdomains of BtuB (Fig. 5a) and FhuA (Supplementary Fig. 6d) both make direct contact with their ligands. As these subdomains are presumably pulled into the periplasmic space during ligand transport, it is possible that the substrate is also pulled through the receptor. Ligand-plug contacts are also present in the substrate-bound crystal structures of FecA55, HasR56, FpvA57 and FptA58, therefore, this could represent a universal feature of TBDTs. It should be noted, however, that vitamin B12 was displaced by only 3Å during both generation and equilibration of the plug-open state in the MD simulations described above (Fig. 5b). This suggests that either diffusion of substrate through the channel occurs over a longer timescale than our simulations, or that other factors play a role in import.
Overall, this work shows that, in addition to the lipid bilayer, membrane proteins can be directly mechanically gated by a protein–protein interaction. More specifically, we have shown that the TonB–Ton box interaction has mechanical properties perfectly tuned for its function: it is strong enough to facilitate the passage of a specific ligand by partially unfolding sufficient residues in the N-terminal plug subdomain to allow transport yet is weaker than the remainder of the plug domain, allowing dissociation before the integrity of the protective OM is compromised.
Methods
Protein expression and purification
TonBΔTMD was cloned from the genome of E. coli BL21 (DE3) (Agilent Technologies). Primers designed to flank the target gene sequence contained a 5′ extension on the forward primer to encode for an NdeI restriction sequence, (His)6 tag for purification and a cysteine residue for AFM surface attachment (forward primer: 5′-AATAATTAACATATGCATCACCATCACCATCACGGCTGTCATCAGGTTATTGAACTACCTGC-3′). The 5′ end of the reverse primer contained a stop codon and a XhoI recognition sequence (reverse primer: 5′-GGAATTTGACTCGAGTTACTGAATTTCGGTGGTGCCGTTA-3′). The primers for TonBCTD were 5′-AATTTACAACATATGCATCACCATCACCATCACGGATGTCCGGT TACCAGTGTGGCTTCA-3′ (forward) and 5′-GGAATTTGACTCGAGTTACTGAATTTCGGTGGTGCCGTTA-3′ (reverse). The forward primer encoded for an NdeI restriction sequence, (His)6 tag for purification and a cysteine residue for AFM surface attachment. The 5′ end of the reverse primer contained a stop codon and a XhoI recognition sequence. PCR (using vent DNA polymerase (NEB)) was used to amplify the gene, and after digestion with NdeI and XhoI, the product was ligated into pET-23a(+) digested with the same restriction endonucleases. After verification of the presence and sequence of the cloned gene by DNA sequencing, pET23a-TonB constructs were transformed into E. coli BL21 (DE3) cells and grown in LB medium (100 μg ml−1 ampicillin) at 37 °C with shaking to an optical density at 600 nm (OD600) of 0.7 before induction with 0.1% (w/v) IPTG. After 16 h of incubation at 37 °C, the cells were pelleted, resuspended in loading buffer (20 mM Tris-HCl pH 8.0, 300 mM NaCl, 20 mM imidazole, 2 mM DTT, 0.025% (w/v) sodium azide, 1 mM PMSF and 2 mM benzamide), disrupted by sonication and the insoluble material pelleted by centrifugation. The supernatant was then used to resuspend 3 ml Ni Sepharose 6 Fast Flow resin (GE Healthcare) and TonB purified (TonBΔTMD) by batch purification. After washing with loading buffer, the protein was eluted (loading buffer plus 400 mM imidazole). The imidazole was then removed by dialysis into 25 mM Tris pH 7.5, 128 mM NaCl, then further purified (TonBΔTMD) by ion-exchange chromatography (Resource S column (6 ml, GE healthcare)) using a 0–50% gradient of 1 M NaCl over four column volumes. For TonBCTD, size exclusion chromatography using a Superdex 75 26/60 gel filtration column (GE Healthcare) equilibrated with 25 mM Tris-HCl pH 7.5, 128 mM NaCl was used. The purified TonB constructs were finally dialysed into 25 mM Tris pH 7.5, 128 mM NaCl using a 5 kDa molecular weight cutoff membrane and stored at −20 °C after flash freezing in liquid nitrogen. All purification steps for TonB constructs were carried out at 4 °C in the presence of 1 mM PMSF, 2 mM benzamidine.
Labelling of TBBtuB peptide with Alexa Fluor 488
The Ton box peptide from BtuB (TBBtuB, sequence: PDTLVVTANRGSWSC (non TBBtuB residues in bold)) was fluorescently labelled with Alexa Fluor 488 C5 maleimide (ThermoFisher) by creating a 1 mg ml−1 solution of the peptide in 25 mM Tris-HCl pH 7.5, 128 mM NaCl and adding 100 μl of 10 mg ml−1 dye dissolved in DMSO in a drop-wise manner. This was then stirred for 16 h at room temperature in the dark. The reaction mixture was then loaded onto a pre-equilibrated Superdex peptide gel filtration column 10/300 (GE Healthcare) pre-equilibrated with 25 mM Tris-HCl pH 7.5, 128 mM NaCl. The chromatogram from the column contained two major peaks, the first peak was analysed by liquid chromatography electrospray ionization mass spectrometry, which confirmed the presence of the labelled peptide (predicted Mw=2303.4 Da, observed=2303.8 Da). The labelling efficiency was estimated using UV-absorbance spectroscopy.
Microscale thermophoresis (MST)
MST was carried out using a Monolith NT.115 series instrument (NanoTemper) using Alexa Fluor-488 as the fluorescent probe and a blue LED filter (excitation 460–480 nm and emission 515–530 nm). The fluorescently labelled TBBtuB peptide was adjusted to a final concentration of 500 nM (optimal fluorescence when diluted to 250 nM). TonB constructs were concentrated to 1 mM using a 3 kDa cutoff membrane Vivaspin column 500 and centrifugation at 15,000g. The concentration was determined by ultraviolet absorbance spectroscopy (280 nm) and an extinction coefficient of 5,500 M−1 cm−1 (calculated using ExPASy ProtParam tool). A serial dilution over 16 concentrations was set-up for TonB in 25 mM Tris-HCl pH 7.5, 128 mM NaCl. This dilution series was mixed with the 500 nM TBBtuB-Alexa fluor 488 1:1 (v/v) and incubated at room temperature for 5 min. The samples were then loaded into capillaries (K002 MonolithTM NT.115 standard treated capillaries) and analysed using a LED power set to 95% and MST power (IR-laser) set to 40%. The normalized fluorescence (equation (1)) from T-jump and thermophoresis (Fnorm):
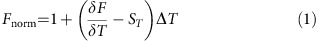 |
(where δF/δT is the fluorescence change due to temperature increase, ΔT is the change in temperature and ST is the Soret coefficient) was then plot against the log10 of the protein concentration and the data were fitted using the Nanotemper analysis software using the law of mass action:
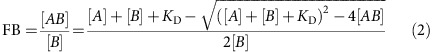 |
where FB is the fraction of bound B. Fnorm from the MST experiment yields the FB59.
AFM cantilever derivatization
Silicon nitride AFM probes (MLCT with reflective gold, Bruker) and surfaces (1 cm2 cut from a silicon nitride disc (Rockwood electronic material)) were oxidized by submersion for 30 s in piranha solution (0.5 M (>95% (v/v) H2SO4 and 30% (v/v) H2O2 mixed 3:1 v-v) followed by washing with excess distilled and de-ionized H2O and drying with a nitrogen flow. The surfaces and AFM probes were then placed under a UV lamp (UVIlite, UVItec) set to 254 nm for 30 min before being transferred into a desiccator along with 80 μl of (3-aminopropyl)triethoxysilane (APTES) and 20 μl of N,-N-diisopropylethylamine held in separate 1.5 ml Eppendorf tube lids. The desiccator was evacuated using a vacuum pump and left to incubate at room temperature for 2 h. After the incubation, the APTES and N,-N-diisopropylethylamine solutions were removed and the derivatized probes/surfaces allowed to cure in an N2 atmosphere for 48 h. The amino-silanized AFM probes and surfaces were then immersed in 1 ml chloroform containing 20 μl of 250 mM NHS-PEG24-maleimide, 250 mM NHS-PEG24-methyl (1:9 ratio in DMSO) and left to incubate at room temperature for 1 h.
Both AFM probes and surfaces were then washed with chloroform and dried under a stream of nitrogen gas. Protein (1 mg ml−1) and peptide (1 mg ml−1) both containing an engineered cysteine residue were deposited over the surfaces and AFM probes and left to incubate in a covered container for 30 min at room temperature. For TonB–TBBtuB dissociation, the peptide was attached to the AFM probe and TonB to the derivatized surface. For TonB–TBDT dissociation, TonB was attached to the AFM probe and the TBDT (inserted into a lipid bilayer) adsorbed to mica (see below). Unreacted protein/oligopeptide was then washed from the surface and AFM probe with buffer (25 mM Tris-HCl, pH 8.0 128 mM NaCl).
Force spectroscopy
The derivatized AFM probe (Bruker MCLT (nominal spring constant: 30 pN nm−1)) was mounted on to a MFP-3D head (Asylum Research) and calibrated using the thermal method60. Force maps of 20 μm2 with 600 approach-retract cycles (starting 1 μm from the substrate surface) were taken to ensure a good coverage of the surface. The approach velocity was kept constant (1 μm s−1) for all experiments while the retraction velocity was varied (0.2–5 μm s−1, see main text). All experiments were conducted in 25 mM Tris-HCl pH 8.0, 128 mM NaCl at 25 °C. All experiments (that is, for each protein variant and/or retraction velocity) were performed at least in triplicate (using a freshly prepared cantilever for each repeat). After filtering and analysis (see below) using IGOR pro 6.32A with an Asylum Research extension (MFP3DXopv30), single Gaussian distributions were generated and fit to unbinding force and contour length histograms in order to determine the most probable force and contour length at rupture for each retraction velocity.
Force curve analysis
Generally less than 1 in 10 approach-retract cycles resulted in a true unbinding event (average hit rates ranging from 5–10%). When a cognate complex is extended via the PEG linkers, there is a large change in the force response and hence a peak in the observed rate of change of the force response. To ensure sufficient surface sampling, 20 μm2 force maps with 600 approach-retract cycles were taken, and the cantilever then repositioned to a new area. A typical data set at a single retraction velocity contained 2–4 force maps, depending on the hit rate. All detected events were then manually fitted to the WLC model30 (equation (3)), where the persistence length was fixed (0.4 nm)61, the hard contact was used to identify the zero distance and the retraction baseline was used to zero the force.
Detected events for tip-sample separation values of less than 10 nm were in general found to be consistent with non-specific tip-sample interactions and displayed a linear force–distance profile. Force–extension profiles were binned for analysis if: (1) the data fitted to the WLC model (single-molecule events should display WLC like behaviour where the force–distance profile is not linear) and (2) the contour length was greater than the length of the PEG24 linkers used. For the TonBΔTMD–TBBtuB experiments, fewer than 0.1% of force curves showed more than one event. This indicates that there are no detectable unfolding events for either protein domain before dissociation of the bound complex.
Loading rates were calculated by fitting a WLC model to the rising edge of each unbinding profile when plotted as force versus tip-sample separation. The instantaneous gradient of this fit at rupture (WLCslope) was calculated by inserting the derived contour length and extension at rupture into a differentiated form of the same equation (equation (4)). The loading rate at rupture was then obtained by multiplying this value by the retraction velocity.
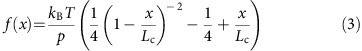 |
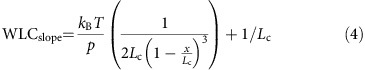 |
where f is the force acting on the WLC, p is the persistence length, Lc is the contour length, x is the extension, kB is Boltzmann's constant and T is the temperature.
Estimation of the contour length
The contour length estimation of the unstructured linker domain of TonBΔTMD was made on the basis of the known length of the PEG24 linkers and the known length of TonBCTD in complex to TBBtuB (PDB: 2GSK), with any additional length observed due to the proline-rich (PR) domain (Supplementary Fig. 2b). The length of a stretched amino acid is 0.4 nm; therefore, if the PR domain (118 amino acids) behaved as an unstructured polypeptide chain, the expected length would be 47.2 nm giving a total contour length at detachment of ∼72 nm. For the TonBCTD construct (lacking the PR domain), the expected length would be ∼24.5 nm.
Purification of BtuB and FhuA
pNGH015 (a pBAD expression plasmid encoding wild-type BtuB, a gift from Professor C. Kleanthous, University of Oxford) or pBAD-FhuA (see below) was transformed into E. coli TNE012 (K12 tsx−ompA−ompB−) cells (also provided by C. Kleanthous) and a single colony then used to inoculate LB medium (100 μg ml−1 ampicillin). The cells were cultured at 37 °C with shaking at 200 r.p.m. to an OD600=0.7 and then induced with 0.15% (w/v) L-(+)-arabinose. After induction, the temperature was decreased to 15 °C and incubated for a further 16 h. The cells were collected by centrifugation(9500g at 4 °C), resuspended in 50 ml 10 g−1 buffer (10 mM Tris-HCl, pH 8.0, 0.25% (w/v) lithium diiodosalicylic acid), disrupted by sonication (on 3 s, off 7 s for total process time of 4 min) and the insoluble material removed by centrifugation (10,000g at 4 °C). The supernatant (containing membranes) was subjected to ultra-centrifugation (158,000g) to collect the total membrane fraction. The membrane pellet was resuspended in 50 ml 10 mM Tris-HCl, pH 8.0, 0.25% (w/v) LIS, 2% (v/v) Triton X-100, homogenized by pipetting and then ultracentrifuged at 158,000g for 1 h at 4 °C. The supernatant was discarded and this step was repeated. The pellet was then resuspend in 56 ml BtuB purification buffer (10 mM Tris-HCl, pH 8.0), homogenized and ultracentrifuged at 158,000g for 1 h, 4 °C and the supernatant was discarded. The pellet was resuspend in 56 ml 10 mM Tris-HCl, pH 8.0, 1% (w/v) n-octyl-β-D-glucopyranoside (β-OG), 5 mM EDTA, homogenized thoroughly using a hand homogenizer and ultracentrifuged at 158,000g for 1 h, 4 °C. The supernatant containing the extracted OM proteins was then loaded onto a 5 ml DEAE-Sepharose column (GE Healthcare) pre-equilibrated in 90% buffer A (50 mM Tris-HCl, pH 7.5, 5 mM EDTA, 0.54% (w/v) β-OG), and 10% buffer B (50 mM Tris-HCl, pH 7.5, 5 mM EDTA, 1 M LiCl, 0.54% (w/v) β-OG) at room temperature using a AKTA prime plus (GE Healthcare). The OM proteins bound to the column were initially washed with 40 ml 90% buffer A, 10% buffer B. A gradient over 65 ml of 10–50% buffer B was then run to remove impurities. Finally, 30 ml of 100% buffer B was run through the column, which causes the target protein to elute from the column. Shortly after elution from the column, pure BtuB (and its variants) forms a white precipitate, which was dissolved into 1% (w/v) β-OG. By contrast to BtuB, FhuA eluted at 22% 1 M LiCl with impurities. A white precipitate formed after elution and was found to contain FhuA by SDS–PAGE, which was isolated and then solubilized in 1% (v/w) β-OG. A Superdex 75 10/300 GL column was used to further purify FhuA in 25 mM Tris-HCl pH 8.0, 1% (w/v) β-OG. The major peak eluted from this column was identified as FhuA by SDS–PAGE and circular dichroism spectroscopy and fluorescence spectroscopy was used to confirm the correct folding.
Force spectroscopy of TBDTs in lipid membranes
Using the method described in ref. 53, TonB-dependent receptors were inserted into a lipid bilayer at a high density by solubilizing the precipitated receptor (0.1 mg) in 200 μl 25 mM Tris-HCl, pH 7.5, 128 mM NaCl, 1% (w/v) β-OG and dialysing in the presence of lipids (0.2 mg) (E. coli polar lipid extract (Avanti)) dried from a chloroform stock (10 mg ml−1) using a nitrogen flow. The ligand for the receptor (cyanocobalamin (Sigma) and ferrichrome (EMC Microcollections) for BtuB and FhuA, respectively) was added to the buffer at a 100-fold molar excess of the receptor. The dried lipids were then mixed with the protein/ligand detergent solution and lipid was solubilized by pipetting. The components were then placed into a 200 μl 12–14 kDa MWCO dialysis bag (D-tube dialyser mini, Merck Millipore) and dialysed against 300 ml of detergent-free buffer (10 mM Tris-HCl, pH 7.5, 300 mM KCl, 400 μM CaCl2, 0.01% (w/v) NaN3) for 7 days at room temperature with daily buffer changes. The sodium azide was omitted for the last dialysis stage. The proteoliposomes were stored at 4 °C. Vesicle formation was confirmed using dynamic light scattering and the presence of TBDT by SDS–PAGE.
To immobilize TBDT-containing liposomes for study by AFM, the vesicles were resuspended by pipetting and then diluted to a final lipid concentration of 0.1 mg ml−1. An aliquot of 200 μl was pooled over a 1 cm diameter disc of freshly cleaved mica and incubated at room temperature for 30 min. The surface was then thoroughly rinsed with 25 mM Tris-HCl pH 8.0, 128 mM NaCl. Force spectroscopy experiments were carried out as described above, using a cantilever derivatized with TonBΔTMD-NHS-PEG24-maleimide, a contact force of 90–120 pN, a dwell of 1 s and a retraction velocity of 1,000 nms−1 over 600 nm unless stated otherwise.
Generation of BtuB variants and cloning of FhuA
Site-directed mutagenesis was performed using the Q5 Site-Directed Mutagenesis kit (NEB). Primers were designed and purchased from Eurofins MWG Operon (Supplementary Table 6). Sequencing was used to confirm the success of the mutagenesis. Wild-type FhuA was cloned from the genome of E. coli JM109 cells (Agilent Technologies) via PCR (Q5 polymerase (NEB)) using the following primers: 5′-CATTAATCACCATGGCGCGTTCCAAAACTGC-3′ (forward) and 5′-TTGAATATTCTCGAGTTAGAAACGGAAGGTTGCGGTTG-3′ (reverse). The amplified product was then inserted into the pBAD vector used for BtuB expression by digesting with NcoI and XhoI restriction enzymes. After ligation (T4 ligase (NEB)), the pBAD-FhuA vector was transformed into TNE012 (K12 tsx−ompA−ompB−) cells and protein expression was induced with arabinose using the same protocol as described for BtuB.
Molecular dynamics simulations
MD simulations were performed using NAMD (v2.10)62 and the CHARMM27 force field63. System preparation was performed using VMD (v1.9.2)64. 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC) was used to model the lipid bilayer, the parameters for vitamin B12 were obtained from ref. 65, whereas the TIP3P force field was used for water66. The X-ray crystal structure of BtuB in complex with TonB and vitamin B12 (PDB 2GSK), including calcium ions and crystal waters, was inserted into the homogenous POPC lipid bilayer placed in the XY plane. The dimensions of the simulation box were 100 × 100 × 300 Å (Supplementary Fig. 4a). A total of 100,211 molecules of TIP3P water, 149 Na+ and 141 Ca2+ were then used to fill the simulation box. The total system comprised 342, 843 atoms. The simulations were conducted using periodic boundary conditions. The bonds between hydrogen and heavy atoms were constrained with SHAKE67. The r-RESPA multiple time step method68 was employed with 2 fs for bonded potentials, 2 fs for short-range non-bonded potentials and 4 fs for long-range non-bonded potentials62. Long-range electrostatic interactions were treated with the particle-mesh-Ewald method69. The distance cutoff for non-bonded interactions was set to 10 Å, and a switching function was applied to smooth interactions between 9 and 10 Å. All simulations were conducted in the NPT ensemble. The temperature was set to 300 K and regulated via a Langevin thermostat70; the pressure was set to 1 atm and regulated via an isotropic Langevin piston manostat71.
A multistage equilibration was used to relax the system. Initially all of the atoms except water and ions were fixed. Initially, 2,000 steps of conjugate gradient geometry optimization were conducted. After energy minimization, the system was simulated for 1 ns to equilibrate the solvent around the protein and membrane. Next, the membrane was relaxed around the protein by removing the restraints on the fatty acid tails for 1 ns, and keeping the protein and vitamin B12 atoms restrained to their initial positions by applying an harmonic potential on the heavy atoms using a force constant of 1 kcal (mol Å2)−1. The protein and ligand was then gradually released over the next 3 ns by decreasing the force constant to 0.5 kcal (mol Å2)−1. Finally, all restraints were removed and the system was equilibrated for 10 ns (Supplementary Fig. 4b). The final structure from the unrestrained MD trajectory was used as the starting point in the pulling simulations (Supplementary Fig. 4a). The pulling was performed by using the steered molecular simulation (SMD) module as implemented in NAMD62. The SMD simulation was performed by restraining the phosphate head groups of the upper bilayer leaflet to its initial coordinates (to prevent the membrane being dragged) and extending a harmonic spring attached to either the Ton box of BtuB (Pro5 Cα) or the centre of mass of TonB (all Cα residues of TonB). The spring constant was set to 0.5 kcal (mol Å2)−1, while the pulling velocity was set to 2.5 Å ns−1 along the z-direction.
BtuB methionine phenotype assay
E. coli RK5016 cells72 (MC4100, metE70, argH, btuB, recA, a gift from Professor C. Kleanthous, University of Oxford) freshly transformed with pAG1 encoding wild-type btuB or its variants were streaked onto agar plates containing Davis and Mingioli minimal medium supplemented with 5 μg ml−1 arginine, 1 μg ml−1 thiamine, 100 μg ml−1 ampicillin, 25 μg ml−1 streptomycin and various concentrations of vitamin B12 (0.1, 1, 10, 100 nM) or 5 μg ml−1 methionine. These were incubated at 37 °C for 48 h before analysis of colony size. Full confluent growth (thus functional BtuB) is indicated by large colonies (diameter 1.5–2 mm) on the vitamin B12-supplemented plates that are comparable with the methionine-supplemented control (Supplementary Fig. 5).
Bacitracin sensitivity assay
Single colonies of RK5016 cells transformed with pAG1 encoding wild-type btuB or its variants were used to inoculate 96-well plate wells containing 200 μl LB supplemented with ampicillin (100 μg ml−1), streptomycin (25 μg ml−1), 200 μg ml−1 bacitracin and for a control where TonB activity is prevented, 200 μg ml−1 Ton box pentapeptide (ETVIV). The plates (Corning Costar assay plate, 96-well, black with clear flat bottom, non-treated, 1 cm path length) were incubated at 37 °C with 200 r.p.m. shaking for 24 h and the absorbance at 600 nm measured every 4 min (CLARIOstar high-performance monochromator multimode microplate reader (BMG LABTECH)).
Data availability
The authors declare that the data supporting the findings of this study are available within the article and its Supplementary Information files and from the corresponding author upon reasonable request. The following PDB structures with accession codes 2GSK, 1NQE and 1XX3 were used in this work.
Additional information
How to cite this article: Hickman, S. J. et al. Gating of TonB-dependent transporters by substrate-specific forced remodelling. Nat. Commun. 8, 14804 doi: 10.1038/ncomms14804 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Material
Supplementary Figures, Supplementary Tables.
Acknowledgments
We thank Dr Nicholas Housden and Professor Colin Kleanthous (University of Oxford) for providing plasmids pNGH015, pAG1 and E. coli strains RK5016 and TNE012 together with advice on purifying BtuB. Thanks to Dr James Gumbart (Georgia Tech) for providing the vitamin B12 parameters in a usable format. We also thank Professor Sheena Radford and members of the Brockwell/Radford groups for useful discussions. This work was supported by the Wellcome Trust Grant 099753/Z/12/Z (to S.J.H.).
Footnotes
The authors declare no competing financial interests.
Author contributions S.J.H. designed and performed all experiments and analysis, R.E.M.C. created and purified the BtuB3A mutant, L.B. set up the MD simulation system, E.P. coordinated the MD simulation experiments, D.J.B. coordinated the entire study, designed experiments and wrote the manuscript with S.J.H.
References
- Koebnik R., Locher K. P. & Van Gelder P. Structure and function of bacterial outer membrane proteins: barrels in a nutshell. Mol. Microbiol. 37, 239–253 (2000). [DOI] [PubMed] [Google Scholar]
- Postle K. & Kadner R. J. Touch and go: tying TonB to transport. Mol. Microbiol. 49, 869–882 (2003). [DOI] [PubMed] [Google Scholar]
- Noinaj N., Guillier M., Barnard T. J. & Buchanan S. K. TonB-dependent transporters: regulation, structure, and function. Ann. Rev. Microbiol. 64, 43–60 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krewulak K. D. & Vogel H. J. TonB or not TonB: is that the question? Biochem. Cell Biol. 89, 87–97 (2011). [DOI] [PubMed] [Google Scholar]
- Schauer K., Rodionov D. A. & de Reuse H. New substrates for TonB-dependent transport: do we only see the ‘tip of the iceberg'? Trends Biochem. Sci. 33, 330–338 (2008). [DOI] [PubMed] [Google Scholar]
- Garcia E. C., Brumbaugh A. R. & Mobley H. L. Redundancy and specificity of Escherichia coli iron acquisition systems during urinary tract infection. Infect. Immun. 79, 1225–1235 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins-Balding D., Ratliff-Griffin M. & Stojiljkovic I. Iron transport systems in Neisseria meningitidis. Microbiol. Mol. Biol. Rev. 68, 154–171 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cascales E. et al. Colicin biology. Microbiol. Mol. Biol. Rev. 71, 158–229 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundrigan M. D. & Kadner R. J. Nucleotide-sequence of the gene for the ferrienterochelin receptor FepA in Escherichia coli—homology among outer-membrane receptors that interact with TonB. J. Biol. Chem. 261, 797–801 (1986). [PubMed] [Google Scholar]
- Fanucci G. E., Cadieux N., Kadner R. J. & Cafiso D. S. Competing ligands stabilize alternate conformations of the energy coupling motif of a TonB-dependent outer-membrane transporter. Proc. Natl Acad. Sci. USA 100, 11382–11387 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanucci G. E., Lee J. Y. & Cafiso D. S. Spectroscopic evidence that osmolytes used in crystallization buffers inhibit a conformation change in a membrane protein. Biochemistry 42, 13106–13112 (2003). [DOI] [PubMed] [Google Scholar]
- Shultis D. D., Purdy M. D., Banchs C. N. & Wiener M. C. Outer-membrane active transport: structure of the BtuB:TonB complex. Science 312, 1396–1399 (2006). [DOI] [PubMed] [Google Scholar]
- Pawelek P. D. et al. Structure of TonB in complex with FhuA, E. coli outer-membrane receptor. Science 312, 1399–1402 (2006). [DOI] [PubMed] [Google Scholar]
- Kohler S. D., Weber A., Howard S. P., Welte W. & Drescher M. The proline-rich domain of TonB possesses an extended polyproline II-like conformation of sufficient length to span the periplasm of Gram-negative bacteria. Protein Sci. 19, 625–630 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celia H. et al. Structural insight into the role of the Ton complex in energy transduction. Nature 538, 60–65 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer E., Gunter K. & Braun V. Involvement of ExbB and TonB in transport across the outer-membrane of Escherichia coli—phenotypic complementation of Exb mutants by overexpressed TonB and physical stabilization of TonB by ExbB. J. Bacteriol. 171, 5127–5134 (1989). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jana B., Manning M. & Postle K. Mutations in the ExbB cytoplasmic carboxy terminus prevent energy-dependent interaction between the TonB and ExbD periplasmic domains. J. Bacteriol. 193, 5649–5657 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun V. et al. Energy-coupled transport across the outer membrane of Escherichia coli: ExbB binds ExbD and TonB in vitro, and leucine 132 in the periplasmic region and aspartate 25 in the transmembrane region are important for ExbD activity. J. Bacteriol. 178, 2836–2845 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson M., Hannavy K. & Higgins C. F. A sequence-specific function for the N-terminal signal-like sequence of the TonB protein. Mol. Microbiol. 8, 379–388 (1993). [DOI] [PubMed] [Google Scholar]
- Braun V. FhuA (TonA) the career of a protein. J. Bacteriol. 191, 3431–3436 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chimento D. P., Kadner R. J. & Wiener M. C. Comparative structural analysis of TonB-dependent outer membrane transporters: implications for the transport cycle. Proteins 59, 240–251 (2005). [DOI] [PubMed] [Google Scholar]
- Gumbart J., Wiener M. C. & Tajkhorshid E. Mechanics of force propagation in TonB-dependent outer membrane transport. Biophys. J. 93, 496–504 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klebba P. E. ROSET model of TonB action in Gram-negative bacterial iron acquisition. J. Bacteriol. 198, 1013–1021 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Z. H., Warfel P., Newton S. M. C. & Klebba P. E. Spectroscopic observations of ferric enterobactin transport. J. Biol. Chem. 278, 1022–1028 (2003). [DOI] [PubMed] [Google Scholar]
- Newton S. M., Trinh V., Pi H. L. & Klebba P. E. Direct measurements of the outer-membrane stage of ferric enterobactin transport postuptake binding. J. Biol. Chem. 285, 17488–17497 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradbeer C. The proton motive force drives the outer-membrane transport of cobalamin in Escherichia coli. J. Bacteriol. 175, 3146–3150 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockwell D. J. et al. Pulling geometry defines the mechanical resistance of a beta-sheet protein. Nat. Struct. Biol. 10, 731–737 (2003). [DOI] [PubMed] [Google Scholar]
- Chen Y., Radford S. E. & Brockwell D. J. Force-induced remodelling of proteins and their complexes. Curr. Opin. Struct. Biol. 30, 89–99 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peacock R. S., Weljie A. M., Howard S. P., Price F. D. & Vogel H. J. The solution structure of the C-terminal domain of TonB and interaction studies with TonB box peptides. J. Mol. Biol. 345, 1185–1197 (2005). [DOI] [PubMed] [Google Scholar]
- Bustamante C., Marko J. F., Siggia E. D. & Smith S. Entropic elasticity of lambda-phage DNA. Science 265, 1599–1600 (1994). [DOI] [PubMed] [Google Scholar]
- Cadieux N., Bradbeer C. & Kadner R. J. Sequence changes in the Ton box region of BtuB affect its transport activities and interaction with TonB protein. J. Bacteriol. 182, 5954–5961 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans E. & Ritchie K. Dynamic strength of molecular adhesion bonds. Biophys. J. 72, 1541–1555 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrance O. E. et al. A force-activated trip switch triggers rapid dissociation of a colicin from its immunity protein. PLoS Biol. 11, e1001489 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwesinger F. et al. Unbinding forces of single antibody-antigen complexes correlate with their thermal dissociation rates. Proc. Natl Acad. Sci. USA 97, 9972–9977 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crampton N. & Brockwell D. J. Unravelling the design principles for single protein mechanical strength. Curr. Opin. Struct. Biol. 20, 508–517 (2010). [DOI] [PubMed] [Google Scholar]
- Hoffmann T., Tych K. M., Hughes M. L., Brockwell D. J. & Dougan L. Towards design principles for determining the mechanical stability of proteins. Phys. Chem. Chem. Phys. 15, 15767–15780 (2013). [DOI] [PubMed] [Google Scholar]
- Noinaj N. et al. Structural basis for iron piracy by pathogenic Neisseria. Nature 483, 53–58 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller K., Mann B. J. & Kadner R. J. Cloning and expression of the gene for the vitamin B12 receptor protein in the outer-membrane of Escherichia coli. J. Bacteriol. 161, 896–903 (1985). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudmundsdottir A., Bell P. E., Lundrigan M. D., Bradbeer C. & Kadner R. J. Point mutations in a conserved region (TonB box) of Escherichia coli outer-membrane protein BtuB affect vitamin B12 transport. J. Bacteriol. 171, 6526–6533 (1989). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toscano W. A. Jr & Storm D. R. Bacitracin. Pharmacol. Ther. 16, 199–210 (1982). [DOI] [PubMed] [Google Scholar]
- Killmann H., Braun M., Herrmann C. & Braun V. FhuA barrel-cork hybrids are active transporters and receptors. J. Bacteriol. 183, 3476–3487 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuckman M. & Osburne M. S. In vivo inhibition of TonB-dependent processes by a TonB box consensus pentapeptide. J. Bacteriol. 174, 320–323 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silhavy T. J., Kahne D. & Walker S. The bacterial cell envelope. Cold Spring Harb. Perspect. Biol. 2, a000414 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sochacki K. A., Shkel I. A., Record M. T. & Weisshaar J. C. Protein diffusion in the periplasm of E. coli under osmotic stress. Biophys. J. 100, 22–31 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullineaux C. W., Nenninger A., Ray N. & Robinson C. Diffusion of green fluorescent protein in three cell environments in Escherichia coli. J. Bacteriol. 188, 3442–3448 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans J. S., Levine B. A., Trayer I. P., Dorman C. J. & Higgins C. F. Sequence-imposed structural constraints in the TonB protein of Escherichia coli. FEBS Lett. 208, 211–216 (1986). [DOI] [PubMed] [Google Scholar]
- Jordan L. D. et al. Energy-dependent motion of TonB in the Gram-negative bacterial inner membrane. Proc. Natl Acad. Sci. USA 110, 11553–11558 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devanathan S. & Postle K. Studies on colicin B translocation: FepA is gated by TonB. Mol. Microbiol. 65, 441–453 (2007). [DOI] [PubMed] [Google Scholar]
- Endriss F., Braun M., Killmann H. & Braun V. Mutant analysis of the Escherichia coli FhuA protein reveals sites of FhuA activity. J. Bacteriol. 185, 4683–4692 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenhauer H. A., Shames S., Pawelek P. D. & Coulton J. W. Siderophore transport through Escherichia coli outer membrane receptor FhuA with disulfide-tethered cork and barrel domains. J. Biol. Chem. 280, 30574–30580 (2005). [DOI] [PubMed] [Google Scholar]
- Jimenez R. H. F. & Cafiso D. S. The N-terminal domain of a TonB-dependent transporter undergoes a reversible stepwise denaturation. Biochemistry 51, 3642–3650 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udho E., Jakes K. S. & Finkelstein A. TonB-dependent transporter FhuA in planar lipid bilayers: partial exit of its plug from the barrel. Biochemistry 51, 6753–6759 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoma J., Bosshart P., Pfreundschuh M. & Muller D. J. Out but not in: the large transmembrane beta-barrel protein FhuA unfolds but cannot refold via beta-hairpins. Structure 20, 2185–2190 (2012). [DOI] [PubMed] [Google Scholar]
- Ferguson A. D. et al. Signal transduction pathway of TonB-dependent transporters. Proc. Natl Acad. Sci. USA 104, 513–518 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson A. D. et al. Structural basis of gating by the outer membrane transporter FecA. Science 295, 1715–1719 (2002). [DOI] [PubMed] [Google Scholar]
- Krieg S. et al. Heme uptake across the outer membrane as revealed by crystal structures of the receptor-hemophore complex. Proc. Natl Acad. Sci. USA 106, 1045–1050 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobessi D. et al. The crystal structure of the pyoverdine outer membrane receptor FpvA from Pseudomonas aeruginosa at 3.6 Å resolution. J. Mol. Biol. 347, 121–134 (2005). [DOI] [PubMed] [Google Scholar]
- Cobessi D., Celia H. & Pattus F. Crystal structure at high resolution of ferric-pyochelin and its membrane receptor FptA from Pseudomonas aeruginosa. J. Mol. Biol. 352, 893–904 (2005). [DOI] [PubMed] [Google Scholar]
- Seidel S. A. et al. Microscale thermophoresis quantifies biomolecular interactions under previously challenging conditions. Methods 59, 301–315 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutter J. L. & Bechhoefer J. Calibration of atomic-force microscope tips. Rev. Sci. Instrum. 64, 1868–1873 (1993). [Google Scholar]
- Farrance O. E., Paci E., Radford S. E. & Brockwell D. J. Extraction of accurate biomolecular parameters from single-molecule force spectroscopy experiments. ACS Nano 9, 1315–1324 (2015). [DOI] [PubMed] [Google Scholar]
- Phillips J. C. et al. Scalable molecular dynamics with NAMD. J. Comput. Chem. 26, 1781–1802 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKerell A. D. et al. All-atom empirical potential for molecular modeling and dynamics studies of proteins. J. Phys. Chem. B 102, 3586–3616 (1998). [DOI] [PubMed] [Google Scholar]
- Humphrey W., Dalke A. & Schulten K. VMD: visual molecular dynamics. J. Mol. Graph. Model. 14, 33–38 (1996). [DOI] [PubMed] [Google Scholar]
- Gumbart J., Wiener M. C. & Tajkhorshid E. Coupling of calcium and substrate binding through loop alignment in the outer-membrane transporter BtuB. J. Mol. Biol. 393, 1129–1142 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen W. L., Chandrasekhar J., Madura J. D., Impey R. W. & Klein M. L. Comparison of simple potential functions for simulating liquid water. J. Chem. Phys. 79, 926–935 (1983). [Google Scholar]
- Ryckaert J. P., Ciccotti G. & Berendsen H. J. C. Numerical-integration of Cartesian equations of motion of a system with constraints—molecular-dynamics of N-alkanes. J. Comput. Phys. 23, 327–341 (1977). [Google Scholar]
- Tuckerman M., Berne B. J. & Martyna G. J. Reversible multiple time scale molecular-dynamics. J. Chem. Phys. 97, 1990–2001 (1992). [Google Scholar]
- Essmann U. et al. A smooth particle mesh Ewald method. J. Chem. Phys. 103, 8577–8593 (1995). [Google Scholar]
- Grest G. S. & Kremer K. Molecular-dynamics simulation for polymers in the presence of a heat bath. Phys. Rev. A 33, 3628–3631 (1986). [DOI] [PubMed] [Google Scholar]
- Feller S. E., Zhang Y. H., Pastor R. W. & Brooks B. R. Constant-pressure molecular-dynamics simulation—the Langevin piston method. J. Chem. Phys. 103, 4613–4621 (1995). [Google Scholar]
- Cadieux N. & Kadner R. J. Site-directed disulfide bonding reveals an interaction site between energy-coupling protein TonB and BtuB, the outer membrane cobalamin transporter. Proc. Natl Acad. Sci. USA 96, 10673–10678 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figures, Supplementary Tables.
Data Availability Statement
The authors declare that the data supporting the findings of this study are available within the article and its Supplementary Information files and from the corresponding author upon reasonable request. The following PDB structures with accession codes 2GSK, 1NQE and 1XX3 were used in this work.