Abstract
Dramatic changes of gene expressions occur in human endometrial stromal cells (ESCs) during decidualization. The changes in gene expression are associated with changes of chromatin structure, which are regulated by histone modifications. Here we investigated genome-wide changes in histone modifications associated with decidualization in human ESCs using chromatin immunoprecipitation combined with next-generation sequencing. ESCs were incubated with estradiol and medroxyprogesterone acetate for 14 days to induce decidualization. The chromatin immunoprecipitation-sequence data showed that induction of decidualization increased H3K27ac and H3K4me3 signals in many genomic regions but decreased in only a few regions. Most of the H3K27ac-increased regions (80%) and half of the H3K4me3-increased regions were located in the distal promoter regions (more than 3 kb upstream or downstream of the transcription start site). RNA sequence showed that induction of decidualization up-regulated 881 genes, 223 of which had H3K27ac- or H3K4me3-increased regions in the proximal and distal promoter regions. Induction of decidualization increased the mRNA levels of these genes more than it increased the mRNA levels of genes without H3K27ac- or H3K4me3-increased regions. Pathway analysis revealed that up-regulated genes with the H3K27ac- or H3K4me3-increased regions were associated with the insulin signaling, which may be involved in glucose uptake that is necessary for ESCs to undergo decidualization. These results show that histone modification statuses on a genome-wide basis change in human ESCs during decidualization. The main changes of histone modifications are increases of H3K27ac and H3K4me3 in both the proximal and distal promoter regions, which are involved in the up-regulation of gene expression that occurs during decidualization.
Human endometrial stromal cells (ESCs) undergo cyclic changes during the menstrual cycle, including proliferation and differentiation that are controlled by estrogen and progesterone. Decidualization is one of the changes induced by progesterone and is characterized by differentiation of fibroblastoid ESCs of the estrogen-primed endometrium into decidual cells. This process is crucial for embryo implantation and maintenance of pregnancy (1). Impaired decidualization of endometrial stroma is associated with recurrent miscarriage, implantation failure, and unexplained infertility (2, 3). ESCs isolated from human endometrium and cultured in the presence of progesterone and estrogen exhibit morphological and functional changes in vitro that mimic in vivo decidual transformation (1). Using the in vitro model of decidualization, many studies have addressed the molecular mechanisms underlying decidual transformation. Genome-wide microarray analyses have identified a number of genes that are up-regulated or down-regulated by decidualization in human ESCs (4–6), suggesting that decidualization is the process of differentiation accompanied by the dramatic changes of cell functions.
Gene expression including transcription involves a change of chromatin structure, which can be regulated by epigenetic mechanisms such as histone modifications (7, 8). Recent studies on histone modifications have reported that acetylation of histone-H3 lysine-27 (H3K27ac) and monomethylation or trimethylation of histone-H3 lysine-4 (H3K4me1, H3K4me3) are associated with activation of gene transcription, whereas trimethylation of histone-H3 lysine-27 (H3K27me3) is associated with transcriptional inactivation (9–12). We have reported that expressions of IGF-binding protein-1 and prolactin (PRL), which are preferentially induced by decidualization in ESCs, are associated with the histone acetylation status of their promoter regions (13, 14). However, little is known about the epigenetic regulation of decidualization-related gene expressions in human ESCs. We hypothesized that the bindings of key transcription factors to the promoter region are linked to the local histone modifications around the promoter regions and that histone modifications regulate gene expressions during decidualization. Recently Grimaldi et al (15) found using chromatin immunoprecipitation (ChIP) combined with a microarray analysis (ChIP-on-chip) that decidualization altered the H3K27me3 status around the transcription start site in human ESCs during decidualization, suggesting that histone modifications are affected by decidualization. However, it is unclear how histone modifications other than H3K27me3 change and whether histone modifications are altered on a genome-wide basis in ESCs during decidualization.
The combination of chromatin immunoprecipitation with next-generation sequencing technology (ChIP-seq) is a powerful method for genome-wide profiling of histone modifications, and offers many advantages over ChIP-on-chip (16). Cell lines or cancer cells have been mainly used in ChIP-seq studies, but little information is available in primary cultured cells derived from normal human tissues (17, 18). In this study, we used two genome-wide approaches, ChIP-seq and RNA sequence (RNA-seq) to investigate the change of histone modifications during decidualization in human primary ESCs and identified a number of genes in which histone modifications statuses and mRNA levels are altered during decidualization. The present study provides important data on the regulation of decidualization-related gene expressions by histone modifications.
Materials and Methods
Reagents
DMEM, L-glutamine, 1× trypsin-EDTA, streptomycin, and penicillin were purchased from Invitrogen. Fetal bovine serum was obtained from Biological Industries Ltd. Estradiol (E) and medroxyprogesterone acetate (MPA) were obtained from Sigma Chemical Co.
ESC isolation and cell culture
Human endometrial tissues were obtained at hysterectomy from patients with a normal menstrual cycle, aged 40–45 years, who underwent surgery for myoma uteri or early stage of cervical cancer. Informed consent was obtained from all participating patients, and ethical approval was obtained from the Institutional Review Board of Yamaguchi University Hospital. Endometrial samples were histologically diagnosed as being in the late proliferative phase according to published criteria (19). Tissue samples were washed with Phenol Red-free DMEM containing 4 mM glutamine, 50 μg/mL streptomycin, and 50 IU/mL penicillin and minced into pieces of less than 1 mm3. ESCs were isolated as reported previously (20). In brief, after enzymatic digestion of minced tissues with 0.2% collagenase in a shaking water bath for 2 hours at 37°C, stromal cells were separated by filtration through a 70-μm nylon mesh. The filtrates were washed three times with the medium, and the number of viable cells was counted by Trypan blue dye exclusion. The homogeneity of the stromal cell preparation was verified by the immunocytochemistry for the stromal-reacting antibody (vimentin) (data not shown). The cells were also verified to be negative for an epithelial cell-reacting antibody (cytokeratin) (data not shown). Cells were seeded at 105 cells/cm2 in 75-cm2 tissue culture flasks and incubated in Phenol Red-free DMEM containing glutamine, antibiotics, and 10% dextran-coated charcoal-stripped fetal bovine serum at 37°C, 95% air and 5% CO2. At confluence, cells were treated with 1× trypsin-EDTA and subcultured into 75-cm2 tissue culture flasks. At 80% confluence after the first passage, the cell culture medium was changed to the treatment medium.
To induce decidualization, ESCs were incubated with or without E (10−8 mol/L) and MPA (10−6 mol/L) for 14 days at 37°C, 95% air, and 5% CO2. The medium was changed every other day. The concentration of ovarian steroids and the period of incubation were based on our previous report (21). Decidualization was confirmed by the induction of mRNA expression of IGF-binding protein-1 and PRL, which are specific markers of decidualization (1). ESCs from two different individuals were separately incubated and used for RNA-seq and ChIP-seq experiments. ESCs from three different individuals were also used for real-time RT-PCR and ChIP-quantitative PCR (qPCR) to validate the results of the RNA-seq and ChIP-seq experiments. To examine the importance of glucose for decidualization, ESCs were cultured in the medium containing normal (24 mM) or low (0 and 5 mM) glucose concentrations, which is based on a previous report (22), in the presence or absence of E (10−8 mol/L) and MPA (10−6 mol/L) for 14 days, and then decidualization was evaluated by PRL mRNA expression. Standard DMEM contains 24 mM glucose. The medium was changed every other day. A single incubation was performed in triplicate on cells isolated from one patient. Three different incubations from three individuals were performed in a single experiment.
RNA sequencing
Total RNA was isolated from cultured cells with Isogen (Wako Pure Chemical Industries Ltd). The library for mRNA-seq was generated by the modified Illumina protocol using a mRNAseq preparation kit (Illumina). Briefly, 1 μg of total RNA was enriched for polyA RNA by two successive rounds of oligodeoxythymidine selection. The polyA RNA was then fragmented, and first-strand cDNA synthesis was performed using random hexamer priming. After the second-strand cDNA synthesis, double-stranded DNA was repaired using T4 DNA polymerase, Klenow enzyme, and T4 polynucleotide kinase (PNK) (New England Biolabs), followed by treatment with Klenow exo− to add an A base to the 3′ end. After ligation of the Solexa adaptor using TaKaRa ligation Mix (TaKaRa), the adaptor-ligated DNAs were amplified using Solexa PCR primers for 18 cycles, and the amplified library was isolated from an agarose gel. The samples were purified using the QIAquick MinElute kit (QIAGEN, Inc) at each preparation step.
After the quality of the library was validated using the Agilent Bioanalyzer 2100 (Agilent Technologies), the samples were subjected to sequencing with a next-generation sequencer (genome analyzer GAIIx; Illumina). Mapping and quantification of gene expression were performed by TopHat (23) (version 2.0.9; with default parameters) followed by cufflinks (24) (version 2.1.1; with G option). Gene expression values were calculated as reads per kilobase of exon unit per million mapped reads (RPKM). For each case, genes in nondecidualized ESCs (non-dESCs; incubated with culture medium) and decidualized ESCs (dESCs; incubated with E and MPA) were classified into three groups (no expression, low level expression, and high level expression) based on the RPKM values for the genes. The no-expression group consisted of the genes with RPKM = 0. The rest of the genes, ie, those with RPKM greater than 0, were sorted by the RPKM values, and the median value was adopted for the cutoff for classifying the genes into low-level expression and high-level expression. Genes whose RPKM values increased or decreased more than 1.4-fold by decidualization stimuli were defined as up-regulated or down-regulated genes by decidualization, and the common genes of the two individuals (case 1 and case 2) were identified. The level of 1.4-fold has been widely used for the cutoff level in genome-wide analyses because 1.4-fold corresponds to 0.5 when it is transformed to log2 ratio (25, 26).
ChIP sequencing
Cells were cross-linked by adding formaldehyde to the medium at a final concentration of 1% and incubated for 10 minutes at 37°C. Cross-linking was terminated by addition of glycine (0.125 M, final concentration). ChIP was performed as described by Odawara et al (27). The following antibodies against histone modifications were used: H3K27ac, H3K4me3, H3K4me1, and H3K27me3 (generous gifts from Dr H. Kimura, Osaka University, Osaka, Japan). An unimmunoprecipitated control (INPUT) and immunoprecipitated DNA (IP) were used to prepare the ChIP-seq library with NEBNext DNA sample prep master mix set 1 (New England BioLabs) and DNA sample prep kit, oligo only (Illumina) according to the manufacturer's instructions. After the quality of the library was validated using the Agilent Bioanalyzer 2100 (Agilent Technology), the ChIP-seq library was sequenced by the genome analyzer GAIIx (Illumina). The obtained reads from the sequencing platform was first applied for quality checking by the fastx quality stats program in the FASTX-Toolkit package (http://hannonlab.cshl.edu/fastx_toolkit/). The reads that passed the quality checking were then mapped to the human reference genome sequence (hg19) by using the bowtie program (28) with the default parameters.
Identification of the regions with altered histone modifications by decidualization
The regions with altered signals of histone modifications by induction of decidualization were identified by comparing the ChIP-seq tag counts between non-dESCs and dESCs as described previously (29). The regions between −10 kb from the transcription start site (TSS) and +10 kb from the transcription termination site (TTS) were scanned with a 1-kb sliding window with a step size of 200 bp. For each sliding window, the number of reads for both IP and INPUT samples were normalized by calculating the fragments per kilobase of the window per million mapped fragments (FPKM). To obtain the signal in each window, the normalized INPUT value was subtracted from the normalized IP value for the corresponding window. We added 1 to the FPKM value before log2 transformation, and then the difference in the signals between non-dESCs and dESCs was evaluated by a scatter plot. Regions showing more than a 2-fold increase or decrease in signals between non-dESCs and dESCs were defined as having increased or decreased histone modifications, respectively.
The common regions of the two individuals (case 1 and case 2) were considered as the regions in which histone modification signals were altered by induction of decidualization. Regions with the altered H3K27ac or H3K4me3 signals (H3K27ac or H3K4me3 altered regions) were classified by the location of the signals: upstream (−10 kb upstream from the TSS), gene body (between TSS and TTS, including introns and exons) and intergenic (+10 kb downstream from the TTS). If the H3K27ac- or H3K4me3-altered region spanned two classes, it was counted in both classes. The distances between the H3K27ac- or H3K4me3-altered regions and the TSS of their adjacent genes were analyzed and classified into 3 groups according to a previous report (29): proximal promoter (within ±3 kb region from the TSS), distal upstream promoter (more than 3 kb upstream from the TSS), and distal downstream promoter (more than 3 kb downstream from the TSS). For a Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analysis, the up-regulated genes with or without H3K27ac- or H3K4me3-increased regions were submitted to the Database for Annotation, Visualization, and Integrated Discovery (DAVID) bioinformatics database (30). Pathways with both a P value and Benjamini value of P < .05 were considered as significantly enriched.
Real-time RT-PCR
Real-time RT-PCR was performed as reported previously (31). First-strand cDNA was synthesized from 2 μg total RNA with reverse transcriptase (Invitrogen) in 20 μL of reaction mixture. Quantitative real-time PCR was performed with sequence-specific primer sets of insulin receptor substrate 1 (IRS1), insulin receptor substrate 2 (IRS2), insulin receptor (INSR), forkhead box O1 (FOXO1), v-akt murine thymoma viral oncogene homolog 3 (AKT3), mitogen-activated protein kinase 10 (MAPK10), PRL, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (Supplemental Table 1).
Chromatin immunoprecipitation-quantitative PCR
ChIP-qPCR was performed using antibodies for H3K27ac, H3K4me3, and normal mouse IgG (Millipore Corp) as reported previously (14). Primers for ChIP-qPCR were designed within the H3K27ac- or H3K4me3-increased region identified by ChIP-seq (Supplemental Table 2). To determine the relative levels of histone modification of each target sequence, real-time PCR analysis was performed and the ratio of IP DNA to the INPUT DNA sample was calculated in non-dESCs and dESCs.
Glucose uptake assay
The levels of glucose uptake in non-dESCs and dESCs were determined using a 2-deoxygulcose (2-DG) uptake assay kit (Cosmobio). Non-dESCs and dESCs were incubated with serum and glucose free-DMEM for 6 hours. Cells were washed three times with Krebs-Ringer-phosphate-HEPES buffer (20 mM HEPES; 5 mM KH2PO4; 1 mM MgSO4; 1 mM CaCl2; 136 mM NaCl; and 4.7 mM KCl, pH 7.4, 37°C) containing 2% BSA and were further incubated at 37°C for 20 minutes with Krebs-Ringer-phosphate-HEPES buffer containing 2% BSA and 0.1 mM 2-DG. Then the cells were washed three times with PBS, collected in 400 μL of 10 mM Tris-HCl (pH 8.0), heated at 80°C for 15 minutes, and centrifuged at 15 000 × g for 20 minutes at 4°C. The supernatants were assayed for a 2-DG uptake measurement. A single incubation was performed in triplicate on cells isolated from one patient. Three different incubations from three individuals were performed in a single experiment.
Statistical analyses
The distributions of fold changes in mRNA levels by decidualization stimuli were plotted in box plots, and the significance of the difference was estimated using the Mann-Whitney U test on fold changes (see Figures 4 and 5). P values were then adjusted for multiple comparisons by Bonferroni correction. Unpaired t tests were applied to analyze differences between 2 groups (see Figure 6 and Figure 8A). One-way ANOVA followed by the Tukey-Kramer test was applied to analyze differences between 6 groups (see Figure 8B). All statistical analyses were performed using SPSS for Windows version 11 (SPSS Inc). Differences were considered significant at P < .05.
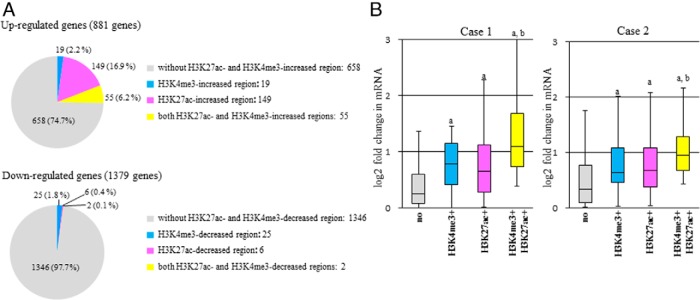
Figure 4. Histone modifications of H3K27ac or H3K4me3 and the up- or down-regulation of gene expression by decidualization.
mRNA levels were analyzed on a genome-wide basis by RNA-seq in dESCs and non-dESCs. Eight hundred eighty-one up-regulated genes and 1379 down-regulated genes (greater than 1.4 fold changes) by decidualization stimuli were identified, which were common in the two individuals (case 1 and case 2). The common regions with altered signals of H3K27ac or H3K4me3 were identified between the two individuals (Table 1). A, Up- or down-regulated genes were classified into 4 groups based on whether they have H3K27ac- or H3K4me3-increased or decreased regions, respectively: 1) genes without H3K27ac- and H3K4me3-altered regions, 2) genes with H3K4me3-altered regions, 3) genes with H3K27ac-altered regions, and 4) genes with both H3K27ac- and H3K4me3-altered regions. The numbers of genes in each group are shown. B, The fold changes in mRNA levels of the up-regulated genes by decidualization stimuli were compared among the 4 groups in each individual. The fold changes in the mRNA levels were calculated by dividing the dESCs (RPKM+1) by the non-dESCs (RPKM+1) obtained from RNA-seq. Box plots show the distribution of log2-transformed fold change value of each group: genes without H3K27ac- and H3K4me3-increased regions (no), genes with H3K4me3-increased regions (H3K4me3+), genes with H3K27ac-increased regions (H3K27ac+), and genes with both H3K27ac- and H3K4me3-altered regions (H3K4me3+, H3K27acl+). Data from each individual (case 1 and case 2) are shown. Solid bars of boxes display the interquartile ranges (25%–75%) with an intersection as the median. The whiskers extending outside the boxes correspond to the lowest and highest data within 1.5-fold interquartile range from the upper and lower quartile. P values are calculated using a Mann-Whitney U test followed by Bonferroni correction. a, P < .01 vs no; b, P < .01 vs H3K27ac+.
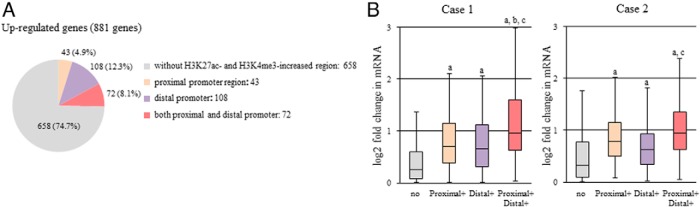
Figure 5. Genomic location of H3K27ac- or H3K4me3-increased regions and the up-regulation of gene expression by decidualization.
A, Up-regulated genes by decidualization stimuli were classified into 4 groups: 1) genes without H3K27ac- and H3K4me3-increased regions, 2) genes with H3K27ac- or H3K4me3-increased regions in the proximal promoter region, 3) genes with H3K27ac- or H3K4me3-increased regions in the distal promoter region, and 4) genes with H3K27ac- or H3K4me3-increased regions in both the proximal and distal promoter regions. The distal promoter region includes both distal upstream promoter (more than 3 kb upstream from the TSS) and distal downstream promoter (more than 3 kb downstream from TSS). The numbers of genes in each group are shown. B, The fold changes in mRNA levels of the up-regulated genes by decidualization stimuli were compared among the 4 groups in each individual. The fold changes in mRNA levels were calculated by dividing the dESCs (RPKM+1) by the non-dESCs (RPKM+1) obtained from RNA-seq. Box plots show the distribution of log2-transformed fold change value of each group: genes without H3K27ac- and H3K4me3-increased regions (no), genes with H3K27ac- or H3K4me3-increased regions in the proximal promoter region (proximal+), in the distal promoter region (distal+), and in both proximal and distal promoter regions (proximal+, distal+). Data from each individual (case 1 and case 2) are shown. Solid bars of boxes display the interquartile ranges (25%–75%) with an intersection as the median. The whiskers extending outside the boxes correspond to the lowest and highest data within 1.5-fold interquartile range from the upper and lower quartile. P values are calculated using a Mann-Whitney U test followed by Bonferroni correction. a, P < .01 vs no; b, P < .05 vs proximal+; c, P < .01 vs distal+.
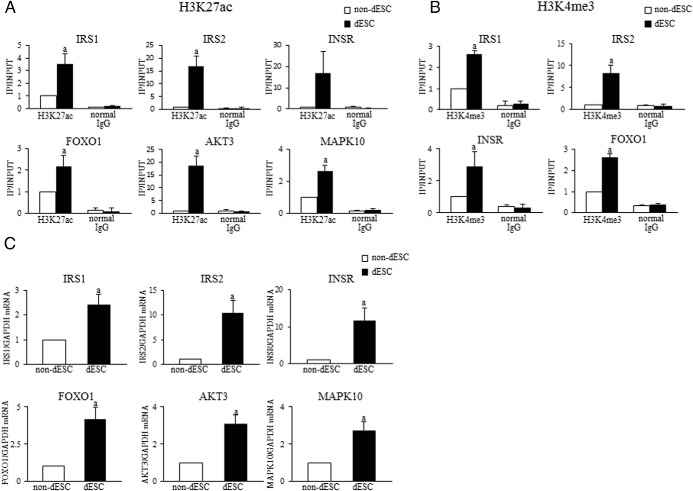
Figure 6. Validation of the results of ChIP-seq and RNA-seq experiments.
ESCs were treated with or without E + MPA for 14 days to induce decidualization and used for the ChIP-qPCR and real time RT-PCR experiments. Six genes (IRS1, IRS2, INSR, FOXO1, AKT3, and MAPK10) were selected as the target genes to validate the result of ChIP-seq and RNA-seq. A and B, H3K27ac and H3K4me3 levels were analyzed by ChIP-qPCR in the promoter regions of insulin signaling genes in non-dESCs and dESCs. Primers for ChIP-qPCR were designed within the H3K27ac- or H3K4me3-increased region identified by ChIP-seq. Normal mouse IgG was used as a negative control. The relative levels of histone modification status were calculated as the ratio of IP DNA to the INPUT DNA sample. Data were expressed as a ratio of the non-dESCs. Values are mean ± SEM of three different incubations. a, P < .05 vs non-dESCs. C, Relative mRNA levels of insulin signaling genes were quantified by real-time RT-PCR. Values of mRNA levels were normalized to those of GAPDH and expressed as a ratio of the non-dESCs. Values are mean ± SEM of three different incubations. a, P < .05 vs non-dESCs (unpaired t test).
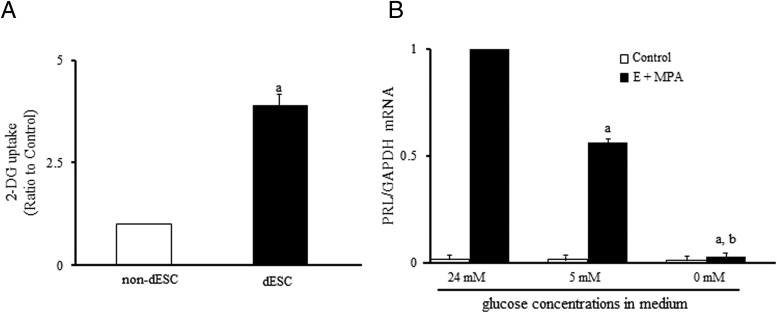
Figure 8. Glucose uptake and decidualization.
A, Effects of decidualization on glucose uptake in ESCs. ESCs were treated with or without E and MPA for 14 days, and glucose uptake was examined by a 2-DG uptake assay in non-dESCs and dESCs. The glucose (2-DG) uptake was expressed as a ratio of the non-dESCs (control). Values are mean ± SEM of three different incubations. a, P < .01 vs non-dESCs (unpaired t test). B, Effects of the low-glucose incubation on decidualization. ESCs were cultured in the medium containing normal (24 mM) or low (0 and 5 mM) glucose concentrations in the presence or absence of E (10−8 mol/l) and MPA (10−6 mol/l) for 14 days, and then decidualization was evaluated by PRL mRNA expression by real-time RT-PCR. Standard DMEM contains 24 mM glucose. Values of PRL mRNA levels were normalized to those of GAPDH and expressed as a ratio of the E + MPA treatment sample under the normal glucose concentration (24 mM). Values are mean ± SEM of three different incubations. a, P < .01 vs E + MPA (24 mM); b, P < .01 vs E+MPA (5 mM) (one way ANOVA followed by the Tukey-Kramer test).
Accession numbers
RNA-seq and ChIP-seq raw data were deposited in the Gene Expression Omnibus (number GSE57010).
Results
Genome-wide distributions of H3K27ac, H3K4me3, H3K4me1, and H3K27me3
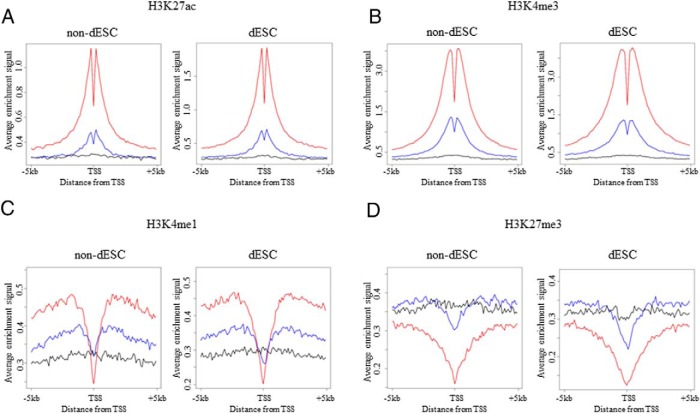
To confirm that ChIP-seq was successfully performed in this study, we first examined whether the distribution profiles of H3K27ac, H3K4me3, H3K4me1, and H3K27me3 in our ChIP-seq assay were consistent with the well-known distribution pattern of each histone modification. For this purpose, composite profiles of each histone modification around the TSS were generated according to their expression levels in non-dESCs (treated with control medium) and dESCs (treated with E and MPA). H3K27ac and H3K4me3 are active marks and are often found in the proximal promoter region (11, 32). Consistent with these reports, H3K27ac and H3K4me3 signals were high around the TSS and positively correlated with the gene expression levels in both non-dESCs and dESCs (Figure 1, A and B). A significant dip in the signal between −200 bp and +200 bp from the TSS (Figure 1, A and B) reflects the nucleosome loss in transcribed genes (11).
Figure 1. Genome-wide distributions of H3K27ac, H3K4me3, H3K4me1, and H3K27me3.
To examine the distribution of histone modifications in non-dESCs (treated with control medium) and dESCs (treated with E and MPA), composite profiles of each histone modification around the TSS were generated according to their expression levels: no expression, low-level expression, and high-level expression based on the mRNA levels. Average ChIP-seq signal profiles for the high-level expression (red), low-level expression (blue), and no expression (black) genes were generated for the indicated histone modifications around the TSS in non-dESCs and dESCs, respectively. Data are representatives from two different individuals.
H3K4me1 is an active enhancer mark that is located in the distal promoter region (10). As expected, the H3K4me1 signal was low around the TSS and peaked around 1 kb upstream or downstream, and the peak signals were positively correlated with the gene expression levels (Figure 1C), which are consistent with previous reports (32, 33). H3K27me3 is a repressive mark that is located in the proximal promoter region (12, 34). The H3K27me3 signal was low around the TSS and negatively correlated with the gene expression levels (Figure 1D), which is also consistent with previous reports (12, 34). No differences of the distribution patterns of these histone modifications were observed between non-dESCs and dESCs. Our finding that the distribution profiles of H3K27ac, H3K4me3, H3K4me1, and H3K27me3 in our ChIP-seq assay were consistent with the well-known distribution pattern of each histone modification clearly confirms the reliability of our ChIP-seq assay.
Genome-wide changes of histone modifications by induction of decidualization
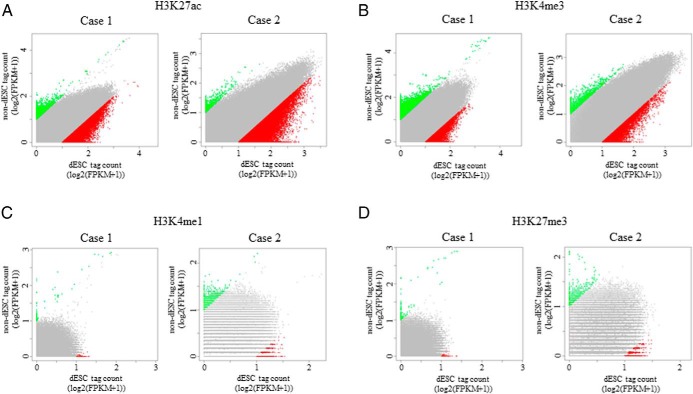
To examine whether histone modification statuses are altered throughout the genome by induction of decidualization in human ESCs, histone modification signals were searched in 1-kb intervals between −10 kb and +10 kb from the TTS, and then the difference in the signals between non-dESCs and dESCs was evaluated by the scatter plot of log2-transformed FPKM values (Figure 2). A number of regions with altered signals of H3K27ac or H3K4me3 (H3K27ac or H3K4me3 altered regions) by decidualization stimuli were observed (Figure 2, A and B). On the other hand, there were few regions in which the signals of H3K4me1 and H3K27me3 were altered by decidualization stimuli (Figure 2, C and D). The common regions of the two individuals (case 1 and case 2) were considered as the regions in which histone modification signals were altered by the induction of decidualization. The number of these regions and their adjacent genes are shown in Table 1. The regions with increased signals of H3K27ac or H3K4me3 (H3K27ac or H3K4me3 increased region) by the induction of decidualization were observed in 3705 and 945 regions, respectively. The regions with decreased signals of H3K27ac or H3K4me3 (H3K27ac or H3K4me3 decreased region) were observed in 42 and 109 regions, respectively. These results show that induction of decidualization alters the H3K27ac and H3K4me3 statuses at many genomic regions and that most of the histone modification changes are the increased signals. The number of the regions with altered signals of H3K4me1 and H3K27me3 by decidualization was quite few.
Figure 2. Changes of genome-wide histone modification statuses by decidualization.
Histone modification signals were searched in 1-kb intervals between −10 kb and +10 kb from the TTS, and then the difference in the signals between non-dESCs and dESCs was evaluated by the scatter plot of log2-transformed FPKM values. Regions showing more than a 2-fold increase (green dots) or decrease (red dots) in signals between non-dESCs and dESCs were defined as having increased or decreased histone modifications, respectively. Gray dots represent the regions showing less than 2-fold changes. Data from two individuals (case 1 and case 2) are shown.
Table 1.
The number of the regions or genes with altered signals of histone modifications by decidualization
| Histone modifications | Regions with increased Histone Modification Signals (gene) | Regions with decreased Histone Modification Signals (gene) |
|---|---|---|
| H3K27ac | 3705 (1846) | 42 (39) |
| H3K4me3 | 945 (847) | 109 (105) |
| H3K4me1 | 3 (3) | 6 (7) |
| H3K27me3 | 2 (2) | 5 (6) |
Histone modification signals (H3K27ac , H3K4me3, H3K4me1, and H3K27me3) were searched every 1 kb genomic region between −10 kb and +10 kb from TTS. The altered (increased or decreased) signals by decidualization were analyzed by the difference in the signals between non-dESC and dESC . The common regions of the two individuals were considered as the regions in which histone modification signals were altered by decidualization. The number of these regions and their adjacent genes are shown.
Genomic locations of H3K27ac- or H3K4me3-altered regions
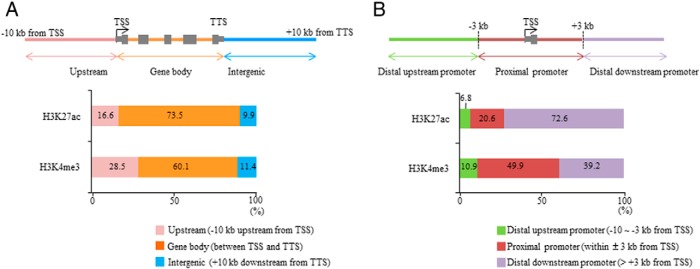
We examined the genomic location of the regions in which H3K27ac and H3K4me3 signals were increased by induction of decidualization. The location of the H3K27ac- and H3K4me3-increased regions was classified as follows (Figure 3A): upstream (−10 kb upstream from the TSS); gene body (between the TSS and TTS, including introns and exons); and intergenic (+10 kb downstream from the TTS). Most of the H3K27ac- or H3K4me3-increased regions were located in the gene body (H3K27ac: 73.5%; H3K4me3: 60.1%) (Figure 3A). The H3K27ac- or H3K4me3-decreased regions showed a similar distribution pattern to the H3K27ac- or H3K4me3-increased regions (data not shown).
Figure 3. Genomic locations of H3K27ac- or H3K4me3-increased regions by decidualization.
A, The H3K27ac- or H3K4me3-increased regions were classified by the location of the signals: upstream (−10 kb upstream from the TSS), gene body (between the TSS and TTS, including introns and exons), and intergenic (+10 kb downstream from the TTS). The results are expressed as a percentage of the total regions. B, The H3K27ac- or H3K4me3-increased regions were classified into 3 groups based on the distance from the TSS: the proximal promoter (within ±3 kb region from the TSS), the distal upstream promoter (more than 3 kb upstream from the TSS), and the distal downstream promoter (more than 3 kb downstream from the TSS). The results are expressed as a percentage of the total regions.
We further examined the location of these regions based on the distance from the TSS of their adjacent genes. The location of the H3K27ac- or H3K4me3-increased regions was classified into 3 groups according to a previous report (29) (Figure 3B): proximal promoter (within ±3 kb region from the TSS); distal upstream promoter (more than 3 kb upstream from the TSS); and distal downstream promoter (more than 3 kb downstream from the TSS). The H3K27ac-increased regions were predominantly located in the distal promoter regions (distal upstream and distal downstream) (79.4%), and half of the H3K4me3-increased regions (50.1%) were also located in the distal promoter regions (Figure 3B). The H3K27ac- or H3K4me3-decreased regions showed similar distribution patterns to the H3K27ac- or H3K4me3-increased regions (data not shown). These results indicate that histone modifications of H3K27ac and H3K4me3 change not only in the proximal promoter region but also in the distal promoter regions by induction of decidualization.
Histone modifications of H3K27ac or H3K4me3 and regulation of gene expression
We investigated the relationship between histone modifications of H3K27ac or H3K4me3 and the up- or down-regulation of gene expression by the induction of decidualization. mRNA levels were analyzed on a genome-wide basis by RNA-seq in dESCs and non-dESCs. We identified 881 up-regulated genes and 1379 down-regulated genes (greater than 1.4 fold changes) by decidualization stimuli, which were common between the two individuals (case 1 and case 2), whereas the common regions with increased signals of H3K27ac and H3K4me3 between the two individuals were observed in 3705 and 945 regions, respectively (Table 1). Because H3K27ac and H3K4me3 are associated with activation of gene transcription, the up-regulated genes were classified into 4 groups, depending on whether they have the H3K27ac- or H3K4me3-increased regions: 1) genes without H3K27ac- and H3K4me3-increased regions, 2) genes with H3K4me3-increased regions, 3) genes with H3K27ac-increased regions, and 4) genes with both H3K27ac- and H3K4me3-increased regions. As shown in Figure 4A, the induction of decidualization up-regulated 881 genes, 223 (25.3%) of which had either or both of the H3K27ac- and H3K4me3-increased region (H3K4me3: 2.2%; H3K27ac: 16.9%; both H3K4me3 and H3K27ac: 6.2%), indicating a possibility that those genes are regulated by the increase of H3K27ac and H3K4me3. On the other hand, most of the down-regulated 1379 genes by decidualization (97.7%) did not have the H3K27ac- or H3K4me3-decreased regions (Figure 4A).
We further investigated whether the increase of H3K27ac or H3K4me3 is associated with the increase in mRNA levels of the up-regulated genes by decidualization. For this purpose, fold changes in the mRNA levels of the up-regulated genes by decidualization stimuli were compared among the 4 groups in each individual: 1) genes without H3K27ac- and H3K4me3-increased regions (658 genes), 2) genes with H3K4me3-increased regions (19 genes), 3) genes with H3K27ac-increased regions (149 genes), and 4) genes with both H3K27ac- and H3K4me3-increased regions (55 genes). As shown in Figure 4B, the induction of decidualization increased the mRNA levels of genes with H3K27ac- or H3K4me3-increased regions more than it increased the mRNA levels of genes without H3K27ac- or H3K4me3-increased regions. This result suggests that the increase of H3K27ac or H3K4me3 is involved in the up-regulation of the gene expression during decidualization.
Genomic location of H3K27ac- or H3K4me3-increased regions and up-regulation of gene expression by decidualization
As shown in Figure 3B, H3K27ac- or H3K4me3-increased regions were found to be located in not only the proximal promoter region but also the distal promoter regions (Figure 3B). The presence of H3K27ac- or H3K4me3-increased regions in the distal promoter regions as well as in the proximal promoter region has been reported to affect gene expression (10, 29, 35). Therefore, we investigated the relationship between the location of the H3K27ac- or H3K4me3-increased regions and the up-regulation of gene expression by decidualization stimuli. Eight hundred eighty-one up-regulated genes by decidualization were classified into 4 groups according to the location of H3K27ac- or H3K4me3-increased regions as described in Figure 3B: 1) genes without H3K27ac- and H3K4me3-increased regions, 2) genes with H3K27ac- or H3K4me3-increased regions in the proximal promoter region, 3) genes with H3K27ac- or H3K4me3-increased regions in the distal promoter region, and 4) genes with H3K27ac- or H3K4me3-increased regions in both proximal and distal promoter regions. The distal promoter region includes both distal upstream promoter region (more than 3 kb upstream from the TSS) and distal downstream promoter region (more than 3 kb downstream from the TSS). As shown in Figure 5A, in the 881 up-regulated genes by decidualization stimuli, 43 (4.9%) had H3K27ac- or H3K4me3-increased regions in the proximal promoter region, 108 (12.3%) in the distal promoter regions, and 72 (8.1%) in both the proximal and distal promoter regions.
We also investigated whether the location of H3K27ac or H3K4me3 is associated with the increase in mRNA levels of the up-regulated genes by decidualization. Fold changes in mRNA levels of the up-regulated genes by decidualization stimuli were compared among the 4 groups described above in each individual. As shown in Figure 5B, induction of decidualization increased the mRNA levels of the genes with H3K27ac- or H3K4me3-increased regions in the proximal or distal promoter regions more than it increased the mRNA levels of genes without H3K27ac- and H3K4me3-increased regions (Figure 5B). The presence of H3K27ac- or H3K4me3-increased regions in both proximal and distal promoter regions showed the synergistic effect on the increase in mRNA levels by decidualization stimuli (Figure 5B). These results suggest that the increase of H3K27ac or H3K4me3 not only in the proximal region but also in the distal promoter regions is involved in the up-regulation of the gene expression during decidualization.
Potential roles of the genes with H3K27ac- or H3K4me3-increased regions by decidualization
To investigate the potential roles of the increase of H3K27ac and H3K4me3 during decidualization, a pathway analysis was performed for the up-regulated genes with or without histone modifications of H3K27ac and H3K4me3. As shown in Figure 4A, 223 of the up-regulated 881 genes by decidualization stimuli were accompanied by the increase of H3K27ac or H3K4me3, but 658 genes were not. All of the 223 genes are listed in Supplemental Table 3. These gene sets were submitted to the DAVID bioinformatics database, and a KEGG pathway enrichment analysis was performed. As shown in Table 2, three pathways were identified as significantly enriched pathways in the 223 up-regulated genes with H3K27ac- or H3K4me3-increased region. On the other hand, very interestingly, there were no significantly enriched pathways in the 658 up-regulated genes without H3K27ac- and H3K4me3-increased regions. Both the type 2 diabetes mellitus pathway and the aldosterone-regulated sodium reabsorption pathway includes the insulin signaling-related genes, which are also included in the insulin signaling pathway. Therefore, we focused on the insulin signaling pathway in this study.
Table 2.
Enriched signaling pathways of up-regulated genes with H3K27ac- or H3K4me3-increased regions by decidualization
| Pathway | Genes | Benjamini P value | P value |
|---|---|---|---|
| Type II diabetes mellitus | IRS1, IRS2, INSR, PIK3CG, MAPK10, HK2 | 0.040308 | 8.23E-04 |
| Insulin signaling pathway | IRS1, IRS2, INSR, PIK3CG, MAPK10, FOXO1, AKT3, PRKAB2, HK2, | 0.041218 | 0.001262 |
| Aldosterone-regulated sodium reabsorption | IRS1, IRS2, INSR, PIK3CG, SGK1, HSD11B1 | 0.042383 | 4.33E-04 |
Three pathways were identified as significantly enriched pathways in the up-regulated 230 genes with H3K27ac- or H3K4me3-increased regions by decidualization (KEGG pathway enrichment analysis).
To validate the results of our ChIP-seq and RNA-seq experiments, ChIP-qPCR and real-time RT-PCR were performed for six genes that belong to the insulin signaling pathway: IRS1, IRS2, INSR, FOXO1, AKT3, and MAPK10. Primers for ChIP-qPCR were designed within the H3K27ac- or H3K4me3-increased regions identified by ChIP-seq. As shown in Figure 6, the H3K27ac and H3K4me3 levels were apparently increased by the induction of decidualization in all selected genes, accompanied by the significant increases in mRNA levels.
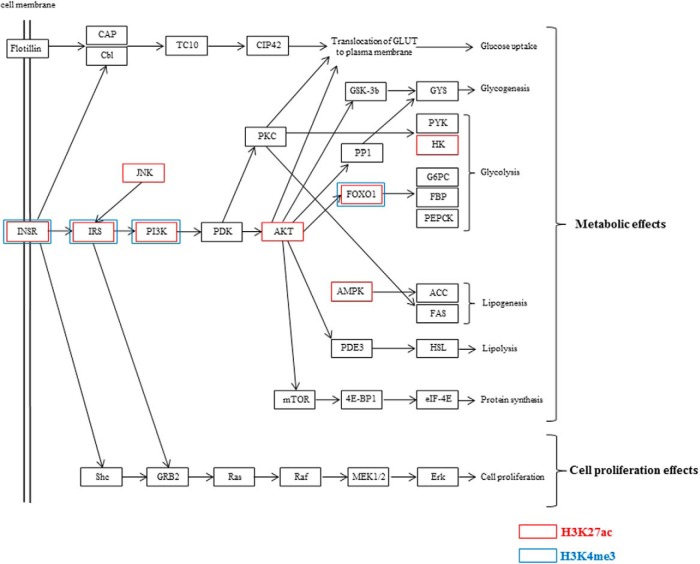
Figure 7 shows the insulin signaling pathway map, which was obtained from DAVID web site. Red boxes indicate the up-regulated genes with H3K27ac-increased regions, and blue boxes indicate the up-regulated genes with H3K4me3-increased regions by decidualization. Most of them are involved in the metabolic actions of insulin. Because those genes are reported to be involved in glucose uptake in other types of cells (36, 37), we examined whether glucose uptake is actually affected in ESCs by decidualization. As shown in Figure 8A, glucose uptake was significantly increased by decidualization. Next, to investigate the role of glucose in decidualization, ESCs were incubated under the environment of low-glucose concentrations (0 and 5 mM) in the presence or absence of E and MPA, and then decidualization was evaluated by mRNA expression of PRL, a specific marker of decidualization. PRL mRNA levels were remarkably increased by E and MPA under the normal-glucose concentration (24 mM), but this increase was significantly inhibited under the low-glucose concentrations (0 and 5 mM) (Figure 8B). These results suggest that E and MPA activate the insulin signaling-related genes by increasing histone modifications of H3K27ac or H3K4me3, which in turn contribute to decidualization through the increase of glucose uptake.
Figure 7. Components of insulin signaling pathway and the up-regulated genes with H3K27ac- or H3K4me3-increased regions by decidualization.
Shown is the modified insulin signaling pathway maps obtained from DAVID web site. Red boxes indicate the up-regulated genes with H3K27ac-increased regions and blue boxes indicate the up-regulated genes with H3K4me3-increased regions by decidualization.
Discussion
In this study, we applied genome-wide approaches to examine the global changes of histone modification statuses during the differentiation of primary human ESCs into decidualized ESCs. The present study demonstrated that histone modification statuses change on a genome-wide basis in human ESCs by the induction of decidualization. The Encyclopedia of DNA Elements project has recently revealed the genome-wide histone modification statuses in a variety of cell types (38). Our study is the first report showing the genome-wide profile of histone modification statuses in human ESCs and its change during decidualization using ChIP-seq.
The main changes of histone modifications that occur during decidualization were increases of H3K27ac and H3K4me3 at a number of genomic regions, whereas decreases of these histone modifications were few. Our results are consistent with the previous finding that induction of decidualization increase global levels of H3K27ac in human ESCs (15). On the other hand, few changes in H3K4me1 and H3K27me3 occurred during decidualization. Changes of H3K4me1 and H3K27me3 statuses on a genome-wide basis occur during the differentiation process of immature cells and stem cells and regulate the expression of genes related to cell differentiation (10, 34, 39). Because human ESCs are well differentiated somatic cells and decidualization is the process of differentiation of ESCs into decidualized ESCs, it is not surprising that the altered pattern of histone modifications in human ESCs by decidualization is different from that in those undifferentiated cells.
Although we observed few changes in H3K27me3 statuses by decidualization, Grimaldi et al (15) observed many H3K27me3 changes. This may be due to differences in method because Grimaldi et al used a ChIP-on-chip assay and induced the decidualization with cAMP and MPA, whereas we used a ChIP-seq and induced decidualization with E and MPA. In fact, in our preliminary ChIP-seq data, the genes with altered histone modifications by E and MPA differed from those by cAMP. One may raise a possibility that our ChIP-seq assay failed to detect H3K4me1 and H3K27me3 statuses. However, the distribution patterns of H3K4me1 and H3K27me3 around the TSS in non-dESCs and dESCs in this study (Figure 1) are consistent with the well-known pattern reported in other ChIP-seq studies (32–34). Therefore, we concluded that there are few regions in which H3K4me1 and H3K27me3 statuses change during decidualization induced with E and MPA in human ESCs.
It is interesting to note that H3K27me3 is well known for marking the bivalent domain together with H3K4me3, which presents a group of genes that are poised to be turned on/off during cell differentiation (40). For example, in the process of embryonic stem cell differentiation, bivalent histone modifications such that H3K27me3 occupies the same nucleosome with H3K4me3 play an important role in the regulation of gene expressions by keeping promoters poised to be transcribed (34). These genes are activated with the loss of H3K27me3 and the gain of H3K4me3 during cell differentiation (34, 40). In this study, the regions with the altered signal of H3K27me3 by decidualization were quite few (Table 1), and the number of the genes with increased mRNA levels, loss of H3K27me3, and gain of H3K4me3 by decidualization was also few, even if analyzed using a less stringent cutoff level (in our preliminary study). Therefore, the mechanism of bivalent histone modifications is unlikely to be involved in the regulation of gene expressions during the decidualization of endometrial stromal cells. Bivalent histone modifications may be specific to the developmental stages of undifferentiated cells such as embryonic stem cells and pluripotent stem cells (34, 40).
We identified a large number of genomic regions in which H3K27ac or H3K4me3 is increased by the induction of decidualization. Most of the increases in H3K27ac and H3K4me3 occurred in the gene body, in agreements with previous reports (29, 39). Eighty percent of the H3K27ac-increased regions were located in the distal promoter regions, which are more than 3 kb upstream or downstream from the TSS. This finding is also consistent with previous reports that H3K27ac changes occur in the distal promoter regions as well as in the proximal promoter region (10, 29, 41) and that it contributes to cell stage-specific gene expressions during the differentiation of embryonic stem cells, adipose stem cells, and monocytes (10, 29, 41). On the other hand, it has been reported that changes in H3K4me3 status occur near the TSS in the proximal promoter region (42). Interestingly, our result showed that half of the H3K4me3-increased regions were located in the distal promoter regions. Similar findings on the location of H3K4me3 have also been observed in other types of cells (35, 39).
RNA-seq revealed that a number of genes are up-regulated or down-regulated in human ESCs by the induction of decidualization (Figure 4A). In the up-regulated genes, approximately 25% (223 genes, Supplemental Table 3) had H3K27ac- or H3K4me3-increased regions, indicating the possibility that they were up-regulated by the increases of H3K27ac and H3K4me3 (Figure 4A). In fact, the genes that have H3K27ac- or H3K4me3-increased regions showed a higher increase in mRNA levels than those without H3K27ac- and H3K4me3-increased regions, suggesting that the increase of H3K27ac or H3K4me3 is involved in the up-regulation of the gene expression during decidualization. On the other hand, interestingly, the number of down-regulated genes with H3K27ac- or H3K4me3-decreased region was quite small. It is therefore likely that gene activation, rather than gene inactivation, is closely associated with the regulation by decidualization-induced histone modifications in human ESCs.
Our results showed that the increase of H3K27ac or H3K4me3 not only in the proximal region but also in the distal promoter regions is associated with the increase in mRNA levels by decidualization stimuli. H3K27ac and H3K4me3 is one of the histone modifications that are highly correlated with the activation of gene transcription. The increases in H3K27ac and H3K4me3 have been found to be involved in the recruitment of a complex consisting of basal transcription factors and RNA polymerase II for triggering transcription (43, 44). Recently not only the regions near the TSS but also the distal promoter regions that are far from the TSS are considered as important regions for transcription. Long-range chromatin interactions, such as distal enhancer-proximal promoter interactions, are recognized as an important mechanism to regulate gene expression levels (45, 46). Changes of H3K27ac and H3K4me3 statuses in the distal promoter region affect gene expression levels during cell differentiation (29, 35, 39). Our finding that the presence of H3K27ac- or H3K4me3-increased region in the distal promoter regions increased the mRNA levels strongly suggests that the interaction between the distal and proximal promoter regions is involved in the up-regulation of gene expression during decidualization in human ESCs.
Regarding a potential role of the increase of H3K27ac and H3K4me3 by decidualization, the KEGG pathway analysis on the up-regulated genes with H3K27ac- or H3K4me3-increased regions revealed that the insulin signaling pathway is one of the significantly enriched pathways. In contrast, very interestingly, no significantly enriched pathways were detected in the up-regulated genes without H3K27ac- or H3K4me3-increased regions by decidualization. These results suggest that histone modifications of H3K27ac and H3K4me3 preferentially occur in the specific gene group such as the insulin signaling pathway-related genes. There are two major pathways in the insulin signaling network. One is a Ras/MAPK pathway, which involves the cell growth or cell proliferation effects of insulin. Another is a phosphatidylinositol 3-kinase (PI3-K)/Akt pathway, which plays pivotal roles in the metabolic action of insulin (Figure 7) (47). Many of the up-regulated genes with H3K27ac- or H3K4me3-increased regions appear to be involved in the metabolism of glucose (Figure 7). Insulin signaling plays important functional roles in reproductive tissues including the ovary and endometrium (48–50). The expression levels of several insulin signaling-related genes such as IRS1, IRS2, INSR, and FOXO1 increase during decidualization in human ESCs (51, 52), which is consistent with our results. In addition, our results clearly showed that glucose uptake in ESCs is increased by decidualization and that glucose is necessary for ESCs to undergo decidualization, which is also consistent with previous reports (22, 53). Taken together, these findings strongly suggest that decidualization stimulus activates the insulin signaling-related genes by increasing histone modifications of H3K27ac or H3K4me3, which in turn contribute to decidualization through the increase of glucose uptake.
Decidualization is a naturally occurring differentiation process in the human endometrium during the menstrual cycle and is accompanied by marked functional changes (1). Gene regulation during the process of cell differentiation is closely associated with the dynamic changes of histone modifications (29, 34, 39, 54). Most of the global chromatin studies so far have focused on the changes of histone modification statuses associated with self-renewal of stem cells or their differentiation process into mature cell types. The present results show that histone modification statuses on a genome-wide basis change, even in well-differentiated somatic cells (primary human ESCs) during decidualization.
Acknowledgments
This work was supported in part by Japan Society for the Promotion of Science KAKENHI (Grants 24592471, 24791704, 24791705, 25293343, 25462559, 25462560, 25861495, 26670726, 26861328, 26861329, 26861330, and 26462492), the Cooperative Research Project Program of the Medical Institute of Bioregulation (Kyushu University), and Takeda Science Foundation.
Disclosure Summary: The authors have nothing to disclose.
Funding Statement
This work was supported in part by Japan Society for the Promotion of Science KAKENHI (Grants 24592471, 24791704, 24791705, 25293343, 25462559, 25462560, 25861495, 26670726, 26861328, 26861329, 26861330, and 26462492), the Cooperative Research Project Program of the Medical Institute of Bioregulation (Kyushu University), and Takeda Science Foundation.
Footnotes
- AKT3
- v-akt murine thymoma viral oncogene homolog 3
- ChIP
- chromatin immunoprecipitation
- ChIP-seq
- ChIP with next-generation sequencing technology
- DAVID
- Database for Annotation, Visualization, and Integrated Discovery
- dESC
- decidualized ESC
- 2-DG
- 2-deoxygulcose
- E
- estradiol
- ESC
- endometrial stromal cell
- FOXO1
- forkhead box O1
- FPKM
- fragments per kilobase of the window per million mapped fragments
- GAPDH
- glyceraldehyde-3-phosphate dehydrogenase
- H3K27ac
- histone-H3 lysine-27
- H3K4me1
- H3K4me3, histone-H3 lysine-4
- H3K27me3
- histone-H3 lysine-27
- INPUT
- unimmunoprecipitated control
- INSR
- insulin receptor
- IP
- immunoprecipitated DNA
- IRS1
- insulin receptor substrate 1
- IRS2
- insulin receptor substrate 2
- KEGG
- Kyoto Encyclopedia of Genes and Genomes
- MPA
- medroxyprogesterone acetate
- non-dESC
- nondecidualized ESC
- PRL
- prolactin
- qPCR
- quantitative PCR
- RNA-seq
- RNA sequence
- RPKM
- reads per kilobase of exon unit per million mapped reads
- TSS
- transcription start site
- TTS
- transcription termination site.
References
- 1. Gellersen B, Brosens J. Cyclic AMP and progesterone receptor cross-talk in human endometrium: a decidualizing affair. J Endocrinol. 2003;178:357–372. [DOI] [PubMed] [Google Scholar]
- 2. Salker M, Teklenburg G, Molokhia M, et al. . Natural selection of human embryos: impaired decidualization of endometrium disables embryo-maternal interactions and causes recurrent pregnancy loss. PLoS One. 2010;5:e10287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Laird SM, Tuckerman EM, Li TC. Cytokine expression in the endometrium of women with implantation failure and recurrent miscarriage. Reprod Biomed Online. 2006;13:13–23. [DOI] [PubMed] [Google Scholar]
- 4. Wang W, Taylor RN, Bagchi IC, Bagchi MK. Regulation of human endometrial stromal proliferation and differentiation by C/EBPβ involves cyclin E-cdk2 and STAT3. Mol Endocrinol. 2012;26:2016–2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Aghajanova L, Horcajadas JA, Weeks JL, et al. . The protein kinase A pathway-regulated transcriptome of endometrial stromal fibroblasts reveals compromised differentiation and persistent proliferative potential in endometriosis. Endocrinology. 2010;151:1341–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Popovici RM, Kao LC, Giudice LC. Discovery of new inducible genes in in vitro decidualized human endometrial stromal cells using microarray technology. Endocrinology. 2000;141:3510–3513. [DOI] [PubMed] [Google Scholar]
- 7. Li B, Carey M, Workman JL. The role of chromatin during transcription. Cell. 2007;128:707–719. [DOI] [PubMed] [Google Scholar]
- 8. Tamura I, Taketani T, Lee L, et al. . Differential effects of progesterone on COX-2 and Mn-SOD expressions are associated with histone acetylation status of the promoter region in human endometrial stromal cells. J Clin Endocrinol Metab. 2011;96:E1073–E1082. [DOI] [PubMed] [Google Scholar]
- 9. Rada-Iglesias A, Bajpai R, Swigut T, Brugmann SA, Flynn RA, Wysocka J. A unique chromatin signature uncovers early developmental enhancers in humans. Nature. 2011;470:279–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Creyghton MP, Cheng AW, Welstead GG, et al. . Histone H3K27ac separates active from poised enhancers and predicts developmental state. Proc Natl Acad Sci USA. 2010;107:21931–21936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Barski A, Cuddapah S, Cui K, et al. . High-resolution profiling of histone methylations in the human genome. Cell. 2007;129:823–837. [DOI] [PubMed] [Google Scholar]
- 12. Zhang JA, Mortazavi A, Williams BA, Wold BJ, Rothenberg EV. Dynamic transformations of genome-wide epigenetic marking and transcriptional control establish T cell identity. Cell. 2012;149:467–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tamura I, Asada H, Maekawa R, et al. . Induction of IGFBP-1 expression by cAMP is associated with histone acetylation status of the promoter region in human endometrial stromal cells. Endocrinology. 2012;153:5612–5621. [DOI] [PubMed] [Google Scholar]
- 14. Tamura I, Sato S, Okada M, et al. . Importance of C/EBPβ binding and histone acetylation status in the promoter regions for induction of IGFBP-1, PRL, and Mn-SOD by cAMP in human endometrial stromal cells. Endocrinology. 2014;155:275–286. [DOI] [PubMed] [Google Scholar]
- 15. Grimaldi G, Christian M, Steel JH, Henriet P, Poutanen M, Brosens JJ. Down-regulation of the histone methyltransferase EZH2 contributes to the epigenetic programming of decidualizing human endometrial stromal cells. Mol Endocrinol. 2011;25:1892–1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Park PJ. ChIP-seq: advantages and challenges of a maturing technology. Nat Rev Genet. 2009;10:669–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wei Y, Chen R, Dimicoli S, et al. . Global H3K4me3 genome mapping reveals alterations of innate immunity signaling and overexpression of JMJD3 in human myelodysplastic syndrome CD34+ cells. Leukemia. 2013;27(11):2177–2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yin P, Roqueiro D, Huang L, et al. . Genome-wide progesterone receptor binding: cell type-specific and shared mechanisms in T47D breast cancer cells and primary leiomyoma cells. PLoS One. 2012;7:e29021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Noyes RW, Hertig AT, Rock J. Dating the endometrial biopsy. Am J Obstet Gynecol. 1975;122:262–263. [DOI] [PubMed] [Google Scholar]
- 20. Sugino N, Karube-Harada A, Sakata A, Takiguchi S, Kato H. Nuclear factor-κB is required for tumor necrosis factor-α-induced manganese superoxide dismutase expression in human endometrial stromal cells. J Clin Endocrinol Metab. 2002;87:3845–3850. [DOI] [PubMed] [Google Scholar]
- 21. Sugino N, Kashida S, Takiguchi S, Nakamura Y, Kato H. Induction of superoxide dismutase by decidualization in human endometrial stromal cells. Mol Hum Reprod. 2000;6:178–184. [DOI] [PubMed] [Google Scholar]
- 22. Frolova AI, Moley KH. Quantitative analysis of glucose transporter mRNAs in endometrial stromal cells reveals critical role of GLUT1 in uterine receptivity. Endocrinology. 2011;152:2123–2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Trapnell C, Pachter L, Salzberg SL. TopHat: discovering splice junctions with RNA-Seq. Bioinformatics. 2009;25:1105–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Trapnell C, Williams BA, Pertea G, et al. . Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat Biotechnol. 2010;28:511–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Spasic M, Friedel CC, Schott J, et al. . Genome-wide assessment of AU-rich elements by the AREScore algorithm. PLoS Genet. 2012;8:e1002433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Li X, Large MJ, Creighton CJ, et al. . COUP-TFII regulates human endometrial stromal genes involved in inflammation. Mol Endocrinol. 2013;27:2041–2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Odawara J, Harada A, Yoshimi T, et al. . The classification of mRNA expression levels by the phosphorylation state of RNAPII CTD based on a combined genome-wide approach. BMC Genomics. 2011;12:516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Langmead B, Trapnell C, Pop M, Salzberg SL. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. 2009;10:R25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pham TH, Benner C, Lichtinger M, et al. . Dynamic epigenetic enhancer signatures reveal key transcription factors associated with monocytic differentiation states. Blood. 2012;119:e161–e171. [DOI] [PubMed] [Google Scholar]
- 30. da Huang W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4:44–57. [DOI] [PubMed] [Google Scholar]
- 31. Matsuoka A, Kizuka F, Lee L, et al. . Progesterone increases manganese superoxide dismutase expression via a cAMP-dependent signaling mediated by noncanonical Wnt5a pathway in human endometrial stromal cells. J Clin Endocrinol Metab. 2010;95:E291–E299. [DOI] [PubMed] [Google Scholar]
- 32. Wang Z, Zang C, Rosenfeld JA, et al. . Combinatorial patterns of histone acetylations and methylations in the human genome. Nat Genet. 2008;40:897–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pei B, Sisu C, Frankish A, et al. . The GENCODE pseudogene resource. Genome Biol. 2012;13:R51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Vastenhouw NL, Schier AF. Bivalent histone modifications in early embryogenesis. Curr Opin Cell Biol. 2012;24:374–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tian Y, Jia Z, Wang J, et al. . Global mapping of H3K4me1 and H3K4me3 reveals the chromatin state-based cell type-specific gene regulation in human Treg cells. PLoS One. 2011;6:e27770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Miura A, Sajan MP, Standaert ML, Bandyopadhyay G, Kahn CR, Farese RV. Insulin substrates 1 and 2 are corequired for activation of atypical protein kinase C and Cbl-dependent phosphatidylinositol 3-kinase during insulin action in immortalized brown adipocytes. Biochemistry. 2004;43:15503–15509. [DOI] [PubMed] [Google Scholar]
- 37. Thirone AC, Huang C, Klip A. Tissue-specific roles of IRS proteins in insulin signaling and glucose transport. Trends Endocrinol Metab. 2006;17:72–78. [DOI] [PubMed] [Google Scholar]
- 38. Bernstein BE, Birney E, Dunham I, Green ED, Gunter C, Snyder M. An integrated encyclopedia of DNA elements in the human genome. Nature. 2012;489:57–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Cui K, Zang C, Roh TY, et al. . Chromatin signatures in multipotent human hematopoietic stem cells indicate the fate of bivalent genes during differentiation. Cell Stem Cell. 2009;4:80–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Fisher CL, Fisher AG. Chromatin states in pluripotent, differentiated, and reprogrammed cells. Curr Opin Genet Dev. 2011;21:140–146. [DOI] [PubMed] [Google Scholar]
- 41. Mikkelsen TS, Xu Z, Zhang X, et al. . Comparative epigenomic analysis of murine and human adipogenesis. Cell. 2010;143:156–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wong P, Hattangadi SM, Cheng AW, Frampton GM, Young RA, Lodish HF. Gene induction and repression during terminal erythropoiesis are mediated by distinct epigenetic changes. Blood. 2011;118:e128–e138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Chen Y, Jorgensen M, Kolde R, et al. . Prediction of RNA Polymerase II recruitment, elongation and stalling from histone modification data. BMC Genomics. 2011;12:544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Auerbach RK, Euskirchen G, Rozowsky J, et al. . Mapping accessible chromatin regions using Sono-Seq. Proc Natl Acad Sci USA. 2009;106:14926–14931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Li G, Ruan X, Auerbach RK, et al. . Extensive promoter-centered chromatin interactions provide a topological basis for transcription regulation. Cell. 2012;148:84–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zhang Y, Wong CH, Birnbaum RY, et al. . Chromatin connectivity maps reveal dynamic promoter-enhancer long-range associations. Nature. 2013;504:306–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Saltiel AR, Kahn CR. Insulin signalling and the regulation of glucose and lipid metabolism. Nature. 2001;414:799–806. [DOI] [PubMed] [Google Scholar]
- 48. Hunzicker-Dunn ME, Lopez-Biladeau B, Law NC, Fiedler SE, Carr DW, Maizels ET. PKA and GAB2 play central roles in the FSH signaling pathway to PI3K and AKT in ovarian granulosa cells. Proc Natl Acad Sci USA. 2012;109:E2979–E2988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Purcell SH, Chi MM, Lanzendorf S, Moley KH. Insulin-stimulated glucose uptake occurs in specialized cells within the cumulus oocyte complex. Endocrinology. 2012;153:2444–2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Lathi RB, Hess AP, Tulac S, Nayak NR, Conti M, Giudice LC. Dose-dependent insulin regulation of insulin-like growth factor binding protein-1 in human endometrial stromal cells is mediated by distinct signaling pathways. J Clin Endocrinol Metab. 2005;90:1599–1606. [DOI] [PubMed] [Google Scholar]
- 51. Ganeff C, Chatel G, Munaut C, Frankenne F, Foidart JM, Winkler R. The IGF system in in vitro human decidualization. Mol Hum Reprod. 2009;15:27–38. [DOI] [PubMed] [Google Scholar]
- 52. Kim JJ, Buzzio OL, Li S, Lu Z. Role of FOXO1A in the regulation of insulin-like growth factor-binding protein-1 in human endometrial cells: interaction with progesterone receptor. Biol Reprod. 2005;73:833–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Frolova A, Flessner L, Chi M, Kim ST, Foyouzi-Yousefi N, Moley KH. Facilitative glucose transporter type 1 is differentially regulated by progesterone and estrogen in murine and human endometrial stromal cells. Endocrinology. 2009;150:1512–1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Orford K, Kharchenko P, Lai W, et al. . Differential H3K4 methylation identifies developmentally poised hematopoietic genes. Dev Cell. 2008;14:798–809. [DOI] [PMC free article] [PubMed] [Google Scholar]