Abstract
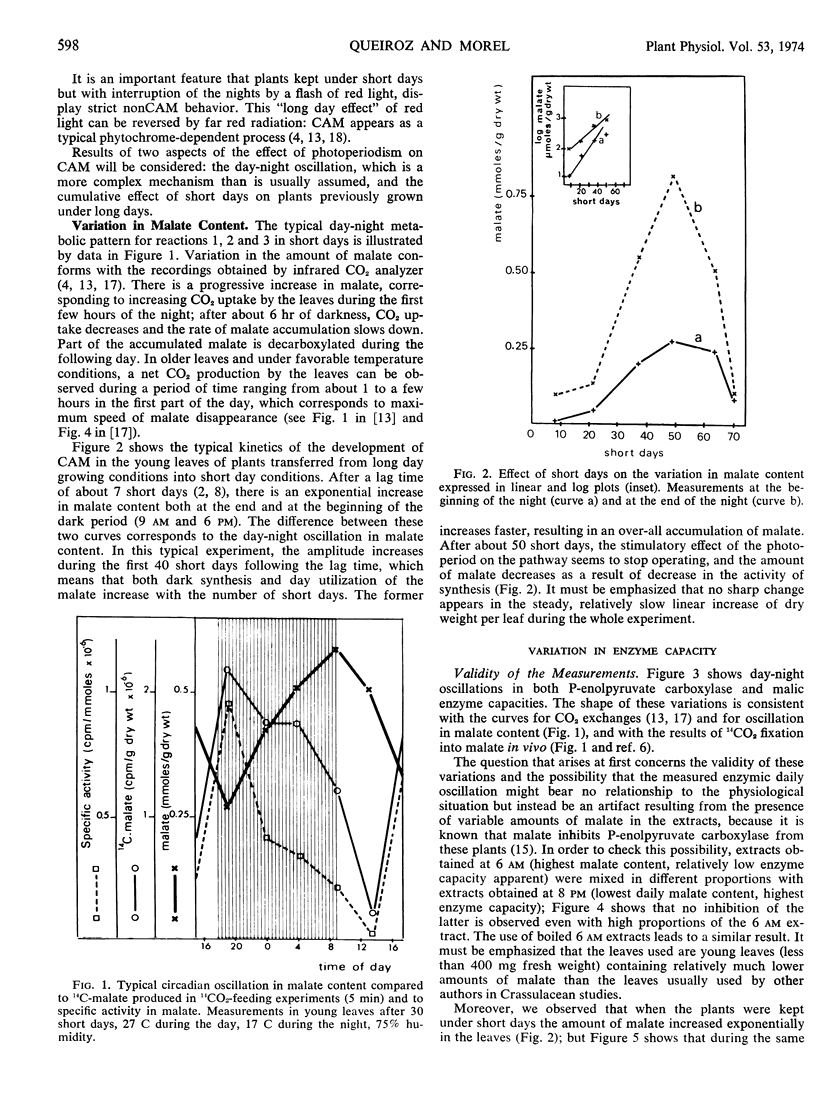
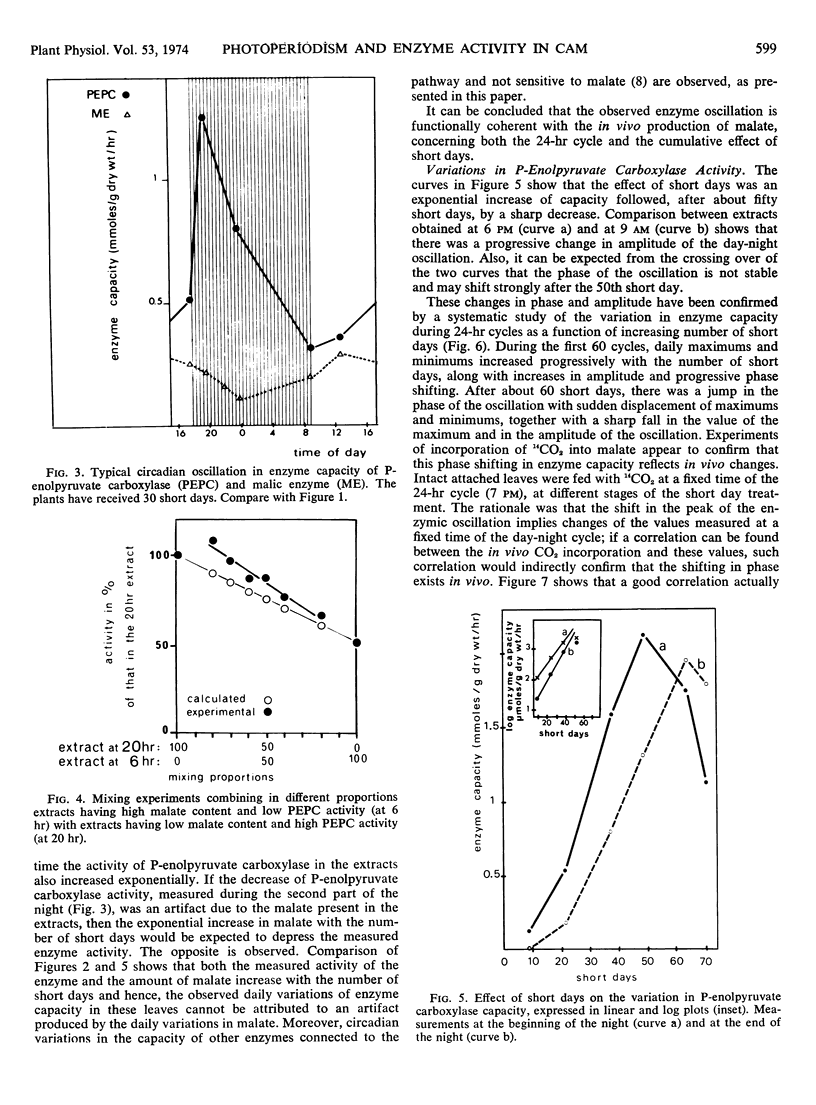
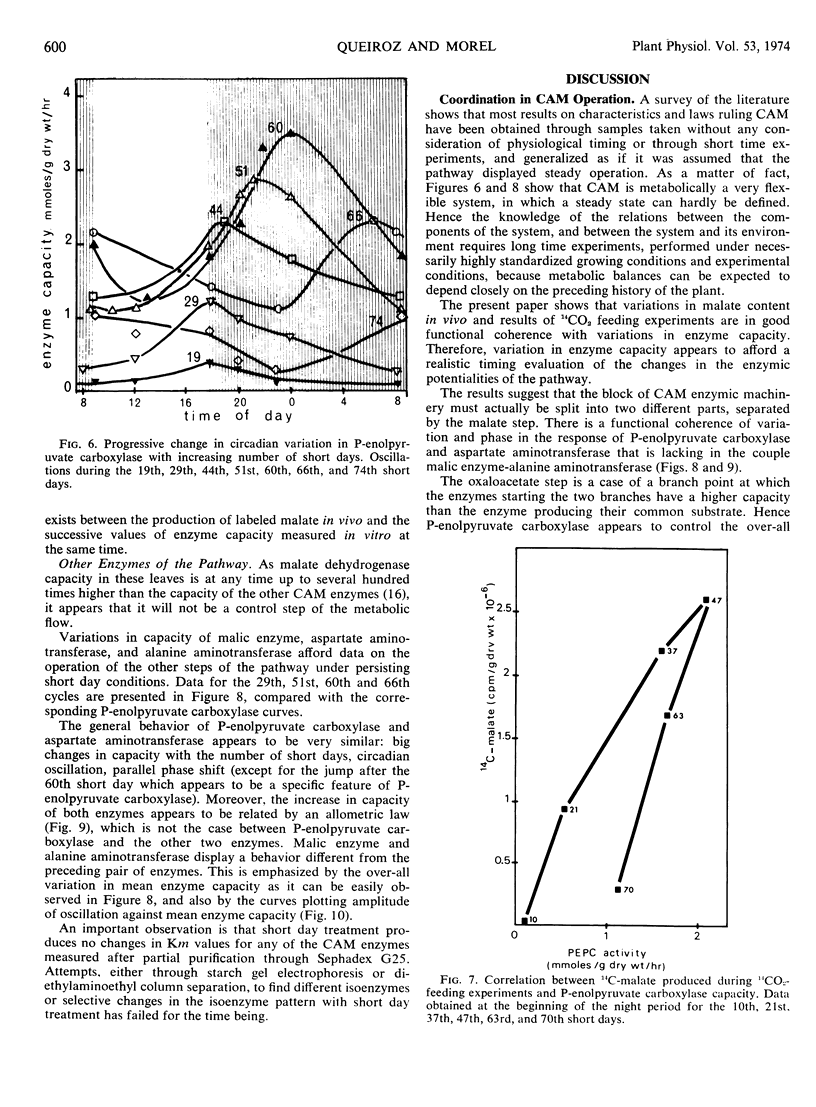
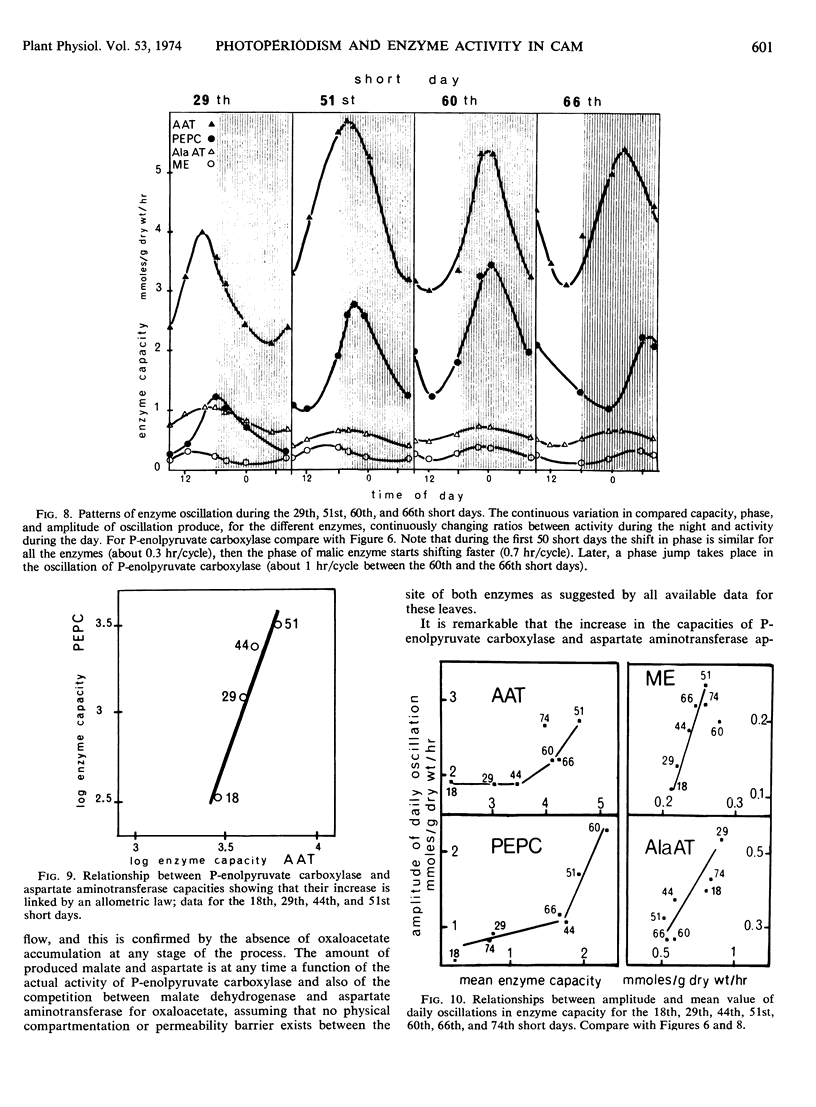
Metabolic readjustments after a change from long days to short days appear, in Kalanchoe blossfeldiana, to be achieved through the operation of two main mechanisms: variation in enzyme capacity, and circadian rhythmicity. After a lag time, capacity in phosphoenolpyruvate carboxylase and capacity in aspartate aminotransferase increase exponentially and appear to be allometrically linked during 50 to 60 short days; then a sudden fall takes place in the activity of the former. Malic enzyme and alanine aminotransferase behave differently. Thus, the operation of the two sections of the pathway (before and after the malate step) give rise to a continuously changing functional compartmentation in the pathway. Circadian rhythmicity, on the other hand, produces time compartmentation through phase shifts and variation in amplitude, independently for each enzyme. These characteristics suggest that the operation of a so-called biological clock would be involved. We propose the hypothesis that feedback regulation would be more accurate and efficient when applied to an already oscillating, clock-controlled enzyme system.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brulfert J., Guerrier D., Queiroz O. Photoperiodism and Enzyme Activity: Balance between Inhibition and Induction of the Crassulacean Acid Metabolism. Plant Physiol. 1973 Jan;51(1):220–222. doi: 10.1104/pp.51.1.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory F. G., Spear I., Thimann K. V. The Interrelation between CO(2) Metabolism and Photoperiodism in Kalanchoë. Plant Physiol. 1954 May;29(3):220–229. doi: 10.1104/pp.29.3.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOLCHINE G. Sur la distribution du 14C dans les molécules d'acide malique synthétisées par fixation de 14CO2 dans les feuilles de Bryophyllum daigremontianum Berger. Bull Soc Chim Biol (Paris) 1959;41(2-3):227–234. [PubMed] [Google Scholar]
- Mukerji S. K. Four-hourly variations in the activities of malate dehydrogenase (decarboxylating) and phosphopyruvate carboxylase in the cactus (Nopalea dejecta) plant. Indian J Biochem. 1968 Jun;5(2):62–64. [PubMed] [Google Scholar]
- Saltman P., Kunitake G., Spolter H., Stitts C. The Dark Fixation of CO(2) by Succulent Leaves: The First Products. Plant Physiol. 1956 Nov;31(6):464–468. doi: 10.1104/pp.31.6.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WALKER D. A. Physiological studies on acid metabolism. 4. Phosphoenolpyruvic carboxylase activity in extracts of Crassulacean plants. Biochem J. 1957 Sep;67(1):73–79. doi: 10.1042/bj0670073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wever R. Virtual synchronization towards the limits of the range of entrainment. J Theor Biol. 1972 Jul;36(1):119–132. doi: 10.1016/0022-5193(72)90181-6. [DOI] [PubMed] [Google Scholar]


