Abstract
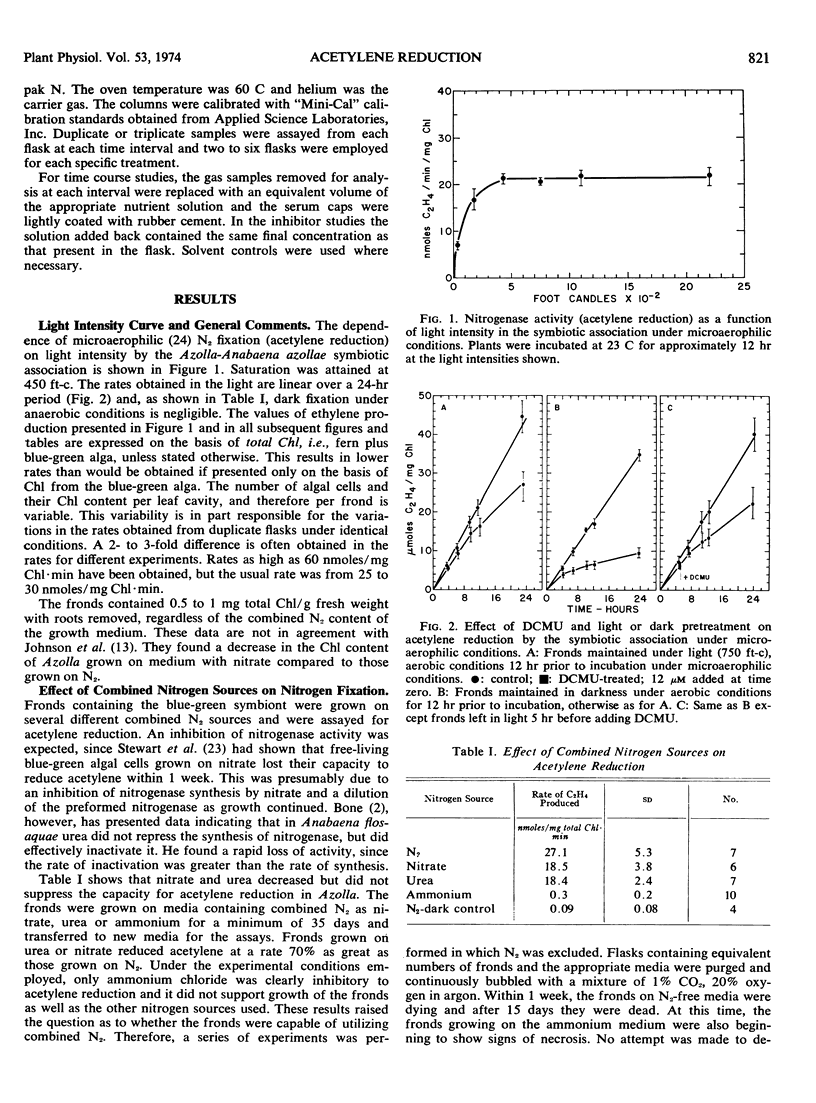
Anaerobic (microaerophilic) acetylene reduction by Azolla caroliniana Willd. was dependent on light and saturated at approximately 450 foot candles. Maximum rates of acetylene reduction were 60 nmoles/mg chlorophyll minute. However, rates of 25 to 30 nmoles/mg chlorophyll minute were more common.
The growth of Azolla for 35 days with nitrate or urea as a nitrogen source decreased the rate of acetylene reduction approximately 30% compared to controls grown on nitrogen. Prolonged growth on nitrate or urea (6-7 months) resulted in a 90% decrease in the rate of acetylene reduction.
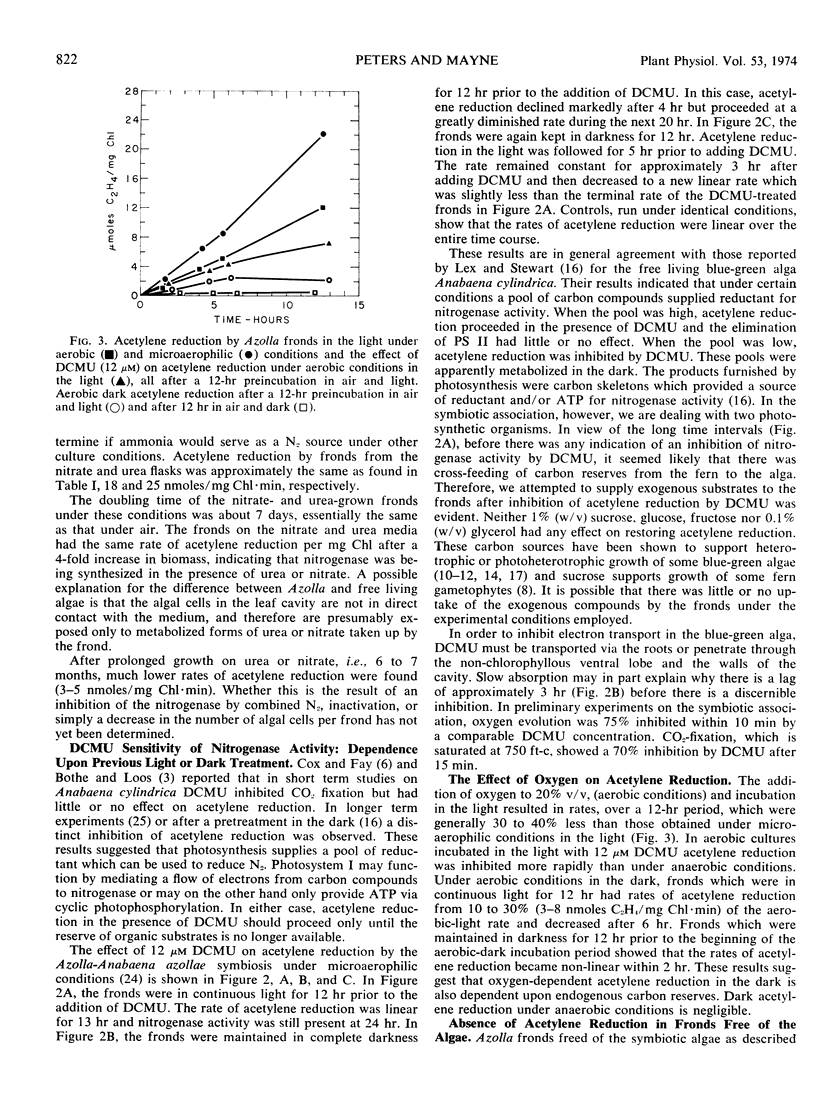
The inhibition of acetylene reduction by 3 (3,4-dichlorophenol) 1,1-dimethylurea (12 μM) was not pronounced until the Azolla became depleted of the reserves formed during photosynthesis. The interval required for this depletion was dependent upon pretreatment and varied from 2 to more than 12 hours. Oxygen evolution was inhibited 75% in 10 minutes by the same concentration of 3 (3,4-dichlorophenol) 1,1-dimethylurea.
The addition of oxygen, 20% volume per volume, resulted in a 30 to 40% decrease in the rate of acetylene reduction and the onsetof 3(3,4-dichlorophenol) 1,1-dimethylurea inhibition was more rapid then under microaerophilic conditions. The aerobic dark reduction of acetylene was from 10 to 30% of the rate of aerobic reduction in the light.
Acetylene reduction activity was absent in fronds freed ofthe symbiotic algae and present in isolated Anabaena azollae. This study shows that the alga is the agent of acetylene reduction and suggests that there is considerable transport of metabolites between the fern and the blue-green alga.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bone D. H. The influence of canavanine, oxygen, and urea on the steady-state levels of nitrogenase in Anabaena flos-aquae. Arch Mikrobiol. 1972;86(1):13–24. doi: 10.1007/BF00412396. [DOI] [PubMed] [Google Scholar]
- Bowyer J. W., Skerman V. B. Production of axemic cultures of soil-borne and endophytic blue-green algae. J Gen Microbiol. 1968 Dec;54(2):299–306. doi: 10.1099/00221287-54-2-299. [DOI] [PubMed] [Google Scholar]
- Cox R. M., Fay P. Special aspects of nitrogen fixation by blue-green algae. Proc R Soc Lond B Biol Sci. 1969 Apr 1;172(1029):357–366. doi: 10.1098/rspb.1969.0026. [DOI] [PubMed] [Google Scholar]
- Donze M., Haveman J., Schiereck P. Absence of photosystem 2 in heterocysts of the blue-green alga Anabaena. Biochim Biophys Acta. 1972 Jan 21;256(1):157–161. doi: 10.1016/0005-2728(72)90170-3. [DOI] [PubMed] [Google Scholar]
- Fay P., Kulasooriya S. A. Tetrazolium reduction and nitrogenase activity in heterocystous blue-green algae. Arch Mikrobiol. 1972;87(4):341–352. doi: 10.1007/BF00409133. [DOI] [PubMed] [Google Scholar]
- Ingram L. O., Van Baalen C., Calder J. A. Role of reduced exogenous organic compounds in the physiology of the blue-green bacteria (algae): photoheterotrophic growth of an "autotrophic" blue-green bacterium. J Bacteriol. 1973 May;114(2):701–705. doi: 10.1128/jb.114.2.701-705.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson G. V., Mayeux P. A., Evans H. J. A Cobalt Requirement for Symbiotic Growth of Azolla filiculoides in the Absence of Combined Nitrogen. Plant Physiol. 1966 May;41(5):852–855. doi: 10.1104/pp.41.5.852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lex M., Stewart W. D. Algal nitrogenase, reductant pools and photosystem I activity. Biochim Biophys Acta. 1973 Feb 22;292(2):436–443. doi: 10.1016/0005-2728(73)90049-2. [DOI] [PubMed] [Google Scholar]
- Miller J. S., Allen M. M. Carbon utilization patterns in the heterotrophic blue-green alga Chlorogloea fritschii. Arch Mikrobiol. 1972;86(1):1–12. doi: 10.1007/BF00412395. [DOI] [PubMed] [Google Scholar]
- Peters G. A., Mayne B. C. The Azolla, Anabaena azollae Relationship: I. Initial Characterization of the Association. Plant Physiol. 1974 Jun;53(6):813–819. doi: 10.1104/pp.53.6.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanier R. Y., Kunisawa R., Mandel M., Cohen-Bazire G. Purification and properties of unicellular blue-green algae (order Chroococcales). Bacteriol Rev. 1971 Jun;35(2):171–205. doi: 10.1128/br.35.2.171-205.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart W. D., Fitzgerald G. P., Burris R. H. Acetylene reduction by nitrogen-fixing blue-green algae. Arch Mikrobiol. 1968;62(4):336–348. doi: 10.1007/BF00425639. [DOI] [PubMed] [Google Scholar]
- Stewart W. D., Lex M. Nitrogenase activity in the blue-green alga Plectonema boryanum strain 594. Arch Mikrobiol. 1970;73(3):250–260. doi: 10.1007/BF00410626. [DOI] [PubMed] [Google Scholar]


