Abstract
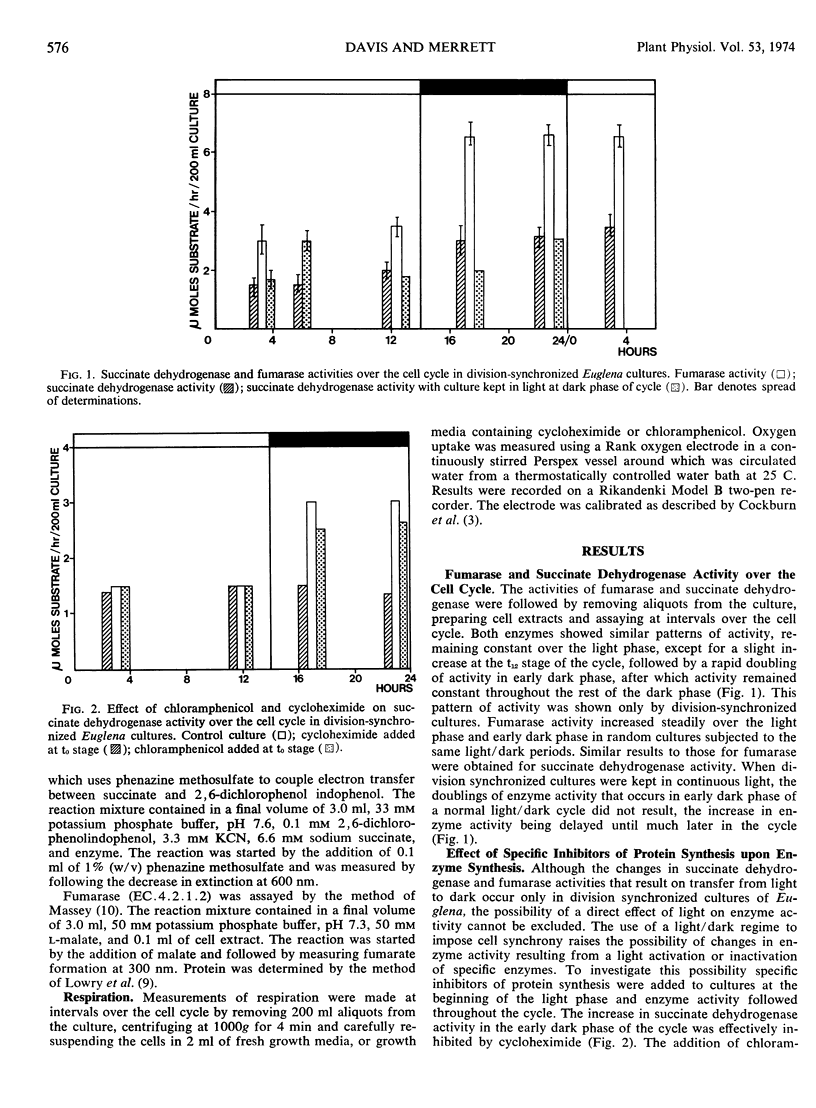
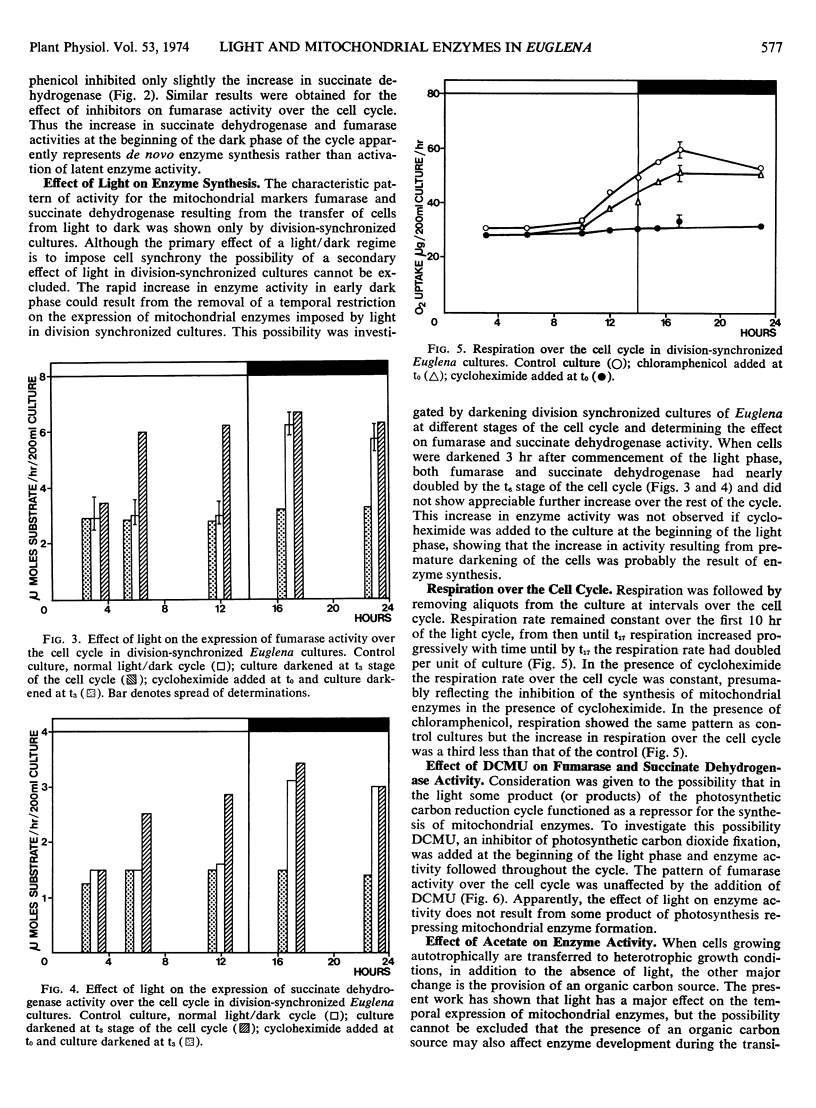
The development of the mitochondrial enzymes fumarase and succinate dehydrogenase has been followed in Euglena cultures division-synchronized by 14-hour light periods alternating with 12-hour dark periods. The activity of both enzymes was unaltered over the light phase, doubled in early dark phase, and thereafter remained constant over the rest of the cycle. The increase in enzyme activity in early dark phase probably represented de novo enzyme synthesis because it was prevented by the addition of cycloheximide at a concentration known to inhibit protein synthesis on Euglena cytoplasmic ribosomes.
When division-synchronized cultures were darkened in early light phase, a doubling of both fumarase and succinate dehydrogenase activity resulted, showing that light was repressing enzyme synthesis. The addition of acetate did not have a similar effect to darkening cultures: enzyme activity being unaltered over the light phase of the cycle. Enzyme expression was also unaffected by the addition of 3-(3,4 dichlorophenyl)-1,1-dimethylurea, a potent inhibitor of photosynthetic carbon dioxide fixation. The addition of 6-methylpurine (an inhibitor of transcription) at the beginning of the light phase inhibited enzyme increase in early dark phase, but when added at a later stage of the light phase (hour 8), increase in enzyme activity in early dark phase was unaffected. We concluded that transcription for these enzymes occurs in early light phase but light exerts a post-transcriptional control so that enzyme synthesis does not result until cells enter the dark phase of the cell cycle.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avadhani N. G., Buetow D. E. Isolation of active polyribosomes from the cytoplasm, mitochondria and chloroplasts of Euglena gracilis. Biochem J. 1972 Jun;128(2):353–365. doi: 10.1042/bj1280353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrispeels M. J., Varner J. E. Hormonal control of enzyme synthesis: on the mode of action of gibberellic Acid and abscisin in aleurone layers of barley. Plant Physiol. 1967 Jul;42(7):1008–1016. doi: 10.1104/pp.42.7.1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockburn W., Walker D. A., Baldry C. W. Photosynthesis by isolated chloroplasts. Reversal of orthophosphate inhibition by Calvin-cycle intermediates. Biochem J. 1968 Mar;107(1):89–95. doi: 10.1042/bj1070089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Codd G. A., Merrett M. J. Photosynthetic products of division synchronized cultures of euglena. Plant Physiol. 1971 May;47(5):635–639. doi: 10.1104/pp.47.5.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Codd G. A., Merrett M. J. The regulation of glycolate metabolism in division synchronized cultures of euglena. Plant Physiol. 1971 May;47(5):640–643. doi: 10.1104/pp.47.5.640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis B., Merrett M. J. Malate dehydrogenase isoenzymes in division synchronized cultures of euglena. Plant Physiol. 1973 Jun;51(6):1127–1132. doi: 10.1104/pp.51.6.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELLS H. A. A colorimetric method for the assay of soluble succinic dehydrogenase and pyridinenucleotide-linked dehydrogenases. Arch Biochem Biophys. 1959 Dec;85:561–562. doi: 10.1016/0003-9861(59)90527-2. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- McCullough W., John P. C. Control of de novo isocitrate lyase synthesis in Chlorella. Nature. 1972 Oct 13;239(5372):402–405. doi: 10.1038/239402a0. [DOI] [PubMed] [Google Scholar]
- Mitchison J. M. Enzyme synthesis in synchronous cultures. Science. 1969 Aug 15;165(3894):657–663. doi: 10.1126/science.165.3894.657. [DOI] [PubMed] [Google Scholar]
- Pain V. M., Clemens M. J. The role of soluble protein factors in the translational control of protein synthesis in eukaryotic cells. FEBS Lett. 1973 Jun 1;32(2):205–212. doi: 10.1016/0014-5793(73)80834-8. [DOI] [PubMed] [Google Scholar]
- Peak M. J., Peak J. G., Ting I. P. Isoenzymes of malate dehydrogenase and their regulation in Euglena gracilis Z. Biochim Biophys Acta. 1972 Sep 19;284(1):1–15. doi: 10.1016/0005-2744(72)90039-3. [DOI] [PubMed] [Google Scholar]
- Schiff J. A., Zeldin M. H., Rubman J. Chlorophyll Formation and Photosynthetic Competence in Euglena During Light-Induced Chloroplast Development in the Presence of 3, (3,4-dichlorophenyl) 1,1-Dimethyl Urea (DCMU). Plant Physiol. 1967 Dec;42(12):1716–1725. doi: 10.1104/pp.42.12.1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smillie R. M., Graham D., Dwyer M. R., Grieve A., Tobin N. F. Evidence for the synthesis in vivo of proteins of the Calvin cycle and of the photosynthetic electron-transfer pathway on chloroplast ribosomes. Biochem Biophys Res Commun. 1967 Aug 23;28(4):604–610. doi: 10.1016/0006-291x(67)90356-7. [DOI] [PubMed] [Google Scholar]
- Tauro P., Halvorson H. O., Epstein R. L. Time of gene expression in relation to centromere distance during the cell cycle of Saccharomyces cereviseae. Proc Natl Acad Sci U S A. 1968 Jan;59(1):277–284. doi: 10.1073/pnas.59.1.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullmann A., Monod J. Cyclic AMP as an antagonist of catabolite repression in Escherichia coli. FEBS Lett. 1968 Nov;2(1):57–60. doi: 10.1016/0014-5793(68)80100-0. [DOI] [PubMed] [Google Scholar]
- Walther W. G., Edmunds L. N. Studies on the Control of the Rhythm of Photosynthetic Capacity in Synchronized Cultures of Euglena gracilis (Z). Plant Physiol. 1973 Feb;51(2):250–258. doi: 10.1104/pp.51.2.250. [DOI] [PMC free article] [PubMed] [Google Scholar]


