Abstract
Successive leaf sections from the base to the tip of rapidly developing leaves of 7-day-old maize (Zea mays var. Kelvedon Glory), grown in the light, utilized acetate for fatty acid biosynthesis in a very divergent manner. Basal regions of the leaf containing proplastids synthesized insignificant proportions of unsaturated fatty acids and appreciable proportions of fatty acids with 20 or more carbon atoms. An increase in the light intensity during incubations with acetate-1-14C resulted in very little enhancement of either total or polyunsaturated fatty acid biosynthesis in this tissue.
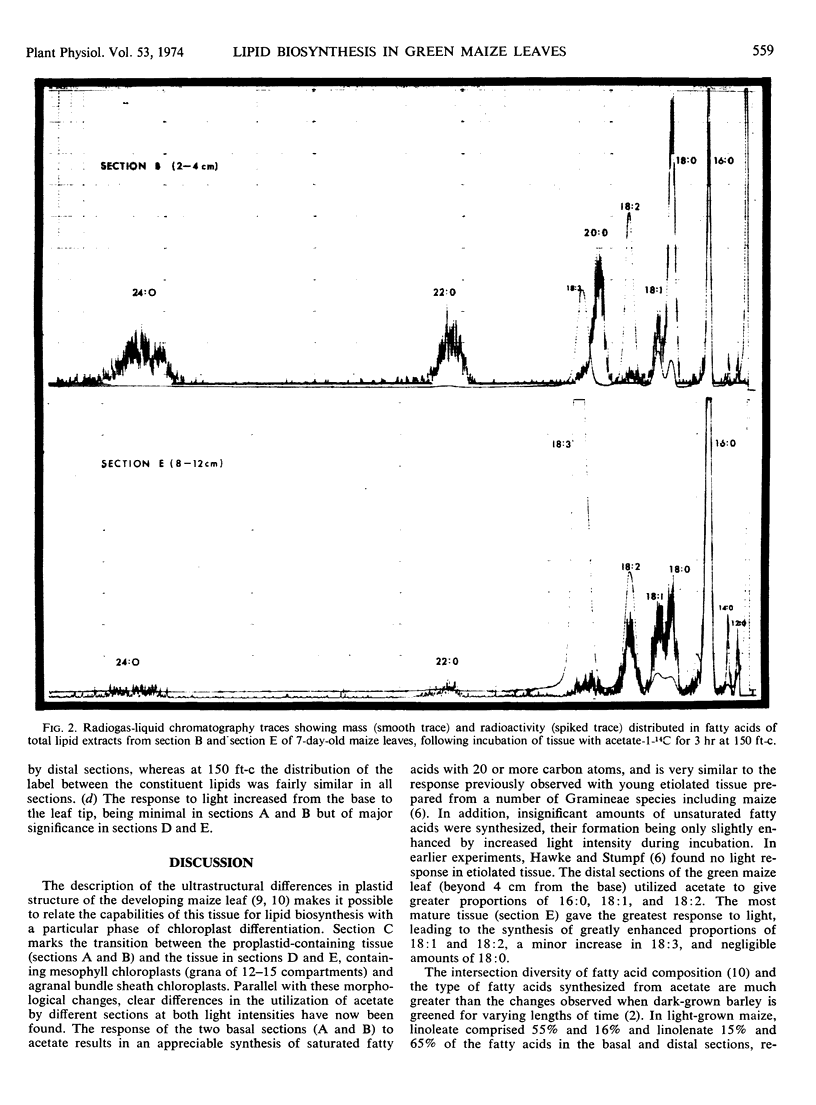
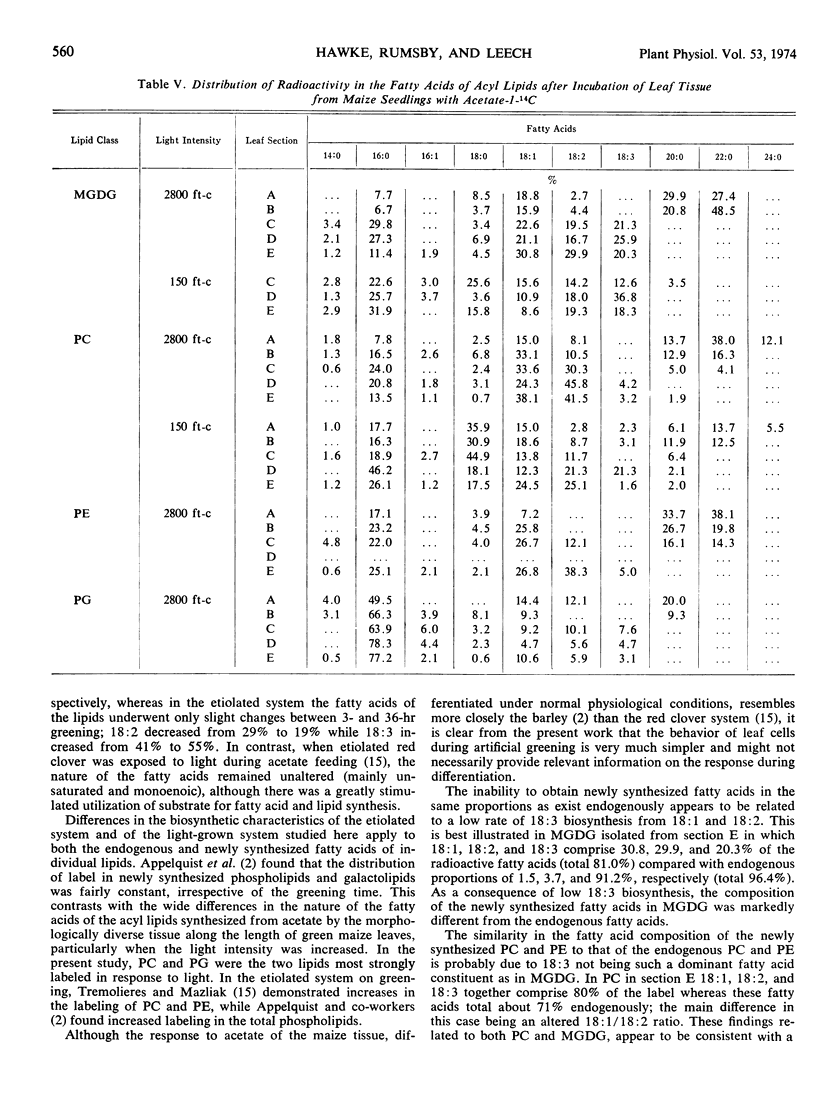
When the distal leaf sections, containing mesophyll chloroplasts with well developed grana and bundle sheath chloroplasts without grana, were incubated with acetate at 150 ft-c and 30 C, approximately 30% of the newly synthesized fatty acids were unsaturated (mainly 18: 1 and 18: 2). At 2800 ft-c and 20 C, 60% of the fatty acids were unsaturated and the total synthesis of fatty acids increased 4-fold. No detectable amount of fatty acids with 20 or more carbon atoms were synthesized in this morphologically mature tissue, and the proportions of newly synthesized fatty acids more closely resembled the endogenous fatty acids in the immature tissue.
Only 4% of the newly synthesized fatty acids were 18: 3 but most of this was incorporated into monogalactolipid. In the distal sections, 20 to 25% of the newly synthesized fatty acids in monogalactolipid were 18: 3 compared with the endogenous proportions of 85%. The differences in the composition of the newly synthesized fatty acids and the endogenous fatty acids appear to be related very largely to the low rate of 18: 3 biosynthesis from 18: 1 and 18: 2. Phosphatidyl choline and phosphatidyl ethanolamine, with lower proportions of 18: 3, contained radioactive fatty acids which resembled the endogenous composition more closely.
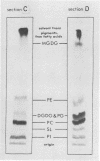
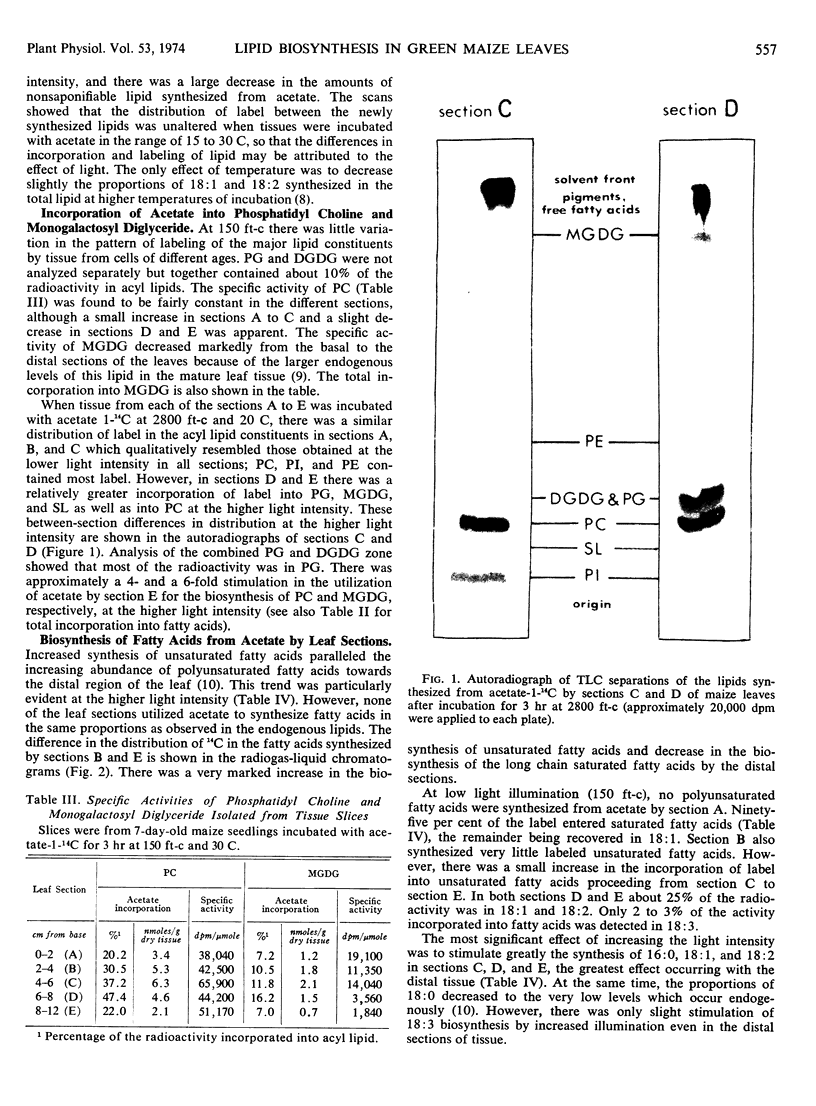
Phosphatidyl choline was quantitatively the most important acyl lipid synthesized under both light conditions. In addition, there was considerable stimulation of acetate incorporation into phosphatidyl glycerol and monogalactolipid, especially in the morphologically most mature regions of the tissue at the higher light intensity.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ABRAMSON D., BLECHER M. QUANTITATIVE TWO-DIMENSIONAL THIN-LAYER CHROMATOGRAPHY OF NATURALLY OCCURRING PHOSPHOLIPIDS. J Lipid Res. 1964 Oct;5:628–631. [PubMed] [Google Scholar]
- Appelqvist L. A., Boynton J. E., Stumpf P. K., von Wettstein D. Lipid biosynthesis in relation to chloroplast development in barley. J Lipid Res. 1968 Jul;9(4):425–436. [PubMed] [Google Scholar]
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
- Gurr M. I., Robinson M. P., James A. T. The mechanism of formation of polyunsaturated fatty acids by photosynthetic tissue. The tight coupling of oleate desaturation with phospholipid synthesis in Chlorella vulgaris. Eur J Biochem. 1969 May 1;9(1):70–78. doi: 10.1111/j.1432-1033.1969.tb00577.x. [DOI] [PubMed] [Google Scholar]
- Hawke J. C., Stumpf P. K. Fat metabolism in higher plants XXVII. Synthesis of long-chain fatty acids by preparations of Hordeum vulgare L. and other graminae. Plant Physiol. 1965 Nov;40(6):1023–1032. doi: 10.1104/pp.40.6.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuiper P. J. Lipids in alfalfa leaves in relation to cold hardiness. Plant Physiol. 1970 Jun;45(6):684–686. doi: 10.1104/pp.45.6.684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leech R. M., Rumsby M. G., Thomson W. W. Plastid differentiation, acyl lipid, and Fatty Acid changes in developing green maize leaves. Plant Physiol. 1973 Sep;52(3):240–245. doi: 10.1104/pp.52.3.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MORRISON W. R., SMITH L. M. PREPARATION OF FATTY ACID METHYL ESTERS AND DIMETHYLACETALS FROM LIPIDS WITH BORON FLUORIDE--METHANOL. J Lipid Res. 1964 Oct;5:600–608. [PubMed] [Google Scholar]
- Roughan P. G. Turnover of the glycerolipids of pumpkin leaves. The importence of phosphatidylcholine. Biochem J. 1970 Mar;117(1):1–8. doi: 10.1042/bj1170001. [DOI] [PMC free article] [PubMed] [Google Scholar]