Abstract
Conventional row crop agriculture for both food and fuel is a source of carbon dioxide (CO2) and nitrous oxide (N2O) to the atmosphere, and intensifying production on agricultural land increases the potential for soil C loss and soil acidification due to fertilizer use. Enhanced weathering (EW) in agricultural soils—applying crushed silicate rock as a soil amendment—is a method for combating global climate change while increasing nutrient availability to plants. EW uses land that is already producing food and fuel to sequester carbon (C), and reduces N2O loss through pH buffering. As biofuel use increases, EW in bioenergy crops offers the opportunity to sequester CO2 while reducing fossil fuel combustion. Uncertainties remain in the long-term effects and global implications of large-scale efforts to directly manipulate Earth's atmospheric CO2 composition, but EW in agricultural lands is an opportunity to employ these soils to sequester atmospheric C while benefitting crop production and the global climate.
Keywords: basalt, carbon sequestration, agriculture, global climate change, silicate weathering, biofuels
1. Introduction
Atmospheric CO2 is regulated on geologic timescales by the natural chemical weathering of silicate rocks, a process that can be accelerated by applying crushed fast-weathering silicate rocks to the land surface as ‘enhanced weathering’ (EW) [1–4]. Conventional row crop agricultural practices result in a net loss of carbon (C) from the soil to the atmosphere and high requirements for fertilizer and lime [5–8]. EW with basalt, a fast-weathering, Ca- and Mg-rich silicate rock, has the potential to create a net C sink in these systems while reducing N loss, counteracting soil acidification, and supplying nutrients through the by-products of the weathering processes. The 10–15 M km2 of global cropland [9] offers a host of environments for deployment of EW substrates, with a potential return of 200–800 kg sequestered CO2 t−1 basalt [10]. In addition, growing interest in biofuels to reduce fossil fuel consumption has increased the proportion of agricultural land producing annual and perennial bioenergy crops, with the potential to expand into marginal lands [7,11–13]. Perennial crops have longer growing seasons than annuals and extensive root systems supporting large biotic communities [8,11,14], which may be more effective than annuals at weathering. In this review, we examine the potential for basalt EW to sequester CO2 and benefit crop yield in conventional and perennial bioenergy agroecosystems.
2. Basalt weathering for C sequestration
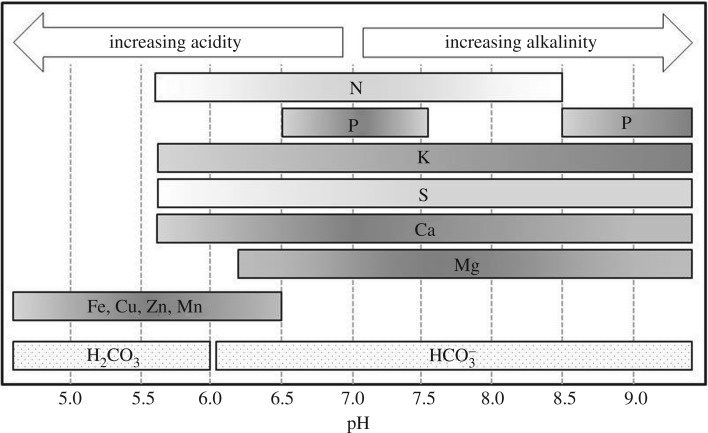
The chemical weathering of silicate rock sequesters CO2 as bicarbonate and carbonate minerals in soils and oceans [1,3,15]. Basalt is being explored for EW due to availability and nutrient content. Basalt weathering occurs at slow natural rates over 6.8 M km2, or 4.6% of terrestrial land area [16]. EW in agricultural lands expands the potential weathering area by 10–15 M km2 [3,15], and offers secondary benefits to agriculture from basalt application as a soil amendment [15]. The use of rock fertilizers is not novel: dolomite and limestone are commercially available, and have three major values beyond C sequestration: buffering soil pH, reducing N loss and providing elemental nutrients [17–20]. The various forms of basalt contain 8–20% Ca and Mg oxides by weight, and 1–2% potassium oxides and phosphates, with small quantities of micronutrients, including Cu, Ni, and Zn (e.g. [21–23]). In an agricultural setting, organic acids produced by plants weather the rock surface, liberating nutrients and dissolving silica [24]. Ca2+ and Mg2+ are among the most easily weathered base cations of basalt [25,26], and react to form soluble bicarbonate compounds [10]. Consumption of H+ ions during the weathering process buffers the soil, increasing the availability of existing soil nutrients, particularly P, which form plant-resistant compounds at low pH (figure 1) [20,27].
Figure 1.
Optimal soil pH ranges for plant-essential nutrient availability, with nutrients supplied by basalt weathering (in grey), and dominant species of dissolved carbonate. Darker shading indicates greater availability. Adapted from Truog [27].
Global rates of rock weathering are directly related to temperature, moisture and interactions with vegetation [4,14,28,29]. Basalts are among the fastest weathered silicate rocks, and in situ weathering of basalt minerals on the Earth's surface currently consumes 179 Mt of CO2 annually [16], approximately 0.5% of annual fossil fuel emissions [30]. This sequestration is limited by basalt quality (Ca +Mg concentration and degree of previous weathering) [4,10] and weathering conditions, such as low temperatures in Siberia or dry conditions in Ethiopia [16], which slow the rate of chemical reactions. Weathering is enhanced by increasing the reactive surface area and by increasing temperature and moisture: EW will proceed most rapidly in warm, wet environments [15,26,31]. Rates of CO2 capture by EW are uncertain, but the most Ca- and Mg-rich silicate rocks have the capacity to sequester >1t CO2 t−1 rock, while basic rocks, including basalts, range from 200–800 kg CO2 t−1 rock [4,10,15]. Plants and rhizosphere microbes, particularly mycorrhizal fungi, accelerate weathering while mining the rocks for nutrients, including P and K, through the production of root exudates and acidification [24,32–34]. The rate of mineral dissolution from ground rock increases 1–5× in the presence of plants [14,18,29,32].
3. Agricultural lands as carbon sinks
Global soils represent a C reservoir of up to 1.5 Pg of organic C and 1 Pg of inorganic C [6], but many agricultural soils are CO2 sources due to soil disturbance and heavy cropping, emitting 5–6 Gt CO2-eq yr−1 [6,7,35–37]. To support the growing human population over the next century, global cropland must expand, or agricultural production must intensify on existing arable land [9,11]. Expansion into natural areas such as tropical forest, or increases in management practices such as tillage and fertilizer application can greatly increase soil C disturbance and N loss to volatilization and runoff [11]. EW has potential to mitigate the effects of agriculture at a global scale and at global locations, without disrupting food production. Earth's surface supports 10–15 M km2 of arable land with potential to deploy EW (7–10% of global land area) [3,15], an area that is expected to expand with growing production requirements in the future (tables 1 and 2). The ubiquity of agricultural lands around the world gives a wide range of temperature and moisture regimes at which EW can be explored, and the weathering rate will differ for each, as will the specific soil chemistry that will determine appropriateness of EW [15,39].
Table 1.
Global population projections and projections of agricultural production of edible crops for fuel through 2050.
| year |
||||
|---|---|---|---|---|
| 2005 | 2030 | 2050 | % increase | |
| population [9] | 6.6B | 8.3B | 9.1B | 37% |
| global arable land [38] | 15 M km2 | 18.5 M km2 | 21 M km2 | 40% |
| cereals [9] | 2.0 Bt | 2.7 Bt | 3.0 Bt | 46% |
| bioenergy/non-food cereals | 65 Mt | 182 Mt | 182 Mt | 180% |
| oils | 139 Mt | 230 Mt | 252 Mt | 81% |
| bioenergy/non-food oils | 7 Mt | 29 Mt | 29 Mt | 314% |
| sugar | 185 Mt | 295 Mt | 333 Mt | 80% |
| bioenergy/non-food sugars | 28 Mt | 81 Mt | 81 Mt | 189% |
Table 2.
Projections of global biodiesel/bioethanol production and 1G bioenergy crop land use.
|
year |
||||
|---|---|---|---|---|
| 2006 | 2010 | 2020 | % increase | |
| bioethanol production [38] | 31 Mt | 67 Mt | 125 Mt | 303% |
| biodiesel production | 6 Mt | 17 Mt | 50 Mt | 733% |
| bioenergy land use | 1.05 M km2 | 2.2 M km2 | 4.8 M km2 | 357% |
Carbon losses from agricultural soils occur due to soil disturbance, crop harvest and microbial activity [6,11]. Crop biomass temporarily sequesters 128–165 Gt of C [6] and contributes roots and litter to slower-turnover organic matter pools in the soil. Liberation of C by tillage, microbial consumption of organic matter and the removal and subsequent destruction of aboveground biomass outweigh C inputs under row crops, and result in a net loss of C [6,12,37]. EW sequesters atmospheric CO2 as inorganic C in soils, and does not directly counteract the organic C loss from agricultural practices, instead reducing net C loss [15]. Alkaline solutions formed in terrestrial reactions may travel through soil water and groundwater to streams and rivers and ultimately to oceans, where vast quantities of C are stored in the shells of marine organisms and precipitated to the sea floor [40].
(a). EW effects on the N cycle in agricultural soils
Much of the increase in agricultural productivity in the past century can be traced back to the widespread adoption of N fertilization, but long-term N fertilizer use has negative effects at both global and local scales. N fertilizer production consumes 1.2% of annual energy produced globally, and represents 1.2% of total greenhouse gas emissions [41,42]. Fertilizers are often applied at rates in excess of biological demand, or in excess of neutralizing soil ions, and lost to volatilization or runoff, resulting in eutrophication of aquatic systems [5]. N2O has a global warming potential approximately 300 times higher than CO2 over a 100-year time period [43], and N fertilizers increase rates of nitrification and/or denitrification [44–46]. Conservation of N in agriculture is critical to reducing the rates of N fertilizer production and application, and N emissions from agricultural soils.
EW of basalt shares some similarities to liming, a practice that alters soil pH with CaCO3 to improve nutrient availability in crops, but liming emits CO2 to the atmosphere as carbonates weather [44]. This CO2 loss is compensated for by reduction of N2O, a more potent greenhouse gas [43], and increased C sequestration in biomass. Logic indicates that increasing soil pH will increase N2O emissions due to increases in microbial N mineralization and nitrification; however, multiple studies have shown a decline in N2O emissions following lime applications [44–46]. The mechanism of N2O reduction through liming is not well understood, but may be a result of increased microbial production of enzymes reducing N2O to N2 at neutral pH [46,47]. Though a representative basalt (approx. 20% CaO+MgO) has half the buffering capacity of limestone (40% CaO by weight), proposed rates of basalt application (2–25× the rate of limestone) [3,48] are adequate to substitute for agricultural lime.
(b). Effects of EW on soil pH and plant nutrition
Approximately 30% of global soils are acidic (pH < 5.5), and continued overuse of ammonia-based N fertilizers adds free protons and lowers soil pH, resulting in the formation of insoluble nutrient compounds that are unusable by plants, nutrient deficiencies, reduced crop yield and water quality degradation [5,49–51]. Plant uptake of base cations further lowers soil pH, and essential nutrients including P, K and S form compounds unavailable to plants as pH decreases. Conversely, plant-availability of Fe, Mn, Cu and Zn increases at low pH, creating potential for metal toxicity (figure 1) [5,50]. EW consumes free protons in the formation of bicarbonate and raises soil pH, and may increase plant-availability of existing nutrients in the soil while adding micronutrients and Si [51,52]. Although EW does not directly sequester organic C from plants, increases in nutrient availability could support greater biomass production, and subsequently lead to increased organic C inputs to the soil system from roots and litter.
Root exudates chemically weather rocks and minerals, and the reactions are enhanced by mycorrhizal acidification of the rhizosphere [14,29,32–34,53]. Root-associating mycorrhizal fungi provide the link between the inorganic C fixation of EW and the organic C cycle of agricultural soils. Mycorrhizal fungi are critical for developing soil structure, which preserves organic matter and resists water erosion [54]. Increases in soil organic matter benefit agriculture by increasing soil water retention and crop yields, both of which amplify weathering by increasing mineral-water contact times and demand for inorganic nutrients, respectively [32].
(c). Potential for increased carbonate formation: a global, millennial effect
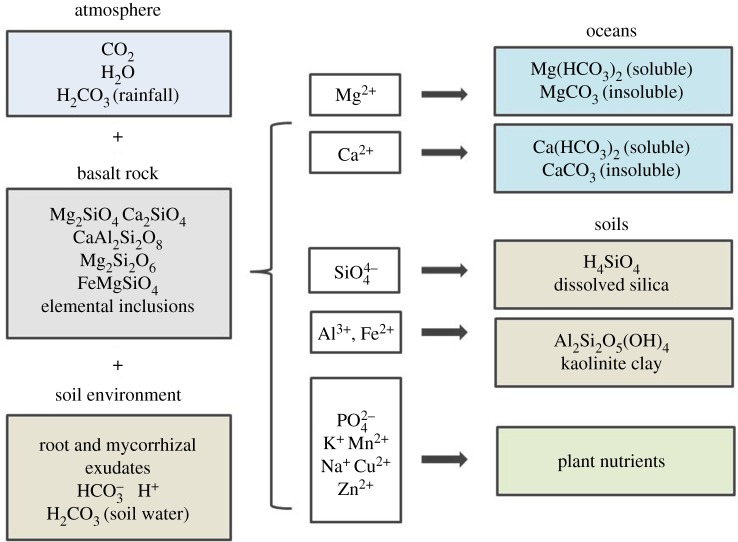
Carbonate precipitates from the soil solution when soils are saturated with Ca2+ and Mg2+ cations, and alkaline soils are a significant terrestrial sink of CO2 [10,55]. Like their acidic counterparts, alkaline soils suffer from nutrient limitations and loss of productivity, and may benefit from the additions of Fe from EW (figure 1) [51]. Alkalinity resulting from EW may travel through the vadose zone to surface and ground waters (figure 2), and eventually to rivers and oceans [10,15,16]. Ocean inputs of base cations are desirable to combat ocean acidification, an effect of the continuing rise of atmospheric CO2 [2,3,37]. Surface coastal oceans provide a major sink for an influx of bicarbonate ions liberated by weathering which, in the presence of adequate Ca2+ or Mg2+ cations, can precipitate biologically (e.g. corals and forams) and on longer timescales abiotically (limestone) [40]. The reaction producing carbonate from bicarbonate liberates CO2 (1 kg kg−1 sequestered); however, the resulting mineral is highly stable and will persist for millions of years in oceans [3,15,40].
Figure 2.
Weathering of basalt minerals by carbonic acid-containing rainfall, soil water, and root and mycorrhizal exudates liberates base cations, metals, and plant-essential nutrients. Basalt components ultimately contribute to plant growth, soil formation and oceanic carbonate storage. (Online version in colour.)
4. Bioenergy crops and the carbon balance
Bioenergy crops have been investigated in both temperate and tropical regions as a means of partially mitigating CO2 emissions from burning fossil fuels. Combustion of bioethanol and biodiesel produces less net CO2 than fossil fuels because bioenergy feedstocks sequester CO2 as biomass and belowground in soil as they grow, recycling C between the atmosphere and the terrestrial C pool [7,12,37]. Crops used to produce first generation biofuels (1G) from sugars and oils including maize, soya beans, and sugar crops, are grown on over 9 M km2 of agricultural land globally, currently with a 90/10 split between food and fuel. In the past 20 years, fuel production from 1G bioenergy crops has increased from near zero in 1990 to 85 million tons of bioethanol and biodiesel in 2010, and the number is expected to grow as countries follow the models of Brazil, the EU and the USA, with subsidies and mandates for fossil fuel reductions (table 2) [38]. 1G bioenergy crops compete with food crops for land area and would benefit from EW in the same manner as those grown for food.
Second generation bioenergy crops (2G), including perennial grasses and woody plants, are grown for cellulose and require additional processing for bioethanol production. 2G crops are intended to spare prime agricultural land and to separate the food and fuel production streams in agriculture [8,11,12,38,56]. Perennial crops have the combined benefits of negative C balance [7,8,12] and high biomass production on marginal land [11,57]. While 2G bioenergy crops have lower nutrient requirements than 1G crops (perennial grasses in the USA range from unfertilized to half the rate of maize) [12], plant-induced weathering of basalt could supply nutrients that improve marginal soils, increasing yields and promoting further organic C sequestration.
5. Limits of agricultural benefits from basalt weathering, questions and uncertainties
Global opportunities to deploy EW are widespread, while feasibility at specific locations is more limited. Basalts account for 6.8 M km2 of Earth's surface, and significantly more beneath the surface and under the oceans [16,58], but mining, processing, and transportation of large amounts of basalt to agricultural areas present financial and logistic challenges to farmers [3,10]. Over 80% of agricultural commodities are consumed locally [9], and areas with limited exports may lack transportation infrastructure needed to import basalt. Remote sources of basalt that do not overlap with arable land, such as outcrops in Siberia or Ethiopia [16], add to the expense of producing the material. In addition to the capital investment in purchasing and transporting basalt, there is a C cost. Fuel consumption and subsequent CO2 release during mining, processing, and transportation reduce gains made by enhanced weathering by an estimated 5–30% of potential C sequestered [15]. Proposed application rates of 10–50 t ha−1 in agricultural soils [3] exceed typical limestone application rates for maize/soya bean in the USA 5- to 25-fold [48], requiring heavy machinery for distribution and restricting deployment of EW in remote or pastoral areas. However, EW in only a portion of global agricultural land area has the potential to offset a significant amount of CO2 production [10,16]. In the USA, with approximately 70 M ha of maize and soya beans planted annually [59], deployment of basalt (10% CaO and 10% MgO, RCO2 = 0.32 [10]) at rates between 10 and 50 t ha−1 represents a theoretical maximum CO2 capture of 0.2–1.1 Gt CO2, up to 13% of the global annual agricultural emissions over the weathering lifespan of the material. This value exceeds the US annual contribution to agricultural emissions (approx. 10% of global) [30,60] before accounting for additional reductions in N2O emissions or fertilizer use. However, the rate of weathering in these soils is unknown, creating uncertainly in predicting how quickly CO2-capture capacity will be reached. Initial deployment to areas of high-intensity agriculture where basalt, road access and heavy machinery are available, such as North America [22] or the UK [10], will be the first test of weathering potential in farmlands.
Widespread adoption of EW will require demonstration of the effectiveness of EW for the global benefit of C sequestration and local benefits of N loss reduction, base cation buffering and nutrient addition that will benefit farmers directly. While C sequestration is of global importance, few farmers will be willing to expend the cost of basalt additions without commensurate improvements in yield or soil fertility, and assurances that basalt application will not negatively influence long-term productivity, crop value, or the health of farm workers, neighbouring landowners, or consumers. Field trials are needed to quantify C capture and demonstrate agricultural benefits of weathering by-products. Additional uncertainties surrounding EW include long-term effects of climate manipulation, varied rates of weathering at different global locations, availability (logistic and financial) of basalt to landowners, both government and landowner perception of the value of C sequestration, and the unforeseen risks and benefits of rock fertilizers.
6. Future of agricultural and bioenergy lands: looking toward 2050
According to FAO estimates [9], the global population will increase to 9.1B by 2050 and world energy demand will rise between 20 and 100% (table 1) [61]. Currently, 37% of global land area is used for agriculture, including both cropland and pasture, and agricultural production is expected to grow at approximately 1% per year through 2050 [9]. By 2050, cereal grain production for food and fuel is expected to increase 46% from 2005 yields, and oils 81% (tables 1 and 2) [9]. Higher productivity requires increased retention and effectiveness of N fertilizers, with consumption expected to increase 1.4% per year between 2012 and 2030 [9]. Biofuel predictions for 2020 (table 2) indicate an increasing demand for biomass for energy production. Bioenergy and non-food crop production are predicted to increase between 71 and 200% by 2050 (table 1) [9,13,62,63], potentially tripling land area in energy crop production. While the development of bioenergy crops and EW were both conceived to combat greenhouse gases and climate change, a shifting climate will exert feedbacks on both. Higher temperatures and rising CO2 concentrations may increase arable land area and crop yields in high latitude regions, but may accelerate organic C decomposition in soils or create desert conditions unfit for agriculture in drier regions [63,64]. Rates of EW may be increased by higher temperatures, but limited by reduced rainfall. The optimal locations for deploying EW will shift, as will agricultural production, in response to climate variability.
7. Conclusion
Strategies for mitigating the effects of atmospheric CO2 in the Earth system as the human population increases are required and our review indicates that EW with basalt has the potential to harness a natural process for C sequestration at globally relevant scales in agroecosystems while benefitting food and fuel production. EW on agricultural lands could combat soil acidification and N loss while providing plant-essential nutrients, two major issues associated with intensive cropland farming. However, caution is required before large-scale deployment can be considered. We need better understanding of potential positive and negative impacts on crop production and feedbacks on soil biogeochemistry and unforeseen consequences. Small scale pilot studies that provide empirical data and build public trust and support are essential next steps.
Data accessibility
This work does not contain any new experimental or observational data.
Competing interests
We have no significant competing financial, professional or personal interest that might have influenced the materials presented in this manuscript.
Funding
This paper is a product of the Leverhulme Centre for Climate Change Mitigation, funded by the Leverhulme Trust through a Research Centre award (RC-2015-029).
References
- 1.Moosdorf N, Hartmann J, Lauerwald R, Hagedorn B, Kempe S. 2011. Atmospheric CO2 consumption by chemical weathering in North America. Geochim. Cosmochim. Acta 75, 7829–7854. ( 10.1016/j.gca.2011.10.007) [DOI] [Google Scholar]
- 2.Hartmann J, West AJ, Renforth P, Kohler P, De La Rocha CL, Wolf-Gladrow DA, Durr HH, Scheffran J. 2013. Enhanced chemical weathering as a geoengineering strategy to reduce atmospheric carbon dioxide, supply nutrients, and mitigate ocean acidification. Rev. Geophys. 52, 113–149. ( 10.1002/rog.20004) [DOI] [Google Scholar]
- 3.Taylor LL, Quirk J, Thorley RMS, Kharecha PA, Hansen J, Ridgwell A, Lomas MR, Banwart SA, Beerling DJ. 2016. Enhanced weathering strategies for stabilizing climate and averting ocean acidification. Nat. Clim. Change 6, 402–406. ( 10.1038/nclimate2882) [DOI] [Google Scholar]
- 4.Schuiling RD, Krijgsman P. 2006. Enhanced weathering: an effective and cheap tool to sequester CO2. Clim. Change 74, 349–354. ( 10.1007/s10584-005-3485-y) [DOI] [Google Scholar]
- 5.Vitousek PM, Aber JD, Howarth RW, Likens GE, Matson PA, Schindler DW, Schlesinger WH, Tilman DG. 1997. Human alteration of the global nitrogen cycle: sources and consequences. Ecol. Appl. 7, 737–750. ( 10.1890/1051-0761(1997)007[0737:haotgn]2.0.co;2) [DOI] [Google Scholar]
- 6.Lal R. 2004. Soil carbon sequestration to mitigate climate change. Geoderma 123, 1–22. ( 10.1016/j.geoderma.2004.01.032) [DOI] [Google Scholar]
- 7.Anderson-Teixeira KJ, Davis SC, Master MD, DeLucia EH. 2009. Changes in soil organic carbon under biofuel crops. Glob. Change Biol. Bioenergy 1, 75–96. ( 10.1111/j.1757-1707.2008.01001.x) [DOI] [Google Scholar]
- 8.Anderson-Teixeira KJ, Masters MD, Black CK, Zeri M, Hussain MZ, Bernacchi CJ, DeLucia EH. 2013. Altered belowground carbon cycling following land-use change to perennial biofuel crops. Ecosystems 16, 508–520. ( 10.1007/s10021-012-9628-x) [DOI] [Google Scholar]
- 9.FAO. 2012. World agriculture: towards 2030/2050: The 2012 Revision. ESA Working Paper No. 12-03. Rome. http://www.fao.org/docrep/016/ap106e/ap106e.pdf.
- 10.Renforth P. 2012. The potential of enhanced weathering in the UK. Int. J. Greenh. Gas Control 10, 229–243. ( 10.1016/j.ijggc.2012.06.011) [DOI] [Google Scholar]
- 11.Anderson-Teixeira KJ, Duval BD, Long SP, DeLucia EH. 2012. Biofuels on the landscape: Is ‘land sharing’ preferable to ‘land sparing’? Ecol. Appl. 22, 2035–2048. ( 10.1890/12-0711.1) [DOI] [PubMed] [Google Scholar]
- 12.Davis SC, Parton WJ, Del Grosso SJ, Keough C, Marx E, Adler PR, DeLucia EH. 2012. Impact of second generation agriculture on greenhouse-gas emissions in the corn-growing regions of the US. Front. Ecol. Environ. 10, 69–74. ( 10.1890/110003) [DOI] [Google Scholar]
- 13.Popp J, Lakner Z, Harangi-Rakos M, Fari M. 2014. The effect of bioenergy expansion: food, energy, and environment. Renew. Sustain. Energy Rev. 32, 559–578. ( 10.1016/j.rser.2014.01.056) [DOI] [Google Scholar]
- 14.Hinsinger P, Barrios ONF, Benedetti MF, Noack Y, Callot G. 2001. Plant-induced weathering of a basaltic rock: experimental evidence. Geochim. Cosmochim. Acta 65, 137–152. ( 10.1016/S0016-7037(00)00524-X) [DOI] [Google Scholar]
- 15.Moosdorf N, Renforth P, Hartmann J. 2014. Carbon dioxide efficiency of terrestrial enhanced weathering. Environ. Sci. Technol. 48, 4809–4816. ( 10.1021/es4052022) [DOI] [PubMed] [Google Scholar]
- 16.Dessert C, Dupre B, Gaillardet J, Francois LM, Allegre CJ. 2003. Basalt weathering laws and the impact of basalt weathering on the global carbon cycle. Chem. Geol. 202, 257–273. ( 10.1016/j.chemgeo.2002.10.001) [DOI] [Google Scholar]
- 17.Islam A, White RE, Chen D. 2006. Nitrification activity in acid soils of north-eastern Victoria, Australia, as affected by liming and phosphorus fertilisation. Aust. J. Soil Res. 44, 739–744. ( 10.1071/SR06058) [DOI] [Google Scholar]
- 18.Nunes JMG, Kautzmann RM, Oliveira C. 2014. Evaluation of the natural fertilizing potential of basalt dust wastes from the mining district of Nova Prata (Brazil). J. Clean. Prod. 84, 649–656. ( 10.1016/j.jclepro.2014.04.032) [DOI] [Google Scholar]
- 19.Shaaban M, Peng Q, Lin S, Wu Y, Zhao J, Hu R. 2014. Nitrous oxide emission from two acidic soils as affected by dolomite application. Soil Res. 52, 841–848. ( 10.1071/SR14129) [DOI] [Google Scholar]
- 20.Shaaban M, Wu Y, Peng Q, Lin S, Mo Y, Wu L, Hu R, Zhou W. 2015. Effects of dicyandiamide and dolomite application on N2O emission from an acidic soil. Environ. Sci. Pollut. Res. 23, 6334–6342. ( 10.1007/s11356-015-5863-y) [DOI] [PubMed] [Google Scholar]
- 21.McDougall I. 1976. Geochemistry and origin of basalt of the Columbia River Group, Oregon and Washington. Geol. Soc. Am. Bull. 87, 777–792. () [DOI] [Google Scholar]
- 22.Marsh J. 1987. Basalt geochemistry and tectonic discrimination within continental flood basalt provinces. J. Volcanol. Geotherm. Res 32, 35–49. ( 10.1016/0377-0273(87)90035-7) [DOI] [Google Scholar]
- 23.Reichow MK, Saunders AD, White RV, Al'Mukhamedov AI, Medvedev AYA. 2005. Geochemistry and petrogenesis of basalts from the Western Siberian Basin: an extension of the Permo-Triassic Siberian Traps, Russia. Lithos 79, 425–452. ( 10.1016/j.lithos.2004.09.011) [DOI] [Google Scholar]
- 24.Berner RA. 1997. The rise of plants and their effect on weathering and atmospheric CO2. Science 276, 544–546. ( 10.1126/science.276.5312.544) [DOI] [Google Scholar]
- 25.Eggleton RA, Foudoulis C, Varkevisser D. 1987. Weathering of basalt: changes in rock chemistry and minerology. Clays Clay Miner. 35, 161–169. ( 10.1346/CCMN.1987.0350301) [DOI] [Google Scholar]
- 26.Gudbrandsson S, Wolff-Boenisch D, Gislason SR, Oelkers EH. 2011. An experimental study of crystalline basalt dissolution from 2<pH<11 and temperatures from 5 to 75°C. Geochim. Cosmochim. Acta 75, 5496–5509. ( 10.1016/j.gca.2011.06.035) [DOI] [Google Scholar]
- 27.Truog E. 1948. Lime in relation to availability of plant nutrients. Soil Sci. 65, 1–8. ( 10.1097/00010694-194801000-00002) [DOI] [Google Scholar]
- 28.Amiotte-Suchet P, Probst JL. 1993. Modelling of atmospheric CO2 consumption by chemical weathering of rocks: applications to the Garonne, Congo and Amazon basins. Chem. Geol. 107, 205–210. ( 10.1016/0009-2541(93)90174-H) [DOI] [Google Scholar]
- 29.Bormann BT, Wang D, Snyder MC, Bormann FH, Benoit G, April R. 1998. Rapid, plant-induced weathering in an aggrading experimental ecosystem. Biogeochemistry 43, 129–155. ( 10.1023/A:1006065620344) [DOI] [Google Scholar]
- 30.Boden TA, Marland G, Andres RJ. 2016. Global, regional, and national fossil-fuel CO2 emissions. Oak Ridge, TN, USA: Carbon Dioxide Information Analysis Center, Oak Ridge National Laboratory, U.S. Department of Energy. [Google Scholar]
- 31.Chadwick OA, Gavenda RT, Kelly EF, Ziegler K, Olson CG, Elliott WC, Hendricks DM. 2002. The impact of climate on the biogeochemical functioning of volcanic soils. Chem. Geol. 202, 195–223. ( 10.1016/j.chemgeo.2002.09.001) [DOI] [Google Scholar]
- 32.Taylor LL, Leake JR, Quirk J, Hardy K, Banwart SA, Beerling DJ. 2009. Biological weathering and the long-term carbon cycle: integrating mycorrhizal evolution and function into the current paradigm. Geobiology 7, 171–191. ( 10.1111/j.1472-4669.2009.00194.x) [DOI] [PubMed] [Google Scholar]
- 33.Quirk J, Beerling DJ, Banwart SA, Kakonyi G, Romero-Gonzales ME, Leake JR. 2012. Evolution of trees and mycorrhizal fungi intensifies silicate mineral weathering. Biol. Lett. 8, 1006–1011. ( 10.1098/rsbl.2012.0503) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Quirk J, Andrews MY, Leake JR, Banwart SA, Beerling DJ. 2014. Ectomycorrhizal fungi and past high CO2 atmospheres enhanced mineral weathering through increased below-ground carbon-energy fluxes. Biol. Lett. 10, 20140375 ( 10.1098/rsbl.2014.0375) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guo JH, Gifford RM. 2002. Soil carbon stocks and land use change: a meta-analysis. Glob. Change Biol. 8, 345–360. ( 10.1046/j.1354-1013.2002.00486.x) [DOI] [Google Scholar]
- 36.Smith P, et al. 2007. Agriculture. In Climate Change 2007: Mitigation. Contribution of Working Group III to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change (eds Metz B, Davidson OR, Bosch PR, Dave R, Meyer LA), pp. 497–540 Cambridge, UK and New York, NY: Cambridge University Press. [Google Scholar]
- 37.Paustian K, Antle JM, Sheehan J, Paul EA. 2006. Agriculture's role in greenhouse gas mitigation. Arlington, VA: Pew Center on Global Climate Change. [Google Scholar]
- 38.Guyomard H, Forslund A, Dronne Y. 2001. Biofuels and world agricultural markets: outlook for 2020 and 2050. In Economic effects of biofuel production. (ed dos Santos Bernardes MA.), pp. 129–162 Rijeka, Croatia: InTech; 2011. ISBN 978-953-307-178-7. ( 10.5772/20581) [DOI] [Google Scholar]
- 39.Hartmann J, Moosdorf N, Lauerwald R, West AJ, Hinderer M. 2014. Global chemical weathering and associated P-release: the role of lithology, temperature and soil properties. Chem. Geol. 363, 145–163. ( 10.1016/j.chemgeo.2013.10.025) [DOI] [Google Scholar]
- 40.Doney SC, Fabry VJ, Feely RA, Kleypas JA. 2009. Ocean acidification: the other CO2 problem. Ann. Rev. Mar. Sci. 1, 169–192. ( 10.1146/annurev.marine.010908.163834) [DOI] [PubMed] [Google Scholar]
- 41.Wood S, Cowie A. 2004. A review of greenhouse gas emission factors for fertilizer production. IEA bioenergy task. Vol. 38. No. 1. See http://ecite.utas.edu.au/87108/1/WoodCowie2004_EmissionsFertiliser.pdf.
- 42.Sutton MA, Oenema O, Erisman JW, Leip A, van Grinsven H, Winiwarter W. 2011. Too much of a good thing. Nature 472, 159–161. ( 10.1038/472159a) [DOI] [PubMed] [Google Scholar]
- 43.Forster P, et al. 2007. Changes in atmospheric constituents and in radiative forcing. In Climate Change 2007: the physical science basis. (eds Solomon S, Qin D, Manning M, Chen Z, Marquis M, Averty KB, Tignor M, Miller HL), pp. 129–234 Cambridge, UK and New York, NY: Cambridge University Press. [Google Scholar]
- 44.Brumme R, Beese F. 1992. Effects of liming and nitrogen fertilization on emissions of CO2 and N2O on temperate forest. J. Geophys. Res. 97, 12 851–12 858. ( 10.1029/92JD01217) [DOI] [Google Scholar]
- 45.Borken W, Brumme R. 1997. Liming practice in temperate forest ecosystems and the effects on CO2, N2O and CH4 fluxes. Soil Use Manage. 13, 251–257. ( 10.1111/j.1475-2743.1997.tb00596.x) [DOI] [Google Scholar]
- 46.Bakken LR, Bergaust L, Liu B, Frostegard A. 2012. Regulation of denitrification at the cellular level: a clue to the understanding of N2O emissions from soils. Phil. Trans. R. Soc. B 367, 1226–1234. ( 10.1098/rstb.2011.0321) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Qu Z, Wang J, Almoy T, Bakken L. 2013. Excessive use of nitrogen in Chinese agriculture results in high N2O/(N2O + N2) product ratio of denitrification, primarily due to acidification of the soils. Glob. Change Biol. 20, 1685–1698. ( 10.1111/gcb.12461) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Iowa State University Extension and Outreach. 2013. A general guide for crop nutrient and limestone recommendations in Iowa. PM 1688. Ames, IA: Iowa State University Cooperative Extension Service.
- 49.Von-Uexkull H, Mutert E. 1995. Global extent, development and economic impact of acid soils. Plant Soil 171, 1–15. ( 10.1007/BF00009558) [DOI] [Google Scholar]
- 50.Barak P, Jobe BO, Kreuger AR, Peterson LA, Laird DA. 1997. Effects of long-term soil acidification due to nitrogen fertilizer inputs in Wisconsin. Plant Soil 197, 61–69. ( 10.1023/A:1004297607070) [DOI] [Google Scholar]
- 51.Barak P, Chen Y, Singer A. 1983. Ground basalt and tuff as iron fertilizers for calcareous soils. Plant Soil 73, 155–158. ( 10.1007/BF02197765) [DOI] [Google Scholar]
- 52.Ma JF, Miyake Y, Takahashi E. 2001. Silicon as a beneficial element for crop plants. Stud. Plant Sci. 8, 17–39. ( 10.1016/S0928-3420(01)80006-9) [DOI] [Google Scholar]
- 53.Burghelea C, Zaharescu DG, Dontsova K, Maier R, Huxman T, Chorover J. 2014. Mineral nutrient mobilization by plants from rock: influence of rock type and arbuscular mycorrhiza. Biogeochemistry 124, 187–203. ( 10.1007/s10533-015-0092-5) [DOI] [Google Scholar]
- 54.Miller RM, Jastrow JD. 2000. Mycorrhizal fungi influence soil structure. In Arbuscular Mycorrhizas: physiology and function (eds Kapulnik Y, Douds DD Jr), pp. 3–18 Dordrecht, The Netherlands: Springer-Science+Business Media. [Google Scholar]
- 55.Xie J, Li Y, Zhai C, Li C, Lan Z. 2009. CO2 absorption by alkaline soils and its implication to the global carbon cycle. Environ. Geol. 56, 953–961. ( 10.1007/s00254-008-1197-0) [DOI] [Google Scholar]
- 56.Dronne Y, Forslund A, Guyomard H. 2011. Les biocarburants de deuxième génération et la compétition pour l'usage des terres. OCL 18, 1–9. ( 10.1051/ocl.2011.0361) [DOI] [Google Scholar]
- 57.Hudiburg TW, Wang WW, Khanna M, Long SP, Dwivedi P, Parton WJ, Hartman M, DeLucia EH. 2016. Impacts of a 32-billion-gallon bioenergy landscape on land and fossil fuel use in the US. Nat. Energy 1, 15005 ( 10.1038/nenergy.2015.5) [DOI] [Google Scholar]
- 58.Coffin MF, Eldholm O. 1994. Large igneous provinces: crustal structure, dimensions, and external consequences. Rev. Geophys. 32, 1–36. ( 10.1029/93RG02508) [DOI] [Google Scholar]
- 59.NASS. 2016. Acreage (June 2016). Agricultural Statistics Board. United States Department of Agriculture; See http://usda.mannlib.cornell.edu/usda/current/Acre/Acre-06-30-2016.pdf. [Google Scholar]
- 60.EPA. 2016. Inventory of US greenhouse gas emissions and sinks: 1990–2014. US Environmental Protection Agency; https://www.epa.gov/sites/production/files/2016-04/documents/us-ghg-inventory-2016-main-text.pdf. [Google Scholar]
- 61.IEA. 2012. World energy outlook. Paris, France: The International Energy Agency. [Google Scholar]
- 62.Erb K-H, Haberl H, Plutzar C. 2012. Dependency of global primary bioenergy crop potentials in 2050 on food systems, yields, biodiversity conservation and political stability. Energy Policy 47, 260–269. ( 10.1016/j.enpol.2012.04.066) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.IPCC. 2001. Climate change: the scientific basis. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 64.Hager HA, Sinasac SE, Gedalof Z, Newman JA. 2014. Predicting potential global distribution of two Miscanthus grasses: implications for horticulture, biofuel production, and invasion. PLoS ONE 9, e100032 ( 10.1371/journal.pone.0100032) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This work does not contain any new experimental or observational data.