Abstract
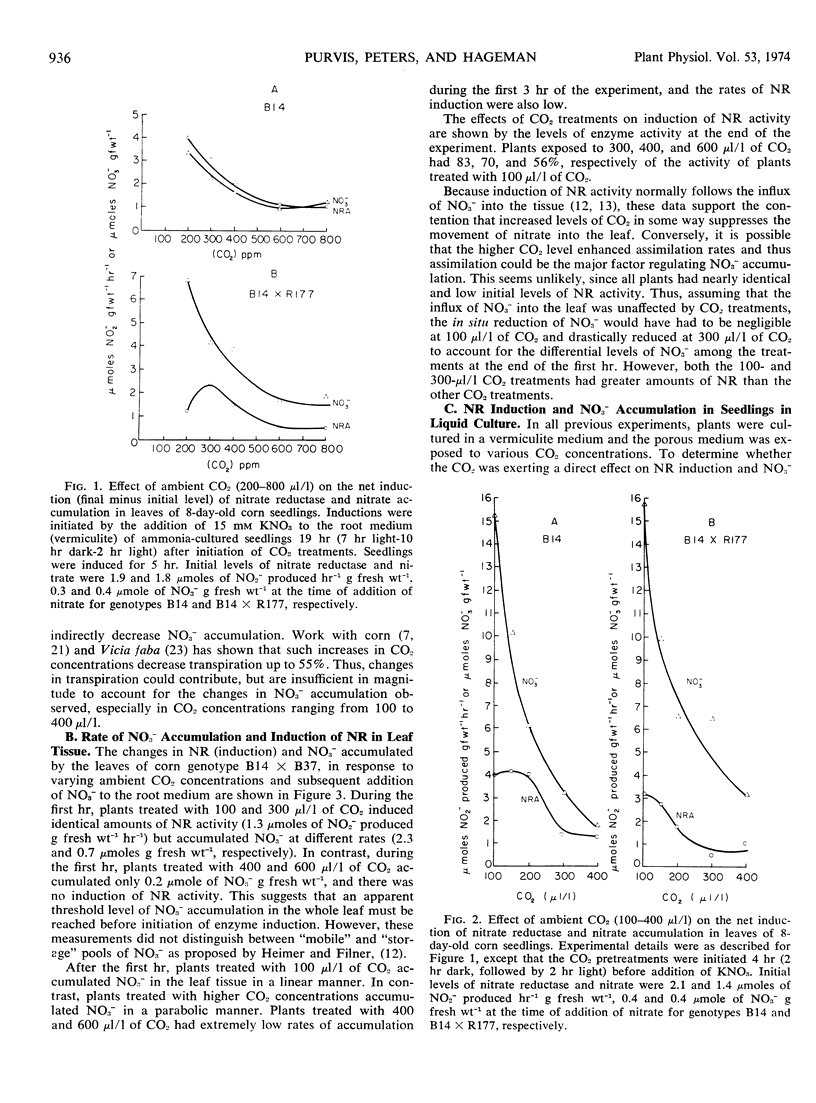
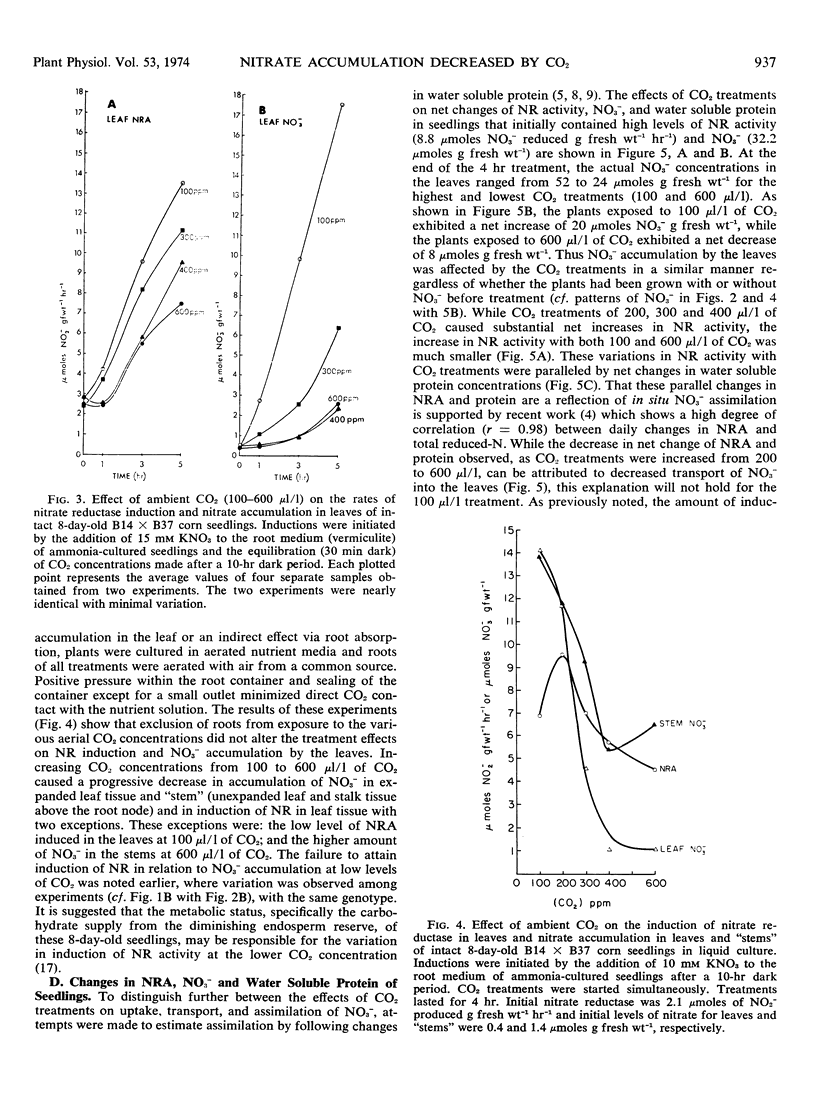
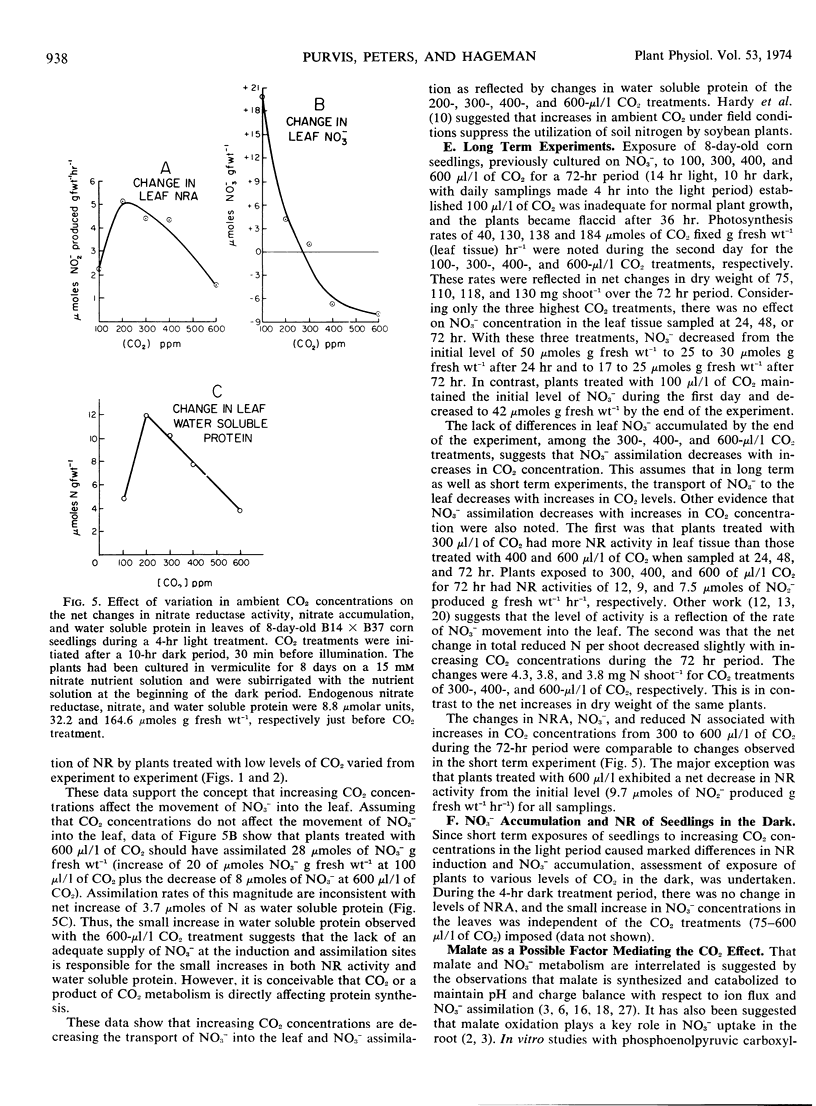
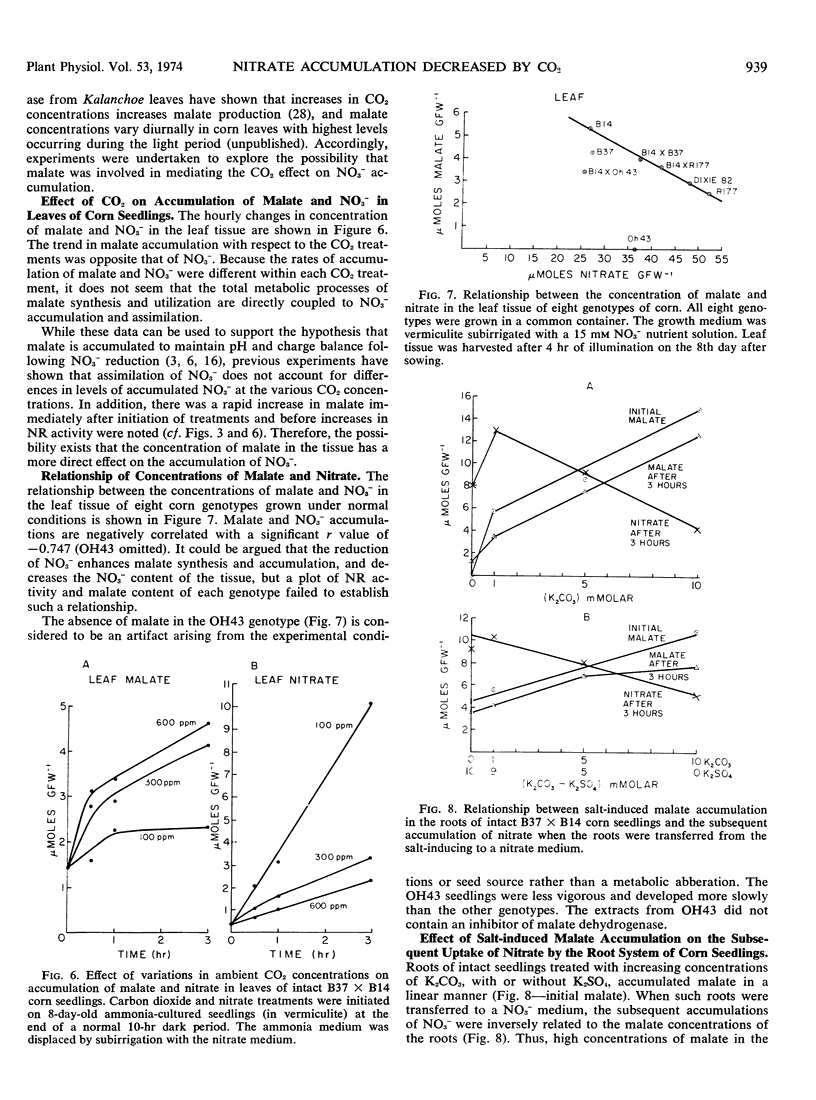
Exposure of the leaf canopy of corn seedlings (Zea mays L.) to atmospheric CO2 levels ranging from 100 to 800 μl/l decreased nitrate accumulation and nitrate reductase activity. Plants pretreated with CO2 in the dark and maintained in an atmosphere containing 100 μl/l CO2 accumulated 7-fold more nitrate and had 2-fold more nitrate reductase activity than plants exposed to 600 μl/l CO2, after 5 hours of illumination. Induction of nitrate reductase activity in leaves of intact corn seedlings was related to nitrate content. Changes in soluble protein were related to in vitro nitrate reductase activity suggesting that in vitro nitrate reductase activity was a measure of in situ nitrate reduction. In longer experiments, levels of nitrate reductase and accumulation of reduced N supported the concept that less nitrate was being absorbed, translocated, and assimilated when CO2 was high. Plants exposed to increasing CO2 levels for 3 to 4 hours in the light had increased concentrations of malate and decreased concentrations of nitrate in the leaf tissue. Malate and nitrate concentrations in the leaf tissue of seven of eight corn genotypes grown under comparable and normal (300 μl/l CO2) environments, were negatively correlated. Exposure of roots to increasing concentrations of potassium carbonate with or without potassium sulfate caused a progressive increase in malate concentrations in the roots. When these roots were subsequently transferred to a nitrate medium, the accumulation of nitrate was inversely related to the initial malate concentrations. These data suggest that the concentration of malate in the tissue seem to be related to the accumulation of nitrate.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chen S., McMahon D., Bogorad L. Early effects of illumination on the activity of some photosynthetic enzymes. Plant Physiol. 1967 Jan;42(1):1–5. doi: 10.1104/pp.42.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hageman R. H., Flesher D. Nitrate Reductase Activity in Corn Seedlings as Affected by Light and Nitrate Content of Nutrient Media. Plant Physiol. 1960 Sep;35(5):700–708. doi: 10.1104/pp.35.5.700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimer Y. M., Filner P. Regulation of the nitrate assimilation pathway in cultured tobacco cells. 3. The nitrate uptake system. Biochim Biophys Acta. 1971 Feb 23;230(2):362–372. doi: 10.1016/0304-4165(71)90223-6. [DOI] [PubMed] [Google Scholar]
- Jackson W. A., Flesher D., Hageman R. H. Nitrate Uptake by Dark-grown Corn Seedlings: Some Characteristics of Apparent Induction. Plant Physiol. 1973 Jan;51(1):120–127. doi: 10.1104/pp.51.1.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacoby B., Laties G. G. Bicarbonate Fixation and Malate Compartmentation in Relation to Salt-induced Stoichiometric Synthesis of Organic Acid. Plant Physiol. 1971 Apr;47(4):525–531. doi: 10.1104/pp.47.4.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klepper L., Flesher D., Hageman R. H. Generation of reduced nicotinamide adenine dinucleotide for nitrate reduction in green leaves. Plant Physiol. 1971 Nov;48(5):580–590. doi: 10.1104/pp.48.5.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Pallas J. E., Wright B. G. Organic Acid Changes in the Epidermis of Vicia faba and Their Implication in Stomatal Movement. Plant Physiol. 1973 Mar;51(3):588–590. doi: 10.1104/pp.51.3.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrader L. E., Ritenour G. L., Eilrich G. L., Hageman R. H. Some characteristics of nitrate reductase from higher plants. Plant Physiol. 1968 Jun;43(6):930–940. doi: 10.1104/pp.43.6.930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WALKER D. A., BROWN J. M. Physiological studies on acid metabolism. 5. Effects of carbon dioxide concentration on phosphoenolpyruvic carboxylase activity. Biochem J. 1957 Sep;67(1):79–83. doi: 10.1042/bj0670079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zschoche W. C., Ting I. P. Malate Dehydrogenases of Pisum sativum: Tissue Distribution and Properties of the Particulate Forms. Plant Physiol. 1973 Jun;51(6):1076–1081. doi: 10.1104/pp.51.6.1076. [DOI] [PMC free article] [PubMed] [Google Scholar]


