Abstract
Excised green leaves of mung bean (Phaseolus aureus L. var. Mungo) were used to determine the effect of light on the rate of endogenous respiration via the tricarboxylic acid cycle. Illumination with white light at an intensity of 0.043 gram calories cm−2min−1 (approximately 8600 lux) of visible radiation (400-700 nm) gave a rate of apparent photosynthesis, measured as net CO2 uptake, of 21 mg CO2 dm−2hr−1 which was about 11-fold greater than the rate of dark respiration. The feeding of 14CO2 or 14C-labeled acids of the tricarboxylic acid cycle in the dark for 2 hours was established as a suitable method for labeling mitochondrial pools of cycle intermediates.
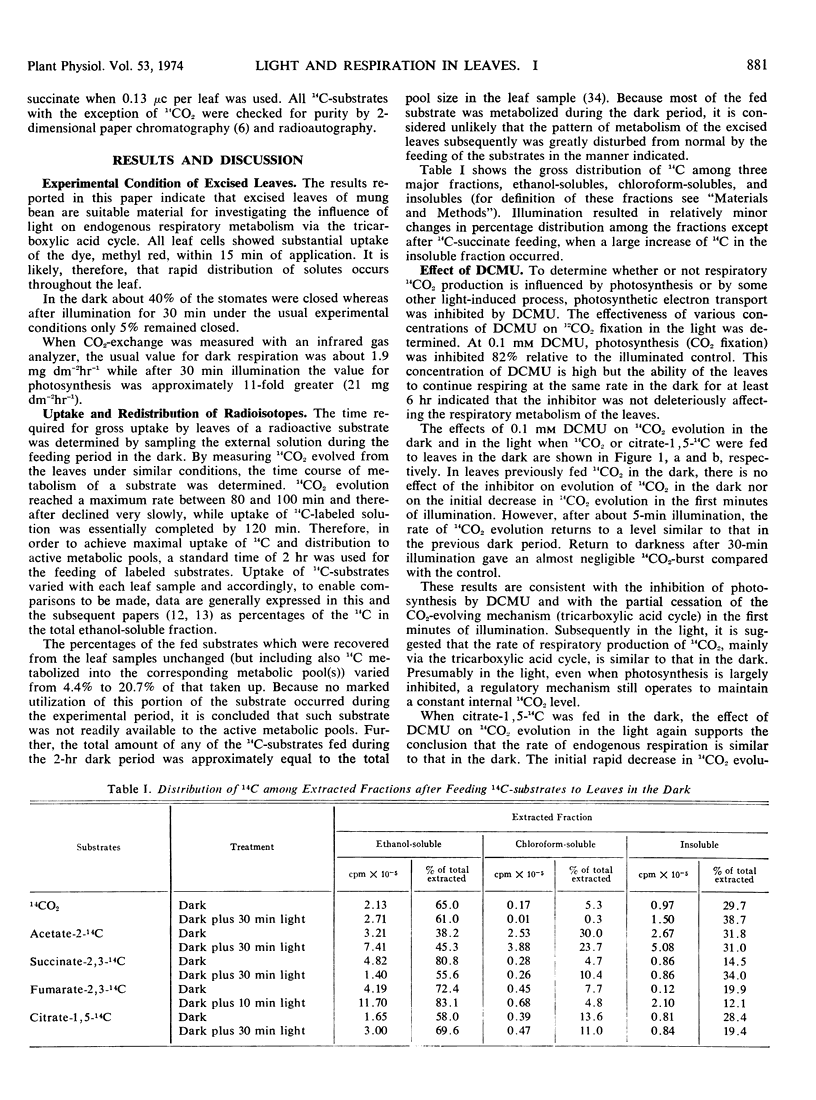
At a concentration of 0.1 mm 3-(3,4-dichlorophenyl)-1,1-dimethylurea, apparent photosynthesis was inhibited 82%, and the refixation of 14CO2 derived internally from endogenous respiration was largely prevented. In the presence of this inhibitor endogenous respiration, measured as 14CO2 evolution, continued in the light at a rate comparable to that in the dark. Consequently, under these conditions light-induced nonphotosynthetic processes have no significant effect on endogenous dark respiration. Inhibitors of the tricarboxylic acid cycle, malonate and fluoroacetate, were used to determine the relative rates of carbon flux through the cycle in the dark and in the light by measuring the rate of accumulation of 14C in either succinate or citrate. Results were interpreted to indicate that the tricarboxylic acid cycle functions in the light at a rate similar to that in the dark except for a brief initial inhibition on transition from dark to light. Evidence was obtained that succinate dehydrogenase as well as aconitase, was inhibited in the presence of fluoroacetate.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BANDURSKI R. S., AXELROD B. The chromatographic identification of some biologically important phosphate esters. J Biol Chem. 1951 Nov;193(1):405–410. [PubMed] [Google Scholar]
- BASSHAM J. A., BENSON A. A., CALVIN M. The path of carbon in photosynthesis. J Biol Chem. 1950 Aug;185(2):781–787. [PubMed] [Google Scholar]
- Baggiolini M., Bickel M. H. A new type of incubation apparatus for the determination of metabolically produced 14CO-2. Anal Biochem. 1966 Feb;14(2):290–295. doi: 10.1016/0003-2697(66)90139-4. [DOI] [PubMed] [Google Scholar]
- Brown A. H., Weis D. Relation Between Respiration and Photosynthesis in the Green Alga, Ankistrodesmus braunii. Plant Physiol. 1959 May;34(3):224–234. doi: 10.1104/pp.34.3.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunt R. V., Eisenthal R., Symons S. A. Inhibitory effects of fluorocitrates on yeast mitochondria. FEBS Lett. 1971 Feb 19;13(2):89–91. doi: 10.1016/0014-5793(71)80205-3. [DOI] [PubMed] [Google Scholar]
- CHANCE B., HOLMES W., HIGGINS J., CONNELLY C. M. Localization of interaction sites in multi-component transfer systems: theorems derived from analogues. Nature. 1958 Nov 1;182(4644):1190–1193. doi: 10.1038/1821190a0. [DOI] [PubMed] [Google Scholar]
- Chapman E. A., Graham D. The Effect of Light on the Tricarboxylic Acid Cycle in Green Leaves: II. Intermediary Metabolism and the Location of Control Points. Plant Physiol. 1974 Jun;53(6):886–892. doi: 10.1104/pp.53.6.886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman E. A., Osmond C. B. The Effect of Light on the Tricarboxylic Acid Cycle in Green Leaves: III. A Comparison between Some C(3) and C(4) Plants. Plant Physiol. 1974 Jun;53(6):893–898. doi: 10.1104/pp.53.6.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Codd G. A. The photoinhibition of malate dehydrogenase. FEBS Lett. 1972 Feb 1;20(2):211–214. doi: 10.1016/0014-5793(72)80797-x. [DOI] [PubMed] [Google Scholar]
- EGGLESTON L. V., HEMS R. Separation of adenosine phosphates by paper chromotography and the equilibrium constant of the myokinase system. Biochem J. 1952 Sep;52(1):156–160. doi: 10.1042/bj0520156. [DOI] [PMC free article] [PubMed] [Google Scholar]
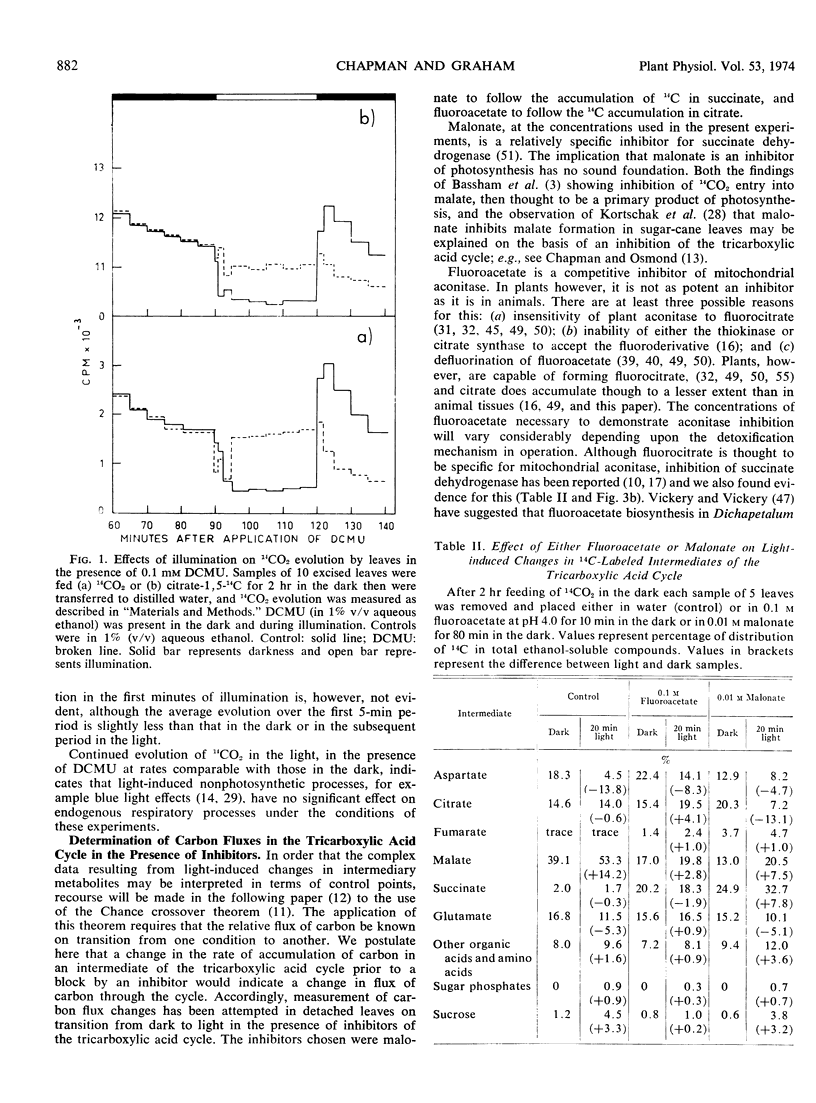
- FANSHIER D. W., GOTTWALD L. K., KUN E. STUDIES ON SPECIFIC ENZYME INHIBITORS. VI. CHARACTERIZATION AND MECHANISM OF ACTION OF THE ENZYME-INHIBITORY ISOMER OF MONOFLUOROCITRATE. J Biol Chem. 1964 Feb;239:425–434. [PubMed] [Google Scholar]
- GIBBS M. Effect of light intensity on the distribution of C14 in sunflower leaf metabolites during photosynthesis. Arch Biochem Biophys. 1953 Jul;45(1):156–160. doi: 10.1016/0003-9861(53)90415-9. [DOI] [PubMed] [Google Scholar]
- GRAHAM D., WALKER D. A. Some effects of light on the interconversion of metabolites in green leaves. Biochem J. 1962 Mar;82:554–560. doi: 10.1042/bj0820554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HANES C. S., ISHERWOOD F. A. Separation of the phosphoric esters on the filter paper chromatogram. Nature. 1949 Dec 31;164(4183):1107-12, illust. doi: 10.1038/1641107a0. [DOI] [PubMed] [Google Scholar]
- HOCH G., OWENS O. V., KOK B. Photosynthesis and respiration. Arch Biochem Biophys. 1963 Apr;101:171–180. doi: 10.1016/0003-9861(63)90547-2. [DOI] [PubMed] [Google Scholar]
- Heber U. W., Santarius K. A. Compartmentation and reduction of pyridine nucleotides in relation to photosynthesis. Biochim Biophys Acta. 1965 Nov 29;109(2):390–408. doi: 10.1016/0926-6585(65)90166-4. [DOI] [PubMed] [Google Scholar]
- ISHERWOOD F. A., HANES C. S. Separation and estimation of organic acids on paper chromatograms. Biochem J. 1953 Dec;55(5):824–830. doi: 10.1042/bj0550824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kortschak H. P., Hartt C. E., Burr G. O. Carbon Dioxide Fixation in Sugarcane Leaves. Plant Physiol. 1965 Mar;40(2):209–213. doi: 10.1104/pp.40.2.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig L. J., Canvin D. T. The Rate of Photorespiration during Photosynthesis and the Relationship of the Substrate of Light Respiration to the Products of Photosynthesis in Sunflower Leaves. Plant Physiol. 1971 Dec;48(6):712–719. doi: 10.1104/pp.48.6.712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MILHAUD G., BENSON A. A., CALVIN M. Metabolism of pyruvic acid-2-C14 and hydroxypyruvic acid-2-C14 in algae. J Biol Chem. 1956 Feb;218(2):599–606. [PubMed] [Google Scholar]
- Maclennan D. H., Beevers H., Harley J. L. 'Compartmentation' of acids in plant tissues. Biochem J. 1963 Nov;89(2):316–327. doi: 10.1042/bj0890316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh H. V., Galmiche J. M., Gibbs M. Effect of Light on the Tricarboxylic Acid Cycle in Scenedesmus. Plant Physiol. 1965 Nov;40(6):1013–1022. doi: 10.1104/pp.40.6.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
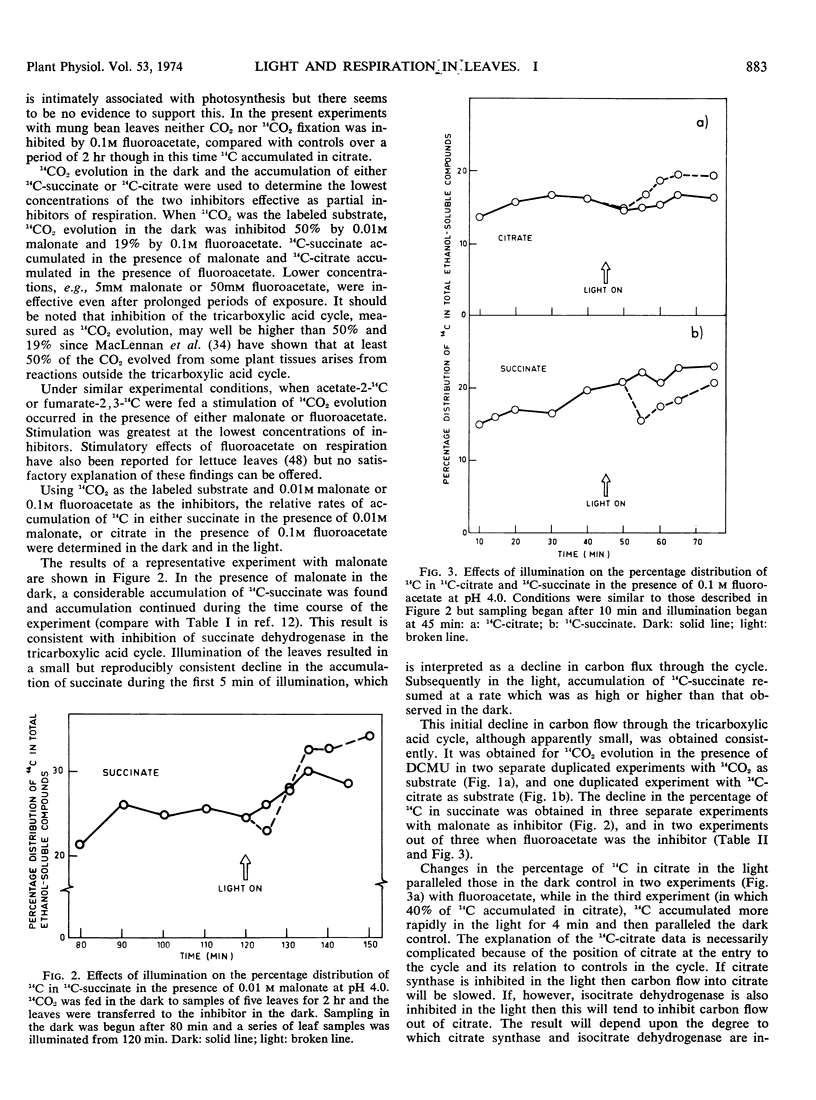
- Ogren W. L., Krogmann D. W. Studies on pyridine nucleotides in photosynthetic tissue. Concentrations, interconversions, and distribution. J Biol Chem. 1965 Dec;240(12):4603–4608. [PubMed] [Google Scholar]
- PATTERSON M. S., GREENE R. C. MEASUREMENT OF LOW ENERGY BETA-EMITTERS IN AQUEOUS SOLUTION BY LIQUID SCINTILLATION COUNTING OF EMULSIONS. Anal Chem. 1965 Jun;37:854–857. doi: 10.1021/ac60226a017. [DOI] [PubMed] [Google Scholar]
- Peters R. A., Shorthouse M. Identification of a volatile constituent formed by homogenates of Acacia georginae exposed to fluoride. Nature. 1971 May 14;231(5298):123–124. doi: 10.1038/231123a0. [DOI] [PubMed] [Google Scholar]
- Santarius K. A., Heber U. Changes in the intracellular levels of ATP, ADP, AMP and P1 and regulatory function of the adenylate system in leaf cells during photosynthesis. Biochim Biophys Acta. 1965 May 25;102(1):39–54. doi: 10.1016/0926-6585(65)90201-3. [DOI] [PubMed] [Google Scholar]
- TREBLE D. H., LAMPORT D. T., PETERS R. A. The inhibition of plant aconitate hydratase (aconitase) by fluorocitrate. Biochem J. 1962 Oct;85:113–115. doi: 10.1042/bj0850113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TYSZKIEWICZ E. An improved solvent system for the paper chromatography of phosphate esters. Anal Biochem. 1962 Feb;3:164–166. doi: 10.1016/0003-2697(62)90107-0. [DOI] [PubMed] [Google Scholar]
- WARD P. F., PETERS R. A. The chemical and biochemical properties of fluorocitric acid. Biochem J. 1961 Mar;78:661–668. doi: 10.1042/bj0780661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward P. F., Huskisson N. S. The metabolism of fluoroacetate by plants. Biochem J. 1969 Jun;113(2):9P–9P. doi: 10.1042/bj1130009pa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward P. F., Huskisson N. S. The metabolism of fluoroacetate in lettuce. Biochem J. 1972 Nov;130(2):575–587. doi: 10.1042/bj1300575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weis D., Brown A. H. Kinetic Relationships Between Photosynthesis and Respiration in the Algal Flagellate, Ochromonas Malhamensis. Plant Physiol. 1959 May;34(3):235–239. doi: 10.1104/pp.34.3.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Withrow R. B., Price L. A Darkroom Safelight for Research in Plant Physiology. Plant Physiol. 1957 May;32(3):244–248. doi: 10.1104/pp.32.3.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Withrow R. B., Price L. Filters for the Isolation of Narrow Regions in the Visible and Near-Visible Spectrum. Plant Physiol. 1953 Jan;28(1):105–114. doi: 10.1104/pp.28.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]


