Abstract
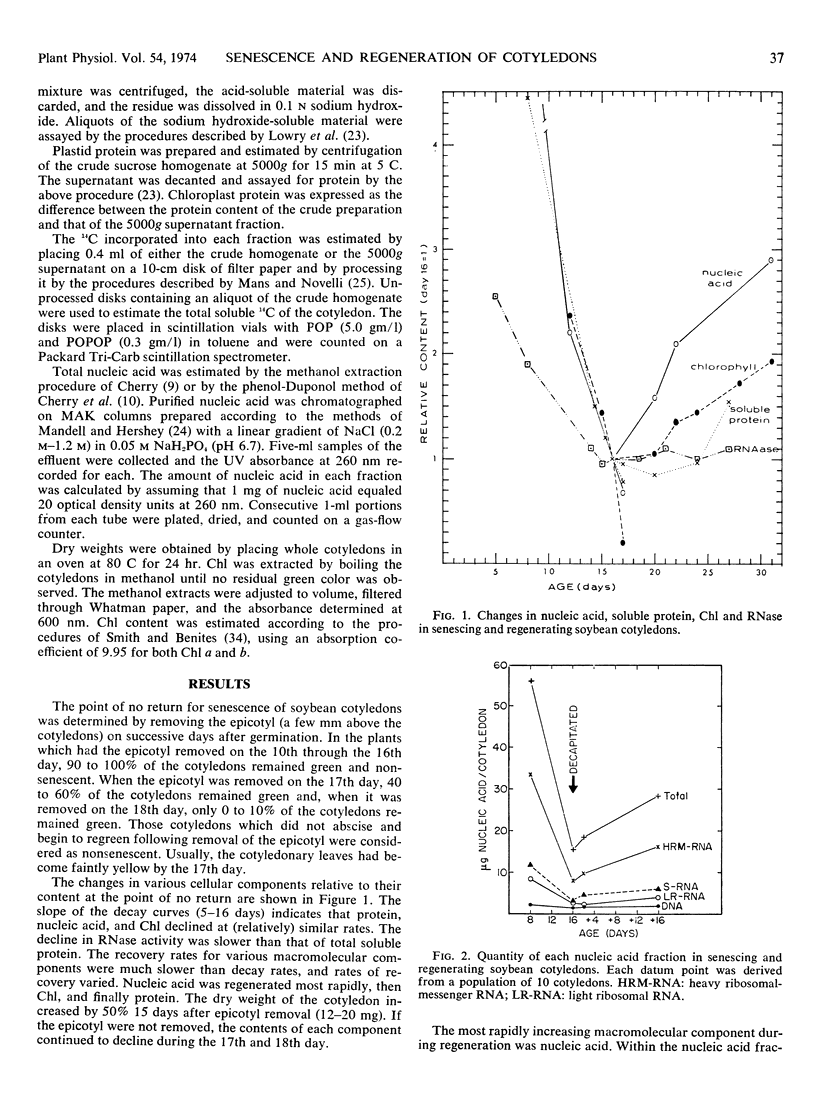
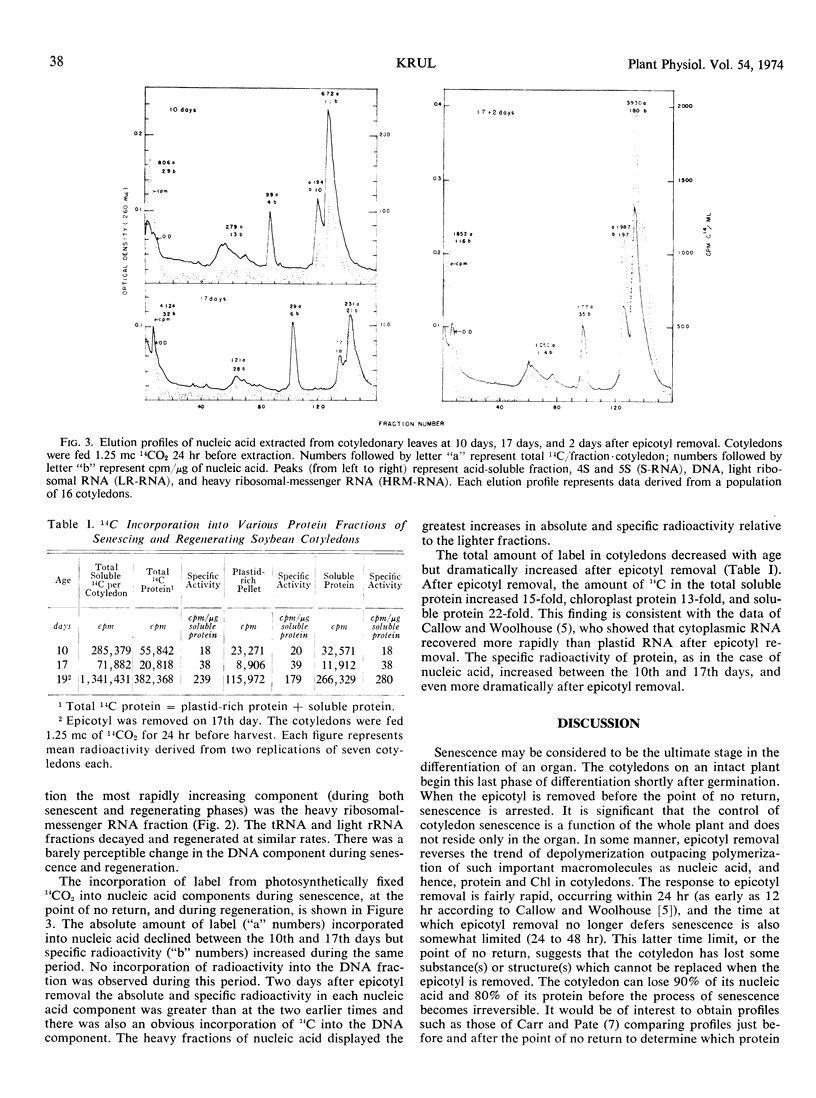
An alternative to the leaf disk system for studies of the metabolism of senescence is described. The progress of senescence of soybean (Glycine max L.) cotyledons is arrested when the epicotyl is removed. Epicotyl removal at 16 or 17 days reversed the decline in nucleic acid, protein, and chlorophyll content in the cotyledon. Epicotyl removal at 18 days did not reverse the decline in the above components, and therefore the progress of cotyledon has passed the point of no return. Cotyledons lost 90% of their nucleic acid and 80% of their protein before senescence became irreversible. The rate of recovery in various macromolecular components after epicotyl removal did not occur in an equal manner. Nucleic acid was regenerated at a faster rate than chlorophyll, which was regenerated at a faster rate than soluble protein. The heavy nucleic acid components (ribosomal and heavy ribosomal messenger fractions) regenerated at greater rates than did the soluble RNA or DNA. No label from 14CO2 was incorporated into DNA of the cotyledons when the epicotyl was present but label was incorporated into DNA after epicotyl removal.
The parallels between the mechanisms of cotyledon senescence and apical dominance are discussed.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carpenter W. J., Cherry J. H. Effects of benzyladenine on accumulation of 32P into nucleic acids of peanut cotyledons. Biochim Biophys Acta. 1966 Mar 21;114(3):640–642. doi: 10.1016/0005-2787(66)90115-8. [DOI] [PubMed] [Google Scholar]
- Carr D. J., Pate J. S. Ageing in the whole plant. Symp Soc Exp Biol. 1967;21:559–599. [PubMed] [Google Scholar]
- Cherry J. H., Chroboczek H., Carpenter W. J., Richmond A. Nucleic Acid Metabolism in Peanut Cotyledons. Plant Physiol. 1965 May;40(3):582–587. doi: 10.1104/pp.40.3.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherry J. H. Nucleic Acid Determination in Storage Tissues of Higher Plants. Plant Physiol. 1962 Sep;37(5):670–678. doi: 10.1104/pp.37.5.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin T. Y., Poulson R., Beevers L. The Influence of Axis Removal on Protein Metabolism in Cotyledons of Pisum sativum L. Plant Physiol. 1972 Apr;49(4):482–489. doi: 10.1104/pp.49.4.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox J. E. Incorporation of a kinin, N, 6-benzyladenine into soluble RNA. Plant Physiol. 1966 Jan;41(1):75–82. doi: 10.1104/pp.41.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KESSLER B., ENGELBERG N. Ribonucleic acid and ribonuclease activity in developing leaves. Biochim Biophys Acta. 1962 Jan 22;55:70–82. doi: 10.1016/0006-3002(62)90932-0. [DOI] [PubMed] [Google Scholar]
- Kende H. The cytokinins. Int Rev Cytol. 1971;31:301–338. doi: 10.1016/s0074-7696(08)60061-3. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Leopold A. C. Senescence in Plant Development: The death of plants or plant parts may be of positive ecological or physiological value. Science. 1961 Dec 1;134(3492):1727–1732. doi: 10.1126/science.134.3492.1727. [DOI] [PubMed] [Google Scholar]
- MANDELL J. D., HERSHEY A. D. A fractionating column for analysis of nucleic acids. Anal Biochem. 1960 Jun;1:66–77. doi: 10.1016/0003-2697(60)90020-8. [DOI] [PubMed] [Google Scholar]
- MANS R. J., NOVELLI G. D. A convenient, rapid and sensitive method for measuring the incorporation of radioactive amino acids into protein. Biochem Biophys Res Commun. 1960 Nov;3:540–543. doi: 10.1016/0006-291x(60)90171-6. [DOI] [PubMed] [Google Scholar]
- Osborne D. J. Effect of Kinetin on Protein & Nucleic Acid Metabolism in Xanthium Leaves During Senescence. Plant Physiol. 1962 Sep;37(5):595–602. doi: 10.1104/pp.37.5.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SETH A., WAREING P. F. INTERACTION BETWEEN AUXINS, GIBBERELLINS AND KININS IN HORMONE-DIRECTED TRANSPORT. Life Sci. 1964 Dec;3:1483–1486. doi: 10.1016/0024-3205(64)90092-x. [DOI] [PubMed] [Google Scholar]
- SRIVASTAVA B. I. EFFECT OF KINETIN ON THE ECTEOLA CELLULOSE ELUTION PROFILE AND OTHER PROPERTIES OF RNA FROM THE EXCISED FIRST SEEDLING LEAVES OF BARLEY. Arch Biochem Biophys. 1965 Apr;110:97–103. doi: 10.1016/0003-9861(65)90159-1. [DOI] [PubMed] [Google Scholar]
- WILSON C. M. Chromatographic separation of ribonucleases in corn. Biochim Biophys Acta. 1963 Feb 26;68:177–184. doi: 10.1016/0006-3002(63)90133-1. [DOI] [PubMed] [Google Scholar]


