ABSTRACT
Platinum-based chemotherapy is usually curative for patients with testicular germ cell tumors (TGCT), but a subset of patients experience disease progression and poor clinical outcomes. Here, we tested whether immune profiling of TGCT could identify novel prognostic markers and therapeutic targets for this patient cohort. We obtained primary and metastatic TGCT samples from one center. We performed immune profiling using multiplexed fluorescence immunohistochemistry (FIHC) for T-cell subsets and immune checkpoints, and targeted gene expression profiling (Nanostring nCounter Immune panel). Publically available data sets were used to validate primary sample analyses. Nearly all samples had some degree of T-cell infiltration and immune checkpoint expression. Seminomas were associated with increased CD3+ T-cell infiltration, decreased Regulatory T-cells, increased PD-L1, and increased PD-1/PD-L1 spatial interaction compared with non-seminomas using FIHC. Gene expression profiling confirmed these findings and also demonstrated increased expression of T-cell markers (e.g., IFNγ, and LAG3) and cancer/testis antigens (e.g., PRAME) in seminomas, whereas non-seminomas demonstrated high neutrophil and macrophage gene signatures. Irrespective of histology, advanced TGCT stage was associated with decreased T-cell and NK-cell signatures, while Treg, neutrophil, mast cell and macrophage signatures increased with advanced stage. Importantly, cancer/testis antigen, neutrophil, and CD8+/regulatory T-cell signatures correlated with recurrence free survival. Thus, deep immune characterization of TGCT using IHC and gene expression profiling identified activated T-cell infiltration which correlated with seminoma histology and good prognosis. These results may provide a rationale for testing of anti-PD-1/PD-L1 agents and suggest prognostic markers.
KEYWORDS: Immune, non-seminoma, PD-1/PD-L1, seminoma, testicular
Introduction
Testicular germ cell tumors (TGCT) represent one of the most common cancers affecting adolescent men and are broadly defined as either seminomas or non-seminomas.1 Both histological subtypes are exquisitely sensitive to platinum-based chemotherapies, and the majority of these patients are cured by either surgery alone or combination approaches with chemotherapies. While frequently curative, chemotherapy is associated with increased risk of infertility and secondary cancers.2 Furthermore, a sub-group of patients present with high risk disease, which may display primary resistance, or patients may experience disease progression after chemotherapy. Treatment options for these therapy resistant patients are generally associated with cure rates <50%, necessitating the development of novel therapies and predictors of prognosis.3-5
The interaction between programmed cell death-1/ligand-1 (PD-1/PD-L1) suppresses antitumor immunity in the tumor microenvironment. Agents blocking this pathway have produced remarkable activity in many cancer types by unleashing repressed immune responses.6 Despite the success of PD-1 agents, responses remain quite heterogeneous between patients, and validated biomarkers and prognostic factors remain elusive. Candidate biomarkers have largely focused on identifying pre-existing immune recognition and exhaustion mediated by PD-1/PD-L1, as well as various tumor intrinsic factors. These include PD-L1 expression, cytokine production, infiltrating immune cells and tumor mutational burden.7-11 Recent evidence suggested that evidence of T-cell activation is present in TGCT, particularly in seminomas.12
Several features make further immune profiling of TGCT particularly intriguing. First, the testes are classically considered a site of immune privilege evading normal systemic immune surveillance. Interestingly, immune infiltrates have been described in both normal testicular tissue and in all stages of TGCT development (particularly in seminomas).13,14 Second, PD-1 and PD-L1 may play a role in modulating testes-specific immune responses.15 Third, cancer/testis antigens (CTAs) are immunogenic molecules restricted to numerous tumor types but also normal male testicular germ cells.16 These CTAs are also highly expressed in TGCT, and are more frequent in seminomas compared with non-seminomas.17,18
Although the presence of immune cell infiltrates in TGCT has been described, the immune landscape of this disease is not well defined. Further, differences between primary and metastatic tumors, effects of cytotoxic chemotherapy, and histologic subtypes are not well characterized. In addition, the activity of anti-PD-1 agents in seminomas and non-seminomas is essentially unknown.
In this paper, we present evidence for a vigorous immune infiltrate in TGCT with histologic and prognostic correlations. Using a combination of multiplexed fluorescence immunohistochemistry and gene expression profiling technologies on primary and metastatic TGCT in a single center cohort, we describe the immune infiltrate, PD-1/PD-L1 interaction, gene signatures for specific immune populations, and expression of cancer testis (CT) antigens. Moreover, we observed a correlation between immune features and patient outcomes, suggesting possible prognostic implications of such immune profiling.
Methods
Patients
Following IRB-approval for this study, we retrospectively identified all patients with a diagnosis of TGCT seen at Vanderbilt University Medical Center since 2005 (Tables S1 and S2). All patients with seminoma and non-seminoma histology were eligible. Of 35 TGCT samples, 11 were seminoma (31%) and 24 non-seminoma (69%); 12 were stage I, 9 stage II, and 14 stage III. All patients who developed metastatic disease or refractory disease following platinum-based chemotherapy with archival samples were included. Many patients received chemotherapy but some patients had only a resected primary tumor without disease recurrence or need for systemic therapy. We collected baseline demographics, risk factors (serum tumor markers, disease stage), systemic therapies, recurrences, and survival.
Multiplexed fluorescence immunohistochemistry
Formalin fixed, paraffin embedded (FFPE) samples were obtained from existing tissue banks. We performed multiplexed fluorescence immunohistochemistry (FIHC) combined with automated quantitative analysis (AQUA; Genoptix, Inc.)19 to assess PD-1, PD-L1, CD3, CD4, CD8, CD25, and FOXP3. FFPE sections were dewaxed and rehydrated through a series of xylene to alcohol washes before incubating in distilled water. FIHC staining was then performed after heat-induced antigen retrieval followed by cooling and Tris-buffer treatment. Following primary antibodies were used: 0.5 µg/mL mouse anti-PD1 (NAT105, Biocare), 3.6 µg/mL rabbit anti-PD-L1 (E1L3N, Cell Signaling Technology), rabbit anti-CD3 (EP41, 1:200, Biocare Medical), mouse anti-CD4 (4B12, 1:50, DAKO), mouse anti-CD8 (C8/144B, 1:400, DAKO), rabbit anti-FOXP3 (D2W8E, 1:200, Cell Signaling Technology), rabbit anti-CD25 (SP176, 1:200, Novus). Following secondary antibodies were used: anti-mouse Envision HRP (DAKO), anti-rabbit Envision HRP (DAKO) plus 4′,6-diamidino-2-phenylindole (DAPI). Following reagents were used to detect secondary antibodies: TSA+Cy3.5 (Perkin Elmer), TSA-Cy5 (Perkin Elmer), TSA-AlexaFluor488 (Life Technologies), TSA-Cy3, and TSA-AlexaFluor488.
FIHC image analysis
Fluorescence images were acquired using the Vectra 2 Intelligent Slide Analysis System using the Vectra software version 2.0.8 (Perkin Elmer). First, monochrome imaging of the slide at 4× magnification using DAPI was conducted. An automated algorithm (developed using inForm software) was used to identify areas of the slide containing tissue. The areas of the slide identified as containing tissue were imaged at 4× magnification for channels associated with DAPI, FITC, and Cy5 to create RGB images. These 4× magnification images were processed using an automated enrichment algorithm (developed using inForm) to identify 20× fields of view according to the highest Cy5 expression. The top 40 fields of view were imaged at 20× magnification across DAPI, FITC, Texas Red, and Cy5 wavelengths. Raw images were reviewed for acceptability, and images that were out of focus, lacked any tumor cells, were highly necrotic, or contained high levels of fluorescence signal not associated with expected antibody localization (i.e., background staining) were rejected before analysis. Accepted images were processed using AQUAduct (Perkin Elmer), wherein each fluorophore was spectrally unmixed into individual channels and saved as a separate file. These files were analyzed using AQUAnalysis™ software. DAPI was used to generate a binary mask of all viable cells in the image. Similarly, the PD-1, PD-L1, CD3, CD4, CD8, CD25, and FoxP3 expression was used in conjunction with the DAPI to create binary masks of all cells expressing these biomarkers of interest respectively. Additionally, the binary mask of CD4+ or CD8+ was further combined with the CD3 mask to limit the analysis to T cells. The total area of CD4 or CD8 cells, measured in pixels, was divided by the total area of the CD3+ cells, measured in pixels, to determine the percent of T cells that were CD4+ or CD8+, respectively. In a similar manner, the binary masks for CD25 and FoxP3 were combined to identify the T cells that were double positive for both biomarkers in conjunction with CD3. The total area of the CD25+FoxP3+ cells was divided by the total area of the CD3 positive cells to determine the percent of CD25+FoxP3+ cells in the sample. The PD-1/PD-L1 interaction score was calculated by measuring the total area of PD-1 positive cells within the proximity of PD-L1 positive cells. This area was then divided by the total area of all nucleated cells in the image and multiplied by a factor of 10,000.
Gene expression analysis
RNA was extracted from banked FFPE slides from 29 patients with testicular germ cell tumors using the RNeasy FFPE Kit (Quiagen) after deparaffinization with Xylene. RNA concentration was assessed with a Nanodrop spectrophotometer and RNA fragmentation was determined by 2200 TapeStation (Agilent). 100 ng of RNA >300 nt from 24 patient samples was used for input into nanoString nCounter hybridization and expression analysis was performed using the nanoString pan-cancer immunology panel.20
TCGA data set and gene-expression data analyses
Volcano plots of all measured genes, CT antigen analyses comparing seminomas and non-seminomas, pathway scores, and immune cell type signatures were produced using the nSolver software 3.0 (nanoString). For selected analyses, the Human Pan Cancer Immunology Advanced Module (nanoString) was used. Algorithms for the nSolver advanced immune profiling analyses were described by Chen et al.21 and immune gene signatures used by nSolver software were described by Bindea et al.22 Recurrence free survival data were obtained after processing Z-score transformation of gene-expression values. TCGA data from 86 selected testicular cancer patients with follow-up duration comparable to internal cohort were accessed through the cBioportal.23 Survival curves were generated for patient groups that were defined by the summative gene signature Z-scores from defined signatures. The same Z-score threshold was used for internal cohort and TCGA data set survival analyses.
Statistical analyses
PFS was calculated from time of chemotherapy start to progression by RECIST 1.1 criteria24 and was compared between groups using the logrank test. Recurrence free survival was calculated as time from initial diagnosis to first relapse. Overall survival was calculated from first diagnosis to date of last follow up or death. All patients were censored for OS and/or PFS at last follow-up. For certain survival analyses, the significance of the predicting factor was tested in a cox proportional hazards model after adjusting for stage and histology (seminoma versus non-seminoma). Statistical analyses were performed using GraphPad Prism (GraphPad software). A p value of < 0.05 was considered significant for all studies.
Results
Seminoma histology is associated with decreased Treg cell infiltration and higher PD-1/PD-L1 interaction
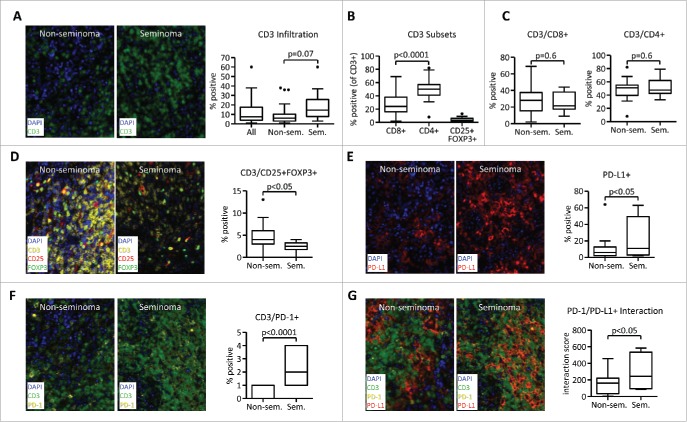
Testicular germ cell tumors are known to be infiltrated with T cells and other immune cells.14 To further investigate the immune infiltration in the two most prevalent histological subgroups, seminomas and non-seminomas, we performed Multiplexed Fluorescence Immunohistochemistry (FIHC) that was combined with Automated Quantitative Analysis (AQUA®) Technology. A CD3 T-cell infiltration was evident in most tumor samples and ranged from 1% to 60% of nuclear cells. We observed a trend toward higher CD3 T-cell infiltration in seminomas compared with non-seminomas (Fig. 1A). Among infiltrating T-cells a higher infiltration with CD4+ T cells compared with CD8+ T-cells was observed in all patients. Furthermore, a median of 3% infiltration with CD3+CD25+FOXP3+ regulatory T (Treg) cells was observed (ranged from 0% to 13%, measured as CD25+FOXP3+ from CD3+ nuclear cells) (Fig. 1B). No difference in CD8+ or CD4+ T-cell infiltration was observed in seminomas versus non-seminomas (Fig. 1C). However, seminoma histology was associated with the decreased presence of CD3+CD25+FOXP3+ Treg cells (Fig. 1D).
Figure 1.
Seminoma histology is associated with decreased Treg cell infiltration and higher PD-1/PD-L1 interaction. Samples from a cohort of 34 testicular germ cell tumor patients were analyzed using Quantitative Multiplexed Fluorescence Immunohistochemistry with Automated Quantitative Analysis (AQUA). Samples were stained with DAPI and antibodies against CD3 (A) or CD3 in combination with CD4, CD8, CD25, and FOXP3 (B–D). (E) Expression of PD-L1 in the tumor microenvironment. (F) Percentage of PD-1+ cells as a ratio from CD3+ DAPI+ cells. (G) PD-1/PD-L1 interaction scores. For data quantification, percentage of all DAPI+ nuclear cells (for CD3 and PDL1) or percentage from parent CD3 population (CD4+, CD8+, and CD25+FOXP3+) was used. PD-1/PD-L1 interaction is a numerical representation of the proportion of PD-1 positive cells within a pre-defined distance to PD-L1. The value is normalized by the total number of DAPI+ cells. All images were obtained using 20× zoom and were scaled digitally. In all images, 1 pixel = 0.5 µm.
PD-1/PD-L1 signaling can induce T-cell suppression,25 and high PD-L1 expression was recently shown to correlate with decreased progression free survival in TGCT.26 Interestingly, we observed expression of PD-L1 that was higher in seminomas compared with non-seminomas (Fig. 1E). Furthermore, the percentage of CD3+ T cells expressing PD-1 (Fig. 1F) and the PD-1/PD-L1 interaction was higher in seminomas (Fig. 1G). Thus, multiplexed FIHC enables detection of seminoma-specific T-cell infiltration that is hallmarked by the decreased presence of Treg cells but higher expression of PD-1 and PD-L1 and increased PD-1/PD-L1 interaction.
Gene expression profiling allows detection of histology-specific differences in expression of immune genes
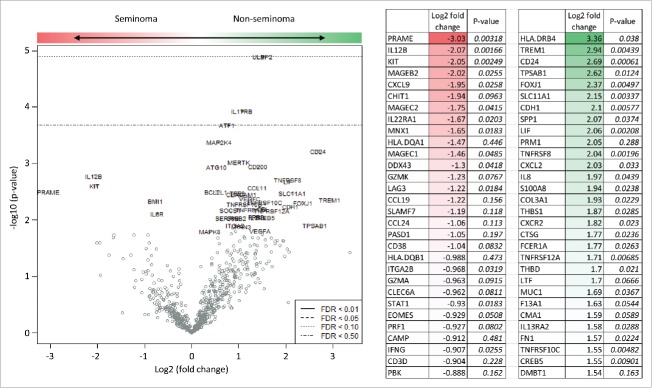
To further investigate the immune infiltration of seminoma and non-seminoma TGCT, we performed broad gene expression profiling with focus on genes associated with immune function (Fig. 2 and Table S3). Expression of a large number of genes was increased in seminomas, most prominently PRAME, IL12B and KIT. Other genes were however associated with non-seminoma histology, most prominently HLA-DRB4, TREM, and CD24. Thus, gene expression profiling allowed deeper exploration of differences between two major TGCT histologies. Furthermore, genes with high relevance to T-cell response such as PRAME or HLA.DRB4 were differentially expressed in seminomas versus non-seminomas.
Figure 2.
Detection of histology-specific differences in expression of immune genes in seminomatous and non-seminomatous testicular germ cell tumors. RNA from 24 patient samples was extracted and analyzed with nCounter station (nanoString) using pan-cancer immune panel detecting expression of 730 immune-associated genes. A total of 30 genes with the highest ratio of non-seminoma/seminoma expression values are shown.
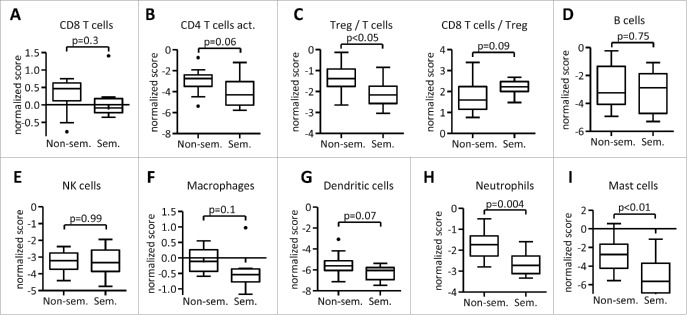
Immune gene signatures confirm immunohistochemistry and allow deeper analysis of immune infiltrates
Antibody-based assays such as immunohistochemistry (IHC) allow detection of a distinct number of markers and are thus limited in the exploration of immune populations. We therefore performed gene expression profiling and used gene expression signatures to describe immune infiltrates in TGCT. Consistent with data obtained with FIHC, no differences in CD8+ T-cell (Fig. 3A) or CD4+ T-cell (Fig. 3B) signatures were observed in seminomas versus non-seminomas. Also consistent with our FIHC data, seminomas showed lower signatures of Treg cells that were associated with an increased CD8/Treg ratio (Fig. 3C). We next analyzed whether expression of genes associated with T-cell functions is different in both histologic sub-types. Interestingly, while some genes associated with T-cell functions were associated with seminoma histology (such as IFNγ, CXCL9, and LAG3), others were preferentially expressed in non-seminomas (such as FAS or DPP4) (Fig. S1).
Figure 3.
Gene-expression based immune gene signatures allow characterization of the immune cell infiltrate of testicular germ cell tumors. Gene-expression data were analyzed by nSolver software (nanoString) and normalized to CD45 expression. Gene signatures for selected immune populations were described previously.22
We next asked whether immune populations other than T-cells are present in TGCT. While no difference in B cell or NK cell signatures was observed comparing seminomas and non-seminomas (Fig. 3D and E), seminomas showed a trend toward decreased macrophage (Fig. 3F) and dendritic cell (Fig. 3G) signatures. Furthermore, seminoma histology was associated with decreased neutrophil (Fig. 3H) and mast cell (Fig. 3I) signatures. Collectively, immune gene signatures allowed detection of differences between seminoma and non-seminoma TGCT that showed decreased Treg, neutrophil, and mast cell signature in seminomas. In addition, expression of several genes associated with T-cell function correlated with seminoma versus non-seminoma histology.
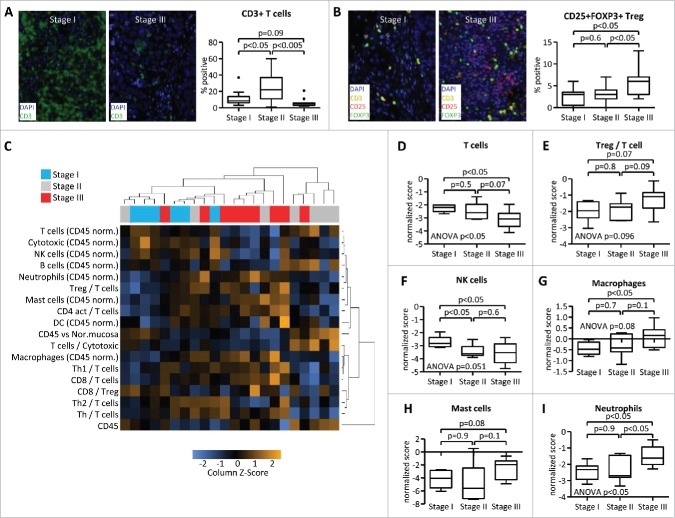
Advanced stage is associated with changes in immune cell infiltration
Current histologic assessments of TGCT that drive patient care are based on seminoma and non-seminoma categories and by clinical staging.27 We therefore asked whether stage of the disease may correlate with the immune infiltrate irrespective of the histologic subtype, and compared stage I (localized disease), stage II (lymph node metastases), and stage III (disseminated metastases) tumors. FIHC analyses revealed that CD3 T-cell infiltration was lowest in stage III patients. Interestingly, samples from stage II patients showed a higher CD3 T-cell infiltration than in stage I or stage III (Fig. 4A). By contrast, the percentage of CD3+CD25+FOXP3+ Treg cells increased with advanced stage (Fig. 4B).
Figure 4.
Advanced stage is associated with changes in immune cell infiltration. Samples from testicular germ cell tumors were analyzed by Quantitative Multiplexed Immunohistochemistry (A, B) and by gene-expression profiling (C–I). Stage I (localized disease), stage II (lymph node metastases), and stage III (disseminated metastases) tumors were analyzed for CD3 expression (A) and CD3/CD25/FOXP3 co-expression (B). Numbers represent percentages of all DAPI+ nuclear cells (A) or of all CD3+ cells (B). (C) Gene-expression data were subjected to unsupervised clustering of both patient samples and scores of selected immune signatures. (D–I) Immune gene signature scores of stage I/II/III tumors were calculated by nSolver software.
Next, gene expression profiling was performed to further describe the immune landscape in TGCT. A broad immune pathway profiling and unsupervised clustering of patient samples as well as analysis of defined immune signatures were performed and showed a general separation of stage I versus stage II and III patients (Fig. 4C). Consistent with FIHC data, T-cell gene signature was decreased in stage III patient samples and Treg signature increased with advanced stage (Fig. 4D and E). Furthermore, advanced stage was associated with decreased NK cell signature (Fig. 4F), increased macrophage signature (Fig. 4G) and increased signature scores of mast cells (Fig. 4H) and neutrophils (Fig. 4I). Interestingly, the patients with low neutrophil gene expression score showed a 100% recurrence free survival, whereas 63% of patients with a high neutrophil score were recurrence free 2 y after diagnosis (Fig. S2). These data demonstrate that the advanced stage is associated with significant changes in immune cell infiltration in testicular germ cell tumors, disfavoring immune populations associated with antitumor immunity such as T-cells and NK cells while enriching for pro-tumor cell populations such as Treg cells and potentially pro-tumor immune populations such as macrophages, mast cells, and neutrophils.28,29
Tumor immune infiltration and CT antigen expression allows risk stratification and correlates with recurrence free survival
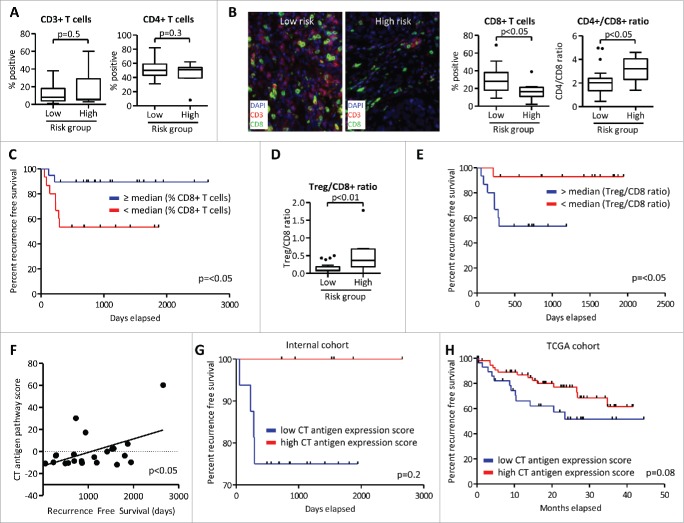
While histological classification or clinical staging allow clinicians to predict prognosis of TGCT patients, other markers to detect high-risk patients are needed. We therefore tested whether features of tumor immune infiltration allow prediction of disease recurrence and duration of recurrence free survival. While no differences in CD3+ T-cell and CD4+ T-cell infiltration were observed in patients without recurrence (low risk) and with disease recurrence (high risk) (Fig. 5A), high-risk patients showed lower percentage of infiltrating CD8+ T-cells, while CD4+ T-cell/CD8+ T-cell ratio was increased in this group (Fig. 5B). Moreover, patients with decreased CD8+ T-cell infiltration also showed decreased recurrence free survival compared with those with higher CD8+ infiltration (53% versus 89% with 2 y recurrence free survival) (Fig. 5C).
Figure 5.
Tumor immune infiltration and CT antigen expression allows risk stratification and correlates with recurrence free survival. Samples from patients without recurrence (low risk) and with disease recurrence (high risk) were analyzed by Quantitative Multiplexed Immunohistochemistry (A–E) and by gene-expression profiling (F–H). (A, B) Samples were stained with CD3, CD4+, and CD8+ antibodies. (C) Recurrence free survival was assessed for patients with high and low CD8+ T-cell infiltration. (D, E) Infiltration of CD3+CD25+FOXP3+ Treg cells relative to infiltration with CD3+CD8+ T cells was assessed and recurrence free survival was calculated as in (C). (F) Expression of CT antigens (defined in Table S4) was measured and correlated with recurrence free survival. p value was calculated using Pearson R correlation. (G) Recurrence free survival of 22 patients from the internal cohort was calculated for samples with a CT antigen expression cumulative Z-score threshold of 2. (H) TCGA data set of patients with seminomatous and non-seminomatous testicular germ cell tumors was analyzed for the expression of CT antigen genes as in (G).
Treg cells can suppress antitumor immunity30 and we observed higher Treg cell infiltration in advanced stage tumors. Accordingly, high-risk patients showed a higher Treg/CD8+ ratio (Fig. 5D). Furthermore, patients with increased Treg/CD8+ ratio demonstrated lower recurrence free survival, as only one patient recurred in the Treg/CD8+ low group, whereas 47% of patients in the Treg/CD8+ high group recurred in the second year after tumor biopsy (Fig. 5E). Importantly, Treg/CD8+ ratio was an independent and significant predictor of RFS (p = 0.041) in a cox proportional hazards model after adjusting for stage and type (seminoma versus non-seminoma). In a sub-group analysis of patients that recurred or were refractory to platinum-based therapies that were provided in addition to surgery, we observed an inversed correlation between recurrence free survival and markers of T-cell dysfunction (Fig. S3A–D).
T-cell infiltration allowed prognostic stratifications in our cohort. Several studies have however highlighted the prognostic relevance of CT antigen expression in testicular cancers31 and suggested that tumor-antigen expression is required for effective antitumor immune response.32 Moreover, CT antigen re-expression has been associated with progression of testicular germ cell tumors and with seminoma histology.33 We therefore measured expression of key CT antigen molecules (Table S4 and Fig. S4) and found a positive correlation between CT antigen signature scores and recurrence free survival (Fig. 5F). Furthermore, all patients from our cohort that had high expression of CT antigens (signature Z-score >2) stayed recurrence free. In contrast, patients with lower CT antigen expression showed decreased progression free survival (Fig. 5G). Next, we selected 86 patient samples from the testicular cancer TCGA data set with both seminoma and non-seminoma histologies. Employment of the CT antigen expression signature revealed trends similar to our cohort as patients with a Z-score >2 showed improved recurrence free survival as compared with patients with lower expression scores (p = 0.08; Fig. 5H). Thus, features associated with tumoral immune infiltration may help stratify patients and predict recurrence free survival in patients with TCGT.
Discussion
While TGCT are generally curable with cytotoxic chemotherapy, some patients ultimately relapse and die of their disease. Markers of poor prognosis, as well as alternative treatment strategies, are thus urgently needed for this subset of patients. In this study, we performed immune profiling of testicular germ cell tumors using RNA and protein analysis in a cohort of patient samples, the first time this type of analysis has been performed to our knowledge. From this effort, we observed that most tumors demonstrate a vigorous inflammatory infiltrate characterized by T-cell infiltration and markers of T-cell exhaustion (including PD-1 and PD-L1). This suggests that anti-PD-1/PD-L1 based approaches could have activity in this disease. Further, this may provide valuable prognostic markers.
Identifying surrogates of pre-existing immune recognition has provided promising markers of anti-PD-1/PD-L1 response in other tumor types.8,9 These markers include infiltrating CD8+ T cells, PD-1/PD-L1 expression, and RNA expression of T-cell activation molecules (IFNγ signatures, granzyme, and MHC expression). While frequently present in this cohort of testicular germ cell tumors, markers of distinct T-cell phenotypes tended to correlate with good prognosis, seminoma histology, and earlier stage. This suggests that immune therapy approaches could have the most value at relatively early time points (e.g., after failure of first-line chemotherapy rather than in a salvage, truly refractory setting. Several patients with metastatic disease refractory to chemotherapy also had a vigorous infiltrate, however, suggesting that anti-PD-1/PD-L1 could be considered even in this setting.
Our study also suggested distinctions in the immune landscape of seminomas and non-seminomas. While similar in terms of CD4+ and CD8+ T-cell infiltration, non-seminomas were characterized by increased infiltration of tumor promoting regulatory T cells. Other immune cell populations associated with impaired antitumor immunity, such as macrophages, neutrophils and mast cells also were associated with non-seminoma histology. By contrast, non-seminomas displayed lower levels of markers associated with T-cell responses, including PD-1 and PD-L1 expression as well as PD1/PDL1 interaction. One could hypothesize that this distinction in immune infiltration could partially explain the improved prognosis of seminomas compared with non-seminomas. Moreover, lower abundance of PD-1 and PD-L1 in the tumor microenvironment and increased presence of immunosuppressive Treg cells may indicate a lower likelihood of response of non-seminoma germ cell tumors to therapies targeting PD-1/PD-L1.
Currently, clinical staging, including serum tumor markers, and histology are the primary prognostic features that are used for patients treated with cytotoxic chemotherapy or those being observed following surgery. No molecular markers have been identified to date to enhance the prognostic value of these clinical and histologic features. In our study, tumors with enhanced CD8+ infiltration, and particularly those with higher CD8+/regulatory T-cell ratios, had seemingly better recurrence free survival, suggesting this metric might have use as a prognostic marker in early stage disease. Ultimately, prospective studies will be needed to confirm these findings.
This study has several weaknesses, including a relatively small sample size, a population including metastatic and chemotherapy-refractory tumors, and a single institution. Ultimately, however, it is the largest comprehensive immune profiling effort performed to our knowledge that synthesizes protein and RNA analyses to characterize these tumors.
In conclusion, testicular germ cell tumors are frequently characterized by T-cell infiltration, PD-1/PD-L1 interaction and specific RNA signatures of immune activation. These markers are most closely correlated with seminoma histology, early stage, and good prognosis, which may lend insight into the biology of this disease. However, immune infiltrate was also detected in a subset of patients with refractory non-seminoma germ cell tumors. These studies provide rationale to consider anti-PD-1/PD-L1 directed therapies in patients with testicular germ cell tumors in the chemotherapy refractory setting, and suggest further explorations of immune prognostic markers in this population.
Supplementary Material
Disclosure of potential conflicts of interest
DBJ is on advisory boards for Bristol Myers Squibb and Genoptix. JB, JYK, BD, and ND are employees of Genoptix, Inc.
Funding
DBJ is on advisory boards for Bristol Myers Squibb and Genoptix. JB, JYK, ND employees of Navigate, BD employee of Genoptix.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin 2016; 66:7-30; PMID:26742998; http://dx.doi.org/ 10.3322/caac.21332 [DOI] [PubMed] [Google Scholar]
- 2.Hashibe M, Abdelaziz S, Al-Temimi M, Fraser A, Boucher KM, Smith K, Lee YA, Rowe K, Rowley B, Daurelle M, et al.. Long-term health effects among testicular cancer survivors. J Cancer Surviv 2016; 10(6):1051-7; PMID:27169992; http://dx.doi.org/ 10.1007/s11764-016-0548-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kondagunta GV, Bacik J, Donadio A, Bajorin D, Marion S, Sheinfeld J, Bosl GJ, Motzer RJ. Combination of paclitaxel, ifosfamide, and cisplatin is an effective second-line therapy for patients with relapsed testicular germ cell tumors. J Clin Oncol 2005; 23:6549-55; PMID:16170162; http://dx.doi.org/ 10.1200/JCO.2005.19.638 [DOI] [PubMed] [Google Scholar]
- 4.Lorch A, Bascoul-Mollevi C, Kramar A, Einhorn L, Necchi A, Massard C, De Giorgi U, Fléchon A, Margolin K, Lotz JP, et al.. Conventional-dose versus high-dose chemotherapy as first salvage treatment in male patients with metastatic germ cell tumors: evidence from a large international database. J Clin Oncol 2011; 29:2178-84; PMID:21444870; http://dx.doi.org/ 10.1200/JCO.2010.32.6678 [DOI] [PubMed] [Google Scholar]
- 5.Motzer RJ, Geller NL, Tan CC, Herr H, Morse M, Fair W, Sheinfeld J, Sogani P, Russo P, Bosl GJ. Salvage chemotherapy for patients with germ cell tumors. The Memorial Sloan-Kettering Cancer Center experience (1979–1989). Cancer 1991; 67:1305-10; PMID:1703917; http://dx.doi.org/ [DOI] [PubMed] [Google Scholar]
- 6.Wolchok JD. PD-1 blockers. Cell 2015; 162:937; PMID:26317459; http://dx.doi.org/ 10.1016/j.cell.2015.07.045 [DOI] [PubMed] [Google Scholar]
- 7.Johnson DB, Estrada MV, Salgado R, Sanchez V, Doxie DB, Opalenik SR, Vilgelm AE, Feld E, Johnson AS, Greenplate AR, et al.. Melanoma-specific MHC-II expression represents a tumour-autonomous phenotype and predicts response to anti-PD-1/PD-L1 therapy. Nat Commun 2016; 7:10582; PMID:26822383; http://dx.doi.org/ 10.1038/ncomms10582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, et al.. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Eng J Med 2012; 366:2443-54; PMID:22658127; http://dx.doi.org/ 10.1056/NEJMoa1200690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tumeh PC, Harview CL, Yearley JH, Shintaku IP, Taylor EJ, Robert L, Chmielowski B, Spasic M, Henry G, Ciobanu V, et al.. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature 2014; 515:568-71; PMID:25428505; http://dx.doi.org/ 10.1038/nature13954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Herbst RS, Soria JC, Kowanetz M, Fine GD, Hamid O, Gordon MS, Sosman JA, McDermott DF, Powderly JD, Gettinger SN, et al.. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature 2014; 515:563-7; PMID:25428504; http://dx.doi.org/ 10.1038/nature14011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hugo W, Zaretsky JM, Sun L, Song C, Moreno BH, Hu-Lieskovan S, Berent-Maoz B, Pang J, Chmielowski B, Cherry G, et al.. Genomic and transcriptomic features of response to Anti-PD-1 therapy in metastatic melanoma. Cell 2016; 165:35-44; PMID:26997480; http://dx.doi.org/ 10.1016/j.cell.2016.02.065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shah S, Ward JE, Bao R, Hall CR, Brockstein BE, Luke JJ. Clinical response of a patient to Anti-PD-1 immunotherapy and the immune landscape of testicular germ cell tumors. Cancer Immunol Res 2016; 4(11):903-09; PMID:27638840; http://dx.doi.org/ 10.1158/2326-6066.CIR-16-0087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yakirevich E, Lefel O, Sova Y, Stein A, Cohen O, Izhak OB, Resnick MB. Activated status of tumour-infiltrating lymphocytes and apoptosis in testicular seminoma. J Pathol 2002; 196:67-75; PMID:11748644; http://dx.doi.org/ 10.1002/path.996 [DOI] [PubMed] [Google Scholar]
- 14.Hvarness T, Nielsen JE, Almstrup K, Skakkebaek NE, Rajpert-De Meyts E, Claesson MH. Phenotypic characterisation of immune cell infiltrates in testicular germ cell neoplasia. J Reprod Immunol 2013; 100:135-45; PMID:24290033; http://dx.doi.org/ 10.1016/j.jri.2013.10.005 [DOI] [PubMed] [Google Scholar]
- 15.Cheng X, Dai H, Wan N, Moore Y, Vankayalapati R, Dai Z. Interaction of programmed death-1 and programmed death-1 ligand-1 contributes to testicular immune privilege. Transplantation 2009; 87:1778-86; PMID:19543053; http://dx.doi.org/ 10.1097/TP.0b013e3181a75633 [DOI] [PubMed] [Google Scholar]
- 16.Scanlan MJ, Gure AO, Jungbluth AA, Old LJ, Chen YT. Cancer/testis antigens: an expanding family of targets for cancer immunotherapy. Immunol Rev 2002; 188:22-32; PMID:12445278; http://dx.doi.org/ 10.1034/j.1600-065X.2002.18803.x [DOI] [PubMed] [Google Scholar]
- 17.Yuasa T, Okamoto K, Kawakami T, Mishina M, Ogawa O, Okada Y. Expression patterns of cancer testis antigens in testicular germ cell tumors and adjacent testicular tissue. J Urol 2001; 165:1790-4; PMID:11342977; http://dx.doi.org/ 10.1016/S0022-5347(05)66415-4 [DOI] [PubMed] [Google Scholar]
- 18.Bode PK, Barghorn A, Fritzsche FR, Riener MO, Kristiansen G, Knuth A, Moch H. MAGEC2 is a sensitive and novel marker for seminoma: a tissue microarray analysis of 325 testicular germ cell tumors. Mod Pathol 2011; 24:829-35; PMID:21780320; http://dx.doi.org/ 10.1038/modpathol.2011.6 [DOI] [PubMed] [Google Scholar]
- 19.Camp RL, Dolled-Filhart M, King BL, Rimm DL. Quantitative analysis of breast cancer tissue microarrays shows that both high and normal levels of HER2 expression are associated with poor outcome. Cancer Res 2003; 63:1445-8; PMID:12670887 [PubMed] [Google Scholar]
- 20.Geiss GK, Bumgarner RE, Birditt B, Dahl T, Dowidar N, Dunaway DL, Fell HP, Ferree S, George RD, Grogan T, et al.. Direct multiplexed measurement of gene expression with color-coded probe pairs. Nat Biotechnol 2008; 26:317-25; PMID:18278033; http://dx.doi.org/ 10.1038/nbt1385 [DOI] [PubMed] [Google Scholar]
- 21.Chen PL, Roh W, Reuben A, Cooper ZA, Spencer CN, Prieto PA, Miller JP, Bassett RL, Gopalakrishnan V, Wani K, et al.. Analysis of immune signatures in longitudinal tumor samples yields insight into biomarkers of response and mechanisms of resistance to immune checkpoint blockade. Cancer Discov 2016; 6:827-37; PMID:27301722; http://dx.doi.org/ 10.1158/2159-8290.CD-15-1545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bindea G, Mlecnik B, Tosolini M, Kirilovsky A, Waldner M, Obenauf AC, Angell H, Fredriksen T, Lafontaine L, Berger A, et al.. Spatiotemporal dynamics of intratumoral immune cells reveal the immune landscape in human cancer. Immunity 2013; 39:782-95; PMID:24138885; http://dx.doi.org/ 10.1016/j.immuni.2013.10.003 [DOI] [PubMed] [Google Scholar]
- 23.Cerami E, Gao J, Dogrusoz U, Gross BE, Sumer SO, Aksoy BA, Jacobsen A, Byrne CJ, Heuer ML, Larsson E, et al.. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov 2012; 2:401-4; PMID:22588877; http://dx.doi.org/ 10.1158/2159-8290.CD-12-0095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, et al.. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009; 45:228-47; PMID:19097774; http://dx.doi.org/ 10.1016/j.ejca.2008.10.026 [DOI] [PubMed] [Google Scholar]
- 25.Chen L, Han X. Anti-PD-1/PD-L1 therapy of human cancer: past, present, and future. J Clin Invest 2015; 125:3384-91; PMID:26325035; http://dx.doi.org/ 10.1172/JCI80011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cierna Z, Mego M, Miskovska V, Machalekova K, Chovanec M, Svetlovska D, Hainova K, Rejlekova K, Macak D, Spanik S, et al.. Prognostic value of programmed-death-1 receptor (PD-1) and its ligand 1 (PD-L1) in testicular germ cell tumors. Ann Oncol 2016; 27:300-5; http://dx.doi.org/ 10.1093/annonc/mdv574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hanna NH, Einhorn LH. Testicular cancer – discoveries and updates. N Eng J Med 2014; 371:2005-16; PMID:25409373; http://dx.doi.org/ 10.1056/NEJMra1407550 [DOI] [PubMed] [Google Scholar]
- 28.Maciel TT, Moura IC, Hermine O. The role of mast cells in cancers. F1000Prime Rep 2015; 7:09; PMID:25705392; http://dx.doi.org/ 10.12703/P7-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jensen HK, Donskov F, Marcussen N, Nordsmark M, Lundbeck F, von der Maase H. Presence of intratumoral neutrophils is an independent prognostic factor in localized renal cell carcinoma. J Clin Oncol 2009; 27:4709-17; PMID:19720929; http://dx.doi.org/ 10.1200/JCO.2008.18.9498 [DOI] [PubMed] [Google Scholar]
- 30.Vignali DA, Collison LW, Workman CJ. How regulatory T cells work. Nat Rev Immunol 2008; 8:523-32; PMID:18566595; http://dx.doi.org/ 10.1038/nri2343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Neuhaus N. PRAME as diagnostic marker and as regulator for cell fate decisions in germ cell cancers. Br J Cancer 2016; 115:401-2; PMID:27441497; http://dx.doi.org/ 10.1038/bjc.2016.217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gjerstorff MF, Andersen MH, Ditzel HJ. Oncogenic cancer/testis antigens: prime candidates for immunotherapy. Oncotarget 2015; 6:15772-87; PMID:26158218; http://dx.doi.org/ 10.18632/oncotarget.4694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bode PK, Thielken A, Brandt S, Barghorn A, Lohe B, Knuth A, Moch H. Cancer testis antigen expression in testicular germ cell tumorigenesis. Mod Pathol 2014; 27:899-905; PMID:24232866; http://dx.doi.org/ 10.1038/modpathol.2013.183 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.