Abstract
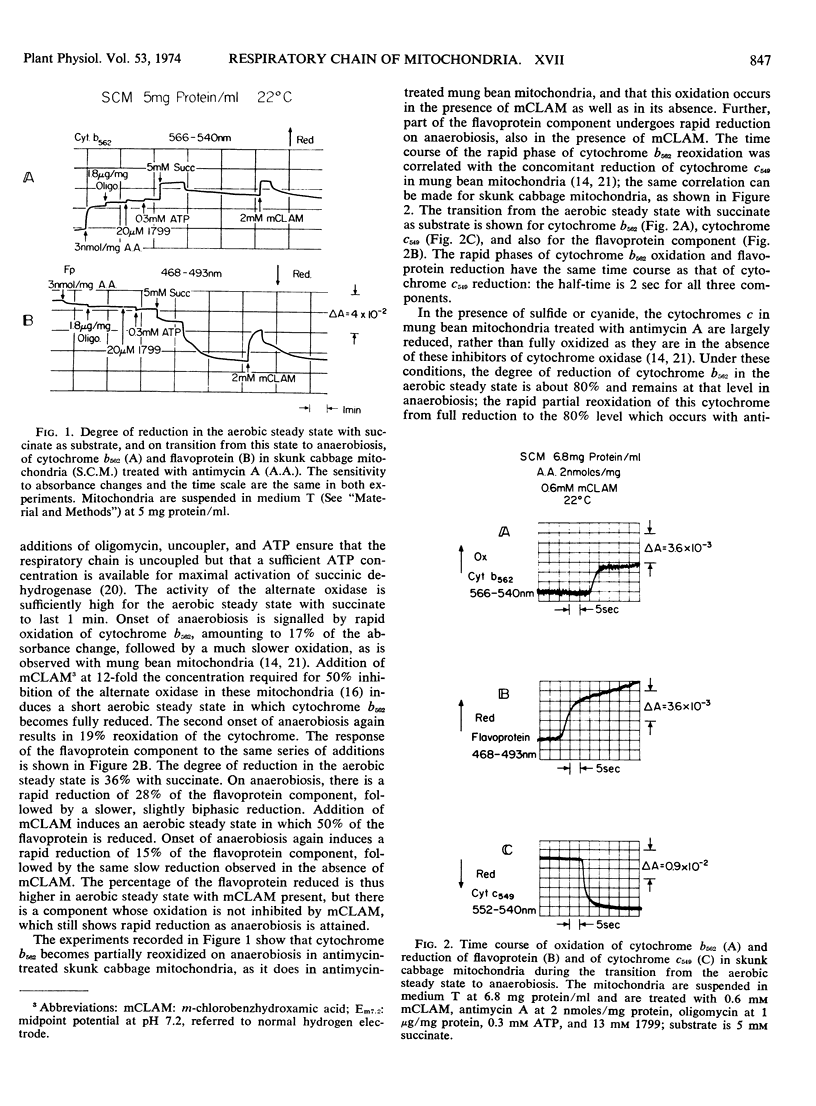
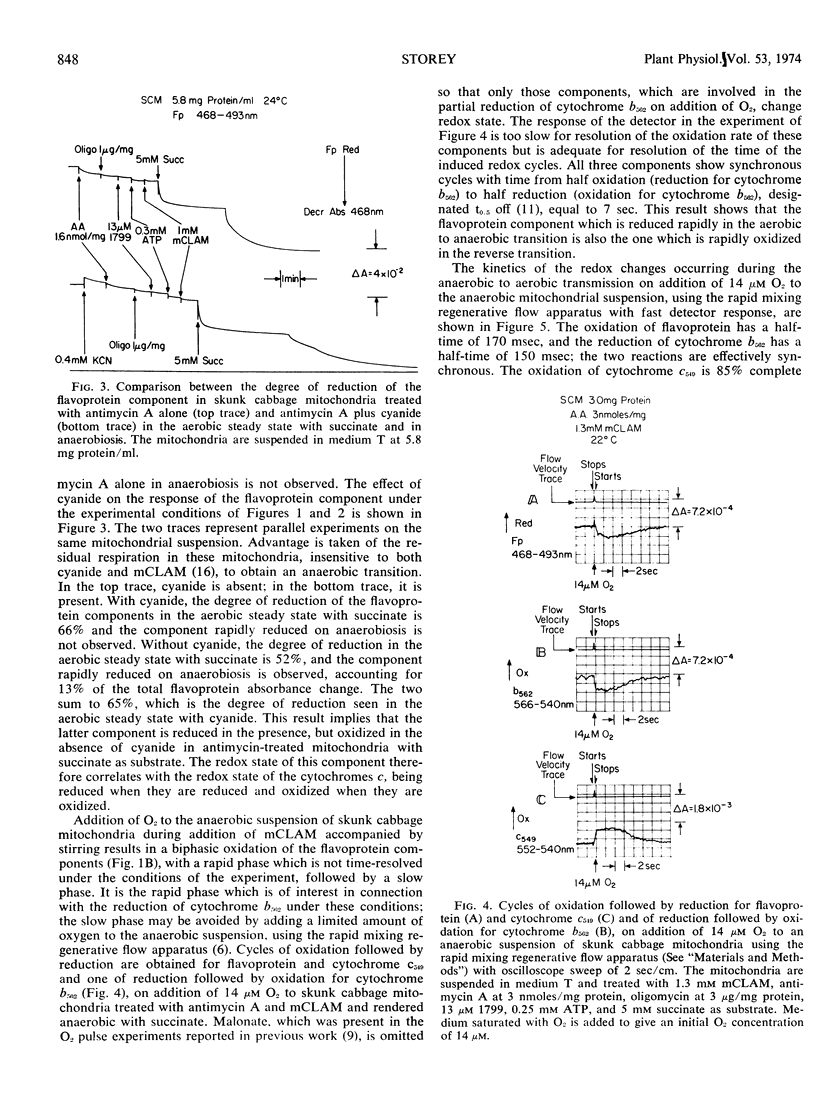
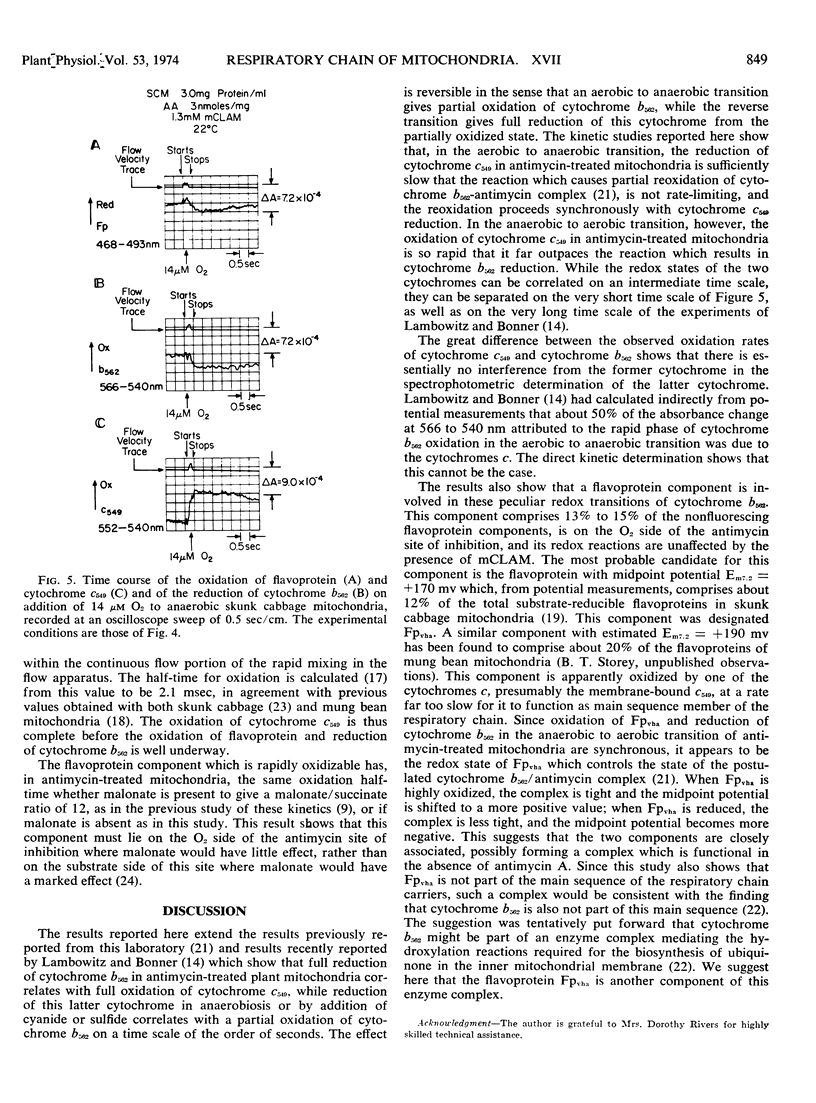
During the transition from the aerobic steady state with succinate as substrate to anaerobiosis, in suspensions of skunk cabbage (Symplocarpus foetidus) mitochondria treated with antimycin A, cytochrome b562 becomes reoxidized to the extent of about 20%, synchronously with the reduction of cytochrome c549. This reoxidation occurs in both the absence and presence of m-chlorobenzhydroxamic acid, a specific inhibitor for the alternate terminal oxidase of plant mitochondria. A flavoprotein component, amounting to 13% to 15% of the total nonfluorescent mitochondrial flavoprotein, undergoes reduction synchronously with the oxidation of cytochrome b562 during the aerobic to anaerobic transition with succinate as substrate in the presence of both antimycin A and m-chlorobenzhydroxamic acid. This flavoprotein component remains reduced in the presence of cyanide. The half-time for reduction of the flavoprotein component and cytochrome c549 and for oxidation of cytochrome b562 during the aerobic to anaerobic transition with succinate as substrate in the presence of both antimycin A and m-chlorobenzhydroxamic acid is 2 seconds. The half-times for oxidation of cytochrome c549 and the flavoprotein component are 2.1 and 170 milliseconds, respectively, during the anaerobic to aerobic transition induced by addition of 14 μm O2 to the mitochondrial suspensions. The half-time for reduction of cytochrome b562 under these conditions is 150 milliseconds, synchronous with the flavoprotein component. The synchrony of the flavoprotein oxidation and of the cytochrome b562 reduction at a rate much slower than that of cytochrome c549 oxidation implies that, in antimycin-treated plant mitochondria, the state of the cytochrome b562/antimycin complex is regulated by the redox state of this flavoprotein component, rather than by cytochrome c549. It is tentatively suggested that these two components are not part of the main sequence of the respiratory chain, but may be part of a multienzyme complex active in the hydroxylation reactions required for ubiquinone biosynthesis in the inner mitochondrial membrane.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Erecinska M., Storey B. T. The Respiratory Chain of Plant Mitochondria: VII. Kinetics of Flavoprotein Oxidation in Skunk Cabbage Mitochondria. Plant Physiol. 1970 Oct;46(4):618–624. doi: 10.1104/pp.46.4.618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HIGGINS J. Analysis of sequential reactions. Ann N Y Acad Sci. 1963 May 10;108:305–321. doi: 10.1111/j.1749-6632.1963.tb13382.x. [DOI] [PubMed] [Google Scholar]
- Ikuma H., Bonner W. D. Properties of Higher Plant Mitochondria. I. Isolation and Some Characteristics of Tightly-coupled Mitochondria from Dark-grown Mung Bean Hypocotyls. Plant Physiol. 1967 Jan;42(1):67–75. doi: 10.1104/pp.42.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambowitz A. M., Bonner W. D., Jr The effect of antimycin on the b-cytochromes of plant mitochondria. Oxidation-reduction behavior of cytochrome b-566. Biochem Biophys Res Commun. 1973 Jun 8;52(3):703–711. doi: 10.1016/0006-291x(73)90994-7. [DOI] [PubMed] [Google Scholar]
- Schonbaum G. R., Bonner W. D., Jr, Storey B. T., Bahr J. T. Specific inhibition of the cyanide-insensitive respiratory pathway in plant mitochondria by hydroxamic acids. Plant Physiol. 1971 Jan;47(1):124–128. doi: 10.1104/pp.47.1.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storey B. T., Bahr J. T. The Respiratory Chain of Plant Mitochondria: XIV. Ordering of Ubiquinone, Flavoproteins, and Cytochromes in the Respiratory Chain. Plant Physiol. 1972 Jul;50(1):95–102. doi: 10.1104/pp.50.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storey B. T., Bahr J. T. The respiratory chain of plant mitochondria. I. Electron transport between succinate and oxygen in skunk cabbage mitochondria. Plant Physiol. 1969 Jan;44(1):115–125. doi: 10.1104/pp.44.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storey B. T. Rate of ubiquinone oxidation in electron transport particles reduced by succinate. Arch Biochem Biophys. 1968 Aug;126(2):585–592. doi: 10.1016/0003-9861(68)90445-1. [DOI] [PubMed] [Google Scholar]
- Storey B. T. The Respiratory Chain of Plant Mitochondria. III. Oxidation Rates of the Cytochromes c and b in Mung Bean Mitochondria Reduced With Succinate. Plant Physiol. 1969 Mar;44(3):413–421. doi: 10.1104/pp.44.3.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storey B. T. The Respiratory Chain of Plant Mitochondria: XI. Electron Transport from Succinate to Endogenous Pyridine Nucleotide in Mung Bean Mitochondria. Plant Physiol. 1971 Dec;48(6):694–701. doi: 10.1104/pp.48.6.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storey B. T. The Respiratory Chain of Plant Mitochondria: XVI. Interaction of Cytochrome b(562) with the Respiratory Chain of Coupled and Uncoupled Mung Bean Mitochondria: Evidence for Its Exclusion from the Main Sequence of the Chain. Plant Physiol. 1974 Jun;53(6):840–845. doi: 10.1104/pp.53.6.840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storey B. T. The respiratory chain of plant mitochondria. 13. Redox state changes of cytochrome b 562 in mung bean seedling mitochondria treated with antimycin A. Biochim Biophys Acta. 1972 Apr 20;267(1):48–64. doi: 10.1016/0005-2728(72)90137-5. [DOI] [PubMed] [Google Scholar]
- Storey B. T. The respiratory chain of plant mitochondria: x. Oxidation-reduction potentials of the flavoproteins of skunk cabbage mitochondria. Plant Physiol. 1971 Oct;48(4):493–497. doi: 10.1104/pp.48.4.493. [DOI] [PMC free article] [PubMed] [Google Scholar]


